LC–HRMS and Chemical Derivatization Strategies for the Structure Elucidation of Caribbean Ciguatoxins: Identification of C-CTX-3 and -4
Abstract
1. Introduction
2. Results and Discussion
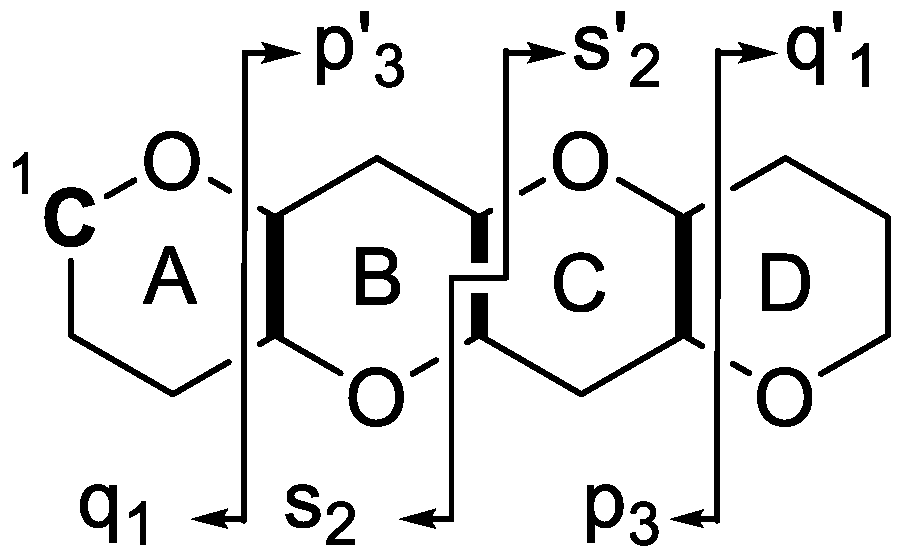
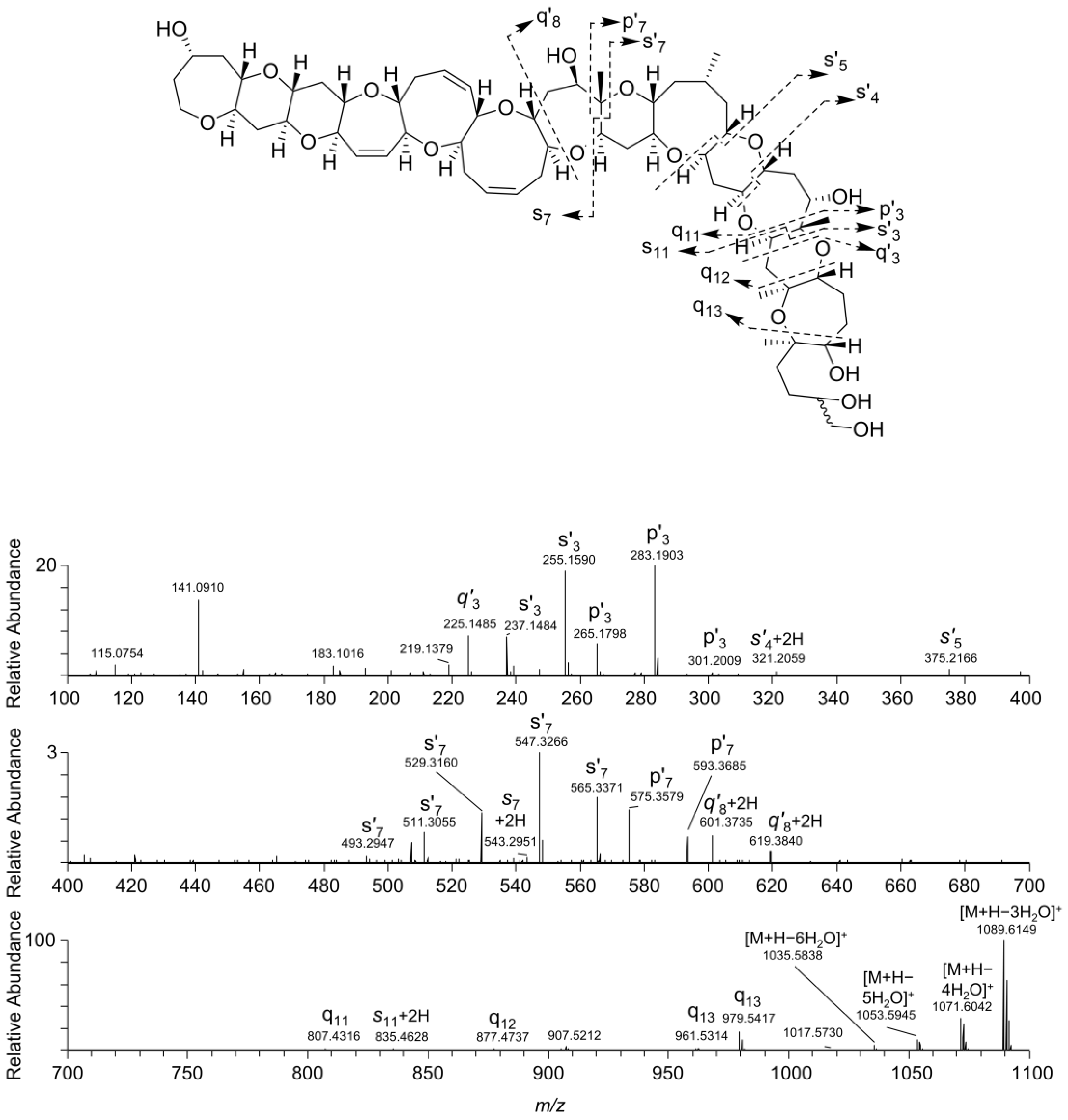
2.1. General Fragmentation Pathways of Ladder Frame Polyether Molecules
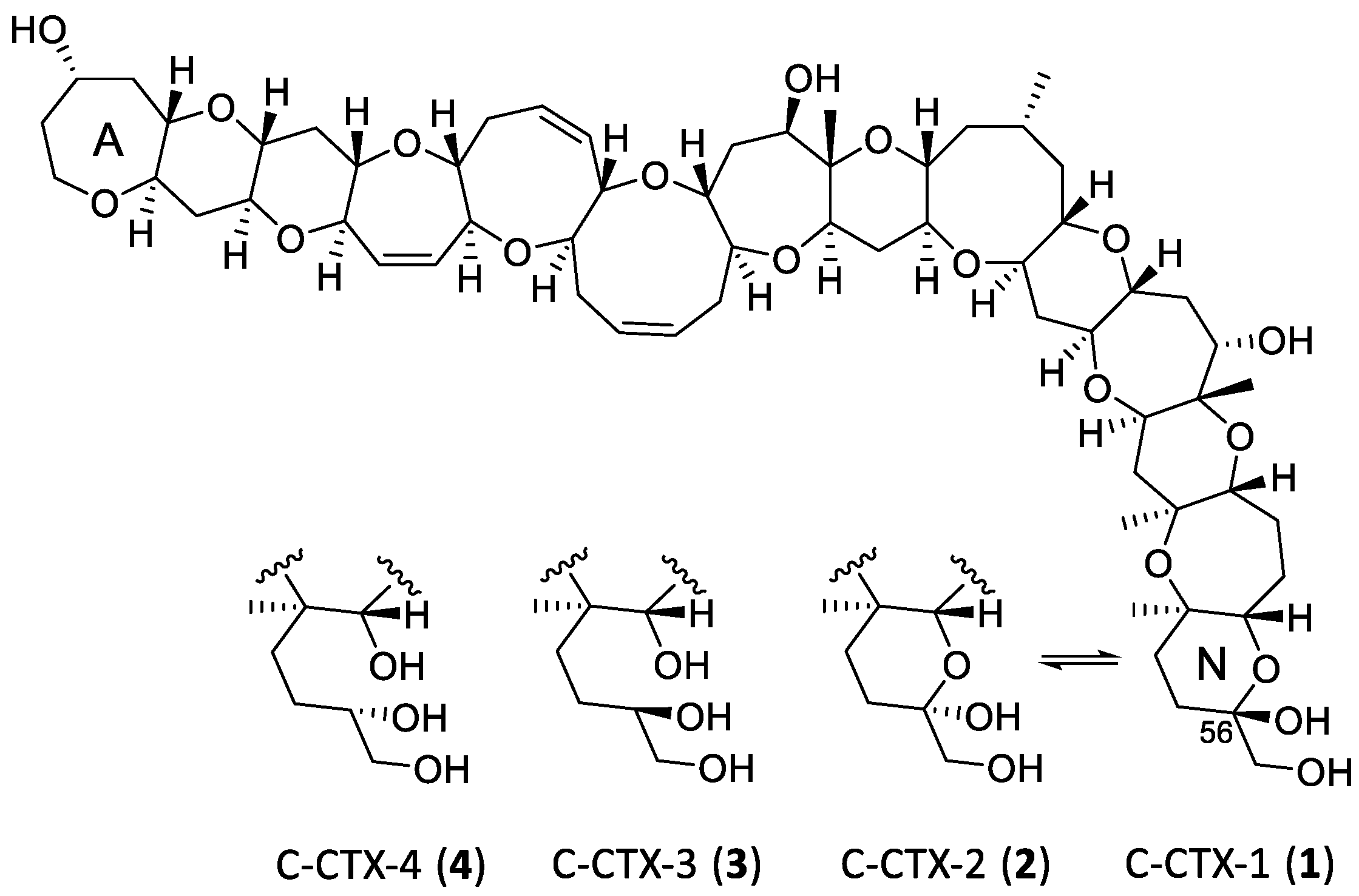
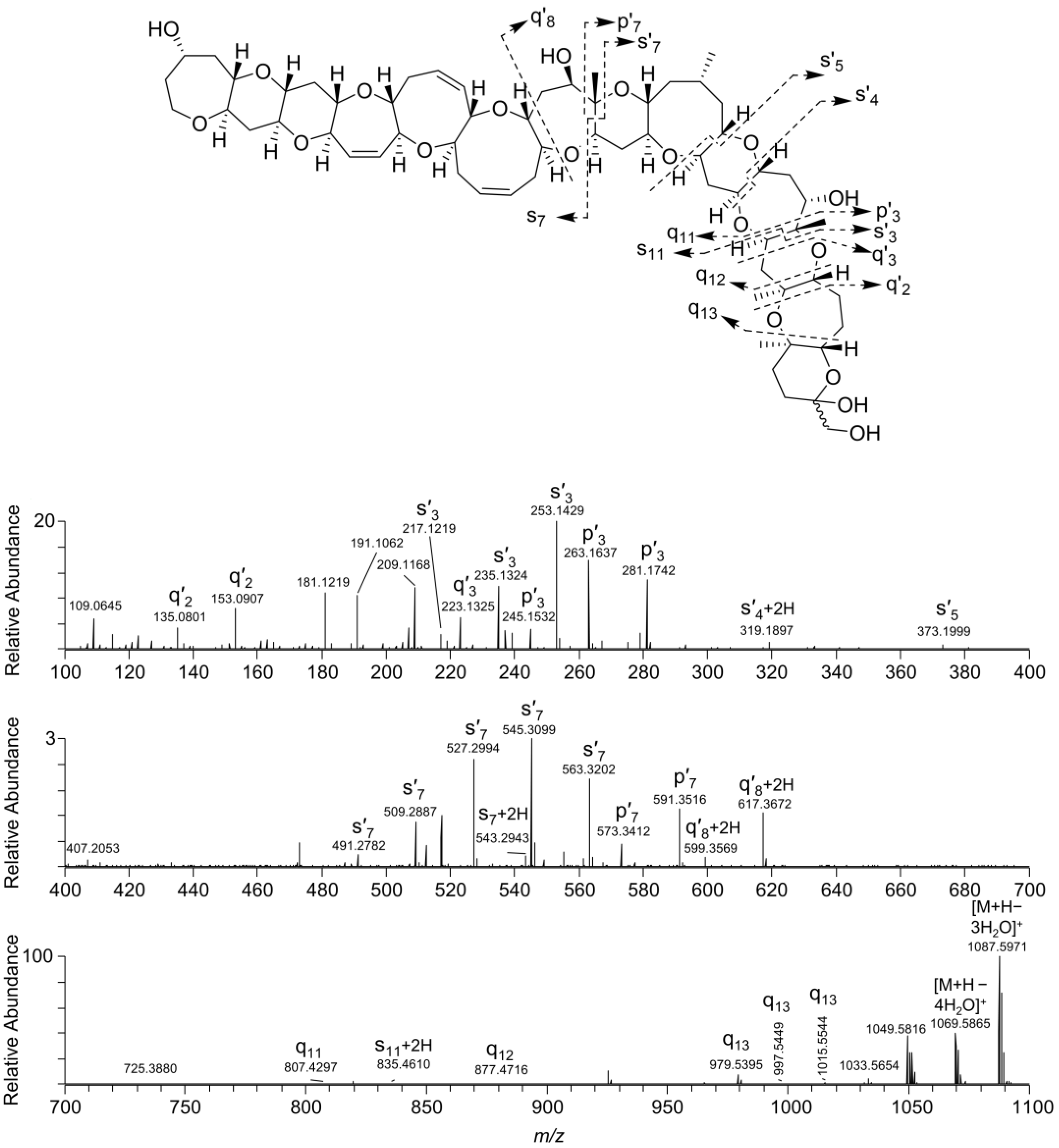
2.2. Interrogation of the MS/MS Spectra of 1–4
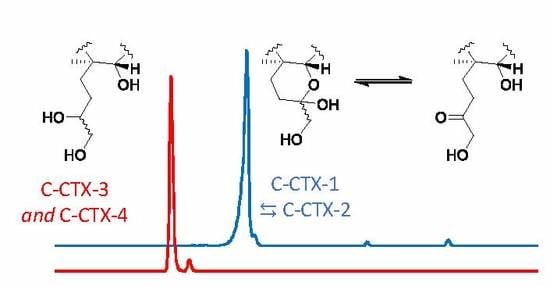
2.3. Borohydride Reduction of Epimers 1 and 2 to Epimers 3 and 4
2.4. Reaction of 1–4 with Periodate
3. Materials and Methods
3.1. Collection of Fish
3.2. Chemicals
3.3. Extraction and Sample Preparation
3.4. Toxicity Assessment for Sample Selection by MTT-Neuroblastoma Assay (MTT-N2A)
3.5. Liquid Chromatography–High-Resolution Mass Spectrometry (LC–HRMS)
3.6. Analytical-Scale Fractionation
3.7. Borohydride Reduction of 1 and 2
3.8. Periodate Oxidation of 1/2 and 3/4
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Friedman, M.A.; Fernandez, M.; Backer, L.C.; Dickey, R.W.; Bernstein, J.; Schrank, K.; Kibler, S.; Stephan, W.; Gribble, M.O.; Bienfang, P.; et al. An updated review of ciguatera fish poisoning: Clinical, epidemiological, environmental, and public health management. Mar. Drugs 2017, 15, 72. [Google Scholar] [CrossRef] [PubMed]
- Yasumoto, T. The chemistry and biological function of natural marine toxins. Chem. Rec. 2001, 1, 228–242. [Google Scholar] [CrossRef] [PubMed]
- Yasumoto, T.; Murata, M. Marine Toxins. Chem. Rev. 1993, 93, 1897–1909. [Google Scholar] [CrossRef]
- Kohli, G.S.; Campbell, K.; John, U.; Smith, K.F.; Fraga, S.; Rhodes, L.L.; Murray, S.A. Role of modular polyketide synthases in the production of polyether ladder compounds in ciguatoxin-producing Gambierdiscus polynesiensis and G. excentricus (Dinophyceae). J. Eukaryot. Microbiol. 2017, 64, 691–706. [Google Scholar] [CrossRef]
- Lewis, R.J.; Sellin, M.; Poli, M.A.; Norton, R.S.; Macleod, J.K.; Sheil, M.M. Purification and characterization of ciguatoxins from moray eel (Lycodontis javanicus, Muraenidae). Toxicon 1991, 29, 1115–1127. [Google Scholar] [CrossRef]
- Murata, M.; Legrand, A.M.; Ishibashi, Y.; Fukui, M.; Yasumoto, T. Structures and configurations of ciguatoxin from the moray eel Gymnothorax javanicus and its likely precursor from the dinoflagellate Gambierdiscus toxicus. J. Am. Chem. Soc. 1990, 112, 4380–4386. [Google Scholar] [CrossRef]
- Murata, M.; Legrand, A.M.; Ishibashi, Y.; Yasumoto, T. Structures of ciguatoxin and its congener. J. Am. Chem. Soc. 1989, 111, 8929–8931. [Google Scholar] [CrossRef]
- Satake, M.; Ishibashi, Y.; Legrand, A.M.; Yasumoto, T. Isolation and structure of ciguatoxin-4A, a new ciguatoxin precursor, from cultures of dinoflagellate Gambierdiscus toxicus and parrotfish Scarus gibbus. Biosci. Biotechnol. Biochem. 1996, 60, 2103–2105. [Google Scholar] [CrossRef]
- Yasumoto, T.; Igarashi, T.; Legrand, A.M.; Cruchet, P.; Chinain, M.; Fujita, T.; Naoki, H. Structural elucidation of ciguatoxin congeners by fast-atom bombardment tandem mass spectroscopy. J. Am. Chem. Soc. 2000, 122, 4988–4989. [Google Scholar] [CrossRef]
- Satake, M.; Fukui, M.; Legrand, A.M.; Cruchet, P.; Yasumoto, T. Isolation and structures of new ciguatoxin analogs, 2,3-dihydroxyCTX3C and 51-hydroxyCTX3C, accumulated in tropical reef fish. Tetrahedron Lett. 1998, 39, 1197–1198. [Google Scholar] [CrossRef]
- Satake, M.; Murata, M.; Yasumoto, T. The Structure of CTX3C, a ciguatoxin congener isolated from cultured Gambierdiscus toxicus. Tetrahedron Lett. 1993, 34, 1975–1978. [Google Scholar] [CrossRef]
- Hamilton, B.; Hurbungs, M.; Jones, A.; Lewis, R.J. Multiple ciguatoxins present in Indian Ocean reef fish. Toxicon 2002, 40, 1347–1353. [Google Scholar] [CrossRef]
- Hamilton, B.; Hurbungs, M.; Vernoux, J.P.; Jones, A.; Lewis, R.J. Isolation and characterisation of Indian Ocean ciguatoxin. Toxicon 2002, 40, 685–693. [Google Scholar] [CrossRef]
- Lewis, R.J.; Vernoux, J.P.; Brereton, I.M. Structure of Caribbean ciguatoxin isolated from Caranx latus. J. Am. Chem. Soc. 1998, 120, 5914–5920. [Google Scholar] [CrossRef]
- Estevez, P.; Leao, J.M.; Yasumoto, T.; Dickey, R.W.; Gago-Martinez, A. Caribbean ciguatoxin-1 stability under strongly acidic conditions: Characterisation of a new C-CTX1 methoxy congener. Food Addit. Contam. A 2019, 37, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Dickey, R. Ciguatera toxins: Chemistry, toxicology, and detection. In Seafood and Freshwater Toxins: Pharmacology, Physiology, and Detection; Botana, L.M., Ed.; CRC Press: Boca Raton, FL, USA, 2008; Volume 22, pp. 489–493. [Google Scholar]
- Pottier, I.; Vernoux, J.P.; Jones, A.; Lewis, R.J. Characterisation of multiple Caribbean ciguatoxins and congeners in individual specimens of horse-eye jack (Caranx latus) by high-performance liquid chromatography/mass spectrometry. Toxicon 2002, 40, 929–939. [Google Scholar] [CrossRef]
- Vernoux, J.P.; Lewis, R.J. Isolation and characterisation of Caribbean ciguatoxins from the horse-eye jack (Caranx latus). Toxicon 1997, 35, 889–900. [Google Scholar] [CrossRef]
- Abraham, A.; Jester, E.L.E.; Granade, H.R.; Plakas, S.M.; Dickey, R.W. Caribbean ciguatoxin profile in raw and cooked fish implicated in ciguatera. Food Chem. 2012, 131, 192–198. [Google Scholar] [CrossRef]
- De Darwent, B.B. Bond Dissociation Energies in Simple Molecules; National Bureau of Standards: Washington, DC, USA, 1970; pp. 18–23. Available online: https://nvlpubs.nist.gov/nistpubs/Legacy/NSRDS/nbsnsrds31.pdf (accessed on 24 February 2020).
- Pottier, I.; Vernoux, J.P.; Jones, A.; Lewis, R.J. Analysis of toxin profiles in three different fish species causing ciguatera fish poisoning in Guadeloupe, French West Indies. Food Addit. Contam. 2002, 19, 1034–1042. [Google Scholar] [CrossRef]
- Robertson, A.; Garcia, A.C.; Quintana, H.A.F.; Smith, T.B.; Castillo, B.F.; Reale-Munroe, K.; Gulli, J.A.; Olsen, D.A.; Hooe-Rollman, J.I.; Jester, E.L.E.; et al. Invasive lionfish (Pterois volitans): A potential human health threat for ciguatera fish poisoning in tropical waters. Mar. Drugs 2014, 12, 88–97. [Google Scholar] [CrossRef]
- Radwan, F.F.; Ramsdell, J.S. Characterization of in vitro oxidative and conjugative metabolic pathways for brevetoxin (PbTx-2). Toxicol. Sci. 2006, 89, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.S.; Cole, R.B. Electrospray ionization tandem mass spectrometry for structural elucidation of protonated brevetoxins in red tide algae. Anal. Chem. 2000, 72, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Roepstorff, P.; Fohlman, J. Proposal for a common nomenclature for sequence ions in mass spectra of peptides. Biomed. Mass Spectrom. 1984, 11, 601. [Google Scholar] [CrossRef] [PubMed]
- Domon, B.; Costello, C.E. A systematic nomenclature for carbohydrate fragmentations in FAB-MS/MS spectra of glycoconjugates. Glycoconj. J. 1988, 5, 397–409. [Google Scholar] [CrossRef]
- Chaikin, S.W.; Brown, W.G. Reduction of aldehydes, ketones and acid chlorides by sodium borohydride. J. Am. Chem. Soc. 1949, 7, 122–125. [Google Scholar] [CrossRef]
- Moriyasu, M.; Kato, A.; Okada, M.Y.; Hashimoto, Y. HPLC separation of sugar anomers in a very low temperature region. Anal. Lett. 1984, 17, 689–699. [Google Scholar] [CrossRef]
- Sklarz, B. Organic chemistry of periodates. Q. Rev. Chem. Soc. 1967, 21, 3–28. [Google Scholar] [CrossRef]
- Murray, J.S.; Selwood, A.I.; Harwood, D.T.; van Ginkel, R.; Puddick, J.; Rhodes, L.L.; Rise, F.; Wilkins, A.L. 44-Methylgambierone, a new gambierone analogue isolated from Gambierdiscus australes. Tetrahedron Lett. 2019, 60, 621–625. [Google Scholar] [CrossRef]
- Baltzer, K.L. Investigating the Behavioral Effects of Sublethal Caribbean Ciguatoxin Exposure in Zebrafish (Danio rerio). Master’s Thesis, University of the Virgin Islands, St. Thomas, ND, USA, 2015. [Google Scholar]
- Lewis, R.J.; Yang, A.; Jones, A. Rapid extraction combined with LC-tandem mass spectrometry (CREM-LC/MS/MS) for the determination of ciguatoxins in ciguateric fish flesh. Toxicon 2009, 54, 62–66. [Google Scholar] [CrossRef]

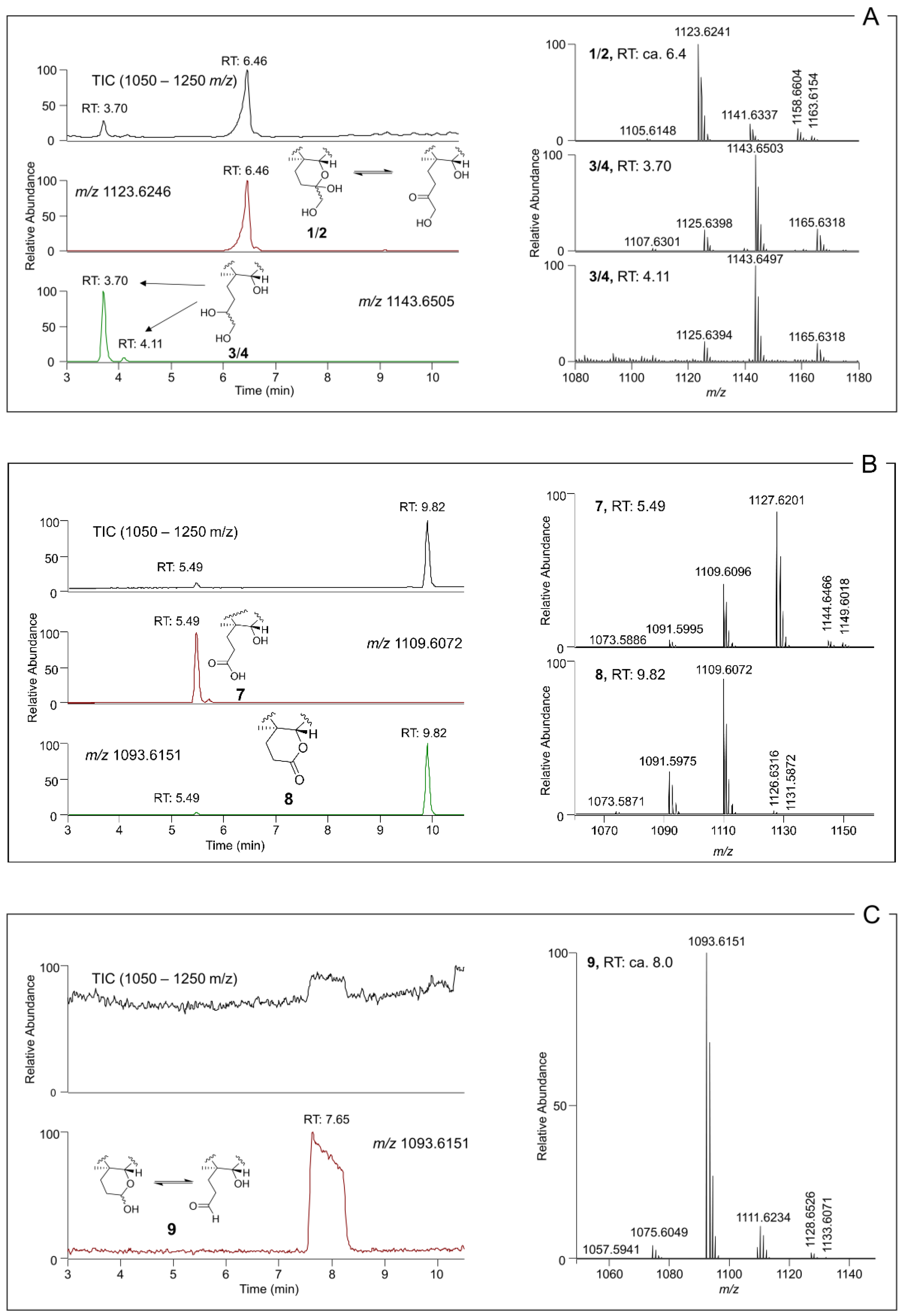
| Retention Time (min) | [M − H2O + H]+ (Δ; Int.) | [M + H]+ (Δ; Int.) | [M + NH4]+ (Δ; Int.) | [M + Na]+ (Δ; Int.) | [M + HCOO]− (Δ; Int.) | Formula (Neutral) | RDBE | |
|---|---|---|---|---|---|---|---|---|
| 1, 2 | ca. 6.4a | 1123.6241 (+3.6; 100) | 1141.6337 (+2.7; 19) | 1158.6604 (+2.8; 20) | 1163.6154 (+2.5; 6) | 1185.6220 (+0.4; 100) | C62H92O19 | 17 |
| 3, 4 | 3.70, 4.11 | 1125.6398 (+3.7; 23) | 1143.6503 (+3.6; 100) | 1160.6767 (+3.4; 5) | 1165.6318 (+3.1; 24) | 1187.6375 (+0.3; 100) | C62H94O19 | 16 |
| 5, 6 | 3.70, 4.11 | 1126.6436 (+1.5; 25) | 1144.6535 (−0.9; 100) | 1161.6798 (+0.7; 3) | 1166.6344 (0.0; 28) | 1188.6446 (+1.0; 100) | C62H93DO19 | 16 |
| 7 | 5.49 | 1109.6096 (+4.7; 47) | 1127.6201 (+4.6; 100) | 1144.6466 (+4.5; 5) | 1149.6018 (4.3; 3) | 1125.6065b (+5.5; 100) | C61H90O19 | 17 |
| 8 | 9.82 | 1091.5975 (+3.4; 32) | 1109.6072 (+2.6; 100) | 1126.6316 (0.6; 2) | 1131.5872 (0.8; 0.3) | n. d. | C61H88O18 | 18 |
| 9 | ca. 8.0a | 1093.6151 (+5.2; 100) | 1111.6234 (+3.1; 11) | 1128.6526 (5.4; 2) | 1133.6071 (4.6; 0.4) | n. d. | C61H90O18 | 17 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kryuchkov, F.; Robertson, A.; Miles, C.O.; Mudge, E.M.; Uhlig, S. LC–HRMS and Chemical Derivatization Strategies for the Structure Elucidation of Caribbean Ciguatoxins: Identification of C-CTX-3 and -4. Mar. Drugs 2020, 18, 182. https://doi.org/10.3390/md18040182
Kryuchkov F, Robertson A, Miles CO, Mudge EM, Uhlig S. LC–HRMS and Chemical Derivatization Strategies for the Structure Elucidation of Caribbean Ciguatoxins: Identification of C-CTX-3 and -4. Marine Drugs. 2020; 18(4):182. https://doi.org/10.3390/md18040182
Chicago/Turabian StyleKryuchkov, Fedor, Alison Robertson, Christopher O. Miles, Elizabeth M. Mudge, and Silvio Uhlig. 2020. "LC–HRMS and Chemical Derivatization Strategies for the Structure Elucidation of Caribbean Ciguatoxins: Identification of C-CTX-3 and -4" Marine Drugs 18, no. 4: 182. https://doi.org/10.3390/md18040182
APA StyleKryuchkov, F., Robertson, A., Miles, C. O., Mudge, E. M., & Uhlig, S. (2020). LC–HRMS and Chemical Derivatization Strategies for the Structure Elucidation of Caribbean Ciguatoxins: Identification of C-CTX-3 and -4. Marine Drugs, 18(4), 182. https://doi.org/10.3390/md18040182