Hidden Diversity in an Antarctic Algal Forest: Metabolomic Profiling Linked to Patterns of Genetic Diversification in the Antarctic Red Alga Plocamium sp.
Abstract
1. Introduction
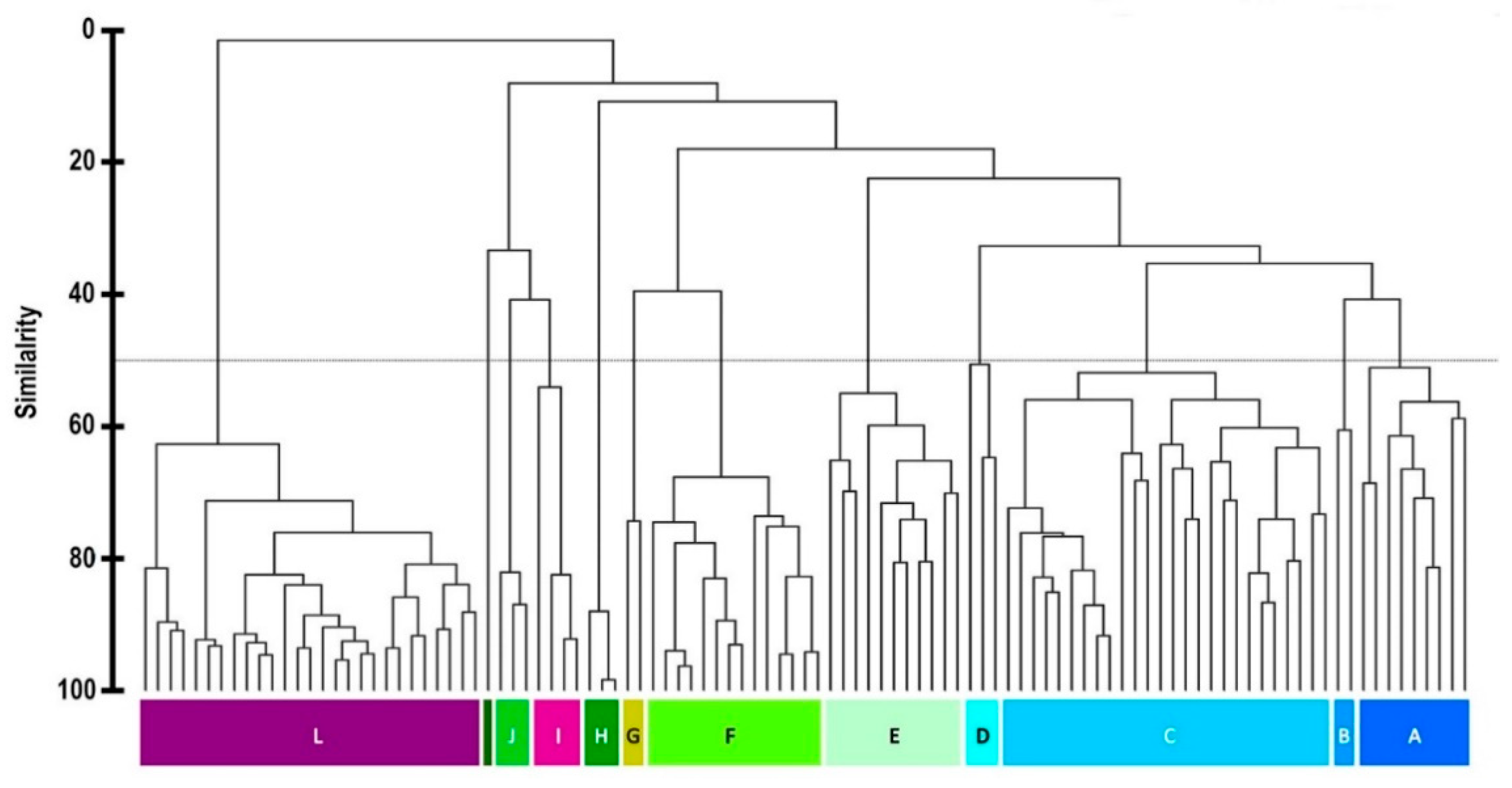
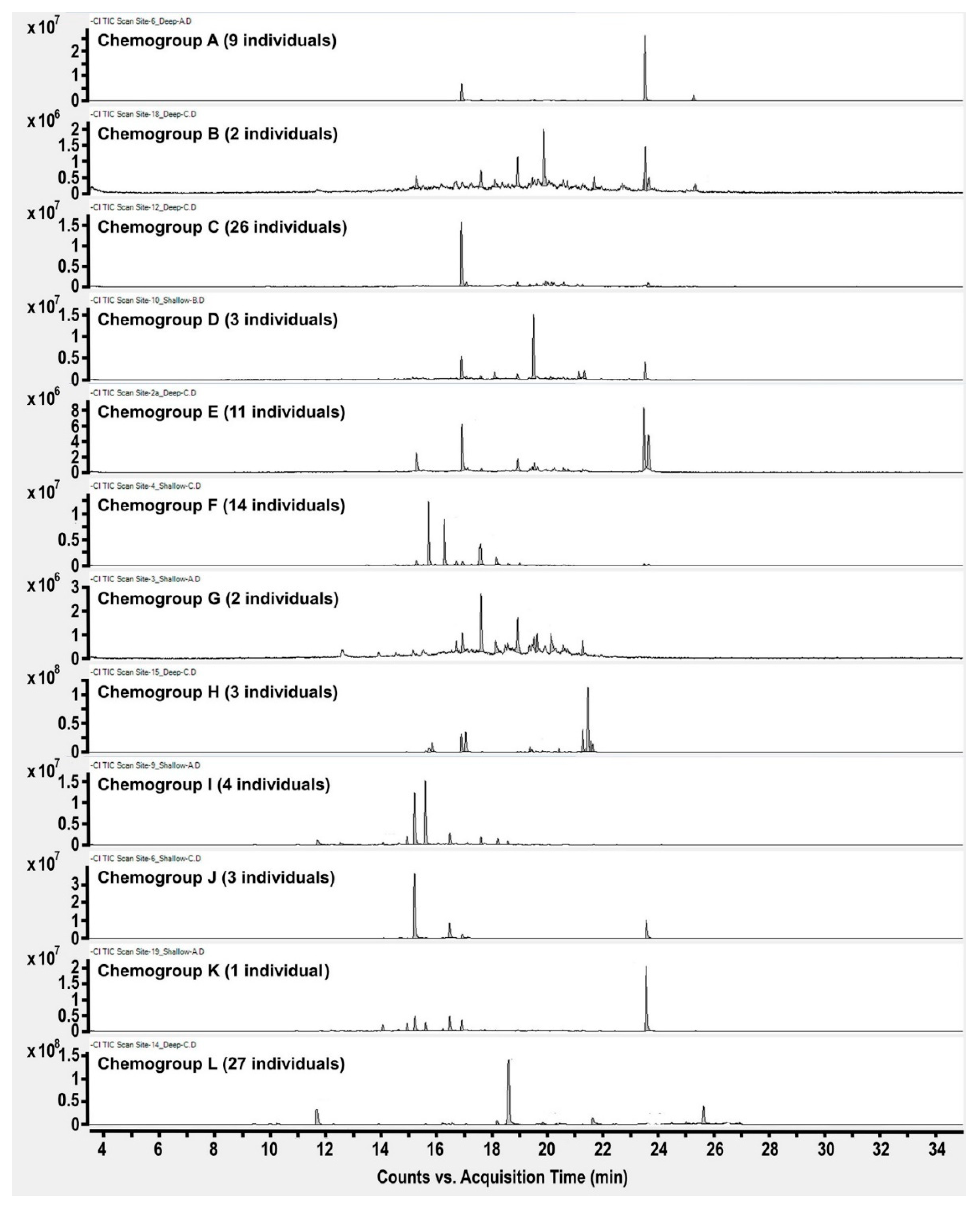
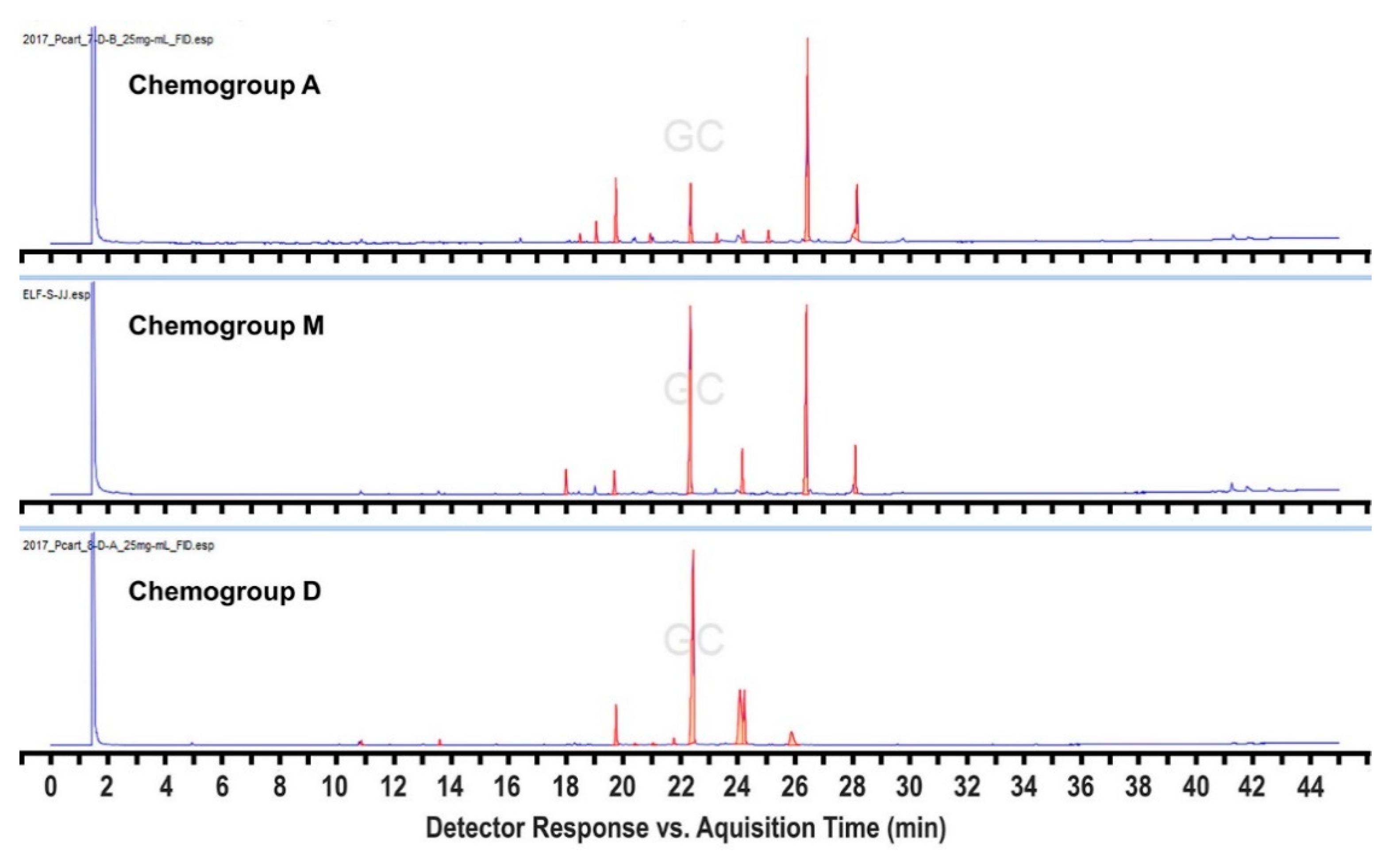
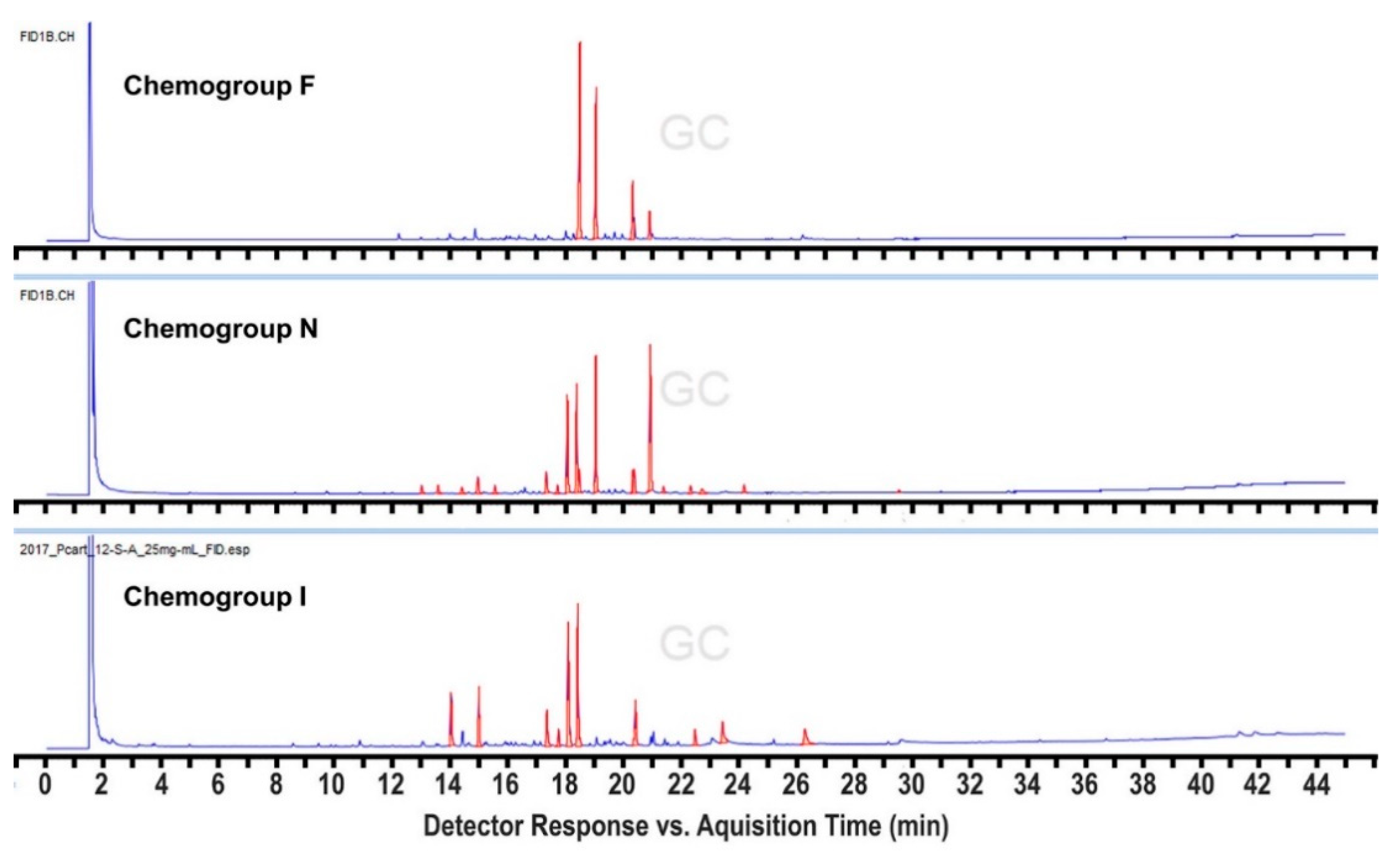
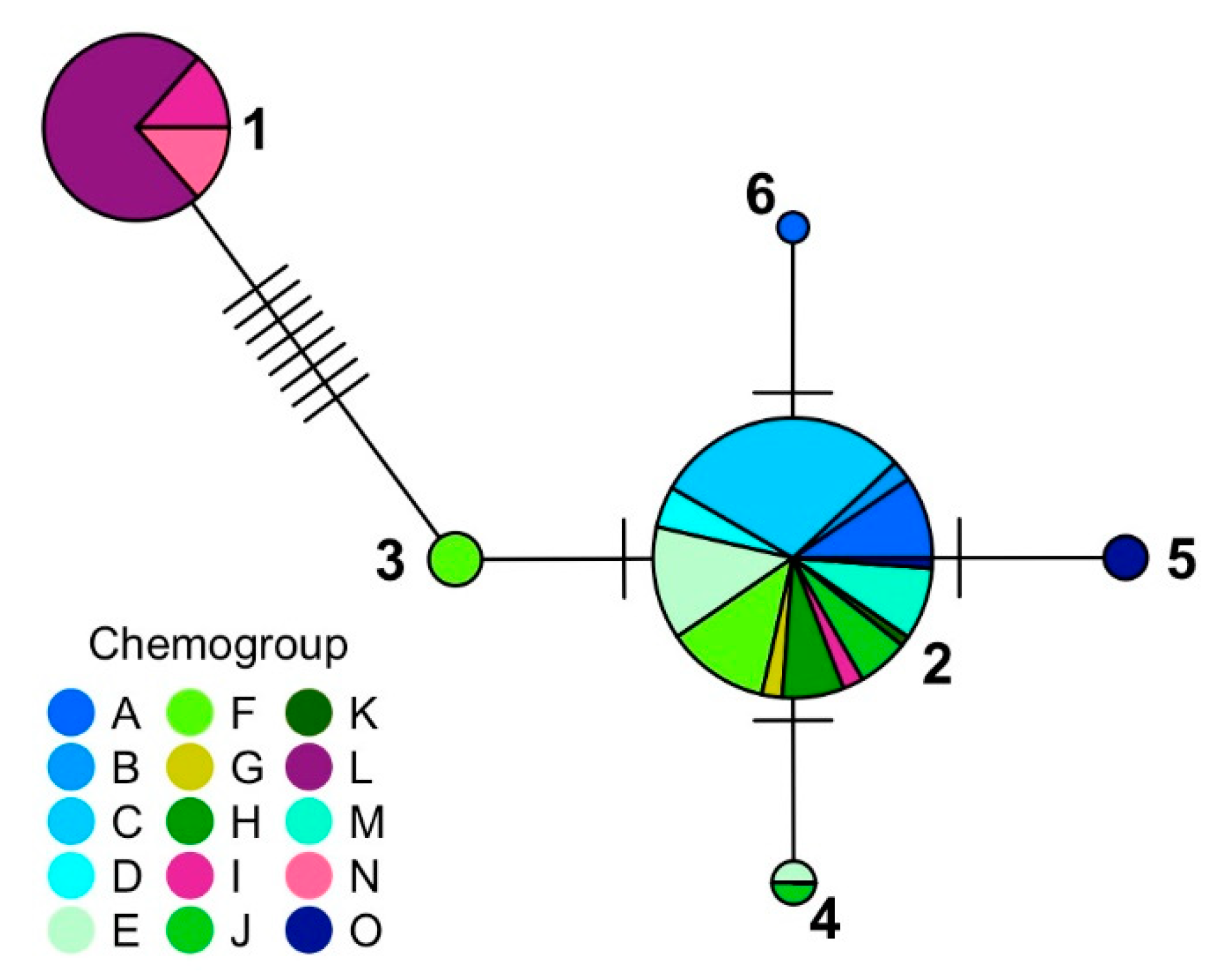
2. Results and Discussion
3. Materials and Methods
3.1. Biological Material
3.2. General Procedures
3.3. Metabolomic Analysis of Plocamium sp.
3.4. Statistical Analysis of Plocamium sp. Metabolomics
3.5. Determination of Haplotypes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McClintock, J.B.; Baker, B.J. Marine Chemical Ecology; CRC Press: Boca Raton, FL, USA, 2001; p. 610. [Google Scholar]
- Kladi, M.; Vagias, C.; Roussis, V. Volatile halogenated metabolites from marine red algae. Phytochem. Rev. 2004, 3, 337–366. [Google Scholar] [CrossRef]
- Crews, P. Monoterpene halogenation by the red alga Plocamium oregonum. J. Org. Chem. 1977, 42, 2634–2636. [Google Scholar] [CrossRef]
- Norton, R.S.; Warren, R.G.; Wells, R.J. Three new polyhalogenated monoterpenes from Plocamium species. Tetrahedron Lett. 1977, 18, 3905–3908. [Google Scholar] [CrossRef]
- Castedo, L.; García, M.L.; Quiñoá, E.; Riguera, R. Halogenated monoterpenes from Plocamium coccineum of northwest Spain. J. Nat. Prod. 1984, 47, 724–726. [Google Scholar] [CrossRef]
- Kazlauskas, R.; Murphy, P.T.; Quinn, R.J.; Wells, R.J.; Schönhlzer, P.; F-Konzern, J.S.; Roche, F.H.-L. Two polyhalogenated monoterpenes from the red alga Plocamium costatum. Tetrahedron Lett. 1976, 17, 4451–4454. [Google Scholar] [CrossRef]
- Saunders, G.W.; Lehmkuhl, K.V. Molecular divergence and morphological diversity among four cryptic species of Plocamium (Plocamiales, Florideophyceae) in northern Europe. Eur. J. Phycol. 2005, 40, 293–312. [Google Scholar] [CrossRef]
- Cremades, J.; Barreiro, R.; Maneiro, I.; Saunders, G.W. A new taxonomic interpretation of the type of Plocamium cartilagineum (Plocamiales, Florideophyceae) and its consequences. Eur. J. Phycol. 2011, 46, 125–142. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M. AlgaeBase. World-Wide Electronic Publication. 2021. Available online: http://www.algaebase.org (accessed on 12 April 2021).
- Crews, P.; Kho, E. Cartilagineal. Unusual monoterpene aldehyde from marine alga. J. Org. Chem. 1974, 39, 3303–3304. [Google Scholar] [CrossRef]
- Cueto, M.; Darias, J.; Rovirosa, J.; San Martin, A. Tetrahydropyran monoterpenes from Plocamium cartilagineum and Pantoneura plocamioides. J. Nat. Prod. 1998, 61, 1466–1468. [Google Scholar] [CrossRef] [PubMed]
- Darias, J.; Rovirosa, J.; San Martin, A.; Díaz, A.-R.; Dorta, E.; Cueto, M. Furoplocamioids A−C, novel polyhalogenated furanoid monoterpenes from Plocamium cartilagineum. J. Nat. Prod. 2001, 64, 1383–1387. [Google Scholar] [CrossRef]
- Wiencke, C.; Amsler, C.D.; Clayton, M.N. Macroalgae. In Biogeographic Atlas of the Southern Ocean; Scientific Committee on Antarctic Research: Cambridge, UK, 2014; pp. 66–73. [Google Scholar]
- Heiser, S.; Amsler, C.D.; McClintock, J.B.; Shilling, A.J.; Baker, B.J. Every rule has an exception: A cheater in the community-wide mutualism in Antarctic seaweed forests. Integr. Comp. Biol. 2020, 60, 1358–1368. [Google Scholar] [CrossRef]
- Neushul, M. Diving observation of sub-tidal Antarctic marine vegetation. Bot. Mar. 1965, 8, 234–243. [Google Scholar] [CrossRef]
- DeLaca, T.E.; Lipps, J.H. Shallow water marine associations, Antarctic Peninsula. Antarct. J. USA 1976, 11, 12–20. [Google Scholar]
- Klöser, H.; Quartino, M.L.; Wiencke, C. Distribution of macroalgae and macroalgal communities in gradients of physical conditions in Potter Cove, King George Island, Antarctica. Hydrobiologia 1996, 333, 1–17. [Google Scholar] [CrossRef]
- Amsler, C.D.; Rowley, R.J.; Laur, D.R.; Quetin, L.B.; Ross, R.M. Vertical distribution of Antarctic Peninsular macroalgae: Cover, biomass, and species composition. Phycologia 1995, 34, 424–430. [Google Scholar] [CrossRef]
- Quartino, M.L.; Boraso de Zaixso, A.L. Summer macroalgal biomass in Potter Cove, South Shetland Islands, Antarctica: Its production and flux to the ecosystem. Polar Biol. 2008, 31, 281–294. [Google Scholar] [CrossRef]
- Wiencke, C.; Amsler, C.D. Seaweeds and their communities in polar regions. In Seaweed Biology: Novel Insights into Ecophysiology, Ecology and Utilization; Wiencke, C., Bischof, K., Eds.; Springer: Berlin, Germany, 2012; pp. 265–294. [Google Scholar]
- Hommersand, M.H.; Moe, R.L.; Amsler, C.D.; Fredericq, S. Notes on the systematics and biogeographical relationships of Antarctic and Sub-Antarctic Rhodophyta with descriptions of four new genera and five new species. Bot. Mar. 2009, 52, 509–534. [Google Scholar] [CrossRef]
- Dubrasquet, H.; Reyes, J.; Sanchez, R.P.; Valdivia, N.; Guillemin, M.-L. Molecular-assisted revision of red macroalgal diversity and distribution along the Western Antarctic Peninsula and South Shetland Islands. Cryptogam. Algol. 2018, 39, 409–429. [Google Scholar] [CrossRef]
- Young, R.M.; von Salm, J.L.; Amsler, M.O.; Lopez-Bautista, J.; Amsler, C.D.; McClintock, J.B.; Baker, B.J. Site-specific variability in the chemical diversity of the Antarctic red alga Plocamium cartilagineum. Mar. Drugs 2013, 11, 2126–2139. [Google Scholar] [CrossRef] [PubMed]
- Guillemin, M.-L.; Dubrasquet, H.; Reyes, J.; Valero, M. Comparative phylogeography of six red algae along the Antarctic Peninsula: Extreme genetic depletion linked to historical bottlenecks and recent expansion. Polar Biol. 2018, 41, 827–837. [Google Scholar] [CrossRef]
- Ankisetty, S.; Nandiraju, S.; Win, H.; Park, Y.C.; Amsler, C.D.; McClintock, J.B.; Baker, J.A.; Diyabalanage, T.K.; Pasaribu, A.; Singh, M.P.; et al. Chemical investigation of predator-deterred macroalgae from the Antarctic Peninsula. J. Nat. Prod. 2004, 67, 1295–1302. [Google Scholar] [CrossRef] [PubMed]
- Stierle, D.B.; Sims, J.J. Marine natural products—XV: Polyhalogenated cyclic monoterpenes from the red alga Plocamium cartilagineum of Antarctica. Tetrahedron 1979, 35, 1261–1265. [Google Scholar] [CrossRef]
- Stierle, D.B.; Wing, R.M.; Sims, J.J. Marine natural products—XVI: Polyhalogenated acyclic monoterpenes from the red alga Plocamium of Antarctica. Tetrahedron 1979, 35, 2855–2859. [Google Scholar] [CrossRef]
- Rovirosa, J.; Soler, A.; Blanc, V.; Leon, R.; San-Martin, A. Bioactive monoterpenes from Antarctic Plocamium cartilagineum. J. Chil. Chem. Soc. 2013, 58, 225–226. [Google Scholar] [CrossRef][Green Version]
- Shilling, A.J.; von Salm, J.L.; Sanchez, A.R.; Kee, Y.; Amsler, C.D.; McClintock, J.B.; Baker, B.J. Anverenes B–E, new polyhalogenated monoterpenes from the Antarctic red alga Plocamium cartilagineum. Mar. Drugs 2019, 17, 230. [Google Scholar] [CrossRef]
- Amsler, C.D.; Iken, K.; McClintock, J.B.; Amsler, M.O.; Peters, K.J.; Hubbard, J.M.; Furrow, F.B.; Baker, B.J. Comprehensive evaluation of the palatability and chemical defenses of subtidal macroalgae from the Antarctic Peninsula. Mar. Ecol. Prog. Ser. 2005, 294, 141–159. [Google Scholar] [CrossRef]
- Knott, M.G.; Mkwananzi, H.; Arendse, C.E.; Hendricks, D.T.; Bolton, J.J.; Beukes, D.R. Plocoralides A–C, polyhalogenated monoterpenes from the marine alga Plocamium corallorhiza. Phytochemistry 2005, 66, 1108–1112. [Google Scholar] [CrossRef]
- Von Salm, J.L.; Schoenrock, K.M.; McClintock, J.B.; Amsler, C.D.; Baker, B.J. The status of marine chemical ecology in Antarctica: Form and function of unique high-latitude chemistry. In Chemical Ecology: The Ecological Impacts of Marine Natural Products; Puglisi-Weening, M.P., Becerro, M.A., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 27–69. [Google Scholar]
- Amsler, C.D.; McClintock, J.B.; Baker, B.J. Chemical mediation of Antarctic macroalga-grazer interactions. In Antarctic Seaweeds: Diversity, Adaptation and Ecosystem Services; Gómez, I., Huovinen, P., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 339–363. [Google Scholar]
- Fenical, W. Halogenation in the Rhodophyta: A review. J. Phycol. 1975, 11, 245–259. [Google Scholar]
- Mynderse, J.S.; Faulkner, D.J. Variations in the halogenated monoterpene metabolites of Plocamium cartilagineum and P. violaceum. Phytochemistry 1978, 17, 237–240. [Google Scholar] [CrossRef]
- San-Martín, A.; Rovirosa, J. Variations in the halogenated monoterpene metabolites of Plocamium cartilagineum of the Chilean coast. Biochem. Syst. Ecol. 1986, 14, 459–461. [Google Scholar] [CrossRef]
- Rovirosa, J.; Moena, J.; San-Martín, A. Two chemical types of the red alga Plocamium cartilagineum from Chile. Biochem. Syst. Ecol. 1988, 16, 593–595. [Google Scholar] [CrossRef]
- Blunt, J.; Bowman, N.; Munro, H.; Parsons, M.; Wright, G.; Kon, Y.K. Polyhalogenated monoterpenes of the New Zealand marine red alga Plocamium cartilagineum. Aust. J. Chem. 1985, 38, 519–525. [Google Scholar] [CrossRef]
- Yang, M.Y.; Kim, M.S. Cryptic species diversity of ochtodenes-producing Portieria species (Gigartinales, Rhodophyta) from the northwest Pacific. Algae 2018, 33, 205–214. [Google Scholar] [CrossRef]
- Payo, D.A.; Colo, J.; Calumpong, H.; de Clerck, O. Variability of non-polar secondary metabolites in the red alga Portieria. Mar. Drugs 2011, 9, 2438–2468. [Google Scholar] [CrossRef]
- Aumack, C.F.; Amsler, C.D.; McClintock, J.B.; Baker, B.J. Chemically mediated resistance to mesoherbivory in finely branched macroalgae along the western Antarctic Peninsula. Eur. J. Phycol. 2010, 45, 19–26. [Google Scholar] [CrossRef]
- Amsler, M.O.; Amsler, C.D.; von Salm, J.L.; Aumack, C.F.; McClintock, J.B.; Young, R.M.; Baker, B.J. Tolerance and sequestration of macroalgal chemical defenses by an Antarctic amphipod: A ‘cheater’ among mutualists. Mar. Ecol. Prog. Ser. 2013, 490, 79–90. [Google Scholar] [CrossRef]
- Becerra, J.X. The impact of herbivore–plant coevolution on plant community structure. Proc. Natl. Acad. Sci. USA 2007, 104, 7483–7488. [Google Scholar] [CrossRef]
- Kursar, T.A.; Dexter, K.G.; Lokvam, J.; Pennington, R.T.; Richardson, J.E.; Weber, M.G.; Murakami, E.T.; Drake, C.; McGregor, R.; Coley, P.D. The evolution of antiherbivore defenses and their contribution to species coexistence in the tropical tree genus Inga. Proc. Natl. Acad. Sci. USA 2009, 106, 18073–18078. [Google Scholar] [CrossRef]
- Sedio, B.E.; Archibold, D.A.; Echeverri, J.C.R.; Debyser, C.; Boya P, C.A.; Wright, S.J. A comparison of inducible, ontogenetic, and interspecific sources of variation in the foliar metabolome in tropical trees. PeerJ 2019, 7, e7536. [Google Scholar] [CrossRef]
- Ehrlich, P.R.; Raven, P.H. Butterflies and plants: A study in coevolution. Evolution 1964, 18, 586–608. [Google Scholar] [CrossRef]
- Vermeij, G.J. The evolutionary interaction among species: Selection, escalation, and coevolution. Ann. Rev. Ecol. Syst. 1994, 25, 219–236. [Google Scholar] [CrossRef]
- Agrawal, A.A. Macroevolution of plant defense strategies. Trends Ecol. Evol. 2007, 22, 103–109. [Google Scholar] [CrossRef]
- Futuyma, D.J.; Agrawal, A.A. Macroevolution and the biological diversity of plants and herbivores. Proc. Natl. Acad. Sci. USA 2009, 106, 18054–18061. [Google Scholar] [CrossRef]
- Saunders, G.W. Applying DNA barcoding to red macroalgae: A preliminary appraisal holds promise for future applications. Philos. Trans. R. Soc. B Biol. Sci. 2005, 360, 1879–1888. [Google Scholar] [CrossRef]
- Saunders, G.W.; McDevit, D.C. Methods for DNA barcoding photosynthetic protists emphasizing the macroalgae and diatoms. In DNA Barcodes: Methods and Protocol; Kress, W.J., Erickson, D.L., Eds.; Springer: New York, NY, USA, 2012; pp. 207–222. [Google Scholar]
- Bandelt, H.J.; Forster, P.; Röhl, A. Median-joining networks for inferring intraspecific phylogenies. Mol. Biol. Evol. 1999, 16, 37–48. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017; Available online: http://www.R-project.org/ (accessed on 12 April 2021).
- Jombart, T. adegenet: A R package for the multivariate analysis of genetic markers. Bioinformatics 2008, 24, 1403–1405. [Google Scholar] [CrossRef] [PubMed]
- Paradis, E.; Schliep, K. ape 5.0: An environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 2019, 35, 526–528. [Google Scholar] [CrossRef]
- Paradis, E. Pegas: An R package for population genetics with an integrated–modular approach. Bioinformatics 2010, 26, 419–420. [Google Scholar] [CrossRef]
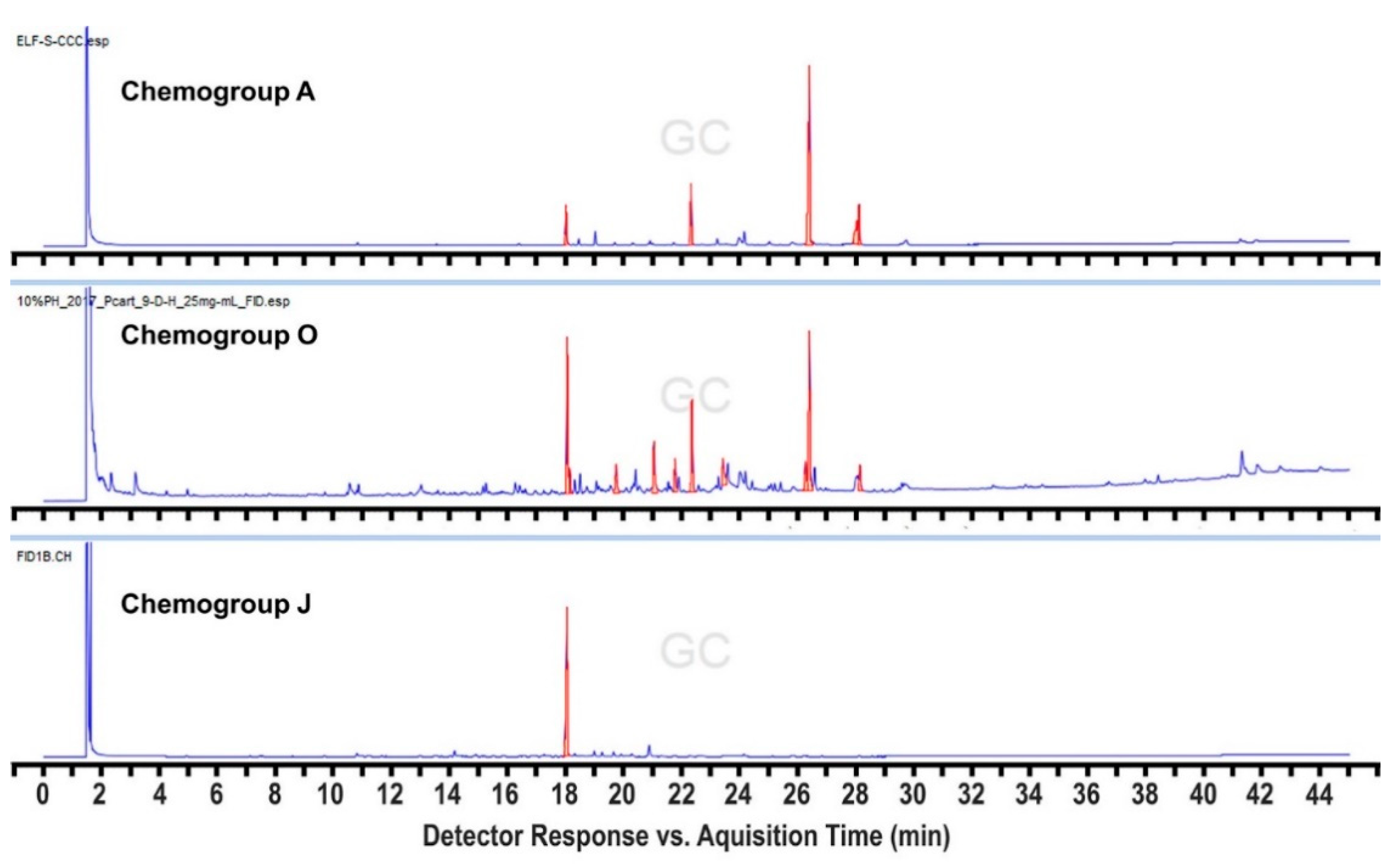
| ID | Structure | Characteristic (Major) Metabolite of Chemogroups | Minor Metabolite in Chemogroups (>5% AUC) | Trace Metabolite in Chemogroups (<5% AUC) |
|---|---|---|---|---|
| 1 | 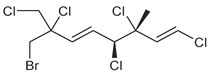 | A, M *, O * | D, L | none |
| 2 | 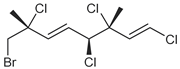 | D, M * | A, G, O | E, N |
| 3 |  | none | D, M | A, N, O |
| oregonene A | 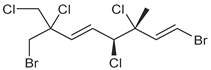 | none | A, M | D |
| anverene A | 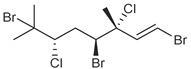 | H | none | none |
| anverene B | 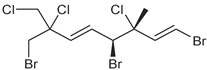 | none | none | A, D, M |
| anverene C | 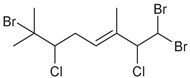 | none | B, C, E | F |
| anverene D | 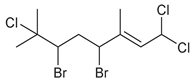 | none | none | B, E, G |
| anverene E | 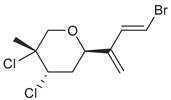 | L | none | none |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shilling, A.J.; Heiser, S.; Amsler, C.D.; McClintock, J.B.; Baker, B.J. Hidden Diversity in an Antarctic Algal Forest: Metabolomic Profiling Linked to Patterns of Genetic Diversification in the Antarctic Red Alga Plocamium sp. Mar. Drugs 2021, 19, 607. https://doi.org/10.3390/md19110607
Shilling AJ, Heiser S, Amsler CD, McClintock JB, Baker BJ. Hidden Diversity in an Antarctic Algal Forest: Metabolomic Profiling Linked to Patterns of Genetic Diversification in the Antarctic Red Alga Plocamium sp. Marine Drugs. 2021; 19(11):607. https://doi.org/10.3390/md19110607
Chicago/Turabian StyleShilling, Andrew J., Sabrina Heiser, Charles D. Amsler, James B. McClintock, and Bill J. Baker. 2021. "Hidden Diversity in an Antarctic Algal Forest: Metabolomic Profiling Linked to Patterns of Genetic Diversification in the Antarctic Red Alga Plocamium sp." Marine Drugs 19, no. 11: 607. https://doi.org/10.3390/md19110607
APA StyleShilling, A. J., Heiser, S., Amsler, C. D., McClintock, J. B., & Baker, B. J. (2021). Hidden Diversity in an Antarctic Algal Forest: Metabolomic Profiling Linked to Patterns of Genetic Diversification in the Antarctic Red Alga Plocamium sp. Marine Drugs, 19(11), 607. https://doi.org/10.3390/md19110607







