Pimarane-Type Diterpenes with Anti-Inflammatory Activity from Arctic-Derived Fungus Eutypella sp. D-1
Abstract
:1. Introduction
2. Results and Discussion
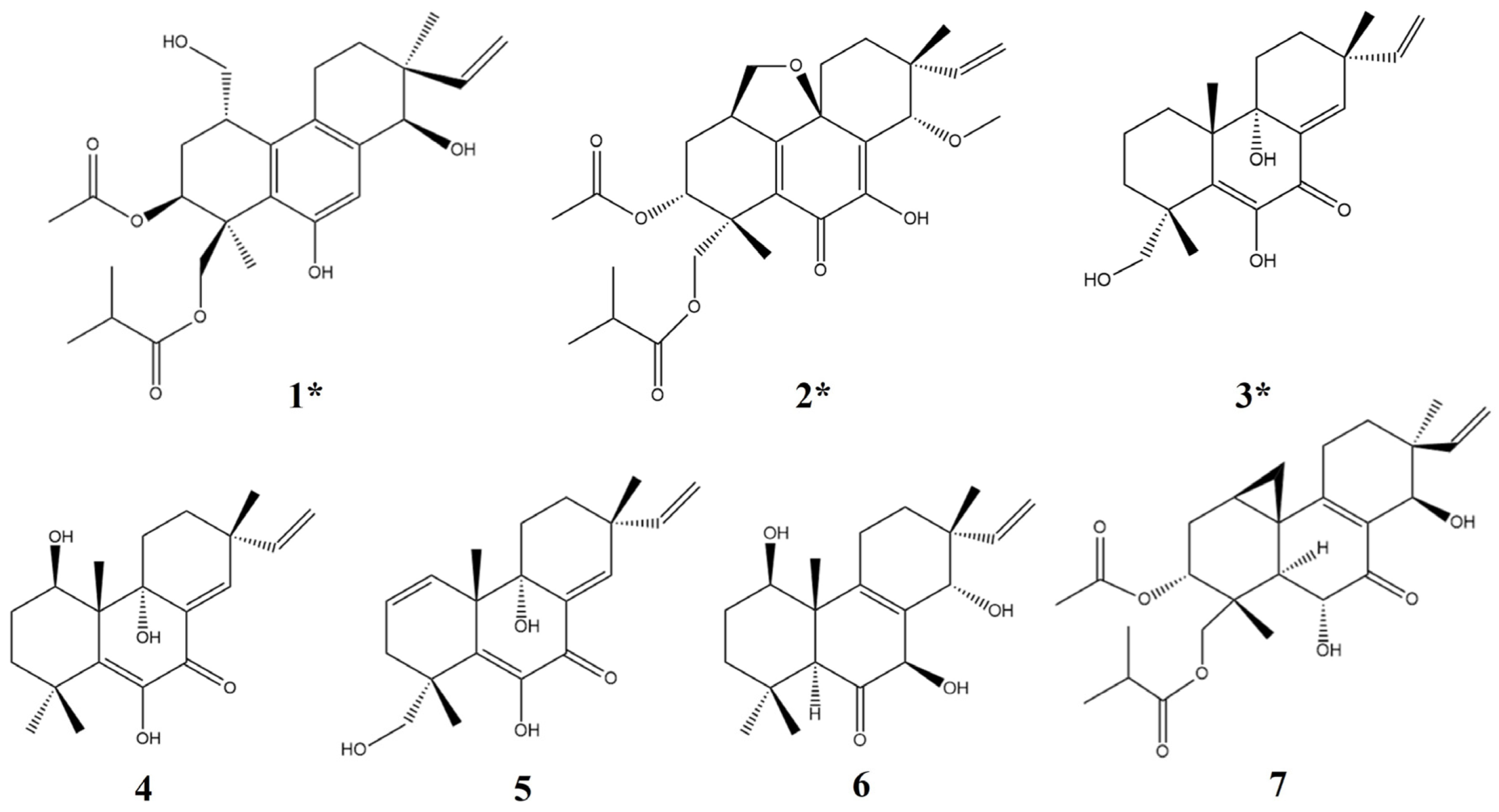
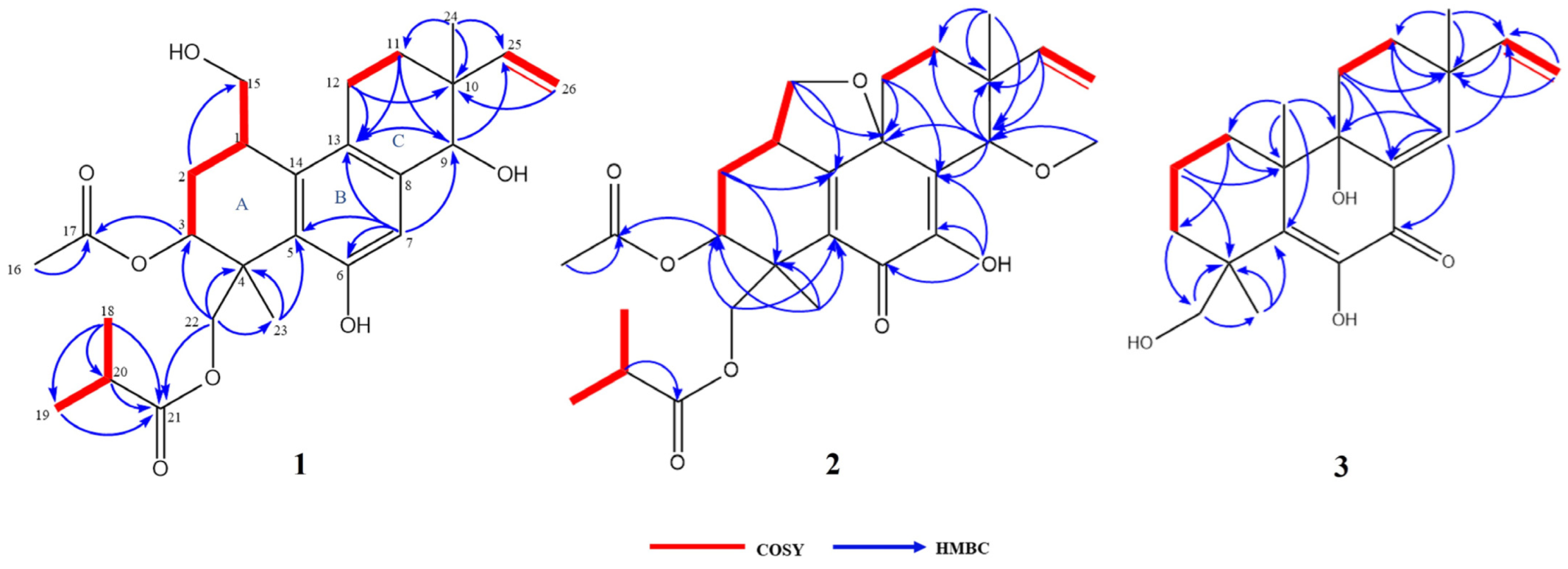
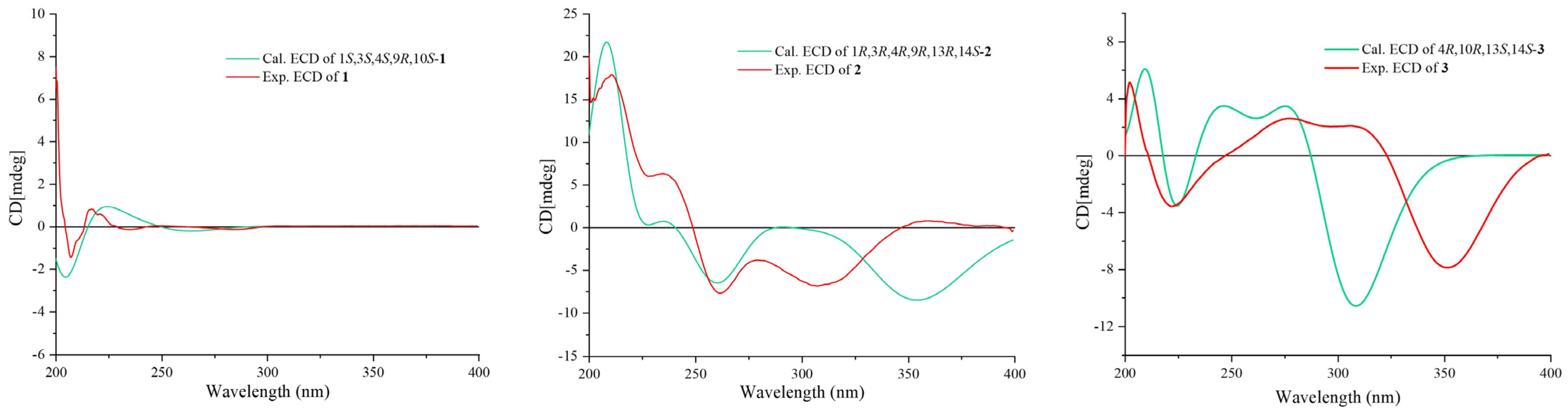
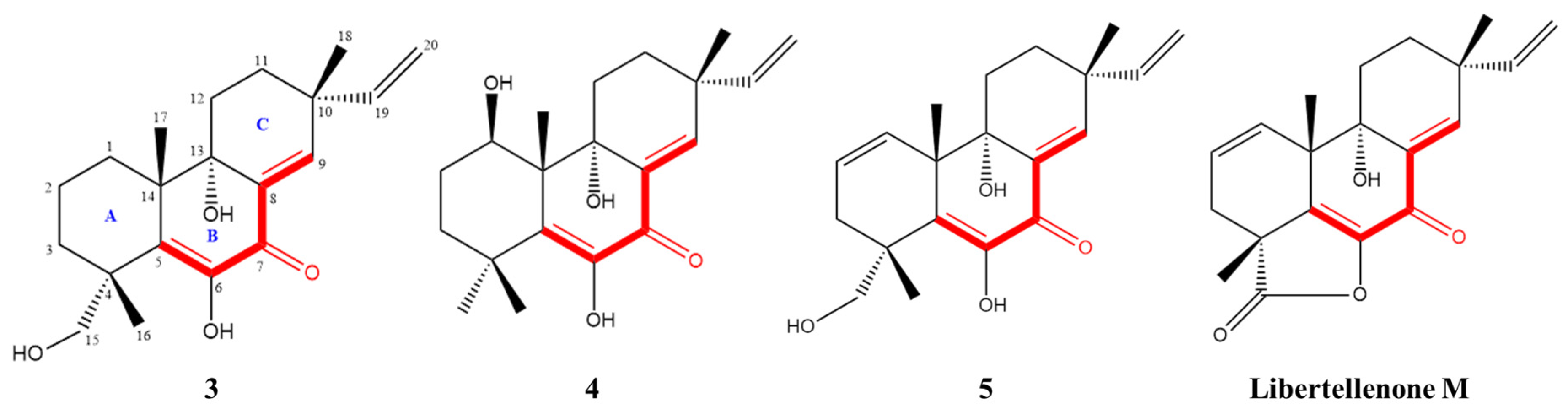
2.1. Structural Analysis of Compounds
2.2. Evaluation of the Inhibitory Effect on the NO Release
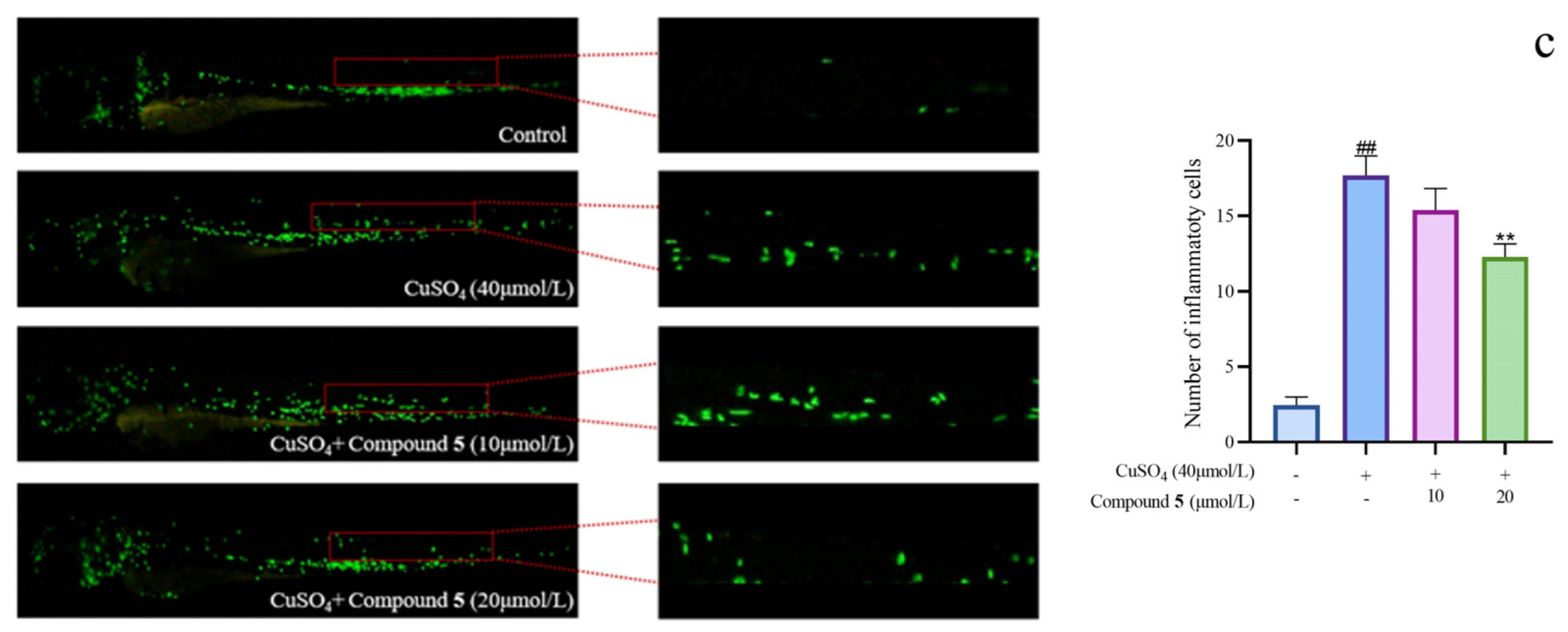
2.3. Evaluation of Anti-Inflammatory Activity Based on Zebrafish Model
3. Materials and Methods
3.1. General Experimrntal Procedures
3.2. Fungal Strain
3.3. Fermentation
3.4. Extraction and Isolation
3.5. ECD Calculations
3.6. Assay of Anti-Inflammatory Activity Based on LPS-Induced RAW264.7 Model
3.7. Assay of Anti-Inflammatory Activity Based on Zebrafish Model
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Fullerton, J.N.; Gilroy, D.W. Resolution of inflammation: A new therapeutic frontier. Nat. Rev. Drug Discov. 2016, 15, 551–567. [Google Scholar] [CrossRef]
- Yeung, Y.T.; Aziz, F.; Guerrero-Castilla, A. Signaling Pathways in Inflammation and Anti-inflammatory Therapies. Curr. Pharm. De. 2018, 24, 1449–1484. [Google Scholar] [CrossRef] [PubMed]
- Boshtam, M.; Asgary, S.; Kouhpayeh, S. Aptamers Against Pro- and Anti-Inflammatory Cytokines: A Review. Inflammation 2017, 40, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Dray, A. Inflammatory mediators of pain. Br. J. Anaesth. 1995, 75, 123–131. [Google Scholar] [CrossRef]
- Hsemian, M.; Owlia, S.; Owlia, M.B. Review of Anti-Inflammatory Herbal Medicines. Adv. Pharmacol. Sci. 2016, 5, 9130979. [Google Scholar]
- Butler, M.S. Natural products to drugs: Natural product derived compounds in clinical trials. Nat. Prod. Rep. 2005, 22, 162–195. [Google Scholar] [CrossRef]
- Aldholmi, M.; Marchand, P.; Ourliac-Garnier, I.; Pape, P.L.; Ganesan, A. A decade of antifungal leads from natural products: 2010–2019. Pharmaceuticals 2019, 12, 182. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Tang, S.Y.; Cao, S.G. Antimicrobial compounds from marine fungi. Phytochem. Rev. 2021, 20, 85–117. [Google Scholar] [CrossRef]
- Yang, A.G.; Si, L.L.; Shi, Z.P.; Tian, L.; Liu, D.; Zhou, D.M.; Proksch, P.; Lin, W.H. Nitrosporeusines A and B, unprecedented thioester-bearing alkaloids from the Arctic Streptomyces nitrosporeus. Org. Lett. 2013, 15, 5366–5369. [Google Scholar] [CrossRef]
- Tian, Y.; Li, Y.L.; Zhao, F.C. Secondary Metabolites from Polar Organisms. Mar. Drugs 2017, 15, 28. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.X.; Zhang, J.P.; Yu, H.B.; Liu, X.Y.; Lu, X.L. A new sesquiterpene lactone from fungus Eutypella sp. D-1. Nat. Prod. Res. 2017, 31, 1676–1681. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.X.; Yu, H.B.; Xu, W.H.; Hu, B.; Guild, A.; Zhang, J.P. Eutypellacytosporins A-D, meroterpenoids from the Arctic fungus Eutypella sp. D-1. J. Nat. Prod. 2019, 82, 3089–3095. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.L.; Liu, J.T.; Liu, X.Y.; Gao, Y.; Zhang, J.P.; Jiao, B.H. Pimarane diterpenes from the Arctic fungus Eutypella sp. D-1. J. Antibiot. 2014, 67, 171–174. [Google Scholar] [CrossRef]
- Yu, H.B.; Wang, X.L.; Zhang, Y.X.; Xu, W.H.; Zhang, J.P.; Zhou, X.Y. Libertellenones O-S and Eutypellenones A and B, pimarane diterpene derivatives from the Arctic fungus Eutypella sp. D-1. J. Nat. Prod. 2018, 81, 1553–1560. [Google Scholar] [CrossRef]
- Yu, H.B.; Wang, X.L.; Xu, W.H.; Zhang, Y.X.; Qian, Y.S.; Zhang, J.P.; Lu, X.L.; Liu, X.Y. Eutypellenoids A–C, New Pimarane Diterpenes from the Arctic Fungus Eutypella sp. D-1. Mar. Drugs 2018, 16, 284. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.R.; Zhu, Y.P.; Yu, H.B.; Liu, X.Y.; Jiao, B.H.; Lu, X.L. Libertellenone H, a natural pimarane diterpenoid, inhibits thioredoxin system and induces ROS-mediated apoptosis in human pancreatic cancer cells. Molecules 2021, 26, 315. [Google Scholar] [CrossRef]
- Shen, C.; Xu, N.; Gao, Y.Y.; Sun, X.Y.; Yin, Y.; Cai, M.H. Stimulatory effect of ethanol on libertellenone H biosynthesis by Arctic fungus Eutypella sp. D-1. Bioprocess Biosyst. Eng. 2016, 39, 353–360. [Google Scholar] [CrossRef]
- Oh, D.C.; Jensen, P.R.; Kauffman, C.A. Libertellenones A-D: Induction of cytotoxic diterpenoid biosynthesis by marine microbial competition. Bioorgan. Med. Chem. 2005, 13, 5267–5273. [Google Scholar] [CrossRef]
- Isaka, M.; Palasarn, S.; Prathumpai, W.; Laksanacharoen, P. Pimarane diterpenes from the endophytic fungus Eutypella sp. BCC 13199. Chem. Pharm. Bull. 2011, 59, 1157–1159. [Google Scholar] [CrossRef]
- Fan, M.M.; Xiang, G.; Chen, J.W. Libertellenone M, a diterpene derived from an endophytic fungus Phomopsis sp. S12, protects against DSS-induced colitis via inhibiting both nuclear translocation of NF-κB and NLRP3 inflammasome activation. Int. Immunopharmacol 2020, 80, 106144. [Google Scholar] [CrossRef]
- Pan, R.; Bai, X.L.; Chen, J.W.; Zhang, H.W.; Wang, H. Exploring structural diversity of microbe secondary metabolites using OSMAC strategy: A literature review. Front. Microbiol. 2019, 10, 294. [Google Scholar] [CrossRef] [PubMed]



| Position | 1 * | 2 * | 3 * | |||
|---|---|---|---|---|---|---|
| δc, type | δH, mult. (J in Hz) | δc, type | δH, mult. (J in Hz) | δc, type | δH, mult. (J in Hz) | |
| 1a | 38.46, CH | 3.30, m | 32.5, CH | 3.26, m | 30.58, CH2 | 1.95, m |
| 1b | 1.52, m | |||||
| 2a | 25.62, CH2 | 2.28, m | 27.0, CH2 | 2.34, m | 17.34, CH2 | 1.69, m |
| 2b | 2.22, dd, (12.62, 4.95) | 1.51, m | 1.65, m | |||
| 3a | 74.90, CH | 5.21, m | 73.0, CH | 5.12, d, (1.1) | 36.36, CH2 | 1.91, m |
| 3b | 1.36, m | |||||
| 4 | 41.51, C | 40.6, C | 41.32, C | |||
| 5 | 125.88, C | 126.5, C | 138.79, C | |||
| 6 | 153.37, C | 179.8, C | 143. 82, C | |||
| 7 | 115.25, CH | 6.86, s | 145.4, C | 181.80, C | ||
| 8 | 137.12, C | 122.3, C | 133.71, C | |||
| 9 | 75.42, CH | 4.29, m | 78.0, C | 147. 97, CH | 7.04, d, (1.86) | |
| 10 | 40.13, C | 167.2, C | 38.73, C | |||
| 11a | 31.62, CH2 | 1.96, m | 35.1, CH2 | 2.04, m | 29.36, CH2 | 1.84, m |
| 11b | 1.77, m | 1.50, m | 1.57, m | |||
| 12a | 22.64, CH2 | 2.74, m | 28.2, CH2 | 1.52, m | 25.66, CH2 | 1.92, m |
| 12b | 2.18, m | 1.80, m | ||||
| 13 | 125.84, C | 44.3, C | 74.86, C | |||
| 14 | 138.70, C | 80.1, CH | 4.40, s | 44.45, C | ||
| 14-OCH3 | 57.5, OCH3 | 3.34, s | ||||
| 15a | 63.96, CH2 | 3.73, m | 143.2, CH | 5.69, dd, (17.8, 11.1) | 70.49, CH2 | 4.48, d, (8.10) |
| 15b | 3.16, d, (11.36) | |||||
| 16a | 21.45, CH3 | 2.17, s | 113.6, CH2 | 5.01, dd, (11.2, 1.0) | 22.38, CH3 | 1.17, s |
| 16b | 5.08, d, (1.0) | |||||
| 17 | 171.50, C | 25.5, CH3 | 1.20, s | 29.73, CH3 | 1.20, s | |
| 18a | 18.78, CH3 | 0.90, d, (7.51) | 65.2, CH2 | 4.35, d, (10.5) | 23.17, CH3 | 1.12, s |
| 18b | 4.79, d, (10.5) | |||||
| 19 | 18.64, CH3 | 0.98, d, (7.51) | 21.0, CH3 | 1.36, s | 145.36, CH | 5.88, q, (6.41) |
| 20a | 34.06, CH | 2.37, m | 72.3, CH2 | 4.42, t, (7.95) | 112.63, CH2 | 5.08, m |
| 20b | 3.75, dd, (8.7, 7.4) | |||||
| 21 | 176.80, C | 170.1, C | ||||
| 22a | 65.42, CH2 | 5.0, d, (11.19) | 21.1, CH3 | 2.0, s | ||
| 22b | 4.30, m | |||||
| 23 | 21.81, CH3 | 1.54, s | 176.6, C | |||
| 24 | 23.06, CH3 | 1.18, s | 34.3, CH | 2.53, m | ||
| 25 | 140.86, CH | 5.83, q, (7.24) | 19.1, CH3 | 1.13, d, (7.0) | ||
| 26 | 116.44, CH2 | 5.21, m | 19.1, CH3 | 1.14, d, (7.0) | ||
| Compound | Inhibition (%) |
|---|---|
| 1 | <20 |
| 2 | <20 |
| 3 | 60.93 |
| 4 | 89.4 |
| 5 | 84.2 |
| 6 | 23.17 |
| 7 | <20 |
| Dexamethasone | 72.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ning, Y.; Zhang, S.; Zheng, T.; Xu, Y.; Li, S.; Zhang, J.; Jiao, B.; Zhang, Y.; Ma, Z.; Lu, X. Pimarane-Type Diterpenes with Anti-Inflammatory Activity from Arctic-Derived Fungus Eutypella sp. D-1. Mar. Drugs 2023, 21, 541. https://doi.org/10.3390/md21100541
Ning Y, Zhang S, Zheng T, Xu Y, Li S, Zhang J, Jiao B, Zhang Y, Ma Z, Lu X. Pimarane-Type Diterpenes with Anti-Inflammatory Activity from Arctic-Derived Fungus Eutypella sp. D-1. Marine Drugs. 2023; 21(10):541. https://doi.org/10.3390/md21100541
Chicago/Turabian StyleNing, Yaodong, Shi Zhang, Te Zheng, Yao Xu, Song Li, Jianpeng Zhang, Binghua Jiao, Yun Zhang, Zengling Ma, and Xiaoling Lu. 2023. "Pimarane-Type Diterpenes with Anti-Inflammatory Activity from Arctic-Derived Fungus Eutypella sp. D-1" Marine Drugs 21, no. 10: 541. https://doi.org/10.3390/md21100541
APA StyleNing, Y., Zhang, S., Zheng, T., Xu, Y., Li, S., Zhang, J., Jiao, B., Zhang, Y., Ma, Z., & Lu, X. (2023). Pimarane-Type Diterpenes with Anti-Inflammatory Activity from Arctic-Derived Fungus Eutypella sp. D-1. Marine Drugs, 21(10), 541. https://doi.org/10.3390/md21100541





