Ferroptosis Related Immunomodulatory Effect of a Novel Extracellular Polysaccharides from Marine Fungus Aureobasidium melanogenum
Abstract
:1. Introduction
2. Results
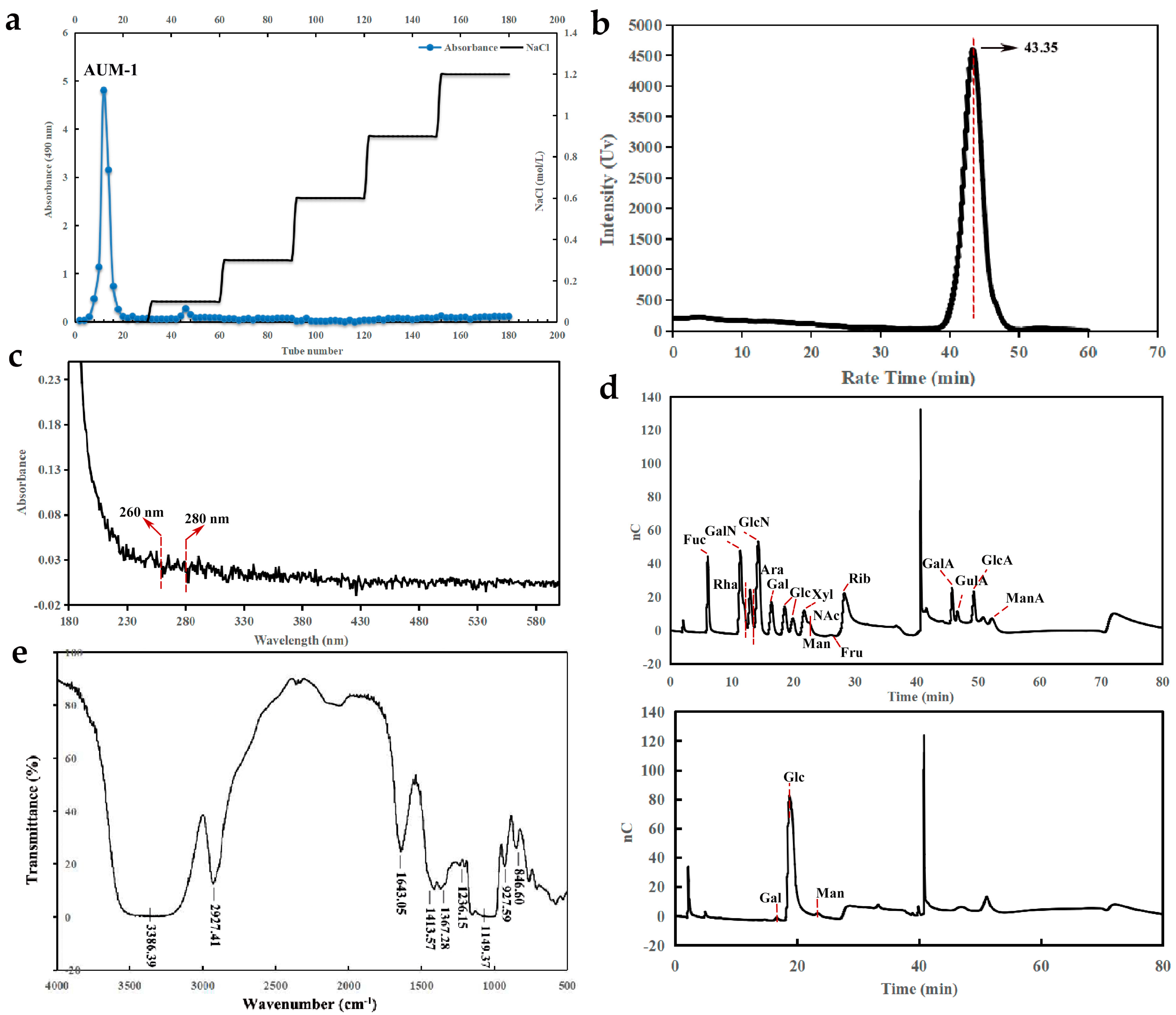
2.1. Preparation and Physicochemical Properties
2.2. Structural Characterization
2.2.1. Monosaccharide Composition Analysis
2.2.2. FT-IR Analysis
2.2.3. Methylation Analysis
2.2.4. NMR Analysis
2.3. Immunomodulatory Effects of AUM-1 on RAW264.7 Cells
2.4. Metabolites Analysis
2.4.1. DAMs Data
2.4.2. KEGG Pathways Analysis
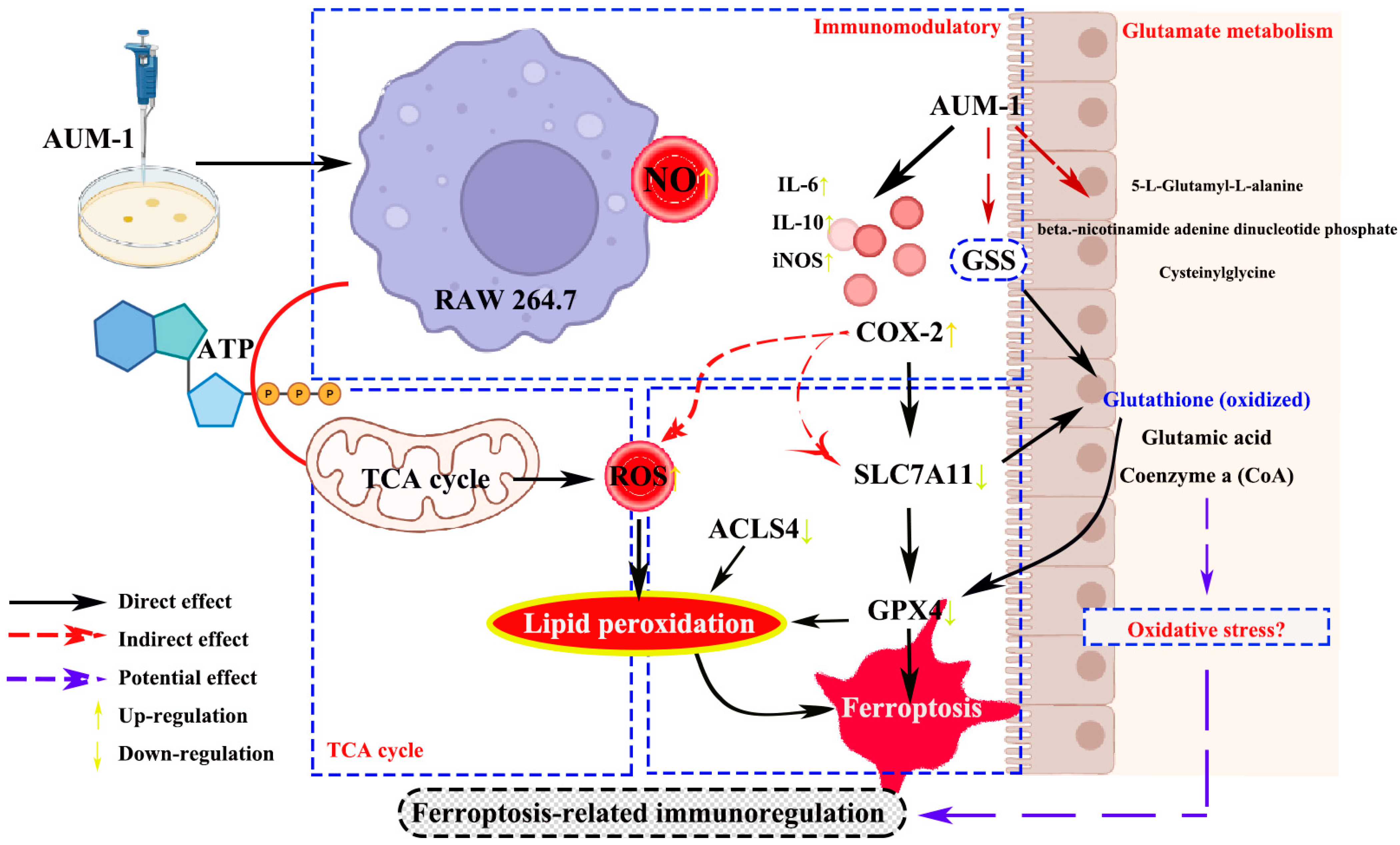
2.5. Verification of Ferroptosis-Related Immunomodulatory
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Polysaccharides
4.3. Physicochemical Characterizations
4.3.1. Molecular Weight and Monosaccharide Composition
4.3.2. UV and FT-IR Spectrum
4.3.3. Methylation
4.3.4. NMR Analysis
4.4. Immunomodulatory Effect
4.4.1. Cell Culture and CCK-8 Assay
4.4.2. NO Assay and Cytokine Production
4.4.3. Immunofluorescence Staining
4.5. Metabolites Analysis
4.6. Western Blot Analysis
4.7. Determination of ROS and Immunofluorescence
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arnosti, C.; Wietz, M.; Brinkhoff, T.; Hehemann, J.H.; Probandt, D.; Zeugner, L.; Amann, R. The Biogeochemistry of Marine Polysaccharides: Sources, Inventories, and Bacterial Drivers of the Carbohydrate Cycle. Annu. Rev. Mar. Sci. 2021, 13, 81–108. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Mao, W.; Gao, Y.; Teng, X.; Zhu, W.; Chen, Y.; Zhao, C.; Li, N.; Wang, C.; Yan, M.; et al. Structural elucidation of an extracellular polysaccharide produced by the marine fungus Aspergillus versicolor. Carbohydr. Polym. 2013, 93, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cao, K.; Cong, P.; Liu, Y.; Cui, H.; Xue, C. Structure characterization and antitumor activity of the extracellular polysaccharide from the marine fungus Hansfordia sinuosae. Carbohydr. Polym. 2018, 190, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Ding, R.; Zhou, Y.; Zhang, X.; Zhu, R.; Gao, X.D. Immunomodulatory effects of polysaccharide from marine fungus Phoma herbarum YS4108 on T cells and dendritic cells. Mediat. Inflamm. 2014, 2014, 738631. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.L.; Mao, W.J.; Tao, H.W.; Zhu, W.M.; Yan, M.X.; Liu, X.; Guo, T.T.; Guo, T. Preparation and characterization of a novel extracellular polysaccharide with antioxidant activity, from the mangrove-associated fungus Fusarium oxysporum. Mar. Biotechnol. 2015, 17, 219–228. [Google Scholar] [CrossRef]
- Cao, M.X.; Xie, X.D.; Wang, X.R.; Hu, W.Y.; Zhao, Y.; Chen, Q.; Ji, L.; Wei, Y.Y.; Yu, M.L.; Hu, T.J. Separation, Purification, Structure Analysis, In Vitro Antioxidant Activity and circRNA-miRNA-mRNA Regulatory Network on PRV-Infected RAW264.7 Cells of a Polysaccharide Derived from Arthrospira platensis. Arthrospira Platensis Antioxid. 2021, 10, 1689. [Google Scholar] [CrossRef]
- Ma, Z.C.; Liu, N.N.; Chi, Z.; Liu, G.L.; Chi, Z.M. Genetic Modification of the Marine-Isolated Yeast Aureobasidium melanogenum P16 for Efficient Pullulan Production from Inulin. Mar. Biotechnol. 2015, 17, 511–522. [Google Scholar] [CrossRef]
- Jiang, H.; Chen, T.J.; Chi, Z.; Hu, Z.; Liu, G.L.; Sun, Y.; Zhang, S.H.; Chi, Z.M. Macromolecular pullulan produced by Aureobasidium melanogenum 13-2 isolated from the Taklimakan desert and its crucial roles in resistance to the stress treatments. Int. J. Biol. Macromol. 2019, 135, 429–436. [Google Scholar] [CrossRef]
- Xue, S.J.; Chen, L.; Jiang, H.; Liu, G.L.; Chi, Z.M.; Hu, Z.; Chi, Z. High pullulan biosynthesis from high concentration of glucose by a hyperosmotic resistant, yeast-like fungal strain isolated from a natural comb-honey. Food Chem. 2019, 286, 123–128. [Google Scholar] [CrossRef]
- Chen, G.; Zhu, Y.; Zhang, G.; Liu, H.; Wei, Y.; Wang, P.; Wang, F.; Xian, M.; Xiang, H.; Zhang, H. Optimization and characterization of pullulan production by a newly isolated high-yielding strain Aureobasidium melanogenum. Prep. Biochem. Biotechnol. 2019, 49, 557–566. [Google Scholar] [CrossRef]
- Liu, N.N.; Chi, Z.; Wang, Q.Q.; Hong, J.; Liu, G.L.; Hu, Z.; Chi, Z.M. Simultaneous production of both high molecular weight pullulan and oligosaccharides by Aureobasdium melanogenum P16 isolated from a mangrove ecosystem. Int. J. Biol. Macromol. 2017, 102, 1016–1024. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, F.; Liu, X.; St Ange, K.; Zhang, A.; Li, Q.; Linhardt, R.J. Isolation of a lectin binding rhamnogalacturonan-I containing pectic polysaccharide from pumpkin. Carbohydr. Polym. 2017, 163, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Chylińska, M.; Szymańska-Chargot, M.; Zdunek, A. FT-IR and FT-Raman characterization of non-cellulosic polysaccharides fractions isolated from plant cell wall. Carbohydr. Polym. 2016, 154, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Foley, S.A.; Szegezdi, E.; Mulloy, B.; Samali, A.; Tuohy, M.G. An unfractionated fucoidan from Ascophyllum nodosum: Extraction, characterization, and apoptotic effects in vitro. J. Nat. Prod. 2011, 74, 1851–1861. [Google Scholar] [CrossRef] [PubMed]
- Jayawardena, T.U.; Wang, L.; Sanjeewa, K.K.A.; Kang, S.I.; Lee, J.S.; Jeon, Y.J. Antioxidant Potential of Sulfated Polysaccharides from Padina boryana; Protective Effect against Oxidative Stress in In Vitro and In Vivo Zebrafish Model. Mar. Drugs 2020, 18, 212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernardi, G.; Springer, G.F. Properties of highly purified fucan. J. Biol. Chem. 1962, 237, 75–80. [Google Scholar] [CrossRef]
- Guo, T.; Yang, Y.; Gao, M.; Qu, Y.; Guo, X.; Liu, Y.; Cui, X.; Wang, C. Lepidium meyenii Walpers polysaccharide and its cationic derivative re-educate tumor-associated macrophages for synergistic tumor immunotherapy. Carbohydr. Polym. 2020, 250, 116904. [Google Scholar] [CrossRef]
- Zhang, S.; Qiao, Z.; Zhao, Z.; Guo, J.; Lu, K.; Mayo, K.H.; Zhou, Y. Comparative study on the structures of intra- and extra-cellular polysaccharides from Penicillium oxalicum and their inhibitory effects on galectins. Int. J. Biol. Macromol. 2021, 181, 793–800. [Google Scholar] [CrossRef]
- Chen, P.; Lin, Y.; Chen, Y.; Chang, Q.; Zheng, B.; Zhang, Y.; Hu, X.; Zeng, H. Structural characterization of a novel mannogalactoglucan from Fortunella margarita and its simulated digestion in vitro. Food Chem. Toxicol. 2019, 133, 110778. [Google Scholar] [CrossRef]
- Zhang, J.; Song, Z.; Li, Y.; Zhang, S.; Bao, J.; Wang, H.; Dong, C.; Ohizumi, Y.; Xu, J.; Guo, Y. Structural analysis and biological effects of a neutral polysaccharide from the fruits of Rosa laevigata. Carbohydr. Polym. 2021, 265, 118080. [Google Scholar] [CrossRef]
- Speciale, I.; Notaro, A.; Garcia-Vello, P.; Di Lorenzo, F.; Armiento, S.; Molinaro, A.; Marchetti, R.; Silipo, A.; De Castro, C. Liquid-state NMR spectroscopy for complex carbohydrate structural analysis: A hitchhiker’s guide. Carbohydr. Polym. 2022, 277, 118885. [Google Scholar] [CrossRef] [PubMed]
- Huifan, L.; Jiaxi, L.; Gengsheng, X.; Lukai, M.; Qin, W. Dendrobine Suppresses Lipopolysaccharide-induced Gut Inflammation in a Co-culture of Intestinal Epithelial Caco-2 Cells and RAW264.7 Macrophages. eFood 2021, 2, 92–99. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, P.; Zhai, B.; Zhang, M.; Xiang, Y.; Fang, J.; Xu, S.; Gao, Y.; Chen, X.; Sui, X.; et al. The emerging role of ferroptosis in inflammation. Biomed. Pharmacother. 2020, 127, 110108. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Kang, R.; Kroemer, G.; Tang, D. Ferroptosis in infection, inflammation, and immunity. J. Exp. Med. 2021, 218. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Monian, P.; Quadri, N.; Ramasamy, R.; Jiang, X. Glutaminolysis and Transferrin Regulate Ferroptosis. Mol. Cell 2015, 59, 298–308. [Google Scholar] [CrossRef] [Green Version]
- Dodson, M.; Castro-Portuguez, R.; Zhang, D.D. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019, 23, 101107. [Google Scholar] [CrossRef]
- Stockwell, B.R.; Jiang, X.; Gu, W. Emerging Mechanisms and Disease Relevance of Ferroptosis. Trends Cell Biol. 2020, 30, 478–490. [Google Scholar] [CrossRef]
- Su, L.J.; Zhang, J.H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.Y. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxid. Med. Cell. Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef] [Green Version]
- Mak, T.W.; Grusdat, M.; Duncan, G.S.; Dostert, C.; Nonnenmacher, Y.; Cox, M.; Binsfeld, C.; Hao, Z.; Brüstle, A.; Itsumi, M.; et al. Glutathione Primes T Cell Metabolism for Inflammation. Immunity 2017, 46, 675–689. [Google Scholar] [CrossRef] [Green Version]
- Mineo, A.; Miguel-Aliaga, I. Defend or reproduce? Muscle-derived glutamate determines an immune-reproductive energetic tradeoff. Cell Metab. 2021, 33, 2307–2309. [Google Scholar] [CrossRef]
- Levine, M. New concepts in the biology and biochemistry of ascorbic acid. N. Engl. J. Med. 1986, 314, 892–902. [Google Scholar] [CrossRef] [PubMed]
- Doll, S.; Proneth, B.; Tyurina, Y.Y.; Panzilius, E.; Kobayashi, S.; Ingold, I.; Irmler, M.; Beckers, J.; Aichler, M.; Walch, A.; et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat. Chem. Biol. 2017, 13, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Izumi, H.; Sato, K.; Kojima, K.; Saito, T.; Saido, T.C.; Fukunaga, K. Oral glutathione administration inhibits the oxidative stress and the inflammatory responses in App(NL-G-F/NL-G-F) knock-in mice. Neuropharmacology 2020, 168, 108026. [Google Scholar] [CrossRef] [PubMed]
- Fu, A.; van Rooyen, L.; Evans, L.; Armstrong, N.; Avizonis, D.; Kin, T.; Bird, G.H.; Reddy, A.; Chouchani, E.T.; Liesa-Roig, M.; et al. Glucose metabolism and pyruvate carboxylase enhance glutathione synthesis and restrict oxidative stress in pancreatic islets. Cell Rep. 2021, 37, 110037. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.M.; Zhao, Y.; Guo, C.; Yang, X.B. A comparative study on the antioxidant activities of an acidic polysaccharide and various solvent extracts derived from herbal Houttuynia cordata. Carbohydr. Polym. 2011, 83, 537–544. [Google Scholar] [CrossRef]
- Dodgson, K.S.; Price, R.G. A note on the determination of the ester sulphate content of sulphated polysaccharides. Biochem. J. 1962, 84, 106–110. [Google Scholar] [CrossRef] [Green Version]
- Hao, H.; Han, Y.; Yang, L.; Hu, L.; Duan, X.; Yang, X.; Huang, R. Structural characterization and immunostimulatory activity of a novel polysaccharide from green alga Caulerpa racemosa var peltata. Int. J. Biol. Macromol. 2019, 134, 891–900. [Google Scholar] [CrossRef]
- Ji, X.; Zhang, F.; Zhang, R.; Liu, F.; Peng, Q.; Wang, M. An acidic polysaccharide from Ziziphus Jujuba cv. Muzao: Purification and structural characterization. Food Chem. 2019, 274, 494–499. [Google Scholar] [CrossRef]
- Ren, F.; Yang, Y.; Wu, K.; Zhao, T.; Shi, Y.; Song, M.; Li, J. The Effects of Dandelion Polysaccharides on Iron Metabolism by Regulating Hepcidin via JAK/STAT Signaling Pathway. Oxid. Med. Cell. Longev. 2021, 2021, 7184760. [Google Scholar] [CrossRef]
- Guo, H.; Guo, H.; Zhang, L.; Tang, Z.; Yu, X.; Wu, J.; Zeng, F. Metabolome and Transcriptome Association Analysis Reveals Dynamic Regulation of Purine Metabolism and Flavonoid Synthesis in Transdifferentiation during Somatic Embryogenesis in Cotton. Int. J. Mol. Sci. 2019, 20, 2070. [Google Scholar] [CrossRef] [Green Version]
- Xia, X.; Hao, H.; Zhang, X.; Wong, I.N.; Chung, S.K.; Chen, Z.; Xu, B.; Huang, R. Immunomodulatory sulfated polysaccharides from Caulerpa racemosa var. peltata induces metabolic shifts in NF-κB signaling pathway in RAW 264.7 macrophages. Int. J. Biol. Macromol. 2021, 182, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Li, J.; Yuan, X.; Wang, Y.L.; Wu, K.X.; Kang, L.X.; Luo, Y.Y.; Zhang, H.M.; Yuan, Z.Q. Dandelion polysaccharides exert anticancer effect on Hepatocellular carcinoma by inhibiting PI3K/AKT/mTOR pathway and enhancing immune response. J. Funct. Foods 2019, 55, 263–274. [Google Scholar] [CrossRef]
- Chiang, H.M.; Chan, S.Y.; Chu, Y.; Wen, K.C. Fisetin Ameliorated Photodamage by Suppressing the Mitogen-Activated Protein Kinase/Matrix Metalloproteinase Pathway and Nuclear Factor-κB Pathways. J. Agric. Food Chem. 2015, 63, 4551–4560. [Google Scholar] [CrossRef] [PubMed]





| Retention Time (min) | Methylated Sugars | Molar Ratios (%) | Mass Fragments (m/z) | Linkage Type |
|---|---|---|---|---|
| 16.330 | 2,3,4,6-Me4-Glcp | 10.98 | 43, 101, 117, 129, 145, 161, 205 | Glcp-1 |
| 20.630 | 3,4,6-Me3-Manp | 3.08 | 43, 87, 99, 101, 129, 161, 189 | Manp-1-2 |
| 20.864 | 3,4,6-Me3-Glcp | 2.75 | 43, 87, 99, 117, 129, 161, 189 | Glcp-1-2 |
| 21.270 | 2,3,6-Me3-Glcp | 61.96 | 43, 87, 99, 117, 129, 161,173, 233 | Glcp-1-4 |
| 22.724 | 2,3,4-Me3-Glcp | 4.82 | 43, 99, 117, 129, 161, 189, 233 | Glcp-1-6 |
| 27.453 | 2,3-Me2-Glcp | 16.42 | 43, 99, 117, 159, 201,261 | Glcp-1-4-6 |
| Chemical Shift, δ (ppm) | |||||||
|---|---|---|---|---|---|---|---|
| Glycosyl Linkage | Residue | H1 | H2 | H3 | H4 | H5 | H6 |
| C1 | C2 | C3 | C4 | C5 | C6 | ||
| 1-α-D-Glcp | A | 5.38 | 3.60 | 3.70 | 3.95 | 3.97 | 3.60 |
| 102.72 | 71.94 | 74.03 | 70.61 | 71.68 | 62.30 | ||
| 1,2-α-D-Manp | B | 4.97 | 3.89 | 3.92 | 3.79 | 3.64 | 3.75 |
| 101.52 | 76.30 | 68.95 | 65.20 | 71.77 | 60.04 | ||
| 1,2-α-D-Glcp | C | 5.26 | 3.60 | 3.72 | 3.29 | 3.62 | 3.92 |
| 102.88 | 77.20 | 71.33 | 73.94 | 63.84 | 62.32 | ||
| 1,4-α-D-Glcp | D | 5.41 | 3.62 | 3.73 | 3.68 | 3.93 | 3.69 |
| 101.69 | 71.96 | 72.22 | 79.68 | 71.08 | 60.86 | ||
| 1,6-β-D-Glcp | E | 4.97 | 3.56 | 4.61 | 3.51 | 3.94 | 3.64 |
| 98.72 | 70.70 | 73.40 | 70.40 | 71.33 | 71.40 | ||
| 1,4,6-α-D-Glcp | F | 4.99 | 3.57 | 3.65 | 3.68 | 3.85 | 3.71 |
| 101.01 | 75.73 | 74.02 | 76.01 | 74.60 | 68.47 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.; Yang, J.; Luo, L.; Zhang, X.; Deng, S.; Chen, X.; Li, Y.; Bekhit, A.E.-D.A.; Xu, B.; Huang, R. Ferroptosis Related Immunomodulatory Effect of a Novel Extracellular Polysaccharides from Marine Fungus Aureobasidium melanogenum. Mar. Drugs 2022, 20, 332. https://doi.org/10.3390/md20050332
Lin Y, Yang J, Luo L, Zhang X, Deng S, Chen X, Li Y, Bekhit AE-DA, Xu B, Huang R. Ferroptosis Related Immunomodulatory Effect of a Novel Extracellular Polysaccharides from Marine Fungus Aureobasidium melanogenum. Marine Drugs. 2022; 20(5):332. https://doi.org/10.3390/md20050332
Chicago/Turabian StyleLin, Yuqi, Jiajia Yang, Lianxiang Luo, Xiaoyong Zhang, Shengyu Deng, Xiaodan Chen, Yiyang Li, Alaa El-Din A. Bekhit, Baojun Xu, and Riming Huang. 2022. "Ferroptosis Related Immunomodulatory Effect of a Novel Extracellular Polysaccharides from Marine Fungus Aureobasidium melanogenum" Marine Drugs 20, no. 5: 332. https://doi.org/10.3390/md20050332
APA StyleLin, Y., Yang, J., Luo, L., Zhang, X., Deng, S., Chen, X., Li, Y., Bekhit, A. E.-D. A., Xu, B., & Huang, R. (2022). Ferroptosis Related Immunomodulatory Effect of a Novel Extracellular Polysaccharides from Marine Fungus Aureobasidium melanogenum. Marine Drugs, 20(5), 332. https://doi.org/10.3390/md20050332











