Marine Chitooligosaccharide Alters Intestinal Flora Structure and Regulates Hepatic Inflammatory Response to Influence Nonalcoholic Fatty Liver Disease
Abstract
:1. Introduction
2. Results
2.1. Changes in Body Weight, Food Intake, and Serum Glucose and Lipid Levels
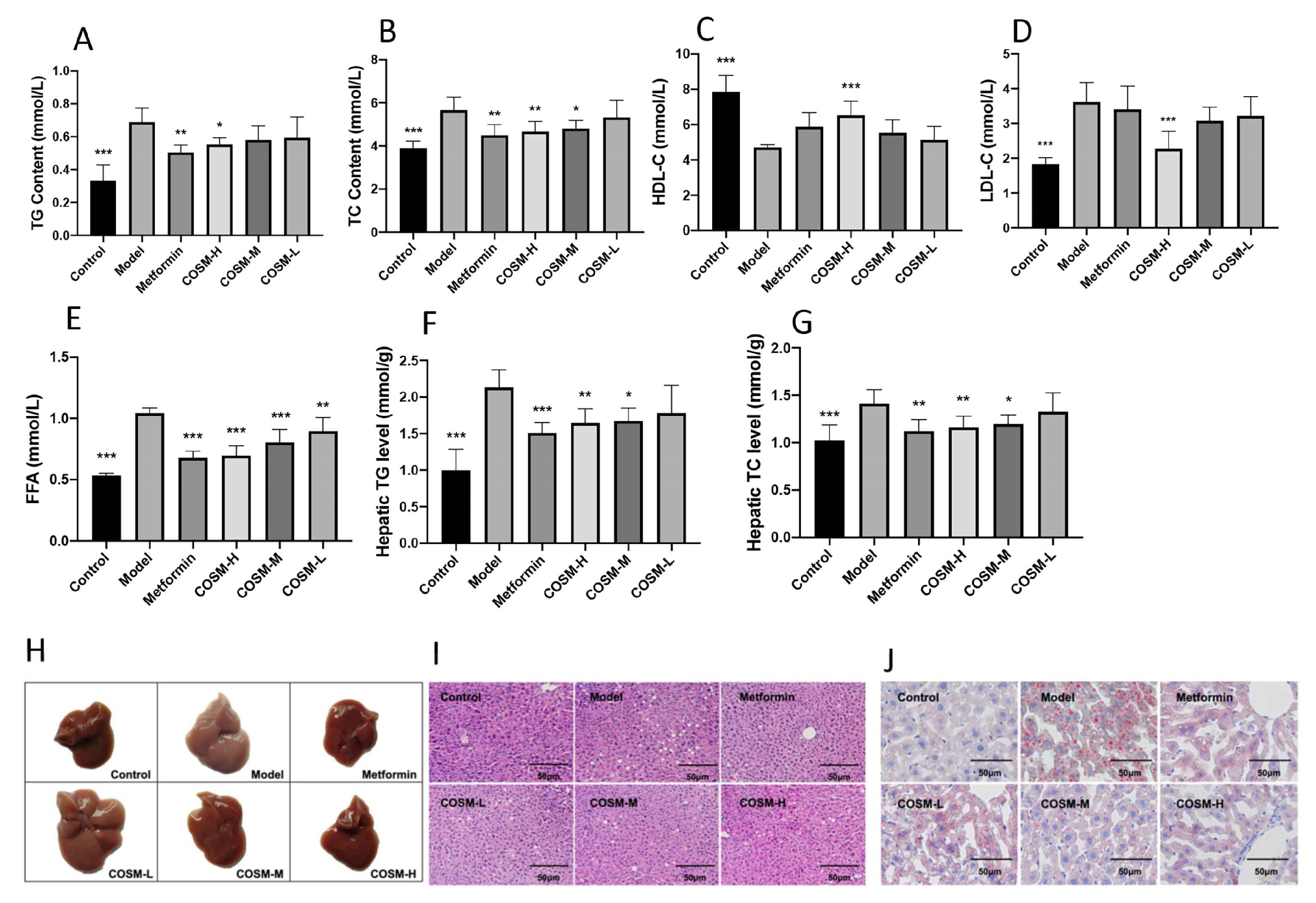
2.2. COSM Could Improve the Lipid Content in the Serum and Liver of HFHSD-Fed Mice
2.3. COSM Could Improve the Liver Function and Antioxidant Capacity of HFHSD-Fed Mice
2.4. COSM Could Improve the Serum Inflammatory Response of HFHSD Mice
2.5. Effects of COSM Intervention on Microflora and the Intestinal Tract
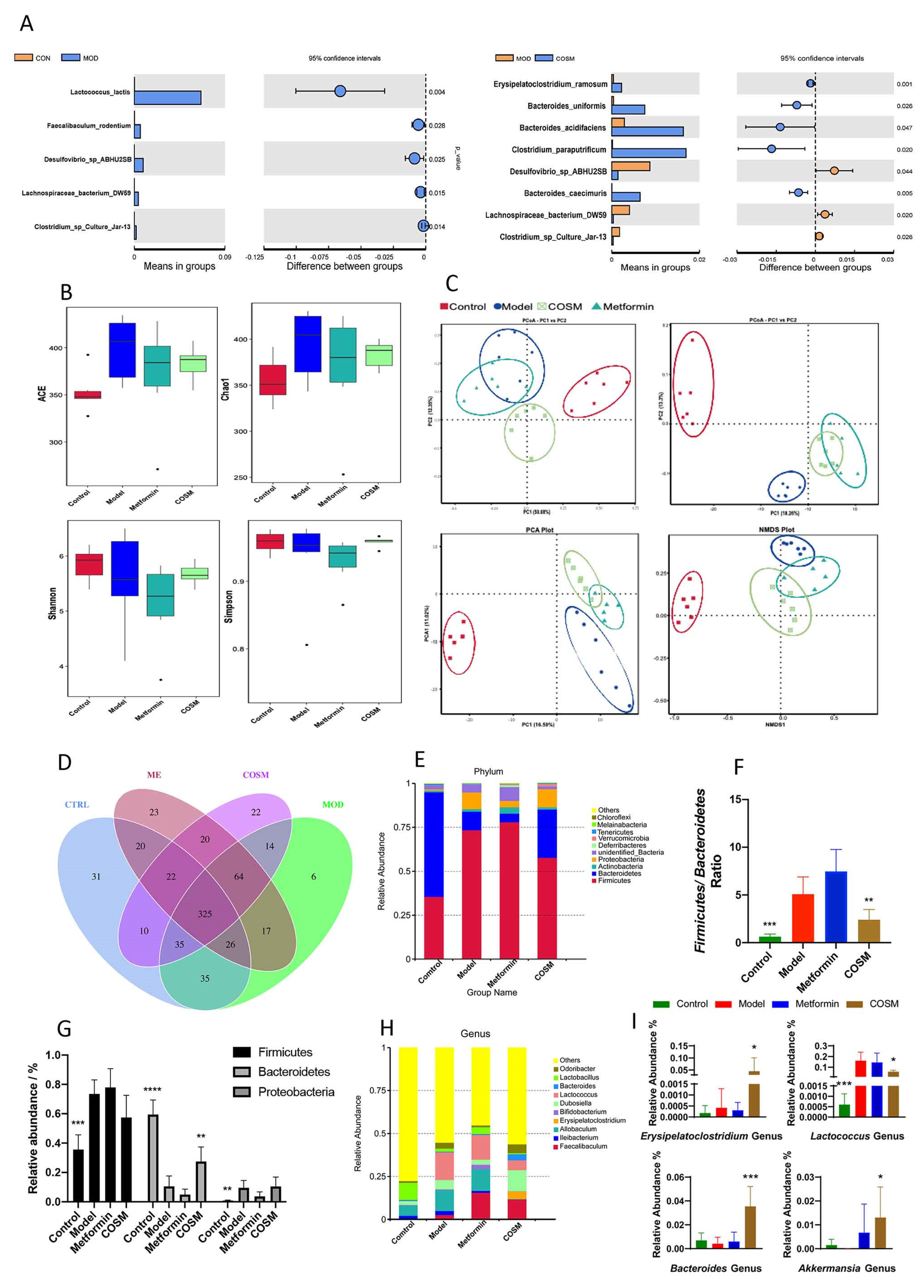
2.5.1. COSM Could Improve the Intestinal Microfloral Structure of HFHSD-Fed Mice
2.5.2. COSM Could Inhibit the Growth of Harmful Intestinal Bacteria and Improve the Abundance of Dominant Bacteria in HFHSD Diet-Fed Mice
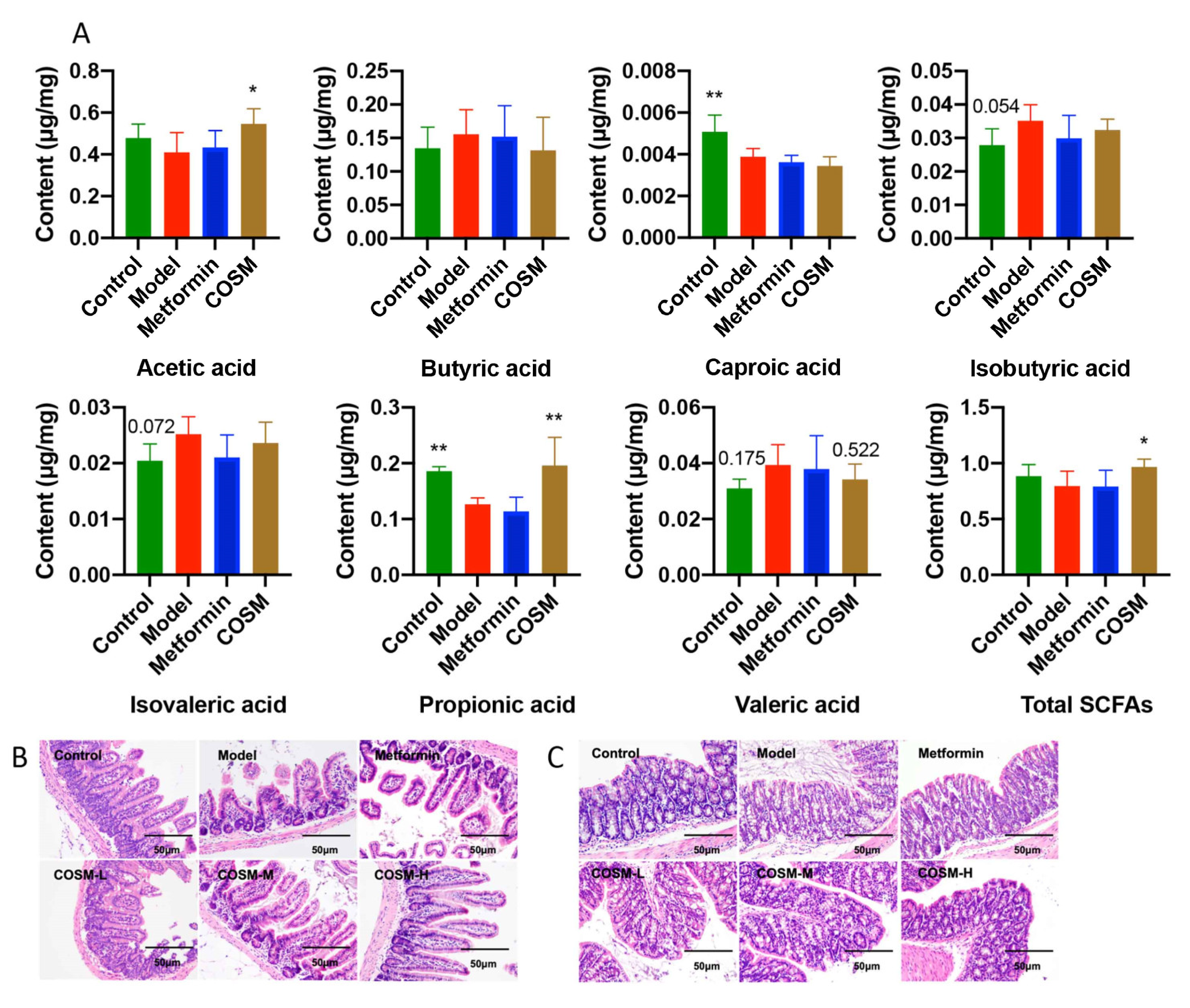
2.5.3. COSM Could Increase the Levels of Acetic Acid, Propionic Acid, and Total SCFAs in the Cecal Contents of HFHSD-Fed Mice
2.5.4. COSM Could Improve the Villus Structure of the Ileum and the Integrity of Colonic Goblet Cells and the Mucosal Layer of HFHSD-Fed Mice
2.6. COSM Improved the Intestinal Wall Barrier Integrity and Endotoxemia in HFHSD-Fed Mice
2.7. COSM Improved the LPS/TLR4/NF-κB Signaling Pathway in the Livers of HFHSD-Fed Mice
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals and Experimental Design
4.3. Serum and Liver Index Analyses
4.4. Histopathological Analysis
4.5. Fluorescence Quantitative PCR (RT-PCR)
4.6. Fecal Microbiota 16S rRNA Analysis
4.7. Short-Chain Fatty Acid (SCFA) Profile Analysis
4.8. Western Blotting
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Antibodies | Company | Catalog No. | Dilution |
|---|---|---|---|
| ZO-1 | abcam | ab216880 | 1:1000 |
| Occludin | abcam | ab167161 | 1:5000 |
| Claudin | abcam | ab180158 | 1:2000 |
| TLR4 | abcam | ab217274 | 1:300 |
| NF-κB | abcam | ab32536 | 1:5000 |
| p-NF-κB | abcam | ab76302 | 1:1000 |
| GAPDH | Bioss | bsm-0978M | 1:2500 |
| Sequencing Area | Primer Name | Sequence Information |
|---|---|---|
| V3 + V4 | 806R | 5′-GGACTACHVGGGTWTCTA-3′ |
| 515F | 5′-GTGCCAGCMGCCGCGGTAA-3′ |
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| TNF-α | CCCACACCGTCAGCCGATTT | GTCTAAGTACTTGGGCAGATTGACC |
| IL-10 | CTTACTGACTGGCATGAGGATCA | GCAGCTCTAGGAGCATGTGG |
| IL-6 | AGACCGCTGCCTGTCTAAAA | TTTGATGTCGTTCACCAGGA |
References
- Yip, T.; Lee, H.; Chan, W.; Wong, G.; Wong, V. Asian perspective on NAFLD-associated HCC. J. Hepatol. 2022, 76, 726–734. [Google Scholar] [CrossRef]
- Younossi, Z.; Koenig, A.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [Green Version]
- Targher, G.; Byrne, C.; Tilg, H. NAFLD and increased risk of cardiovascular disease: Clinical associations, pathophysiological mechanisms and pharmacological implications. Gut 2020, 69, 1691–1705. [Google Scholar] [CrossRef]
- Chauhan, M.; Singh, K.; Thuluvath, P. Bariatric Surgery in NAFLD. Dig. Dis. Sci. 2022, 67, 408–422. [Google Scholar] [CrossRef]
- Wan, M.; Qin, W.; Lei, C.; Li, Q.; Meng, M.; Fang, M.; Song, W.; Chen, J.; Tay, F.; Niu, L. Biomaterials from the sea: Future building blocks for biomedical applications. Bioact. Mater. 2021, 6, 4255–4285. [Google Scholar] [CrossRef]
- Lee, Y.; Ahn, E.; Park, Y. Shape-dependent cytotoxicity and cellular uptake of gold nanoparticles synthesized using green tea extract. Nanoscale Res. Lett. 2019, 14, 129. [Google Scholar] [CrossRef]
- Jiang, Y.; Fu, C.; Liu, G.; Guo, J.; Su, Z. Cholesterol-lowering effects and potential mechanisms of chitooligosaccharide capsules in hyperlipidemic rats. Food Nutr. Res. 2018, 62. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Hu, C.; Deng, X.; Bai, Y.; Cao, H.; Guo, J.; Su, Z. Therapeutic Effect of Chitooligosaccharide Tablets on Lipids in High-Fat Diets Induced Hyperlipidemic Rats. Molecules 2019, 24, 514. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Chen, J.; Cao, P.; Pan, H.; Ding, C.; Xiao, T.; Zhang, P.; Guo, J.; Su, Z. Anti-obese effect of glucosamine and chitosan oligosaccharide in high-fat diet-induced obese rats. Mar. Drugs 2015, 13, 2732–2756. [Google Scholar] [CrossRef]
- Wang, J.; He, W.; Yang, D.; Cao, H.; Bai, Y.; Guo, J.; Su, Z. Beneficial Metabolic Effects of Chitosan and Chitosan Oligosaccharide on Epididymal WAT Browning and Thermogenesis in Obese Rats. Molecules 2019, 24, 4455. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; Yuan, X.; Cheng, G.; Jiao, S.; Feng, C.; Zhao, X.; Yin, H.; Du, Y.; Liu, H. Chitosan oligosaccharides improve the disturbance in glucose metabolism and reverse the dysbiosis of gut microbiota in diabetic mice. Carbohydr. Polym. 2018, 190, 77–86. [Google Scholar] [CrossRef]
- Guan, G.; Wang, H.; Peng, H.; Li, G. Low Dosage of Chitosan Supplementation Improves Intestinal Permeability and Impairs Barrier Function in Mice. BioMed. Res. Int. 2016, 2016, 4847296. [Google Scholar] [CrossRef]
- Qian, M.; Lyu, Q.; Liu, Y.; Hu, H.; Wang, S.; Pan, C.; Duan, X.; Gao, Y.; Qi, L.; Liu, W.; et al. Chitosan Oligosaccharide Ameliorates Nonalcoholic Fatty Liver Disease (NAFLD) in Diet-Induced Obese Mice. Mar. Drugs 2019, 17, 391. [Google Scholar] [CrossRef] [Green Version]
- Angoa-Pérez, M.; Zagorac, B.; Francescutti, D.; Winters, A.; Greenberg, J.; Ahmad, M.; Manning, S.; Gulbransen, B.; Theis, K.; Kuhn, D. Effects of a high fat diet on gut microbiome dysbiosis in a mouse model of Gulf War Illness. Sci. Rep. 2020, 10, 9529. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef] [Green Version]
- Mao, J.; Tang, H.; Zhao, T.; Tan, X.; Bi, J.; Wang, B.; Wang, Y. Intestinal mucosal barrier dysfunction participates in the progress of nonalcoholic fatty liver disease. Int. J. Clin. Exp. Pathol. 2015, 8, 3648–3658. [Google Scholar]
- Duan, Y.; Zeng, L.; Zheng, C.; Song, B.; Li, F.; Kong, X.; Xu, K. Inflammatory Links Between High Fat Diets and Diseases. Front. Immunol. 2018, 9, 2649. [Google Scholar] [CrossRef] [Green Version]
- Ryu, J.; Kim, E.; Kang, M.; Song, D.; Shin, E.; Lee, H.; Jung, J.; Nam, S.; Kim, J.; Kim, H.; et al. Differential TM4SF5-mediated SIRT1 modulation and metabolic signaling in nonalcoholic steatohepatitis progression. J. Pathol. 2021, 253, 55–67. [Google Scholar] [CrossRef]
- Luo, L.; Du, T.; Zhang, J.; Zhao, W.; Cheng, H.; Yang, Y.; Wu, Y.; Wang, C.; Men, K.; Gou, M. Efficient inhibition of ovarian cancer by degradable nanoparticle-delivered survivin T34A gene. Int. J. Nanomed. 2016, 11, 501–512. [Google Scholar] [CrossRef] [Green Version]
- Athinarayanan, S.; Wei, R.; Zhang, M.; Bai, S.; Traber, M.; Yates, K.; Cummings, O.; Molleston, J.; Liu, W.; Chalasani, N. Genetic polymorphism of cytochrome P450 4F2, vitamin E level and histological response in adults and children with nonalcoholic fatty liver disease who participated in PIVENS and TONIC clinical trials. PLoS ONE 2014, 9, e95366. [Google Scholar] [CrossRef] [Green Version]
- Yoon, H.; Cho, C.; Yun, M.; Jang, S.; You, H.; Kim, J.; Han, D.; Cha, K.; Moon, S.; Lee, K.; et al. Akkermansia muciniphila secretes a glucagon-like peptide-1-inducing protein that improves glucose homeostasis and ameliorates metabolic disease in mice. Nat. Microbiol. 2021, 6, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Sivamaruthi, B.; Kesika, P.; Suganthy, N.; Chaiyasut, C. A Review on Role of Microbiome in Obesity and Antiobesity Properties of Probiotic Supplements. BioMed. Res. Int. 2019, 2019, 3291367. [Google Scholar] [CrossRef]
- Li, Y.; Wen, H.; Chen, L.; Yin, T. Succession of bacterial community structure and diversity in soil along a chronosequence of reclamation and re-vegetation on coal mine spoils in China. PLoS ONE 2014, 9, e115024. [Google Scholar] [CrossRef] [PubMed]
- Rocchini, D. Algorithmic foundation of spectral rarefaction for measuring satellite imagery heterogeneity at multiple spatial scales. Sensors 2009, 9, 303–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, W.; Lv, L.; Shi, D.; Ye, J.; Fang, D.; Guo, F.; Li, Y.; He, X.; Li, L. Protective Effect of Akkermansia muciniphila against Immune-Mediated Liver Injury in a Mouse Model. Front Microbiol. 2017, 8, 1804. [Google Scholar] [CrossRef]
- Chen, Y.; Lv, J.; Jia, Y.; Wang, R.; Zhang, Z.; Liu, J.; Jia, C. Effect of Moxibustion on the Intestinal Flora of Rats with Knee Osteoarthritis Induced by Monosodium Iodoacetate. Evid. Based Complement. Altern. Med. 2020, 2020, 3196427. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Wei, K.; He, J.; Ding, N.; Hua, J.; Zhou, T.; Niu, F.; Zhou, G.; Shi, T.; et al. Review: Effect of Gut Microbiota and Its Metabolite SCFAs on Radiation-Induced Intestinal Injury. Front. Cell. Infect. Microbiol. 2021, 11, 577236. [Google Scholar] [CrossRef]
- Deng, M.; Qu, F.; Chen, L.; Liu, C.; Zhang, M.; Ren, F.; Guo, H.; Zhang, H.; Ge, S.; Wu, C.; et al. SCFAs alleviated steatosis and inflammation in mice with NASH induced by MCD. J. Endocrinol. 2020, 245, 425–437. [Google Scholar] [CrossRef]
- Boutagy, N.; McMillan, R.; Frisard, M.; Hulver, M. Metabolic endotoxemia with obesity: Is it real and is it relevant? Biochimie 2016, 124, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Cani, P.; Amar, J.; Iglesias, M.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.; Fava, F.; Tuohy, K.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef] [Green Version]
- Cani, P.; Possemiers, S.; Van de Wiele, T.; Guiot, Y.; Everard, A.; Rottier, O.; Geurts, L.; Naslain, D.; Neyrinck, A.; Lambert, D.; et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 2009, 58, 1091–1103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Xie, T.; Sun, S.; Wang, K.; Liu, B.; Wu, X.; Ding, W. DNase-1 Treatment Exerts Protective Effects in a Rat Model of Intestinal Ischemia-Reperfusion Injury. Sci. Rep. 2018, 8, 17788. [Google Scholar] [CrossRef] [Green Version]
- Orlando, A.; Linsalata, M.; Notarnicola, M.; Tutino, V.; Russo, F. Lactobacillus GG restoration of the gliadin induced epithelial barrier disruption: The role of cellular polyamines. BMC Microbiol. 2014, 14, 19. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Guo, R.; Li, W.; Yu, B.; Han, B.; Liu, L.; Han, D. The role of intestinal endotoxemia in a rat model of aluminum neurotoxicity. Mol. Med. Rep. 2017, 16, 1878–1884. [Google Scholar] [CrossRef] [Green Version]
- Deng, X.; Ye, Z.; Cao, H.; Bai, Y.; Che, Q.; Guo, J.; Su, Z. Chitosan oligosaccharide ameliorated obesity by reducing endoplasmic reticulum stress in diet-induced obese rats. Food Funct. 2020, 11, 6285–6296. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Zhao, M.; Chen, Q.; Fan, L.; Gao, F.; Zhao, L. Absorption Characteristics of Chitobiose and Chitopentaose in the Human Intestinal Cell Line Caco-2 and Everted Gut Sacs. J. Agric. Food Chem. 2019, 67, 4513–4523. [Google Scholar] [CrossRef] [PubMed]
- Muanprasat, C.; Chatsudthipong, V. Chitosan oligosaccharide: Biological activities and potential therapeutic applications. Pharmacol. Ther. 2017, 170, 80–97. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.; Hoffmann, C.; Mota, J. The human gut microbiota: Metabolism and perspective in obesity. Gut Microbes 2018, 9, 308–325. [Google Scholar] [CrossRef] [Green Version]
- Le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.; Kennedy, S.; et al. Richness of human gut microbiome correlates with metabolic markers. Nature 2013, 500, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Hersoug, L.; Møller, P.; Loft, S. Role of microbiota-derived lipopolysaccharide in adipose tissue inflammation, adipocyte size and pyroptosis during obesity. Nutr. Res. Rev. 2018, 31, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Kishi, M.; Fushimi, T.; Kaga, T. Acetic acid upregulates the expression of genes for fatty acid oxidation enzymes in liver to suppress body fat accumulation. J. Agric. Food Chem. 2009, 57, 5982–5986. [Google Scholar] [CrossRef] [PubMed]
- Ruan, N.; Tribble, J.; Peterson, A.; Jiang, Q.; Wang, J.; Chu, X. Acid-Sensing Ion Channels and Mechanosensation. Int. J. Mol. Sci. 2021, 22, 4810. [Google Scholar] [CrossRef]
- De Vadder, F.; Kovatcheva-Datchary, P.; Goncalves, D.; Vinera, J.; Zitoun, C.; Duchampt, A.; Bäckhed, F.; Mithieux, G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 2014, 156, 84–96. [Google Scholar] [CrossRef] [Green Version]
- Hernández, M.; Canfora, E.; Jocken, J.; Blaak, E. The Short-Chain Fatty Acid Acetate in Body Weight Control and Insulin Sensitivity. Nutrients 2019, 11, 1943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Chen, J.; Tan, Q.; Deng, X.; Tsai, P.; Chen, P.; Ye, M.; Guo, J.; Su, Z. Nondigestible Oligosaccharides with Anti-Obesity Effects. J. Agric. Food Chem. 2020, 68, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Hu, Z.; Cui, A.; Liu, Z.; Ma, F.; Xue, Y.; Liu, Y.; Zhang, F.; Zhao, Z.; Yu, Y.; et al. Post-translational regulation of lipogenesis via AMPK-dependent phosphorylation of insulin-induced gene. Nat. Commun. 2019, 10, 623. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Hu, Y.; Liu, L.; Wang, Q.; Zeng, J.; Chen, C. PM2.5 exposure perturbs lung microbiome and its metabolic profile in mice. Sci. Total Environ. 2020, 721, 137432. [Google Scholar] [CrossRef]
- Fallatah, H.; Akbar, H.; Fallatah, A. Fibroscan Compared to FIB-4, APRI, and AST/ALT Ratio for Assessment of Liver Fibrosis in Saudi Patients With Nonalcoholic Fatty Liver Disease. Hepat. Mon. 2016, 16, e38346. [Google Scholar] [CrossRef] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, J.; Liu, Y.; Chen, J.; Bai, Y.; He, J.; Cao, H.; Che, Q.; Guo, J.; Su, Z. Marine Chitooligosaccharide Alters Intestinal Flora Structure and Regulates Hepatic Inflammatory Response to Influence Nonalcoholic Fatty Liver Disease. Mar. Drugs 2022, 20, 383. https://doi.org/10.3390/md20060383
Feng J, Liu Y, Chen J, Bai Y, He J, Cao H, Che Q, Guo J, Su Z. Marine Chitooligosaccharide Alters Intestinal Flora Structure and Regulates Hepatic Inflammatory Response to Influence Nonalcoholic Fatty Liver Disease. Marine Drugs. 2022; 20(6):383. https://doi.org/10.3390/md20060383
Chicago/Turabian StyleFeng, Jiayao, Yongjian Liu, Jiajia Chen, Yan Bai, Jincan He, Hua Cao, Qishi Che, Jiao Guo, and Zhengquan Su. 2022. "Marine Chitooligosaccharide Alters Intestinal Flora Structure and Regulates Hepatic Inflammatory Response to Influence Nonalcoholic Fatty Liver Disease" Marine Drugs 20, no. 6: 383. https://doi.org/10.3390/md20060383
APA StyleFeng, J., Liu, Y., Chen, J., Bai, Y., He, J., Cao, H., Che, Q., Guo, J., & Su, Z. (2022). Marine Chitooligosaccharide Alters Intestinal Flora Structure and Regulates Hepatic Inflammatory Response to Influence Nonalcoholic Fatty Liver Disease. Marine Drugs, 20(6), 383. https://doi.org/10.3390/md20060383






