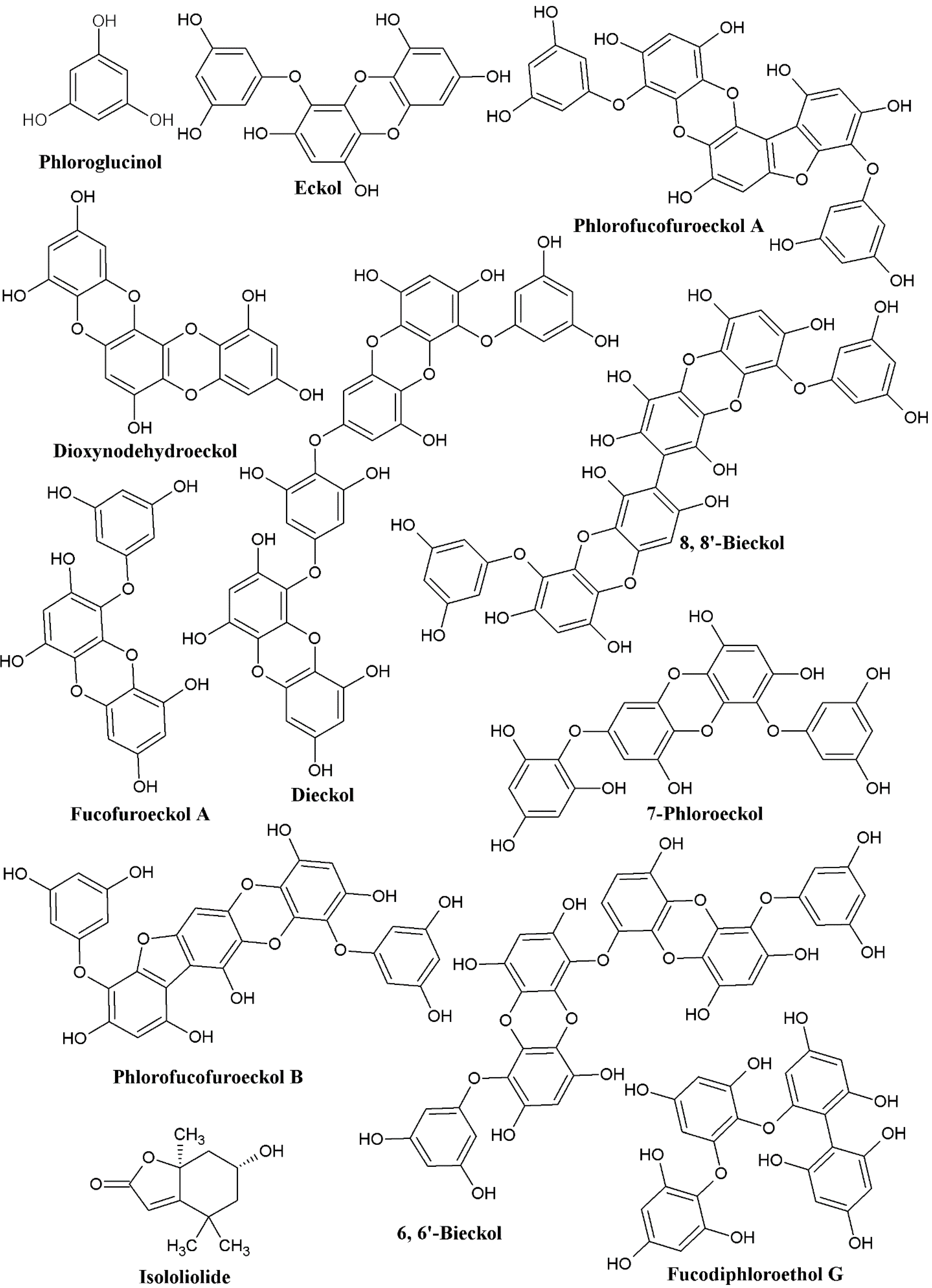
Seaweed-Derived Phlorotannins: A Review of Multiple Biological Roles and Action Mechanisms
Abstract
:1. Introduction
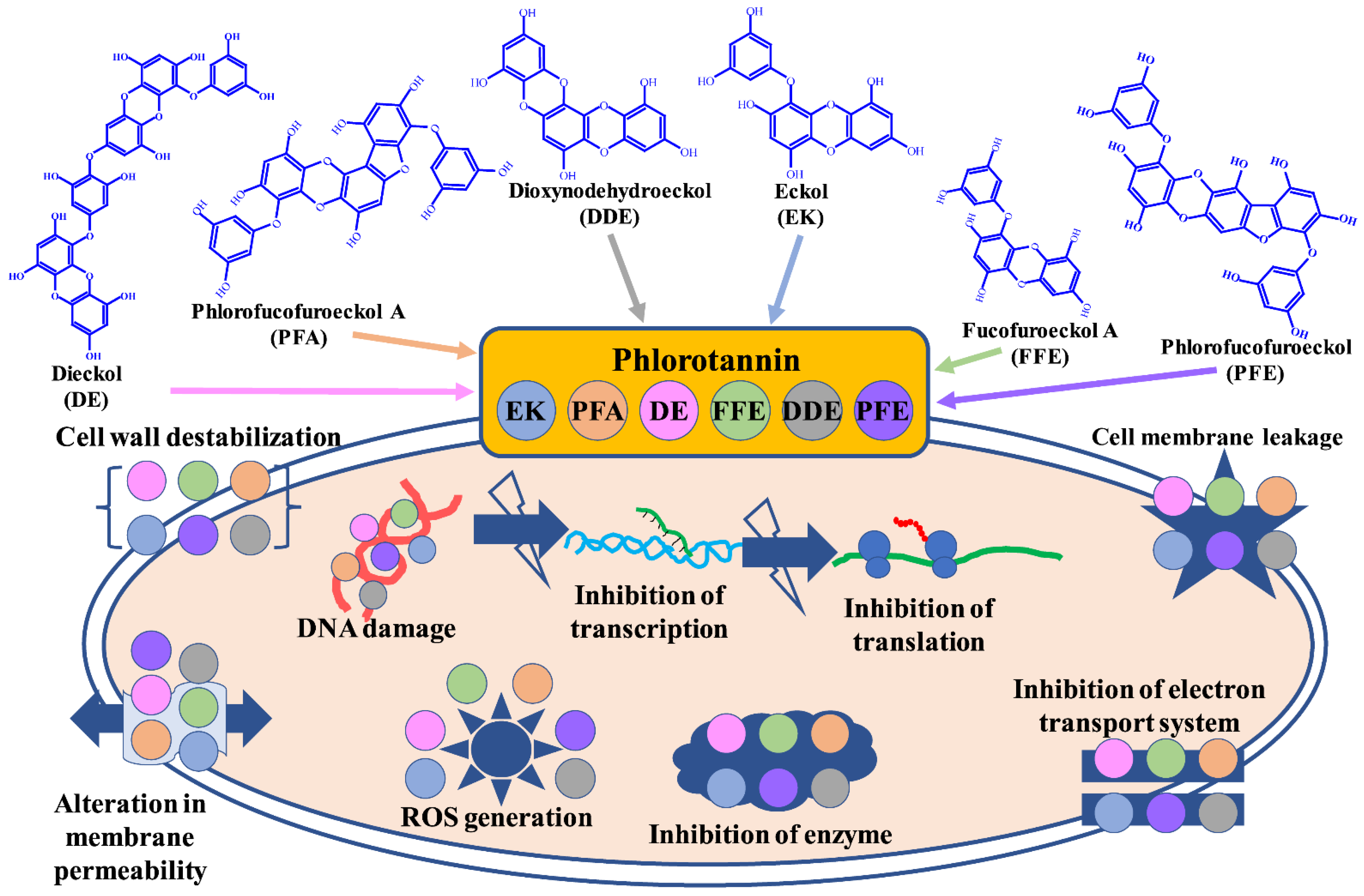
2. Phlorotannin against Pathogenic Bacterial and Fungal Species
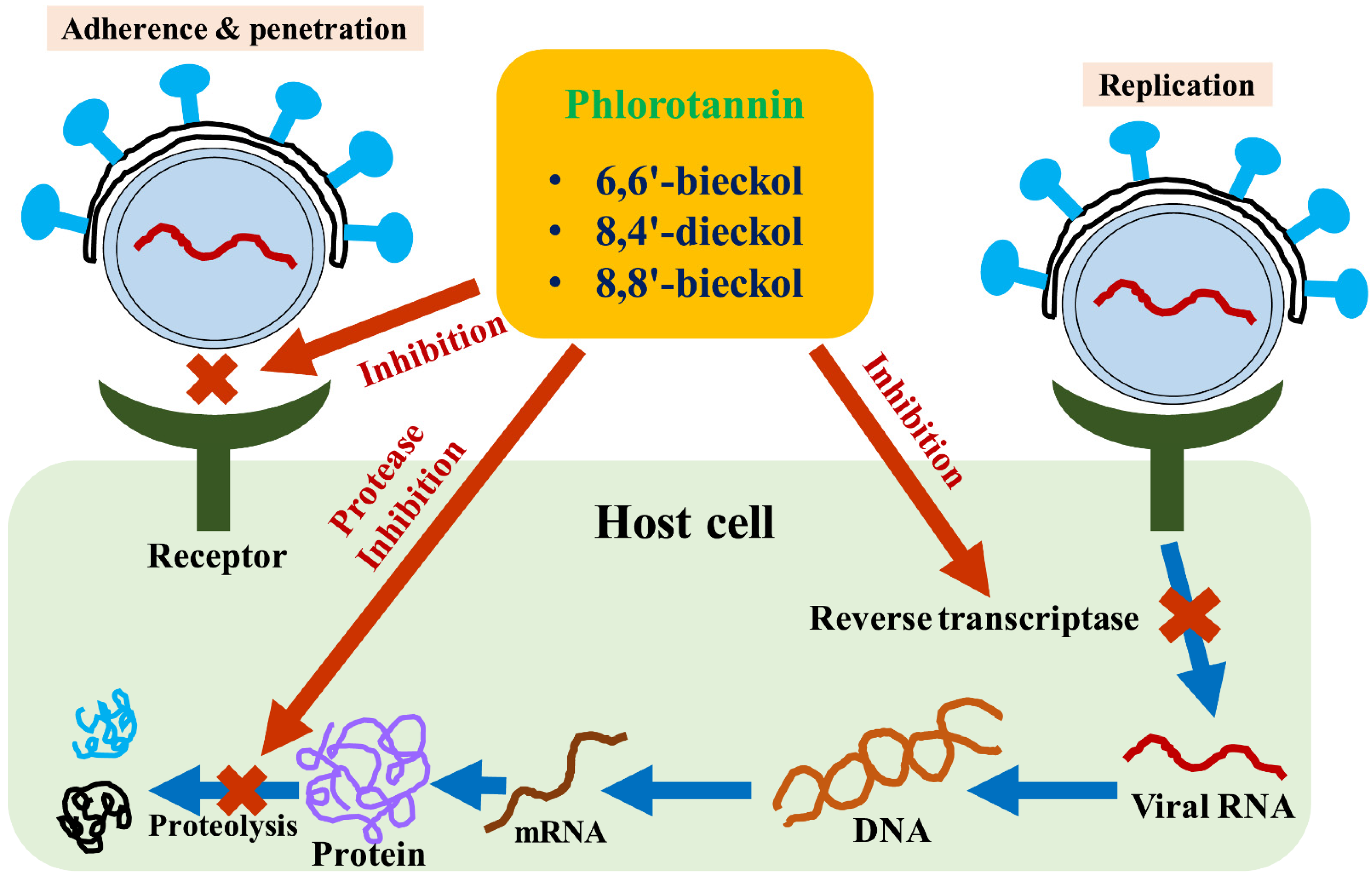
3. Phlorotannins as a Natural Adjuvant of Antiviral Therapy
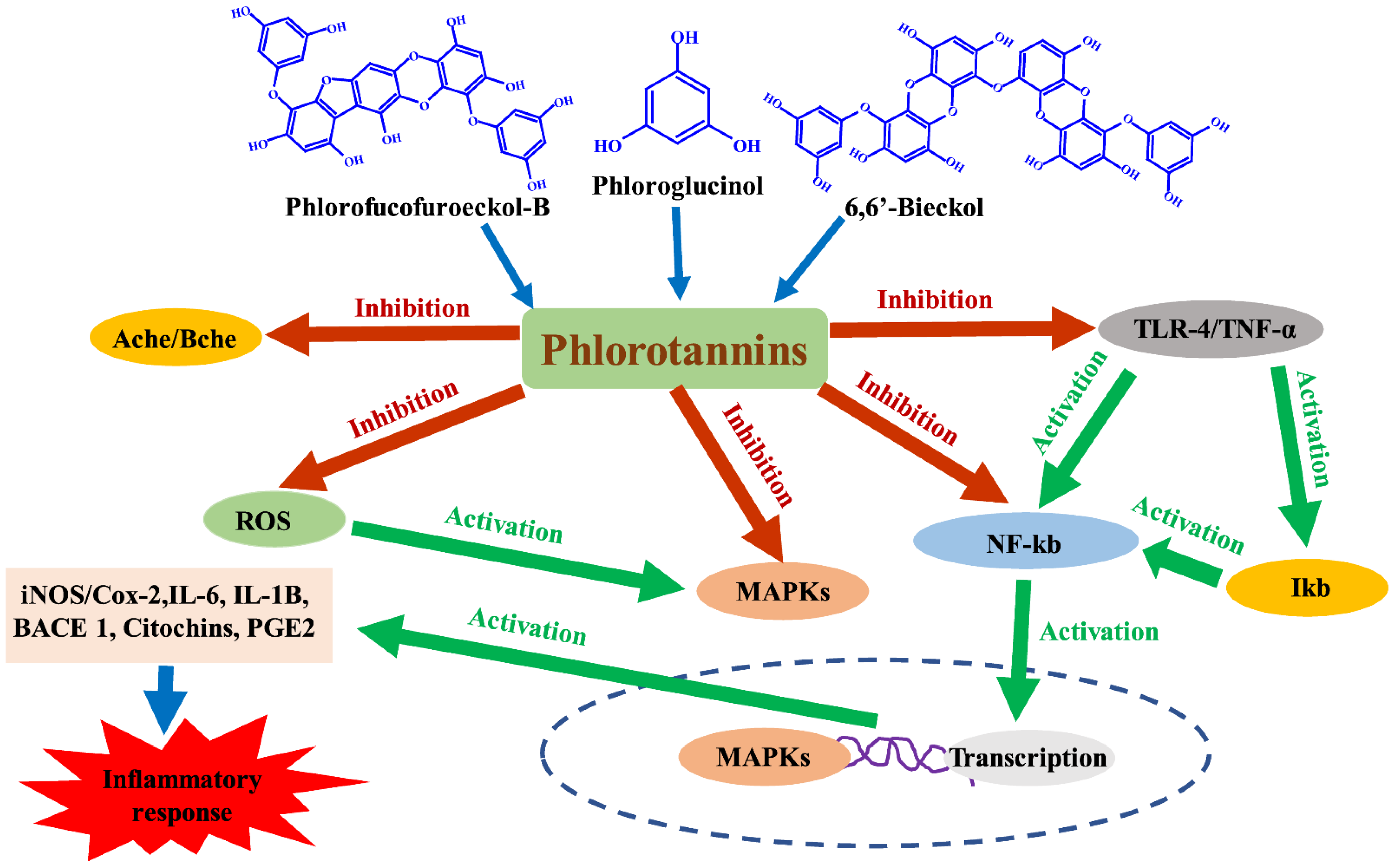
4. Anti-Inflammatory and Immunomodulatory Properties of Phlorotannins
5. Phlorotaninns Having Anticancer Potential
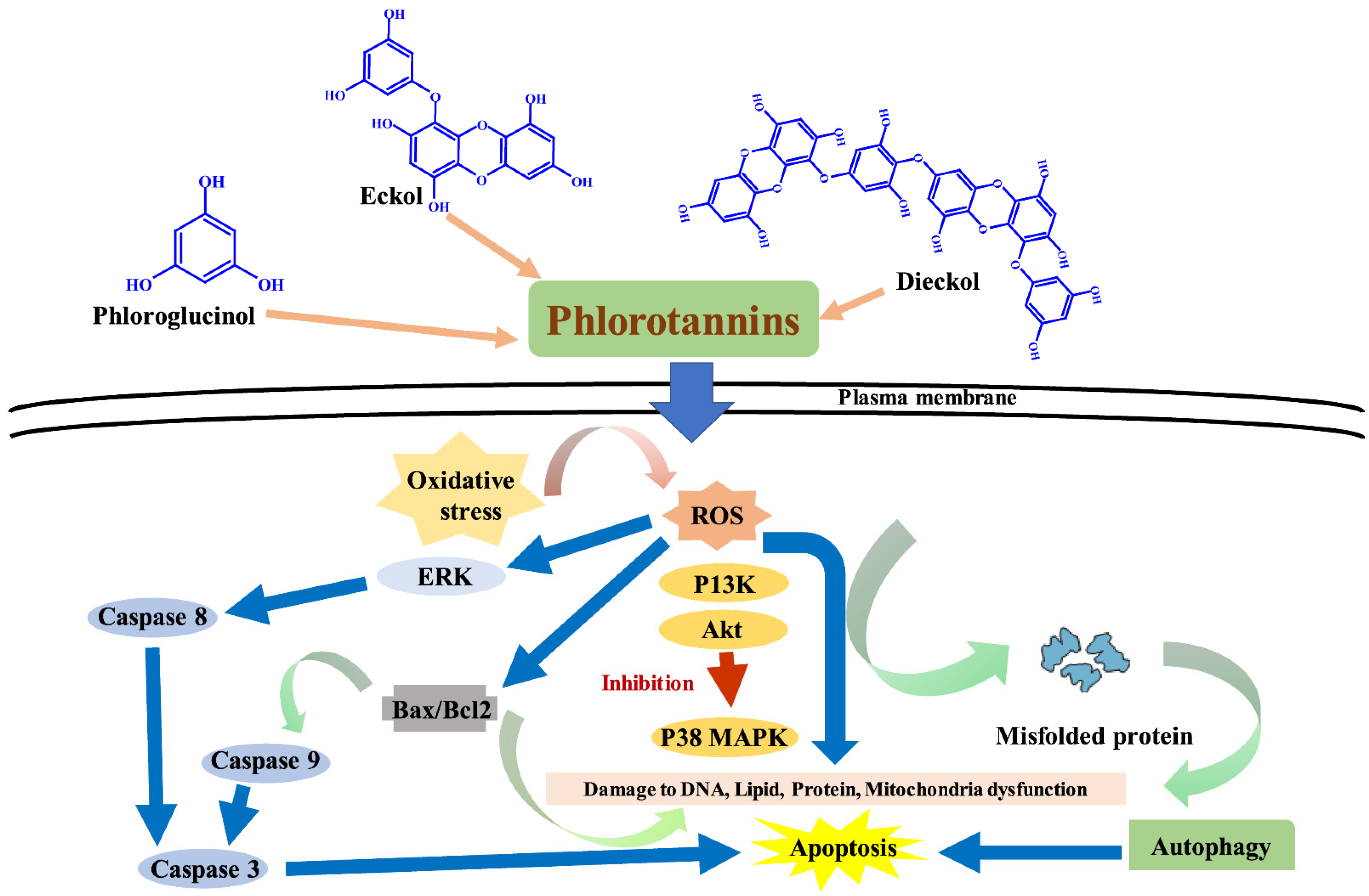
6. Antioxidant Abilities of Phlorotannins in Modulating Oxidative Stress
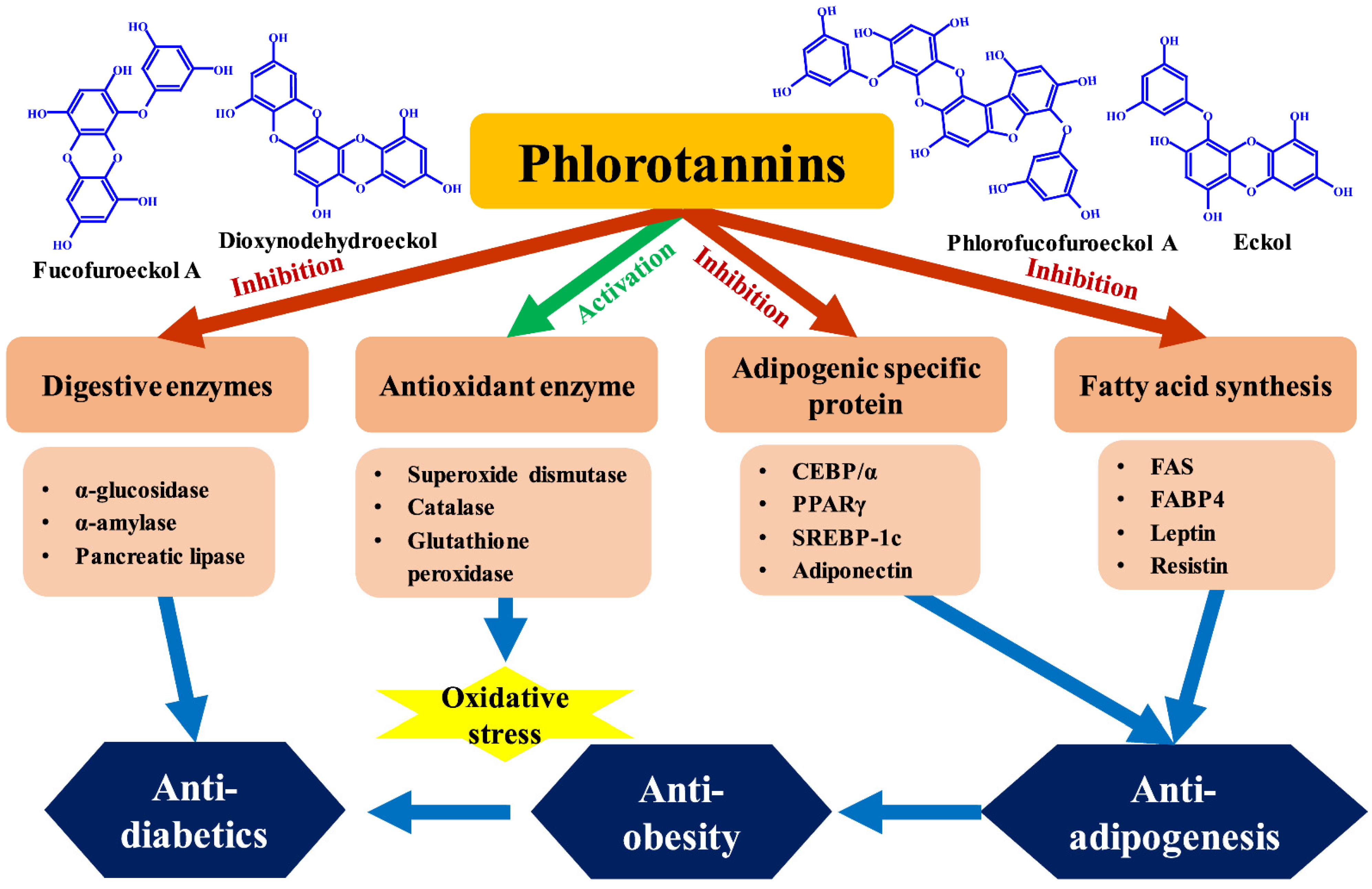
7. Phlorotannins with Anti-Adipogenesis, Antidiabetic, and Anti-Obesity Activities
8. Limitations Related to the Phlorotannin Isolation, Purification, Characterization, and Application
| Pure Phlorotannin or Extracts | Sources | Purification Methodology | Microbial Pathogens | Active Concentration | Types of the Testing Method | Action Mechanism | Reference |
|---|---|---|---|---|---|---|---|
| Antibacterial | |||||||
| Ecklonia kurome | Silica acid chromatography |
|
| Broth microdilution method | ND | [21] |
| Eisenia bicyclis | Folin-Ciocâlteu method | Listeria monocytogenes |
| Micro-dilution method | ND | [36] |
| Crude methanolic extract |
| Folin-Ciocâlteu method | L. monocytogenes, S. abony, Enterococcus faecalis, and P. aeruginosa | 2.21% to 100% bacterial inhibition | Two-fold dilution method | ND | [37] |
| Phlorotannins | Hizikia fusiforme | ND |
|
| Two-fold dilution method |
| [38] |
| E. bicyclis | ND | MRSA | MIC (32 to 64 µg/mL) | Two-fold dilution method | ND | [39] |
| E. cava | Sephadex LH-20 column chromatography | Propinibacterium acnes | MIC (39 µg/mL) | Broth microdilution assay | ND | [40] |
| E. cava | Silica gel column chromatography |
| MIC (125 to 250 µg/mL) | Broth microdilution method | ND | [42] |
| E. cava | Reversed-phase column chromatography |
| MIC 128 to 256 µg/mL | Micro broth dilution method | ND | [44] |
| E. arborea | Column chromatography | V. parahaemolyticus |
| Broth dilution method | Develop antibiotic agents for medicated shrimp feed additive | [45] |
| Dieckol | E. stolonifera | Sephadex LH-20 column chromatography | MRSA | MIC 32 to 64 μg/mL | Two-fold dilution method | ND | [46] |
| Dieckol Phlorofucofuroeckol-A | E. bicyclis | ND |
| MIC 128 −256 μg/mL | Micro-dilution method | ND | [41] |
| Phlorotannin | Sargassum thunbergii | ND | V. parahaemolyticus | 900 μg/mL inhibited biofilm formation | Micro-dilution method | ND | [47] |
| Laminaria digitata | Electron micrograph | Mixed bacterial culture | Biofilm inhibition | Batch assay | ND | [48] |
| Crude phlorotannins | Cymbella spp. | Thin-layer chromatography |
| MIC value of 1.56, 1.56, 3.12, >3.12, 3.12, 1.56, 1.56 mg/mL respectively | Micro-dilution method | Inactivated microbial adhesions, enzymes, and cell envelope transport proteins | [134] |
| Antifungal | |||||||
|
| Crude extraction | Candida albicans ATCC 10231 | MIC 15.6,31.3,31.3 mg/mL for C. nodicaulis, C. usneoides, and F. spiralis, respectively | Broth microdilution method |
| [22] |
| E. cava | Silica-gel chromatography | Trichophyton rubrum | MIC 200 µM | Micro broth dilution assay | Changed cytoplasmic integrity | [49] |
| E. bicyclis | RP-18 open column chromatography and Sephadex LH-20 | C. albicans | MIC 512 µg/mL | Broth microdilution assay |
| [50] |
| Anti-viral | |||||||
| E. cava | Silica-gel chromatography | HIV-1 | EC50 1.72 µM (syncytia production) EC50 1.26 µM (antigen production) | Western blot analysis Cell viability assay |
| [57] |
| 8,4′-Dieckol | E. cava | Silica-gel chromatography | HIV-1 | 91% Inhibition of reverse transcriptase at 50 µM | Reverse transcriptase assay |
| [57] |
| E. cava | ND | HIV-1 | 50% Inhibition of reverse transcriptase at 0.51 µM | Reverse transcriptase assay | Inhibited reverse transcriptase enzyme activity | [58] |
| E. cava | Silica-gel chromatography | SARS-CoV 3CL | IC50 2.7 to >200 µM |
| Inhibited 3CLpro hydrolysis | [23] |
| Phlorofucofuroeckol | E. cava | Silica-gel column chromatography | Influenza A virus | IC50 4.5 µM | Chemiluminescent neuraminidase inhibition assay | ND | [59] |
| Pure Phlorotannin or Extracts | Sources | Purification Methodology | Biological Activity | Active Concentration | Types of the Testing Method | Action Mechanism | Reference |
|---|---|---|---|---|---|---|---|
| Anticancer | |||||||
| Ecklonia cava | ND | Ovarian cancer cells undergo apoptosis |
|
|
| [78] |
| E. cava | Silica-gel column chromatography | Human breast cancer cells |
| Cell proliferation assay |
| [79] |
| Phloroglucinol | Brown seaweeds | ND | MDA-MB231 breast cancer cells | IC50 50 µM of migratory and invasive ability of MDA-MB231 breast cancer cells |
|
| [80] |
| Phloroglucinol | Seaweeds | ND | Human colon cancer cells HT-29 | 12.5 µg/mL caused fragmented nuclei and cell shrinkage |
|
| [135] |
| Phlorotannins | Cystoseira sedoides | Reversed-phase column chromatography | MCF-7 cells (human breast cancer cells) | In MCF-7 cells, the IC50 for inducing apoptosis was 78 µg/mL | Double Annexin V-FITC/PI test | Prevented spheroid growth | [82] |
| Dieckol | E. cava | Sephadex LH-20 column chromatography | Protective efficacy against N-nitrosdiethylamine -induced rat hepatocarcinogenesis | Alkaline phosphatase, lactate dehydrogenase, transaminase, gamma-glutamyl transferase, total bilirubin, and a-fetoprotein activities increased in NDEA-induced rats given dieckol water (10–40 mg/kg body weight) for 15 weeks | Serum marker enzymes analysis |
| [83] |
| Isololiolide | C. tamariscifolia | Reverse phase preparative HPLC | Anti-proliferative activity |
| MTT colorimetric assay |
| [136] |
| Anti-inflammatory | |||||||
| Eisenia bicyclis | Chromatography on silica gel column | Cell viability and NO production (LPS-induced RAW264.7 cells) |
| MTT assay |
| [64] |
| Phloroglucinol | E. cava | ND | Inhibition of oxidative stress and inflammation |
|
|
| [66] |
| Phlorotannins | Bifurcaria bifurcata | Reversed-phase column chromatography | Cytotoxic effect on ATDC-5 mouse model cell lines | 50% inhibition of cell growth in ATDC-5 cells at 100 µM | MTT assay | ND | [68] |
| Phlorotannins | Padina tetrastromatica | Reversed-phase column chromatography | Effect on THP-1 cell viability | From 1.5 to 50.0 µg/mL | MTT assay |
| [69] |
| Phloroglucinol | Brown algae | ND | Anti-inflammatory effect and oxidative stress on RAW264.7 and HT1080 cells | From 1 to 100 µM | MTT assay |
| [66] |
| Phlorotannins |
| Aqueous acetone extraction | Anti-inflammatory and toxicity capability in RAW 264.7 macrophages and cell-free systems |
| MTT assay | Function in inflammatory conditions, acting on both enzymatic and non-enzymatic inflammatory targets | [65] |
| Phlorofucofuroeckol B (PFF-B) | E. stolonifera | High-performance chromatography | PFF-B inhibits the generation of inflammatory mediators induced by LPS |
|
|
| [70] |
| 6,6′-Bieckol | E. cava | ND | LPS-stimulated macrophage RAW 264.7 cells have anti-inflammatory effects |
|
| Downregulation of COX-2, iNOS, and pro-inflammatory cytokines in LPS-stimulated macrophages via the NF-κB pathway | [71] |
| Phlorofucofuroeckol B | Myagropsis myagroides | High-performance chromatography | Anti-inflammatory activity |
|
| In LPS-stimulated macrophage cells, the NF-κB pathway was inhibited by inhibiting the phosphorylation of ERKs and Akt | [67] |
| Antidiabetic | |||||||
| E. bicyclis | Chromatography and nuclear magnetic resonance | Antidiabetic activity |
| Enzymatic inhibitory assay | Inhibition of α-amylase and α-glucosidase enzyme activities | [106] |
| E. cava | Column chromatography using silica gel | antidiabetic activity | IC50 10.75 to 49.49 µmol/mL for α-glucosidase and >500 to 124.98 µmol/L for α-amylase | Enzymatic inhibitory assay | Inhibition of α-amylase and α-glucosidase enzymes activities | [107] |
| Phlorotannins | C. compressa | UPLC-MS/MS | Antidiabetic activity | After four weeks of diabetes induction, diabetics were treated with 60 mg/kg of phlorotannin extract. | MTT assay |
| [108] |
| Ethylacetate fraction of acetone extract | F. vesiculosus | Mass spectroscopy (UHPLC-MS) | Antidiabetic and anti-obesity activity |
| α-amylase, α-glucosidase, pancreatic lipase inhibitory assay |
| [109] |
| Ishophloroglucin A | Ishige okamurae | Semipreparative HPLC column | Anti-α-glucosidase activity | IC50 value of 54.97 µM in α-glucosidase inhibition | α-Glucosidase inhibitory assay | Inhibited α-glucosidase | [110] |
| Phlorofucofuroeckol A | E. cava | Electrospray ionization-multistage tandem mass spectrometry and HPLC | Antidiabetic activity |
|
| Inhibition of α-amylase and α-glucosidase enzymes activities | [111] |
| Dieckol | E. cava | Reversed-phase HPLC | Antidiabetic activity |
|
| Activates both the AMPK and PKB signaling cascades | [112] |
| Antioxidant | |||||||
| Dichloromethane fraction of methanolic extraction |
| Silica-gel column chromatography | Antioxidant activity | 69.62% radical scavenging activity | DPPH radical scavenging | ND | [89] |
| E. curome | Sequential chromatography on two reverse phase column | Antioxidant activity | IC50 10, 11, 110, 10 µM respectively | DPPH radical scavenging | Reduced intracellular reactive oxygen species | [90] |
| E. cava | Sephadex LH-20 column chromatography | Antioxidant activity |
|
|
| [91] |
| Sargassum inggoldianm | Matrix-assisted laser desorption ionization time-of-flight mass spectroscopy | Antioxidant activity | IC50 1.0 µg/mL | Electron spin resonance spectrometry | Showed superoxide anion radical scavenging activity | [92] |
| Phlorotannins | S. dupplicatum | Sephadex LH-20 column chromatography | Antioxidant activity |
|
| ND | [93] |
| Phlorofucofuroeckol-A | E. cava | Centrifugal partition chromatography | Antioxidant activity |
|
|
| [94] |
| E. cava | HPLC | Neuroprotective against H2O2-induced cellular damage in HT22 neuronal cells from the murine hippocampus |
|
|
| [96] |
| E. cava | Sephadex LH-20 column chromatography | The ability of phlorotannins to scavenge ROS in AAPH-induced zebrafish embryos | Reduced intracellular ROS buildup to DCF-DA intensity of 1568, 2346, 1703, 1540, and 2262, respectively (50 µM phlorotannins + 25 mM AAPH) | ROS generation in AAPH-induced zebrafish embryos | Antioxidant efficacy against AAPH-mediated toxicity | [95] |
| Anti-obesity | |||||||
| E. cava | Sephadex LH-20 column chromatography | Inhibition of adipogenesis | Inhibited lipid accumulation 60%, 40% 20% at 100 µL respectively | Oil-Red O staining method | Downregulated adipogenic specific genes (SREBP-1, C/EBPα, FABP4, and PPARγ) | [116] |
| E. cava | Silica-gel column chromatography | Inhibition of adipogenesis | Inhibited adipogenesis 20 µM | Oil-Red O staining method |
| [113] |
| E. cava | Sephadex LH-20 column chromatography | Inhibition of melanogenesis (UV-B radiation-induced cell damage protection effect) | 92.7% (dieckol) and 62.4% (eckol) inhibitory effect on tyrosinase at 100 µM |
|
| [114] |
| Anti-adipogenesis | |||||||
| E. stolonifera | Sephadex LH-20 column chromatography | Inhibition of adipogenesis | Inhibited adipogenesis (12.5–100 µM) | Oil Red O staining | Downregulated adipogenic specific genes of 3T3-L1 (C/EBPα and PPARγ) | [100] |
| Dieckol | Laminaria japonica | Silica gel resin absorption | Anti-fatty liver activity | Body weight gain, plasma lipid profiles, visceral fat index, liver index, and hepatic fat deposition were improved in high-fat diet mice given a dieckol-enriched extract (50 mg/kg/day) for four weeks | Histopathological analysis |
| [115] |
| Dieckol | E. cava | Reverse-phase high-performance liquid chromatography | Inhibitory effect on adipogenesis |
| MTT assay | Activated AMP-activated protein kinase | [116] |
| Other biological activities | |||||||
| E. cava | Sephadex LH-20 column chromatography | Cytoprotective effect against oxidative stress-induced cell damage in (V79-4) Chinese hamster lung fibroblast |
|
|
| [137] |
| E. cava | Sephadex LH-20 column chromatography | Radioprotective effect of cells against γ-ray radiation-induced oxidative damage |
|
|
| [138] |
| Phloroglucinol | Seaweeds | ND | UVB-induced photoaging of human HaCaT keratinocytes |
|
|
| [139] |
| Phlorotannins | E. radiata | High-performance counter-current chromatography | Neuroprotective activity |
|
|
| [140] |
| Phlorotannins | E. cava | Sephadex LH-20 column chromatography | MG63 cell survival and calcium deposition on polycaprolactone (PCL/Ph) micro nanofibres |
| MTT assay | Enhanced bone tissue growth | [116] |
| Dioxynodehydroeckol | E. cava | Silica-gel column chromatography | UVB-induced apoptosis prevention in human keratinocyte (HaCaT) cells | Reduced by 1.83% at 20 µM of DHE compared to 13.31% in cells exposed to UVB | Flowcytometry following Annexin V and PI labeling | ND | [141] |
| Dieckol | E. cava | Sephadex LH-20 with MeOH | Anti-proliferative and anti-angiogenic effect on EA. hy926 cell lines induced with vascular endothelial growth factor |
|
| Cell migration was inhibited by lowering the level of protein and gene expression of matrix metalloproteinases such as MMP-2 and -9 | [142] |
9. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Ghosh, S.; Sarkar, T.; Pati, S.; Kari, Z.A.; Edinur, H.A.; Chakraborty, R. Novel Bioactive Compounds from Marine Sources as a Tool for Functional Food Development. Front. Mar. Sci. 2022, 9, 832957. [Google Scholar] [CrossRef]
- Bamunuarachchi, N.I.; Khan, F.; Kim, Y.M. Antimicrobial Properties of Actively Purified Secondary Metabolites Isolated from Different Marine Organisms. Curr. Pharm. Biotechnol. 2021, 22, 920–944. [Google Scholar] [CrossRef] [PubMed]
- Lomartire, S.; Gonçalves, A.M.M. An Overview of Potential Seaweed-Derived Bioactive Compounds for Pharmaceutical Applications. Marine Drugs 2022, 20, 141. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.P.; Sidana, J. Chapter 5—Phlorotannins. In Functional Ingredients from Algae for Foods and Nutraceuticals; Domínguez, H., Ed.; Woodhead Publishing: Cambridge, UK, 2013; pp. 181–204. [Google Scholar]
- Yang, H.; Zeng, M.; Dong, S.; Liu, Z.; Li, R. Anti-proliferative activity of phlorotannin extracts from brown algae Laminaria japonica Aresch. Chin. J. Oceanol. Limnol. 2010, 28, 122–130. [Google Scholar] [CrossRef]
- Isaza Martínez, J.H.; Torres Castañeda, H.G. Preparation and Chromatographic Analysis of Phlorotannins. J. Chromatogr. Sci. 2013, 51, 825–838. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.; Fang, D.; Lv, Q.; Wang, Z.; Liu, Y. Targeted Therapeutic Strategies in the Battle Against Pathogenic Bacteria. Front. Pharmacol. 2021, 12, 673239. [Google Scholar] [CrossRef]
- Spitzer, M.; Robbins, N.; Wright, G.D. Combinatorial strategies for combating invasive fungal infections. Virulence 2017, 8, 169–185. [Google Scholar] [CrossRef] [Green Version]
- Meganck, R.M.; Baric, R.S. Developing therapeutic approaches for twenty-first-century emerging infectious viral diseases. Nat. Med. 2021, 27, 401–410. [Google Scholar] [CrossRef]
- Ahmad, B.; Serpell, C.J.; Fong, I.L.; Wong, E.H. Molecular Mechanisms of Adipogenesis: The Anti-adipogenic Role of AMP-Activated Protein Kinase. FFront. Mol. Biosci. 2020, 7, 76. [Google Scholar] [CrossRef]
- Hurt, R.T.; Kulisek, C.; Buchanan, L.A.; McClave, S.A. The obesity epidemic: Challenges, health initiatives, and implications for gastroenterologists. Gastroenterol. Hepatol. 2010, 6, 780–792. [Google Scholar]
- Chakraborty, S.; Rahman, T. The difficulties in cancer treatment. Ecancermedicalscience 2012, 6, ed16. [Google Scholar] [PubMed]
- Yang, X.; Yang, H.; Zhou, G.; Zhao, G.-P. Infectious Disease in the Genomic Era. Annu. Rev. Genom. Hum. Genet. 2008, 9, 21–48. [Google Scholar] [CrossRef] [PubMed]
- Reedy, J.L.; Bastidas, R.J.; Heitman, J. The Virulence of Human Pathogenic Fungi: Notes from the South of France. Cell Host Microbe 2007, 2, 77–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heise, M.T. Viral Pathogenesis. In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Peterson, E.; Kaur, P. Antibiotic Resistance Mechanisms in Bacteria: Relationships Between Resistance Determinants of Antibiotic Producers, Environmental Bacteria, and Clinical Pathogens. Front. Microbiol. 2018, 9, 2928. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, S.; Rahman, N.A.A.; Peile, E.; Rahman, M.; Sartelli, M.; Hassali, M.A.; Islam, T.; Islam, S.; Haque, M. Microbial Resistance Movements: An Overview of Global Public Health Threats Posed by Antimicrobial Resistance, and How Best to Counter. Front. Public Health 2020, 8, 535668. [Google Scholar] [CrossRef]
- van Duin, D.; Paterson, D.L. Multidrug-Resistant Bacteria in the Community: Trends and Lessons Learned. Infect. Dis. Clin N. Am. 2016, 30, 377–390. [Google Scholar] [CrossRef] [Green Version]
- Arendrup, M.C.; Patterson, T.F. Multidrug-Resistant Candida: Epidemiology, Molecular Mechanisms, and Treatment. J. Infect. Dis. 2017, 216 (Suppl. 3), S445–S451. [Google Scholar] [CrossRef] [Green Version]
- Villa, T.G.; Feijoo-Siota, L.; Rama, J.L.R.; Ageitos, J.M. Antivirals against animal viruses. Biochem. Pharmacol. 2017, 133, 97–116. [Google Scholar] [CrossRef]
- Nagayama, K.; Iwamura, Y.; Shibata, T.; Hirayama, I.; Nakamura, T. Bactericidal activity of phlorotannins from the brown alga Ecklonia kurome. J. Antimicrob. Chemother. 2002, 50, 889–893. [Google Scholar] [CrossRef] [Green Version]
- Lopes, G.; Pinto, E.; Andrade, P.B.; Valentão, P. Antifungal activity of phlorotannins against dermatophytes and yeasts: Approaches to the mechanism of action and influence on Candida albicans virulence factor. PLoS ONE 2013, 8, e72203. [Google Scholar] [CrossRef]
- Park, J.-Y.; Kim, J.H.; Kwon, J.M.; Kwon, H.-J.; Jeong, H.J.; Kim, Y.M.; Kim, D.; Lee, W.S.; Ryu, Y.B. Dieckol, a SARS-CoV 3CL(pro) inhibitor, isolated from the edible brown algae Ecklonia cava. Bioorganic Med. Chem. 2013, 21, 3730–3737. [Google Scholar] [CrossRef] [PubMed]
- Klil-Drori, A.J.; Azoulay, L.; Pollak, M.N. Cancer, obesity, diabetes, and antidiabetic drugs: Is the fog clearing? Nat. Rev. Clin. Oncol. 2017, 14, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Scully, T.; Ettela, A.; LeRoith, D.; Gallagher, E.J. Obesity, Type 2 Diabetes, and Cancer Risk. Front. Oncol. 2021, 10, 615375. [Google Scholar] [CrossRef] [PubMed]
- Zatterale, F.; Longo, M.; Naderi, J.; Raciti, G.A.; Desiderio, A.; Miele, C.; Beguinot, F. Chronic Adipose Tissue Inflammation Linking Obesity to Insulin Resistance and Type 2 Diabetes. Front. Physiol. 2020, 10, 1607. [Google Scholar] [CrossRef] [PubMed]
- Ediriweera, M.K.; Tennekoon, K.H.; Samarakoon, S.R. A review on ethnopharmacological applications, pharmacological activities, and bioactive compounds of Mangifera indica (Mango). Evid.-Based Complementary Altern. Med. 2017, 2017, 6949835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E.; Gilbert, D.; Rice, L.B.; Scheld, M.; Spellberg, B.; Bartlett, J. Bad bugs, no drugs: No ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 1334. [Google Scholar] [CrossRef] [Green Version]
- Zacchino, S.A.; Butassi, E.; Di Liberto, M.; Raimondi, M.; Postigo, A.; Sortino, M.J.P. Plant phenolics and terpenoids as adjuvants of antibacterial and antifungal drugs. Phytomedicine 2017, 37, 27–48. [Google Scholar] [CrossRef]
- Da Silva, C.M.; da Silva, D.L.; Modolo, L.V.; Alves, R.B.; de Resende, M.A.; Martins, C.V.; de Fátima, Â.J. Schiff bases: A short review of their antimicrobial activities. J. Adv. Res. 2011, 2, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Li, L.; Du, F.; Sun, L.; Shi, J.; Long, M.; Chen, Z.J.M. Activity and Mechanism of Action of Antifungal Peptides from Microorganisms: A Review. Molecules 2021, 26, 3438. [Google Scholar] [CrossRef]
- Dedeurwaerdere, S.; Friedman, A.; Fabene, P.F.; Mazarati, A.; Murashima, Y.L.; Vezzani, A.; Baram, T.Z. Finding a better drug for epilepsy: Antiinflammatory targets. Epilepsia 2012, 53, 1113–1118. [Google Scholar] [CrossRef]
- Shannon, E.; Abu-Ghannam, N. Antibacterial derivatives of marine algae: An overview of pharmacological mechanisms and applications. Mar. Drugs 2016, 14, 81. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Zhang, W.; Smid, S.D. Phlorotannins: A review on biosynthesis, chemistry and bioactivity. Food Biosci. 2021, 39, 100832. [Google Scholar] [CrossRef]
- Besednova, N.N.; Andryukov, B.G.; Zaporozhets, T.S.; Kryzhanovsky, S.P.; Kuznetsova, T.A.; Fedyanina, L.N.; Makarenkova, I.D.; Zvyagintseva, T.N. Algae polyphenolic compounds and modern antibacterial strategies: Current achievements and immediate prospects. Biomedicines 2020, 8, 342. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Dasagrandhi, C.; Kim, S.-H.; Kim, B.-G.; Eom, S.-H.; Kim, Y.-M. In vitro Antibacterial Activity of Phlorotannins from Edible Brown Algae, Eisenia bicyclis Against Streptomycin-Resistant Listeria monocytogenes. Indian J. Microbiol. 2018, 58, 105–108. [Google Scholar] [CrossRef]
- Cox, S.; Abu-Ghannam, N.; Gupta, S. An assessment of the antioxidant and antimicrobial activity of six species of edible Irish seaweeds. Int. Food Res. J. 2010, 17, 205–220. [Google Scholar]
- Tang, J.; Wang, W.; Chu, W. Antimicrobial and Anti-Quorum Sensing Activities of Phlorotannins From Seaweed (Hizikia fusiforme). Front. Cell. Infect. Microbiol. 2020, 10, 586750. [Google Scholar] [CrossRef]
- Eom, S.-H.; Kim, D.-H.; Lee, S.-H.; Yoon, N.-Y.; Kim, J.H.; Kim, T.H.; Chung, Y.-H.; Kim, S.-B.; Kim, Y.-M.; Kim, H.-W.; et al. In vitro Antibacterial Activity and Synergistic Antibiotic Effects of Phlorotannins Isolated from Eisenia bicyclis Against Methicillin-Resistant Staphylococcus aureus. Phytother. Res. 2013, 27, 1260–1264. [Google Scholar] [CrossRef]
- Choi, J.-S.; Lee, K.; Lee, B.-B.; Kim, Y.-C.; Kim, Y.D.; Hong, Y.-K.; Cho, K.K.; Choi, I.S. Antibacterial activity of the phlorotannins dieckol and phlorofucofuroeckol-A from Ecklonia cava against Propionibacterium acnes. Bot. Sci. 2014, 92, 425–431. [Google Scholar] [CrossRef]
- Lee, J.-H.; Eom, S.-H.; Lee, E.-H.; Jung, Y.-J.; Kim, H.-J.; Jo, M.-R.; Son, K.-T.; Lee, H.-J.; Kim, J.H.; Lee, M.-S.; et al. In vitro antibacterial and synergistic effect of phlorotannins isolated from edible brown seaweed Eisenia bicyclis against acne-related bacteria. Algae 2014, 29, 47–55. [Google Scholar] [CrossRef]
- Choi, J.G.; Kang, O.H.; Brice, O.O.; Lee, Y.S.; Chae, H.S.; Oh, Y.C.; Sohn, D.H.; Park, H.; Choi, H.G.; Kim, S.G.; et al. Antibacterial activity of Ecklonia cava against methicillin-resistant Staphylococcus aureus and Salmonella spp. Foodborne Pathog. Dis. 2010, 7, 435–441. [Google Scholar] [CrossRef]
- Mittal, N.; Tesfu, H.H.; Hogan, A.M.; Cardona, S.T.; Sorensen, J.L. Synthesis and antibiotic activity of novel acylated phloroglucinol compounds against methicillin-resistant Staphylococcus aureus. J. Antibiot. 2019, 72, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Kim, S.-B.; Hwang, H.-J.; Kim, Y.-M.; Lee, M.-S. Antibacterial Property of Ecklonia cava Extract against Marine Bacterial Pathogens. J. Food Hyg. Saf. 2016, 31, 380–385. [Google Scholar] [CrossRef]
- González-Colunga, D.; Antunes-Ricardo, M.; Gutiérrez-Uribe, J.A.; Cruz-Suárez, L.E. Bioactivity-guided identification of anti-AHPND (acute hepatopancreatic necrosis disease) metabolites of Ecklonia arborea. J. Appl. Phycol. 2019, 31, 3189–3199. [Google Scholar] [CrossRef]
- Lee, D.-S.; Kang, M.-S.; Hwang, H.-J.; Eom, S.-H.; Yang, J.-Y.; Lee, M.-S.; Lee, W.-J.; Jeon, Y.-J.; Choi, J.-S.; Kim, Y.-M. Synergistic effect between dieckol from Ecklonia stolonifera and β-lactams against methicillin-resistant Staphylococcus aureus. Biotechnol. Bioprocess Eng. 2008, 13, 758–764. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, Q.; Xu, C.; Yu, J.; Zhao, L.; Guo, Q. Damage to the Membrane Permeability and Cell Death of Vibrio parahaemolyticus Caused by Phlorotannins with Low Molecular Weight from Sargassum thunbergii. J. Aquat. Food Prod. Technol. 2016, 25, 323–333. [Google Scholar] [CrossRef]
- Hierholtzer, A.; Chatellard, L.; Kierans, M.; Akunna, J.C.; Collier, P.J. The impact and mode of action of phenolic compounds extracted from brown seaweed on mixed anaerobic microbial cultures. J. Appl. Microbiol. 2013, 114, 964–973. [Google Scholar] [CrossRef]
- Lee, M.H.; Lee, K.B.; Oh, S.M.; Lee, B.H.; Chee, H.Y. Antifungal activities of dieckol isolated from the marine brown alga Ecklonia cava against Trichophyton rubrum. J. Korean Soc. Appl. Biol. Chem. 2010, 53, 504–507. [Google Scholar] [CrossRef]
- Kim, K.-H.; Yu, D.; Eom, S.-H.; Kim, H.-J.; Kim, D.-H.; Song, H.-S.; Kim, D.-M.; Kim, Y.-M. Fucofuroeckol-A from edible marine alga Eisenia bicyclis to restore antifungal activity of fluconazole against fluconazole-resistant Candida albicans. J. Appl. Phycol. 2018, 30, 605–609. [Google Scholar] [CrossRef]
- Balakrishnan, D.; Kandasamy, D.; Nithyanand, P. A review on antioxidant activity of marine organisms. Int. J. Chem. Technol. Res. 2014, 6, 3431–3436. [Google Scholar]
- Ma, L.; Yao, L.J.M. Antiviral effects of plant-derived essential oils and their components: An updated review. Molecules 2020, 25, 2627. [Google Scholar] [CrossRef]
- Kausar, S.; Said Khan, F.; Ishaq Mujeeb Ur Rehman, M.; Akram, M.; Riaz, M.; Rasool, G.; Hamid Khan, A.; Saleem, I.; Shamim, S.; Malik, A. A review: Mechanism of action of antiviral drugs. Int. J. Immunopathol. Pharmacol. 2021, 35. [Google Scholar] [CrossRef] [PubMed]
- Riccio, G.; Ruocco, N.; Mutalipassi, M.; Costantini, M.; Zupo, V.; Coppola, D.; de Pascale, D.; Lauritano, C.J.B. Ten-year research update review: Antiviral activities from marine organisms. Biomolecules 2020, 10, 1007. [Google Scholar] [CrossRef] [PubMed]
- Besednova, N.N.; Andryukov, B.G.; Zaporozhets, T.S.; Kryzhanovsky, S.P.; Fedyanina, L.N.; Kuznetsova, T.A.; Zvyagintseva, T.N.; Shchelkanov, M.Y. Antiviral effects of polyphenols from marine algae. Biomolecules 2021, 9, 200. [Google Scholar] [CrossRef] [PubMed]
- Sansone, C.; Brunet, C.; Noonan, D.M.; Albini, A.J.A. Marine algal antioxidants as potential vectors for controlling viral diseases. Antioxidants 2020, 9, 392. [Google Scholar] [CrossRef]
- Karadeniz, F.; Kang, K.-H.; Park, J.W.; Park, S.-J.; Kim, S.-K. Anti-HIV-1 activity of phlorotannin derivative 8,4‴-dieckol from Korean brown alga Ecklonia cava. Biosci. Biotechnol. Biochem. 2014, 78, 1151–1158. [Google Scholar] [CrossRef]
- Ahn, M.J.; Yoon, K.D.; Min, S.Y.; Lee, J.S.; Kim, J.H.; Kim, T.G.; Kim, S.H.; Kim, N.G.; Huh, H.; Kim, J. Inhibition of HIV-1 reverse transcriptase and protease by phlorotannins from the brown alga Ecklonia cava. Biol. Pharm. Bull. 2004, 27, 544–547. [Google Scholar] [CrossRef] [Green Version]
- Ryu, Y.B.; Jeong, H.J.; Yoon, S.Y.; Park, J.Y.; Kim, Y.M.; Park, S.J.; Rho, M.C.; Kim, S.J.; Lee, W.S. Influenza virus neuraminidase inhibitory activity of phlorotannins from the edible brown alga Ecklonia cava. J. Agric. Food Chem. 2011, 59, 6467–6473. [Google Scholar] [CrossRef]
- Afroz, M.; Zihad, S.N.K.; Uddin, S.J.; Rouf, R.; Rahman, M.S.; Islam, M.T.; Khan, I.N.; Ali, E.S.; Aziz, S.; Shilpi, J.A.; et al. A systematic review on antioxidant and antiinflammatory activity of Sesame (Sesamum indicum L.) oil and further confirmation of antiinflammatory activity by chemical profiling and molecular docking. Phytother. Res. 2019, 33, 2585–2608. [Google Scholar] [CrossRef]
- Beg, S.; Swain, S.; Hasan, H.; Barkat, M.A.; Hussain, M.S.J.P.R. Systematic review of herbals as potential anti-inflammatory agents: Recent advances, current clinical status and future perspectives. Pharmacogn. Rev. 2011, 5, 120. [Google Scholar] [CrossRef] [Green Version]
- Patel, S.S.; Savjani, J.K. Systematic review of plant steroids as potential antiinflammatory agents: Current status and future perspectives. J. Phytopharm. 2015, 4, 121–125. [Google Scholar] [CrossRef]
- Barbosa, M.; Lopes, G.; Andrade, P.B.; Valentão, P. Technology, Bioprospecting of brown seaweeds for biotechnological applications: Phlorotannin actions in inflammation and allergy network. Trends Food Sci. Technol. 2019, 86, 153–171. [Google Scholar] [CrossRef]
- Jung, H.A.; Jin, S.E.; Ahn, B.R.; Lee, C.M.; Choi, J.S. Anti-inflammatory activity of edible brown alga Eisenia bicyclis and its constituents fucosterol and phlorotannins in LPS-stimulated RAW264.7 macrophages. Food Chem. Toxicol. 2013, 59, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, M.; Lopes, G.; Ferreres, F.; Andrade, P.B.; Pereira, D.M.; Gil-Izquierdo, Á.; Valentão, P. Phlorotannin extracts from Fucales: Marine polyphenols as bioregulators engaged in inflammation-related mediators and enzymes. Algal Res. 2017, 28, 1–8. [Google Scholar] [CrossRef]
- Kim, M.-M.; Kim, S.-K. Effect of phloroglucinol on oxidative stress and inflammation. Food Chem. Toxicol. 2010, 48, 2925–2933. [Google Scholar] [CrossRef] [PubMed]
- Joung, E.J.; Lee, M.S.; Choi, J.W.; Kim, J.S.; Shin, T.; Jung, B.M.; Kim, J.I.; Kim, H.R. Anti-inflammatory effects of phlorofucofuroeckol B-rich ethyl acetate fraction obtained from Myagropsis myagroides on lipopolysaccharide-stimulated RAW 264.7 cells and mouse edema. Int. Immunopharmacol. 2012, 14, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves-Fernández, C.; Sineiro, J.; Moreira, R.; Gualillo, O. Extraction and characterization of phlorotannin-enriched fractions from the Atlantic seaweed Bifurcaria bifurcata and evaluation of their cytotoxic activity in murine cell line. J. Appl. Phycol. 2019, 31, 2573–2583. [Google Scholar] [CrossRef]
- Nair, D.; Vanuopadath, M.; Balasubramanian, A.; Iyer, A.; Ganesh, S.; Anil, A.N.; Vikraman, V.; Pillai, P.; Bose, C.; Nair, B.G.; et al. Phlorotannins from Padina tetrastromatica: Structural characterisation and functional studies. J. Appl. Phycol. 2019, 31, 3131–3141. [Google Scholar] [CrossRef]
- Yu, D.-K.; Lee, B.; Kwon, M.; Yoon, N.; Shin, T.; Kim, N.-G.; Choi, J.-S.; Kim, H.-R. Phlorofucofuroeckol B suppresses inflammatory responses by down-regulating nuclear factor κB activation via Akt, ERK, and JNK in LPS-stimulated microglial cells. Int. Immunopharmacol. 2015, 28, 1068–1075. [Google Scholar] [CrossRef]
- Yang, Y.-I.; Shin, H.-C.; Kim, S.H.; Park, W.-Y.; Lee, K.-T.; Choi, J.-H. 6,6′-Bieckol, isolated from marine alga Ecklonia cava, suppressed LPS-induced nitric oxide and PGE2 production and inflammatory cytokine expression in macrophages: The inhibition of NFκB. Int. Immunopharmacol. 2012, 12, 510–517. [Google Scholar] [CrossRef]
- Hamed, I.; Özogul, F.; Özogul, Y.; Regenstein, J.M. Marine bioactive compounds and their health benefits: A review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 446–465. [Google Scholar] [CrossRef]
- Bratchkova, A.; Kroumov, A.D. Microalgae as producers of biologically active compounds with antibacterial, antiviral, antifungal, antialgal, antiprotozoal, antiparasitic and anticancer activity. Acta Microbiol. Bulg. 2020, 36, 79–89. [Google Scholar]
- Manganyi, M.C.; Ateba, C.N.J.M. Untapped potentials of endophytic fungi: A review of novel bioactive compounds with biological applications. Microorganisms 2020, 8, 1934. [Google Scholar] [CrossRef] [PubMed]
- Jose, G.M. Biological Responses of Algal Derived Sulfated Polysaccharides: An Emphasis on Cancer Prophylaxis. Trends Biomater. Artif. Organs 2015, 29, 64–85. [Google Scholar]
- Hussain, E.; Wang, L.-J.; Jiang, B.; Riaz, S.; Butt, G.Y.; Shi, D.-Y. A review of the components of brown seaweeds as potential candidates in cancer therapy. RSC Adv. 2016, 6, 12592–12610. [Google Scholar] [CrossRef]
- Meng, W.; Mu, T.; Sun, H.; Garcia-Vaquero, M. Phlorotannins: A review of extraction methods, structural characteristics, bioactivities, bioavailability, and future trends. Algal Res. 2021, 60, 102484. [Google Scholar] [CrossRef]
- Ahn, J.-H.; Yang, Y.-I.; Lee, K.-T.; Choi, J.-H. Dieckol, isolated from the edible brown algae Ecklonia cava, induces apoptosis of ovarian cancer cells and inhibits tumor xenograft growth. J. Cancer Res. Clin. Oncol. 2015, 141, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.-S.; Kim, J.-A.; Yoon, N.-Y.; Kim, S.-K. Induction of apoptosis by phloroglucinol derivative from Ecklonia Cava in MCF-7 human breast cancer cells. Food Chem. Toxicol. 2009, 47, 1653–1658. [Google Scholar] [CrossRef]
- Kim, R.-K.; Suh, Y.; Yoo, K.-C.; Cui, Y.-H.; Hwang, E.; Kim, H.-J.; Kang, J.-S.; Kim, M.-J.; Lee, Y.Y.; Lee, S.-J. Phloroglucinol suppresses metastatic ability of breast cancer cells by inhibition of epithelial-mesenchymal cell transition. Cancer Sci. 2015, 106, 94–101. [Google Scholar] [CrossRef] [Green Version]
- Kang, M.-H.; Kim, I.-H.; Nam, T.-J. Phloroglucinol induces apoptosis via apoptotic signaling pathways in HT-29 colon cancer cells. Oncol. Rep. 2014, 32, 1341–1346. [Google Scholar] [CrossRef] [Green Version]
- Abdelhamid, A.; Lajili, S.; Elkaibi, M.A.; Ben Salem, Y.; Abdelhamid, A.; Muller, C.D.; Majdoub, H.; Kraiem, J.; Bouraoui, A. Optimized Extraction, Preliminary Characterization and Evaluation of the in vitro Anticancer Activity of Phlorotannin-Rich Fraction from the Brown Seaweed, Cystoseira sedoides. J. Aquat. Food Prod. Technol. 2019, 28, 892–909. [Google Scholar] [CrossRef]
- Sadeeshkumar, V.; Duraikannu, A.; Ravichandran, S.; Fredrick, W.S.; Sivaperumal, R.; Kodisundaram, P. Protective effects of dieckol on N-nitrosodiethylamine induced hepatocarcinogenesis in rats. Biomed. Pharmacother. 2016, 84, 1810–1819. [Google Scholar] [CrossRef] [PubMed]
- Antolovich, M.; Prenzler, P.D.; Patsalides, E.; McDonald, S.; Robards, K. Methods for testing antioxidant activity. Analyst 2002, 127, 183–198. [Google Scholar] [CrossRef] [PubMed]
- Fubini, B.; Hubbard, A. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) generation by silica in inflammation and fibrosis. Free. Radic. Biol. Med. 2003, 34, 1507–1516. [Google Scholar] [CrossRef]
- Nigam, V.; Sodhi, J.S. Some medicinal plants with antioxidant activity—A review. Int. J. Pharm. Biol. Sci. 2014, 4, 173–178. [Google Scholar]
- Diniz do Nascimento, L.; De Moraes, A.A.B.; Da Costa, K.S.; Galúcio, J.M.P.; Taube, P.S.; Costa, C.M.L.; Cruz, J.N.; de Aguiar Andrade, E.H.; De Faria, L.J.G. Bioactive Natural Compounds and Antioxidant Activity of Essential Oils from Spice Plants: New Findings and Potential Applications. Biomolecules 2020, 10, 988. [Google Scholar] [CrossRef] [PubMed]
- Chaves, N.; Santiago, A.; Alías, J.C. Quantification of the Antioxidant Activity of Plant Extracts: Analysis of Sensitivity and Hierarchization Based on the Method Used. Antioxidants 2020, 9, 76. [Google Scholar] [CrossRef] [Green Version]
- Sathya, R.; Kanaga, N.; Sankar, P.; Jeeva, S. Antioxidant properties of phlorotannins from brown seaweed Cystoseira trinodis (Forsskål) C. Agardh. Arab. J. Chem. 2017, 10, S2608–S2614. [Google Scholar] [CrossRef] [Green Version]
- Yotsu-Yamashita, M.; Kondo, S.; Segawa, S.; Lin, Y.-C.; Toyohara, H.; Ito, H.; Konoki, K.; Cho, Y.; Uchida, T. Isolation and Structural Determination of Two Novel Phlorotannins from the Brown Alga Ecklonia kurome Okamura, and Their Radical Scavenging Activities. Marine Drugs 2013, 11, 165–183. [Google Scholar] [CrossRef] [Green Version]
- Ahn, G.-N.; Kim, K.-N.; Cha, S.-H.; Song, C.-B.; Lee, J.; Heo, M.-S.; Yeo, I.-K.; Lee, N.-H.; Jee, Y.-H.; Kim, J.-S.; et al. Antioxidant activities of phlorotannins purified from Ecklonia cava on free radical scavenging using ESR and H2O2-mediated DNA damage. Eur. Food Res. Technol. 2007, 226, 71–79. [Google Scholar] [CrossRef]
- Nakai, M.; Kageyama, N.; Nakahara, K.; Miki, W. Phlorotannins as Radical Scavengers from the Extract of Sargassum ringgoldianum. Mar. Biotechnol. 2006, 8, 409–414. [Google Scholar] [CrossRef]
- Boi, V.N.; Trang, N.T.M.; Cuong, D.X.; Ha, H.T. Antioxidant Phlorotannin from Brown Algae Sargassum dupplicatum: Enzyme-assissted Extraction and Purification. World J. Food Sci. Technol. 2020, 4, 62–68. [Google Scholar] [CrossRef]
- Lee, J.-H.; Ko, J.-Y.; Oh, J.-Y.; Kim, E.-A.; Kim, C.-Y.; Jeon, Y.-J. Evaluation of phlorofucofuroeckol-A isolated from Ecklonia cava (Phaeophyta) on anti-lipid peroxidation in vitro and in vivo. Algae 2015, 30, 313–323. [Google Scholar] [CrossRef] [Green Version]
- Kang, M.-C.; Cha, S.H.; Wijesinghe, W.A.J.P.; Kang, S.-M.; Lee, S.-H.; Kim, E.-A.; Song, C.B.; Jeon, Y.-J. Protective effect of marine algae phlorotannins against AAPH-induced oxidative stress in zebrafish embryo. Food Chem. 2013, 138, 950–955. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-M.; Cha, S.-H.; Ko, J.-Y.; Kang, M.-C.; Kim, D.; Heo, S.-J.; Kim, J.-S.; Heu, M.S.; Kim, Y.-T.; Jung, W.-K.; et al. Neuroprotective effects of phlorotannins isolated from a brown alga, Ecklonia cava, against H2O2-induced oxidative stress in murine hippocampal HT22 cells. Environ. Toxicol. Pharmacol. 2012, 34, 96–105. [Google Scholar] [CrossRef]
- Kang, X.; Liang, H.; Luo, Y.; Li, Z.; He, F.; Han, X.; Zhang, L. Anti-adipogenesis and metabolism-regulating effects of heat-inactivated Streptococcus thermophilus MN-ZLW-002. Lett. Appl. Microbiol. 2021, 72, 677–687. [Google Scholar] [CrossRef]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef]
- Tsai, Y.-C.; Yang, B.-C.; Peng, W.-H.; Lee, Y.-M.; Yen, M.-H.; Cheng, P.-Y. Heme oxygenase-1 mediates anti-adipogenesis effect of raspberry ketone in 3T3-L1 cells. Phytomedicine 2017, 31, 11–17. [Google Scholar] [CrossRef]
- Jung, H.A.; Jung, H.J.; Jeong, H.Y.; Kwon, H.J.; Ali, M.Y.; Choi, J.S. Phlorotannins isolated from the edible brown alga Ecklonia stolonifera exert anti-adipogenic activity on 3T3-L1 adipocytes by downregulating C/EBPα and PPARγ. Fitoterapia 2014, 92, 260–269. [Google Scholar] [CrossRef]
- Karadeniz, F.; Ahn, B.-N.; Kim, J.; Seo, Y.; Jang, M.-S.; Nam, K.-H.; Kim, M.; Lee, S.-H.; Kong, C.-S. Phlorotannins suppress adipogenesis in pre-adipocytes while enhancing osteoblastogenesis in pre-osteoblasts. Arch. Pharmacal Res. 2015, 38, 2172–2182. [Google Scholar] [CrossRef]
- Seo, Y.-J.; Kim, K.-J.; Koh, E.-J.; Choi, J.; Lee, B.-Y. Anti-adipogenesis mechanism of pterostilbene through the activation of heme oxygenase-1 in 3T3-L1 cells. Phytomedicine 2017, 33, 7–13. [Google Scholar] [CrossRef]
- Guo, L.; Li, K.; Kang, J.S.; Kang, N.J.; Son, B.G.; Choi, Y.W. Strawberry fermentation with Cordyceps militaris has anti-adipogenesis activity. Food Biosci. 2020, 35, 100576. [Google Scholar] [CrossRef]
- Kong, C.S.; Kim, H.; Seo, Y. Edible Brown Alga Ecklonia cava Derived Phlorotannin-Induced Anti-Adipogenic Activity in vitro. J. Food Biochem. 2015, 39, 1–10. [Google Scholar] [CrossRef]
- Hu, X.; Tao, N.; Wang, X.; Xiao, J.; Wang, M. Marine-derived bioactive compounds with anti-obesity effect: A review. J. Funct. Foods 2016, 21, 372–387. [Google Scholar] [CrossRef]
- Eom, S.H.; Lee, S.H.; Yoon, N.Y.; Jung, W.K.; Jeon, Y.J.; Kim, S.K.; Lee, M.S.; Kim, Y.M. α-Glucosidase-and α-amylase-inhibitory activities of phlorotannins from Eisenia bicyclis. J. Sci. Food Agric. 2012, 92, 2084–2090. [Google Scholar] [CrossRef]
- Lee, S.-H.; Yong, L.; Karadeniz, F.; Kim, M.-M.; Kim, S.-K. α-Glucosidase and α-amylase inhibitory activities of phloroglucinal derivatives from edible marine brown alga, Ecklonia cava. J. Sci. Food Agric. 2009, 89, 1552–1558. [Google Scholar] [CrossRef]
- Gheda, S.; Naby, M.A.; Mohamed, T.; Pereira, L.; Khamis, A. Antidiabetic and antioxidant activity of phlorotannins extracted from the brown seaweed Cystoseira compressa in streptozotocin-induced diabetic rats. Environ. Sci. Pollut. Res. 2021, 28, 22886–22901. [Google Scholar] [CrossRef]
- Catarino, M.D.; Silva, A.; Mateus, N.; Cardoso, S.M. Optimization of phlorotannins extraction from Fucus vesiculosus and evaluation of their potential to prevent metabolic disorders. Marine Drugs 2019, 17, 162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryu, B.; Jiang, Y.; Kim, H.-S.; Hyun, J.-M.; Lim, S.-B.; Li, Y.; Jeon, Y.-J. Ishophloroglucin A, a novel phlorotannin for standardizing the anti-α-glucosidase activity of Ishige okamurae. Marine Drugs 2018, 16, 436. [Google Scholar] [CrossRef] [Green Version]
- You, H.-N.; Lee, H.-A.; Park, M.-H.; Lee, J.-H.; Han, J.-S. Phlorofucofuroeckol A isolated from Ecklonia cava alleviates postprandial hyperglycemia in diabetic mice. Eur. J. Pharmacol. 2015, 752, 92–96. [Google Scholar] [CrossRef]
- Kang, M.-C.; Wijesinghe, W.A.J.P.; Lee, S.-H.; Kang, S.-M.; Ko, S.-C.; Yang, X.; Kang, N.; Jeon, B.-T.; Kim, J.; Lee, D.-H.; et al. Dieckol isolated from brown seaweed Ecklonia cava attenuates type II diabetes in db/db mouse model. Food Chem. Toxicol. 2013, 53, 294–298. [Google Scholar] [CrossRef]
- Kim, S.-K.; Kong, C.-S. Anti-adipogenic effect of dioxinodehydroeckol via AMPK activation in 3T3-L1 adipocytes. Chem.-Biol. Interact. 2010, 186, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.-J.; Ko, S.-C.; Cha, S.-H.; Kang, D.-H.; Park, H.-S.; Choi, Y.-U.; Kim, D.; Jung, W.-K.; Jeon, Y.-J. Effect of phlorotannins isolated from Ecklonia cava on melanogenesis and their protective effect against photo-oxidative stress induced by UV-B radiation. Toxicol. Vitr. 2009, 23, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, D.; Liu, G.-M.; Chen, Q.; Lu, Z. Ameliorative effect of dieckol-enriched extraction from Laminaria japonica on hepatic steatosis induced by a high-fat diet via β-oxidation pathway in ICR mice. J. Funct. Foods 2019, 58, 44–55. [Google Scholar] [CrossRef]
- Ko, S.-C.; Lee, M.; Lee, J.-H.; Lee, S.-H.; Lim, Y.; Jeon, Y.-J. Dieckol, a phlorotannin isolated from a brown seaweed, Ecklonia cava, inhibits adipogenesis through AMP-activated protein kinase (AMPK) activation in 3T3-L1 preadipocytes. Environ. Toxicol. Pharmacol. 2013, 36, 1253–1260. [Google Scholar] [CrossRef]
- Filippini, M.; Baldisserotto, A.; Menotta, S.; Fedrizzi, G.; Rubini, S.; Gigliotti, D.; Valpiani, G.; Buzzi, R.; Manfredini, S.; Vertuani, S. Heavy metals and potential risks in edible seaweed on the market in Italy. Chemosphere 2021, 263, 127983. [Google Scholar] [CrossRef] [PubMed]
- Rajaram, R.; Rameshkumar, S.; Anandkumar, A. Health risk assessment and potentiality of green seaweeds on bioaccumulation of trace elements along the Palk Bay coast, Southeastern India. Mar. Pollut. Bull. 2020, 154, 111069. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Pan, X.-D.; Huang, B.-F.; Han, J.-L. Distribution of metals and metalloids in dried seaweeds and health risk to population in southeastern China. Sci. Rep. 2018, 8, 3578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassani, L.; Gomez-Zavaglia, A.; Jimenez-Lopez, C.; Lourenço-Lopes, C.; Prieto, M.A.; Simal-Gandara, J. Seaweed-based natural ingredients: Stability of phlorotannins during extraction, storage, passage through the gastrointestinal tract and potential incorporation into functional foods. Food Res. Int. 2020, 137, 109676. [Google Scholar] [CrossRef] [PubMed]
- Corona, G.; Ji, Y.; Anegboonlap, P.; Hotchkiss, S.; Gill, C.; Yaqoob, P.; Spencer, J.P.; Rowland, I. Gastrointestinal modifications and bioavailability of brown seaweed phlorotannins and effects on inflammatory markers. Br. J. Nutr. 2016, 115, 1240–1253. [Google Scholar] [CrossRef] [Green Version]
- Rajha, H.N.; Paule, A.; Aragonès, G.; Barbosa, M.; Caddeo, C.; Debs, E.; Dinkova, R.; Eckert, G.P.; Fontana, A.; Gebrayel, P. Recent Advances in Research on Polyphenols: Effects on Microbiota, Metabolism, and Health. Mol. Nutr. Food Res. 2022, 66, 2100670. [Google Scholar] [CrossRef]
- Rajan, D.K.; Mohan, K.; Zhang, S.; Ganesan, A.R. Dieckol: A brown algal phlorotannin with biological potential. Biomed. Pharmacother. 2021, 142, 111988. [Google Scholar] [CrossRef] [PubMed]
- Michalak, I.; Chojnacka, K. Algae as production systems of bioactive compounds. Eng. Life Sci. 2015, 15, 160–176. [Google Scholar] [CrossRef]
- Stengel, D.B.; Connan, S. Marine algae: A source of biomass for biotechnological applications. In Natural Products from Marine Algae; Springer: New York, NY, USA; Humana Press: New York, NY, USA, 2015; pp. 1–37. [Google Scholar]
- Dang, T.T.; Bowyer, M.C.; Van Altena, I.A.; Scarlett, C.J. Optimum conditions of microwave-assisted extraction for phenolic compounds and antioxidant capacity of the brown alga Sargassum vestitum. Sep. Sci. Technol. 2018, 53, 1711–1723. [Google Scholar] [CrossRef]
- Kadam, S.U.; Tiwari, B.K.; O’Donnell, C.P. Application of novel extraction technologies for bioactives from marine algae. J. Agric. Food Chem. 2013, 61, 4667–4675. [Google Scholar] [CrossRef] [PubMed]
- Thiyagarasaiyar, K.; Goh, B.-H.; Jeon, Y.-J.; Yow, Y.-Y. Algae metabolites in cosmeceutical: An overview of current applications and challenges. Mar. Drugs 2020, 18, 323. [Google Scholar] [CrossRef]
- Quitério, E.; Soares, C.; Ferraz, R.; Delerue-Matos, C.; Grosso, C. Marine Health-Promoting Compounds: Recent Trends for Their Characterization and Human Applications. Foods 2021, 10, 3100. [Google Scholar] [CrossRef]
- Corsetto, P.A.; Montorfano, G.; Zava, S.; Colombo, I.; Ingadottir, B.; Jonsdottir, R.; Sveinsdottir, K.; Rizzo, A.M. Characterization of antioxidant potential of seaweed extracts for enrichment of convenience food. Antioxidants 2020, 9, 249. [Google Scholar] [CrossRef] [Green Version]
- Santos, S.A.; Félix, R.; Pais, A.; Rocha, S.M.; Silvestre, A.J. The quest for phenolic compounds from macroalgae: A review of extraction and identification methodologies. Biomolecules 2019, 9, 847. [Google Scholar] [CrossRef] [Green Version]
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction methods, antioxidative action, bioavailability and anticarcinogenic effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef]
- Buedenbender, L.; Astone, F.A.; Tasdemir, D. Bioactive molecular networking for mapping the antimicrobial constituents of the baltic brown alga fucus vesiculosus. Mar. Drugs 2020, 18, 311. [Google Scholar] [CrossRef]
- Al-Mola, H.F. Antibacterial activity of crude extracts and phlorotannin isolated from the diatom Cymbella spp. J. Pharm. Res 2009, 2, 304–308. [Google Scholar]
- Kang, M.-H.; Kim, I.-H.; Nam, T.-J. Phloroglucinol induces apoptosis through the regulation of insulin-like growth factor 1 receptor signaling pathways in human colon cancer HT-29 cells. Int. J. Oncol. 2014, 45, 1036–1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vizetto-Duarte, C.; Custódio, L.; Gangadhar, K.N.; Lago, J.H.; Dias, C.; Matos, A.M.; Neng, N.; Nogueira, J.M.; Barreira, L.; Albericio, F.; et al. Isololiolide, a carotenoid metabolite isolated from the brown alga Cystoseira tamariscifolia, is cytotoxic and able to induce apoptosis in hepatocarcinoma cells through caspase-3 activation, decreased Bcl-2 levels, increased p53 expression and PARP cleavage. Phytomedicine 2016, 23, 550–557. [Google Scholar] [PubMed]
- Kang, K.A.; Lee, K.H.; Chae, S.; Zhang, R.; Jung, M.S.; Ham, Y.M.; Baik, J.S.; Lee, N.H.; Hyun, J.W. Cytoprotective effect of phloroglucinol on oxidative stress induced cell damage via catalase activation. J. Cell. Biochem. 2006, 97, 609–620. [Google Scholar] [CrossRef]
- Kang, K.A.; Zhang, R.; Chae, S.; Lee, S.J.; Kim, J.; Kim, J.; Jeong, J.; Lee, J.; Shin, T.; Lee, N.H.; et al. Phloroglucinol (1,3,5-trihydroxybenzene) protects against ionizing radiation-induced cell damage through inhibition of oxidative stress in vitro and in vivo. Chem.-Biol. Interact. 2010, 185, 215–226. [Google Scholar] [CrossRef]
- Piao, M.J.; Zhang, R.; Lee, N.H.; Hyun, J.W. Phloroglucinol Attenuates Ultraviolet B Radiation-Induced Matrix Metalloproteinase-1 Production in Human Keratinocytes via Inhibitory Actions against Mitogen-Activated Protein Kinases and Activator Protein-1. Photochem. Photobiol. 2012, 88, 381–388. [Google Scholar] [CrossRef]
- Shrestha, S.; Johnston, M.R.; Zhang, W.; Smid, S.D. A phlorotannin isolated from Ecklonia radiata, Dibenzodioxin-fucodiphloroethol, inhibits neurotoxicity and aggregation of β-amyloid. Phytomed. Plus 2021, 1, 100125. [Google Scholar] [CrossRef]
- Ryu, B.; Ahn, B.-N.; Kang, K.-H.; Kim, Y.-S.; Li, Y.-X.; Kong, C.-S.; Kim, S.-K.; Kim, D.G. Dioxinodehydroeckol protects human keratinocyte cells from UVB-induced apoptosis modulated by related genes Bax/Bcl-2 and caspase pathway. J. Photochem. Photobiol. B Biol. 2015, 153, 352–357. [Google Scholar] [CrossRef]
- Li, Y.X.; Li, Y.; Je, J.Y.; Kim, S.K. Dieckol as a novel anti-proliferative and anti-angiogenic agent and computational anti-angiogenic activity evaluation. Environ. Toxicol. Pharmacol. 2015, 39, 259–270. [Google Scholar] [CrossRef]
- Khan, F.; Oh, D.; Chandika, P.; Jo, D.M.; Bamunarachchi, N.I.; Jung, W.K.; Kim, Y.M. Inhibitory activities of phloroglucinol-chitosan nanoparticles on mono- and dual-species biofilms of Candida albicans and bacteria. Colloids Surf. B Biointerfaces 2022, 211, 112307. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, F.; Jeong, G.-J.; Khan, M.S.A.; Tabassum, N.; Kim, Y.-M. Seaweed-Derived Phlorotannins: A Review of Multiple Biological Roles and Action Mechanisms. Mar. Drugs 2022, 20, 384. https://doi.org/10.3390/md20060384
Khan F, Jeong G-J, Khan MSA, Tabassum N, Kim Y-M. Seaweed-Derived Phlorotannins: A Review of Multiple Biological Roles and Action Mechanisms. Marine Drugs. 2022; 20(6):384. https://doi.org/10.3390/md20060384
Chicago/Turabian StyleKhan, Fazlurrahman, Geum-Jae Jeong, Mohd Sajjad Ahmad Khan, Nazia Tabassum, and Young-Mog Kim. 2022. "Seaweed-Derived Phlorotannins: A Review of Multiple Biological Roles and Action Mechanisms" Marine Drugs 20, no. 6: 384. https://doi.org/10.3390/md20060384
APA StyleKhan, F., Jeong, G.-J., Khan, M. S. A., Tabassum, N., & Kim, Y.-M. (2022). Seaweed-Derived Phlorotannins: A Review of Multiple Biological Roles and Action Mechanisms. Marine Drugs, 20(6), 384. https://doi.org/10.3390/md20060384







