Bioactivities of Lipid Extracts and Complex Lipids from Seaweeds: Current Knowledge and Future Prospects
Abstract
1. Introduction
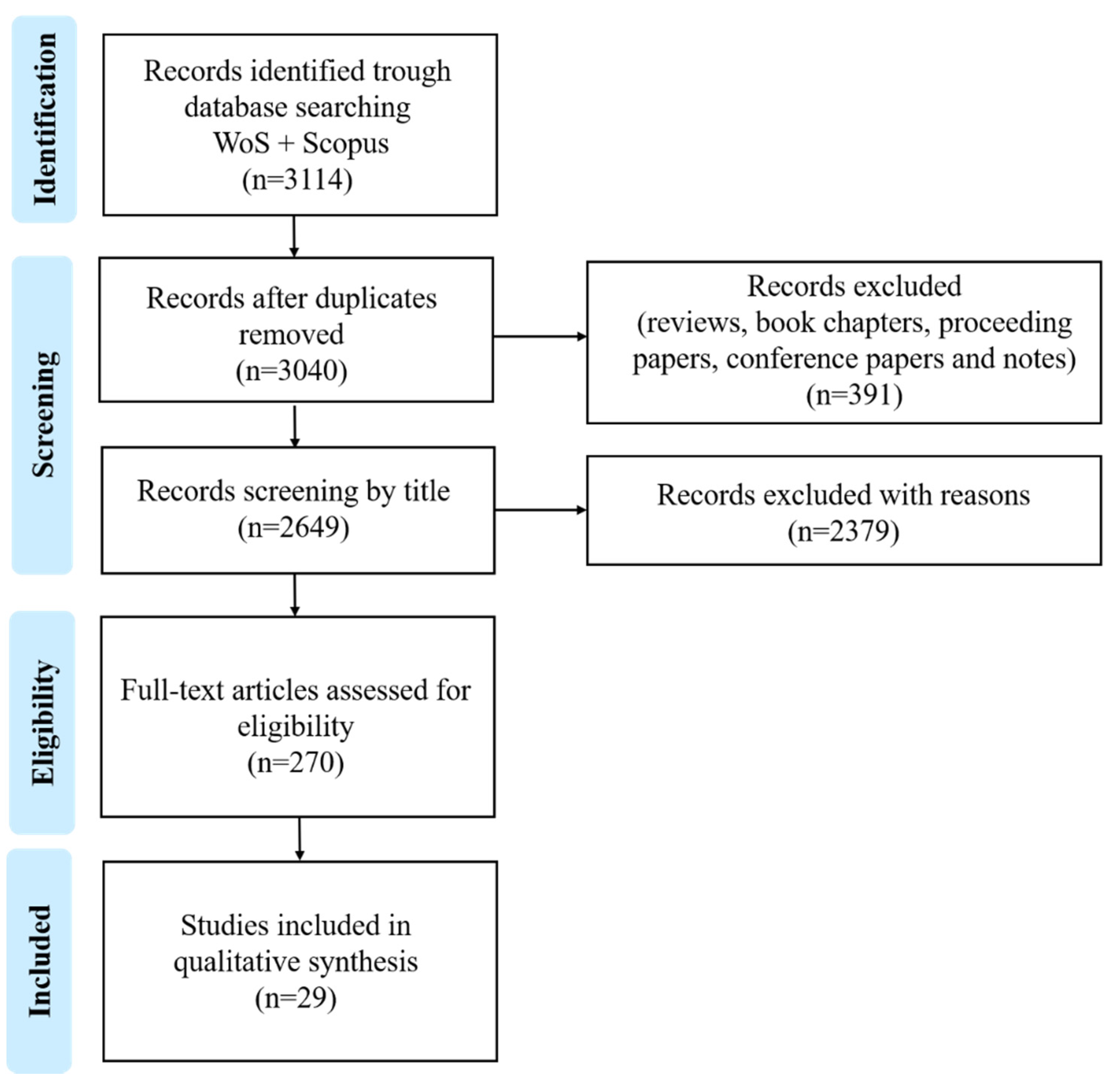
2. Methods
Selection of Eligibility and Exclusion Criteria
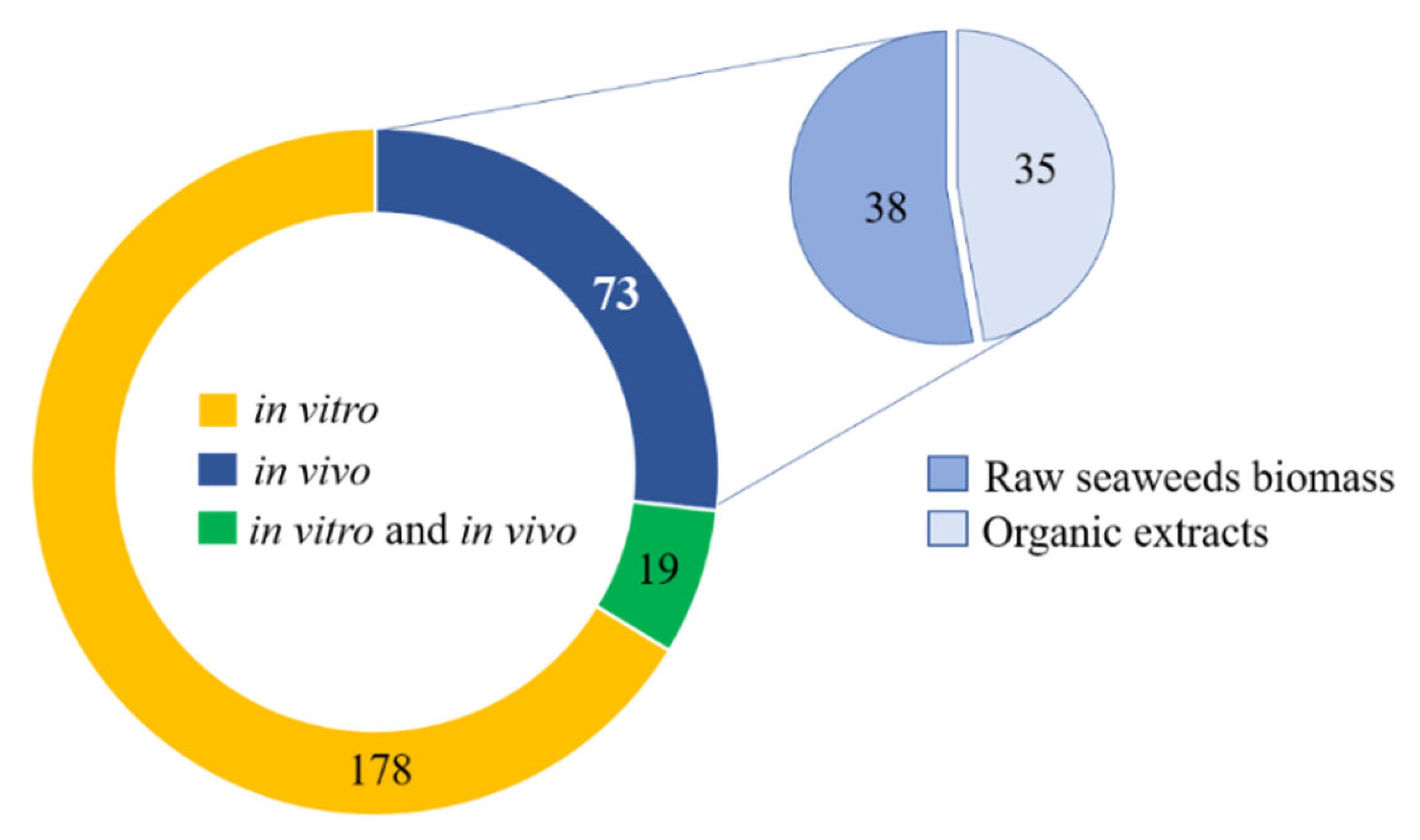
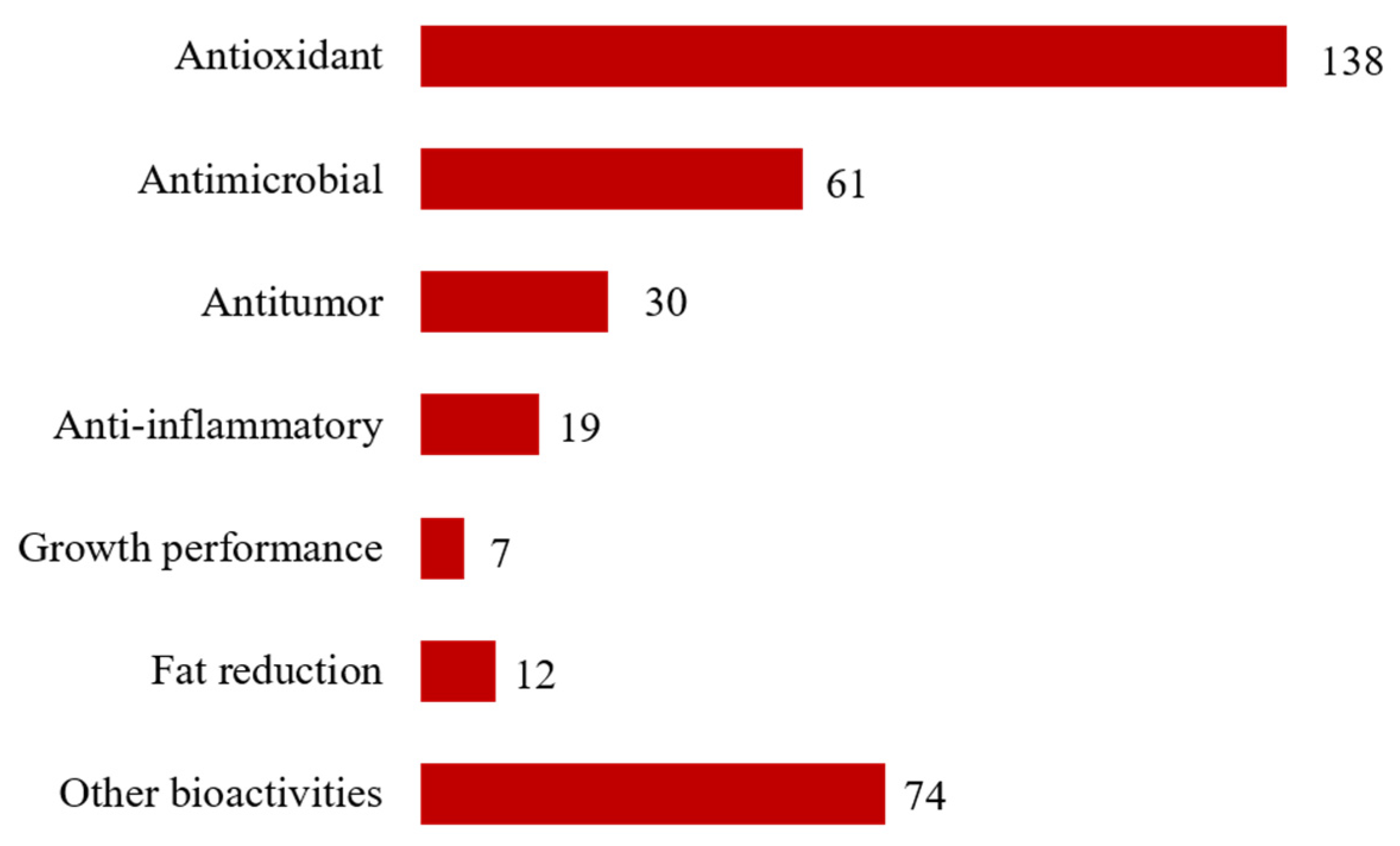
3. Results and Discussion
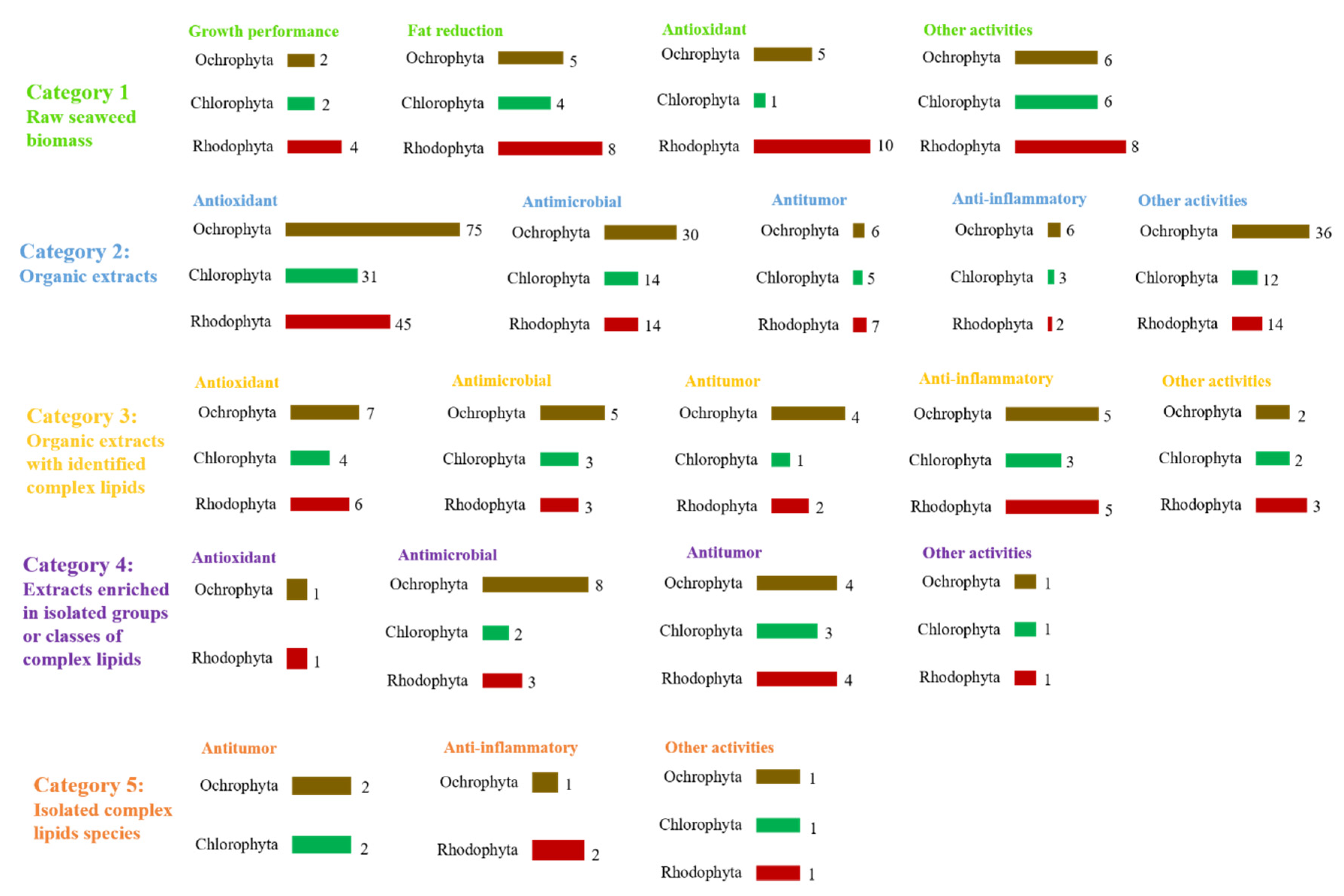
3.1. The Complex Lipids of Seaweeds as Derived Bioactive Phytochemicals
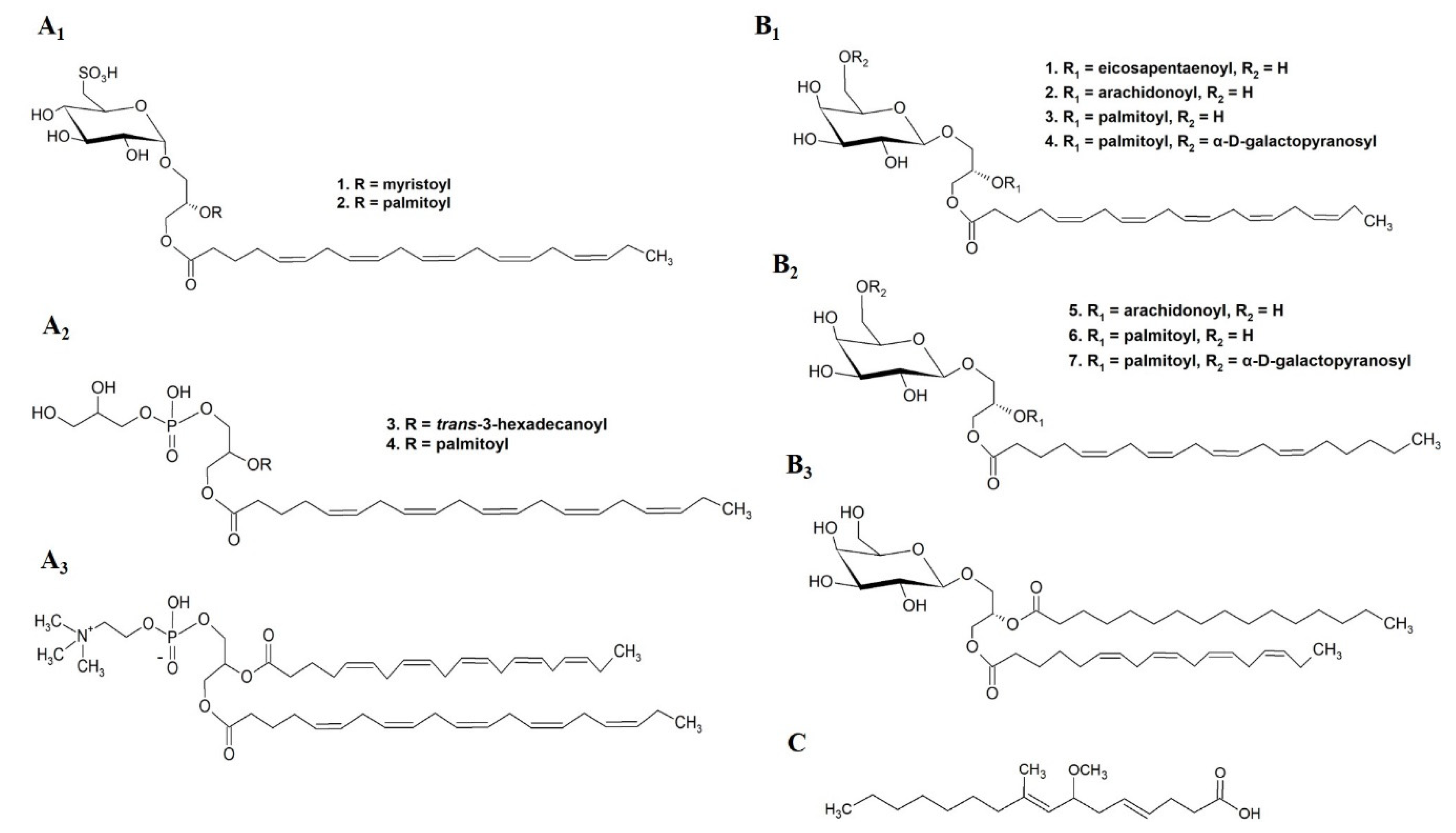
3.1.1. Antitumor Activity
| Study Category | Seaweed Species | Phylum | Lipid Species | Model and Obtained Results | Extraction Procedure | Identification/ Characterization | Ref. |
|---|---|---|---|---|---|---|---|
| Category 5 | Fucus evanescens | Ochrophyta | MGDG (20:5/18:4) | Melanoma (SK-MEL-28), IC50 of 104 µΜ, (MTS assay) | Extraction solvents: ethanol (40 °C); deionized water, aqueous ethanol (70%), chloroform. Fractionation/isolation: silica gel, Sephadex LH-20 column chromatography | TLC; ESI-MS; 1H-, 13C-NMR; GC-FID, GC-MS | [62] |
| Category 5 | Petalonia binghamiae | Ochrophyta | GDG (16:0/18:1) | Inhibition of DNA polymerase α, IC50 of 54 μM, (WST-1 assay) | Extraction solvents: acetone; ethyl acetate and water. Fractionation/isolation: silica gel, SephadexLH-20 column chromatography | GC-FID; EI mass; FABHR mass; 1H-, 13C- and DEPT- NMR | [63] |
| Category 4 | Sargassum horneri | Ochrophyta | DGDD, SQDG, | Colon carcinoma (Caco-2), inhibition effect at 100 µΜ, (action improved with 1.0 mM NaBT) | Extraction solvents: chloroform, methanol, water (Bligh and Dyer). Fractionation: silica gel column chromatography | TLC, GC-FID | [60] |
| Category 4 | Sargassum marginatum | Ochrophyta | PL | Human pro-myelocytic leukemia (HL-60), inhibition >70% at 40 µg mL−1 (trypan blue dye exclusion assay) | Extraction solvents: methanol, chloroform: methanol (1:1), water. Fractionation: silica gel column chromatography | GC-FID; GC-MS | [59] |
| Category 4 | Gracilaria corticata | Rhodophyta | SQDG | Epithelioid cervix carcinoma (HeLa), IC50 of 106.88 μg mL–1 (MTT assay) | Extraction solvents: ethyl acetate/methanol (1:1), n-hexane, dichloromethane, butanol, water. Fractionation: silica gel column chromatography | GC-MS, TLC, 1H-13C-NMR (1H-1H COSY, DEPT, HSQC, HMBC spectra), HPLC | [57] |
| Category 5 | Hydrolithon reinboldii | Rhodophyta | MGDG (20:4/16:0) (designated as Lithonoside) | Colon cancer (HCT116), prostate cancer (PC-3, LNcap-FGC, Du145), ovarian cancer (A2780/DDP-S), lung cancer (NCI-H446, SHP-77), leukemia (CCRF-CEM), breast cancer (BT-549, DU4475, MDA-MB-468, MDA-MB-231), average IC50 of 19.8 μM (MTS assay) | Extraction solvents: methanol, methanol: dichloromethane (1:1), methanol: water (9:1), hexane, ethyl acetate, butanol. Fractionation/isolation: semi-preparative reversed-phase HPLC, C18 HPLC | HPLC(C18)-Q-TOF-MS; 1H-, 13C-NMR (DEPT, COSY, HSQC, HMBC spectra) | [58] |
| Category 4 | Porphyra crispata | Rhodophyta | SQDG | Liver carcinoma (HepG2), IC50 of 126 μg mL−1 (MTT assay) | Extraction solvents: ethanol. Fractionation: HP-20 column, DEAE-cellulose acetate column, TLC | GC-FID; TLC, normal-phase HPLC-ELSD | [55] |
| Category 5 | Avrainvillea nigricans | Chlorophyta | Nigricanoside A | Breast adenocarcinoma (MCF-7) colon cancer (HCT-116) antimitotic activity, IC50 of 3 nM | Extraction solvents: methanol, water, ethyl acetate, hexane, dichloromethane. Fractionation/isolation: normal phase flash, Sephadex LH-20, reversed-phase flash column chromatographies, reversed-phase HPLC | HRESIMS; 1H-, 13C-NMR (DEPT, COSY, HSQC, HMBC spectra) | [66] |
| Category 4 | Solieria chordalis; Ulva armoricana | Rhodophyta Chlorophyta | MGDG (14:0_16:1) DGDG (14:0_18:3) | Bronchopulmonary carcinoma (NSCLC-N6), IC50 of 23.5 μg mL−1 for MGDG (14:0_16:1) and IC50 of 24.0 μg mL−1 for DGDG (14:0_18:3) (MTT assay) | Extraction solvents: chloroform/methanol (1:1), water, dichloromethane, acetone, methanol. Fractionation: flash column chromatography | GC-MS; TLC; LC-MS | [61] |
| Category 4 | Dilophus fasciola; Galaxaura cylindrica; Laurencia popillosa; Taonia atomaria; Ulva fasciata, | Rhodophyta Chlorophyta | Sulfolipid class | Hepato cellular carcinoma (Hep G2), IC50 in a range of 0.60 to 2.75 μg mL–1, Breast adenocarcinoma (MCF-7), IC50 in a range of 0.40 to 0.67 μg mL–1 (SRB assay) | Extraction solvents: methanol/chloroform (2:1). Fractionation: DEAE-cellulose column chromatography | IR; GC-FID; GC-MS; LC-MS/MS | [56] |
| Category 5 | Unknown algal species | sn-1,2-dipalmitoyl-3-(N-palmitoyl-6-deoxy-6-amino-α-d-glucosyl)-glycerol; sn-1-palmitoyl-2-myristoyl-3-(N-stearyl-6-deoxy-6-aminoglucosyl)-glycerol | Inhibition of MYT1 kinase, IC50 of 0.12 and 0.43 μg mL−1 | Extraction solvents: methanol, water, n-hexane, dichloromethane, butanol. Fractionation/isolation: Sephadex LH-20; RP-18 reverse phase silica gel; | 1H-, 13C-NMR; MALDI-TOF-MS | [64] |
3.1.2. Anti-Inflammatory Activity
| Study Category | Seaweed Species | Phylum | Lipid Species | Model and Obtained Results | Compounds Extraction | Identification/Characterization | Ref. |
|---|---|---|---|---|---|---|---|
| Category 5 | Chondrus crispus | Rhodophyta | MGDG(20:5/20:5) MGDG(20:5/20:4) MGDG(18:4/16:0) MGDG(20:5/16:0) MGDG(20:4/20:4) MGDG(20:4/16:0) DGDG(20:5/16:0) DGDG(20:4/16:0) | Raw 264.7 cells NO inhibition at 100 μM | Extraction solvents: methanol, water, ethyl acetate. Fractionation/isolation: SPE, HPLC (synergy MAX-RP column), semi-preparative HPLC | LC/MS; 1H-, 13C-NMR; GC; HRMS | [71] |
| Ishige okamurae | Ochrophyta | MMHDA | in vitro inhibition of PLA2, IC50 of 1.9 μg mL−1 in vivo inhibition of oedema, IC50 of 3.5 mg mL−1 in vivo inhibition of erythema, IC50 of 4.6 mg mL−1 | Extraction solvents: methanol, chloroform. Fractionation/isolation: Sephadex LH-20 column, silica gel column, reverse-phase HPLC, μBondapak C-18 column | HPLC (C18); GC-MS-QP5050A; EIMS; | [74] | |
| Palmaria palmata | Rhodophyta | SQDG(20:5/14:0) SQDG(20:5/16:0) PG(20:5/trans-16:1) PG(20:5/16:1) PC(20:5/20:5) | Raw 264.7 cells NO inhibition SQDG(20:5/14:0), IC50 of 36.5 µM SQDG(20:5/16:0), IC50 of 11.0 µM PG(20:5/trans-16:1), IC50 of 16.7 µM PG(20:5/16:0), IC50 of 42.9 µM PC(20:5/20:5), IC50 of 43.5 µM All species reduced iNOS expression >85% at 100 µM | Extraction solvents: methanol: chloroform (1:1), water, ethyl acetate. Fractionation/isolation: silica gel column chromatography; semi-preparative HPLC | ESI-MS; 1H-, 13C-NMR (COSY, HSQC HMBC spectra) | [70] |
3.1.3. Antimicrobial Activity
| Study Category | Seaweed Species | Phylum | Lipid Species | Activity (Microorganisms) and Obtained Results | Extraction | Identification/ Characterization | Ref. |
|---|---|---|---|---|---|---|---|
| Category 4 | Fucus evanescens | Ochrophyta | MGDG, DGDG, SQDG classes | Antibacterial and antifungal (Candida albicans, Fusarium oxysporum, Staphylococcus aureus, Escherichia coli) Paper disk assay Unknown concentration | Extraction solvents: ethanol; ethanol:acetone (1:1); chloroform:ethanol (1:1); chloroform; water. Fractionation: silica gel column chromatography | TLC; GC-MS | [78] |
| Laminaria cichorioides | Ochrophyta | MGDG, DGDG, SQDG classes | Antibacterial and antifungal (Safale S04, Candida albicans, Fusarium oxysporum, Aspergillus niger, Staphyllococcus aureus, Escherichia coli) Paper disk assay 3 mg mL−1 | Extraction solvents: 96% ethanol, chloroform, water. Fractionation: silica gel column chromatography | TSC | [77] | |
| Lobophora variegata | Ochrophyta | Mixture of SQDG(16:0/14:0), SQDG16:0/16:0) and SQDG(16:0/18:1) species | Antiprotozoal (Giardia intestinalis, Entamoeba histolytica (Eh), Trichomonas vaginalis) Susceptibility assays IC50 of 3.9 μg mL−1 for E. histolytica, IC50 of 8.0 μg mL−1 for T. vaginalis, IC50 of 20.9 μg mL−1 for G. intestinalis | Extraction solvents: dichloromethane/methanol (7:3), methanol/water (9:1), hexane, chloroform, ethyl acetate and n-butanol. Fractionation: Sephadex LH20 column chromatograph | FAB-MS; 1H-13C-NMR (COSY, TOCSY, DEPT, HSQC and HMQC spectra) | [81] | |
| Saccharina cichorioides | Ochrophyta | Glycolipids (GL) group MGDG class | Antibacterial GL group: activity against Staphylococcus aureus, Escherichia coli, Fusarium oxysporum, and Aspergillus niger MGDG class: activity against S. aureus and oxysporum Paper disk assay Unknown concentration | Extraction solvents: ethanol: acetone (1:1, v/v), chloroform:ethanol (1:1, v/v). Fractionation: silica gel column | TLC | [79] | |
| Sargassum vulgare | Ochrophyta | Isolated SQDG fraction, identified SQDG(14:0/16:0), SQDG (16:0/16:0), SQDG (17:0/16:0), SQDG(18:1/ 16:0), SQDG(19:0/16:0), SQDG(23:0/17:0) species | Antiviral Inhibition HSV-1 and HSV-2 with maximum non-toxic concentrations (MNTC) of 50 μg mL−1 and viral inhibition index (VII) in a range of 96 (minimum) to 99.9% (maximum) Titer reduction assay | Extraction solvents: chloroform/methanol (2:1 and 1:2). Fractionation: silica column chromatography. | TLC; ESI-MS; 1H-, 13C-NMR | [85] | |
| Sargassum vulgare | Ochrophyta | Fraction enriched in MGDG (16:0/19:1) DGDG (16:0/16:1) SQDG (16:0/19:0) species | Antifouling Biofilm-forming marine bacteria (Pseudoalteromonas elyakovii, Halomonas marina, Shewanella putrefaciens and Polaribacter irgensii) and marine microalgae (Chlorarachnion reptans, Pleurochrysis roscoffensis, Exanthemachrysis gayraliae, Cylindrotheca closterium, and Navicula jeffreyi) MIC in a range of 0.01 to >10 μg/mL | Extraction solvents: chloroform/methanol (2:1 and 1:2), water. Fractionation: silica gel column chromatography. | HPTLC silica gel; TLC; LC-MS | [83] | |
| Alaria fistulosa, Laminaria bongardiana, Laminaria longipes, Laminaria yezoensis | Ochrophyta | MGDG, DGDG, SQDG classes | Antibacterial (Staphylococcus aureus, Escherichia coli) and antifungal (Candida albicans, Fusarium oxysporum), Paper disk assay Unknown concentration | Extraction solvents: ethanol; ethanol:acetone (1:1); chloroform:ethanol (1:1); chloroform; water. Fractionation: silica gel column chromatography. | TLC; GC-MS | [76] | |
| Chondria armata | Rhodophyta | Glycolipids (GL) group, identified the species 1-oleoyl-2-palmitoyl-3-O-(linolenyl-6′-galactosyl)-glycerol; 2-O-palmitoyl-3-O-(6′-sulfoquinovopyranosyl)-glycerol and 3-digalactosyl-2-palmitoyl glycerol | Antibacterial (Klebsiella sp., Shigella flexineri, Vibrio cholerae) and antifungal (Candida albicans, Cryptococcus neoformans, Aspergillus fumigatus) Paper disk assay impregnated with extract in a range of 65–130 μg/disk | Extraction solvents: methanol, chloroform, n-butanol and water. Fractionation: Sephadex LH20 for gel filtration, silica gel column chromatography, RP-18 column | TLC; QSTARXL MS/MS; 1H-, 13C-NMR (COSY, HMQC, and HMBC spectra) | [80] | |
| Osmundaria obtusiloba | Rhodophyta | SQDG class | Antiviral Inhibition HSV-1 and HSV-2 with 50% of effective concentration (EC50) values of 42 μg mL−1 to HSV-1 and 12 μg mL−1 to HSV-2 Titer reduction assay | Extraction solvents: acetone, chloroform/methanol (2:1 and 1:2). Fractionation: silica gel column chromatography, preparative TLC. | TLC; ESI-MS; 1H-, 13C-NMR (HSQC, COSY and TOCSY spectra) | [84] | |
| Ulva prolifera | Chlorophyta | Enriched subfraction on MGMG(18:0) MGMG(16:0) MGDG(16:0/18:1) | Antialgal: Inhibition > 50% of red tide microalgae (Karenia mikimitoi, Skeletonema costatum, Alexandrium tamarense, Heterosigma akashiwo, Prorocentrum donghaiense) at concentration of 28.8 μg mL−1 | Extraction solvents: methanol, water, ethyl acetate Fractionation: silica gel column, Sephadex LH-20, preparative TLC. | TLC | [82] | |
| Dilophys fasciola, Galaxoura cylindriea, Laurencia popillosa, Taonia atomaria, Ulva fasciata | Ochrophyta Rhodophyta Chlorophyta | Sulfolipids classes | Antiviral: Inhibition HSV-1, IC50 ranged from 15 to 25 μg mL–1 (plaque reduction assay) Antibacterial (Bacillus subtilis, Escherichia coli) with MIC in a range of 40 to 80 μg mL–1 for G. cylindriea, U. fasciata, and T. atomaria (agar diffusion assay) | Extraction solvents: methanol:chloroform (2:1, v/v) Fractionation: DEAE-cellulose column chromatography. | IR, GC MS/MS, LC-MS/MS. | [56] |
3.1.4. Other Bioactivities Attribute to Seaweed Lipids
| Study Category | Seaweed Species | Phylum | Lipid Species | Activity and Action | Extraction | Identification/Characterization | Ref. |
|---|---|---|---|---|---|---|---|
| Category 4 | Solieria chordalis, Sargassum muticum | Rhodophyta Ochrophyta | Glycolipids (GLs) and Phospholipids (PLs) groups | Antioxidant through DPPH free radical scavenging activity Solieria chordalis: GL with EC50 in a range of 0.9 to >5 mg mL−1 PL with EC50 in a range of 1.1 to >5 mg mL−1 Sargassum muticum GL with EC50 in a range of 0.9 to 4.1 mg mL−1 PL with EC50 in a range of 1 to 4.8 mg mL−1 | Extraction solvents: chloroform/methanol (1/1) or supercritical carbon dioxide pure or with 2% or 8% of ethanol. Fractionation: silica gel column chromatography. | No characterization | [86] |
| Ahnfeltia tobuchiensis, Laminaria japonica, Sargassum pallidum, Ulva fenestrata | Rhodophyta Ochrophyta Chlorophyta | MGDG class | Regulation of the immunogenicity of protein antigen in the content of TI-complexes | Extraction solvents: chloroform, methanol. Fractionation: silica gel column chromatography, purified by preparative silica TLC. | GC-FID | [87] | |
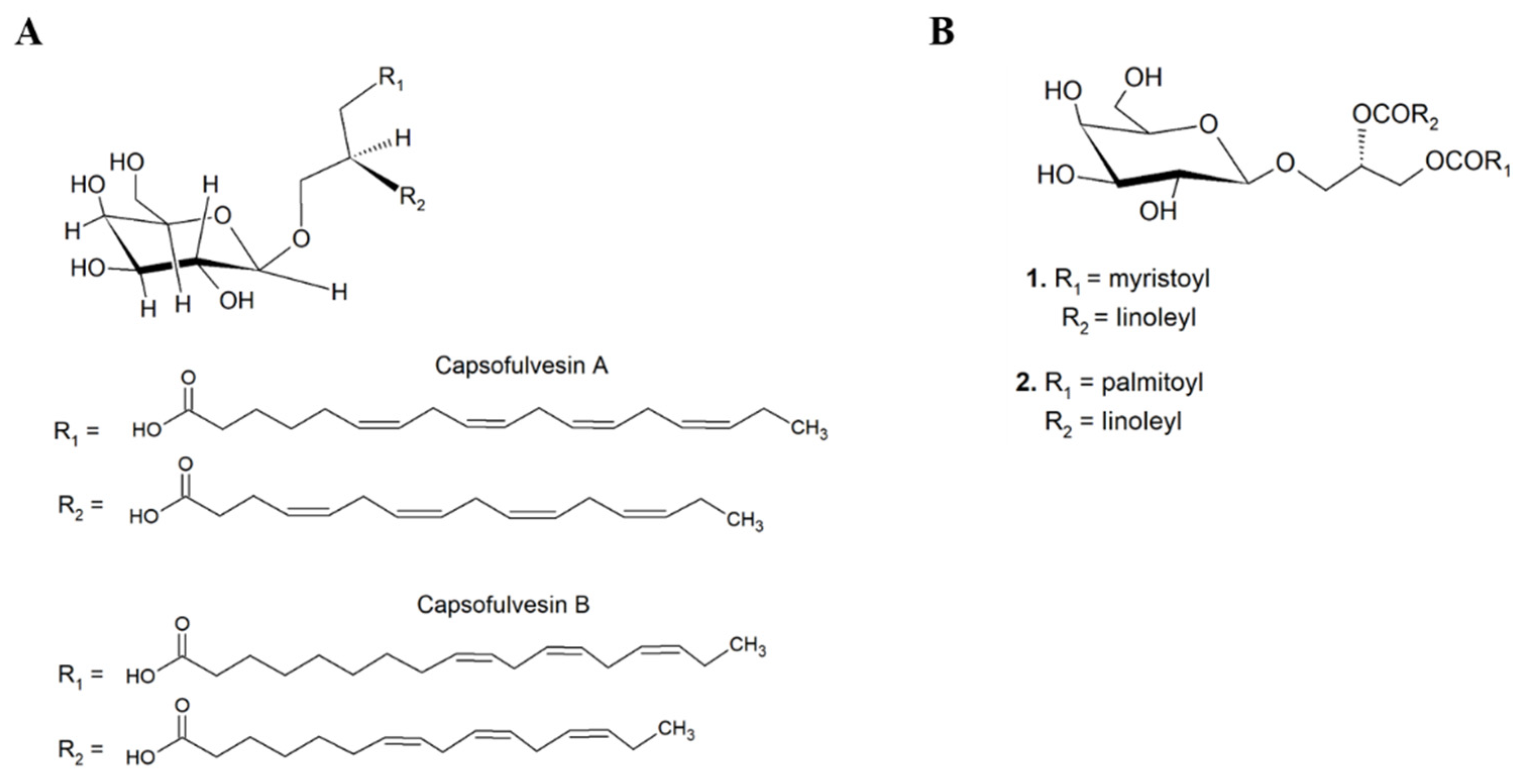
| Category 5 | Capsosiphon fulvescens | Chlorophyta | Capsofulvesin A and B | Anti-diabetic Rat lens aldose reductase (RLAR) inhibitory assay capsofulvesin A: IC50 of 52.53 μM capsofulvesin B: IC50 of 101.92 μM | Extraction solvents: 95% ethanol at 80 °C, water, partitioned dichloromethane, ethyl acetate, and n-butanol. Fractionation/isolation: silica gel column chromatography, reversed-phase (RP-C18) chromatography. | 1H-, 13C-NMR | [88] |
| Sargassum horneri | Ochrophyta | MGDG(14:0/18:2) MGDG(16:0/18:2) | Inhibitory effects on triglyceride and free fatty acids accumulation in 3T3-L1 adipocytes at concentration of 10 μM | Extraction: 70% alcohol, ethyl acetate. Fractionation/isolation: vacuum liquid chromatography (VLC) over silica gel, Sephadex LH-20, flash silica gel column chromatography | TLC; 1H-, 13C-NMR; GC-FID; HPLC−MS/MS | [89] | |
| Gelidiella acerosa | Rhodophyta | SQDG (S-ACT-1) | Human sperm motility stimulating activity | Extraction: dichloromethane: methanol (1:1). Fractionation/isolation: Sephadex LH-20 | TLC; 1H-, 13C-NMR, IR | [90] |
4. Concluding Remarks and Future Prospects
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mahadevan, K. Chapter 13—Seaweeds: A Sustainable Food Source; Tiwari, B.K., Troy, D.J.B.T.-S.S., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 347–364. [Google Scholar]
- European Union. Farm to Fork Strategy: For a Fair, Healthy and Environmentally-Friendly Food System; EU: Maastricht, The Netherlands, 2020. [Google Scholar]
- WHO. Sustainable Healthy Diets: Guiding Principles; Food and Agriculture Organization of the United Nations: Rome, Italy, 2019; p. 37. [Google Scholar]
- Collins, K.G.; Fitzgerald, G.F.; Stanton, C.; Ross, R.P. Looking beyond the terrestrial: The potential of seaweed derived bioactives to treat non-communicable diseases. Mar. Drugs 2016, 14, 60. [Google Scholar] [CrossRef]
- Wan-Loy, C.; Siew-Moi, P. Marine algae as a potential source for anti-obesity agents. Mar. Drugs 2016, 14, 222. [Google Scholar] [CrossRef] [PubMed]
- Shannon, E.; Abu-Ghannam, N. Antibacterial Derivatives of Marine Algae: An Overview of Pharmacological Mechanisms and Applications. Mar. Drugs 2016, 14, 81. [Google Scholar] [CrossRef] [PubMed]
- Alves, E.; Dias, M.; Lopes, D.; Almeida, A.; Domingues, M.D.R.; Rey, F. Antimicrobial lipids from plants and marine organisms: An overview of the current state-of-the-art and future prospects. Antibiotics 2020, 9, 441. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.; Critchley, A.T. The COVID 19 novel coronavirus pandemic 2020: Seaweeds to the rescue? Why does substantial, supporting research about the antiviral properties of seaweed polysaccharides seem to go unrecognized by the pharmaceutical community in these desperate times? J. Appl. Phycol. 2020, 32, 1875–1877. [Google Scholar] [CrossRef] [PubMed]
- Vincent, A.; Stanley, A.; Ring, J. Hidden Champion of the Ocean: Seaweed as a Growth Engine for a Sustainable European Future. 2020. Available online: https://www.seaweedeurope.com/wp-content/uploads/2020/10/Seaweed_for_Europe-Hidden_Champion_of_the_ocean-Report.pdf (accessed on 5 November 2021).
- Leal, M.C.; Munro, M.H.G.; Blunt, J.W.; Puga, J.; Jesus, B.; Calado, R.; Rosa, R.; Madeira, C. Biogeography and biodiscovery hotspots of macroalgal marine natural products. Nat. Prod. Rep. 2013, 30, 1380–1390. [Google Scholar] [CrossRef]
- Vieira, H.; Leal, M.C.; Calado, R. Fifty Shades of Blue: How Blue Biotechnology is Shaping the Bioeconomy. Trends Biotechnol. 2020, 38, 940–943. [Google Scholar] [CrossRef]
- Gomez-Zavaglia, A.; Prieto Lage, M.A.; Jimenez-Lopez, C.; Mejuto, J.C.; Simal-Gandara, J. The Potential of Seaweeds as a Source of Functional Ingredients of Prebiotic and Antioxidant Value. Antioxidants 2019, 8, 406. [Google Scholar] [CrossRef]
- Pereira, L. Seaweeds as Source of Bioactive Substances and Skin Care Therapy—Cosmeceuticals, Algotheraphy, and Thalassotherapy. Cosmetics 2018, 5, 68. [Google Scholar] [CrossRef]
- Rengasamy, K.R.R.; Mahomoodally, M.F.; Aumeeruddy, M.Z.; Zengin, G.; Xiao, J.; Kim, D.H. Bioactive compounds in seaweeds: An overview of their biological properties and safety. Food Chem. Toxicol. 2020, 135, 111013. [Google Scholar] [CrossRef]
- van Ginneken, V.J.; Helsper, J.P.; de Visser, W.; van Keulen, H.; Brandenburg, W.A. Polyunsaturated fatty acids in various macroalgal species from north Atlantic and tropical seas. Lipids Health Dis. 2011, 10, 1–8. [Google Scholar] [CrossRef]
- Cyberlipid. Available online: http://cyberlipid.gerli.com/description/complex-lipids/ (accessed on 5 November 2021).
- Kagan, M.L.; Levy, A.; Leikin-Frenkel, A. Comparative study of tissue deposition of omega-3 fatty acids from polar-lipid rich oil of the microalgae Nannochloropsis oculata with krill oil in rats. Food Funct. 2015, 6, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Che, H.; Zhou, M.; Zhang, T.; Zhang, L.; Ding, L.; Yanagita, T.; Xu, J.; Xue, C.; Wang, Y. EPA enriched ethanolamine plasmalogens significantly improve cognition of Alzheimer’s disease mouse model by suppressing β-amyloid generation. J. Funct. Foods 2018, 41, 9–18. [Google Scholar] [CrossRef]
- Drouin, G.; Catheline, D.; Guillocheau, E.; Gueret, P.; Baudry, C.; Le Ruyet, P.; Rioux, V.; Legrand, P. Comparative effects of dietary n-3 docosapentaenoic acid (DPA), DHA and EPA on plasma lipid parameters, oxidative status and fatty acid tissue composition. J. Nutr. Biochem. 2019, 63, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Lordan, R.; Tsoupras, A.; Zabetakis, I. Phospholipids of animal and marine origin: Structure, function, and anti-inflammatory properties. Molecules 2017, 22, 1964. [Google Scholar] [CrossRef] [PubMed]
- Haq, M.; Suraiya, S.; Ahmed, S.; Chun, B.-S. Phospholipids from marine source: Extractions and forthcoming industrial applications. J. Funct. Foods 2021, 80, 104448. [Google Scholar] [CrossRef]
- Kalisch, B.; Dörmann, P.; Hölzl, G. DGDG and Glycolipids in Plants and Algae BT—Lipids in Plant and Algae Development; Nakamura, Y., Li-Beisson, Y., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 51–83. [Google Scholar]
- Kumari, P.; Kumar, M.; Reddy, C.R.K.; Jha, B. Algal lipids, fatty acids and sterols. In Functional Ingredients from Algae for Foods and Nutraceuticals; Woodhead Publishing: Sawston, UK, 2013; pp. 87–134. [Google Scholar]
- Shen, L.; Yang, Y.; Ou, T.; Key, C.-C.C.; Tong, S.H.; Sequeira, R.C.; Nelson, J.M.; Nie, Y.; Wang, Z.; Boudyguina, E.; et al. Dietary PUFAs attenuate NLRP3 inflammasome activation via enhancing macrophage autophagy. J. Lipid Res. 2017, 58, 1808–1821. [Google Scholar] [CrossRef]
- Barros, R.; Moreira, A.; Fonseca, J.; Delgado, L.; Graça Castel-Branco, M.; Haahtela, T.; Lopes, C.; Moreira, P. Dietary intake of α-linolenic acid and low ratio of n-6: N-3 PUFA are associated with decreased exhaled NO and improved asthma control. Br. J. Nutr. 2011, 106, 441–450. [Google Scholar] [CrossRef]
- Lopes, D.; Melo, T.; Rey, F.; Meneses, J.; Monteira, F.L.; Helguero, L.A.; Abreu, M.H.; Lillebø, A.I.; Calado, R.; Domingues, M.R. Valuing bioactive lipids from green, red and brown macroalgae from aquaculture, to foster functionality and biotechnological applications. Molecules 2020, 25, 3883. [Google Scholar] [CrossRef]
- Lopes, D.; Melo, T.; Meneses, J.; Abreu, M.H.; Pereira, R.; Domingues, P.; Lillebø, A.I.; Calado, R.; Rosário Domingues, M. A new look for the red macroalga Palmaria palmata: A seafood with polar lipids rich in EPA and with antioxidant properties. Mar. Drugs 2019, 17, 533. [Google Scholar] [CrossRef]
- Plouguerné, E.; da Gama, B.A.P.; Pereira, R.C.; Barreto-Bergter, E. Glycolipids from seaweeds and their potential biotechnological applications. Front. Cell. Infect. Microbiol. 2014, 4, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Wang, P.; Xiao, L.; Liang, Y.; Huang, Y.; Ye, H.; Wu, K.; Lu, Y. Enrichment of lipids from agar production wastes of Gracilaria lemaneiformis by ultrasonication: A green sustainable process. Biomass Convers. Biorefinery 2020, 11, 2899–2908. [Google Scholar] [CrossRef]
- do-Amaral, C.C.F.; Pacheco, B.S.; Segatto, N.V.; Paschoal, J.D.F.; Santos, M.A.Z.; Seixas, F.K.; Pereira, C.M.P.; Astorga-España, M.S.; Mansilla, A.; Collares, T. Lipidic profile of sub-Antarctic seaweed Mazzaella laminarioides (Gigartinales, Rhodophyta) in distinct developmental phases and cell cytotoxicity in bladder cancer. Algal Res. 2020, 48, 101936. [Google Scholar] [CrossRef]
- Pacheco, B.S.; dos Santos, M.A.Z.; Schultze, E.; Martins, R.M.; Lund, R.G.; Seixas, F.K.; Colepicolo, P.; Collares, T.; Paula, F.R.; De Pereira, C.M.P. Cytotoxic activity of fatty acids from Antarctic macroalgae on the growth of human breast cancer cells. Front. Bioeng. Biotechnol. 2018, 6, 185. [Google Scholar] [CrossRef] [PubMed]
- Lopes, D.; Moreira, A.S.P.; Rey, F.; da Costa, E.; Melo, T.; Maciel, E.; Rego, A.; Abreu, M.H.; Domingues, P.; Calado, R.; et al. Lipidomic signature of the green macroalgae Ulva rigida farmed in a sustainable integrated multi-trophic aquaculture. J. Appl. Phycol. 2019, 31, 1369–1381. [Google Scholar] [CrossRef]
- Rey, F.; Cartaxana, P.; Melo, T.; Calado, R.; Pereira, R.; Abreu, H.; Domingues, P.; Cruz, S.; Rosário Domingues, M. Domesticated populations of Codium tomentosum display lipid extracts with lower seasonal shifts than conspecifics from the wild-relevance for biotechnological applications of this green seaweed. Mar. Drugs 2020, 18, 188. [Google Scholar] [CrossRef] [PubMed]
- Melo, T.; Alves, E.; Azevedo, V.; Martins, A.S.; Neves, B.; Domingues, P.; Calado, R.; Abreu, M.H.; Domingues, M.R. Lipidomics as a new approach for the bioprospecting of marine macroalgae—Unraveling the polar lipid and fatty acid composition of chondrus crispus. Algal Res. 2015, 8, 181–191. [Google Scholar] [CrossRef]
- da Costa, E.; Azevedo, V.; Melo, T.; Rego, A.M.; Evtuguin, D.V.; Domingues, P.; Calado, R.; Pereira, R.; Abreu, M.H.; Domingues, M.R. High-resolution lipidomics of the early life stages of the red seaweed Porphyra dioica. Molecules 2018, 23, 187. [Google Scholar] [CrossRef]
- Da Costa, E.; Melo, T.; Moreira, A.S.P.; Bernardo, C.; Helguero, L.; Ferreira, I.; Cruz, M.T.; Rego, A.M.; Domingues, P.; Calado, R.; et al. Valorization of lipids from Gracilaria sp. through lipidomics and decoding of antiproliferative and anti-inflammatory activity. Mar. Drugs 2017, 15, 62. [Google Scholar] [CrossRef]
- da Costa, E.; Domingues, P.; Melo, T.; Coelho, E.; Pereira, R.; Calado, R.; Abreu, H.M.; Domingues, R.M. Lipidomic signatures reveal seasonal shifts on the relative abundance of high-valued lipids from the brown algae Fucus vesiculosus. Mar. Drugs 2019, 17, 335. [Google Scholar] [CrossRef]
- Rey, F.; Lopes, D.; Maciel, E.; Monteiro, J.; Skjermo, J.; Funderud, J.; Raposo, D.; Domingues, P.; Calado, R.; Domingues, M.R. Polar lipid profile of Saccharina latissima, a functional food from the sea. Algal Res. 2019, 39, 101473. [Google Scholar] [CrossRef]
- Santos, F.; Monteiro, J.P.; Duarte, D.; Melo, T.; Lopes, D.; da Costa, E.; Domingues, M.R. Unraveling the Lipidome and Antioxidant Activity of Native Bifurcaria bifurcata and Invasive Sargassum muticum Seaweeds: A Lipid Perspective on How Systemic Intrusion May Present an Opportunity. Antioxidants 2020, 9, 642. [Google Scholar] [CrossRef] [PubMed]
- Lopes, D.; Melo, T.; Rey, F.; Costa, E.; Moreira, A.S.P.; Abreu, M.H.; Domingues, P.; Lillebø, A.I.; Calado, R.; Rosário Domingues, M. Insights of species-specific polar lipidome signatures of seaweeds fostering their valorization in the blue bioeconomy. Algal Res. 2021, 55, 102242. [Google Scholar] [CrossRef]
- Araújo, R.; Vázquez Calderón, F.; Sánchez López, J.; Azevedo, I.C.; Bruhn, A.; Fluch, S.; Garcia Tasende, M.; Ghaderiardakani, F.; Ilmjärv, T.; Laurans, M. Current status of the algae production industry in Europe: An emerging sector of the Blue Bioeconomy. Front. Mar. Sci. 2021, 7, 1247. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Prisma Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Kindleysides, S.; Quek, S.-Y.; Miller, M.R. Inhibition of fish oil oxidation and the radical scavenging activity of New Zealand seaweed extracts. Food Chem. 2012, 133, 1624–1631. [Google Scholar] [CrossRef]
- Francavilla, M.; Franchi, M.; Monteleone, M.; Caroppo, C. The red seaweed Gracilaria gracilis as a multi products source. Mar. Drugs 2013, 11, 3754–3776. [Google Scholar] [CrossRef] [PubMed]
- Nagappan, T.; Vairappan, C.S. Nutritional and bioactive properties of three edible species of green algae, genus Caulerpa (Caulerpaceae). J. Appl. Phycol. 2014, 26, 1019–1027. [Google Scholar] [CrossRef]
- Rodeiro, I.; Olguín, S.; Santes, R.; Herrera, J.A.; Pérez, C.L.; Mangas, R.; Hernández, Y.; Fernández, G.; Hernández, I.; Hernández-Ojeda, S.; et al. Gas Chromatography-Mass Spectrometry Analysis of Ulva fasciata (Green Seaweed) Extract and Evaluation of Its Cytoprotective and Antigenotoxic Effects. Evid.-Based Complement. Altern. Med. 2015, 2015, 520598. [Google Scholar] [CrossRef]
- Farvin, K.H.S.; Jacobsen, C. Antioxidant activity of seaweed extracts: In vitro assays, evaluation in 5% fish oil-in-water emulsions and characterization. J. Am. Oil Chem. Soc. 2015, 92, 571–587. [Google Scholar] [CrossRef]
- Honold, P.J.; Jacobsen, C.; Jónsdóttir, R.; Kristinsson, H.G.; Hermund, D.B. Potential seaweed-based food ingredients to inhibit lipid oxidation in fish-oil-enriched mayonnaise. Eur. Food Res. Technol. 2016, 242, 571–584. [Google Scholar] [CrossRef]
- Trigui, M.; Gasmi, L.; Zouari, I.; Tounsi, S. Seasonal variation in phenolic composition, antibacterial and antioxidant activities of Ulva rigida (Chlorophyta) and assessment of antiacetylcholinesterase potential. J. Appl. Phycol. 2013, 25, 319–328. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Marine Natural Products and Related Compounds in Clinical and Advanced Preclinical Trials. J. Nat. Prod. 2004, 67, 1216–1238. [Google Scholar] [CrossRef]
- Cragg, G.M.; Newman, D.J. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2013, 1830, 3670–3695. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Vong, C.T.; Chen, H.; Gao, Y.; Lyu, P.; Qiu, L.; Zhao, M.; Liu, Q.; Cheng, Z.; Zou, J.; et al. Naturally occurring anti-cancer compounds: Shining from Chinese herbal medicine. Chin. Med. 2019, 14, 48. [Google Scholar] [CrossRef]
- Cragg, G.M.; Kingston, D.G.I.; Newman, D.J. Anticancer Agents from Natural Products; CRC Press: Boca Raton, FL, USA; Taylor & Francis: Abingdon, UK, 2005. [Google Scholar]
- Newman, D.J.; Cragg, G.M.; Snader, K.M. Natural Products as Sources of New Drugs over the Period 1981–2002. J. Nat. Prod. 2003, 66, 1022–1037. [Google Scholar] [CrossRef]
- Tsai, C.-J.; Sun Pan, B. Identification of sulfoglycolipid bioactivities and characteristic fatty acids of marine macroalgae. J. Agric. Food Chem. 2012, 60, 8404–8410. [Google Scholar] [CrossRef]
- El Baz, F.K.; El Baroty, G.S.; Abd El Baky, H.H.; Abd El-Salam, O.I.; Ibrahim, E.A. Structural characterization and biological activity of sulfolipids from selected marine algae. Grasas Aceites 2013, 64, 561–571. [Google Scholar]
- Akbari, V.; Abedi, M.; Yegdaneh, A. Bioassay-guided isolation of glycolipids from the seaweed Gracilaria corticata. Res. Pharm. Sci. 2020, 15, 473–480. [Google Scholar] [CrossRef]
- Jiang, R.-W.; Hay, M.E.; Fairchild, C.R.; Prudhomme, J.; Le Roch, K.; Aalbersberg, W.; Kubanek, J. Antineoplastic unsaturated fatty acids from Fijian macroalgae. Phytochemistry 2008, 69, 2495–2500. [Google Scholar] [CrossRef]
- Bhaskar, N.; Hosakawa, M.; Miyashita, K. Growth inhibition of human pro-myelocytic leukemia (HL-60) cells by lipid extracts of marine alga Sargassum marginatum (Fucales, Phaeophyta) harvested of Goa (west coast of India) with special reference to fatty acid composition. Indian J. Mar. Sci. 2004, 33, 355–360. [Google Scholar]
- Hossain, Z.; Kurihara, H.; Hosokawa, M.; Takahashi, K. Growth inhibition and induction of differentiation and apoptosis mediated by sodium butyrate in Caco-2 cells with algal glycolipids. Vitr. Cell. Dev. Biol.-Anim. 2005, 41, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Kendel, M.; Wielgosz-collin, G.; Bertrand, S.; Roussakis, C.; Bourgougnon, N.; Bedoux, G. Lipid Composition, Fatty Acids and Sterols in the Seaweeds Ulva armoricana, and Solieria chordalis from Brittany (France): An Analysis from Nutritional, Chemotaxonomic, and Antiproliferative Activity Perspectives. Mar. Drugs 2015, 13, 5606–5628. [Google Scholar] [CrossRef] [PubMed]
- Imbs, T.I.; Ermakova, S.P.; Fedoreyev, S.A.; Anastyuk, S.D.; Zvyagintseva, T.N. Isolation of Fucoxanthin and Highly Unsaturated Monogalactosyldiacylglycerol from Brown Alga Fucus evanescens C Agardh and in vitro Investigation of Their Antitumor Activity. Mar. Biotechnol. 2013, 15, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Mizushina, Y.; Sugiyama, Y.; Yoshida, H.; Hanashima, S.; Yamazaki, T.; Kamisuki, S.; Ohta, K.; Takemura, M.; Yamaguchi, T.; Matsukage, A.; et al. Galactosyldiacylglycerol, a Mammalian DNA Polymerase Alpha-Specific Inhibitor from a Sea Alga, Petalonia bingbamiae. Biol. Pharm. Bull. 2001, 24, 982–987. [Google Scholar] [CrossRef][Green Version]
- Zhou, B.-N.; Tang, S.; Johnson, R.K.; Mattern, M.P.; Lazo, J.S.; Sharlow, E.R.; Harich, K.; Kingston, D.G.I. New glycolipid inhibitors of Myt1 kinase. Tetrahedron 2005, 61, 883–887. [Google Scholar] [CrossRef]
- Göllner, C.; Philipp, C.; Dobner, B.; Sippl, W.; Schmidt, M. First total synthesis of 1,2-dipalmitoyl-3-(N-palmitoyl-6′-amino-6′-deoxy-α-d-glucosyl)-sn-glycerol—A glycoglycerolipid of a marine alga with a high inhibitor activity against human Myt1-kinase. Carbohydr. Res. 2009, 344, 1628–1631. [Google Scholar] [CrossRef]
- Williams, D.E.; Sturgeon, C.M.; Roberge, M.; Andersen, R.J. Nigricanosides A and B, antimitotic glycolipids isolated from the green alga Avrainvillea nigricans collected in Dominica. J. Am. Chem. Soc. 2007, 129, 5822–5823. [Google Scholar] [CrossRef]
- Abdulkhaleq, L.A.; Assi, M.A.; Abdullah, R.; Zamri-Saad, M.; Taufiq-Yap, Y.H.; Hezmee, M.N.M. The crucial roles of inflammatory mediators in inflammation: A review. Vet. World 2018, 11, 627. [Google Scholar] [CrossRef]
- Kamali, A.N.; Noorbakhsh, S.M.; Hamedifar, H.; Jadidi-Niaragh, F.; Yazdani, R.; Bautista, J.M.; Azizi, G. A role for Th1-like Th17 cells in the pathogenesis of inflammatory and autoimmune disorders. Mol. Immunol. 2019, 105, 107–115. [Google Scholar] [CrossRef]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef]
- Banskota, A.H.; Stefanova, R.; Sperker, S.; Lall, S.P.; Craigie, J.S.; Hafting, J.T.; Critchley, A.T. Polar lipids from the marine macroalga Palmaria palmata inhibit lipopolysaccharide-induced nitric oxide production in RAW264.7 macrophage cells. Phytochemistry 2014, 101, 101–108. [Google Scholar] [CrossRef]
- Banskota, A.H.; Stefanova, R.; Sperker, S.; Lall, S.; Craigie, J.S.; Hafting, J.T. Lipids isolated from the cultivated red alga Chondrus crispus inhibit nitric oxide production. J. Appl. Phycol. 2014, 26, 1565–1571. [Google Scholar] [CrossRef]
- Jang, Y.; Kim, M.; Hwang, S.W. Molecular mechanisms underlying the actions of arachidonic acid-derived prostaglandins on peripheral nociception. J. Neuroinflammation 2020, 17, 30. [Google Scholar] [CrossRef]
- Burke, J.E.; Dennis, E.A. Phospholipase A2 structure/function, mechanism, and signaling. J. Lipid Res. 2009, 50, S237–S242. [Google Scholar] [CrossRef]
- Cho, J.Y.; Gyawali, Y.P.; Ahn, S.H.; Khan, M.N.A.; Kong, I.S.; Hong, Y.K. A methoxylated fatty acid isolated from the brown seaweed Ishige okamurae inhibits bacterial phospholipase A2. Phyther. Res. 2008, 22, 1070–1074. [Google Scholar] [CrossRef] [PubMed]
- Cos, P.; Vlietinck, A.J.; Berghe, D.V.; Maes, L. Anti-infective potential of natural products: How to develop a stronger in vitro ‘proof-of-concept’. J. Ethnopharmacol. 2006, 106, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Gerasimenko, N.I.; Martyyas, E.A.; Busarova, N.G. Composition of lipids and biological activity of lipids and photosynthetic pigments from algae of the families Laminariaceae and Alariaceae. Chem. Nat. Compd. 2012, 48, 737–741. [Google Scholar] [CrossRef]
- Gerasimenko, N.I.; Chaykina, E.L.; Busarova, N.G.; Anisimov, M.M. Antimicrobic and hemolytic activity of low-molecular metabolits of brown seaweed Laminaria cichorioides (Miyabe). Appl. Biochem. Microbiol. 2010, 46, 426–430. [Google Scholar] [CrossRef]
- Gerasimenko, N.I.; Busarova, N.G.; Martyyas, E.A. Composition of lipids from Fucus evanescens (Seas of Okhotsk and Japan) and biological activity of lipids and photosynthetic pigments. Chem. Nat. Compd. 2012, 48, 742–747. [Google Scholar] [CrossRef]
- Martyyas, E.A.; Gerasimenko, N.I.; Busarova, N.G.; Yurchenko, E.A.; Skriptsova, A.V.; Anisimov, M.M. Seasonal changes in biological activity of lipids and photosynthetic pigments of Saccharina cichorioides (Miyabe) (Laminariaceae Family). Russ. J. Bioorganic Chem. 2013, 39, 720–727. [Google Scholar] [CrossRef]
- Al-Fadhli, A.; Wahidulla, S.; D’Souza, L. Glycolipids from the red alga Chondria armata (Kütz.) Okamura. Glycobiology 2006, 16, 902–915. [Google Scholar] [CrossRef]
- Cantillo-Ciau, Z.; Moo-Puc, R.; Quijano, L.; Freile-Pelegrín, Y. The tropical brown alga Lobophora variegata: A source of antiprotozoal compounds. Mar. Drugs 2010, 8, 1292–1304. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.Y.; Wang, H.; Guo, G.L.; Pu, Y.F.; Yan, B.L.; Wang, C.H. Isolation, purification, and identification of antialgal substances in green alga Ulva prolifera for antialgal activity against the common harmful red tide microalgae. Environ. Sci. Pollut. Res. 2016, 23, 1449–1459. [Google Scholar] [CrossRef] [PubMed]
- Plouguerné, E.; de Souza, L.M.; Sassaki, G.L.; Hellio, C.; Trepos, R.; da Gama, B.A.P.; Pereira, R.C.; Barreto-Bergter, E. Glycoglycerolipids From Sargassum vulgare as Potential Antifouling Agents. Front. Mar. Sci. 2020, 7, 116. [Google Scholar] [CrossRef]
- De Souza, L.M.; Sassaki, G.L.; Romanos, M.T.V.; Barreto-Bergter, E. Structural characterization and anti-HSV-1 and HSV-2 activity of glycolipids from the marine algae Osmundaria obtusiloba isolated from Southeastern Brazilian coast. Mar. Drugs 2012, 10, 918–931. [Google Scholar] [CrossRef]
- Plouguerné, E.; De Souza, L.M.; Sassaki, G.L.; Cavalcanti, J.F.; Romanos, M.T.V.; Da Gama, B.A.P.; Pereira, R.C.; Barreto-Bergter, E. Antiviral sulfoquinovosyldiacylglycerols (SQDGs) from the Brazilian brown seaweed Sargassum vulgare. Mar. Drugs 2013, 11, 4628–4640. [Google Scholar] [CrossRef] [PubMed]
- Terme, N.; Boulho, R.; Kucma, J.-P.; Bourgougnon, N.; Bedoux, G. Radical scavenging activity of lipids from seaweeds isolated by solid-liquid extraction and supercritical fluids. OCL 2018, 25, D505. [Google Scholar] [CrossRef]
- Sanina, N.M.; Kostetsky, E.Y.; Shnyrov, V.L.; Tsybulsky, A.V.; Novikova, O.D.; Portniagina, O.Y.; Vorobieva, N.S.; Mazeika, A.N.; Bogdanov, M. V The influence of monogalactosyldiacylglycerols from different marine macrophytes on immunogenicity and conformation of protein antigen of tubular immunostimulating complex. Biochimie 2012, 94, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.N.; Choi, S.H.; Moon, H.E.; Park, J.J.; Jung, H.A.; Woo, M.H.; Woo, H.C.; Choi, J.S. The inhibitory activities of the edible green alga Capsosiphon fulvescens on rat lens aldose reductase and advanced glycation end products formation. Eur. J. Nutr. 2014, 53, 233–242. [Google Scholar] [CrossRef]
- Ma, A.-C.; Chen, Z.; Wang, T.; Song, N.; Yan, Q.; Fang, Y.-C.; Guan, H.-S.; Liu, H.-B. Isolation of the Molecular Species of Monogalactosyldiacylglycerols from Brown Edible Seaweed Sargassum horneri and Their Inhibitory Effects on Triglyceride Accumulation in 3T3-L1 Adipocytes. J. Agric. Food Chem. 2014, 62, 11157–11162. [Google Scholar] [CrossRef] [PubMed]
- Premakumara, G.A.; Ratnasooriya, W.D.; Tillekeratne, L.M.; Amarasekare, A.S. Human sperm motility stimulating activity of a sulfono glycolipid isolated from Sri Lankan marine red alga Gelidiella acerosa. Asian J. Androl. 2001, 3, 27–31. [Google Scholar] [PubMed]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Mohamed, S.; Hashim, S.N.; Rahman, H.A. Seaweeds: A sustainable functional food for complementary and alternative therapy. Trends Food Sci. Technol. 2012, 23, 83–96. [Google Scholar] [CrossRef]
- Wijesinghe, W.A.J.P.; Jeon, Y.-J. Biological activities and potential cosmeceutical applications of bioactive components from brown seaweeds: A review. Phytochem. Rev. 2011, 10, 431–443. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes, D.; Rey, F.; Leal, M.C.; Lillebø, A.I.; Calado, R.; Domingues, M.R. Bioactivities of Lipid Extracts and Complex Lipids from Seaweeds: Current Knowledge and Future Prospects. Mar. Drugs 2021, 19, 686. https://doi.org/10.3390/md19120686
Lopes D, Rey F, Leal MC, Lillebø AI, Calado R, Domingues MR. Bioactivities of Lipid Extracts and Complex Lipids from Seaweeds: Current Knowledge and Future Prospects. Marine Drugs. 2021; 19(12):686. https://doi.org/10.3390/md19120686
Chicago/Turabian StyleLopes, Diana, Felisa Rey, Miguel C. Leal, Ana I. Lillebø, Ricardo Calado, and Maria Rosário Domingues. 2021. "Bioactivities of Lipid Extracts and Complex Lipids from Seaweeds: Current Knowledge and Future Prospects" Marine Drugs 19, no. 12: 686. https://doi.org/10.3390/md19120686
APA StyleLopes, D., Rey, F., Leal, M. C., Lillebø, A. I., Calado, R., & Domingues, M. R. (2021). Bioactivities of Lipid Extracts and Complex Lipids from Seaweeds: Current Knowledge and Future Prospects. Marine Drugs, 19(12), 686. https://doi.org/10.3390/md19120686










