Evaluation of Intestinal Absorption of Dietary Halocynthiaxanthin, a Carotenoid from the Sea Squirt Halocynthia roretzi
Abstract
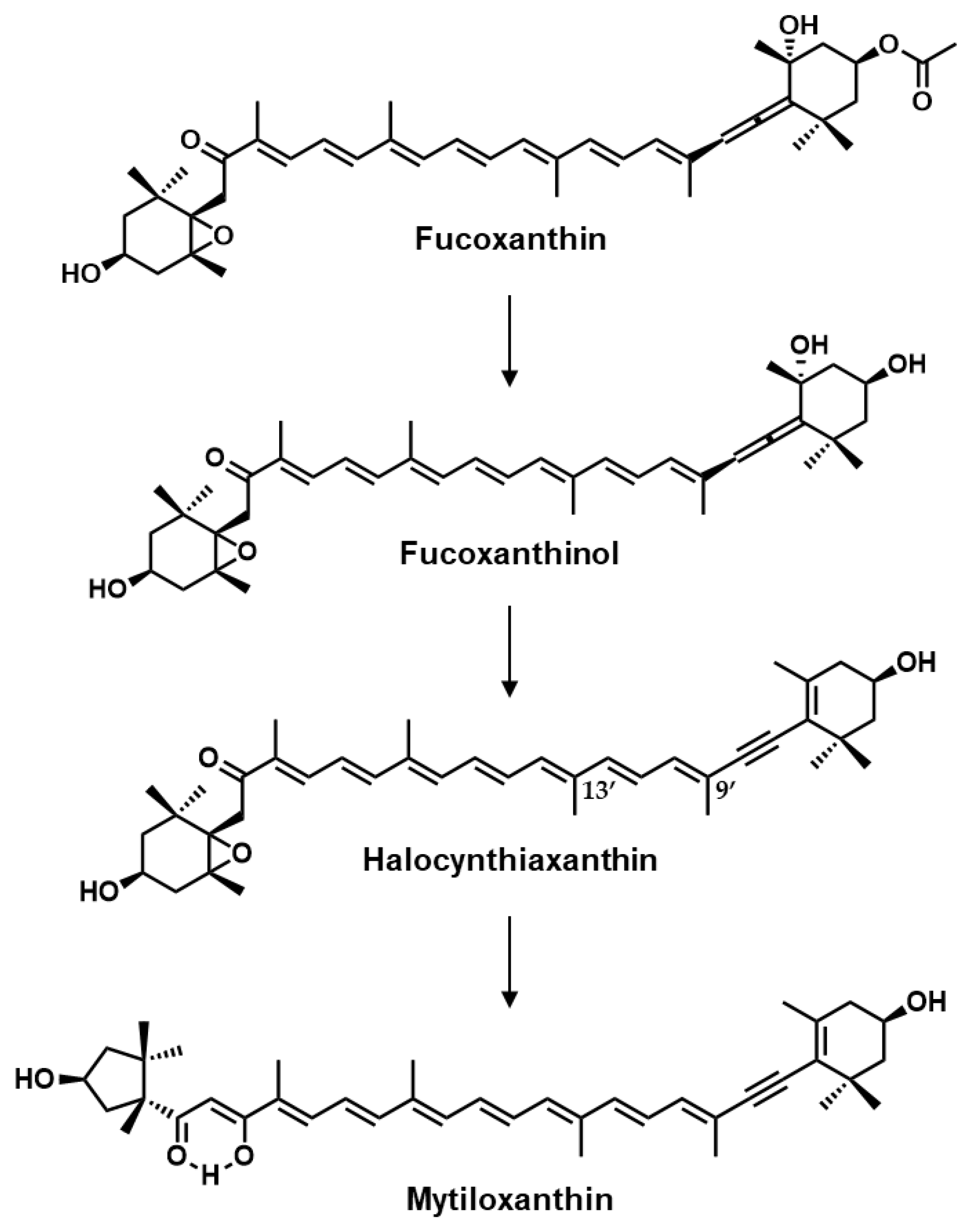
1. Introduction
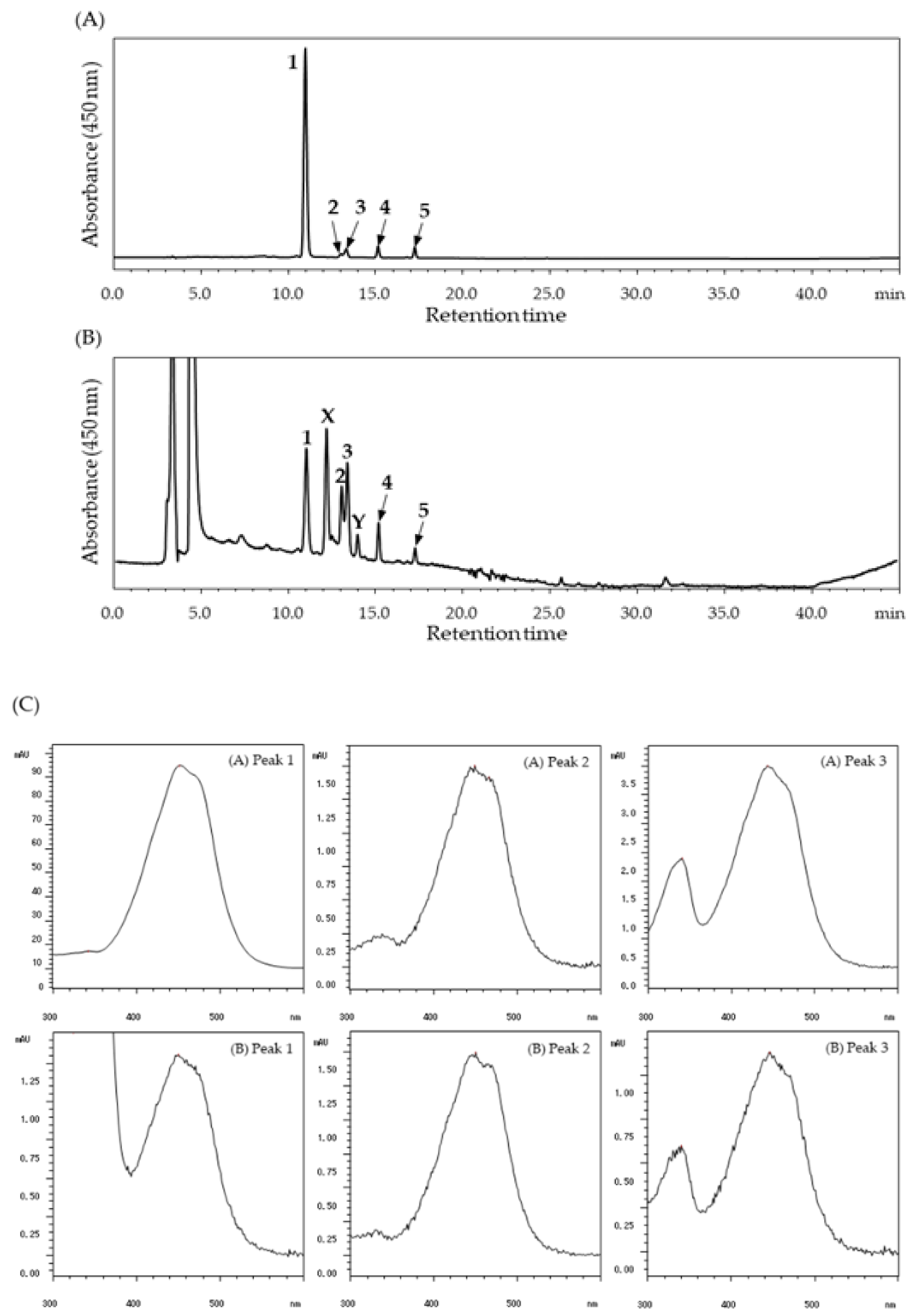
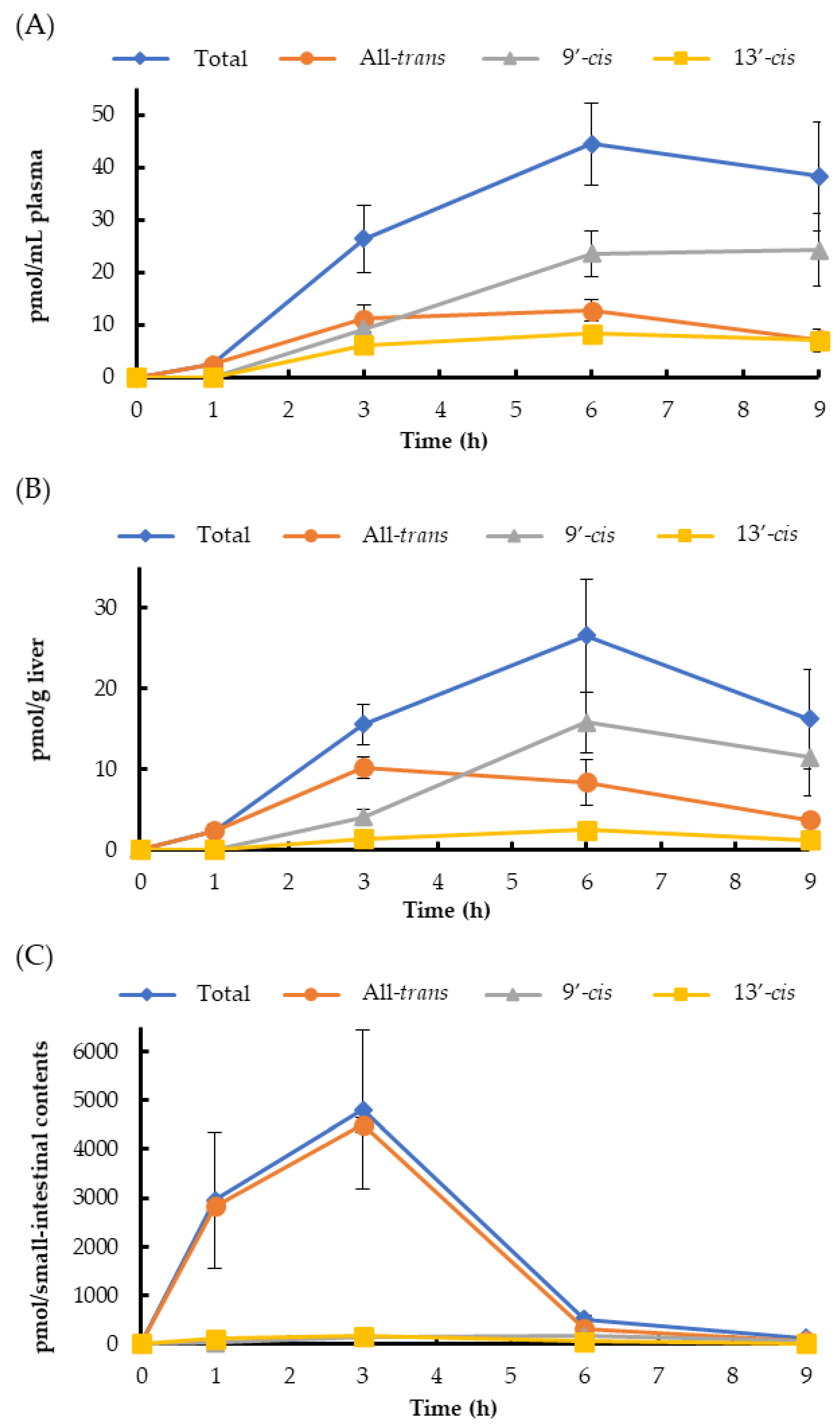
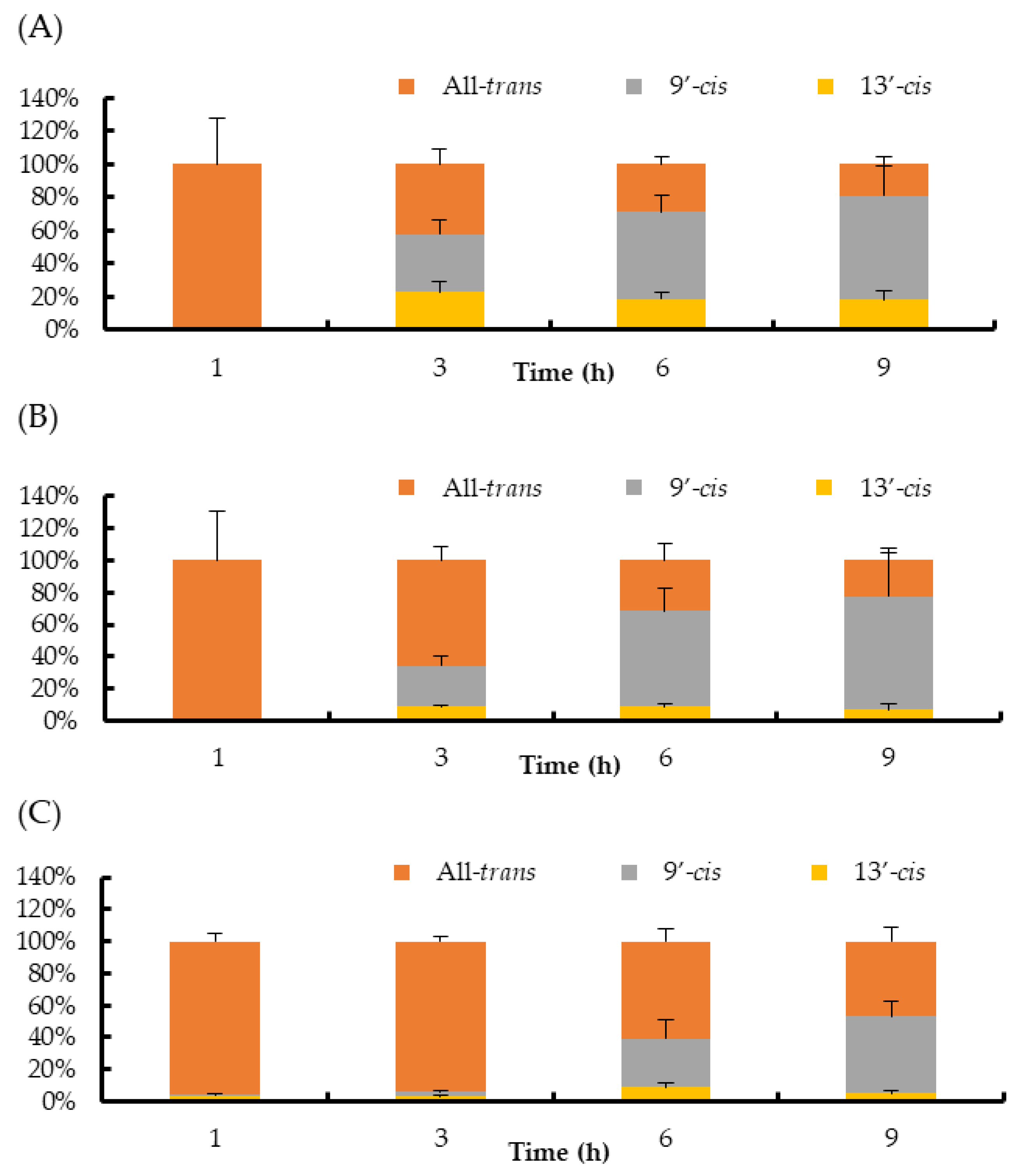
2. Results
3. Discussion
4. Materials and Methods
4.1. Preparation of Halocynthiaxanthin
4.2. Animal Studies
4.3. Lipid Extraction
4.4. HPLC Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Britton, G.; Liaaen-Jensen, S.; Pfander, H. Carotenoids Handbook; Birkhäuser: Basel, Switzerland, 2004. [Google Scholar]
- Maoka, T. Recent progress in structural studies of carotenoids in animals and plants. Arch. Biochem. 2009, 48, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Maoka, T. Carotenoids as natural functional pigments. J. Nat. Med. 2019, 74, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Funayama, K.; Miyashita, K. Fucoxanthin from edible seaweed, Undaria pinnatifida, shows antiobesity effect through UCP1 expression in white adipose tissues. Biochem. Biophys. Res. Commun. 2005, 332, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Shiratori, K.; Ohgami, K.; Ilieva, I.; Jin, X.; Koyama, Y.; Miyashita, K.; Yoshida, K.; Kase, S.; Ohno, S. Effects of fucoxanthin on lipopolysaccharide-induced inflammation in vitro and in vivo. Exp. Eye Res. 2005, 81, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, T.; Ganesan, P.; Li, Z.; Manabe, Y.; Hirata, T. Siphonaxanthin, a green algal carotenoid, as a novel functional compound. Mar. Drugs. 2014, 12, 3660–3668. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, P.; Noda, K.; Manabe, Y.; Ohkubo, T.; Tanaka, Y.; Maoka, T.; Sugawara, T.; Hirata, T. Siphonaxanthin, a marine carotenoid from green algae, effectively induces apoptosis in human leukemia (HL-60) cells. Biochim. Biophys. Acta. 2011, 1810, 497–503. [Google Scholar] [CrossRef]
- Ganesan, P.; Matsubara, K.; Ohkubo, T.; Tanaka, Y.; Noda, K.; Sugawara, T.; Hirata, T. Anti-angiogenic effect of siphonaxanthin from green alga, Codium fragile. Phytomedicine 2010, 17, 1140–1144. [Google Scholar] [CrossRef]
- Ganesan, P.; Matsubara, K.; Sugawara, T.; Hirata, T. Marine algal carotenoids inhibit angiogenesis by down-regulating FGF-2-mediated intracellular signals in vascular endothelial cells. Mol. Cell. Biochem. 2013, 380, 1–9. [Google Scholar] [CrossRef]
- Li, Z.; Noda, K.; Fujita, E.; Manabe, Y.; Hirata, T.; Sugawara, T. The green algal carotenoid siphonaxanthin inhibits adipogenesis in 3T3-L1 preadipocytes and the accumulation of lipids in white adipose tissue of KK-Ay mice. J. Nutr. 2015, 145, 490–498. [Google Scholar] [CrossRef]
- Manabe, Y.; Hirata, T.; Sugawara, T. Suppressive effects of carotenoids on the antigen-induced degranulation in RBL-2H3 rat basophilic leukemia cells. J. Oleo. Sci. 2014, 63, 291–294. [Google Scholar] [CrossRef]
- Matsuno, T.; Ookubo, M. A new carotenoid, halocynthiaxanthin from the sea squirt, Halocynthia roretzi. Tetrahedron Lett. 1981, 22, 4659–4660. [Google Scholar] [CrossRef]
- Matsuno, T.; Ookubo, M.; Nishizara, T.; Shimizu, I. Carotenoids of sea squirts. I. New marine carotenoids, halocynthiaxanthin and mytiloxanthinone from Halocynthia roretzi. Chem. Pharm. Bull. 1984, 32, 4309–4315. [Google Scholar] [CrossRef]
- Palanisamy, S.K.; Trisciuoglio, D.; Zwergel, C.; Del Bufalo, D.; Mai, A. Metabolite profiling of ascidian Styela plicata using LC–MS with multivariate statistical analysis and their antitumor activity. J. Enzyme Inhib. Med. 2017, 32, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Matsuno, T.; Ookubo, M. Carotenoids of sea squirts: II. Comparative biochemical studies of carotenoids in sea squirts. Comp. Biochem. Physiol. 1985, 81, 137–141. [Google Scholar]
- Maoka, T. Carotenoids in Marine Animals. Mar. Drugs. 2011, 9, 278–293. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, T.; Baskaran, V.; Tsuzuki, W.; Nagao, A. Brown algae fucoxanthin is hydrolyzed to fucoxanthinol during absorption by Caco-2 human intestinal cells and mice. J. Nutr. 2002, 132, 946–951. [Google Scholar] [CrossRef] [PubMed]
- Nishino, H.; Tsushima, M.; Matsuno, T.; Tanaka, Y.; Okuzumi, J.; Murakoshi, M.; Satomi, Y.; Takayasu, J.; Tokuda, H.; Nishino, A.; et al. Anti-neoplastic effect of halocynthiaxanthin, a metabolite of fucoxanthin. Anticancer Drugs. 1992, 3, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Konishi, I.; Hosokawa, M.; Sashima, T.; Kobayashi, H.; Miyashita, K. Halocynthiaxanthin and fucoxanthinol isolated from Halocynthia roretzi induce apoptosis in human leukemia, breast and colon cancer cells. Comp. Biochem. Physiol. 2006, 142, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Tsushima, M.; Maoka, T.; Katsuyama, M.; Kozuka, M.; Matsuno, T.; Tokuda, H.; Nishino, H.; Iwashima, A. Inhibitory effect of natural carotenoids on Epstein-Barr virus activation activity of a tumor promoter in Raji cells. A screening study for anti-tumor promoters. Biol. Pharm. Bull. 1995, 18, 227–233. [Google Scholar] [CrossRef]
- Murakami, A.; Nakashima, M.; Koshiba, T.; Maoka, T.; Nishino, H.; Yano, M.; Sumida, T.; Kim, O.; Koshimizu, K.; Ohigashi, H. Modifying effects of carotenoids on superoxide and nitric oxide generation from stimulated leucocytes. Cancer Lett. 2000, 149, 115–123. [Google Scholar] [CrossRef]
- Manabe, Y.; Hirata, T.; Sugawara, T. Inhibitory effect of carotenoids on ligand-induced lipid raft translocation of immunoreceptors. J. Oleo Sci. 2019, 68, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Yonekura, L.; Nagao, A. Intestinal absorption of dietary carotenoids. Mol. Nutr. Food. Res. 2007, 51, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Kotake-Nara, A.; Kim, S.J.; Kobori, M.; Miyashita, K.; Nagao, A. Acyclo-retinoic acid induces apoptosis in human prostate cancer cells. Anticancer Res. 2002, 22, 689–695. [Google Scholar] [PubMed]
- Asai, A.; Sugawara, T.; Ono, H.; Nagao, A. Biotransformation of fucoxanthinol into amarouciaxanthin A in mice and HepG2 cells: Formation and cytotoxicity of fucoxanthin metabolites. Drug Metab. Dispos. 2004, 32, 205–211. [Google Scholar] [CrossRef]
- Hashimoto, T.; Ozaki, Y.; Taminato, M.; Das, S.K.; Mizuno, M.; Yoshimura, K.; Maoka, T.; Kanazawa, K. The distribution and accumulation of fucoxanthin and its metabolites after oral administration in mice. Br. J. Nutr. 2009, 102, 242–248. [Google Scholar] [CrossRef]
- Zhou, Q.; Xu, J.; Yang, L.; Gu, C.; Xue, C. Thermal stability and oral absorbability of astaxanthin esters from Haematococcus pluvialis in Balb/c mice. J. Sci. Food. Agric. 2019, 99, 3662–3671. [Google Scholar] [CrossRef]
- Baskaran, V.; Sugawara, T.; Nagao, A. Phospholipids affect intestinal absorption of carotenoids in mice. Lipids 2003, 38, 705–711. [Google Scholar] [CrossRef]
- Clinton, S.K. Lycopene: Chemistry, biology, and implications for human health and disease. Nutr. Rev. 1998, 56, 35–51. [Google Scholar] [CrossRef]
- Honest, K.; Wei Zhang, H.; Zhang, L. Lycopene: Isomerization effects on bioavailability and bioactivity properties. Food Rev. Int. 2011, 27, 248–258. [Google Scholar] [CrossRef]
- Nakazawa, Y.; Sashima, T.; Hosokawa, M.; Miyashita, K. Comparative evaluation of growth inhibitory effect of stereoisomers of fucoxanthin in human cancer cell lines. J. Funct. Foods 2009, 1, 88–97. [Google Scholar] [CrossRef]
- Liu, X.; Osawa, T. Cis astaxanthin and especially 9-cis astaxanthin exhibits a higher antioxidant activity in vitro compared to the all-trans isomer. Biochem. Biophys. Res. Commun. 2007, 357, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Böhm, V.; Puspitasari-Nienaber, N.; Ferruzzi, M.; Schwartz, S. Trolox equivalent antioxidant capacity of different geometrical isomers of α-carotene, β-carotene, lycopene, and zeaxanthin. J. Agric. Food Chem. 2002, 50, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zheng, J.; Luo, X.; Manabe, Y.; Hirata, T.; Sugawara, T. Absorption and Tissue Distribution of Siphonaxanthin from Green Algae. Mar. Drugs 2020, 18, 291. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikeda, C.; Manabe, Y.; Tomonaga, N.; Wada, T.; Maoka, T.; Sugawara, T. Evaluation of Intestinal Absorption of Dietary Halocynthiaxanthin, a Carotenoid from the Sea Squirt Halocynthia roretzi. Mar. Drugs 2020, 18, 588. https://doi.org/10.3390/md18120588
Ikeda C, Manabe Y, Tomonaga N, Wada T, Maoka T, Sugawara T. Evaluation of Intestinal Absorption of Dietary Halocynthiaxanthin, a Carotenoid from the Sea Squirt Halocynthia roretzi. Marine Drugs. 2020; 18(12):588. https://doi.org/10.3390/md18120588
Chicago/Turabian StyleIkeda, Chiaki, Yuki Manabe, Nami Tomonaga, Tatsuya Wada, Takashi Maoka, and Tatsuya Sugawara. 2020. "Evaluation of Intestinal Absorption of Dietary Halocynthiaxanthin, a Carotenoid from the Sea Squirt Halocynthia roretzi" Marine Drugs 18, no. 12: 588. https://doi.org/10.3390/md18120588
APA StyleIkeda, C., Manabe, Y., Tomonaga, N., Wada, T., Maoka, T., & Sugawara, T. (2020). Evaluation of Intestinal Absorption of Dietary Halocynthiaxanthin, a Carotenoid from the Sea Squirt Halocynthia roretzi. Marine Drugs, 18(12), 588. https://doi.org/10.3390/md18120588




