Astaxanthin as a King of Ketocarotenoids: Structure, Synthesis, Accumulation, Bioavailability and Antioxidant Properties
Abstract
1. Introduction
2. Cultivation and Accumulation of Astaxanthin in Haematococcus lacustris
2.1. Biochemical Pathway
2.2. Wastewater as Growth Medium for Haematococcus
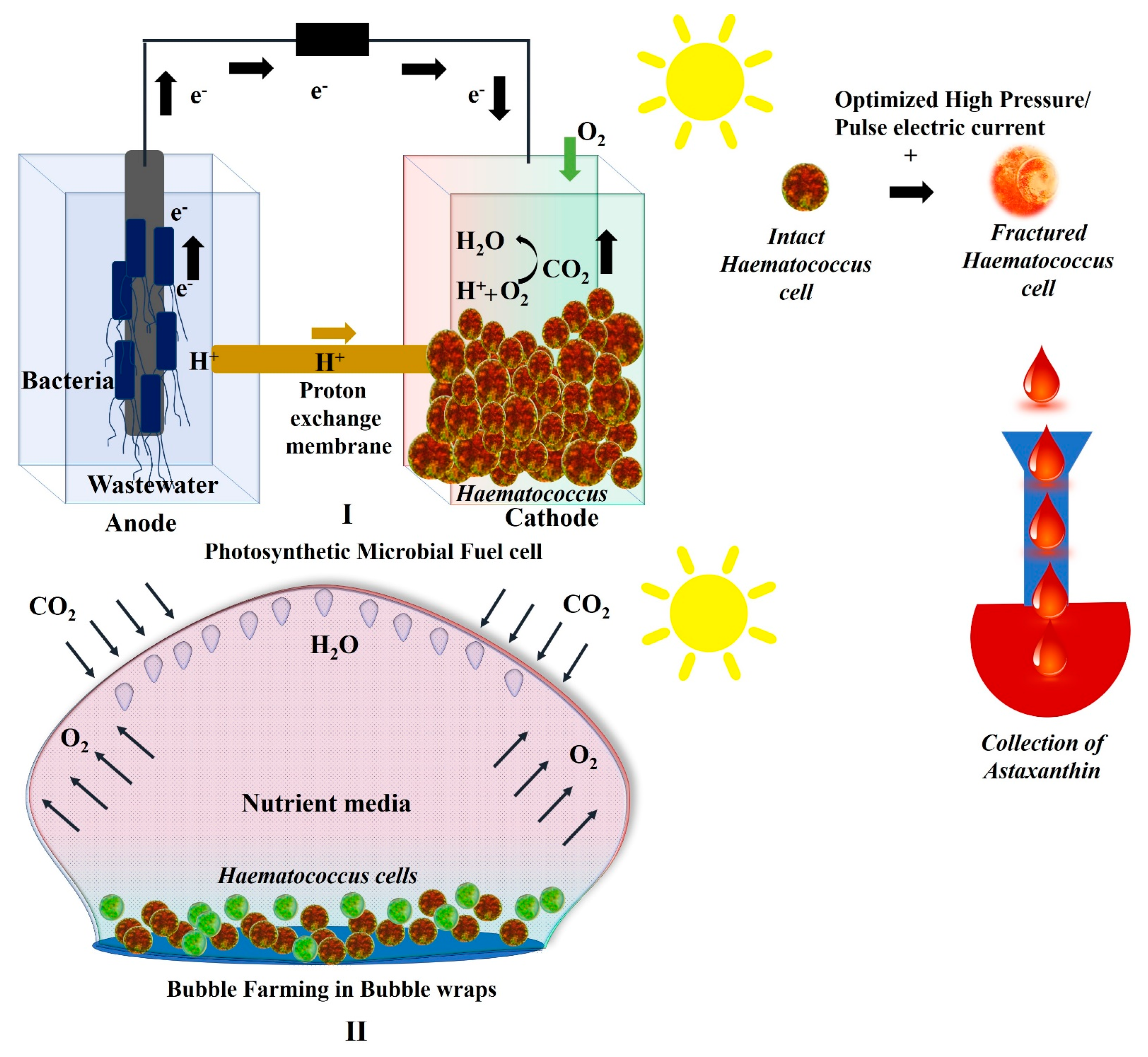
2.3. Growth of Haematococcus in Photosynthetic Microbial Fuel Cells
2.4. Growth of Haematococcus in Plastic Bubble Wraps
2.5. Stress Factors to Induce Astaxanthin Production in Haematococcus
3. Astaxanthin Harvesting and Its Bioavailability
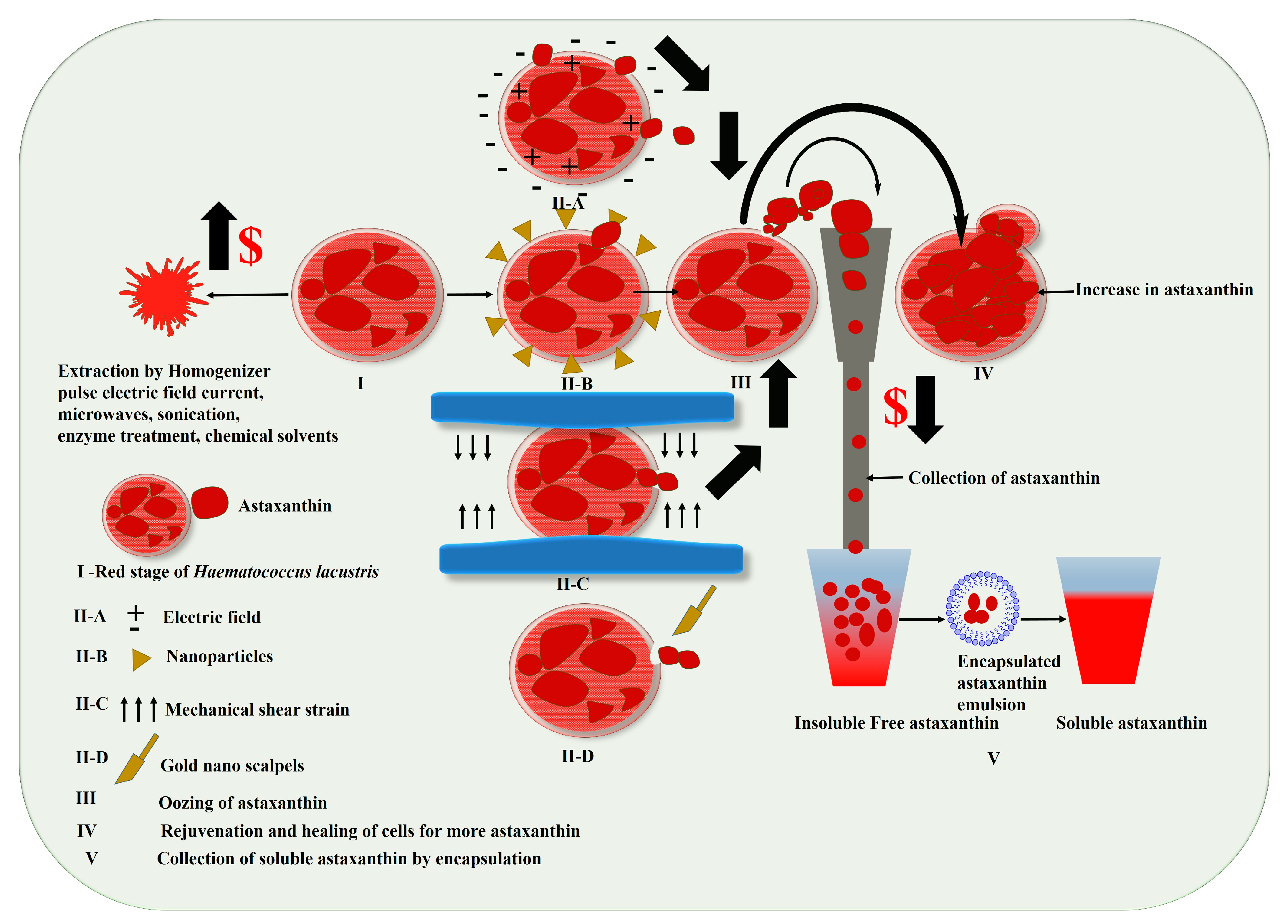
3.1. Harvesting of Haematococcus for Astaxanthin
3.2. Astaxanthin Bioavailability
4. Anti-Oxidative Properties of Astaxanthin versus Other Carotenoids
4.1. Immune System and Inflammation
4.2. Treatment of Skin Conditions and Protection of Eyes Health
5. Safety Measures to Fight against Infectious Diseases Using Astaxanthin
6. Future Perspectives of Astaxanthin as a Novel Drug
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schoefs, B.T. Chlorophyll and carotenoid analysis in food products. Properties of the pigments and methods of analysis. Trends Food Sci. Technol. 2002, 13, 361–371. [Google Scholar] [CrossRef]
- Takaichi, S. Carotenoids in phototrophic microalgae: Distributions and biosynthesis. In Pigments from Microalgae Handbook; Springer: Berlin/Heidelberg, Germany, 2020; pp. 19–41. [Google Scholar]
- Pagano, M.; Corrêa, E.; Duarte, N.; Yelikbayev, B. Pharmacological Potential of Pigments. In Biomolecules from Natural Sources: Advances and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2022; pp. 101–112. [Google Scholar]
- Britton, G. Structure and properties of carotenoids in relation to function. FASEB J. 1995, 9, 1551–1558. [Google Scholar] [CrossRef] [PubMed]
- Christaki, E.; Bonos, E.; Giannenas, I.; Florou-Paneri, P. Functional properties of carotenoids originating from algae. J. Sci. Food Agric. 2013, 93, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, Y.; Schoefs, B. Secondary ketocarotenoid astaxanthin biosynthesis in algae: A multifunctional response to stress. Photosynth. Res. 2010, 106, 155–177. [Google Scholar] [CrossRef]
- Maoka, T. Carotenoids as natural functional pigments. J. Nat. Med. 2020, 74, 1–16. [Google Scholar] [CrossRef]
- Haugan, J.A. Algal carotenoids 54. Carotenoids of brown algae (Phaeophyceae). Biochem. Syst. Ecol. 1994, 22, 31–41. [Google Scholar] [CrossRef]
- Goodwin, T.W. Carotenoids and reproduction. Biol. Rev. 1950, 25, 391–413. [Google Scholar] [CrossRef]
- Riaz, M.; Zia-Ul-Haq, M.; Dou, D. Chemistry of carotenoids. In Carotenoids: Structure and Function in the Human Body; Springer: Berlin/Heidelberg, Germany, 2021; pp. 43–76. [Google Scholar]
- Mordi, R.C.; Ademosun, O.T.; Ajanaku, C.O.; Olanrewaju, I.O.; Walton, J.C. Free radical mediated oxidative degradation of carotenes and xanthophylls. Molecules 2020, 25, 1038. [Google Scholar] [CrossRef]
- Miller, N.J.; Sampson, J.; Candeias, L.P.; Bramley, P.M.; Rice-Evans, C.A. Antioxidant activities of carotenes and xanthophylls. FEBS Lett. 1996, 384, 240–242. [Google Scholar] [CrossRef]
- Ahirwar, A.; Kesharwani, K.; Deka, R.; Muthukumar, S.; Khan, M.J.; Rai, A.; Vinayak, V.; Varjani, S.; Joshi, K.B.; Morjaria, S. Microalgal drugs: A promising therapeutic reserve for the future. J. Biotechnol. 2022, 349, 32–46. [Google Scholar] [CrossRef]
- Régnier, P.; Bastias, J.; Rodriguez-Ruiz, V.; Caballero-Casero, N.; Caballo, C.; Sicilia, D.; Fuentes, A.; Maire, M.; Crepin, M.; Letourneur, D. Astaxanthin from Haematococcus pluvialis prevents oxidative stress on human endothelial cells without toxicity. Mar. Drugs 2015, 13, 2857–2874. [Google Scholar] [CrossRef] [PubMed]
- Scarsini, M.; Marchand, J.; Schoefs, B. Carotenoid overproduction in microalgae: Biochemical and genetic engineering. In Pigments from Microalgae Handbook; Jacob-Lopes, E., Queiroz, M.I., Zepka, L.Q., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 81–126. [Google Scholar] [CrossRef]
- Stachowiak, B.; Szulc, P. Astaxanthin for the food industry. Molecules 2021, 26, 2666. [Google Scholar] [CrossRef]
- Ahirwar, A.; Meignen, G.; Khan, M.J.; Sirotiya, V.; Scarsini, M.; Roux, S.; Marchand, J.; Schoefs, B.; Vinayak, V. Light modulates transcriptomic dynamics upregulating astaxanthin accumulation in Haematococcus: A review. Bioresour. Technol. 2021, 340, 125707. [Google Scholar] [CrossRef] [PubMed]
- Vinayak, V.; Manoylov, K.M.; Gateau, H.; Blanckaert, V.; Herault, J.; Pencreac’h, G.; Marchand, J.; Gordon, R.; Schoefs, B. Diatom milking: A review and new approaches. Mar. Drugs 2015, 13, 2629–2665. [Google Scholar] [CrossRef]
- Li, J.; Zhu, D.; Niu, J.; Shen, S.; Wang, G. An economic assessment of astaxanthin production by large scale cultivation of Haematococcus pluvialis. Biotechnol. Adv. 2011, 29, 568–574. [Google Scholar] [CrossRef]
- Olaizola, M. Commercial production of astaxanthin from Haematococcus pluvialis using 25,000-liter outdoor photobioreactors. J. Appl. Phycol. 2000, 12, 499–506. [Google Scholar] [CrossRef]
- Fei, Z.; Fan, F.; Liao, J.; Wan, M.; Bai, W.; Wang, W.; He, M.; Li, Y. Improving astaxanthin production of Haematococcus pluvialis on the outdoor large scale cultivation by optimizing the disinfection strategy of photobioreactor. Algal Res. 2022, 64, 102708. [Google Scholar] [CrossRef]
- Chekanov, K. Diversity and Distribution of Carotenogenic Algae in Europe: A Review. Mar. Drugs 2023, 21, 108. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, R.; Sörensen, N.A. Über astaxanthin und ovoverdin. Ber. Der Dtsch. Chem. Ges. A B Ser. 1938, 71, 1879–1888. [Google Scholar] [CrossRef]
- Seybold, A.; Goodwin, T. Occurrence of astaxanthin in the flower petals of Adonis annua L. Nature 1959, 184, 1714–1715. [Google Scholar] [CrossRef]
- Egger, K.; Kleinig, H. Die ketocarotinoide in Adonis annua L.—II.: Zur struktur der ester. Phytochemistry 1967, 6, 437–440. [Google Scholar] [CrossRef]
- Fucikova, K.; Lewis, L.A. Intersection of Chlorella, Muriella and Bracteacoccus: Resurrecting the genus Chromochloris kol et chodat (Chlorophyceae, Chlorophyta). Fottea 2012, 12, 83–93. [Google Scholar] [CrossRef]
- Kopecky, J.; Schoefs, B.; Loest, K.; Stys, D.; Pulz, O. Microalgae as a source for secondary carotenoid production: A screening study. Algol. Stud. 2000, 98, 153–168. [Google Scholar] [CrossRef]
- Chekanov, K.; Litvinov, D.; Fedorenko, T.; Chivkunova, O.; Lobakova, E. Combined production of astaxanthin and β-carotene in a new strain of the microalga Bracteacoccus aggregatus BM5/15 (IPPAS C-2045) cultivated in photobioreactor. Biology 2021, 10, 643. [Google Scholar] [CrossRef]
- Ali, H.E.A.; Vorisek, F.; Dowd, S.E.; Kesner, S.; Song, Y.; Qian, D.; Crocker, M. Formation of Lutein, β-Carotene and Astaxanthin in a Coelastrella sp. Isolate. Molecules 2022, 27, 6950. [Google Scholar] [CrossRef]
- Viala, G. Recherches sur le Chlamydomonas nivalis Wille dans les Pyrénées. Bull. De La Société Bot. De Fr. 1967, 114, 75–79. [Google Scholar] [CrossRef]
- Yuan, J.P.; Peng, J.; Yin, K.; Wang, J.H. Potential health-promoting effects of astaxanthin: A high-value carotenoid mostly from microalgae. Mol. Nutr. Food Res. 2011, 55, 150–165. [Google Scholar] [CrossRef]
- Tsubokura, A.; Yoneda, H.; Mizuta, H. Paracoccus carotinifaciens sp. nov., a new aerobic gram-negative astaxanthin-producing bacterium. Int. J. Syst. Evol. Microbiol. 1999, 49, 277–282. [Google Scholar] [CrossRef]
- Lemoine, Y.; Rmiki, N.-E.; Créach, A.; Rachidi, J.B.S. Cytoplasmic accumulation of astaxanthin by the green alga Haematococcus pluvialis (Flotow). In Plant Cell Compartments; Schoefs, B., Ed.; Research Signpost: Kerala, India, 2008; pp. 251–284. [Google Scholar]
- WongCarter, K.; Llansola-Portoles, M.J.; Kodis, G.; Gust, D.; Moore, A.L.; Moore, T.A.; Croce, R.; van Grondelle, R.; van Amerongen, H.; van Stokkum, I. Light harvesting, photoregulation, and photoprotection in selected artificial photosynthetic systems. In Light Harvesting in Photosynthesis; CRC Press: Boca Raton, FL, USA, 2018; pp. 485–510. [Google Scholar]
- Qiao, X.; Yang, L.; Hu, X.; Cao, Y.; Li, Z.; Xu, J.; Xue, C. Characterization and evaluation of inclusion complexes between astaxanthin esters with different molecular structures and hydroxypropyl-β-cyclodextrin. Food Hydrocoll. 2021, 110, 106208. [Google Scholar] [CrossRef]
- Jafari, Z.; Bigham, A.; Sadeghi, S.; Dehdashti, S.M.; Rabiee, N.; Abedivash, A.; Bagherzadeh, M.; Nasseri, B.; Karimi-Maleh, H.; Sharifi, E. Nanotechnology-Abetted astaxanthin formulations in multimodel therapeutic and biomedical applications. J. Med. Chem. 2021, 65, 2–36. [Google Scholar] [CrossRef]
- Yang, L.; Qiao, X.; Gu, J.; Li, X.; Cao, Y.; Xu, J.; Xue, C. Influence of molecular structure of astaxanthin esters on their stability and bioavailability. Food Chem. 2021, 343, 128497. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Lee, P.-C.; Wu, Y.-L.; Liu, L.-Y. In vivo effects of free form astaxanthin powder on anti-oxidation and lipid metabolism with high-cholesterol diet. PLoS ONE 2015, 10, e0134733. [Google Scholar] [CrossRef] [PubMed]
- Sapone, V.; Iannone, A.; Alivernini, A.; Cicci, A.; Jessop, P.G.; Bravi, M. An innovative simplified one-pot process for Astaxanthin purification from Paracoccus carotinifaciens. Sep. Purif. Technol. 2023, 308, 122843. [Google Scholar] [CrossRef]
- Panis, G.; Carreon, J.R. Commercial astaxanthin production derived by green alga Haematococcus pluvialis: A microalgae process model and a techno-economic assessment all through production line. Algal Res. 2016, 18, 175–190. [Google Scholar] [CrossRef]
- Liu, X.; Luo, Q.; Cao, Y.; Goulette, T.; Liu, X.; Xiao, H. Mechanism of different stereoisomeric astaxanthin in resistance to oxidative stress in Caenorhabditis elegans. J. Food Sci. 2016, 81, H2280–H2287. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Yan, J.; Dong, H.; Gao, K.; Yu, K.; He, C.; Mao, X. Astaxanthin with different configurations: Sources, activity, post-modification and application in foods. Curr. Opin. Food Sci. 2022, 49, 100955. [Google Scholar] [CrossRef]
- Gateau, H.; Solymosi, K.; Marchand, J.; Schoefs, B. Carotenoids of microalgae used in food industry and medicine. Mini Rev. Med. Chem. 2017, 17, 1140–1172. [Google Scholar] [CrossRef] [PubMed]
- Capelli, B.; Talbott, S.; Ding, L. Astaxanthin sources: Suitability for human health and nutrition. Funct. Foods Health Dis. 2019, 9, 430–445. [Google Scholar] [CrossRef]
- Capelli, B.; Bagchi, D.; Cysewski, G.R. Synthetic astaxanthin is significantly inferior to algal-based astaxanthin as an antioxidant and may not be suitable as a human nutraceutical supplement. Nutrafoods 2013, 12, 145–152. [Google Scholar] [CrossRef]
- Onorato, C.; Rösch, C. Comparative life cycle assessment of astaxanthin production with Haematococcus pluvialis in different photobioreactor technologies. Algal Res. 2020, 50, 102005. [Google Scholar] [CrossRef]
- Khoo, K.S.; Lee, S.Y.; Ooi, C.W.; Fu, X.; Miao, X.; Ling, T.C.; Show, P.L. Recent advances in biorefinery of astaxanthin from Haematococcus pluvialis. Bioresour. Technol. 2019, 288, 121606. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yue, C.; Geng, S.; Ning, D.; Ma, T.; Yu, X. Role of media composition in biomass and astaxanthin production of Haematococcus pluvialis under two-stage cultivation. Bioprocess Biosyst. Eng. 2019, 42, 593–602. [Google Scholar] [CrossRef]
- Schoefs, B.t.; Rmiki, N.-E.; Rachadi, J.; Lemoine, Y. Astaxanthin accumulation in Haematococcus requires a cytochrome P450 hydroxylase and an active synthesis of fatty acids. Febs Lett. 2001, 500, 125–128. [Google Scholar] [CrossRef]
- Xu, Y.; Ibrahim, I.M.; Wosu, C.I.; Ben-Amotz, A.; Harvey, P.J. Potential of new isolates of Dunaliella salina for natural β-carotene production. Biology 2018, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K. [34] Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1987; Volume 148, pp. 350–382. [Google Scholar]
- Luo, Q.; Bian, C.; Tao, M.; Huang, Y.; Zheng, Y.; Lv, Y.; Li, J.; Wang, C.; You, X.; Jia, B. Genome and transcriptome sequencing of the astaxanthin-producing green microalga, Haematococcus pluvialis. Genome Biol. Evol. 2019, 11, 166–173. [Google Scholar] [CrossRef]
- Schoefs, B. The protochlorophyllide–chlorophyllide cycle. Photosynth. Res. 2001, 70, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Yu, C.; Zhong, D.-b.; Zhao, Y.; Yu, X. Melatonin and calcium act synergistically to enhance the coproduction of astaxanthin and lipids in Haematococcus pluvialis under nitrogen deficiency and high light conditions. Bioresour. Technol. 2020, 305, 123069. [Google Scholar] [CrossRef]
- Li, K.; Cheng, J.; Ye, Q.; He, Y.; Zhou, J.; Cen, K. In vivo kinetics of lipids and astaxanthin evolution in Haematococcus pluvialis mutant under 15% CO2 using Raman microspectroscopy. Bioresour. Technol. 2017, 244, 1439–1444. [Google Scholar] [CrossRef] [PubMed]
- Del Campo, J.A.; García-González, M.; Guerrero, M.G. Outdoor cultivation of microalgae for carotenoid production: Current state and perspectives. Appl. Microbiol. Biotechnol. 2007, 74, 1163–1174. [Google Scholar] [CrossRef] [PubMed]
- Waissman-Levy, N.; Leu, S.; Khozin-Goldberg, I.; Boussiba, S. Manipulation of trophic capacities in Haematococcus pluvialis enables low-light mediated growth on glucose and astaxanthin formation in the dark. Algal Res. 2019, 40, 101497. [Google Scholar] [CrossRef]
- Nahidian, B.; Ghanati, F.; Shahbazi, M.; Soltani, N. Effect of nutrients on the growth and physiological features of newly isolated Haematococcus pluvialis TMU1. Bioresour. Technol. 2018, 255, 229–237. [Google Scholar] [CrossRef]
- Kaloudas, D.; Pavlova, N.; Penchovsky, R. Phycoremediation of wastewater by microalgae: A review. Environ. Chem. Lett. 2021, 19, 2905–2920. [Google Scholar] [CrossRef]
- Ledda, C.; Tamiazzo, J.; Borin, M.; Adani, F. A simplified process of swine slurry treatment by primary filtration and Haematococcus pluvialis culture to produce low cost astaxanthin. Ecol. Eng. 2016, 90, 244–250. [Google Scholar] [CrossRef]
- Stevčić, Č.; Pulkkinen, K.; Pirhonen, J. Screening of microalgae and LED grow light spectra for effective removal of dissolved nutrients from cold-water recirculating aquaculture system (RAS) wastewater. Algal Res. 2019, 44, 101681. [Google Scholar] [CrossRef]
- Kang, C.D.; An, J.Y.; Park, T.H.; Sim, S.J. Astaxanthin biosynthesis from simultaneous N and P uptake by the green alga Haematococcus pluvialis in primary-treated wastewater. Biochem. Eng. J. 2006, 31, 234–238. [Google Scholar] [CrossRef]
- Pan, M.; Zhu, X.; Pan, G.; Angelidak, I. Integrated valorization system for simultaneous high strength organic wastewater treatment and astaxanthin production from Haematococcus pluvialis. Bioresour. Technol. 2021, 326, 124761. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial fuel cells: Methodology and technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef]
- Logan, B.E. Exoelectrogenic bacteria that power microbial fuel cells. Nat. Rev. Microbiol. 2009, 7, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Ahirwar, A.; Das, S.; Das, S.; Yang, Y.-H.; Bhatia, S.K.; Vinayak, V.; Ghangrekar, M.M. Photosynthetic microbial fuel cell for bioenergy and valuable production: A review of circular bio-economy approach. Algal Res. 2023, 70, 102973. [Google Scholar] [CrossRef]
- Vinayak, V.; Khan, M.J.; Varjani, S.; Saratale, G.D.; Saratale, R.G.; Bhatia, S.K. Microbial fuel cells for remediation of environmental pollutants and value addition: Special focus on coupling diatom microbial fuel cells with photocatalytic and photoelectric fuel cells. J. Biotechnol. 2021, 338, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.J.; Rai, A.; Ahirwar, A.; Sirotiya, V.; Mourya, M.; Mishra, S.; Schoefs, B.; Marchand, J.; Bhatia, S.K.; Varjani, S. Diatom microalgae as smart nanocontainers for biosensing wastewater pollutants: Recent trends and innovations. Bioengineered 2021, 12, 9531–9549. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.J.; Singh, N.; Mishra, S.; Ahirwar, A.; Bast, F.; Varjani, S.; Schoefs, B.; Marchand, J.; Rajendran, K.; Banu, J.R. Impact of light on microalgal photosynthetic microbial fuel cells and removal of pollutants by nanoadsorbent biopolymers: Updates, challenges and innovations. Chemosphere 2022, 288, 132589. [Google Scholar] [CrossRef] [PubMed]
- Deka, R.; Shreya, S.; Mourya, M.; Sirotiya, V.; Rai, A.; Khan, M.J.; Ahirwar, A.; Schoefs, B.; Bilal, M.; Saratale, G.D. A techno-economic approach for eliminating dye pollutants from industrial effluent employing microalgae through microbial fuel cells: Barriers and perspectives. Environ. Res. 2022, 212, 113454. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, P.; Ghangrekar, M. Design of clayware separator-electrode assembly for treatment of wastewater in microbial fuel cells. Appl. Biochem. Biotechnol. 2014, 173, 378–390. [Google Scholar] [CrossRef]
- Khan, M.J.; Das, S.; Vinayak, V.; Pant, D.; Ghangrekar, M. Live diatoms as potential biocatalyst in a microbial fuel cell for harvesting continuous diafuel, carotenoids and bioelectricity. Chemosphere 2022, 291, 132841. [Google Scholar] [CrossRef]
- Rai, A.; Sirotiya, V.; Mourya, M.; Khan, M.J.; Ahirwar, A.; Sharma, A.K.; Kawatra, R.; Marchand, J.; Schoefs, B.; Varjani, S. Sustainable treatment of dye wastewater by recycling microalgal and diatom biogenic materials: Biorefinery perspectives. Chemosphere 2022, 305, 135371. [Google Scholar] [CrossRef]
- Sirohi, R.; Sim, S.J.; Pandey, A. Photobioreactors: An introduction. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2023; pp. 3–10. [Google Scholar]
- Khan, M.J.; Gordon, R.; Varjani, S.; Vinayak, V. Employing newly developed plastic bubble wrap technique for biofuel production from diatoms cultivated in discarded plastic waste. Sci. Total Environ. 2022, 823, 153667. [Google Scholar] [CrossRef]
- Rai, I.; Ahirwar, A.; Rai, A.; Varjani, S.; Vinayak, V. Biowaste recycling strategies for regenerative life support system: An overview. Sustain. Energy Technol. Assess. 2022, 53, 102525. [Google Scholar] [CrossRef]
- Sirotiya, V.; Ahirwar, A.; Mourya, M.; Khan, M.J.; Rai, A.; Kwatra, R.; Sharma, A.K.; Schoefs, B.; Marchand, J.; Varjani, S. Astaxanthin bioaccumulation in microalgae under environmental stress simulated in industrial effluents highlighting prospects of Haematococcus pluvialis: Knowledge gaps and prospective approaches. Phytochem. Rev. 2022, 1–26. [Google Scholar] [CrossRef]
- Cui, D.; Hu, C.; Zou, Z.; Sun, X.; Shi, J.; Xu, N. Comparative transcriptome analysis unveils mechanisms underlying the promoting effect of potassium iodide on astaxanthin accumulation in Haematococcus pluvialis under high light stress. Aquaculture 2020, 525, 735279. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, H.-P.; Han, B.; Yu, X. Coupling of abiotic stresses and phytohormones for the production of lipids and high-value by-products by microalgae: A review. Bioresour. Technol. 2019, 274, 549–556. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, Y.; Ding, W.; Han, B.; Geng, S.; Ning, D.; Ma, T.; Yu, X. Gamma-aminobutyric acid facilitates the simultaneous production of biomass, astaxanthin and lipids in Haematococcus pluvialis under salinity and high-light stress conditions. Bioresour. Technol. 2021, 320, 124418. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Li, Y.; Wu, G.; Li, G.; Sun, H.; Deng, S.; Shen, Y.; Chen, G.; Zhang, R.; Meng, C. Transcriptome analysis in Haematococcus pluvialis: Astaxanthin induction by salicylic acid (SA) and jasmonic acid (JA). PLoS ONE 2015, 10, e0140609. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Meng, C.; Zhang, X.; Xu, D.; Miao, X.; Wang, Y.; Yang, L.; Lv, H.; Chen, L.; Ye, N. Induction of salicylic acid (SA) on transcriptional expression of eight carotenoid genes and astaxanthin accumulation in Haematococcus pluvialis. Enzym. Microb. Technol. 2012, 51, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Hu, C.; Sun, X.; Xu, N. Transcriptome analysis reveals pathways responsible for the promoting effect of sucrose on astaxanthin accumulation in Haematococcus pluvialis under high light condition. Aquaculture 2021, 530, 735757. [Google Scholar] [CrossRef]
- Cheng, J.; Li, K.; Zhu, Y.; Yang, W.; Zhou, J.; Cen, K. Transcriptome sequencing and metabolic pathways of astaxanthin accumulated in Haematococcus pluvialis mutant under 15% CO2. Bioresour. Technol. 2017, 228, 99–105. [Google Scholar] [CrossRef]
- Hu, C.; Cui, D.; Sun, X.; Shi, J.; Song, L.; Li, Y.; Xu, N. Transcriptomic analysis unveils survival strategies of autotrophic Haematococcus pluvialis against high light stress. Aquaculture 2019, 513, 734430. [Google Scholar] [CrossRef]
- Che, H.; Li, Q.; Zhang, T.; Wang, D.; Yang, L.; Xu, J.; Yanagita, T.; Xue, C.; Chang, Y.; Wang, Y. Effects of astaxanthin and docosahexaenoic-acid-acylated astaxanthin on Alzheimer’s disease in APP/PS1 double-transgenic mice. J. Agric. Food. Chem. 2018, 66, 4948–4957. [Google Scholar] [CrossRef]
- Ma, R.; Thomas-Hall, S.R.; Chua, E.T.; Alsenani, F.; Eltanahy, E.; Netzel, M.E.; Netzel, G.; Lu, Y.; Schenk, P.M. Gene expression profiling of astaxanthin and fatty acid pathways in Haematococcus pluvialis in response to different LED lighting conditions. Bioresour. Technol. 2018, 250, 591–602. [Google Scholar] [CrossRef]
- Gao, Z.; Meng, C.; Zhang, X.; Xu, D.; Zhao, Y.; Wang, Y.; Lv, H.; Yang, L.; Chen, L.; Ye, N. Differential expression of carotenogenic genes, associated changes on astaxanthin production and photosynthesis features induced by JA in H. pluvialis. PLoS ONE 2012, 7, e42243. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, H.-P.; Yu, C.; Ding, W.; Han, B.; Geng, S.; Ning, D.; Ma, T.; Yu, X. Integration of physiological and metabolomic profiles to elucidate the regulatory mechanisms underlying the stimulatory effect of melatonin on astaxanthin and lipids coproduction in Haematococcus pluvialis under inductive stress conditions. Bioresour. Technol. 2021, 319, 124150. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Cui, J.; Li, Q.; Qiao, T.; Zhong, D.-b.; Zhao, P.; Yu, X. A joint strategy comprising melatonin and 3-methyladenine to concurrently stimulate biomass and astaxanthin hyperaccumulation by Haematococcus pluvialis. Bioresour. Technol. 2021, 341, 125784. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Hou, L.; Dong, M.; Shi, J.; Huang, X.; Ding, Y.; Cong, X.; Zhang, F.; Zhang, X.; Zang, X. Transcriptome analysis in Haematococcus pluvialis: Astaxanthin induction by high light with acetate and Fe2+. Int. J. Mol. Sci. 2018, 19, 175. [Google Scholar] [CrossRef]
- Lee, C.; Ahn, J.-W.; Kim, J.-B.; Kim, J.Y.; Choi, Y.-E. Comparative transcriptome analysis of Haematococcus pluvialis on astaxanthin biosynthesis in response to irradiation with red or blue LED wavelength. World J. Microbiol. Biotechnol. 2018, 34, 1–14. [Google Scholar] [CrossRef]
- Lu, Z.; Dai, J.; Zheng, L.; Teng, Z.; Zhang, Q.; Qiu, D.; Song, L. Disodium 2-oxoglutarate promotes carbon flux into astaxanthin and fatty acid biosynthesis pathways in Haematococcus. Bioresour. Technol. 2020, 299, 122612. [Google Scholar] [CrossRef]
- Wang, X.; Meng, C.; Zhang, H.; Xing, W.; Cao, K.; Zhu, B.; Zhang, C.; Sun, F.; Gao, Z. Transcriptomic and proteomic characterizations of the molecular response to blue light and salicylic acid in Haematococcus pluvialis. Mar. Drugs 2021, 20, 1. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cui, D.; Zhuo, P.; Zhang, L.; Sun, X.; Xu, N. A new approach to promote astaxanthin accumulation via Na2WO4 in Haematococcus pluvialis. J. Oceanol. Limnol. 2019, 37, 176–185. [Google Scholar] [CrossRef]
- Xu, R.; Zhang, L.; Yu, W.; Liu, J. A strategy for interfering with the formation of thick cell walls in Haematococcus pluvialis by down-regulating the mannan synthesis pathway. Bioresour. Technol. 2022, 362, 127783. [Google Scholar] [CrossRef]
- Wang, Q.; Oshita, K.; Takaoka, M.; Shiota, K. Influence of water content and cell disruption on lipid extraction using subcritical dimethyl ether in wet microalgae. Bioresour. Technol. 2021, 329, 124892. [Google Scholar] [CrossRef]
- Talebi, S.; Edalatpour, A.; Tavakoli, O. Algal biorefinery: A potential solution to the food–energy–water–environment nexus. Sustain. Energy Fuels 2022, 6, 2623–2664. [Google Scholar] [CrossRef]
- Nitsos, C.; Filali, R.; Taidi, B.; Lemaire, J. Current and novel approaches to downstream processing of microalgae: A review. Biotechnol. Adv. 2020, 45, 107650. [Google Scholar] [CrossRef]
- Ahirwar, A.; Meignen, G.; Khan, M.J.; Khan, N.; Rai, A.; Schoefs, B.; Marchand, J.; Varjani, S.; Vinayak, V. Nanotechnological approaches to disrupt the rigid cell walled microalgae grown in wastewater for value-added biocompounds: Commercial applications, challenges, and breakthrough. Biomass Convers. Biorefinery 2021, 1–26. [Google Scholar] [CrossRef]
- Ahirwar, A.; Khan, M.J.; Sirotiya, V.; Mourya, M.; Rai, A.; Schoefs, B.; Marchand, J.; Varjani, S.; Vinayak, V. Pulsed Electric Field–Assisted Cell Permeabilization of Microalgae (Haematococcus pluvialis) for Milking of Value-Added Compounds. BioEnergy Res. 2022, 16, 1–14. [Google Scholar] [CrossRef]
- Yao, J.; Kim, H.S.; Kim, J.Y.; Choi, Y.-E.; Park, J. Mechanical stress induced astaxanthin accumulation of H. pluvialis on a chip. Lab A Chip 2020, 20, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.J.; Bawra, N.; Verma, A.; Kumar, V.; Pugazhendhi, A.; Joshi, K.B.; Vinayak, V. Cultivation of diatom Pinnularia saprophila for lipid production: A comparison of methods for harvesting the lipid from the cells. Bioresour. Technol. 2021, 319, 124129. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Narasimhan, A.L.; Moon, G.; Kim, Y.-E.; Park, M.; Kim, B.; Mahadi, R.; Chung, S.; Oh, Y.-K. Room-Temperature Cell Disruption and Astaxanthin Recovery from Haematococcus lacustris Cysts Using Ultrathin α-Quartz Nanoplates and Ionic Liquids. Appl. Sci. 2022, 12, 2210. [Google Scholar] [CrossRef]
- Praveenkumar, R.; Gwak, R.; Kang, M.; Shim, T.S.; Cho, S.; Lee, J.; Oh, Y.-K.; Lee, K.; Kim, B. Regenerative astaxanthin extraction from a single microalgal (Haematococcus pluvialis) cell using a gold nano-scalpel. ACS Appl. Mater. Interfaces 2015, 7, 22702–22708. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Li, Y.; Zhang, H.; An, N.; Wei, Y.; Wang, L.; Tian, C.; Yuan, M.; Sun, Y.; Xing, Y. Oxidative stress-mediated blood-brain barrier (BBB) disruption in neurological diseases. Oxidative Med. Cell. Longev. 2020, 2020, 1–27. [Google Scholar] [CrossRef]
- Reboul, E. Mechanisms of carotenoid intestinal absorption: Where do we stand? Nutrients 2019, 11, 838. [Google Scholar] [CrossRef]
- Kidd, P. Astaxanthin, cell membrane nutrient with diverse clinical benefits and anti-aging potential. Altern Med Rev 2011, 16, 355–364. [Google Scholar]
- Okada, Y.; Ishikura, M.; Maoka, T. Bioavailability of astaxanthin in Haematococcus algal extract: The effects of timing of diet and smoking habits. Biosci. Biotechnol. Biochem. 2009, 73, 1928–1932. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, R.; Guo, Z.; Li, C.; Li, P. The preparation and stability of the inclusion complex of astaxanthin with β-cyclodextrin. Food Chem. 2007, 101, 1580–1584. [Google Scholar] [CrossRef]
- Martínez-Álvarez, Ó.; Calvo, M.M.; Gómez-Estaca, J. Recent advances in astaxanthin micro/nanoencapsulation to improve its stability and functionality as a food ingredient. Mar. Drugs 2020, 18, 406. [Google Scholar] [CrossRef]
- Edelman, R.; Engelberg, S.; Fahoum, L.; Meyron-Holtz, E.G.; Livney, Y.D. Potato protein-based carriers for enhancing bioavailability of astaxanthin. Food Hydrocoll. 2019, 96, 72–80. [Google Scholar] [CrossRef]
- Li, C.; Song, Q.; Yin, X.; Song, R.; Chen, G. Preparation, Characterization, and In Vitro Anticancer Activity Evaluation of Broccoli-Derived Extracellular Vesicle-Coated Astaxanthin Nanoparticles. Molecules 2022, 27, 3955. [Google Scholar] [CrossRef]
- Jiang, G.-L.; Zhu, M.-J. Preparation of astaxanthin-encapsulated complex with zein and oligochitosan and its application in food processing. Lwt 2019, 106, 179–185. [Google Scholar] [CrossRef]
- Pan, L.; Wang, H.; Gu, K. Nanoliposomes as vehicles for astaxanthin: Characterization, in vitro release evaluation and structure. Molecules 2018, 23, 2822. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, L.; Li, D.; Wang, Y.; Chen, Z.; Zou, C.; Liu, W.; Ma, Y.; Cao, M.-J.; Liu, G.-M. Re-assembled oleic acid-protein complexes as nano-vehicles for astaxanthin: Multispectral analysis and molecular docking. Food Hydrocoll. 2020, 103, 105689. [Google Scholar] [CrossRef]
- Hu, Q.; Hu, S.; Fleming, E.; Lee, J.-Y.; Luo, Y. Chitosan-caseinate-dextran ternary complex nanoparticles for potential oral delivery of astaxanthin with significantly improved bioactivity. Int. J. Biol. Macromol. 2020, 151, 747–756. [Google Scholar] [CrossRef]
- Zhang, X.; Yin, W.; Qi, Y.; Li, X.; Zhang, W.; He, G. Microencapsulation of astaxanthin in alginate using modified emulsion technology: Preparation, characterization, and cytostatic activity. Can. J. Chem. Eng. 2017, 95, 412–419. [Google Scholar] [CrossRef]
- Liu, X.; Xie, J.; Zhou, L.; Zhang, J.; Chen, Z.; Xiao, J.; Cao, Y.; Xiao, H. Recent advances in health benefits and bioavailability of dietary astaxanthin and its isomers. Food Chem. 2022, 404, 134605. [Google Scholar] [CrossRef] [PubMed]
- Liaqat, F.; Khazi, M.I.; Bahadar, A.; He, L.; Aslam, A.; Liaquat, R.; Agathos, S.N.; Li, J. Mixotrophic cultivation of microalgae for carotenoid production. Rev. Aquac. 2023, 15, 35–61. [Google Scholar] [CrossRef]
- Rao, A.R.; Sindhuja, H.; Dharmesh, S.M.; Sankar, K.U.; Sarada, R.; Ravishankar, G.A. Effective inhibition of skin cancer, tyrosinase, and antioxidative properties by astaxanthin and astaxanthin esters from the green alga Haematococcus pluvialis. J. Agric. Food Chem. 2013, 61, 3842–3851. [Google Scholar] [CrossRef] [PubMed]
- Visioli, F.; Artaria, C. Astaxanthin in cardiovascular health and disease: Mechanisms of action, therapeutic merits, and knowledge gaps. Food Funct. 2017, 8, 39–63. [Google Scholar] [CrossRef]
- Wu, D.; Xu, H.; Chen, J.; Zhang, L. Effects of astaxanthin supplementation on oxidative stress. Int. J. Vitam. Nutr. Res. 2019, 90, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Conti, P.; Ronconi, G.; Caraffa, A.; Gallenga, C.; Ross, R.; Frydas, I.; Kritas, S. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): Anti-inflammatory strategies. J. Biol. Regul Homeost Agents 2020, 34, 327–331. [Google Scholar]
- Zhang, C.; Wu, Z.; Li, J.-W.; Zhao, H.; Wang, G.-Q. Cytokine release syndrome in severe COVID-19: Interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int. J. Antimicrob. Agents 2020, 55, 105954. [Google Scholar] [CrossRef]
- Shah, M.M.R.; Liang, Y.; Cheng, J.J.; Daroch, M. Astaxanthin-producing green microalga Haematococcus pluvialis: From single cell to high value commercial products. Front. Plant Sci. 2016, 7, 531. [Google Scholar] [CrossRef]
- Fassett, R.G.; Coombes, J.S. Astaxanthin: A potential therapeutic agent in cardiovascular disease. Mar. Drugs 2011, 9, 447–465. [Google Scholar] [CrossRef]
- Miyachi, M.; Matsuno, T.; Asano, K.; Mataga, I. Anti-inflammatory effects of astaxanthin in the human gingival keratinocyte line NDUSD-1. J. Clin. Biochem. Nutr. 2015, 56, 171–178. [Google Scholar] [CrossRef]
- Dhinaut, J.; Balourdet, A.; Teixeira, M.; Chogne, M.; Moret, Y. A dietary carotenoid reduces immunopathology and enhances longevity through an immune depressive effect in an insect model. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Palozza, P.; Krinsky, N.I. Astaxanthin and canthaxanthin are potent antioxidants in a membrane model. Arch. Biochem. Biophys. 1992, 297, 291–295. [Google Scholar] [CrossRef]
- Ambati, R.R.; Siew Moi, P.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Osawa, T. Cis astaxanthin and especially 9-cis astaxanthin exhibits a higher antioxidant activity in vitro compared to the all-trans isomer. Biochem. Biophys. Res. Commun. 2007, 357, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Chyun, J.H.; Kim, Y.K.; Line, L.L.; Chew, B.P. Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutr. Metab. 2010, 7, 1–10. [Google Scholar] [CrossRef]
- Fakhri, S.; Yosifova Aneva, I.; Farzaei, M.H.; Sobarzo-Sánchez, E. The Neuroprotective Effects of Astaxanthin: Therapeutic Targets and Clinical Perspective. Molecules 2019, 24, 2640. [Google Scholar] [CrossRef]
- Chang, M.X.; Xiong, F. Astaxanthin and its effects in inflammatory responses and inflammation-associated diseases: Recent advances and future directions. Molecules 2020, 25, 5342. [Google Scholar] [CrossRef]
- Fakhri, S.; Nouri, Z.; Moradi, S.Z.; Farzaei, M.H. Astaxanthin, COVID-19 and immune response: Focus on oxidative stress, apoptosis and autophagy. Phytother. Res. 2020, 34, 2790. [Google Scholar] [CrossRef]
- Kammeyer, A.; Luiten, R. Oxidation events and skin aging. Ageing Res. Rev. 2015, 21, 16–29. [Google Scholar] [CrossRef]
- Honda, M.; Murakami, K.; Osawa, Y.; Kawashima, Y.; Hirasawa, K.; Kuroda, I. Z-Isomers of astaxanthin exhibit greater bioavailability and tissue accumulation efficiency than the all-E-isomer. J. Agric. Food Chem. 2021, 69, 3489–3495. [Google Scholar] [CrossRef]
- Cao, Y.; Yang, L.; Qiao, X.; Xue, C.; Xu, J. Dietary astaxanthin: An excellent carotenoid with multiple health benefits. Crit. Rev. Food Sci. Nutr. 2021, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, Y. Oxidative stress in the light-exposed retina and its implication in age-related macular degeneration. Redox Biol. 2020, 37, 101779. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Qi, X. The putative role of astaxanthin in neuroinflammation modulation: Mechanisms and therapeutic potential. Front. Pharmacol. 2022, 13, 916653. [Google Scholar] [CrossRef]
- Harada, F.; Morikawa, T.; Lennikov, A.; Mukwaya, A.; Schaupper, M.; Uehara, O.; Takai, R.; Yoshida, K.; Sato, J.; Horie, Y. Protective effects of oral astaxanthin nanopowder against ultraviolet-induced photokeratitis in mice. Oxidative Med. Cell. Longev. 2017, 2017, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Yasunori, N.; Miharu, M.; Jiro, T.; Akitoshi, K.; Yoshiharu, H.; Yuri, S.; Hiroki, T. The effect of astaxanthin on retinal capillary blood flow in normal volunteers. J. Clin. Ther. Med 2005, 21, 537–542. [Google Scholar]
- Karppi, J.; Rissanen, T.H.; Nyyssonen, K.; Kaikkonen, J.; Olsson, A.G.; Voutilainen, S.; Salonen, J.T. Effects of astaxanthin supplementation on lipid peroxidation. Int. J. Vitam. Nutr. Res. 2007, 77, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Sorrenti, V.; Davinelli, S.; Scapagnini, G.; Willcox, B.J.; Allsopp, R.C.; Willcox, D.C. Astaxanthin as a putative geroprotector: Molecular basis and focus on brain aging. Mar. Drugs 2020, 18, 351. [Google Scholar] [CrossRef]
- Liu, N.; Chen, J.; Gao, D.; Li, W.; Zheng, D. Astaxanthin attenuates contrast agent-induced acute kidney injury in vitro and in vivo via the regulation of SIRT1/FOXO3a expression. Int. Urol. Nephrol. 2018, 50, 1171–1180. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, F.; Hu, X.; Chen, J.; Wen, X.; Sun, Y.; Liu, Y.; Tang, R.; Zheng, K.; Song, Y. Inhibition of inflammation by astaxanthin alleviates cognition deficits in diabetic mice. Physiol. Behav. 2015, 151, 412–420. [Google Scholar] [CrossRef]
- Ohgami, K.; Shiratori, K.; Kotake, S.; Nishida, T.; Mizuki, N.; Yazawa, K.; Ohno, S. Effects of astaxanthin on lipopolysaccharide-induced inflammation in vitro and in vivo. Investig. Ophthalmol. Vis. Sci. 2003, 44, 2694–2701. [Google Scholar] [CrossRef]
- Solomonov, Y.; Hadad, N.; Levy, R. The combined anti-inflammatory effect of astaxanthin, lyc-O-mato and carnosic acid in vitro and in vivo in a mouse model of peritonitis. J. Nutr. Food Sci. 2018, 8, 1000653. [Google Scholar] [CrossRef]
- Liu, G.; Shi, Y.; Peng, X.; Liu, H.; Peng, Y.; He, L. Astaxanthin attenuates adriamycin-induced focal segmental glomerulosclerosis. Pharmacology 2015, 95, 193–200. [Google Scholar] [CrossRef]
- Qiu, X.; Fu, K.; Zhao, X.; Zhang, Y.; Yuan, Y.; Zhang, S.; Gu, X.; Guo, H. Protective effects of astaxanthin against ischemia/reperfusion induced renal injury in mice. J. Transl. Med. 2015, 13, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Davinelli, S.; Melvang, H.M.; Andersen, L.P.; Scapagnini, G.; Nielsen, M.E. Astaxanthin from shrimp cephalothorax stimulates the immune response by enhancing IFN-γ, IL-10, and IL-2 secretion in splenocytes of Helicobacter pylori-infected mice. Mar. Drugs 2019, 17, 382. [Google Scholar] [CrossRef]
- Park, J.H.; Yeo, I.J.; Han, J.H.; Suh, J.W.; Lee, H.P.; Hong, J.T. Anti-inflammatory effect of astaxanthin in phthalic anhydride-induced atopic dermatitis animal model. Exp. Dermatol. 2018, 27, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Bennedsen, M.; Wang, X.; Willén, R.; Wadström, T.; Andersen, L.P. Treatment of H. pylori infected mice with antioxidant astaxanthin reduces gastric inflammation, bacterial load and modulates cytokine release by splenocytes. Immunol. Lett. 2000, 70, 185–189. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Hou, C.; Li, J.; Peng, H.; Wang, Q. The effect of astaxanthin on inflammation in hyperosmolarity of experimental dry eye model in vitro and in vivo. Exp. Eye Res. 2020, 197, 108113. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, K.; Kiko, T.; Miyazawa, T.; Burdeos, G.C.; Kimura, F.; Satoh, A.; Miyazawa, T. Antioxidant effect of astaxanthin on phospholipid peroxidation in human erythrocytes. Br. J. Nutr. 2011, 105, 1563–1571. [Google Scholar] [CrossRef]
- Ye, Q.; Huang, B.; Zhang, X.; Zhu, Y.; Chen, X. Astaxanthin protects against MPP+-induced oxidative stress in PC12 cells via the HO-1/NOX2 axis. BMC Neurosci. 2012, 13, 1–13. [Google Scholar] [CrossRef]
- Haider, S.; Saleem, S.; Perveen, T.; Tabassum, S.; Batool, Z.; Sadir, S.; Liaquat, L.; Madiha, S. Age-related learning and memory deficits in rats: Role of altered brain neurotransmitters, acetylcholinesterase activity and changes in antioxidant defense system. Age 2014, 36, 1291–1302. [Google Scholar] [CrossRef]
- Al-Amin, M.M.; Akhter, S.; Hasan, A.T.; Alam, T.; Nageeb Hasan, S.; Saifullah, A.; Shohel, M. The antioxidant effect of astaxanthin is higher in young mice than aged: A region specific study on brain. Metab. Brain Dis. 2015, 30, 1237–1246. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-H.; Chen, C.-Y.; Chiou, J.-Y.; Peng, R.Y.; Peng, C.-H. Astaxanthine secured apoptotic death of PC12 cells induced by β-amyloid peptide 25–35: Its molecular action targets. J. Med. Food 2010, 13, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Grimmig, B.; Daly, L.; Hudson, C.; Nash, K.; Bickford, P. Astaxanthin attenuates neurotoxicity in a mouse model of Parkinson’s disease. Funct. Foods Health Dis. 2017, 7, 562–576. [Google Scholar] [CrossRef]
- Kim, H.A.; Miller, A.A.; Drummond, G.R.; Thrift, A.G.; Arumugam, T.V.; Phan, T.G.; Srikanth, V.K.; Sobey, C.G. Vascular cognitive impairment and Alzheimer’s disease: Role of cerebral hypoperfusion and oxidative stress. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2012, 385, 953–959. [Google Scholar] [CrossRef]
- Lennikov, A.; Kitaichi, N.; Fukase, R.; Murata, M.; Noda, K.; Ando, R.; Ohguchi, T.; Kawakita, T.; Ohno, S.; Ishida, S. Amelioration of ultraviolet-induced photokeratitis in mice treated with astaxanthin eye drops. Mol. Vis. 2012, 18, 455. [Google Scholar] [PubMed]
- Dong, L.-Y.; Jin, J.; Lu, G.; Kang, X.-L. Astaxanthin attenuates the apoptosis of retinal ganglion cells in db/db mice by inhibition of oxidative stress. Mar. Drugs 2013, 11, 960–974. [Google Scholar] [CrossRef] [PubMed]
- Janani, R.; Anitha, R.E.; Perumal, M.K.; Divya, P.; Baskaran, V. Astaxanthin mediated regulation of VEGF through HIF1α and XBP1 signaling pathway: An insight from ARPE-19 cell and streptozotocin mediated diabetic rat model. Exp. Eye Res. 2021, 206, 108555. [Google Scholar] [CrossRef]
- Yeh, P.-T.; Huang, H.-W.; Yang, C.-M.; Yang, W.-S.; Yang, C.-H. Astaxanthin inhibits expression of retinal oxidative stress and inflammatory mediators in streptozotocin-induced diabetic rats. PLoS ONE 2016, 11, e0146438. [Google Scholar] [CrossRef]
- Kuraji, M.; Matsuno, T.; Satoh, T. Astaxanthin affects oxidative stress and hyposalivation in aging mice. J. Clin. Biochem. Nutr. 2016, 59, 79–85. [Google Scholar] [CrossRef]
- Xue, Y.; Qu, Z.; Fu, J.; Zhen, J.; Wang, W.; Cai, Y.; Wang, W. The protective effect of astaxanthin on learning and memory deficits and oxidative stress in a mouse model of repeated cerebral ischemia/reperfusion. Brain Res. Bull. 2017, 131, 221–228. [Google Scholar] [CrossRef]
- Lee, D.-H.; Lee, Y.J.; Kwon, K.H. Neuroprotective effects of astaxanthin in oxygen-glucose deprivation in SH-SY5Y cells and global cerebral ischemia in rat. J. Clin. Biochem. Nutr. 2010, 47, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Taksima, T.; Chonpathompikunlert, P.; Sroyraya, M.; Hutamekalin, P.; Limpawattana, M.; Klaypradit, W. Effects of astaxanthin from shrimp shell on oxidative stress and behavior in animal model of Alzheimer’s disease. Mar. Drugs 2019, 17, 628. [Google Scholar] [CrossRef] [PubMed]
- Waldvogel, F.A. Infectious diseases in the 21st century: Old challenges and new opportunities. Int. J. Infect. Dis. 2004, 8, 5–12. [Google Scholar] [CrossRef]
- Mohammed Ali, H.S.; Altayb, H.N.; Bayoumi, A.A.M.; El Omri, A.; Firoz, A.; Chaieb, K. In silico screening of the effectiveness of natural compounds from algae as SARS-CoV-2 inhibitors: Molecular docking, ADMT profile and molecular dynamic studies. J. Biomol. Struct. Dyn. 2022, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, A.-R.; Ayazi-Nasrabadi, R. Astaxanthin protective barrier and its ability to improve the health in patients with COVID-19. Iran. J. Microbiol. 2021, 13, 434. [Google Scholar] [CrossRef] [PubMed]
- Honda, M.; Kageyama, H.; Hibino, T.; Osawa, Y.; Kawashima, Y.; Hirasawa, K.; Kuroda, I. Evaluation and improvement of storage stability of astaxanthin isomers in oils and fats. Food Chem. 2021, 352, 129371. [Google Scholar] [CrossRef]
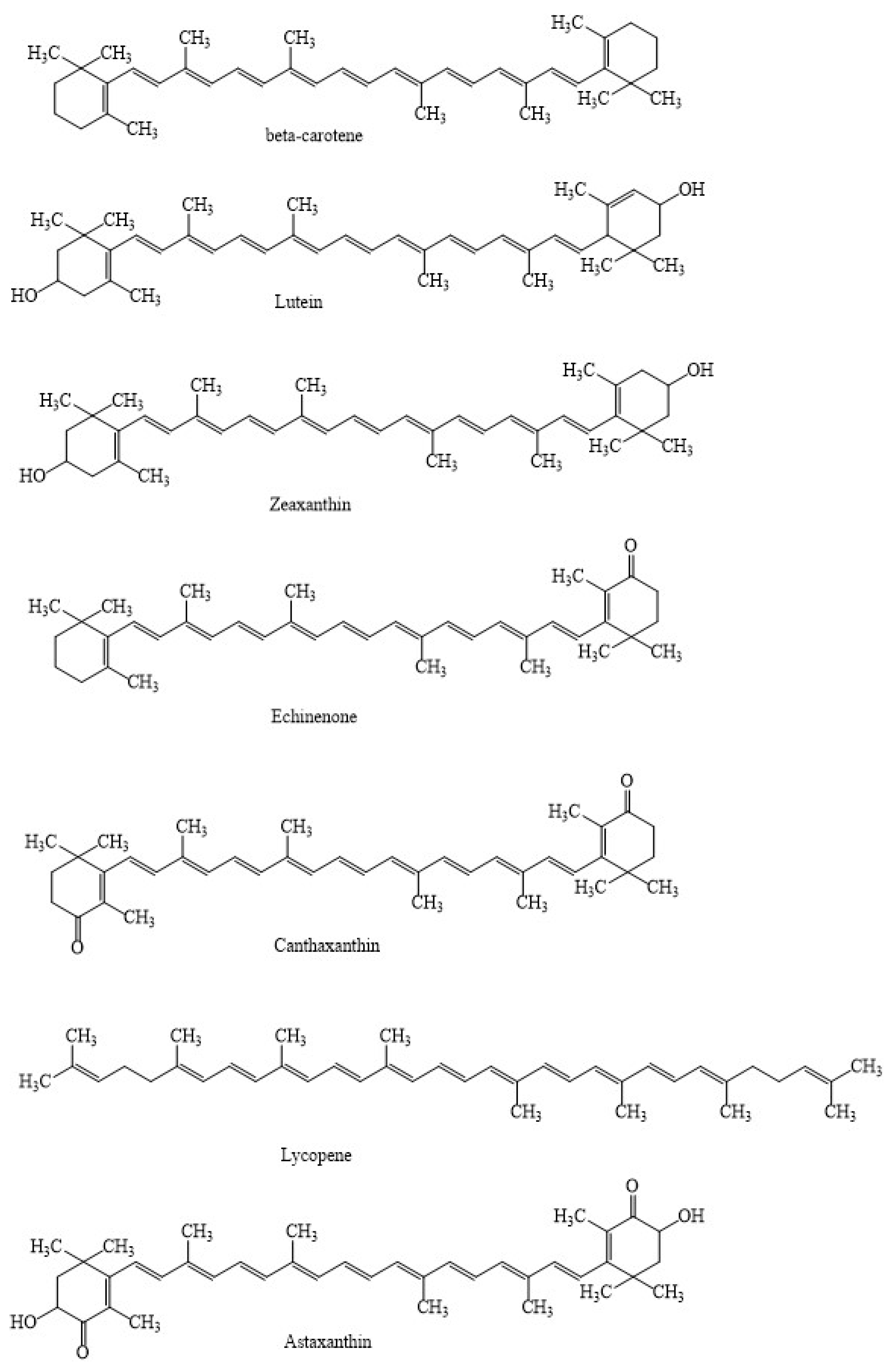
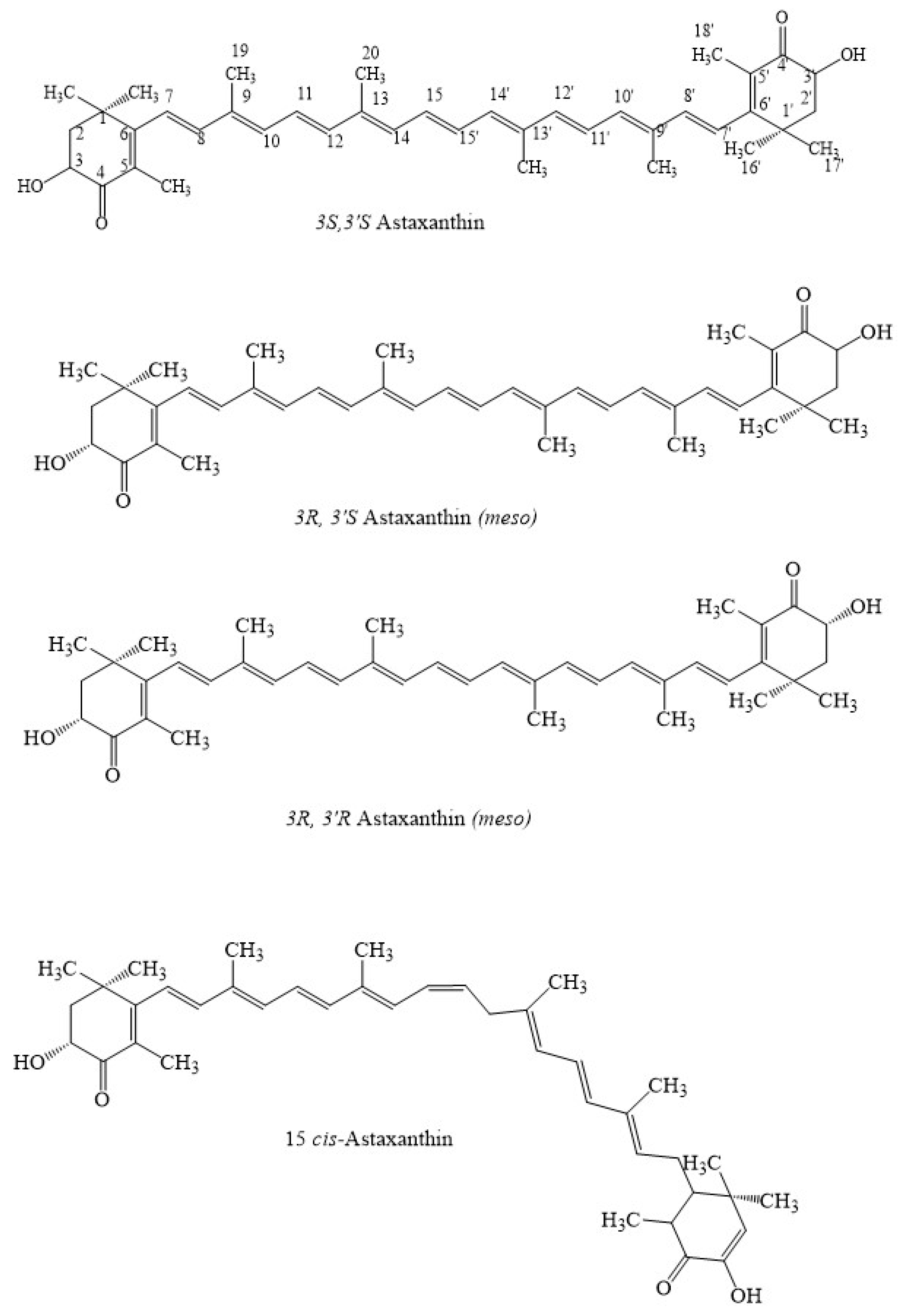
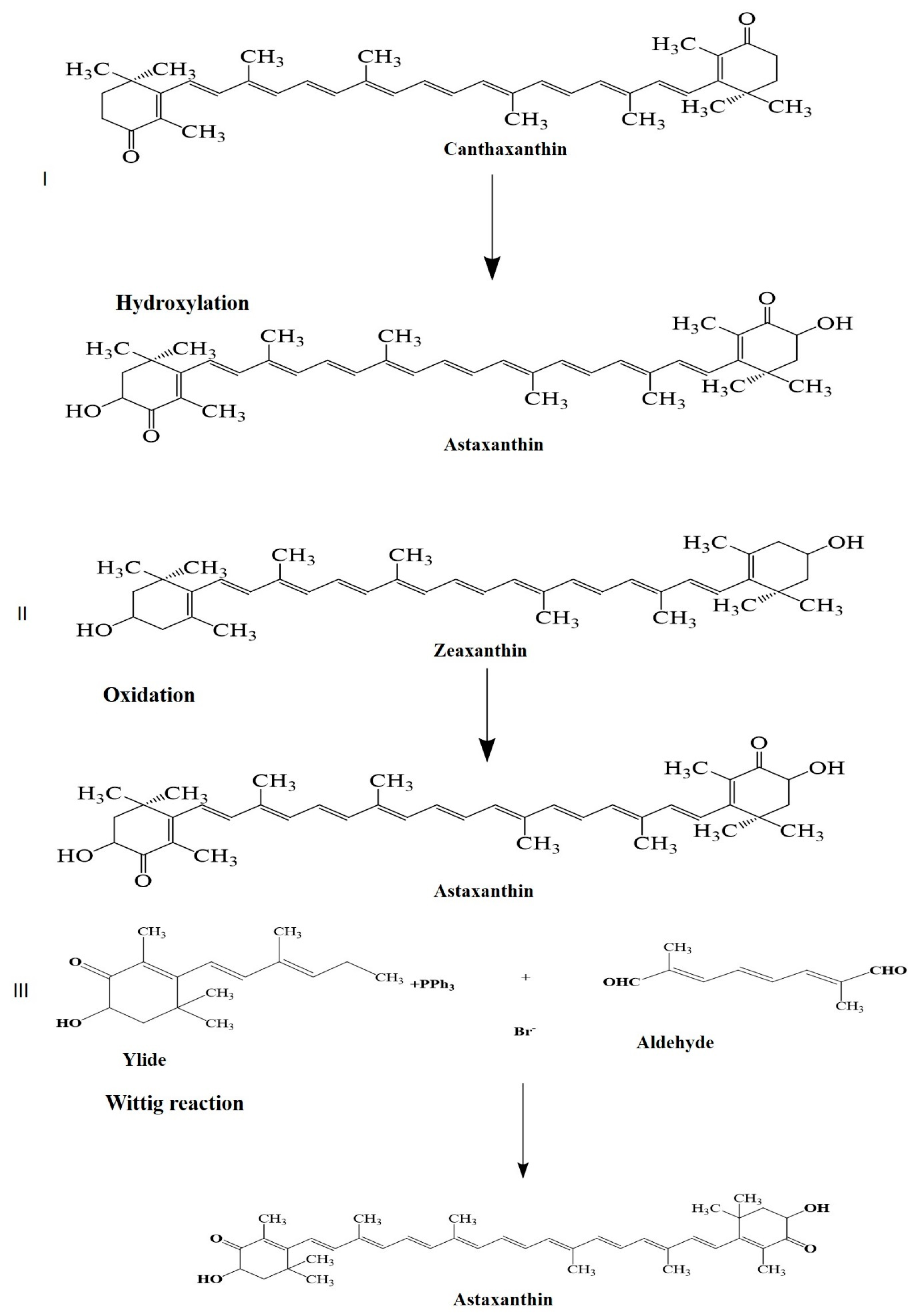
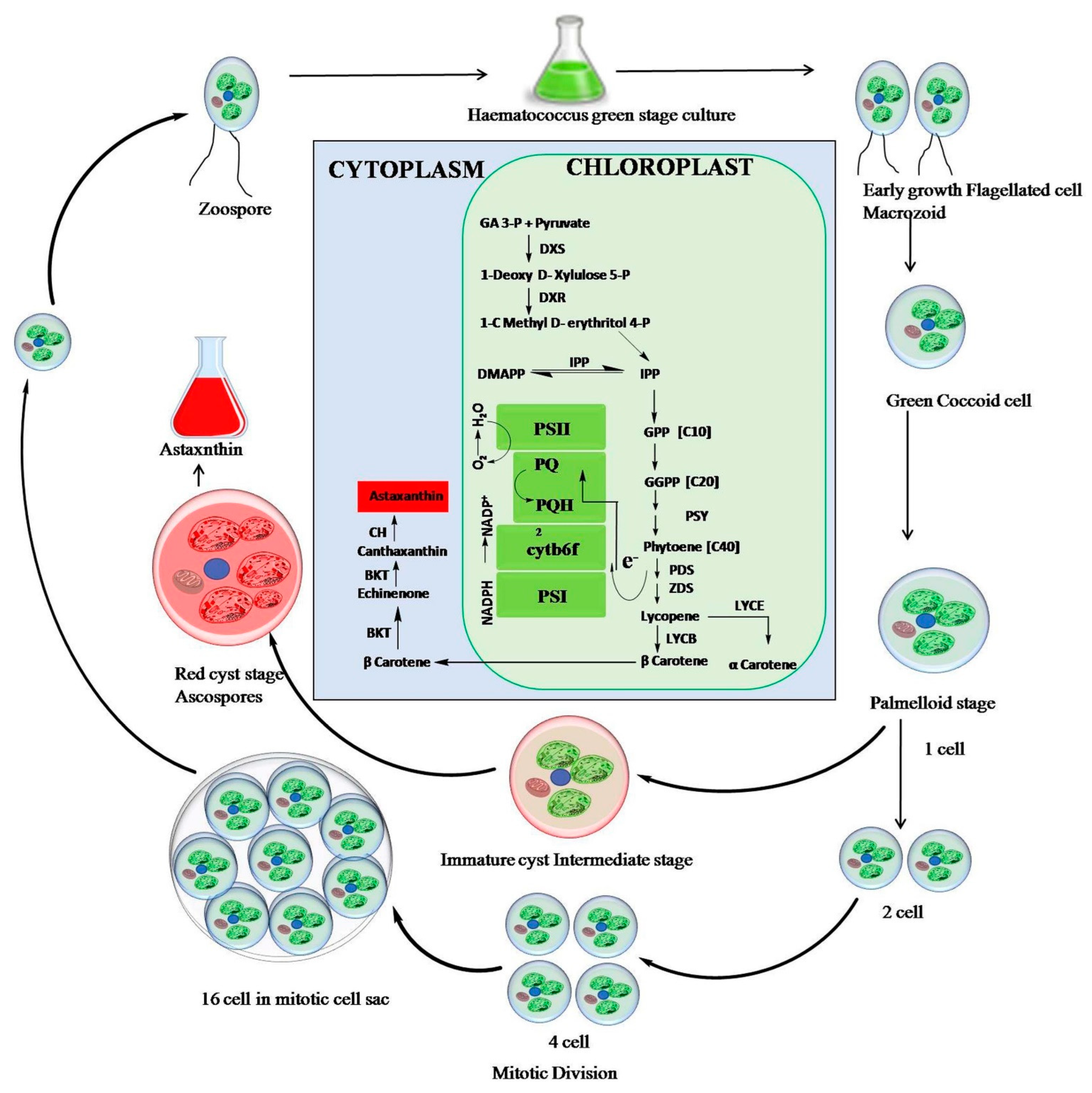

| Factors/Chemicals | Astaxanthin Biosynthesis and Gene Expression | Reference |
|---|---|---|
| Salicylic acid and jasmonic acid | Up-regulation of carotenogenic genes of psy, pds, zds and crtR-B | [81] |
| Salicylic acid | Up-regulation by ipi-1, ipi-2, psy, crtR-B, bkt and crtO genes at transcriptional level. lyc gene at post-transcriptional level and pds gene at both. | [82] |
| Potassium iodide and high light | Upregulation of transcription levels of 15-cis-phytoene/all-transphytoene synthase gene (CrtB) and 15-cis-phytoene desaturase gene (PDS). | [78] |
| Trisodium citrate | Up-regulation and the expression of genes encoding beta-ring hydroxylase (LUT5), beta-carotene/zeaxanthin 4-ketolase (CrtW) and beta-carotene 3-hydroxylase (CrtZ). | [83] |
| CO2 | Upregulation of genes involved in β-carotene biosynthesis such as PSY, ZDS and lcyB and β-carotene conversion like crtZ. | [84] |
| High light stress | Up-regulation and the expression of genes in MEP and astaxanthin biosynthesis pathway genes (ISPF, GGPS, PDS, CrtW and CrtZ) and concurrent down-regulation the expression of SPS, CHLP and CrtL-e. | [85] |
| Low temperature plasma | Expression of IPT9 and CYP735A1 involved in zeatin synthesis, amidase gene (AMI1), aldehyde dehydrogenase gene (ALDH7A1) in the indole-3-acetic acid (IAA) synthesis pathway, acyl-CoA oxidase gene (ACX1) in methyl Jasmonates synthesis pathway | [86] |
| High intensity blue and white LED light | Under white light upregulation of transcripts of astaxanthin biosynthesis genes psy, crtO, and bkt2. and under blue light upregulation of genes psy, lcy, crtO, and crtR-B. | [87] |
| Jasmonic acid | Astaxanthin biosynthesis up-regulation by psy, pds, crtR-B, lyc, bkt2 and crtO at the transcriptional level and ipi-1, ipi-2 at both transcriptional and post-transcriptional levels. | [88] |
| Blue, white, and red light | Blue light receptor gene upregulation of the biosynthesis pathway genes psy and pds, as well as dgat1 and dgat2d. | [87] |
| Melatonin | Upregulation of the major metabolites of the TCA cycle and the GABA shunt. | [89] |
| Melatonin + 3-methyladenine | The gene encoding lycopene β-cyclase (LCY), which catalyses lycopene to β-carotene which acts as the direct precursor for the accumulation of astaxanthin, which is further catalysed by β-carotene hydroxylase (CHY). | [90] |
| Melatonin + Calcium | Up-regulation and the expression levels of astaxanthin biosynthetic genes (lcy, lycopene β-cyclase; bkt, β-carotene ketolase; chy, β-carotene hydroxylase) | [54] |
| Acetate and Fe2+ | PDS, crtISO, LUT1, LUT5, lcyB, lcyE, crtZ, CCD8, ZEP | [91] |
| Under the monochromatic red (660 nm) or blue (450 nm) light-emitting diode (LED) irradiation | Upregulation and expression of gene IPP (encoding isopentenyl pyrophosphate), PSY (encoding phytoene synthase), PDS (encoding phytoene desaturase), LYC (encoding lycopene beta cyclase), BKT (encoding beta-carotene ketolase), CHY (encoding carotenoid hydroxylase), and CBR (encoding carotene biosynthesis-related protein (chlorophyll a-b binding protein) | [92] |
| Disodium 2-oxoglutarate (2-OG-2Na) | Up-regulation of genes ipi, bkt and crtR-b. | [93] |
| Sucrose | The expression of genes encoding phytoene synthase (CrtB), beta-carotene/zeaxanthin 4-ketolase (CrtW, BKT) and beta-carotene 3- hydroxylase (CrtZ). | [83] |
| Blue light and salicylic acid | Under blue light the expression of fpps was significantly regulated, and blue light reduced the expression of genes involved in astaxanthin synthesis. | [94] |
| Na2 WO4 | Expression of ipi, psy and bkt for astaxanthin biosynthesis | [95] |
| Test Parameter | Model | Astaxanthin Concentration | Target Gene/Biomarker | Reference |
|---|---|---|---|---|
| Anti-inflammation | Mice | 25 mgkg−1 day−1 | NF-κB, TNF-α | [147] |
| Rats | 1, 10 or 100 mgkg−1 | TNF-α, PGE2, IL-1βp-IKKα, p-IκBα | [148] | |
| BV-2 cells | 50 µM | NF-κB p65, IL-6, MAPK | [149] | |
| Male Balb/c mice | 50 mgkg−1 | Nrf2, NLRP3, IL-1β, IL-18 | [150] | |
| Male ICR mice | 5 mgkg−1 day−1 | IL-1β, IL-6, TNF-α | [151] | |
| BALB/c female mice | 10 or 40 mg d−1 | IL-2 and IL-10, IFN-γ | [152] | |
| HR-1 mice | 10 µg or 20 µgcm−2 | NF-κB, IL-1β, IL-6, TNF-α, IgE, COX-2, iNOS | [153] | |
| Balb/cA mice | 200 mg kg−1 body weight day−1 | IL-2, IFN-γ, IL-4 | [154] | |
| BALB/c mice | 1 µL drop of 5 µM | PI3K/Akt, HMGB1, TNF-α, IL-1β | [155] | |
| Antioxidant | Human | 6 or 12 mgd−1 | PLOOH | [156] |
| PC12 cells | 5, 10, 20 µM | NOX2, Sp1/NR, NFR2, HO-1 | [157] | |
| Mice | 2 mg kg−1 | APOP, SOD, GSH, MDA | [158] | |
| PC12 cells | 0.1 µM | NF-κB, Bax, IL-1β, TNFα | [159] | |
| Primary hippocampal neurons | 0.1 µM | RyR2, NFATc4 | [160] | |
| SH-SY5Y cells | 100 nM | CYTc, PARP | [161] | |
| Motor neurons | 100 nM | SOD1 | [162] | |
| Ocular health | C57BL/6J mice | 1/0.1/ 0.01 ng ml−1 by eye drop | NF-κB | [163] |
| Db/db rats | 25/5 mg kg−1 (oral gavage) | 8-OHdG, SOD, MDA | [164] | |
| C57BL/6J mice | 50 mg.kg−1 | Bax, Bcl-2, Nrf2. HO1, ROS | [165] | |
| Wistar rats | 0.6/3 mg kg−1 | acrolein, 8-OHdG, NO, MCP-1, ICAM-1 | [166] | |
| ddY mice | 100 mg kg−1 | 4-HNE, 8-OHdG | [167] | |
| Neuroprotective | Rats | 10 mg kg−1 | MVA, Nef2, SOD | [168] |
| SH-SY5Y cells and Rats | 10 to 50 µM (cells) 30 mg kg−1 (rats) | HSPs, iNOS | [162] | |
| Mice | 20 mg kg−1 | SOD, GHS, Casp3, Cyt C | [169] | |
| Alzheimer’s Disease | Wistar rats | 10 mg kg−1 body weight | Oxidative markers | [170] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nair, A.; Ahirwar, A.; Singh, S.; Lodhi, R.; Lodhi, A.; Rai, A.; Jadhav, D.A.; Harish; Varjani, S.; Singh, G.; et al. Astaxanthin as a King of Ketocarotenoids: Structure, Synthesis, Accumulation, Bioavailability and Antioxidant Properties. Mar. Drugs 2023, 21, 176. https://doi.org/10.3390/md21030176
Nair A, Ahirwar A, Singh S, Lodhi R, Lodhi A, Rai A, Jadhav DA, Harish, Varjani S, Singh G, et al. Astaxanthin as a King of Ketocarotenoids: Structure, Synthesis, Accumulation, Bioavailability and Antioxidant Properties. Marine Drugs. 2023; 21(3):176. https://doi.org/10.3390/md21030176
Chicago/Turabian StyleNair, Anagha, Ankesh Ahirwar, Shashikala Singh, Reeta Lodhi, Aishwarya Lodhi, Anshuman Rai, Dipak A Jadhav, Harish, Sunita Varjani, Gurpreet Singh, and et al. 2023. "Astaxanthin as a King of Ketocarotenoids: Structure, Synthesis, Accumulation, Bioavailability and Antioxidant Properties" Marine Drugs 21, no. 3: 176. https://doi.org/10.3390/md21030176
APA StyleNair, A., Ahirwar, A., Singh, S., Lodhi, R., Lodhi, A., Rai, A., Jadhav, D. A., Harish, Varjani, S., Singh, G., Marchand, J., Schoefs, B., & Vinayak, V. (2023). Astaxanthin as a King of Ketocarotenoids: Structure, Synthesis, Accumulation, Bioavailability and Antioxidant Properties. Marine Drugs, 21(3), 176. https://doi.org/10.3390/md21030176









