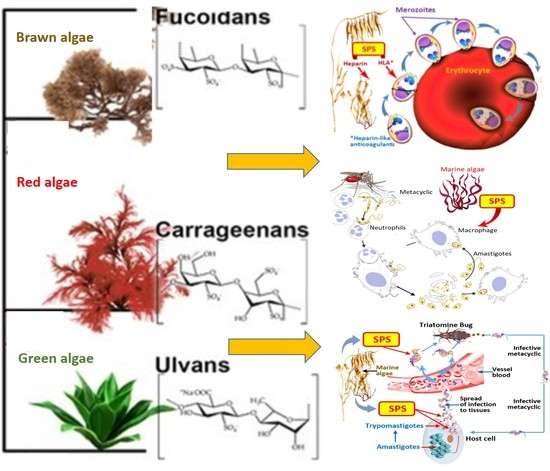
Antiparasitic Effects of Sulfated Polysaccharides from Marine Hydrobionts
Abstract
1. Introduction
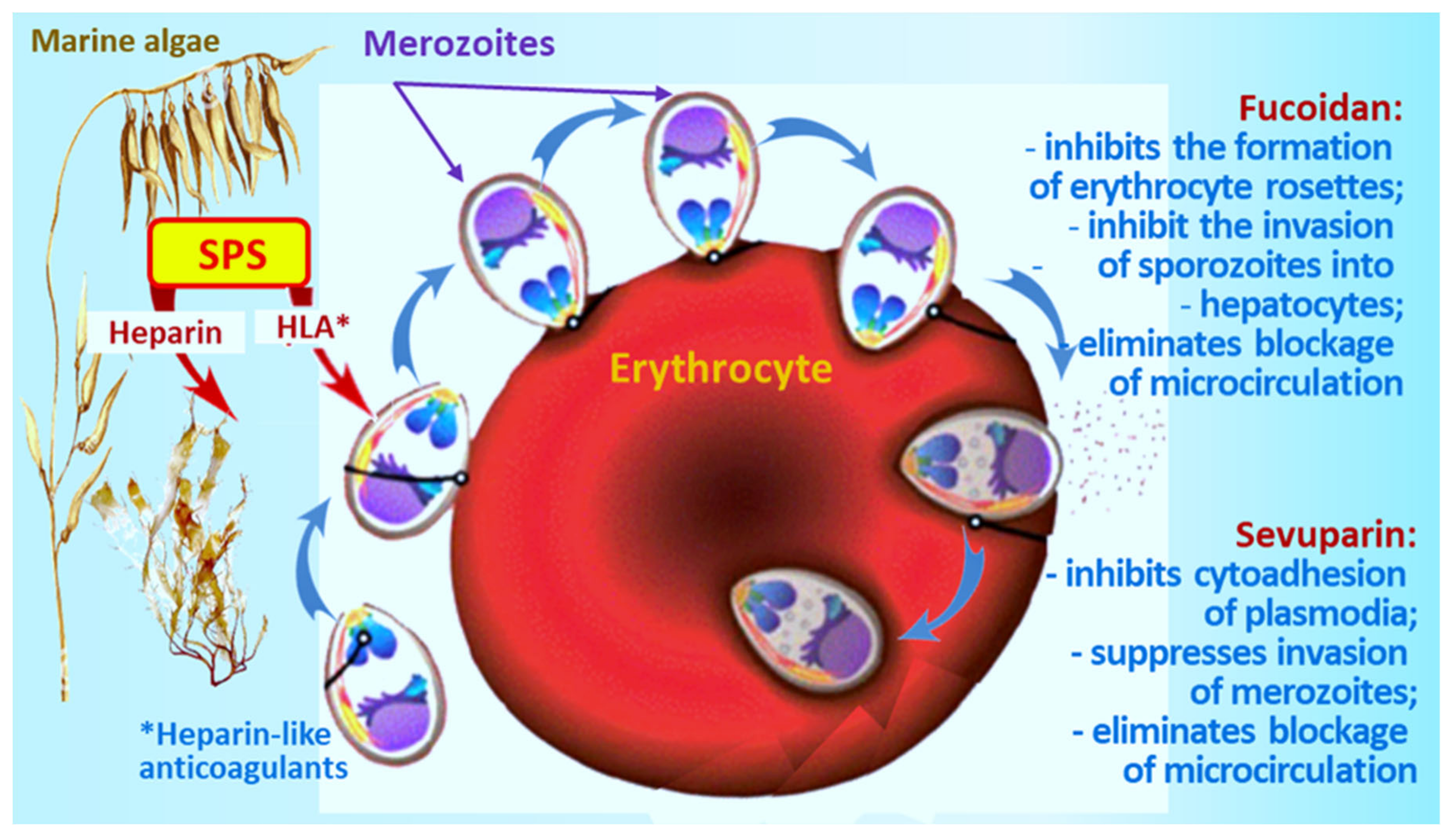
2. Malaria
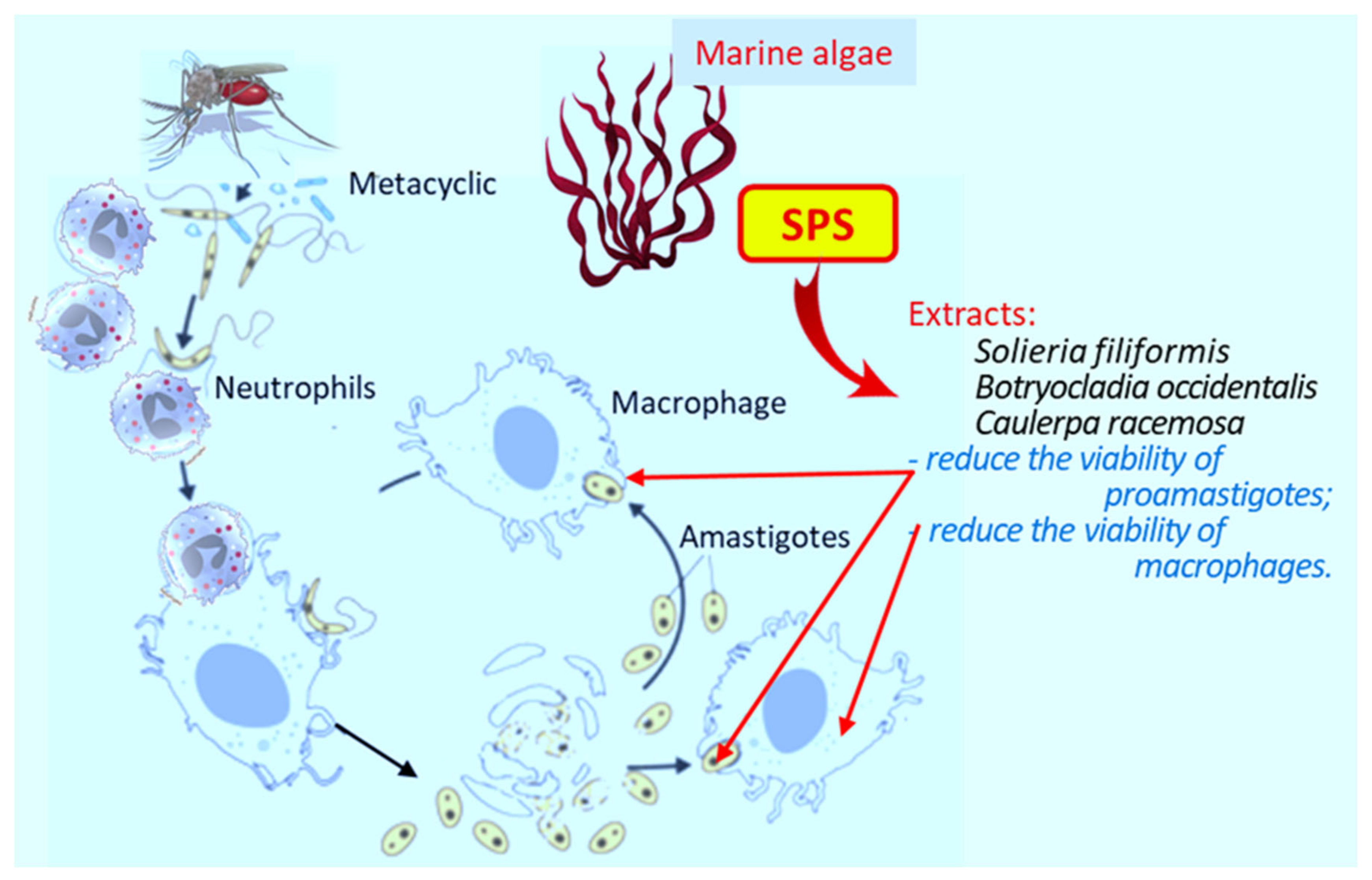
3. Leishmaniasis
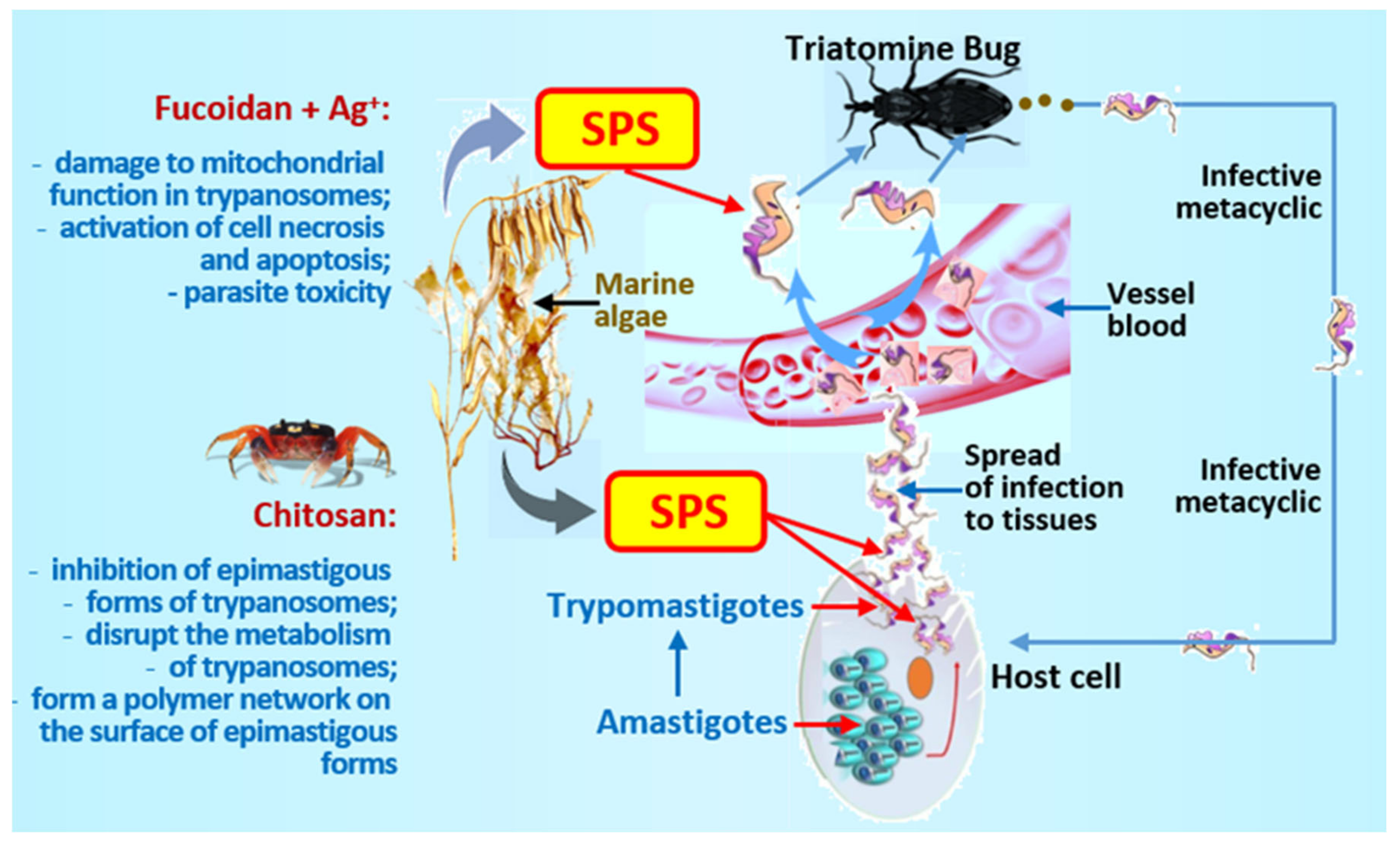
4. Trypanosomiasis
5. Schistosomiasis
6. Cryptosporidiosis
7. Trichomoniasis
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fletcher, S.; Caprarelli, G.; Merif, J.; Andresen, D.; van Hal, S.; Stark, D.; Ellis, J. Epidemiology and geographical distribution of enteric protozoan infections in Sydney, Australia. J. Public Health Res. 2014, 3, 298. [Google Scholar] [CrossRef]
- Henry, N.B.; Sermé, S.S.; Siciliano, G.; Sombié, S.; Diarra, A.; Sagnon, N.; Traoré, A.S.; Sirima, S.B.; Soulama, I.; Alano, P. Biology of Plasmodium falciparum gametocyte sex ratio and implications in malaria parasite transmission. Malar. J. 2019, 18, 70. [Google Scholar] [CrossRef] [PubMed]
- Szempruch, A.J.; Dennison, L.; Kieft, R.; Harrington, J.M.; Hajduk, S.L. Sending a message: Extracellular vesicles of pathogenic protozoan parasites. Nat. Rev. Genet. 2016, 14, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Okwor, I.; Uzonna, J. Social and economic burden of human leishmaniasis. Am. J. Trop. Med. Hyg. 2016, 94, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Strasen, J.; Williams, T.; Ertl, G.; Zoller, T.; Stich, A.; Ritter, O. Epidemiology of Chagas disease in Europe: Many calculations, little knowledge. Clin. Res. Cardiol. 2014, 103, 1–10. [Google Scholar] [CrossRef]
- Kim, S.-B.; Paulmurugan, R. Bioluminescent imaging systems for assay developments. Anal. Sci. 2021, 37, 233–247. [Google Scholar] [CrossRef]
- Álvarez-Bardón, M.; Pérez-Pertejo, Y.; Ordóñez, C.; Sepúlveda-Crespo, D.; Carballeira, N.M.; Tekwani, B.L.; Murugesan, S.; Martinez-Valladares, M.; García-Estrada, C.; Reguera, R.M.; et al. Screening marine natural products for new drug leads against trypanosomatids and malaria. Mar. Drugs 2020, 18, 187. [Google Scholar] [CrossRef] [PubMed]
- Stein, É.M.; Machado, L.P.; Roffato, H.K.; Miyasato, P.A.; Nakano, E.; Colepicolo, P.; Andreguetti, D.X. Antischistosomal activity from Brazilian marine algae. Rev. Bras. Farm. 2015, 25, 663–667. [Google Scholar] [CrossRef]
- Vonthron-Sénécheau, C.; Kaiser, M.; Devambez, I.; Vastel, A.; Mussio, I.; Rusig, A.-M. Antiprotozoal activities of organic extracts from french marine seaweeds. Mar. Drugs 2011, 9, 922–933. [Google Scholar] [CrossRef] [PubMed]
- Powers, J.L.; Zhang, X.; Kim, C.Y.; Abugri, D.A.; Witola, W.H. Activity of green algae extracts against Toxoplasma gondii. Med. Aromat. Plants 2017, 6, 3. [Google Scholar] [CrossRef]
- Torres, F.A.; Passalacqua, T.G.; Velásquez, A.M.A.; de Souza, R.A.; Colepicolo, P.; Graminha, M.A. New drugs with antiprotozoal activity from marine algae: A review. Rev. Bras. Farm. 2014, 24, 265–276. [Google Scholar] [CrossRef]
- Moo-Puc, R.; Robledo, D.; Freile-Pelegrin, Y. Evaluation of selected tropical seaweeds for in vitro anti-trichomonal activity. J. Ethnopharmacol. 2008, 120, 92–97. [Google Scholar] [CrossRef]
- Yamthe, L.R.T.; Appiah-Opong, R.; Fokou, P.V.T.; Tsabang, N.; Boyom, F.F.; Nyarko, A.K.; Wilson, M.D. Marine algae as source of novel antileishmanial drugs: A review. Mar. Drugs 2017, 15, 323. [Google Scholar] [CrossRef] [PubMed]
- Hutson, K.S.; Mata, L.; Paul, N.A.; de Nys, R. Seaweed extracts as a natural control against the monogenean ectoparasite, Neobenedenia sp., infecting farmed barramundi (Lates calcarifer). Int. J. Parasitol. 2012, 42, 1135–1141. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, T.K.; Viswanathan, K.; Ganguly, T.; Elankumaran, S.; Smith, S.; Pelzer, K.; Lansing, J.; Sriranganathan, N.; Zhao, G.; Galcheva-Gargova, Z.; et al. Contaminated heparin associated with adverse clinical events and activation of the contact system. N. Engl. J. Med. 2008, 358, 2457–2467. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, M.; Beccati, D.; Shriver, Z.; Naggi, A.M.; Viswanathan, K.; Bisio, A.; Capila, I.; Lansing, J.C.; Guglieri, S.; Fraser, B.; et al. Oversulfated chondroitin sulfate is a contaminant in heparin associated with adverse clinical events. Nat. Biotechnol. 2008, 26, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Arlov, Ø.; Rütsche, D.; Korayem, M.A.; Öztürk, E.; Zenobi-Wong, M. Engineered sulfated polysaccharides for biomedical applications. Adv. Funct. Mater. 2021, 31, 2010732. [Google Scholar] [CrossRef]
- Zeng, K.; Groth, T.; Zhang, K. Recent advances in artificially sulfated polysaccharides for applications in cell growth and differentiation, drug delivery, and tissue engineering. ChemBioChem 2019, 20, 737–746. [Google Scholar] [CrossRef]
- Gandhi, N.S.; Mancera, R.L. The structure of glycosaminoglycans and their interactions with proteins. Chem. Biol. Drug Des. 2008, 72, 455–482. [Google Scholar] [CrossRef] [PubMed]
- Zaporozhets, T.; Besednova, N. Prospects for the therapeutic application of sulfated polysaccharides of brown algae in diseases of the cardiovascular system: Review. Pharm. Biol. 2016, 54, 3126–3135. [Google Scholar] [CrossRef] [PubMed]
- Meneghetti, M.C.Z.; Hughes, A.; Rudd, T.; Nader, H.B.; Powell, A.K.; Yates, E.A.; Lima, M.A. Heparan sulfate and heparin interactions with proteins. J. R. Soc. Interface 2015, 12, 20150589. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, W.; Wang, Y.; Ma, Y.; Huang, L.; Zou, C.; Li, D.; Cao, M.-J.; Liu, G.-M. Inhibitory effect of depolymerized sulfated galactans from marine red algae on the growth and adhesion of diarrheagenic Escherichia coli. Mar. Drugs 2019, 17, 694. [Google Scholar] [CrossRef] [PubMed]
- Kwon, P.S.; Oh, H.; Kwon, S.-J.; Jin, W.; Zhang, F.; Fraser, K.; Hong, J.J.; Linhardt, R.J.; Dordick, J.S. Sulfated polysaccharides effectively inhibit SARS-CoV-2 In Vitro. Cell Discov. 2020, 6, 50. [Google Scholar] [CrossRef] [PubMed]
- Irhimeh, M.R.; Fitton, J.H.; Lowenthal, R.M.; Kongtawelert, P. A quantitative method to detect fucoidan in human plasma using a novel antibody. Methods Find. Exp. Clin. Pharmacol. 2005, 27, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Varo, R.; Chaccour, C.; Bassat, Q. Update on malaria. Medicina Clínica 2020, 155, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Graumans, W.; Jacobs, E.; Bousema, T.; Sinnis, P. When is a plasmodium-infected mosquito an infectious mosquito? Trends Parasitol. 2020, 36, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Center for Disease Control and Prevention. Malaria. Available online: https://www.cdc.gov/parasites/malaria/ (accessed on 1 October 2021).
- Goerdeler, F.; Seeberger, P.H.; Moscovitz, O. Unveiling the Sugary Secrets of Plasmodium Parasites. Front Microbiol. 2021, 12, 712538. [Google Scholar] [CrossRef]
- Sato, S. Plasmodium—A brief introduction to the parasites causing human malaria and their basic biology. J. Physiol. Anthr. 2021, 40, 1. [Google Scholar] [CrossRef]
- Weiss, G.E.; Gilson, P.R.; Taechalertpaisarn, T.; Tham, W.-H.; de Jong, N.; Harvey, K.L.; Fowkes, F.; Barlow, P.N.; Rayner, J.C.; Wright, G.; et al. Revealing the sequence and resulting cellular morphology of receptor-ligand interactions during Plasmodium falciparum invasion of erythrocytes. PLoS Pathog. 2015, 11, e1004670. [Google Scholar] [CrossRef]
- Venugopal, K.; Hentzschel, F.; Valkiūnas, G.; Marti, M. Plasmodium asexual growth and sexual development in the haematopoietic niche of the host. Nat. Rev. Genet. 2020, 18, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Maier, A.G.; Cooke, B.M.; Cowman, A.F.; Tilley, L. Malaria parasite proteins that remodel the host erythrocyte. Nat. Rev. Genet. 2009, 7, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Counihan, N.A.; Modak, J.K.; de Koning-Ward, T.F. How malaria parasites acquire nutrients from their host. Front. Cell Dev. Biol. 2021, 9, 649184. [Google Scholar] [CrossRef] [PubMed]
- Marques, J.; Valle-Delgado, J.J.; Urban, P.; Baró, E.; Prohens, R.; Mayor, A.; Cisteró, P.; Delves, M.; Sinden, R.E.; Grandfils, C.; et al. Adaptation of targeted nanocarriers to changing requirements in antimalarial drug delivery. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.S.; Uboldi, A.D.; Epp, C.; Bujard, H.; Tsuboi, T.; Czabotar, P.; Cowman, A.F. Multiple Plasmodium falciparum merozoite surface protein 1 complexes mediate merozoite binding to human erythrocytes. J. Biol. Chem. 2016, 291, 7703–7715. [Google Scholar] [CrossRef] [PubMed]
- Dijkman, P.M.; Marzluf, T.; Zhang, Y.; Chang, S.-Y.S.; Helm, D.; Lanzer, M.; Bujard, H.; Kudryashev, M. Structure of the merozoite surface protein 1 from Plasmodium falciparum. Sci. Adv. 2021, 7, eabg0465. [Google Scholar] [CrossRef] [PubMed]
- Counihan, N.A.; Kalanon, M.; Coppel, R.; de Koning-Ward, T. Plasmodium rhoptry proteins: Why order is important. Trends Parasitol. 2013, 29, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Lingelbach, K.; Joiner, K. The parasitophorous vacuole membrane surrounding Plasmodium and Toxoplasma: An unusual compartment in infected cells. J. Cell Sci. 1998, 111, 1467–1475. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Kaneko, O.; Thongkukiatkul, A.; Tachibana, M.; Otsuki, H.; Gao, Q.; Tsuboi, T.; Torii, M. Rhoptry neck protein RON2 forms a complex with microneme protein AMA1 in Plasmodium falciparum merozoites. Parasitol. Int. 2009, 58, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.H.; Ackerman, H.C.; Su, X.; Wellems, T.E. Malaria biology and disease pathogenesis: Insights for new treatments. Nat. Med. 2013, 19, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-C.; Russel, B.; Renia, L. Sticking for a cause: The falciparum malaria parasites cytoadherence paradigm. Front. Immunol. 2019, 10, 1444. [Google Scholar] [CrossRef]
- Takala-Harrison, S.; Jacob, C.G.; Arze, C.; Cummings, M.P.; Silva, J.C.; Dondorp, A.M.; Fukuda, M.M.; Hien, T.T.; Mayxay, M.; Noedl, H.; et al. Independent emergence of artemisinin resistance mutations among Plasmodium falciparum in southeast Asia. J. Infect. Dis. 2015, 211, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Shibeshi, M.A.; Kifle, Z.D.; Atnafie, S.A. Antimalarial drug resistance and novel targets for antimalarial drug discovery. Infect. Drug Resist. 2020, 13, 4047–4060. [Google Scholar] [CrossRef]
- Belete, T.M. Recent progress in the development of new antimalarial drugs with novel targets. Drug Des. Dev. Ther. 2020, 14, 3875–3889. [Google Scholar] [CrossRef]
- Dans, M.G.; Weiss, G.E.; Wilson, D.; Sleebs, B.E.; Crabb, B.S.; de Koning-Ward, T.F.; Gilson, P.R. Screening the medicines for malaria venture pathogen box for invasion and egress inhibitors of the blood stage of Plasmodium falciparum reveals several inhibitory compounds. Int. J. Parasitol. 2020, 50, 235–252. [Google Scholar] [CrossRef] [PubMed]
- Beeson, J.G.; Drew, D.R.; Boyle, M.; Feng, G.; Fowkes, F.; Richards, J.S. Merozoite surface proteins in red blood cell invasion, immunity and vaccines against malaria. FEMS Microbiol. Rev. 2016, 40, 343–372. [Google Scholar] [CrossRef] [PubMed]
- Lantero, E.; Aláez-Versón, C.; Romero, P.; Sierra, T.; Fernàndez-Busquets, X. Repurposing heparin as antimalarial: Evaluation of multiple modifications toward In Vivo application. Pharmaceutics 2020, 12, 825. [Google Scholar] [CrossRef]
- Kobayashi, K.; Takano, R.; Takemae, H.; Sugi, T.; Ishiwa, A.; Gong, H.; Recuenco, F.C.; Iwanaga, T.; Horimoto, T.; Akashi, H.; et al. Analyses of interactions between heparin and the apical surface proteins of Plasmodium falciparum. Sci. Rep. 2013, 3, 3178. [Google Scholar] [CrossRef] [PubMed]
- Leitgeb, A.M.; Nde, P.; Cho-Ngwa, F.; Titanji, V.; Samje, M.; Blomqvist, K.; Wahlgren, M. Low anticoagulant heparin disrupts Plasmodium falciparum rosettes in fresh clinical isolates. Am. J. Trop. Med. Hyg. 2011, 84, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Saiwaew, S.; Sritabal, J.; Piaraksa, N.; Keayarsa, S.; Ruengweerayut, R.; Utaisin, C.; Sila, P.; Niramis, R.; Udomsangpetch, R.; Charunwatthana, P.; et al. Effects of sevuparin on rosette formation and cytoadherence of Plasmodium falciparum infected erythrocytes. PLoS ONE 2017, 12, e0172718. [Google Scholar] [CrossRef] [PubMed]
- McQuaid, F.; Rowe, J.A. Rosetting revisited: A critical look at the evidence for host erythrocyte receptors in Plasmodium falciparum rosetting. Parasitology 2020, 147, 1–11. [Google Scholar] [CrossRef]
- Boyle, M.J.; Skidmore, M.; Dickerman, B.; Cooper, L.; Devlin, A.; Yates, E.; Horrocks, P.; Freeman, C.; Chai, W.; Beeson, J.G. Identification of heparin modifications and polysaccharide inhibitors of Plasmodium falciparum merozoite invasion that have potential for novel drug development. Antimicrob. Agents Chemother. 2017, 61, 00709–00717. [Google Scholar] [CrossRef] [PubMed]
- Marques, J.; Vilanova, E.; Mourão, P.A.S.; Fernàndez-Busquets, X. Marine organism sulfated polysaccharides exhibiting significant antimalarial activity and inhibition of red blood cell invasion by Plasmodium. Sci. Rep. 2016, 6, 24368. [Google Scholar] [CrossRef]
- Mourão, P.A.S.; Pereira, M.S.; Pavão, M.S.G.; Mulloy, B.; Tollefsen, D.M.; Mowinckel, M.-C.; Abildgaard, U. Structure and anticoagulant activity of a fucosylated chondroitin sulfate from echinoderm. J. Biol. Chem. 1996, 271, 23973–23984. [Google Scholar] [CrossRef]
- Chen, S.; Hu, Y.; Ye, X.; Li, G.; Yu, G.; Xue, C.; Chai, W. Sequence determination and anticoagulant and antithrombotic activities of a novel sulfated fucan isolated from the sea cucumber Isostichopus badionotus. Biochim. Biophys. Acta Gen. Subj. 2012, 1820, 989–1000. [Google Scholar] [CrossRef] [PubMed]
- Rowe, A.; Berendt, A.; Marsh, K.; Newbold, C. Plasmodium falciparum: A family of sulfated glycoconjugates disrupts erythrocyte rosettes. Exp. Parasitol. 1994, 79, 506–516. [Google Scholar] [CrossRef]
- Chen, J.-H.; Lim, J.D.; Sohn, E.-H.; Choi, Y.-S.; Han, E.-T. Growth-Inhibitory effect of a fucoidan from brown seaweed Undaria pinnatifida on Plasmodium parasites. Parasitol. Res. 2008, 104, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Melo, F.R.; Pereira, M.S.; Foguel, D.; Mourão, P.A.S. Antithrombin-Mediated anticoagulant activity of sulfated polysaccharides: Different mechanisms for heparin and sulfated galactans. J. Biol. Chem. 2004, 279, 20824–20835. [Google Scholar] [CrossRef] [PubMed]
- Boddey, J.A.; O’Neill, M.T.; Lopaticki, S.; Carvalho, T.; Hodder, A.N.; Nebl, T.; Wawra, S.; Van West, P.; Ebrahimzadeh, Z.; Richard, D.; et al. Export of malaria proteins requires co-translational processing of the PEXEL motif independent of phosphatidylinositol-3-phosphate binding. Nat. Commun. 2016, 7, 10470. [Google Scholar] [CrossRef] [PubMed]
- Warncke, J.D.; Vakonakis, I.; Beck, H.-P. Plasmodium Helical Interspersed Subtelomeric (PHIST) proteins, at the center of host cell remodeling. Microbiol. Mol. Biol. Rev. 2016, 80, 905–927. [Google Scholar] [CrossRef] [PubMed]
- Tarr, S.J.; Moon, R.W.; Hardege, I.; Osborne, A.R. A conserved domain targets exported PHISTb family proteins to the periphery of Plasmodium infected erythrocytes. Mol. Biochem. Parasitol. 2014, 196, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Mutisya, J.M.; Mobegi, V.A.; Kinyua, J.K.; Kivecu, M.N.; Okoth, R.O.; Chemwor, G.C.; Mwakio, E.W.; Cheruiyot, A.C.; Yeda, R.A.; Okello, C.O.; et al. Characterization of sulfated polysaccharide activity against virulent Plasmodium falciparum PHISTb/RLP1 protein. F1000Research 2020, 9, 1268. [Google Scholar] [CrossRef]
- Bastos, M.F.; Albrecht, L.; Kozlowski, E.O.; Lopes, S.C.P.; Blanco, Y.C.; Carlos, B.C.; Castiñeiras, C.; Vicente, C.P.; Werneck, C.C.; Wunderlich, G.; et al. Fucosylated chondroitin sulfate inhibits Plasmodium falciparum cytoadhesion and merozoite invasion. Antimicrob. Agents Chemother. 2014, 58, 1862–1871. [Google Scholar] [CrossRef] [PubMed]
- Burns, A.L.; Dans, M.G.; Balbin, J.M.; de Koning-Ward, T.F.; Gilson, P.R.; Beeson, J.G.; Boyle, M.J.; Wilson, D.W. Targeting malaria parasite invasion of red blood cells as an antimalarial strategy. FEMS Microbiol Rev. 2019, 43, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Bastos, M.F.; Albrecht, L.; Gomes, A.M.; Lopes, S.; Vicente, C.P.; De Almeida, R.P.; Cassiano, G.C.; Fonseca, R.J.C.; Werneck, C.C.; Pavão, M.S.; et al. A new heparan sulfate from the mollusk Nodipecten nodosus inhibits merozoite invasion and disrupts rosetting and cytoadherence of Plasmodium falciparum. Memórias Inst. Oswaldo Cruz 2019, 114, e190088. [Google Scholar] [CrossRef] [PubMed]
- Eun, Y.; Tae, H. Pharmaceutical Composition for Preventing or Treating Malaria, Containing Fucoidan as Active. International Patent Application No. WO2021060862, 24 September 2020. [Google Scholar]
- Rahmah, Z.; Indriana, N.; Astari, L.F.; Sutikno, A.M. The combined effect of extract seaweed and DHP on placental malaria. Adv. Soc. Sci. Educ. Humanit. Res. 2020, 59, 452–458. [Google Scholar] [CrossRef]
- Atun, R.A.; Bennett, S.; Duran, A. When do Vertical (Stand-Alone) Programmes Have a Place in Health Systems? WHO POLICY Brief. 2008. Available online: https://www.who.int/management/district/services/WhenDoVerticalProgrammesPlaceHealthSystems.pdf (accessed on 12 May 2021).
- Rahi, M.; Chaturvedi, R.; Das, P.; Sharma, A. India can consider integration of three eliminable disease control programmes on malaria, lymphatic filariasis, and visceral leishmaniasis. PLoS Pathog. 2021, 17, e1009492. [Google Scholar] [CrossRef]
- Sharma, G.; Kar, S.; Ball, W.B.; Ghosh, K.; Das, P.K. The curative effect of fucoidan on visceral leishmaniasis is mediated by activation of MAP kinases through specific protein kinase C isoforms. Cell. Mol. Immunol. 2014, 11, 263–274. [Google Scholar] [CrossRef]
- Tasdemir, D.; Kaiser, M.; Brun, R.; Yardley, V.; Schmidt, T.J.; Tosun, F.; Rüedi, P. Antitrypanosomal and antileishmanial activities of flavonoids and their analogues: In Vitro, In Vivo, Structure-activity relationship, and quantitative structure-activity relationship studies. Antimicrob. Agents Chemother. 2006, 50, 1352–1364. [Google Scholar] [CrossRef]
- Berbert, T.R.N.; De Mello, T.F.P.; Nassif, P.W.; Mota, C.A.; Silveira, A.V.; Duarte, G.C.; Demarchi, I.G.; Aristides, S.M.A.; Lonardoni, M.V.C.; Teixeira, J.J.V.; et al. Pentavalent antimonials combined with other therapeutic alternatives for the treatment of cutaneous and mucocutaneous leishmaniasis: A systematic review. Dermatol. Res. Pract. 2018, 2018, 9014726. [Google Scholar] [CrossRef] [PubMed]
- De Castro Côrtes, L.M.; de Souza Pereira, M.C.; da Silva, F.S.; Pereira, B.A.S.; de Oliveira Junior, F.O.; de Araújo Soares, R.O.; Brazil, R.P.; Toma, L.; Vicente, C.M.; Nader, H.B.; et al. Participation of heparin binding proteins from the surface of Leishmania (Viannia) braziliensis promastigotes in the adhesion of parasites to Lutzomyia longipalpis cells (Lulo) In Vitro. Parasites Vectors 2012, 5, 142. [Google Scholar] [CrossRef] [PubMed]
- Maciej-Hulme, M.L.; Skidmore, M.; Price, H.P. The role of heparan sulfate in host macrophage infection by Leishmania species. Biochem. Soc. Trans. 2018, 46, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Pires, C.L.; Rodrigues, S.D.; Bristot, D.; Gaeta, H.H.; Toyama, D.d.; Farias, W.R.L.; Toyama, M.H. Evaluation of macroalgae sulfated polysaccharides on the Leishmania amazoensis promastigote. Mar. Drugs. 2013, 11, 934–943. [Google Scholar] [CrossRef]
- Minicante, S.A.; Michelet, S.; Bruno, F.; Castelli, G.; Vitale, F.; Sfriso, A.; Morabito, M.; Genovese, G. Bioactivity of phycocolloids against the mediterranean protozoan Leishmania infantum: An inceptive study. Sustainability 2016, 8, 1131. [Google Scholar] [CrossRef]
- Kar, S.; Sharma, G.; Das, P.K. Fucoidan cures infection with both antimony-susceptible and -resistant strains of Leishmania donovani through Th1 response and macrophage-derived oxidants. J. Antimicrob. Chemother. 2011, 66, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Shadab, M.; Ali, N. Evasion of host defence by Leishmania donovani: Subversion of signaling pathways. Mol. Biol. Int. 2011, 2011, 1–10. [Google Scholar] [CrossRef][Green Version]
- Olivier, M.; Brownsey, R.W.; Reiner, N.E. Defective stimulus-response coupling in human monocytes infected with Leishmania donovani is associated with altered activation and translocation of protein kinase C. Proc. Natl. Acad. Sci. USA 1992, 89, 7481–7485. [Google Scholar] [CrossRef] [PubMed]
- Solano-Gálvez, S.-G.; Álvarez-Hernández, D.-A.; Gutiérrez-Kobeh, L.; Vázquez-López, R. Leishmania: Manipulation of signaling pathways to inhibit host cell apoptosis. Ther. Adv. Infect. Dis. 2021, 8, 20499361211014977. [Google Scholar] [CrossRef] [PubMed]
- Soulat, D.; Bogdan, C. Function of macrophage and parasite phosphatases in leishmaniasis. Front. Immunol. 2017, 8, 1838. [Google Scholar] [CrossRef] [PubMed]
- De Martini, C.C.; De Andrade, J.T.; De Almeida, S.K.M.; Silva, K.L.O.; Eugenio, F.D.R.; Dos Santos, P.S.P.; De Lima, V.M.F. Cellular apoptosis and nitric oxide production in PBMC and spleen from dogs with visceral leishmaniasis. Comp. Immunol. Microbiol. Infect. Dis. 2018, 57, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hoseini, M.H.M.; Moradi, M.; Alimohammadian, M.H.; Shahgoli, V.K.; Darabi, H.; Rostami, A. Immunotherapeutic effects of chitin in comparison with chitosan against Leishmania major infection. Parasitol. Int. 2016, 65, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Riezk, A.; Raynes, J.G.; Yardley, V.; Murdan, S.; Croft, S.L. Activity of chitosan and its derivatives against Leishmania major and Leishmania mexicana In Vitro. Antimicrob. Agents Chemother. 2020, 64, 01772-19. [Google Scholar] [CrossRef]
- Antinori, S.; Galimberti, L.; Bianco, R.; Grande, R.; Galli, M.; Corbellino, M. Chagas disease in Europe: A review for the internist in the globalized world. Eur. J. Intern. Med. 2017, 43, 6–15. [Google Scholar] [CrossRef]
- Rassi, A.; Marin-Neto, J.A. Chagas Disease. Negl. Trop. Dis. 2015, 375, 45–71. [Google Scholar] [CrossRef]
- Varikuti, S.; Jha, B.K.; Volpedo, G.; Ryan, N.; Halsey, G.; Hamza, O.M.; McGwire, B.S.; Satoskar, A.R. Host-directed drug therapies for neglected tropical diseases caused by protozoan parasites. Front. Microbiol. 2018, 9, 2655. [Google Scholar] [CrossRef] [PubMed]
- Stillwaggon, E.; Perez-Zetune, V.; Bialek, S.R.; Montgomery, S.P. Congenital chagas disease in the United States: Cost savings through maternal screening. Am. J. Trop. Med. Hyg. 2018, 98, 1733–1742. [Google Scholar] [CrossRef] [PubMed]
- Jackson, Y.; Alirol, E.; Getaz, L.; Wolff, H.; Combescure, C.; Chappuis, F. Tolerance and safety of nifurtimox in patients with chronic chagas disease. Clin. Infect. Dis. 2010, 51, e69–e75. [Google Scholar] [CrossRef] [PubMed]
- Leal, D.; Mansilla, A.; Matsuhiro, B.; Moncada-Basualto, M.; Lapier, M.; Maya, J.; Olea-Azar, C.; De Borggraeve, W. Chemical structure and biological properties of sulfated fucan from the sequential extraction of sub Antarctic Lessonia sp. (Phaeophyceae). Carbohydr. Polym. 2018, 199, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jayawardena, T.U.; Yang, H.-W.; Lee, H.G.; Kang, M.-C.; Sanjeewa, K.K.A.; Oh, J.Y.; Jeon, Y.-J. Isolation, characterization, and antioxidant activity evaluation of a fucoidan from an enzymatic digest of the edible seaweed, Hizikia fusiforme. Antioxidants 2020, 9, 363. [Google Scholar] [CrossRef] [PubMed]
- Sousa, W.M.; Silva, R.O.; Bezerra, F.F.; Bingana, R.D.; Barros, F.C.N.; Costa, L.E.C.; Sombra, V.G.; Soares, P.M.G.; Feitosa, J.P.A.; de Paula, R.C.M.; et al. Sulfated polysaccharide fraction from marine algae Solieria filiformis: Structural characterization, gastroprotective and antioxidant effects. Carbohydr. Polym. 2016, 152, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Usoltseva, R.V.; Anastyuk, S.D.; Ishina, I.; Isakov, V.V.; Zvyagintseva, T.N.; Thinh, P.D.; Zadorozhny, P.A.; Dmitrenok, P.S.; Ermakova, S.P. Structural characteristics and anticancer activity in vitro of fucoidan from brown alga Padina boryana. Carbohydr. Polym. 2018, 184, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Barroso, E.M.A.; Costa, L.S.; Medeiros, V.P.; Cordeiro, S.L.; Costa, M.S.S.P.; Franco, C.R.C.; Nader, H.B.; Leite, E.L.; Rocha, H.A.O. A non-anticoagulant heterofucan has antithrombotic activityin vivo. Planta Medica 2008, 74, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.; Araújo, N.; Daniele-Silva, A.; Oliveira, J.; Medeiros, J.; Araújo, R.; Ferreira, L.; Rocha, H.; Silva-Junior, A.; Silva, M.; et al. Antimicrobial activity of chitosan oligosaccharides with special attention to antiparasitic potential. Mar. Drugs 2021, 19, 110. [Google Scholar] [CrossRef] [PubMed]
- Yan, N.; Chen, X. Don’t waste seafood waste: Turning cast-off shells into nitrogen-rich chemicals would benefit economics and the environment. Nature 2015, 524, 155–157. [Google Scholar] [CrossRef] [PubMed]
- Šimat, V.; ElAbed, N.; Kulawik, P.; Ceylan, Z.; Jamroz, E.; Yazgan, H.; Čagalj, M.; Regenstein, J.M.; Özogul, F. Recent advances in marine-based nutraceuticals and their health benefits. Mar. Drugs 2020, 18, 627. [Google Scholar] [CrossRef] [PubMed]
- Ke, C.-L.; Deng, F.-S.; Chuang, C.-Y.; Lin, C.-H. Antimicrobial actions and applications of chitosan. Polymers 2021, 13, 904. [Google Scholar] [CrossRef] [PubMed]
- McManus, D.P.; Dunne, D.; Sacko, M.; Utzinger, J.; Vennervald, B.J.; Zhou, X.-N. Schistosomiasis. Nat. Rev. Dis. Prim. 2018, 4, 13. [Google Scholar] [CrossRef] [PubMed]
- Chuah, C.; Jones, M.; Burke, M.; McManus, D.P.; Gobert, G.N. Cellular and chemokine-mediated regulation in schistosome-induced hepatic pathology. Trends Parasitol. 2014, 30, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Colley, D.G.; Bustinduy, A.L.; Secor, W.E.; King, C.H. Human schistosomiasis. Lancet 2014, 383, 2253–2264. [Google Scholar] [CrossRef]
- Sanjeewa, K.K.A.; Jayawardena, T.U.; Kim, H.-S.; Kim, S.-Y.; Fernando, I.S.; Wang, L.; Abetunga, D.; Kim, W.-S.; Lee, D.-S.; Jeon, Y.-J. Fucoidan isolated from Padina commersonii inhibit LPS-induced inflammation in macrophages blocking TLR/NF-κB signal pathway. Carbohydr. Polym. 2019, 224, 115195. [Google Scholar] [CrossRef]
- Yu, H.-H.; Ko, E.C.; Chang, C.-L.; Yuan, K.S.-P.; Wu, A.T.H.; Shan, Y.-S.; Wu, S.-Y. Fucoidan inhibits radiation-induced pneumonitis and lung fibrosis by reducing inflammatory cytokine expression in lung tissues. Mar. Drugs 2018, 16, 392. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Li, M.; Wang, X.; Chang, H.; Ni, Y.; Li, C.; He, K.; Wang, H.; Yang, Y.; Tian, T.; et al. Therapeutic potential of fucoidan in the reduction of hepatic pathology in murine schistosomiasis japonica. Parasites Vectors 2020, 13, 451. [Google Scholar] [CrossRef]
- Clode, P.L.; Koh, W.H.; Thompson, R.A. Life without a host cell: What is cryptosporidium? Trends Parasitol. 2015, 31, 614–624. [Google Scholar] [CrossRef] [PubMed]
- Gunasekera, S.; Zahedi, A.; O’Dea, M.; King, B.; Monis, P.; Thierry, B.; Carr, J.M.; Ryan, U. Organoids and bioengineered intestinal models: Potential solutions to the cryptosporidium culturing dilemma. Microorganisms 2020, 8, 715. [Google Scholar] [CrossRef]
- Mammeri, M.; Chevillot, A.; Chenafi, I.; Thomas, M.; Julien, C.; Vallée, I.; Polack, B.; Follet, J.; Adjou, K.T. Molecular characterization of Cryptosporidium isolates from diarrheal dairy calves in France. Veter. Parasitol. Reg. Stud. Rep. 2019, 18, 100323. [Google Scholar] [CrossRef]
- Li, X.; Nguyen, T.; Xiao, C.; Levy, A.; Akagi, Y.; Silkie, S.; Atwill, E.R. Prevalence and genotypes of cryptosporidium in wildlife populations co-located in a protected watershed in the pacific northwest, 2013 to 2016. Microorganisms 2020, 8, 914. [Google Scholar] [CrossRef]
- Merga, Y.; Campbell, B.J.; Rhodes, J.M. mucosal barrier, bacteria and inflammatory bowel disease: Possibilities for therapy. Dig. Dis. 2014, 32, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Ishiwa, A. The role of carbohydrates in infection strategies of enteric pathogens. Trop. Med. Health 2015, 43, 41–52. [Google Scholar] [CrossRef] [PubMed]
- McGuckin, M.A.; Lindén, S.K.; Sutton, P.; Florin, T.H. Mucin dynamics and enteric pathogens. Nat. Rev. Genet. 2011, 9, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Barria, E.; Boothroyd, J.C. A toxoplasma lectin-like activity specific for sulfated polysaccharides is involved in host cell infection. J. Biol. Chem. 1999, 274, 1267–1276. [Google Scholar] [CrossRef]
- Ishiwa, A.; Kobayashi, K.; Takemae, H.; Sugi, T.; Gong, H.; Recuenco, F.C.; Murakoshi, F.; Inomata, A.; Horimoto, T.; Kato, K. Effects of dextran sulfates on the acute infection and growth stages of Toxoplasma gondii. Parasitol. Res. 2013, 112, 4169–4176. [Google Scholar] [CrossRef] [PubMed]
- Recuenco, F.C.; Kobayashi, K.; Ishiwa, A.; Enomoto-Rogers, Y.; Fundador, N.G.V.; Sugi, T.; Takemae, H.; Iwanaga, T.; Murakoshi, F.; Gong, H.; et al. Gellan sulfate inhibits Plasmodium falciparum growth and invasion of red blood cells In Vitro. Sci. Rep. 2014, 4, 4723. [Google Scholar] [CrossRef] [PubMed]
- Inomata, A.; Murakoshi, F.; Ishiwa, A.; Takano, R.; Takemae, H.; Sugi, T.; Recuenco, F.; Horimoto, T.; Kato, K. Heparin interacts with elongation factor 1α of Cryptosporidium parvum and inhibits invasion. Sci. Rep. 2015, 5, 11599. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, H.; Tanaka, M.; Hashimoto, M.; Inoue, M.; Sasahara, T. The suppressive effect of Mekabu fucoidan on an attachment of Cryptosporidium parvum oocysts to the intestinal epithelial cells in neonatal mice. Life Sci. 2007, 80, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Mammeri, M.; Chevillot, A.; Thomas, M.; Polack, B.; Julien, C.; Marden, J.-P.; Auclair, E.; Vallee, I.; Adjou, K.T. Efficacy of chitosan, a natural polysaccharide, against Cryptosporidium parvum In Vitro and In Vivo in neonatal mice. Exp. Parasitol. 2018, 194, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mérou, N.; Lecadet, C.; Pouvreau, S.; Arzul, I. An eDNA/eRNA-based approach to investigate the life cycle of non-cultivable shellfish micro-parasites: The case of Bonamia ostreae, a parasite of the European flat oyster Ostrea edulis. Microb. Biotechnol. 2020, 13, 1807–1818. [Google Scholar] [CrossRef] [PubMed]
- Paulish-Miller, T.E.; Augostini, P.; Schuyler, J.A.; Smith, W.L.; Mordechai, E.; Adelson, M.E.; Gygax, S.E.; Secor, W.E.; Hilbert, D.W. Trichomonas vaginalis metronidazole resistance is associated with single nucleotide polymorphisms in the nitroreductase genes ntr4Tv and ntr6Tv. Antimicrob. Agents Chemother. 2014, 58, 2938–2943. [Google Scholar] [CrossRef] [PubMed]
- Bouchemal, K.; Bories, C.; Loiseau, P. Strategies for prevention and treatment of Trichomonas vaginalis infections. Clin. Microbiol. Rev. 2017, 30, 811–825. [Google Scholar] [CrossRef]
- Telles, C.B.S.; Mendes-Aguilar, C.; Fidelis, G.P.; Frasson, A.P.; Pereira, W.O.; Scortecci, K.C.; Camara, R.B.G.; Nobre, L.T.D.B.; Costa, L.S.; Tasca, T.; et al. Immunomodulatory effects and antimicrobial activity of heterofucans from Sargassum filipendula. J. Appl. Phycol. 2018, 30, 569–578. [Google Scholar] [CrossRef]
- Asker, M.S.; Kady, E.M.; Mahmoud, M.G. New trends of the polysaccharides as a drug. World J. Agric. Soil Sci. 2019, 3, 114–119. [Google Scholar] [CrossRef]
- Sanjeewa, K.; Jeon, Y.-J. Fucoidans as scientifically and commercially important algal polysaccharides. Mar. Drugs 2021, 19, 284. [Google Scholar] [CrossRef] [PubMed]
- Andrew, M.; Jayaraman, G. Marine sulfated polysaccharides as potential antiviral drug candidates to treat Corona Virus disease (COVID-19). Carbohydr. Res. 2021, 505, 108326. [Google Scholar] [CrossRef] [PubMed]
- Azzouz, N.; Kamena, F.; Laurino, P.; Kikkeri, R.; Mercier, C.; Cesbron-Delauw, M.-F.; Dubremetz, J.-F.; De Cola, L.; Seeberger, P.H. Toxoplasma gondii secretory proteins bind to sulfated heparin structures. Glycobiology 2013, 23, 106–120. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liu, Y.; Cao, M.-J.; Liu, G.-M.; Chen, Q.; Sun, L.; Chen, H. Antibacterial activity and mechanisms of depolymerized fucoidans isolated from Laminaria japonica. Carbohydr. Polym. 2017, 172, 294–305. [Google Scholar] [CrossRef]
- Palanisamy, S.; Vinosha, M.; Rajasekar, P.; Anjali, R.; Sathiyaraj, G.; Marudhupandi, T.; Selvam, S.; Prabhu, N.M.; You, S. Antibacterial efficacy of a fucoidan fraction (Fu-F2) extracted from Sargassum polycystum. Int. J. Biol. Macromol. 2019, 125, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Choi, G.-W.; Lee, Y.-B.; Cho, H.-Y. Interpretation of non-clinical data for prediction of human pharmacokinetic parameters: In Vitro-In Vivo extrapolation and allometric scaling. Pharmaceutics 2019, 11, 168. [Google Scholar] [CrossRef] [PubMed]
- Silchenko, A.S.; Rasin, A.B.; Kusaykin, M.I.; Malyarenko, O.S.; Shevchenko, N.M.; Zueva, A.O.; Kalinovsky, A.I.; Zvyagintseva, T.N.; Ermakova, S.P. Modification of native fucoidan from Fucus evanescens by recombinant fucoidanase from marine bacteria Formosa algae. Carbohydr. Polym. 2018, 193, 189–195. [Google Scholar] [CrossRef]
- Belik, A.; Silchenko, A.; Malyarenko, O.; Rasin, A.; Kiseleva, M.; Kusaykin, M.; Ermakova, S. Two new alginate lyases of PL7 and PL6 families from polysaccharide-degrading bacterium Formosa algae KMM 3553T: Structure, properties, and products analysis. Mar. Drugs 2020, 18, 130. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Besednova, N.N.; Zaporozhets, T.S.; Andryukov, B.G.; Kryzhanovsky, S.P.; Ermakova, S.P.; Kuznetsova, T.A.; Voronova, A.N.; Shchelkanov, M.Y. Antiparasitic Effects of Sulfated Polysaccharides from Marine Hydrobionts. Mar. Drugs 2021, 19, 637. https://doi.org/10.3390/md19110637
Besednova NN, Zaporozhets TS, Andryukov BG, Kryzhanovsky SP, Ermakova SP, Kuznetsova TA, Voronova AN, Shchelkanov MY. Antiparasitic Effects of Sulfated Polysaccharides from Marine Hydrobionts. Marine Drugs. 2021; 19(11):637. https://doi.org/10.3390/md19110637
Chicago/Turabian StyleBesednova, Natalya N., Tatyana S. Zaporozhets, Boris G. Andryukov, Sergey P. Kryzhanovsky, Svetlana P. Ermakova, Tatyana A. Kuznetsova, Anastasia N. Voronova, and Mikhail Y. Shchelkanov. 2021. "Antiparasitic Effects of Sulfated Polysaccharides from Marine Hydrobionts" Marine Drugs 19, no. 11: 637. https://doi.org/10.3390/md19110637
APA StyleBesednova, N. N., Zaporozhets, T. S., Andryukov, B. G., Kryzhanovsky, S. P., Ermakova, S. P., Kuznetsova, T. A., Voronova, A. N., & Shchelkanov, M. Y. (2021). Antiparasitic Effects of Sulfated Polysaccharides from Marine Hydrobionts. Marine Drugs, 19(11), 637. https://doi.org/10.3390/md19110637