Abstract
Eight new scalarane sesterterpenes, phyllofenones F–M (1–8), together with two known analogues, carteriofenones B and A (9–10), were isolated from the marine sponge Phyllospongia foliascens collected from the South China Sea. The structures of these compounds were determined based on extensive spectroscopic and quantum chemical calculation analysis. The antibacterial and cytotoxic activity of these compounds was evaluated. Among them, only compounds 4 and 6 displayed weak inhibitory activity against Staphylococcus aureus and Escherichia coli, with MIC values of 16 μg/mL and 8 μg/mL, respectively. Compounds 1–10 exhibited cytotoxic activity against the HeLa, HCT-116, H460, and SW1990 cancer cell lines, with IC50 values ranging from 3.4 to 19.8 μM.
1. Introduction
Marine organisms have gained increasing attention as potential sources of interesting secondary metabolites with broad-spectrum activity and novel chemical structures [1]. Scalaranes, a family of bioactive marine sesterterpenoids, possess a 6/6/6/6 tetracyclic or 6/6/6/6/5 pentacyclic fused ring system [2]. Scalaranes can be divided into homoscalaranes (methylated at C-20 or C-24) and bishomoscalaranes (methylated at both C-20 and C-24) [2,3]. They are exclusively obtained from nudibranchs and their food chain, marine sponges [3,4]. The majority (around 90%) of scalaranes have been isolated from various marine sponges, including Hyrtios sp., Phyllospongia (previously identified as Carteriospongia) sp., Dysidea sp., Lendenfeldia sp., hippospongia sp., Scalarispongia sp., Spongia sp., Psammocinia sp., Ircinia sp., Euryspongia sp., Hyatella sp., Hyattlla sp., Coscinoderma sp., Smenospongia sp., and Collospongia sp. [2,4]. Since the first scalarane, scalarin, was reported in 1972 [5], approximately 500 scalaranes have been identified [2,3]. Moreover, extensive research has been conducted on the synthesis or semi-synthesis of scalarane derivatives due to their diverse bioactivity, including cytotoxic [6], anti-inflammatory [7], antimicrobial [4,8], and enzyme-inhibitory activity [9].
During our ongoing research on bioactive secondary metabolites from marine sponges in the South China Sea [4,7,10], we discovered that an extract obtained from the sponge P. foliascens demonstrated potent cytotoxic activity against human cancer cells. Following a comprehensive chemical investigation of the bioactive extracts, we successfully isolated eight new scalaranes, phyllofenones F–M (1–8), along with two known analogues, carteriofenones B and A (9–10) (Figure 1). Herein, we report the isolation, structure elucidation, and bioactivity of these scalaranes.
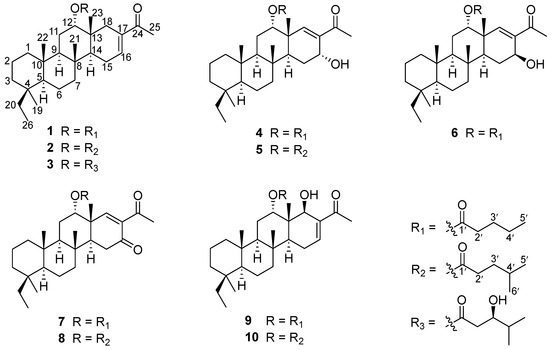
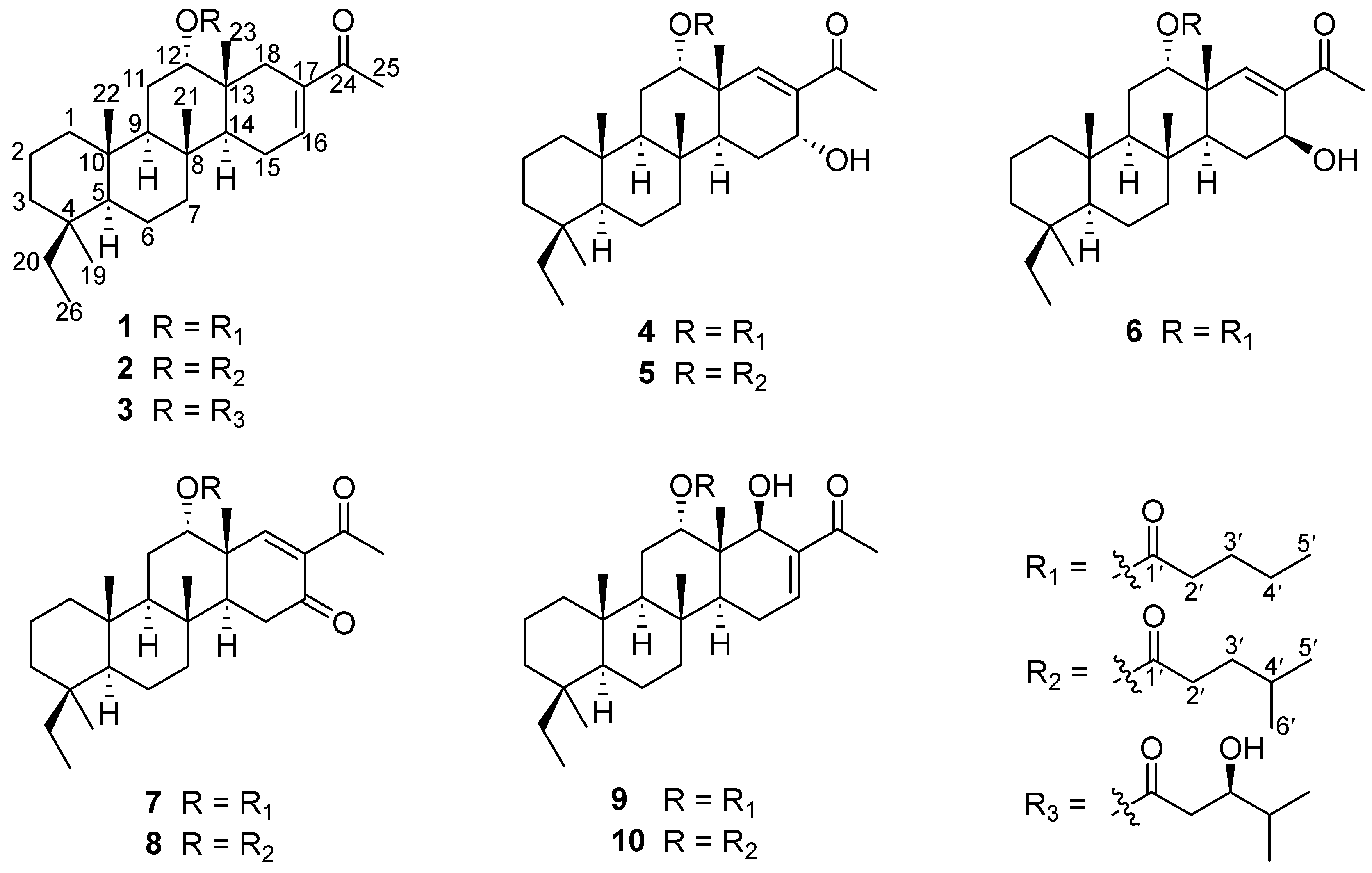
Figure 1.
Structures of the isolated compounds 1–10.
2. Results
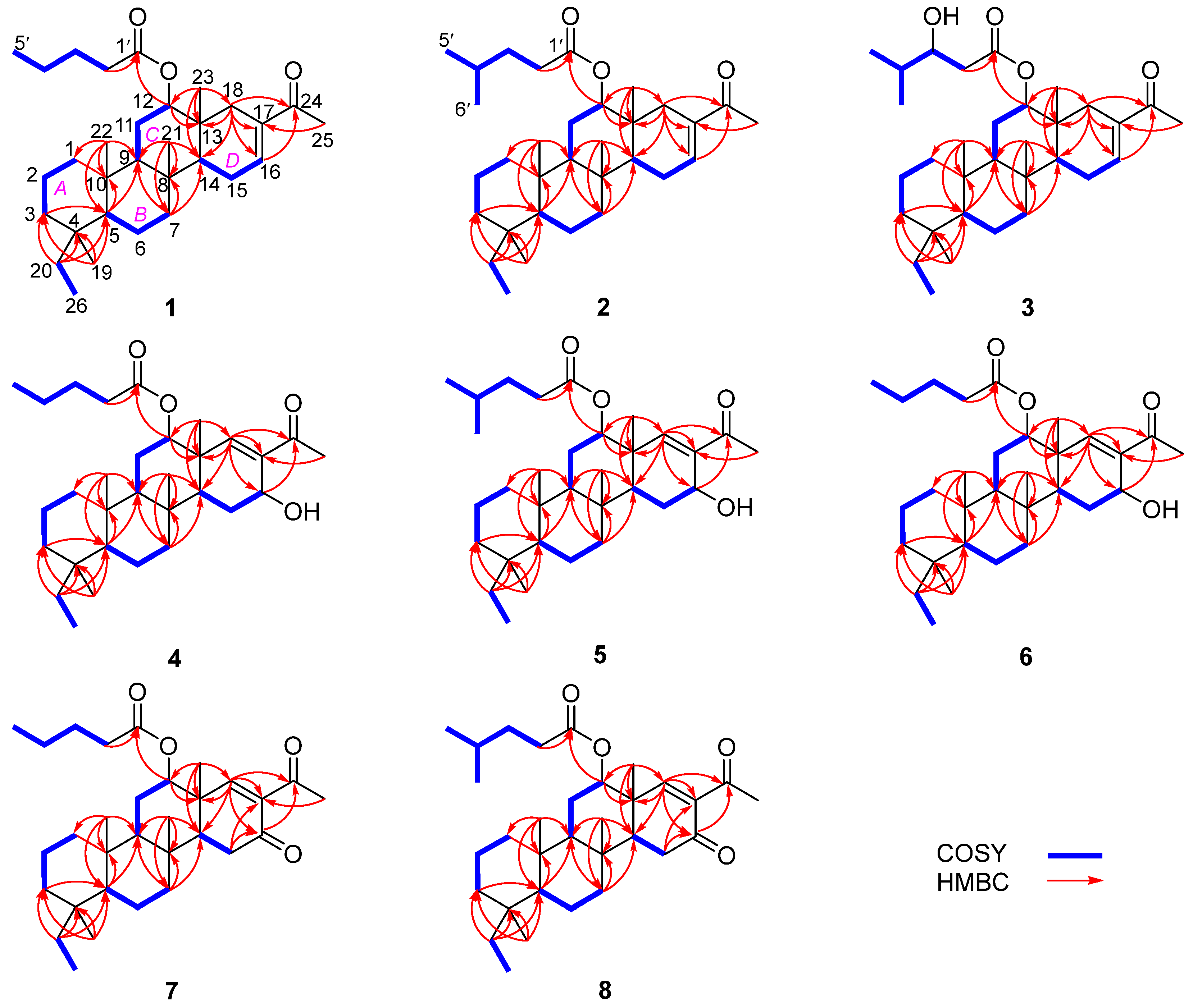
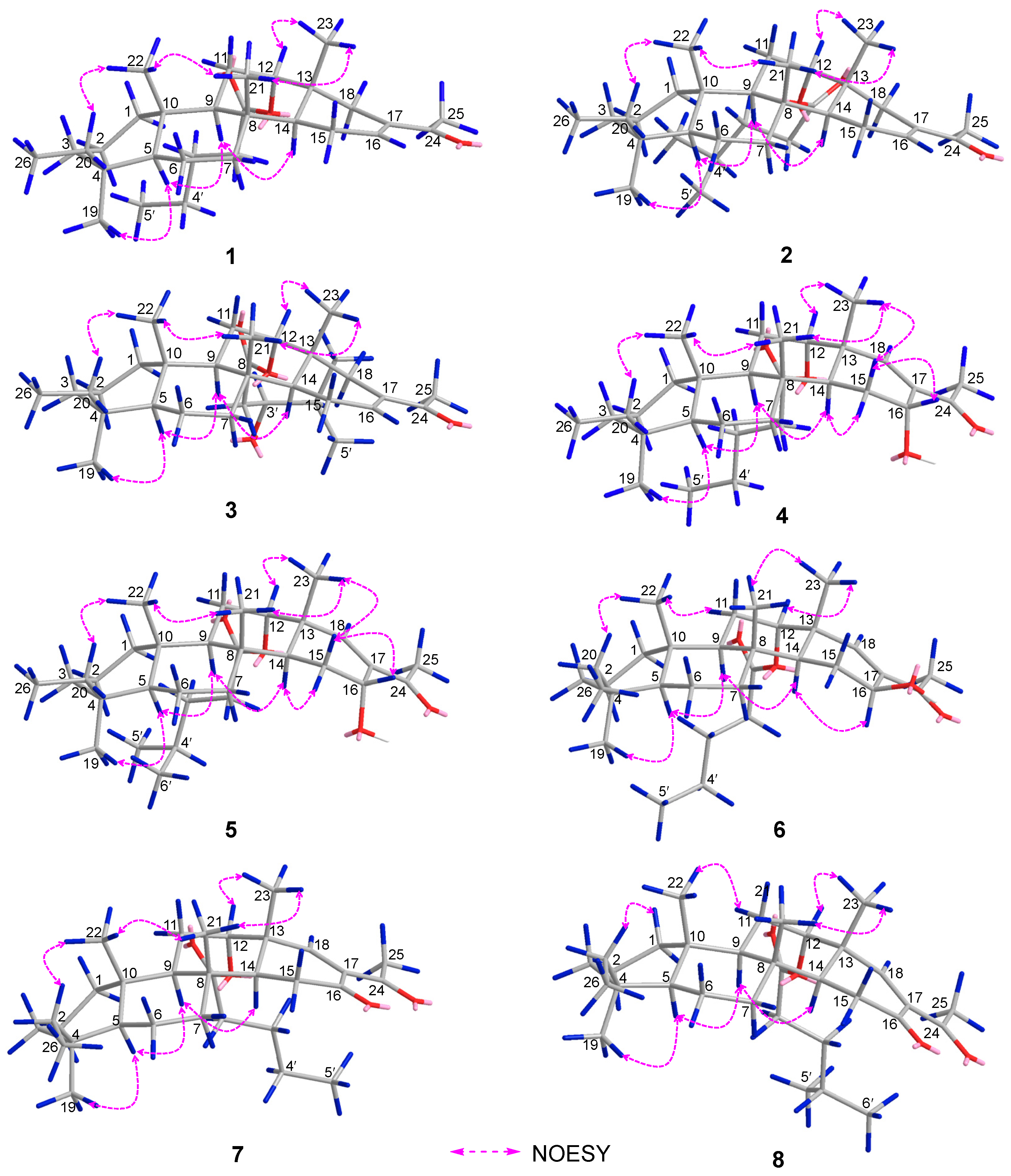
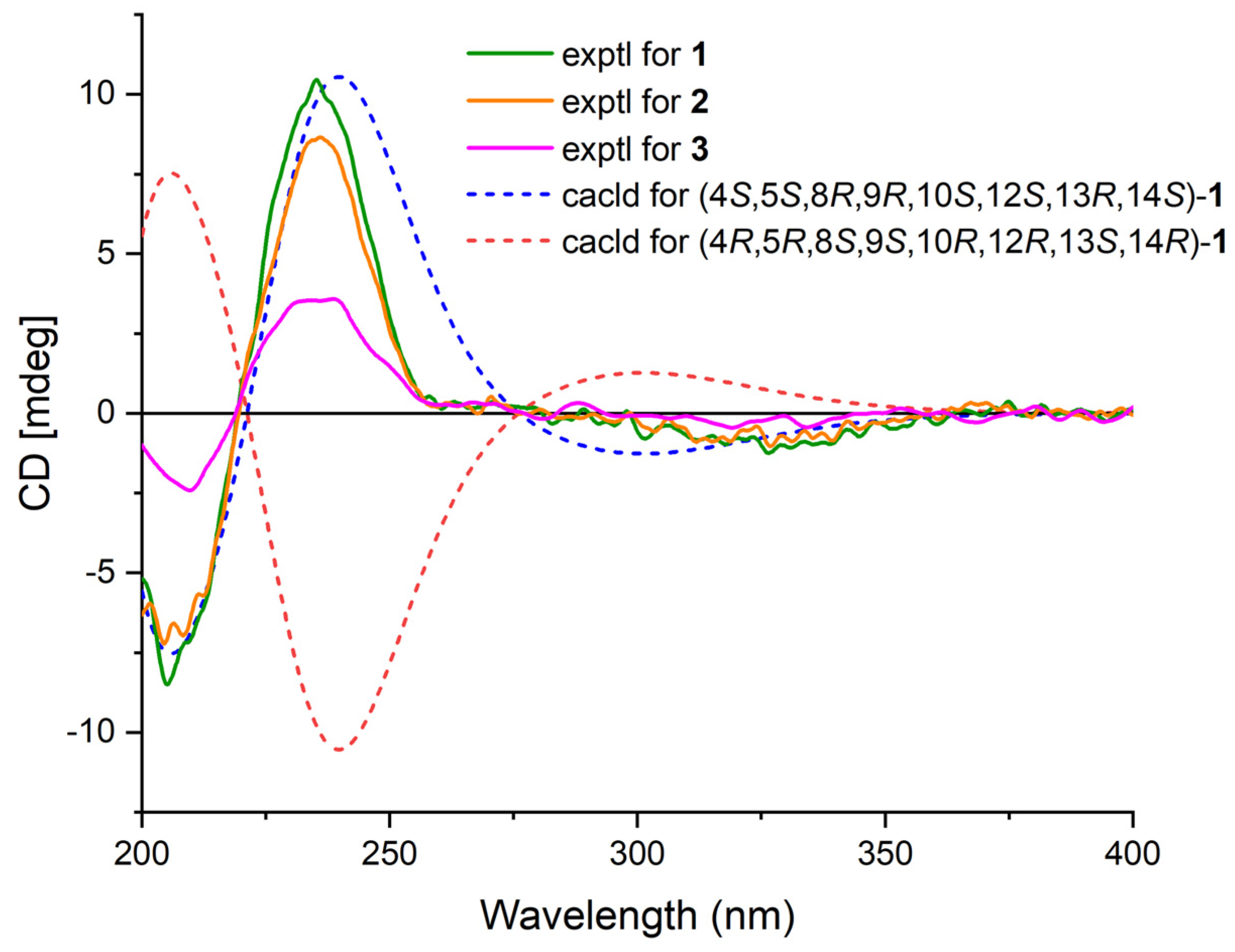
Phyllofenone F (1) was obtained as a white powder. Its molecular formula was determined to be C31H50O3 based on the molecular ion peak at [M + Na]+ m/z 493.3652, indicating the presence of seven degrees of unsaturation. The existence of a carbonyl group was confirmed by the IR absorption at 1723 cm–1 [4]. In the 1H NMR spectrum of 1 (Table 1), five methyl singlets were observed at δH 0.80, 0.83, 0.85, 0.92, and 2.28, along with two methyl triplets at δH 0.74 (t, 7.5) and 0.92 (t, 7.5) and one olefinic methine multiplet at δH 6.85. The 13C NMR and 135 DEPT spectra indicated the presence of seven methyl groups (including one ketone methyl at δC 25.3), 12 methylene groups, five methine groups (including one oxygenated at δC 76.7 and one olefinic at δC 139.5), and seven quaternary carbons (including one olefinic at δC 137.7, one ester carbonyl δC 172.9, and one ketone carbonyl at δC 199.0). The NMR data accounted for three degrees of unsaturation, suggesting a tetracyclic core structure in 1. The analysis of the 1D NMR data revealed that the C26 20,24-bishomo-25-norscalarane sesterterpene skeleton of 1 was closely related to the known compound phyllofenone A (Figure S85) [11]. The HMBC correlations (Figure 2) from H3-19 and H2-20 to C-3, C-4, and C-5, from H3-21 to C-7, C-8, C-9, and C-14, from H3-22 to C-1, C-5, C-9, and C-10, from H3-23 to C-12, C-13, C-14, and C-18, and from H-18α and H-18β to C-16 and C-17, together with the COSY correlations (Figure 2) of H-1α/H-2α/H-3α, H-5/H2-6/H2-7, H-9/H2-11/H-12, and H-14/H2-15/H-16, provided further evidence of the presence of an A/B/C/D ring scalarane system in 1 [11,12]. The COSY correlations from H2-2′ to H2-3′, H2-3′/H2-4′, and H2-4′/H3-5′ and HMBC correlations from H-12 and H2-2′ to C-1′ suggested that a valerate group was connected to ring C via the downfield shift carbon C-12 (δC 76.7). Additionally, HMBC correlations from H3-25 (δH 2.28) to C-17 and C-24 (δC 199.0) indicated the presence of an acetyl group at C-17. Therefore, the planar structure of 1 was established as a 6/6/6/6 tetracyclic scalarane sesterterpene. The relative configuration of 1 was deduced from a NOESY experiment. The NOESY correlations between H2-20/H3-22, H3-21/H3-22, H-12/H3-23, and H3-21/H3-23 indicated their β-orientation, whereas the NOESY correlations between H-5/H3-19, H-5/H-9, and H-9/H-14 suggested their α-orientation and therefore that all junctures for rings were trans A/B/C/D (Figure 3). Furthermore, the β-orientation of H2-19 and H-12 was also deduced from the 13C NMR chemical shift of CH2-20 (δC 24.5) and the small J-value (J = 2.5 Hz) observed for H-12 (δH 4.79), respectively [4,11]. Therefore, the relative configuration of 1 was determined. The CD spectrum of 1 exhibited a characteristic positive Cotton effect at 237 nm, and its specific rotation ( +73.5, c 0.1, MeCN) was almost identical to that of phyllofenone A (Figure S85) (CD 237 nm +6.2 MeCN; +8.0, c 0.3, MeOH) [11]. These findings indicated that 1 possessed the same absolute configuration as phyllofenone A, 4S, 5S, 8R, 9R, 10S, 12S, 13R, 14S, which was also supported by the comparison of the experimental and calculated ECD spectra (Figure 4).

Table 1.
1H (500 MHz) and 13C NMR (125 MHz) spectroscopic data of 1–3 in CDCl3.
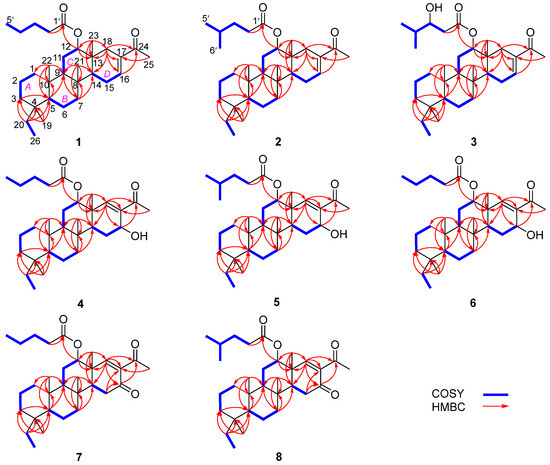
Figure 2.
Key COSY and HMBC correlations of 1–8.
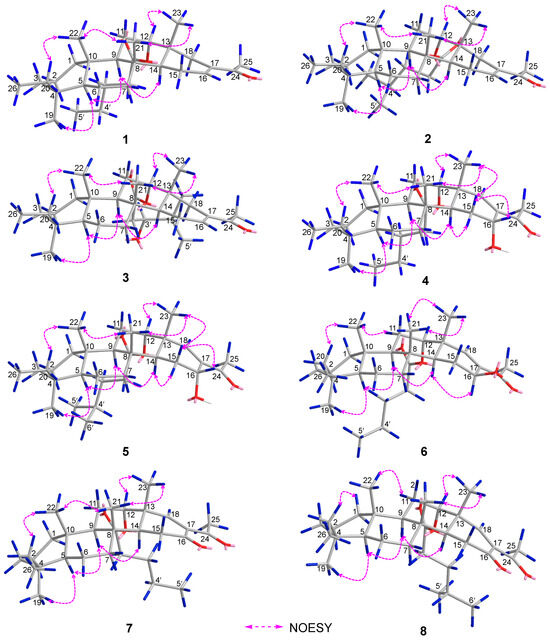
Figure 3.
Key NOESY correlations of 1–8.
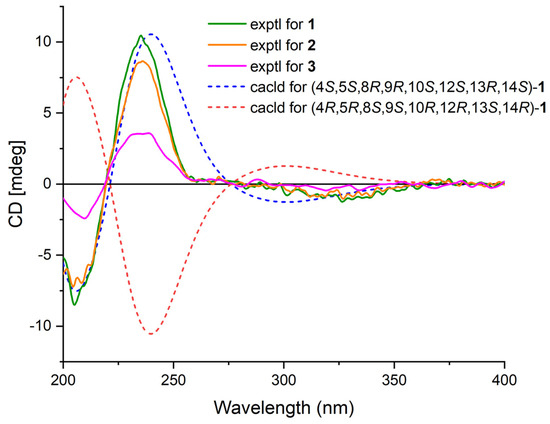
Figure 4.
Experimental ECD spectra of 1–3 and calculated ECD spectra of 1.
Phyllofenone G (2) was also isolated as a white powder. Its molecular formula was determined to be C32H52O3, with 11 degrees of unsaturation, based on the HRESIMS ion at m/z 507.3815 [M + Na]+. A thorough analysis of the 1D NMR data (Table 1) of 2 indicated that it belonged to the same class of scalarane skeletons as 1. However, notable differences between 1 and 2 were observed. Compound 2 exhibited additional methyl and methine signals, while one methylene resonance was absent, indicating that C-12 in 2 was substituted by a 4-methylpentanoate subunit. This was confirmed by the COSY correlations of H2-2′ (δH 2.32)/H2-3′ (δH 1.54), H2-3′/H-4′ (δH 1.60), H-4′/H3-5′ (δH 0.90), and H-4′/H3-6′ (δH 0.90), as well as the HMBC correlations from H2-2′ and H-12 (δH 4.80) to C-1′ (δC 173.1) (Figure 2). The similarity observed in the NOESY correlations (Figure 3), the coupling constant of H-12, and the CD spectra (Figure 4) between 1 and 2 indicated that 2 possessed the same absolute configuration as 1 [4,11].
Phyllofenone H (3), isolated as a white powder, showed a molecular formula of C32H52O4 based on HRESIMS, which is larger than that of 2 by 16 amu. The NMR data (Table 1) of 2 were almost identical to those of 3, supporting the presence of the same scalarane core. However, an obvious difference was noted, with 3 containing a 3-OH-4-methylpentanoate subunit instead of the 4-methylpentanoate subunit found in 2. The presence of the 3-OH-4-methylpentanoate group was confirmed by the COSY correlations of H2-2′a (δH 2.41)/H2-3′ (δH 3.79), H2-3′/H-4′ (δH 1.71), H-4′/H3-5′ (δH 0.97), and H-4′/H3-6′ (δH 0.93) and HMBC correlations from H2-2′a and H-12 (δH 4.85) to C-1′ (δC 172.9) (Figure 2), along the molecular formula and 13C NMR chemical shift of C-3′ (δC 72.7). The relative configuration of 3 was identical to that of 2 in the comparison of their chemical shifts, the coupling constant of H-12, NOESY correlations (Figure 3), and CD spectra (Figure 4) [4,11]. The 3′R configuration was determined by comparing the specific rotations of (S)-3-hydroxy-4-methylpentanoic acid ( −25.3, CHCl3, c 1.2) [13] with (R)-3-hydroxy-4-methylpentanoic acid ( +39.8, CHCl3, c 1.0) [14,15] and those of 3 ( +78.1, MeCN, c 0.1) with 2 ( +60.2, MeCN, c 0.1), respectively. Additionally, the R configuration for C-3′ was also confirmed by the specific rotation of the 3-hydroxy-4-methylpentanoic acid subunit ( +2.1, CHCl3, c 0.1), which was derived from the hydrolysis of 3 and subsequent purification by small-scale column chromatography on silica gel. The absolute configuration of 3 was determined as 4S, 5S, 8R, 9R, 10S, 12S, 13R, 14S, 3′R.
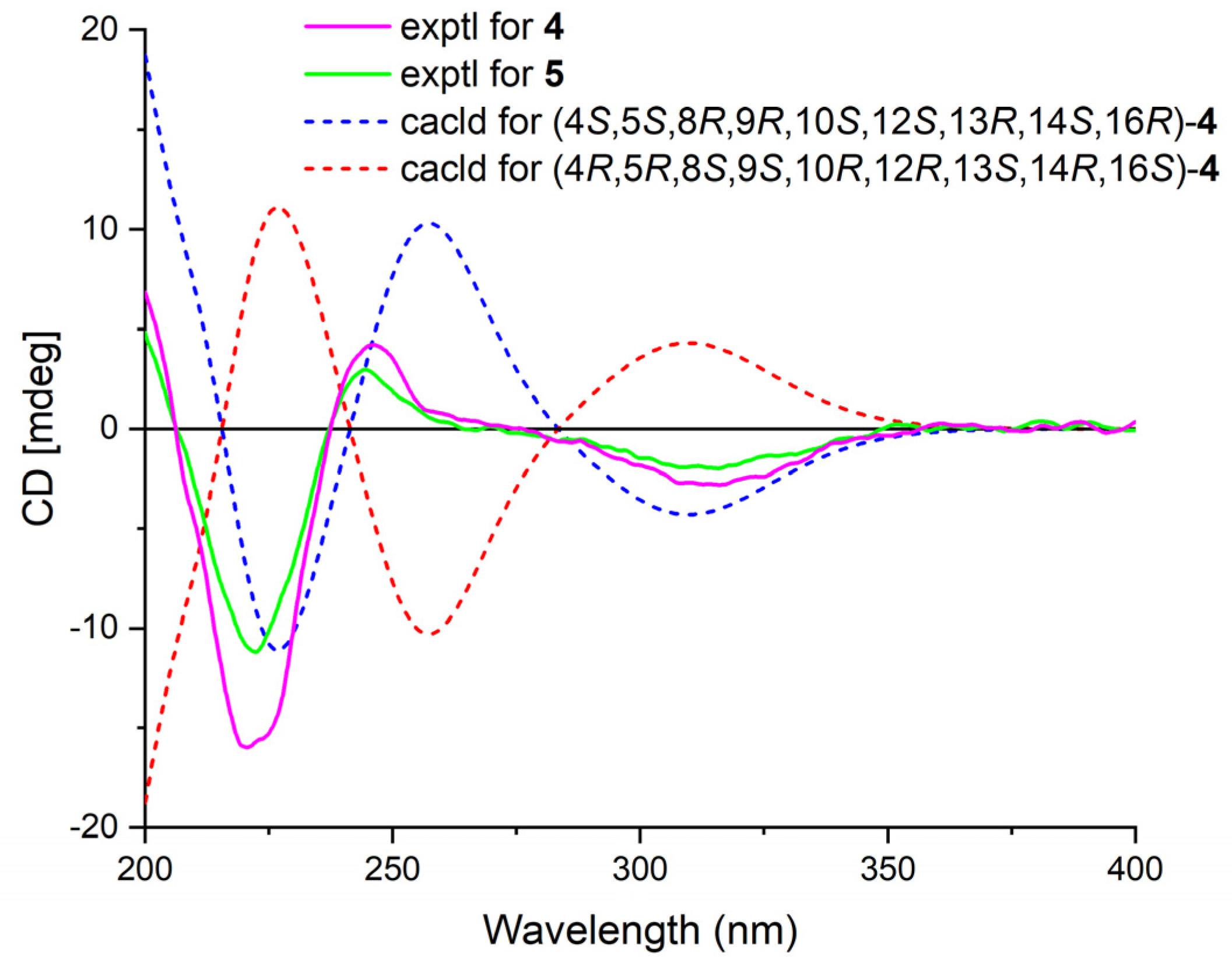
Phyllofenone I (4) was also isolated as a white powder, and its molecular formula was determined as C31H50O4 based on HRESIMS data (Table 2); it was 16 amu larger than that of 1. The IR spectrum indicated the presence of hydroxy (3535 cm−1) and carbonyl (1729 cm−1) groups [16]. Careful analysis of the NMR data revealed a 6/6/6/6 fused scalarane sesterterpene for 4, similar to 1 [16,17]. A notable distinction between these two compounds was the downshift of a single olefinic carbon from δC 139.5 in 1 to δC 152.2 in 4, as well as the presence of an oxygenated methine (δC 63.3, δH 4.56) in 4 instead of the methylene (δC 35.5, δH 2.21) found in 1. These differences suggested the existence of a C-17/C-18 double bond in ring D with hydroxy substitution at C-16. Additional HMBC correlations from H-16 to C-17 and C-14, from H-18 to C-13, C-17, and C-24, and from H3-23 to C-13, C-14, and C-18, along with consecutive COSY correlations of H-14/H2-15/H-16, confirmed this hypothesis (Figure 2). The NOESY correlations of H-5/H-9 and H3-19, H-7α/H-9 and H-14, and H-14/H-15α indicated that these protons were on the same face of the molecule. Other sets of NOESY correlations of H3-22/H3-21, H-20β, and H3-23, H3-23/H-12 and H-15β, and H-15β/H-16 and H3-21 indicated that these protons were located on the other face of the molecule (Figure 3). Based on the chemical shifts, the coupling constant of H-12, and the above NOESY data [4,11], the relative configuration of 4 was determined as depicted. Moreover, the quantum chemical electronic circular dichroism (ECD) calculation method was employed to determine the whole absolute configurations of 4. The calculated ECD spectrum of the 4S, 5S, 8R, 9R, 10S, 12S, 13R, 14S, 16R enantiomer approximately matched the Cotton effects observed in the experimental ECD spectrum of 4 (Figure 5), enabling the assignment of the absolute configuration as shown.

Table 2.
1H (500 MHz) and 13C NMR (125 MHz) spectroscopic data of 4–6 in CDCl3.
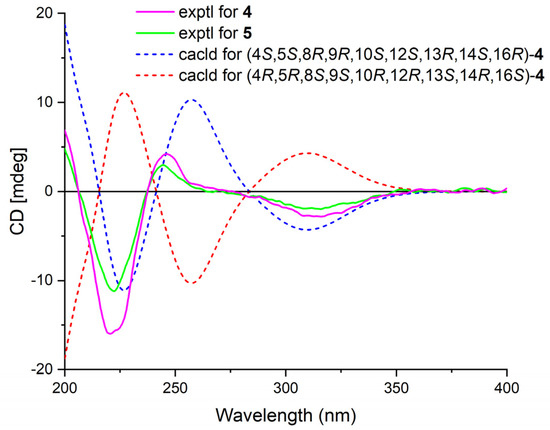
Figure 5.
Experimental ECD spectra of 4–5 and calculated ECD spectra of 4.
Phyllofenone J (5) was obtained as a white powder, and its molecular formula was determined to be C32H52O4 by HRESIMS analysis. The NMR data of 5 (Table 2) were almost identical to those for 4 except for the different substituent at C-12. In compound 5, a 4-methylpentanoate group was attached to C-12 instead of the valerate group present in 4. This was supported by the HMBC correlations from H-12 (δH 5.05) and H2-2′ (δH 1.48) to C-1′ (δC 173.8), as well as COSY correlations of H2-2′/H2-3′ (δH 2.30)/H-4′ (δH 1.55)/H3-5′ (δH 0.87) and H-4′/H3-6′ (δH 0.88) (Figure 2). Furthermore, the similarity of their NOESY correlations (Figure 3), the coupling constant of H-12, and the CD spectra (Figure 5) between 4 and 5 suggested that compound 5 possessed the same absolute configuration as 4 [4,11].
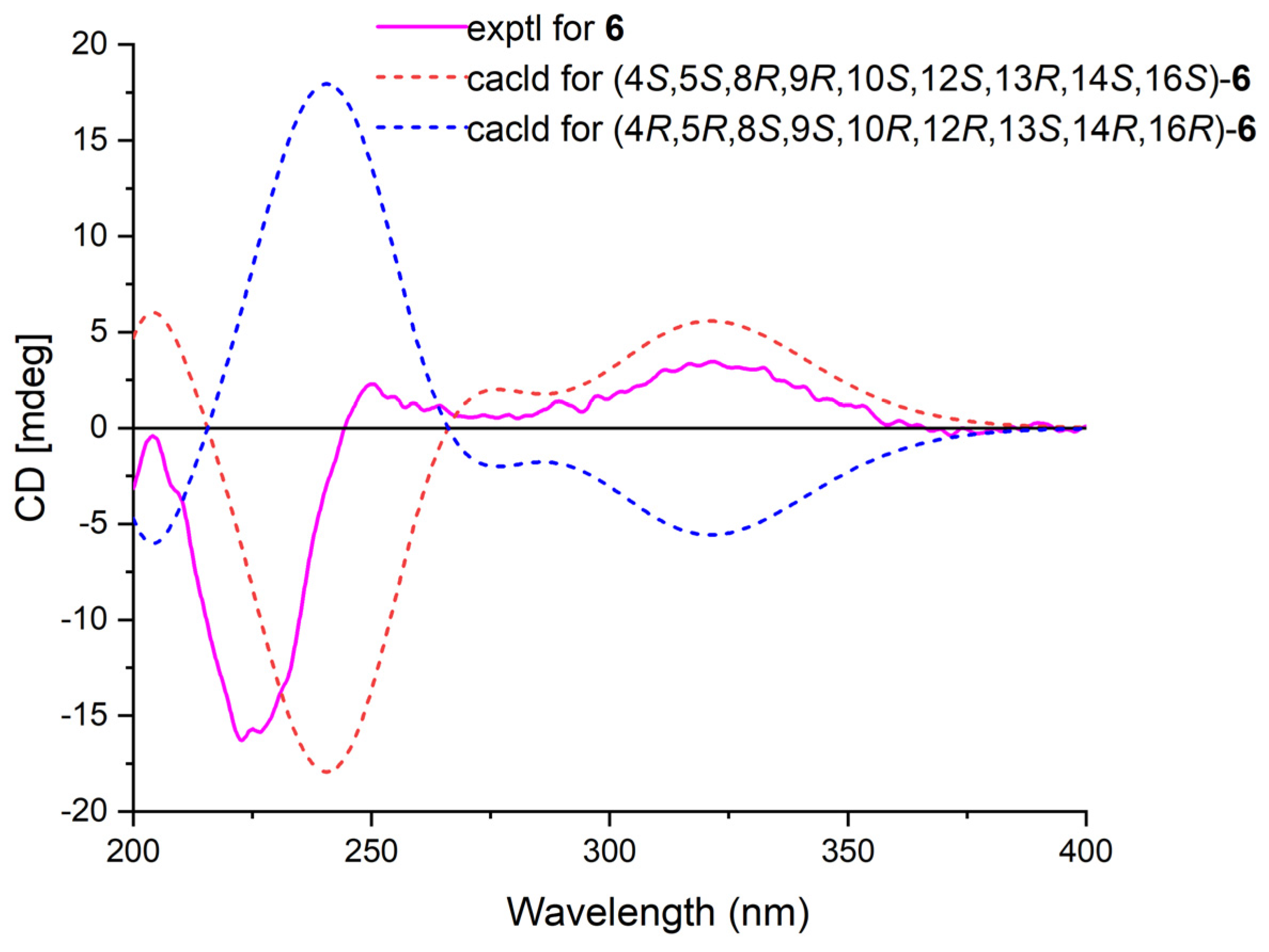
Phyllofenone K (6) was isolated as a white powder and assigned the molecular formula of C31H50O4 based on HRESIMS data. The NMR data of 6 (Table 2) closely resembled those of 4, suggesting that they shared the same planar scalarane structure. This hypothesis was confirmed by the HMBC and COSY correlations, as shown in Figure 2. Significant differences between 4 and 6 were observed in the downfield shifts of C-14 (from δC 43.8 in 4 to δC 47.3 in 6) and C-16 (from δC 63.3 in 4 to δC 68.0 in 6). These data indicated that these two compounds were a pair of epimers. Moreover, two groups of NOESY correlations, H-5/H-6α, H-5/H3-19, H-6α/H-9, H-7α/H-9, H-7α/H-14, H-7α/H-15α, and H-7α/H-16 and H2-20/H3-21, H2-20/H3-22, H-12/H3-23, H-15β/H3-23, and H3-21/H3-23, were detected from the NOESY spectrum (Figure 3). These two sets of correlations revealed the cofacial orientation of H-4, H-10, H-14, H-16, and H3-19, as well as H-12, H2-20, H3-21, H3-22, and H3-23, respectively. Subsequently, the absolute configuration of 6 was determined as 4S, 5S, 8R, 9R, 10S, 12S, 13R, 14S, 16S by comparing its calculated and experimental ECD spectra (Figure 6).
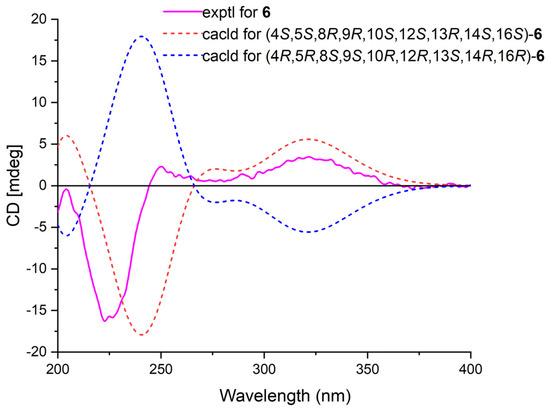
Figure 6.
Calculated and experimental ECD spectra of 6.
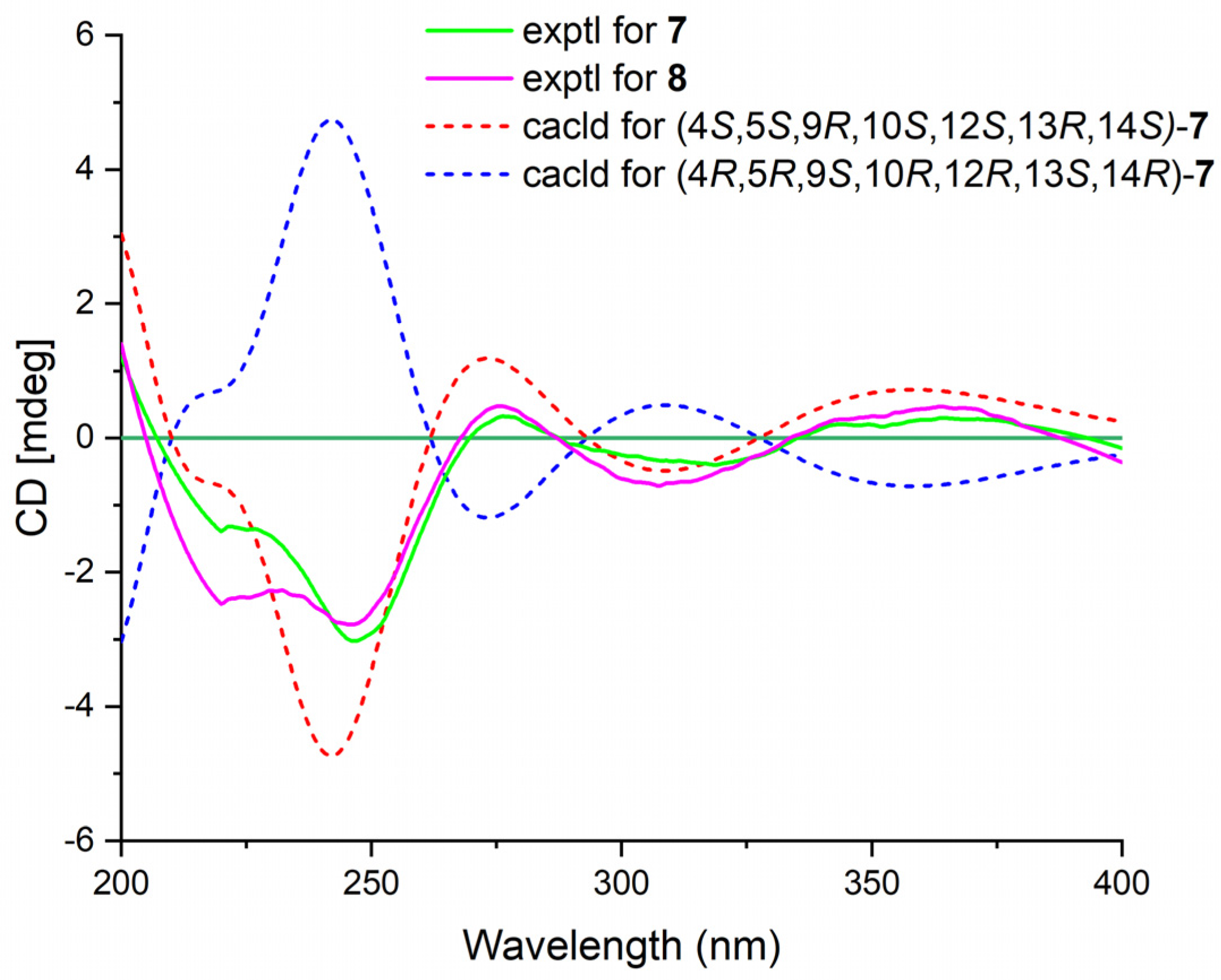
Phyllofenone L (7) was obtained as a white power. Its molecular formula was determined to be C31H48O4 based on the analysis of the HRESIMS spectrum. A detailed comparison of the NMR data (Table 3) between 7 and 4 indicated that they shared an almost identical scalarane skeleton. The main distinction between 4 and 7 was the absence of an oxygenated methine carbon (δC 63.3) and the presence of a carbonyl carbon (δC 197.8) at C-16 in 7. This deduction was supported by the HMBC correlations from H-18 and H3-25 to C-17 and C-24, from H2-15 to C-13, C-14, C-16, and C-17, and from H3-23 to C-13, C-14, and C-18 (Figure 2). The absolute configuration of 7 was determined to be 4S, 5S, 9R, 10S, 12S, 13R, 14S through CD spectral comparison (Figure 7), followed by analysis of the NOESY correlations, the coupling constant of H-12, and 13C NMR chemical shifts [4,11].

Table 3.
1H (500 MHz) and 13C NMR (125 MHz) spectroscopic data of 1 in CDCl3.
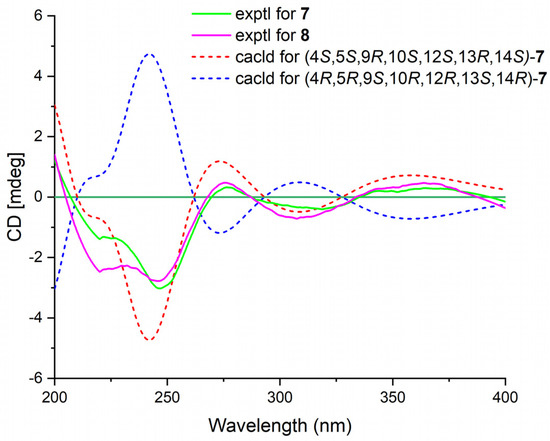
Figure 7.
Experimental ECD spectra of 7–8 and calculated ECD spectra of 7.
Phyllofenone M (8) was also obtained as a white power. The molecular formula, C32H50O4, was deduced from its HRESIMS data (m/z 521.3608 [M + Na]+). Compound 8 showed chemical shifts (Table 3) that were nearly identical to those of 7, with the only differences being similar to those found between 4 and 5. Correlations observed in the 2D NMR spectra (Figure 2) confirmed the same scalarane core between 7 and 8, with the 4-methylpentanoate group substituted at C-12 in 8 instead of the valerate group in 7. By comparing the CD spectra (Figure 7) and specific rotation values between 7 and 8, the absolute configuration of 8 was unequivocally established as 4S, 5S, 9R, 10S, 12S, 13R, 14S.
The known compounds carteriofenones B and A (9–10) were also isolated from P. foliascens and were completely characterized via a comparison of their NMR data with those previously reported [18].
The cytotoxic activity of compounds 1–10 was tested against HeLa, HCT-116, H460, and SW1990 by the CCK-8 method. Among them, only compound 5 exhibited significant cytotoxic activity against the above cancer cell lines, with IC50 values ranging from 3.4 to 7.3 μM (Table 4). Comparing the relative potency of these scalaranes revealed that the substitution of a 4-methylpentanoate group at C-12 increased the cytotoxicity compared to a valerate group at C-12. This observation is consistent with previously reported results [4]. Additionally, the α-OH substitution at C-16 in 4, compared to the β-OH substitution at C-16 in 6, led to increased cytotoxic activity. Furthermore, these derivatives were also assayed for antibacterial activity against Vibrio parahaemolyticus, V. alginolyticus, V. cholerae, V. vulnificus, Staphylococcus aureus, and Escherichia coli by the minimum inhibitory concentration (MIC) method. Only compounds 4 and 6 displayed weak activity against S. aureus and E. coli, with MIC values of 16 μg/mL and 8 μg/mL, respectively.

Table 4.
Cytotoxicity of compounds 1−10 to human cancer cell lines (IC50 in μM).
3. Materials and Methods
3.1. General Experimental Procedures
Optical rotations were recorded on a Perkin-Elmer model 341 polarimeter (Perkin-Elmer Inc., Waltham, MA, USA). The UV and CD spectra were recorded on a UV-8000 spectrophotometer (Shanghai Metash instruments Co., Ltd., Shanghai, China) and a Jasco J-715 spectropolarimeter in MeOH (JASCO, Tokyo, Japan), respectively. The IR spectra were obtained on a VERTEX 70v FT-IR spectrometer (Bruker Biospin Corp., Billerica, MA, USA). The NMR experiments were conducted on a Bruker AMX-500 instrument (Bruker Biospin Corp., Billerica, MA, USA). HRESIMS spectra were recorded on an AB SCIEX Triple Tof-4600 spectrometer (ABSciex, Vaughan ON, Canada). Column chromatographic separations were carried out using silica gel (200–300 mesh, Qingdao Ocean Chemical Co., Ltd., Qingdao, China) and ODS (50 μm, YMC Co., Ltd., Kyoto, Japan). MPLC was carried out on a SepaBean machine (Santai Technology Co., Ltd., Changzhou, China). RP HPLC was performed on a YMC-Pack Pro C18 column (250 × 10 mm, 5 mm, YMC Co., Ltd., Kyoto, Japan) using a Waters 1525 binary HPLC pump with a Waters 2998 photodiode array detector (Waters Corp., Milford, MA, USA). Analytical thin-layer chromatography was performed on silica gel HSGF254 plates and visualized by spraying with anisaldehyde–H2SO4 reagent.
3.2. Sponge Material
The marine sponge was collected off the Woody Island in the South China Sea in December 2021 and authenticated by Prof. Prof. Hou-Wen Lin. A voucher specimen (XS-2021.12) has been deposited at the Naval Medical Center of PLA, Naval Medical University.
3.3. Extraction and Isolation
The sponge (0.6 kg, dry weight) was extracted three times with MeOH/CH2Cl2 (v/v 1:1, 1 L). The crude extract (29.7 g), obtained after evaporating the MeOH/CH2Cl2 was separated into 11 fractions (Fr. A−K) by a silica gel column with gradient petroleum ether/EtOAc (100:1, 50:1, 25:1, 10:1, 5:1, 3:1, 1:1, 0:1, v/v). Fraction F (3.72 g) was subjected to a silica gel column with a gradient elution of petroleum ether/EtOAc (1:0, 20:1, 15:1, 10:1, 5:1, 0:1, v/v) to afford six subfractions (Fr. F1−F6). Fr. F6 (128.4 mg) was further purified by reversed-phase semi-preparative HPLC (95% CH3OH/5% H2O, 2.0 mL/min, 236 nm), yielding 1 (5.3 mg, tR = 32.0 min) and 2 (4.5 mg, tR = 36.0 min). Fraction G (4.26 g) was subjected to a silica gel column eluted with a gradient of petroleum ether/EtOAc (100:1, 80:1, 50:1, 30:1, 20:1, 10:1, 5:1, 0:1, v/v) to obtain eight fractions (Fr. G1−G8). Fr. G3 (64.5 mg) was purified with 90% CH3OH/10% H2O by HPLC (254 nm, 2.0 mL/min) to afford 7 (5.5 mg, tR = 39.8 min) and 8 (6.3 mg, tR = 45.9 min). Fr. G4 (444.1 mg) was chromatographed over ODS using a gradient elution of MeOH/H2O (from 40% to 100%) to give eight fractions, G4a−G4h. Fr. G4b (33.4 mg) was purified by semi-preparative HPLC (90% CH3OH/10% H2O, 2 mL/min, 225 nm) to provide 5 (2.5 mg, tR = 32.0 min). Fr. G4c (60.1 mg) was separated by HPLC (90% CH3OH/10% H2O, 2 mL/min, 235 nm) to obtain 9 (5.0 mg, tR = 26.6 min) and 10 (2.6 mg, tR = 28.9 min). Fr. G4d (54.2 mg) was purified with 88% CH3CN/12% H2O via HPLC (2.0 mL/min, 234 nm) to afford 6 (2.2 mg, tR = 48.0 min) and 3 (2.8 mg, tR = 54.0 min). Fr. G4g (33.0 mg) was further purified with 95% CH3CN via HPLC (2.0 mL/min, 238 nm) to afford 4 (2.1 mg, tR = 41.0 min).
Phyllofenone F (1): white powder; +73.5 (c 0.1, MeCN); UV (MeCN) λmax 209 (3.61), 232 (3.93) nm; CD (MeCN) (Δε) 205 (−12.1), 235 (+14.9); IR (KBr) vmax 2956, 922, 2853, 1723, 1644, 1456, 1383, 1289, 1259, 1240, 1170, 1087, 1033, 956, 817, 801, 732, 604 cm–1; HRESIMS m/z [M + Na]+ 493.3653 (Calcd. C31H50O3Na, 493.3652, Δ = 0.2 ppm); for 1H and 13C NMR data, see Table 1.
Phyllofenone G (2): white powder; +60.2 (c 0.1, MeCN); UV (MeCN) λmax 209 (3.46), 233 (3.85) nm; CD (MeCN) (Δε) 205 (−10.5), 237 (+12.6); IR (KBr) vmax 2955, 2924, 2870, 2850, 1729, 1666, 1645, 1465, 1348, 1258, 1166, 1122, 1099, 1070, 1033, 1006, 991, 954, 935, 817, 601, 574 cm–1; HRESIMS m/z [M + Na]+ 507.3815 (Calcd. C32H52O3Na, 507.3809, Δ = 1.2 ppm); for 1H and 13C NMR data, see Table 1.
Phyllofenone H (3): white powder; +78.1 (c 0.1, MeCN); UV (MeCN) λmax 229 (3.73) nm; CD (MeCN) (Δε) 208 (−3.5), 235 (+5.3); IR (KBr) vmax 3459, 2956, 2925, 2871, 2852, 1731, 1668, 1462, 1425, 1384, 1350, 1301, 1257, 1167, 1130, 1099, 1033, 1005, 959, 860, 818, 721, 668, 630, 599, 574 cm–1; HRESIMS m/z [M + Na]+ 523.3757 (Calcd. C32H52O4Na, 523.3758, Δ = −0.2 ppm); for 1H and 13C NMR data, see Table 1.
Phyllofenone I (4): white powder; −53.2 (c 0.1, MeCN); UV (MeCN) λmax 205 (4.09), 224 (4.27) nm; CD (MeCN) (Δε) 220 (−23.6), 246 (+6.2), 313 (−4.1); IR (KBr) vmax 3535, 2957, 2930, 2872, 1729, 1661, 1461, 1381, 1252, 1175, 1093, 1034, 1015, 966, 931, 867, 568 cm–1; HRESIMS m/z [M + Na]+ 509.3599 (Calcd. C31H50O4Na, 509.3601, Δ = −0.6 ppm); for 1H and 13C NMR data, see Table 2.
Phyllofenone J (5): white powder; −55.6 (c 0.1, MeCN); UV (MeCN) λmax 202 (3.73), 226 (4.07) nm; CD (MeCN) (Δε) 223 (−16.9), 245 (+4.5), 313 (−2.9); IR (KBr) vmax 3540, 2956, 2929, 2871, 1728, 1660, 1462, 1384, 1268, 1225, 1177, 1100, 1034, 1013, 963, 931, 885, 867, 776, 713, 632, 567, 461 cm–1; HRESIMS m/z [M + Na]+ 523.3759 (Calcd. C32H52O4Na, 523.3758 Δ = 0.1 ppm); for 1H and 13C NMR data, see Table 2.
Phyllofenone K (6): white powder; −14.9 (c 0.1, MeCN); UV (MeCN) λmax 208 (3.94), 228 (4.10) nm; CD (MeCN) (Δε) 223 (−24.0), 250 (+3.4), 322 (+5.1); IR (KBr) vmax 3235, 2956, 2927, 2871, 1730, 1655, 1462, 1370, 1294, 1252, 1172, 1093, 1077, 1052, 1033, 1004, 885, 675, 632, 604, 517 cm–1; HRESIMS m/z [M + Na]+ 509.3605 (Calcd. C31H50O4Na, 509.3601, Δ = 0.8 ppm); for 1H and 13C NMR data, see Table 2.
Phyllofenone L (7): white powder; +14.8 (c 0.1, MeCN); UV (MeCN) λmax 211 (3.65), 224 (3.68) nm; CD (MeCN) (Δε) 247 (−4.4), 276 (+0.4), 310 (−1.0), 365 (+0.4); IR (KBr) vmax 2957, 2929, 2872, 1770, 1733, 1692, 1678, 1601, 1461, 1418, 1381, 1356, 1288, 1272, 1243, 1227, 1168, 1129, 1107, 1087, 1048, 1034, 1004, 984, 962, 925 cm–1; HRESIMS m/z [M + Na]+ 507.3438 (Calcd. C31H48O4Na, 507.3445, Δ = −1.5 ppm); for 1H and 13C NMR data, see Table 3.
Phyllofenone M (8): white powder; +21.9 (c 0.1, MeCN); UV (MeCN) λmax 211 (3.54), 225 (3.58) nm; CD (MeCN) (Δε) 247 (−4.2), 276 (0.7), 309 (−1.0), 365 (+0.7); IR (KBr) vmax 2956, 2925, 2871, 2852, 1735, 1693, 1679, 1601, 1465, 1417, 1357, 1272, 1243, 1227, 1165, 1110, 1034 cm–1; HRESIMS m/z [M + Na]+ 521.3608 (Calcd. C32H50O4Na, 521.3601, Δ = 1.4 ppm); for 1H and 13C NMR data, see Table 3.
3.4. Acid Hydrolysis of Phyllofenone H (3)
A solution of phyllofenone H (3) (1.0 mg in 6 M HCl, 1 mL) was heated to 110 °C in a sealed vial and maintained at 110 °C for 12 h. Subsequently, the hydrolysate was evaporated to dryness under a stream of dry N2 and subjected to purification through a small column (6 × 70 mm) on silica gel (200 mesh) eluted with CH2Cl2/MeOH (20:1, v/v) to give (R)-3-hydroxy-4-methylpentanoic acid [19].
(R)-3-hydroxy-4-methylpentanoic acid: colorless oil; +2.1 (c 0.10, CHCl3); ESIMS m/z 155.15 [M + Na]+; 1H NMR (500 MHz, CDCl3): δH 3.81 (1H, m), 2.49 (1H, dd, 16.5, 3.5), 2.59 (1H, 16.5, 9.0), 1.72 (1H, m), 0.96 (3H, d, 6.5 Hz), 0.95 (3H, d, 6.5 Hz).
3.5. Biological Assays
The cytotoxicity of compounds 1–10 against the HeLa, HCT-116, H460, and SW1990 human cancer cell lines was determined using the CCK-8 method [4,20], with cisplatin used as a positive control. The antimicrobial activity of compounds 1–10 against Vibrio parahaemolyticus, V. alginolyticus, V. cholerae, V. vulnificus, Staphylococcus aureus, and Escherichia coli was evaluated as previously described [4], with levofloxacin used as a positive control.
4. Conclusions
Eight new scalarane sesterterpenes, phyllofenones F–M (1–8), along with two known analogues, carteriofenones B and A (9–10), were isolated from a South China Sea sponge, P. foliascens. In conjunction with our previous chemical study of the same organism, we discovered that a 4-methylpentanoate group substituted at C-12 positively affected the activity. Additionally, compound 5 displayed notable cytotoxicity, highlighting the significance of the α-OH substitution at C-16 for its cytotoxic properties. Collectively, this research contributes to the expansion of the chemical molecular diversity of the scalarane sesterterpene family.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/md21100507/s1, S1. Quantum chemical CD calculation of compound 1; S2. Quantum chemical CD calculation of compound 4; S3. Quantum chemical CD calculation of compound 6; S4. Quantum chemical CD calculation of compound 7; S5. 1H NMR spectrum of phyllofenone F (1) in CDCl3; S6. 13C NMR spectrum of phyllofenone F (1) in CDCl3; S7. DEPT135 spectrum of phyllofenone F (1) in CDCl3; S8. HSQC spectrum of phyllofenone F (1) in CDCl3; S9. COSY spectrum of phyllofenone F (1) in CDCl3; S10. HMBC spectrum of phyllofenone F (1) in CDCl3; S11. NOESY spectrum of phyllofenone F (1) in CDCl3; S12. HRESIMS of phyllofenone F (1); S13. UV spectrum of phyllofenone F (1); S14. IR spectrum of phyllofenone F (1) (KBr); S15. 1H NMR spectrum of phyllofenone G (2) in CDCl3; S16. 13C NMR spectrum of phyllofenone G (2) in CDCl3; S17. DEPT135 spectrum of phyllofenone G (2) in CDCl3; S18. HSQC spectrum of phyllofenone G (2) in CDCl3; S19. COSY spectrum of phyllofenone G (2) in CDCl3; S20. HMBC spectrum of phyllofenone G (2) in CDCl3; S21. NOESY spectrum of phyllofenone G (2) in CDCl3; S22. HRESIMS of phyllofenone G (2); S23. UV spectrum of phyllofenone G (2) in MeOH; S24 IR spectrum of phyllofenone G (2) (KBr); S25. 1H NMR spectrum of phyllofenone H (3) in CDCl3; S26. 13C NMR spectrum of phyllofenone H (3) in CDCl3; S27. DEPT135 spectrum of phyllofenone H (3) in CDCl3; S28. HSQC spectrum of phyllofenone H (3) in CDCl3; S29. COSY spectrum of phyllofenone H (3) in CDCl3; S30. HMBC spectrum of phyllofenone H (3) in CDCl3; S31. NOESY spectrum of phyllofenone H (3) in CDCl3; S32. HRESIMS of phyllofenone H (3); S33. UV spectrum of phyllofenone H (3). S34. IR spectrum of phyllofenone H (3) (KBr); S35. 1H NMR spectrum of phyllofenone I (4) in CDCl3; S36. 13C NMR spectrum of phyllofenone I (4) in CDCl3; S37. DEPT135 spectrum of phyllofenone I (4) in CDCl3; S38. HSQC spectrum of phyllofenone I (4) in CDCl3; S39. COSY spectrum of phyllofenone I (4) in CDCl3; S40. HMBC spectrum of phyllofenone I (4) in CDCl3; S41. NOESY spectrum of phyllofenone I (4) in CDCl3; S42. HRESIMS of phyllofenone I (4); S43. UV spectrum of phyllofenone I (4); S44. IR spectrum of phyllofenone I (4) (KBr); S45. 1H NMR spectrum of phyllofenone J (5) in CDCl3; S46. 13C NMR spectrum of phyllofenone J (5) in CDCl3; S47. DEPT135 spectrum of phyllofenone J (5) in CDCl3; S48. HSQC spectrum of phyllofenone J (5) in CDCl3; S49. COSY spectrum of phyllofenone J (5) in CDCl3; S50. HMBC spectrum of phyllofenone J (5) in CDCl3; S51. NOESY spectrum of phyllofenone J (5) in CDCl3; S52. HRESIMS of phyllofenone J (5); S53. UV spectrum of phyllofenone J (5); S54. IR spectrum of phyllofenone J (5) (KBr); S55. 1H NMR spectrum of phyllofenone K (6) in CDCl3; S56. 13C NMR spectrum of phyllofenone K (6) in CDCl3; S57. DEPT135 spectrum of phyllofenone K (6) in CDCl3; S58. HSQC spectrum of phyllofenone K (6) in CDCl3; S59. COSY spectrum of phyllofenone K (6) in CDCl3; S60. HMBC spectrum of phyllofenone K (6) in CDCl3; S61. NOESY spectrum of phyllofenone K (6) in CDCl3; S62. HRESIMS of phyllofenone K (6); S63. UV spectrum of phyllofenone K (6); S64. IR spectrum of phyllofenone K (6) (KBr); S65. 1H NMR spectrum of phyllofenone L (7) in CDCl3; S66. 13C NMR spectrum of phyllofenone L (7) in CDCl3; S67. DEPT135 spectrum of phyllofenone L (7) in CDCl3; S68. HSQC spectrum of phyllofenone L (7) in CDCl3; S69. COSY spectrum of phyllofenone L (7) in CDCl3; S70. HMBC spectrum of phyllofenone L (7) in CDCl3; S71. NOESY spectrum of phyllofenone L (7) in CDCl3; S72. HRESIMS of phyllofenone L (7); S73. UV spectrum of phyllofenone L (7) in MeOH; S74. IR spectrum of phyllofenone L (7) (KBr); S75. 1H NMR spectrum of phyllofenone M (8) in CDCl3; S76. 13C NMR spectrum of phyllofenone M (8) in CDCl3; S77. DEPT135 spectrum of phyllofenone M (8) in CDCl3; S78. HSQC spectrum of phyllofenone M (8) in CDCl3; S79. COSY spectrum of phyllofenone M (8) in CDCl3; S80. HMBC spectrum of phyllofenone M (8) in CDCl3; S81. NOESY spectrum of phyllofenone M (8) in CDCl3; S82. HRESIMS of phyllofenone M (8); S83. UV spectrum of phyllofenone M (8); S84. IR spectrum of phyllofenone M (8) (KBr); S85. The structure of phyllofenone A.
Author Contributions
H.-B.Y. designed this study and drafted the work. H.-B.Y., B.H. and X.-L.M. performed the collection, extraction, isolation, and structure elucidation. Z.N. and B.H. performed the bioactivity evaluation. Y.H. and Z.-F.Y. contributed to checking the isolation process. B.-H.J. checked the structure elucidation process. H.-W.L. and X.-Y.L. supervised the laboratory work and contributed to the critical proofreading and revision of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the National Key Research and Development Project (Nos. 2022YFC2804500 and 2022YFC2804105), the Natural Science Foundation of Shanghai (Nos. 21ZR1479500 and 20ZR1470600), and the Shanghai Pujiang Program (No. 2020PJD082).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gaudêncio, S.P.; Bayram, E.; Lukić Bilela, L.; Cueto, M.; Díaz-Marrero, A.R.; Haznedaroglu, B.Z.; Jimenez, C.; Mandalakis, M.; Pereira, F.; Reyes, F.; et al. Advanced Methods for Natural Products Discovery: Bioactivity Screening, Dereplication, Metabolomics Profiling, Genomic Sequencing, Databases and Informatic Tools, and Structure Elucidation. Mar. Drugs 2023, 21, 308–373. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.A. Scalarane sesterterpenoids. Curr. Bioact. Compd. 2010, 6, 178–206. [Google Scholar] [CrossRef]
- Yu, H.-B.; Chen, H.-Y.; Duan, S.; Zhu, Y.-P.; Hu, B.; He, Y.; Cheng, S.-T.; Jiao, B.-H.; Liu, X.-Y. Bioactive Scalarane-type Sesterterpenoids from Marine Sources. Chem. Biodivers. 2022, 19, e202200049. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.-B.; Hu, B.; Wu, G.-F.; Ning, Z.; He, Y.; Jiao, B.-H.; Liu, X.-Y.; Lin, H.-W. Phyllospongianes A–E, Dinorscalarane Sesterterpenes from the Marine Sponge Phyllospongia foliascens. J. Nat. Prod. 2023, 86, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
- Fattorusso, E.; Magno, S.; Santacroce, C.; Sica, D. Scalarin, a new pentacyclic C-25 terpenoid from the sponge Cacospongia scalaris. Tetrahedron 1972, 28, 5993–5997. [Google Scholar] [CrossRef]
- Zhou, M.; Peng, B.-R.; Tian, W.; Su, J.-H.; Wang, G.; Lin, T.; Zeng, D.; Sheu, J.-H.; Chen, H. Deacetyl-12-epi-scalaradial, a scalarane sesterterpenoid from a marine sponge Hippospongia sp., induces HeLa cells apoptosis via MAPK/ERK pathway and modulates nuclear receptor Nur77. Mar. Drugs 2020, 18, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.-B.; Hong, L.-L.; Shang, R.-Y.; Liu, H.-Y.; Zhang, L.; Liu, L.-Y.; Zhao, L.; Zhang, W.; Sun, F.; Jiao, W.-H.; et al. Dysiscalarones A–E, scalarane sesterterpenoids with nitric oxide production inhibitory activity from marine sponge Dysidea granulosa. Bioorg. Chem. 2021, 111, 104791–104799. [Google Scholar] [CrossRef] [PubMed]
- Hertzer, C.; Kehraus, S.; Boehringer, N.; Kaligis, F.; Bara, R.; Erpenbeck, D.; Woerheide, G.; Schaeberle, T.F.; Waegele, H.; Koenig, G.M. Antibacterial scalarane from Doriprismatica stellata nudibranchs (Gastropoda, Nudibranchia), egg ribbons, and their dietary sponge Spongia cf. agaricina (Demospongiae, Dictyoceratida). Beilstein J. Org. Chem. 2020, 16, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Abdjul, D.B.; Takahashi, O.; Kirikoshi, R.; Namikoshi, M.; Yamazaki, H.; Mangindaan, R.E.P. Two new protein tyrosine phosphatase 1B inhibitors, hyattellactones A and B, from the Indonesian marine sponge Hyattella sp. Bioorg. Med. Chem. Lett. 2015, 25, 904–907. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.-H.; Hong, L.-L.; Jiao, W.-H.; Lin, H.-W. Natural sesquiterpene quinone/quinols: Chemistry, biological activity, and synthesis. Nat. Prod. Rep. 2023, 40, 718–749. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Fu, X.; Su, J.; Chen, S.; Snyder, J.K. Phyllofenone A, a New Scalarane Sesterterpene from the Sponge Phyllospongia Foliascens (Pallas). Chem. Res. Chin. Univ. 1991, 7, 100–106. [Google Scholar]
- Hassan, M.H.A.; Rateb, M.E.; Hetta, M.; Abdelaziz, T.A.; Sleim, M.A.; Jaspars, M.; Mohammed, R. Scalarane sesterterpenes from the Egyptian Red Sea sponge Phyllospongia lamellosa. Tetrahedron 2015, 71, 577–583. [Google Scholar] [CrossRef]
- Pellicena, M.; Solsona, J.G.; Romea, P.; Urpí, F. Stereoselective titanium-mediated aldol reactions of α-benzyloxy methyl ketones. Tetrahedron 2012, 68, 10338–10350. [Google Scholar] [CrossRef]
- Lorente, A.; Pellicena, M.; Romea, P.; Urpí, F. Stereoselective acetate aldol reactions of α-silyloxy ketones. Tetrahedron 2015, 71, 1023–1035. [Google Scholar] [CrossRef]
- Orsini, F.; Sello, G.; Manzo, A.M.; Lucci, E.M. (1S,2S)-1-Amino-2-hydroxy-1,2,3,4-tetrahydronaphthalene: A new chiral auxiliary for asymmetric Reformatsky reactions. Tetrahedron Asymmetr. 2005, 16, 1913–1918. [Google Scholar] [CrossRef]
- Lan, W.-J.; Li, H.-J. New sesterterpenoids from the marine sponge Phyllospongia papyracea. Helv. Chim. Acta 2007, 90, 1218–1222. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, Y.; Yang, L.; Luo, X.; de Voogd, N.J.; Tang, X.; Li, P.; Li, G. Bishomoscalarane Sesterterpenoids from the Sponge Dysidea granulosa Collected in the South China Sea. J. Nat. Prod. 2020, 83, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Wu, Z.-H.; Shao, C.-L.; Pang, S.; Liang, X.-Y.; de Voogd, N.J.; Wang, C.-Y. Cytotoxic scalarane sesterterpenoids from the South China Sea sponge Carteriospongia foliascens. Org. Biomol. Chem. 2015, 13, 4016–4024. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.-B.; Glukhov, E.; Li, Y.; Iwasaki, A.; Gerwick, L.; Dorrestein, P.C.; Jiao, B.-H.; Gerwick, W.H. Cytotoxic Microcolin Lipopeptides from the Marine Cyanobacterium Moorea producens. J. Nat. Prod. 2019, 82, 2608–2619. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.-B.; Gu, B.-B.; Iwasaki, A.; Jiang, W.-L.; Ecker, A.; Wang, S.-P.; Yang, F.; Lin, H.-W. Dactylospenes A–E, Sesterterpenes from the Marine Sponge Dactylospongia elegans. Mar. Drugs 2020, 18, 491–501. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).