Marine Sulfated Polysaccharides: Preventive and Therapeutic Effects on Metabolic Syndrome: A Review
Abstract
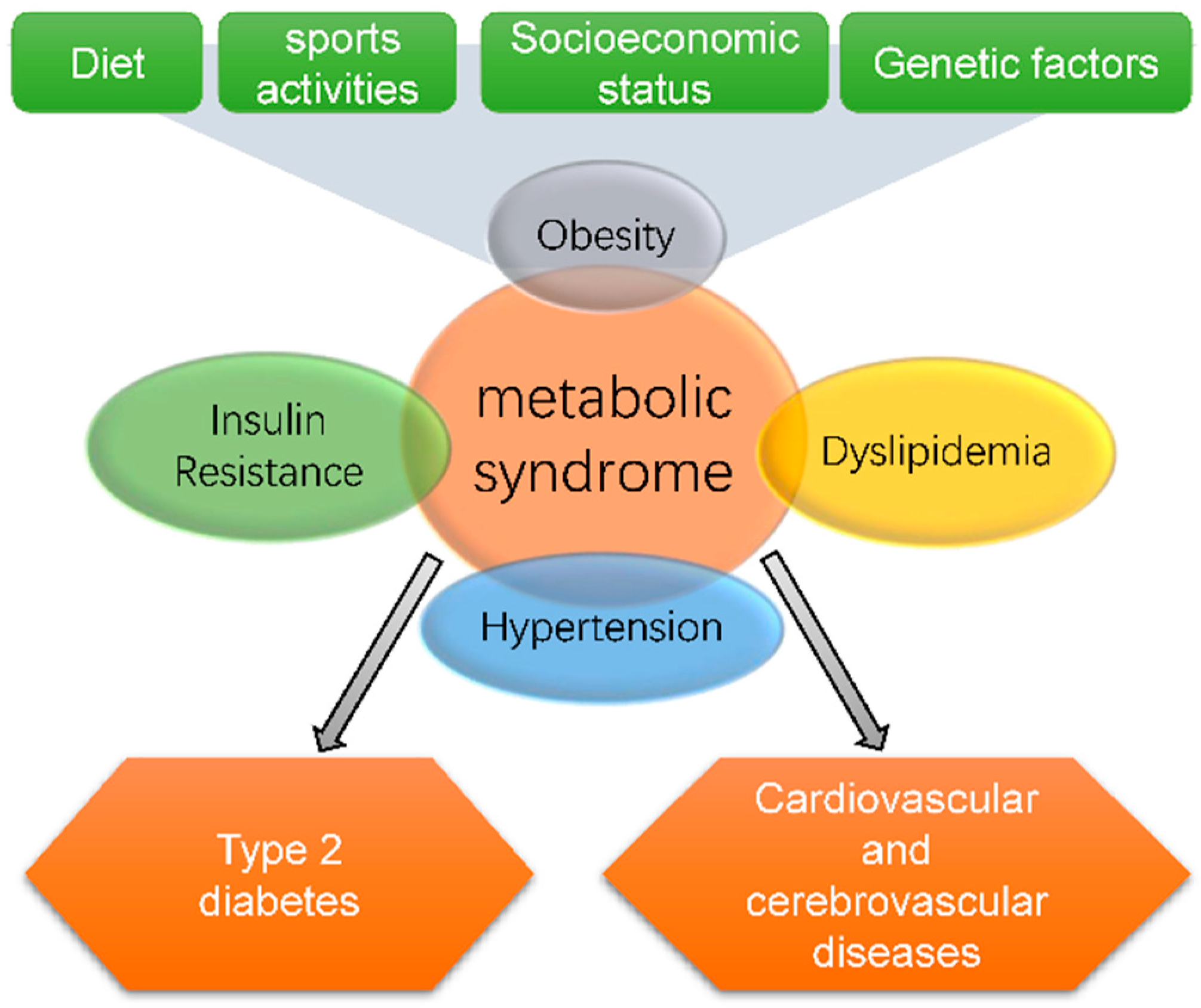
:1. Introduction
2. Effects of MSPs on Metabolic Syndrome
2.1. Obesity
2.2. Insulin Resistance
2.3. Dyslipidemia
2.4. Hypertension
3. Mechanism of MSPs in Treatment/Prevention of Metabolic Syndrome
3.1. Regulating Glucose Metabolism
3.2. Regulating Lipid Metabolism
4. Regulation of MSPs on Gut Microbiota
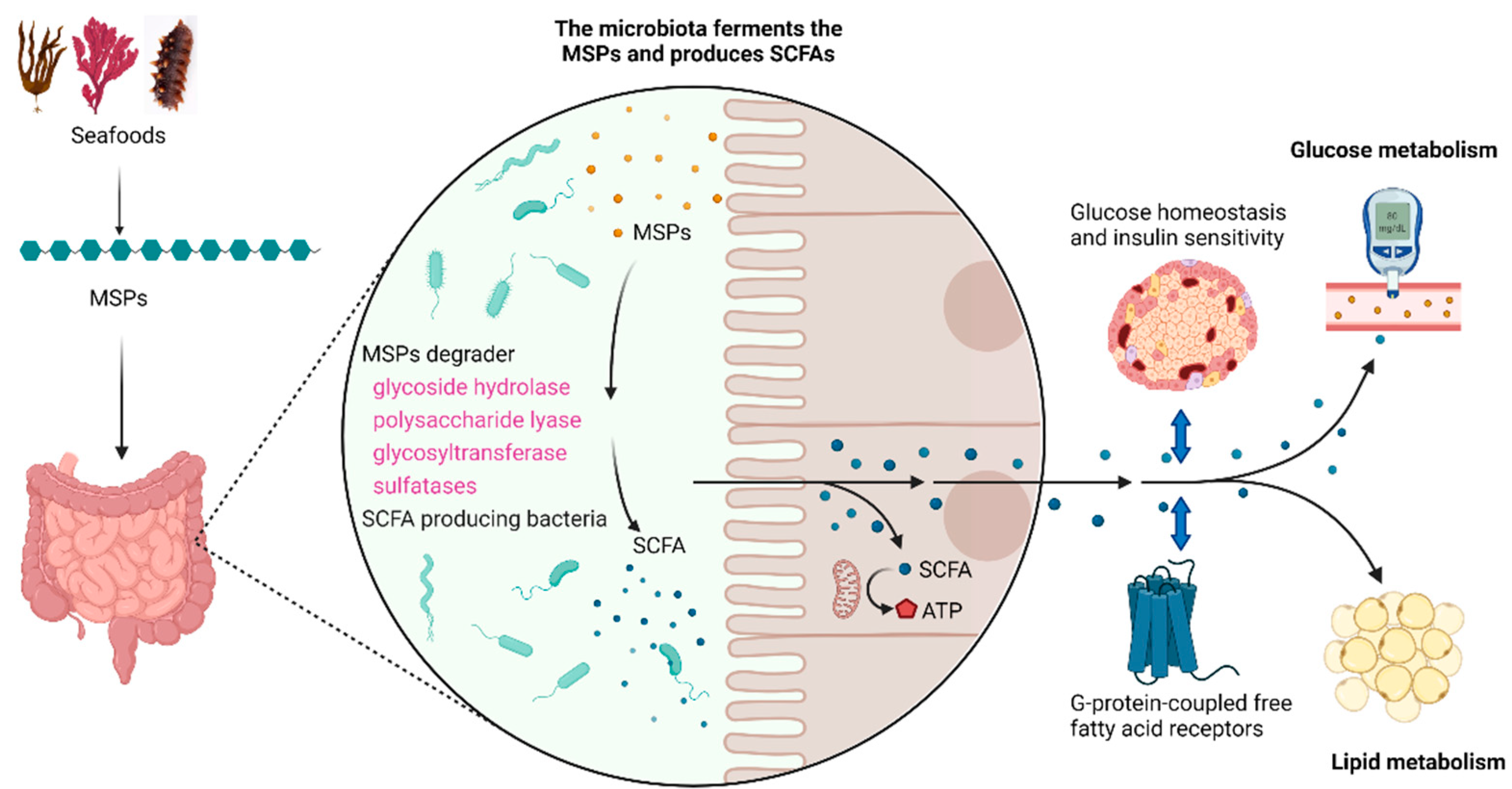
5. Utilization of MSPs by Gut Microbiota
6. Role of Sulfate Group and Sulphation Pattern
7. Therapeutic Methods and Dietary Intervention
8. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Abubakar, I.I.; Tillmann, T.; Banerjee, A. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 385, 117–171. [Google Scholar]
- Huang, P.L. A comprehensive definition for metabolic syndrome. Dis. Model. Mech. 2009, 2, 231–237. [Google Scholar] [CrossRef] [Green Version]
- Castro-Barquero, S.; Ruiz-León, A.M.; Sierra-Pérez, M.; Estruch, R.; Casas, R. Dietary strategies for metabolic syndrome: A comprehensive review. Nutrients 2020, 12, 2983. [Google Scholar] [CrossRef]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luthuli, S.; Wu, S.; Cheng, Y.; Zheng, X.; Wu, M.; Tong, H. Therapeutic effects of fucoidan: A review on recent studies. Mar. Drugs 2019, 17, 487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, P.; Zheng, W.; Sun, X.; Jiang, G.; Wu, S.; Xu, Y.; Song, S.; Ai, C. Sulfated polysaccharides from Undaria pinnatifida improved high fat diet-induced metabolic syndrome, gut microbiota dysbiosis and inflammation in BALB/c mice. Int. J. Biol. Macromol. 2021, 167, 1587–1597. [Google Scholar] [CrossRef] [PubMed]
- Muthukumar, J.; Chidambaram, R.; Sukumaran, S. Sulfated polysaccharides and its commercial applications in food industries-A review. J. Food Sci. Technol. 2021, 58, 2453–2466. [Google Scholar] [CrossRef] [PubMed]
- Pomin, V.H. Structural and functional insights into sulfated galactans: A systematic review. Glycoconj. J. 2010, 27, 1–12. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Park, S.; Sathiyaseelan, A.; Mariadoss, A.V.A.; Park, S.; Kim, S.J.; Wang, M.H. Isolation of Polysaccharides from Trichoderma harzianum with Antioxidant, Anticancer, and Enzyme Inhibition Properties. Antioxidants 2021, 10, 1372. [Google Scholar] [CrossRef]
- Gao, N.; Chen, R.; Mou, R.; Xiang, J.; Zhou, K.; Li, Z.; Zhao, J. Purification, structural characterization and anticoagulant activities of four sulfated polysaccharides from sea cucumber Holothuria fuscopunctata. Int. J. Biol. Macromol. 2020, 164, 3421–3428. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Wu, H.; Liu, R. Overview on biological activities and molecular characteristics of sulfated polysaccharides from marine green algae in recent years. Mar. Drugs 2014, 12, 4984–5020. [Google Scholar] [CrossRef]
- Wu, N.; Zhang, Y.; Ye, X.; Hu, Y.; Ding, T.; Chen, S. Sulfation pattern of fucose branches affects the anti-hyperlipidemic activities of fucosylated chondroitin sulfate. Carbohydr. Polym. 2016, 147, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Ai, C.; Duan, M.; Ma, N.; Sun, X.; Yang, J.; Wen, C.; Sun, Y.; Zhao, N.; Song, S. Sulfated polysaccharides from pacific abalone reduce diet-induced obesity by modulating the gut microbiota. J. Funct. Foods 2018, 47, 211–219. [Google Scholar] [CrossRef]
- Li, Y.; Qin, J.; Cheng, Y.; Ai, Y.; Han, Z.; Li, M.; Qi, Y.; Zhao, Q.; Li, Z. Polysaccharide from Patinopecten yessoensis Skirt Boosts Immune Response via Modulation of Gut Microbiota and Short-Chain Fatty Acids Metabolism in Mice. Foods 2021, 10, 2478. [Google Scholar] [CrossRef]
- Zhu, Z.; Han, Y.; Ding, Y.; Zhu, B.; Song, S.; Xiao, H. Health effects of dietary sulfated polysaccharides from seafoods and their interaction with gut microbiota. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2882–2913. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Jiang, H.; Cai, C.; Li, G.; Hao, J.; Yu, G. Marine polysaccharides attenuate metabolic syndrome by fermentation products and altering gut microbiota: An overview. Carbohydr. Polym. 2018, 195, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef] [Green Version]
- Schluter, J.; Peled, J.U.; Taylor, B.P.; Markey, K.A.; Smith, M.; Taur, Y.; Niehus, R.; Staffas, A.; Dai, A.; Fontana, E.; et al. The gut microbiota is associated with immune cell dynamics in humans. Nature 2020, 588, 303–307. [Google Scholar] [CrossRef]
- Adak, A.; Khan, M.R. An insight into gut microbiota and its functionalities. Cell. Mol. Life Sci. 2019, 76, 473–493. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Ben Abdallah Kolsi, R.; Ben Gara, A.; Chaaben, R.; El Feki, A.; Patti, F.P.; El Feki, L.; Belghith, K. Anti-obesity and lipid lowering effects of Cymodocea nodosa sulphated polysaccharide on high cholesterol-fed-rats. Arch. Physiol. Biochem. 2015, 121, 210–217. [Google Scholar] [CrossRef]
- Chen, L.; Xu, W.; Chen, D.; Chen, G.; Liu, J.; Zeng, X.; Shao, R.; Zhu, H. Digestibility of sulfated polysaccharide from the brown seaweed Ascophyllum nodosum and its effect on the human gut microbiota in vitro. Int. J. Biol. Macromol. 2018, 112, 1055–1061. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, N.; Sun, X.; Duan, M.; Luo, T.; Jiang, P.; Jiang, G.; Song, S.; Ai, C. Effect of intake pattern of sulfated polysaccharides on its biological activity in high fat diet-fed mice. Int. J. Biol. Macromol. 2019, 132, 9–16. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhu, B.; Sun, Y.; Ai, C.; Wang, L.; Wen, C.; Yang, J.; Song, S.; Liu, X. Sulfated Polysaccharide from Sea Cucumber and its Depolymerized Derivative Prevent Obesity in Association with Modification of Gut Microbiota in High-Fat Diet-Fed Mice. Mol. Nutr. Food Res. 2018, 62, e1800446. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, J.; Yan, L.; Cheng, Y.; Li, Q.; Wu, S.; Chen, L.; Thring, R.W.; Yang, Y.; Gao, Y.; et al. Sargassum fusiforme Fucoidan Alleviates High-Fat Diet-Induced Obesity and Insulin Resistance Associated with the Improvement of Hepatic Oxidative Stress and Gut Microbiota Profile. J. Agric. Food Chem. 2020, 68, 10626–10638. [Google Scholar] [CrossRef]
- Wright, C.M.; Bezabhe, W.; Fitton, J.H.; Stringer, D.N.; Bereznicki, L.R.E.; Peterson, G.M. Effect of a fucoidan extract on insulin resistance and cardiometabolic markers in obese, nondiabetic subjects: A randomized, controlled trial. J. Altern. Complement. Med. 2019, 25, 346–352. [Google Scholar] [CrossRef]
- Zhu, Q.; Lin, L.; Zhao, M. Sulfated fucan/fucosylated chondroitin sulfate-dominated polysaccharide fraction from low-edible-value sea cucumber ameliorates type 2 diabetes in rats: New prospects for sea cucumber polysaccharide based-hypoglycemic functional food. Int. J. Biol. Macromol. 2020, 159, 34–45. [Google Scholar] [CrossRef]
- Yokota, T.; Nomura, K.; Nagashima, M.; Kamimura, N. Fucoidan alleviates high-fat diet-induced dyslipidemia and atherosclerosis in ApoE(shl) mice deficient in apolipoprotein E expression. J. Nutr. Biochem. 2016, 32, 46–54. [Google Scholar] [CrossRef]
- Kolsi, R.B.A.; Salah, H.B.; Jardak, N.; Chaaben, R.; Jribi, I.; Feki, A.E.; Rebai, T.; Jamoussi, K.; Allouche, N.; Blecker, C.; et al. Sulphated polysaccharide isolated from Sargassum vulgare: Characterization and hypolipidemic effects. Carbohydr. Polym. 2017, 170, 148–159. [Google Scholar] [CrossRef]
- Li, S.; Li, J.; Zhi, Z.; Hu, Y.; Ge, J.; Ye, X.; Tian, D.; Linhardt, R.J.; Chen, S. 4-O-Sulfation in sea cucumber fucodians contribute to reversing dyslipidiaemia caused by HFD. Int. J. Biol. Macromol. 2017, 99, 96–104. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Jiang, N.; Li, B.; Wan, M.; Chang, X.; Liu, H.; Zhang, L.; Yin, S.; Qi, H.; Liu, S. Antioxidant activity of purified ulvan in hyperlipidemic mice. Int. J. Biol. Macromol. 2018, 113, 971–975. [Google Scholar] [CrossRef]
- Cui, W.; Zheng, Y.; Zhang, Q.; Wang, J.; Wang, L.; Yang, W.; Guo, C.; Gao, W.; Wang, X.; Luo, D. Low-molecular-weight fucoidan protects endothelial function and ameliorates basal hypertension in diabetic Goto-Kakizaki rats. Lab. Investig. 2014, 94, 382–393. [Google Scholar] [CrossRef]
- Liang, Z.; Zheng, Y.; Wang, J.; Zhang, Q.; Ren, S.; Liu, T.; Wang, Z.; Luo, D. Low molecular weight fucoidan ameliorates streptozotocin-induced hyper-responsiveness of aortic smooth muscles in type 1 diabetes rats. J. Ethnopharmacol. 2016, 191, 341–349. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Li, Z.; Sang, Y.; Niu, Y.; Zhang, Q.; Ding, H.; Yin, S. Fucoidan from Undaria pinnatifida prevents vascular dysfunction through PI3K/Akt/eNOS-dependent mechanisms in the l-NAME-induced hypertensive rat model. Food Funct. 2016, 7, 2398–2408. [Google Scholar] [CrossRef] [PubMed]
- du Preez, R.; Paul, N.; Mouatt, P.; Majzoub, M.E.; Thomas, T.; Panchal, S.K.; Brown, L. Carrageenans from the red seaweed Sarconema filiforme attenuate symptoms of diet-induced metabolic syndrome in rats. Mar. Drugs 2020, 18, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, B.N.; Liu, X.T.; Liang, Z.H.; Wang, J.H. Gut microbiota in obesity. World J. Gastroenterol. 2021, 27, 3837–3850. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Farrokhi, F.R.; Butler, A.E.; Sahebkar, A. Insulin resistance: Review of the underlying molecular mechanisms. J. Cell. Physiol. 2019, 234, 8152–8161. [Google Scholar] [CrossRef]
- Hill, M.A.; Yang, Y.; Zhang, L.; Sun, Z.; Jia, G.; Parrish, A.R.; Sowers, J.R. Insulin resistance, cardiovascular stiffening and cardiovascular disease. Metabolism 2021, 119, 154766. [Google Scholar] [CrossRef]
- Heeba, G.H.; Morsy, M.A. Fucoidan ameliorates steatohepatitis and insulin resistance by suppressing oxidative stress and inflammatory cytokines in experimental non-alcoholic fatty liver disease. Environ. Toxicol. Pharmacol. 2015, 40, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hu, S.; Jiang, W.; Song, W.; Cai, L.; Wang, J. Fucoidan from sea cucumber may improve hepatic inflammatory response and insulin resistance in mice. Int. Immunopharmacol. 2016, 31, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Kopin, L.; Lowenstein, C. Dyslipidemia. Ann. Intern. Med. 2017, 167, Itc81–itc96. [Google Scholar] [CrossRef] [PubMed]
- Shan, X.; Liu, X.; Hao, J.; Cai, C.; Fan, F.; Dun, Y.; Zhao, X.; Liu, X.; Li, C.; Yu, G. In vitro and in vivo hypoglycemic effects of brown algal fucoidans. Int. J. Biol. Macromol. 2016, 82, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Pozharitskaya, O.N.; Obluchinskaya, E.D.; Shikov, A.N. Mechanisms of bioactivities of fucoidan from the brown seaweed Fucus vesiculosus L. of the barents sea. Mar. Drugs 2020, 18, 275. [Google Scholar] [CrossRef]
- Havale, S.H.; Pal, M. Medicinal chemistry approaches to the inhibition of dipeptidyl peptidase-4 for the treatment of type 2 diabetes. Bioorg. Med. Chem. 2009, 17, 1783–1802. [Google Scholar] [CrossRef]
- Ye, H.; Shen, Z.; Cui, J.; Zhu, Y.; Li, Y.; Chi, Y.; Wang, J.; Wang, P. Hypoglycemic activity and mechanism of the sulfated rhamnose polysaccharides chromium(III) complex in type 2 diabetic mice. Bioorg. Chem. 2019, 88, 102942. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.W.; Tian, Y.Y.; Chang, Y.G.; Li, Z.J.; Xue, C.H.; Wang, Y.M. Fucosylated chondroitin sulfate from sea cucumber improves glucose metabolism and activates insulin signaling in the liver of insulin-resistant mice. J. Med. Food 2014, 17, 749–757. [Google Scholar] [CrossRef]
- Jeong, Y.T.; Kim, Y.D.; Jung, Y.M.; Park, D.C.; Lee, D.S.; Ku, S.K.; Li, X.; Lu, Y.; Chao, G.H.; Kim, K.J.; et al. Low molecular weight fucoidan improves endoplasmic reticulum stress-reduced insulin sensitivity through AMP-activated protein kinase activation in L6 myotubes and restores lipid homeostasis in a mouse model of type 2 diabetes. Mol. Pharmacol. 2013, 84, 147–157. [Google Scholar] [CrossRef]
- Zhong, Q.W.; Zhou, T.S.; Qiu, W.H.; Wang, Y.K.; Xu, Q.L.; Ke, S.Z.; Wang, S.J.; Jin, W.H.; Chen, J.W.; Zhang, H.W.; et al. Characterization and hypoglycemic effects of sulfated polysaccharides derived from brown seaweed Undaria pinnatifida. Food Chem. 2021, 341, 128148. [Google Scholar] [CrossRef]
- Goto, M.; Azuma, K.; Arima, H.; Kaneko, S.; Higashi, T.; Motoyama, K.; Michihara, A.; Shimizu, T.; Kadowaki, D.; Maruyama, T.; et al. Sacran, a sulfated polysaccharide, suppresses the absorption of lipids and modulates the intestinal flora in non-alcoholic steatohepatitis model rats. Life Sci. 2021, 268, 118991. [Google Scholar] [CrossRef] [PubMed]
- Han, L.K.; Sumiyoshi, M.; Takeda, T.; Chihara, H.; Nishikiori, T.; Tsujita, T.; Kimura, Y.; Okuda, H. Inhibitory effects of chondroitin sulfate prepared from salmon nasal cartilage on fat storage in mice fed a high-fat diet. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 1131–1138. [Google Scholar] [CrossRef] [Green Version]
- Ben Gara, A.; Ben Abdallah Kolsi, R.; Chaaben, R.; Hammami, N.; Kammoun, M.; Paolo Patti, F.; El Feki, A.; Fki, L.; Belghith, H.; Belghith, K. Inhibition of key digestive enzymes related to hyperlipidemia and protection of liver-kidney functions by Cystoseira crinita sulphated polysaccharide in high-fat diet-fed rats. Biomed. Pharmacother. 2017, 85, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Jia, Z.; Li, C.; Chen, J.; Fang, T. Hypolipidemic and anti-atherogenic activities of crude polysaccharides from abalone viscera. Food Sci. Nutr. 2020, 8, 2524–2534. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, M.; Sivakumar, K.; Mariadoss, A.V.; Suresh, K. Modulating Effect of Hypnea musciformis (Red Seaweed) on Lipid Peroxidation, Antioxidants and Biotransforming Enzymes in 7,12-Dimethylbenz (a) Anthracene Induced Mammary Carcinogenesis in Experimental Animals. Pharmacogn. Res. 2017, 9, 108–115. [Google Scholar]
- Ren, R.; Gong, J.; Zhao, Y.; Zhuang, X.; Ye, Y.; Lin, W. Sulfated polysaccharides from Enteromorpha prolifera suppress SREBP-2 and HMG-CoA reductase expression and attenuate non-alcoholic fatty liver disease induced by a high-fat diet. Food Funct. 2017, 8, 1899–1904. [Google Scholar] [CrossRef]
- Yang, Z.; Yin, J.; Wang, Y.; Wang, J.; Xia, B.; Li, T.; Yang, X.; Hu, S.; Ji, C.; Guo, S. The fucoidan A3 from the seaweed Ascophyllum nodosum enhances RCT-related genes expression in hyperlipidemic C57BL/6J mice. Int. J. Biol. Macromol. 2019, 134, 759–769. [Google Scholar] [CrossRef]
- Yin, J.; Wang, J.; Li, F.; Yang, Z.; Yang, X.; Sun, W.; Xia, B.; Li, T.; Song, W.; Guo, S. The fucoidan from the brown seaweed Ascophyllum nodosum ameliorates atherosclerosis in apolipoprotein E-deficient mice. Food Funct. 2019, 10, 5124–5139. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, J.; Chang, Y.; Xu, J.; Wang, Y.; Long, T.; Xue, C. Fucoidan from the sea cucumber Acaudina molpadioides exhibits anti-adipogenic activity by modulating the Wnt/β-catenin pathway and down-regulating the SREBP-1c expression. Food Funct. 2014, 5, 1547–1555. [Google Scholar] [CrossRef]
- Shang, Q.; Jiang, H.; Cai, C.; Hao, J.; Li, G.; Yu, G. Gut microbiota fermentation of marine polysaccharides and its effects on intestinal ecology: An overview. Carbohydr. Polym. 2018, 179, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Festi, D.; Schiumerini, R.; Eusebi, L.H.; Marasco, G.; Taddia, M.; Colecchia, A. Gut microbiota and metabolic syndrome. World J. Gastroenterol. 2014, 20, 16079–16094. [Google Scholar] [CrossRef]
- Wang, P.X.; Deng, X.R.; Zhang, C.H.; Yuan, H.J. Gut microbiota and metabolic syndrome. Chin. Med. J. 2020, 133, 808–816. [Google Scholar] [CrossRef]
- Shang, Q.; Shi, J.; Song, G.; Zhang, M.; Cai, C.; Hao, J.; Li, G.; Yu, G. Structural modulation of gut microbiota by chondroitin sulfate and its oligosaccharide. Int. J. Biol. Macromol. 2016, 89, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, J.; Mao, G.; Wu, T.; Lin, D.; Hu, Y.; Ye, X.; Tian, D.; Chai, W.; Linhardt, R.J.; et al. Fucosylated chondroitin sulfate from Isostichopus badionotus alleviates metabolic syndromes and gut microbiota dysbiosis induced by high-fat and high-fructose diet. Int. J. Biol. Macromol. 2019, 124, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Mou, J.; Li, Q.; Shi, W.; Qi, X.; Song, W.; Yang, J. Chain conformation, physicochemical properties of fucosylated chondroitin sulfate from sea cucumber Stichopus chloronotus and its in vitro fermentation by human gut microbiota. Carbohydr. Polym. 2020, 228, 115359. [Google Scholar] [CrossRef] [PubMed]
- Shang, Q.; Shan, X.; Cai, C.; Hao, J.; Li, G.; Yu, G. Dietary fucoidan modulates the gut microbiota in mice by increasing the abundance of Lactobacillus and Ruminococcaceae. Food Funct. 2016, 7, 3224–3232. [Google Scholar] [CrossRef]
- Wu, Q.; Wu, S.; Cheng, Y.; Zhang, Z.; Mao, G.; Li, S.; Yang, Y.; Zhang, X.; Wu, M.; Tong, H. Sargassum fusiforme fucoidan modifies gut microbiota and intestinal metabolites during alleviation of hyperglycemia in type 2 diabetic mice. Food Funct. 2021, 12, 3572–3585. [Google Scholar] [CrossRef]
- Cheng, Y.; Sibusiso, L.; Hou, L.; Jiang, H.; Chen, P.; Zhang, X.; Wu, M.; Tong, H. Sargassum fusiforme fucoidan modifies the gut microbiota during alleviation of streptozotocin-induced hyperglycemia in mice. Int. J. Biol. Macromol. 2019, 131, 1162–1170. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Cui, X.; Duan, M.; Ai, C.; Song, S.; Chen, X. In vitro fermentation of κ-carrageenan oligosaccharides by human gut microbiota and its inflammatory effect on HT29 cells. J. Funct. Foods 2019, 59, 80–91. [Google Scholar] [CrossRef]
- Mi, Y.; Chin, Y.X.; Cao, W.X.; Chang, Y.G.; Lim, P.E.; Xue, C.H.; Tang, Q.J. Native κ-carrageenan induced-colitis is related to host intestinal microecology. Int. J. Biol. Macromol. 2020, 147, 284–294. [Google Scholar] [CrossRef]
- Seong, H.; Bae, J.-H.; Seo, J.S.; Kim, S.-A.; Kim, T.-J.; Han, N.S. Comparative analysis of prebiotic effects of seaweed polysaccharides laminaran, porphyran, and ulvan using in vitro human fecal fermentation. J. Funct. Foods 2019, 57, 408–416. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, N.; Li, Z.; Wang, X.; Shi, H.; Xue, C.; Li, R.W.; Tang, Q. Chondroitin sulfate disaccharides modified the structure and function of the murine gut microbiome under healthy and stressed conditions. Sci. Rep. 2017, 7, 6783. [Google Scholar] [CrossRef]
- Menni, C.; Lin, C.; Cecelja, M.; Mangino, M.; Matey-Hernandez, M.L.; Keehn, L.; Mohney, R.P.; Steves, C.J.; Spector, T.D.; Kuo, C.F.; et al. Gut microbial diversity is associated with lower arterial stiffness in women. Eur. Heart J. 2018, 39, 2390–2397. [Google Scholar] [CrossRef] [Green Version]
- Zhai, Q.; Feng, S.; Arjan, N.; Chen, W. A next generation probiotic, Akkermansia muciniphila. Crit. Rev. Food Sci. Nutr. 2019, 59, 3227–3236. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Xue, L.; Devkota, S.; Chang, E.; Morris, S.; Tobacman, J.K. Carrageenan-induced colonic inflammation is reduced in Bcl10 null mice and increased in IL-10-deficient mice. Mediat. Inflamm. 2013, 2013, 397642. [Google Scholar] [CrossRef] [Green Version]
- Shannon, E.; Conlon, M.; Hayes, M. Seaweed components as potential modulators of the gut microbiota. Mar. Drugs 2021, 19, 358. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gotteland, M.; Riveros, K.; Gasaly, N.; Carcamo, C.; Magne, F.; Liabeuf, G.; Beattie, A.; Rosenfeld, S. The Pros and Cons of Using Algal Polysaccharides as Prebiotics. Front. Nutr. 2020, 7, 163. [Google Scholar] [CrossRef] [PubMed]
- Michel, C.; Macfarlane, G.T. Digestive fates of soluble polysaccharides from marine macroalgae: Involvement of the colonic microflora and physiological consequences for the host. J. Appl. Bacteriol. 1996, 80, 349–369. [Google Scholar] [CrossRef] [PubMed]
- Kong, Q.; Dong, S.; Gao, J.; Jiang, C. In vitro fermentation of sulfated polysaccharides from E. prolifera and L. japonica by human fecal microbiota. Int. J. Biol. Macromol. 2016, 91, 867–871. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Liu, Y.; Jiang, P.; Song, S.; Ai, C. Interaction of sulfated polysaccharides with intestinal Bacteroidales plays an important role in its biological activities. Int. J. Biol. Macromol. 2021, 168, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Chang, Y.; Gao, Y.; Wang, X.; Chen, X.; Wang, Y.; Xue, C.; Tang, Q. Dietary fucoidan of Acaudina molpadioides alters gut microbiota and mitigates intestinal mucosal injury induced by cyclophosphamide. Food Funct. 2017, 8, 3383–3393. [Google Scholar] [CrossRef]
- Cantarel, B.L.; Lombard, V.; Henrissat, B. Complex carbohydrate utilization by the healthy human microbiome. PLoS ONE 2012, 7, e28742. [Google Scholar] [CrossRef] [Green Version]
- Grondin, J.M.; Tamura, K.; Déjean, G.; Abbott, D.W.; Brumer, H. Polysaccharide utilization loci: Fueling microbial communities. J. Bacteriol. 2017, 199, e00860-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottière, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and functional importance in the gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhu, B.; Sun, Y.; Ai, C.; Wu, S.; Wang, L.; Song, S.; Liu, X. Sulfated polysaccharide from sea cucumber modulates the gut microbiota and its metabolites in normal mice. Int. J. Biol. Macromol. 2018, 120, 502–512. [Google Scholar] [CrossRef]
- Hu, S.; Wang, J.; Xu, Y.; Yang, H.; Wang, J.; Xue, C.; Yan, X.; Su, L. Anti-inflammation effects of fucosylated chondroitin sulphate from Acaudina molpadioides by altering gut microbiota in obese mice. Food Funct. 2019, 10, 1736–1746. [Google Scholar] [CrossRef]
- Green, M.; Arora, K.; Prakash, S. Microbial medicine: Prebiotic and probiotic functional foods to target obesity and metabolic syndrome. Int. J. Mol. Sci. 2020, 21, 2890. [Google Scholar] [CrossRef]
- Canfora, E.E.; Jocken, J.W.; Blaak, E.E. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol. 2015, 11, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Imbs, T.I.; Zvyagintseva, T.N.; Ermakova, S.P. Is the transformation of fucoidans in human body possible? Int. J. Biol. Macromol. 2020, 142, 778–781. [Google Scholar] [CrossRef] [PubMed]
- Kadena, K.; Tomori, M.; Iha, M.; Nagamine, T. Absorption Study of Mozuku Fucoidan in Japanese Volunteers. Mar. Drugs 2018, 16, 254. [Google Scholar] [CrossRef] [Green Version]
- Irhimeh, M.R.; Fitton, J.H.; Lowenthal, R.M.; Kongtawelert, P. A quantitative method to detect fucoidan in human plasma using a novel antibody. Methods Find. Exp. Clin. Pharmacol. 2005, 27, 705–710. [Google Scholar] [CrossRef]
- Pozharitskaya, O.N.; Shikov, A.N.; Faustova, N.M.; Obluchinskaya, E.D.; Kosman, V.M.; Vuorela, H.; Makarov, V.G. Pharmacokinetic and Tissue Distribution of Fucoidan from Fucus vesiculosus after Oral Administration to Rats. Mar. Drugs 2018, 16, 132. [Google Scholar] [CrossRef] [Green Version]
- Guo, S.; Wang, J.; He, C.; Wei, H.; Ma, Y.; Xiong, H. Preparation and antioxidant activities of polysaccharides obtained from abalone viscera by combination of enzymolysis and multiple separation methods. J. Food Sci. 2020, 85, 4260–4270. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, J.; Song, S.; Zhou, D.; Qiao, W.; Zhu, C.; Liu, S.; Zhu, B. Anticoagulant activity and structural characterization of polysaccharide from abalone (Haliotis discus hannai Ino) gonad. Molecules 2016, 21, 697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.; Liu, Y.; Jiang, P.; Xu, Y.; Zheng, W.; Song, S.; Ai, C. Effect of sulfate group on sulfated polysaccharides-induced improvement of metabolic syndrome and gut microbiota dysbiosis in high fat diet-fed mice. Int. J. Biol. Macromol. 2020, 164, 2062–2072. [Google Scholar] [CrossRef]
- Barton, L.L.; Ritz, N.L.; Fauque, G.D.; Lin, H.C. Sulfur Cycling and the Intestinal Microbiome. Dig. Dis. Sci. 2017, 62, 2241–2257. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.B.; Lin, H.C. Hydrogen Sulfide in Physiology and Diseases of the Digestive Tract. Microorganisms 2015, 3, 866–889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, R.; Azuma, Y.; Ojima, T.; Hashimoto, T.; Mizuno, M.; Nishitani, Y.; Yoshida, M.; Azuma, T.; Kanazawa, K. Modulation of platelet aggregation-related eicosanoid production by dietary F-fucoidan from brown alga Laminaria japonica in human subjects. Br. J. Nutr. 2013, 110, 880–890. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Corona, D.M.; Martínez-Abundis, E.; González-Ortiz, M. Effect of fucoidan administration on insulin secretion and insulin resistance in overweight or obese adults. J. Med. Food 2014, 17, 830–832. [Google Scholar] [CrossRef] [PubMed]
- Shih, P.H.; Shiue, S.J.; Chen, C.N.; Cheng, S.W.; Lin, H.Y.; Wu, L.W.; Wu, M.S. Fucoidan and Fucoxanthin Attenuate Hepatic Steatosis and Inflammation of NAFLD through Modulation of Leptin/Adiponectin Axis. Mar. Drugs 2021, 19, 148. [Google Scholar] [CrossRef] [PubMed]
- Feferman, L.; Bhattacharyya, S.; Oates, E.; Haggerty, N.; Wang, T.; Varady, K.; Tobacman, J.K. Carrageenan-free diet shows improved glucose tolerance and insulin signaling in prediabetes: A randomized, pilot clinical trial. J. Diabetes Res. 2020, 2020, 8267980. [Google Scholar] [CrossRef]
- Wanyonyi, S.; du Preez, R.; Brown, L.; Paul, N.A.; Panchal, S.K. Kappaphycus alvarezii as a food supplement prevents diet-induced metabolic syndrome in rats. Nutrients 2017, 9, 1261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabbia, D.; De Martin, S. Brown seaweeds for the management of metabolic syndrome and associated diseases. Molecules 2020, 25, 4182. [Google Scholar] [CrossRef] [PubMed]

| MSPs | Compounds/Extract | Source | Molecular Weight | Model | Positive/Negative Controls | Administration | Effects | References |
|---|---|---|---|---|---|---|---|---|
| sulphated polysaccharide | extract | Cymodocea nodosa | - | male Wistar rats with high fat diet | BC: normal rats were fed a standard laboratory diet PC: the rats received high-fat diet and treated with Orlistat NC: rats were fed a high-fat diet | gastric gavages route (200 mg/kg of body weight/daily) for 42 days | body weight ↓ lipase activity ↓ superoxide dismutase activity ↑ catalase activity ↑ glutathione peroxidase activity ↑ Thiobarbituric acid reactive substances levels ↓ | [21] |
| fucoidan | extract | Ascophyllum nodosum | - | the human gut microbiota in vitro | PC: fermentation system with fructooligosaccharide BC: fermentation system without any addition | 1 mL of the fecal inoculum was mixed with 9 mL of basal nutrient medium containing 90 mg of fucoidan | modulated the composition of the gut microbiota the relative abundance of Bacteroidetes and Firmicutes ↑ the total SCFA content ↑ | [22] |
| sulfated polysaccharide | compound | abalone gonad | 9 kDa | male BALB/c mice with high fat diet | BC: fed with a normal diet NC: fed with high fat diet | orally or gavage administrated with 2 mg the polysaccharide as drinking water (5 mL/day) | weight gain ↓ fat accumulation ↓ lipid droplets ↓ the level of Bacteroidetes ↑ the level of Firmicutes ↓ SCFAs production ↑ the expression of GPR43, aP2, UCP2, and LPL ↓ | [23] |
| sulfated polysaccharide corresponding to fucosylated chondroitin sulfate and fucoidan | compound | Stichopus japonicus | 179.4 kDa and >670 kDa, | diet-induced obese BALB/c mice | BC: fed a standard maintenance diet MC: fed with high fat diet | oral dosage of 300 mg/kg·d the sulfated polysaccharide | body weight ↓ fat and liver hypertrophy ↓ insulin resistance ↓ serum lipid ↓ inflammatory cytokine levels ↓ gut tissue index ↑ LPS-binding protein ↓ SCFA ↑ microbiota diversity ↑ probiotic Akkermansia ↑ endotoxin-bearing proteobacteria ↑ | [24] |
| fucoidan | extract | Sargassum fusiforme | - | male ICR mice with high fat diet | BC: fed a standard maintenance diet MC: fed with high fat diet | physiological saline with fucoidan at 200 mg/kg intragastrically once per day for 6 weeks | blood glucose and insulin resistance index ↓ the levels of MDA and 4-HNE-modified protein ↓ GSH/GSSG ratio ↑ antioxidant enzymes ↑ activated Nrf2 signaling the abundance and diversity of gut microbiota ↑ improve intestinal integrity and inflammation | [25] |
| fucoidan | extract | Fucus vesiculosus | - | obese, with no history of diabetes, and subjects of ages between 18 and 65 years | BC: taken placebo capsules twice daily | active fucoidan 500 mg twice daily for 90 days | no marked effect on insulin resistance in obese, nondiabetic subjects | [26] |
| Sulfated fucan-dominated polysaccharide fraction fucosylated chondroitin sulfate-dominated polysaccharide fraction | extract | Thelenota ananas Cucumaria frondosa | - | high fat diet and streptozotocin induced type 2 diabetes male Sprague Dawley rat model | BC: fed with a normal diet MC: fed with high fat diet PC: fed with high fat diet and administration with 250 mg/kg/d metformin | 200 or 400 mg/kg/d by daily oral gavage for 8 weeks | ameliorate hyperglycemia restore hypertriglyceridemia and hypercholesterolemia inflammatory status and oxidative stress ↓ protect against liver injury improve insulin resistance accumulation of hepatic glycogen ↑ activate IRS/PI3K/AKT signaling regulate GSK-3β gene expression | [27] |
| fucoidan | extract | brown seaweeds | - | spontaneously hyperlipidemic mice with high fat diet | BC: fed with a normal diet NC: fed with high fat diet | fed with 1% or 5% fucoidan | tissue weight (liver and white adipose tissue) ↓ blood lipid ↓ total cholesterol ↓ triglyceride ↓ non-high-density lipoprotein cholesterol ↓ glucose levels ↓ plasma lipoprotein lipase activity ↑ HDL-C levels ↑ hepatic steatosis levels (liver size, TC and TG levels, and lipid peroxidation) ↓ white adipose tissue LPL activity ↑ | [28] |
| sulphated polysaccharide | extract | Sargassum vulgare | - | obese male Wistar rats | BC: fed a standard laboratory diet MC: fed a high-fat diet PC: fed a high-fat diet and treated with Orlistat (30 mg/kg, body weight/daily) | gastric gavages route (200 mg/kg of bodyweight/daily) for 6 weeks | body weight ↓ lipase activity ↓ antioxidant enzymes activities ↑ lipid peroxidation ↓ protect liver-kidney functions the levels of toxicity parameters in blood ↓ | [29] |
| 4-O-sulfation pattern fucodian | compound | Pearsonoturia graeffei | 320 kDa | Sprague–Dawley rats with high fat diet | BC: fed a standard laboratory diet MC: fed a high-fat diet PC: fed a high-fat diet and treated with simvastatin (5 mg/kg) | oral administration (40 mg/kg) for 28 days by gavage | body weight regulate lipid disorder improve liver function adiponectin level ↑ | [30] |
| ulvan | compound | Ulva pertusa | 143 kDa | the model of hyperlipidemic Kunming mice | BC: fed a standard laboratory diet MC: fed a high-fat diet PC: fed a high-fat diet and treated with colestyramine (500 mg/kg) | oral administration (250 mg/kg body weight) for 28 days | antioxidant activity ↑ malondialdehyde ↓ superoxide dismutase ↑ catalase ↑ | [31] |
| fucoidan | compound | Laminaria japonica | 7 kDa | Goto-Kakizaki type 2 diabetic rats | BC: Wistar control rats MC: Goto-Kakizaki diabetic rats PC: Goto-Kakizaki diabetic rats treated with probucol (100 mg/kg) | fucoidan (50, 100, or 200 mg/kg/day) were given by intragastric administration for 12 weeks | basal hypertension ↓ ameliorate impairment of endothelium-dependent relaxation in the aorta, as well as mesenteric and paw arteries in diabetic rats eNOS phosphorylation at Ser1177 ↑ eNOS expression ↑ NO production ↑ | [32] |
| fucoidan | extract | Laminaria japonica | 6.5 kDa | instreptozotocin-induced type 1 diabetic rats | BC: streptozotocin-induced diabetic rats PC: streptozotocin-induced diabetic rats treated with probucol (100 mg/kg/day) | intragastric administration of fucoidan (50 or 100 mg/kg/day) for 12 weeks | body weight-loss ↑ hypertension ↓ hyperlipidemia ↓ serum level of total cholesterol, triglyceride, and low-density lipoprotein cholesterol ↓ ameliorate STZ-elicited hyper-responsiveness and oxidative stress in aortic smooth muscles superoxide level ↓ glutathione content and higher superoxide dismutase activity ↑ prevent cyclooxygenase-2 stimulation thromboxane synthase ↑ 6-keto-PGF1α ↓ | [33] |
| fucoidan | compound | Undaria pinnatifida | 54 kDa | eNOS inhibition-induced hypertensive Sprague-Dawley rats | BC: the normotensive group placed on a basal diet MC: L-NAME-induced hypertension rats PC: L-NAME-induced hypertension rats treated with nifedipine (5 mg/kg) | administered at 20 mg/kg/day or 100 mg/kg/day by daily gavage for four weeks | hypertension ↓ NO production ↑ activate eNOS and Akt phosphorylation protect against vascular structure damage enhance endothelium-independent vascular function and inhibit abnormal proliferation of smooth muscle cells vascular inflammation and oxidative stress ↓ | [34] |
| carrageenans | extract | Sarconema filiforme | - | high-carbohydrate, high-fat diet-fed male Wistar rats | BC: fed either corn starch or high carbohydrate, high-fat diets | supplemented with 5% S. filiforme power for the last 8 weeks | body weight ↓ abdominal and liver fat ↓ systolic blood pressure ↓ plasma total cholesterol concentrations ↓ plasma activities of alanine transaminase and aspartate transaminase ↓ modulate gut microbiota without changing the Firmicutes to Bacteroidetes ratio infiltration of inflammatory cells into organs ↓ | [35] |
| MSPs | Source | Compounds /Extract | Molecular Weight | Model | Positive/Negative Controls | Administration | Effects | References |
|---|---|---|---|---|---|---|---|---|
| Chondroitin sulfate | - | compounds | 24 kDa and 130 kDa | Kunming mice | NC1: male mice oral administrated with normal saline NC2: female mice oral administrated with normal saline | administration of 150 mg/kg by gavage once a day for 6 weeks | sex-dependent effect on gut microbiota | [61] |
| Fucosylated chondroitin sulfate | Isostichopus badionotus | compounds | 10.9 kDa | C57BL/6 mice with high-fat and high sucrose diet | BC: mice were fed on regular chow NC: mice were fed on a high-fat and high sucrose diet | administration of 20 or 40 mg/kg/day by metallic gavage needle for 6 weeks | alleviate obesity, hyperlipidemia, hyperglycemia, inflammation, liver steatosis, and adipocyte hypertrophy ratio of Firmicutes to Bacteroidetes ↓ Lachnospiraceae and Allobaculum ↓ Porphyromonadaceae, Barnesiella, and Bacteroides ↑ | [62] |
| Fucosylated chondroitin sulfate | S. chloronotus | compounds | 63.2 kDa | in vitro fermentation with fecal slurry | PC: fructo-oligosaccharide were dissolved in culture medium at 10 mg/mL NC: blank culture medium | - | absolute abundance of microbiota ↑ Megamonas, Bacteroides, Fusobacterium, Parabacteroides, Prevotella, Faecalibacterium ↑ short-chain fatty acids ↑ | [63] |
| fucoidan | Ascophyllum nodosum and Laminaria japonica | extract | - | specific pathogen-free male C57BL/6 mice | NC: oral administration of normal saline | 100 mg/kg/day by gavage for 6 weeks | Lactobacillus and Ruminococcaceae ↑ a more balanced composition of gut microbiota serum lipopolysaccharide-binding protein levels ↓ | [64] |
| fucoidan | Sargassum fusiforme | extract | 205.8 kDa | Pathogen-free male ICR mice with high-fat diet and streptozotocin induced mouse model of type 2 diabetes | BC: healthy mice fed with a normal chow diet NC: diabetic mice administered normal saline | 100 mg/kg/day by gavage for 4 weeks | blood glucose ↓ diet and water intake ↓ hyperlipidemia ↓ glucose tolerance ↑ epididymal fat deposition ↓ pathological changes in heart and liver tissues ↓ oxidative stress ↓ Bacteroides, Faecalibacterium and Blautia ↑ levels of (R)-carnitine and choline in the colon ↑ | [65] |
| fucoidan | Sargassum fusiforme | extract | 205.8 kDa | male ICR mice with streptozotocin-induced hyperglycemia | BC: normal mice treated with distilled water NC: diabetic mice with distilled water | 100 mg/kg/day by gavage for 6 weeks | blood glucose ↓ diet and water intake ↓ the pathological change in the heart and liver ↓ improve the liver function suppress oxidative stress decrease the relative abundances of the diabetes-related intestinal bacteria | [66] |
| Carrageenan oligosaccharides | Lubao Biochemistry, China | compounds | - | in vitro fermentation with fecal slurry | PC: the medium contained 10 μg LPS NC: basal nutrient medium | - | pro-inflammatory bacteria Prevotella ↑ anti-inflammatory bacteria Bacteroides and Parabacteroides ↓ KO3 (larger degrees of polymerization): Bifidobacterium and Lactobacillius ↑ and SCFAs ↑ KO6 (smaller degrees of polymerization): Prevotellaceae ↑ and SCFAs ↓ | [67] |
| Carrageenan oligosaccharides | Lubao Biochemistry, China | compounds | - | HT29 cells | BC: the cells were treated with fresh DMEM medium for 24 h. PC: the cells were treated with culture medium containing LPS (1 μg/mL) for 24 h | Treatment at 50, 100, and 200 μL/mL for 24 h. | KO6: IL-1β, TNF-α, SIgA and mucin2 ↑ | [67] |
| carrageenan | Kappaphycus alvareziiwere | extract | 365 kDa | male SPF C57BL/6J mice with high fat diet | BC: low fat diet + normal water NC: high fat diet + normal water | administration of 0.5% or 5% carrageenan in drinking water for 6 weeks | disease activity index ↑ myeloperoxidase activity ↑ mRNA expression of Toll-like receptor 4 in colon ↑ inflammatory-causing bacteria Alistipes finegoldii and Bacteroides acidifaciens ↑ | [68] |
| laminaran | Carbosynth, UK | compounds | - | in vitro fermentation with fecal slurry | NC: basal culture medium | - | Bifidobacteria and Bacteroides ↑ acetate and propionate ↑ | [69] |
| Ulvan | Carbosynth, UK | compounds | - | in vitro fermentation with fecal slurry | NC: basal culture medium | - | Bifidobacteria and Lactobacillus ↑ lactate and acetate ↑ | [69] |
| porphyran | Carbosynth, UK | - | in vitro fermentation with fecal slurry | NC: basal culture medium | - | little prebiotic effect | [69] |
| MSPs | The Number of Patients | Disease | Dosing Regimen | Timing | Results | References |
|---|---|---|---|---|---|---|
| fucoidan | 24 | healthy participants | 1 mg fucoxanthin (6 participants), 400 mg fucoidan (9 participants), and both (9 participants) administered to volunteers | 5 weeks | significantly shortened lysis time of the thrombu H2O2 ↑ the secretion of prostacyclin ↑ | [91] |
| 25 | obese or overweight | 13 patients received an oral dose of 500 mg of fucoidan once daily before breakfast and 12 patients received placebo | 3 months | Diastolic blood pressure ↓ low-density lipoprotein cholesterol ↓ insulin ↑ HOMA β-cell ↑ HOMA insulin resistance ↑ | [92] | |
| 20 | obese, with no history of diabetes | active fucoidan 500 mg or placebo capsules twice daily | 90 days | no differences the mean change in HOMA scores was 0 for the placebo and −0.1 for the active groups | [25] | |
| 42 | non-alcoholic fatty liver disease | 275 mg fucoidan and 275 mg fucoxanthin twice per day in the treatment group, or placebo (550 mg/capsule cellulose powder) in the control group | 24 weeks | alanine aminotransferase ↓ aspartate aminotransferase ↓ total cholesterol ↓ triglyceride ↓ fasting blood glucose ↓ hemoglobin a1c ↓ the scores of controlled attenuation parameter ↓ adiponectin and leptin expression ↑ | [93] | |
| carrageenan | 13 | prediabetes | 8 patients were provided all meals with no carrageenan. 5 patients received a similar diet with carrageenan (total estimated to be 250 mg/day) | 12 weeks | no significant declines in Hemoglobin a1c or HOMA-IR C-peptide ↑ phospho-serine-insulin receptor substrate 1 ↑ phospho-serine-protein kinase 1 ↓ mononuclear cell arylsulfatase B ↓ | [94] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Qin, J.; Cheng, Y.; Lv, D.; Li, M.; Qi, Y.; Lan, J.; Zhao, Q.; Li, Z. Marine Sulfated Polysaccharides: Preventive and Therapeutic Effects on Metabolic Syndrome: A Review. Mar. Drugs 2021, 19, 608. https://doi.org/10.3390/md19110608
Li Y, Qin J, Cheng Y, Lv D, Li M, Qi Y, Lan J, Zhao Q, Li Z. Marine Sulfated Polysaccharides: Preventive and Therapeutic Effects on Metabolic Syndrome: A Review. Marine Drugs. 2021; 19(11):608. https://doi.org/10.3390/md19110608
Chicago/Turabian StyleLi, Ying, Juan Qin, Yinghui Cheng, Dong Lv, Meng Li, Yanxia Qi, Jing Lan, Qiancheng Zhao, and Zhibo Li. 2021. "Marine Sulfated Polysaccharides: Preventive and Therapeutic Effects on Metabolic Syndrome: A Review" Marine Drugs 19, no. 11: 608. https://doi.org/10.3390/md19110608
APA StyleLi, Y., Qin, J., Cheng, Y., Lv, D., Li, M., Qi, Y., Lan, J., Zhao, Q., & Li, Z. (2021). Marine Sulfated Polysaccharides: Preventive and Therapeutic Effects on Metabolic Syndrome: A Review. Marine Drugs, 19(11), 608. https://doi.org/10.3390/md19110608






