Dietary Supplementation of Astaxanthin Improved the Growth Performance, Antioxidant Ability and Immune Response of Juvenile Largemouth Bass (Micropterus salmoides) Fed High-Fat Diet
Abstract
1. Introduction
2. Results
2.1. Growth Performance, Feed Utilization and Somatic Parameters
2.2. Whole Body and Muscle Proximate Composition
2.3. Plasma and Hepatic Biochemical Indexes
2.4. Lipid Accumulation in the Liver
2.5. Lipid Metabolism Related Gene Expression in Liver
2.6. Oxidative Stress and Anti-Oxidative Related Parameters in Liver and Plasma
2.7. Immune Response Related Gene Expression in Liver
3. Discussion
4. Materials and Methods
4.1. Diet Preparation
4.2. Fish Rearing and Experimental Conditions
4.3. Sample Collection and Biochemical Composition Analysis
4.4. Proximate Analysis of Diets and Body Composition
4.5. Antioxidative Related Parameters Analysis and Biochemical Index Assays
4.6. Quantitative Real-Time PCR Analysis
| Gene | Primer Sequence | Products | Sources |
|---|---|---|---|
| EF1α F | GGCTGGTATCTCCAAGAACG | 239 | [60] |
| EF1α R | GTCTCCAGCATGTTGTCWCC | ||
| GSH-px F | GGGGCTCCACCTGCTTCTTG | / | FJ030930 |
| GSH-px R | ACCCCTCTGCTCAGGCATTT | ||
| SOD F | TGGCAAGAACAAGAACCACA | 167 | FJ030929 |
| SOD R | CCTCTGATTTCTCCTGTCACC | ||
| FXR F | AGAAATGGCAACAAGTCAA | 77 | KT827789.1 |
| FXR R | CACGGTCCAGAGAGAGAAA | ||
| PPARα F | CCACCGCAATGGTCGATATG | 161 | [61] |
| PPARα R | TGCTGTTGATGGACTGGGAAA | ||
| HBP F | AAATCCAAATCCCACGAC | 134 | KF652241.1 |
| HBP R | CACCCTCTCTACAGCACG | ||
| PPAR-γ F | CCTGTGAGGGCTGTAAGGGTTT | 118 | [61] |
| PPAR-γ R | TTGTTGCGGGACTTCTTGTGA | ||
| IL-15 F | GTATGCTGCTTCTGTGCCTGG | 82 | [61] |
| IL-15 R | AGCGTCAGATTTCTCAATGGTGT | ||
| TGF-β F | GCTCAAAGAGAGCGAGGATG | 118 | [61] |
| TGF-β R | TCCTCTACCATTCGCAATCC | ||
| Caspase3 F | GCTTCATTCGTCTGTGTTC | 98 | [61] |
| Caspase3 R | CGAAAAAGTGATGTGAGGTA | ||
| Caspase9 F | CTGGAATGCCTTCAGGAGACGGG | 125 | [61] |
| Caspase9 R | GGGAGGGGCAAGACAACAGGGTG | ||
| Caspase10 F | CAAACCACTCACAGCGTCTACAT | 146 | [61] |
| Caspase10 R | TGGTTGGTTGAGGACAGAGAGGG | ||
| HSP 70 F | CAGTGATGAAGACAAGCAGAAGA | 163 | [62] |
| HSP 70 R | GCCACCAGCACTCTGATACA | ||
| Bcl-2 F | CCATCCACGACGAACCTG | 75 | / |
| Bcl-2 R | GGCGTATCGCTGCTCAAACT | ||
| Bcl-xL F | CATCCTCCTTGGCTCTGG | 141 | / |
| Bcl-xL R | GGGTCTGTTTGCCTTTGG | ||
| RXRα F | AGCAGAGCAGGCAGTGGA | 144 | KT827793.1 |
| RXRα R | CGTTGGGCGAGTTGGAT | ||
| HMGCR F | GGTGGAGTGCTTAGTAATCGG | 125 | / |
| HMGCR R | ACGCAGGGAAGAAAGTCAT | ||
| BAD F | CACATTTCGGATGCCACTAT | 116 | / |
| BAD R | TTCTGCTCTTCTGCGATTGA | ||
| P53 F | AGATTGAATGGTGGTGGG | 144 | / |
| P53 R | GTTCTGGCGGACTGGA |
4.7. Oil Red O Staining
4.8. Calculations and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BAD | Bcl-2-associated death protein |
| Bcl-2 | B-cell lymphoma-2 |
| Caspase3 | cysteine-aspartic proteases-3 |
| Caspase9 | cysteine-aspartic proteases-9 |
| Caspase10 | cysteine-aspartic proteases-10 |
| CF | condition factor |
| EF1α | elongation factor 1α |
| FBW | final body weight |
| FXR | farnesoid X receptor |
| HDL-C | high-density lipoprotein cholesterol |
| HFD | high fat diet |
| HIS | hepatosomatic index |
| HBP | lipoprotein binding protein |
| HMGCR | 3-hydroxy-3-methylglutaryl-coenzyme A reductase |
| HSP70 | Heat shock protein 70 |
| IBW | initial body weight |
| IPF | intraperitoneal fat ratio |
| LDL-C | low-density lipoprotein cholesterol |
| IL15 | interleukin 15 |
| MDA | malondialdehyde |
| PER | protein efficiency ratio |
| PPARα | peroxisome proliferator activating receptor α |
| PPARγ | proliferator activating receptor γ |
| RXR | retinoid X receptor |
| SOD | superoxide dismutase |
| TC | total cholesterol |
| TG | total triglyceride |
| TGFβ | transforming growth factor-β |
| VSI | viscerosomatic index |
| WG | weight gain |
References
- Ministry of Agriculture Fishery and Fishery Administration Bureau. China Fisheries Statistics Yearbook; China Agriculture Press: Beijing, China, 2020.
- Zhang, Y.; Xie, S.; Wei, H.; Zheng, L.; Liu, Z.; Fang, H.; Xie, J.; Liao, S.; Tian, L.; Liu, Y.; et al. High dietary starch impaired growth performance, liver histology and hepatic glucose metabolism of juvenile largemouth bass, Micropterus salmoides. Aquac. Nutr. 2020, 26, 1083–1095. [Google Scholar] [CrossRef]
- Ma, H.-J.; Mou, M.-M.; Pu, D.-C.; Lin, S.-M.; Chen, Y.-J.; Luo, L. Effect of dietary starch level on growth, metabolism enzyme and oxidative status of juvenile largemouth bass, Micropterus salmoides. Aquaculture 2019, 498, 482–487. [Google Scholar] [CrossRef]
- Glencross, B.D. Exploring the nutritional demand for essential fatty acids by aquaculture species. Rev. Aquac. 2009, 1, 71–124. [Google Scholar] [CrossRef]
- Huang, D.; Wu, Y.; Lin, Y.; Chen, J.; Karrow, N.; Ren, X.; Wang, Y. Dietary Protein and Lipid Requirements for Juvenile Largemouth Bass, Micropterus salmoides. J. World Aquac. Soc. 2017, 48, 782–790. [Google Scholar] [CrossRef]
- Sagada, G.; Chen, J.; Shen, B.; Huang, A.; Sun, L.; Jiang, J.; Jin, C. Optimizing protein and lipid levels in practical diet for juvenile northern snakehead fish (Channa argus). Anim. Nutr. 2017, 3, 156–163. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, W.; Gladstone, S.; Ng, W.-K.; Zhang, J.; Shao, Q. Effects of isoenergetic diets with varying protein and lipid levels on the growth, feed utilization, metabolic enzymes activities, antioxidative status and serum biochemical parameters of black sea bream (Acanthopagrus schlegelii). Aquaculture 2019, 513, 734397. [Google Scholar] [CrossRef]
- Chatzifotis, S.; Panagiotidou, M.; Papaioannou, N.; Pavlidis, M.; Nengas, I.; Mylonas, C.C. Effect of dietary lipid levels on growth, feed utilization, body composition and serum metabolites of meagre (Argyrosomus regius) juveniles. Aquaculture 2010, 307, 65–70. [Google Scholar] [CrossRef]
- Morais, S.; Bell, J.; Robertson, D.A.; Roy, W.J.; Morris, P.C. Protein/lipid ratios in extruded diets for Atlantic cod (Gadus morhua L.): Effects on growth, feed utilisation, muscle composition and liver histology. Aquaculture 2001, 203, 101–119. [Google Scholar] [CrossRef]
- Wang, J.-T.; Liu, Y.-J.; Tian, L.-X.; Mai, K.-S.; Du, Z.-Y.; Wang, Y.; Yang, H.-J. Effect of dietary lipid level on growth performance, lipid deposition, hepatic lipogenesis in juvenile cobia (Rachycentron canadum). Aquaculture 2005, 249, 439–447. [Google Scholar] [CrossRef]
- Bright, L.A.; Coyle, S.D.; Tidwell, J.H. Effect of Dietary Lipid Level and Protein Energy Ratio on Growth and Body Composition of Largemouth Bass Micropterus salmoides. J. World Aquac. Soc. 2005, 36, 129–134. [Google Scholar] [CrossRef]
- Yin, P.; Xie, S.; Zhuang, Z.; He, X.; Tang, X.; Tian, L.; Liu, Y.; Niu, J. Dietary supplementation of bile acid attenuate adverse effects of high-fat diet on growth performance, antioxidant ability, lipid accumulation and intestinal health in juvenile largemouth bass (Micropterus salmoides). Aquaculture 2021, 531, 735864. [Google Scholar] [CrossRef]
- Zhou, Y.-L.; Guo, J.-L.; Tang, R.-J.; Ma, H.-J.; Chen, Y.-J.; Lin, S.-M. High dietary lipid level alters the growth, hepatic metabolism enzyme, and anti-oxidative capacity in juvenile largemouth bass Micropterus salmoides. Fish Physiol. Biochem. 2019, 46, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, S.; Abbaszadeh, F.; Dargahi, L.; Jorjani, M. Astaxanthin: A mechanistic review on its biological activities and health benefits. Pharmacol. Res. 2018, 136, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Fang, N.; Wang, C.; Liu, X.; Zhao, X.; Liu, Y.; Liu, X.; Du, Y.; Zhang, Z.; Zhang, H. De novo synthesis of astaxanthin: From organisms to genes. Trends Food Sci. Technol. 2019, 92, 162–171. [Google Scholar] [CrossRef]
- Yi, X.; Xu, W.; Zhou, H.; Zhang, Y.; Luo, Y.; Zhang, W.; Mai, K. Effects of dietary astaxanthin and xanthophylls on the growth and skin pigmentation of large yellow croaker Larimichthys croceus. Aquaculture 2014, 433, 377–383. [Google Scholar] [CrossRef]
- Choubert, G.; Mendes-Pinto, M.M.; Morais, R. Pigmenting efficacy of astaxanthin fed to rainbow trout Oncorhynchus mykiss: Effect of dietary astaxanthin and lipid sources. Aquaculture 2006, 257, 429–436. [Google Scholar] [CrossRef]
- Palma, J.; Andrade, J.; Bureau, D. The impact of dietary supplementation with astaxanthin on egg quality and growth of long snout seahorse (Hippocampus guttulatus) juveniles. Aquac. Nutr. 2017, 23, 304–312. [Google Scholar] [CrossRef]
- Li, M.; Sun, L.; Niu, X.; Chen, X.; Tian, J.; Kong, Y.; Wang, G.Q. Astaxanthin protects lipopolysaccharide-induced inflammatory response in Channa argus through inhibiting NF-κB and MAPKs signaling pathways. Fish Shellfish Immunol. 2019, 86, 280–286. [Google Scholar] [CrossRef]
- Xie, S.; Fang, W.; Wei, D.; Liu, Y.; Yin, P.; Niu, J.; Tian, L.-X. Dietary supplementation of Haematococcus pluvialis improved the immune capacity and low salinity tolerance ability of post-larval white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 2018, 80, 452–457. [Google Scholar] [CrossRef]
- Jiang, X.; Zu, L.; Wang, Z.; Cheng, Y.; Yang, Y.; Wu, X. Micro-algal astaxanthin could improve the antioxidant capability, immunity and ammonia resistance of juvenile Chinese mitten crab, Eriocheir sinensis. Fish Shellfish Immunol. 2020, 102, 499–510. [Google Scholar] [CrossRef]
- Wang, W.; Ishikawa, M.; Koshio, S.; Yokoyama, S.; Hossain, S.; Moss, A.S. Effects of dietary astaxanthin supplementation on juvenile kuruma shrimp, Marsupenaeus japonicus. Aquaculture 2018, 491, 197–204. [Google Scholar] [CrossRef]
- Xie, J.; Fang, H.; He, X.; Liao, S.; Liu, Y.; Tian, L.; Niu, J. Study on mechanism of synthetic astaxanthin and Haematococcus pluvialis improving the growth performance and antioxidant capacity under acute hypoxia stress of golden pompano (Trachinotus ovatus) and enhancing anti-inflammatory by activating Nrf2-ARE pathway to antagonize the NF-κB pathway. Aquaculture 2020, 518, 734657. [Google Scholar]
- Lim, K.C.; Yusoff, F.M.; Shariff, M.; Kamarudin, M.S.; Nagao, N. Dietary supplementation of astaxanthin enhances hemato-biochemistry and innate immunity of Asian seabass, Lates calcarifer (Bloch, 1790). Aquaculture 2019, 512, 734339. [Google Scholar] [CrossRef]
- Yang, Y.; Pham, T.X.; Wegner, C.J.; Kim, B.; Ku, C.S.; Park, Y.-K.; Lee, J.-Y. Astaxanthin lowers plasma TAG concentrations and increases hepatic antioxidant gene expression in diet-induced obesity mice. Br. J. Nutr. 2014, 112, 1797–1804. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Wu, C.; Kim, J.; Kim, B.; Lee, S.-J. Astaxanthin reduces hepatic lipid accumulations in high-fat-fed C57BL/6J mice via activation of peroxisome proliferator-activated receptor (PPAR) alpha and inhibition of PPAR gamma and Akt. J. Nutr. Biochem. 2016, 28, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.-Y.; Liu, Y.-J.; Tian, L.-X.; Wang, J.-T.; Wang, Y.; Liang, G.-Y. Effect of dietary lipid level on growth, feed utilization and body composition by juvenile grass carp (Ctenopharyngodon idella). Aquac. Nutr. 2005, 11, 139–146. [Google Scholar] [CrossRef]
- Han, T.; Li, X.; Wang, J.; Hu, S.; Jiang, Y.; Zhong, X. Effect of dietary lipid level on growth, feed utilization and body composition of juvenile giant croaker Nibea japonica. Aquaculture 2014, 434, 145–150. [Google Scholar] [CrossRef]
- Jia, R.; Cao, L.-P.; Du, J.-L.; He, Q.; Gu, Z.-Y.; Jeney, G.; Xu, P.; Yin, G. Effects of high-fat diet on antioxidative status, apoptosis and inflammation in liver of tilapia (Oreochromis niloticus) via Nrf2, TLRs and JNK pathways. Fish Shellfish Immunol. 2020, 104, 391–401. [Google Scholar] [CrossRef]
- Dai, Y.-J.; Cao, X.-F.; Zhang, D.-D.; Li, X.-F.; Liu, W.-B.; Jiang, G. Chronic inflammation is a key to inducing liver injury in blunt snout bream (Megalobrama amblycephala) fed with high-fat diet. Dev. Comp. Immunol. 2019, 97, 28–37. [Google Scholar] [CrossRef]
- Guo, J.-L.; Zhou, Y.-L.; Zhao, H.; Chen, W.-Y.; Chen, Y.-J.; Lin, S.-M. Effect of dietary lipid level on growth, lipid metabolism and oxidative status of largemouth bass, Micropterus salmoides. Aquaculture 2019, 506, 394–400. [Google Scholar] [CrossRef]
- Kalinowski, C.T.; Robaina, L.E.; Izquierdo, M. Effect of dietary astaxanthin on the growth performance, lipid composition and post-mortem skin colouration of red porgy Pagrus pagrus. Aquac. Int. 2011, 19, 811–823. [Google Scholar] [CrossRef]
- Ikeuchi, M.; Koyama, T.; Takahashi, J.; Yazawa, K. Effects of Astaxanthin in Obese Mice Fed a High-Fat Diet. Biosci. Biotechnol. Biochem. 2007, 71, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Tang, N.; Kord-Varkaneh, H.; Low, T.Y.; Tan, S.C.; Wu, X.; Zhu, Y. The effects of astaxanthin supplementation on obesity, blood pressure, CRP, glycemic biomarkers, and lipid profile: A meta-analysis of randomized controlled trials. Pharmacol. Res. 2020, 161, 105113. [Google Scholar] [CrossRef] [PubMed]
- Ursoniu, S.; Sahebkar, A.; Serban, M.-C.; Banach, M. Lipid profile and glucose changes after supplementation with astaxanthin: A systematic review and meta-analysis of randomized controlled trials. Arch. Med. Sci. AMS 2015, 11, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Ning, L.-J.; He, A.-Y.; Li, J.-M.; Lu, D.-L.; Jiao, J.-G.; Li, L.-Y.; Zhang, M.-L.; Chen, L.; Du, Z.-Y. Mechanisms and metabolic regulation of PPARα activation in Nile tilapia (Oreochromis niloticus). Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2016, 1861, 1036–1048. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Yin, P.; Tian, L.; Liu, Y.; Niu, J. Lipid metabolism and plasma metabolomics of juvenile largemouth bass Micropterus salmoides were affected by dietary oxidized fish oil. Aquaculture 2020, 522, 735158. [Google Scholar] [CrossRef]
- Vial, G.; Dubouchaud, H.; Couturier, K.; Cottet-Rousselle, C.; Taleux, N.; Athias, A.; Galinier, A.; Casteilla, L.; Leverve, X.M. Effects of a high-fat diet on energy metabolism and ROS production in rat liver. J. Hepatol. 2011, 54, 348–356. [Google Scholar] [CrossRef]
- Weihong, C.; Bin, C.; JianFeng, Y. Transmembrane protein 126B protects against high fat diet (HFD)-induced renal injury by suppressing dyslipidemia via inhibition of ROS. Biochem. Biophys. Res. Commun. 2019, 509, 40–47. [Google Scholar] [CrossRef]
- Tang, T.; Hu, Y.; Peng, M.; Chu, W.; Hu, Y.; Zhong, L. Effects of high-fat diet on growth performance, lipid accumulation and lipid metabolism-related MicroRNA/gene expression in the liver of grass carp (Ctenopharyngodon idella). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2019, 234, 34–40. [Google Scholar] [CrossRef]
- Lesser, M.P. Oxidative Stress in Marine Environments: Biochemistry and Physiological Ecology. Annu. Rev. Physiol. 2006, 68, 253–278. [Google Scholar] [CrossRef]
- Jagruthi, C.; Yogeshwari, G.; Anbazahan, S.M.; Mari, L.S.S.; Arockiaraj, J.; Mariappan, P.; Sudhakar, G.R.L.; Balasundaram, C.; Harikrishnan, R. Effect of dietary astaxanthin against Aeromonas hydrophila infection in common carp, Cyprinus carpio. Fish Shellfish Immunol. 2014, 41, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Liu, Y.; Yin, P.; Zhou, W.; Tian, L.-X.; Liu, Y.; Xu, D.; Niu, J. Astaxanthin Attenuates Fish Oil-Related Hepatotoxicity and Oxidative Insult in Juvenile Pacific White Shrimp (Litopenaeus vannamei). Mar. Drugs 2020, 18, 218. [Google Scholar] [CrossRef] [PubMed]
- Koruk, M.; Taysi, S.; Savas, M.C.; Yilmaz, O.; Akçay, F.; Karakök, M. Oxidative stress and enzymatic antioxidant status in patients with nonalcoholic steatohepatitis. Ann. Clin. Lab. Sci. 2004, 34, 57–62. [Google Scholar] [PubMed]
- Bhuvaneswari, S.; Yogalakshmi, B.; Sreeja, S.; Anuradha, C.V. Astaxanthin reduces hepatic endoplasmic reticulum stress and nuclear factor-κB-mediated inflammation in high fructose and high fat diet-fed mice. Cell Stress Chaperones 2014, 19, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stokes, J.; Singh, U.P.; Gunn, K.S.; Acharya, A.; Manne, U.; Mishra, M.K. Targeting Hsp70: A possible therapy for cancer. Cancer Lett. 2016, 374, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Breckenridge, D.G.; Xue, D. Regulation of mitochondrial membrane permeabilization by BCL-2 family proteins and caspases. Curr. Opin. Cell Biol. 2004, 16, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Cheng, E.H.-Y.; Wei, M.C.; Weiler, S.; Flavell, R.; Mak, T.W.; Lindsten, T.; Korsmeyer, S.J. BCL-2, BCL-XL Sequester BH3 Domain-Only Molecules Preventing BAX- and BAK-Mediated Mitochondrial Apoptosis. Mol. Cell 2001, 8, 705–711. [Google Scholar] [CrossRef]
- Fridman, J.S.; Lowe, S.W. Control of apoptosis by p53. Oncogene 2003, 22, 9030–9040. [Google Scholar] [CrossRef]
- Riedl, S.J.; Shi, Y. Molecular mechanisms of caspase regulation during apoptosis. Nat. Rev. Mol. Cell Biol. 2004, 5, 897–907. [Google Scholar] [CrossRef]
- Fogarty, C.E.; Bergmann, A. Killers creating new life: Caspases drive apoptosis-induced proliferation in tissue repair and disease. Cell Death Differ. 2017, 24, 1390–1400. [Google Scholar] [CrossRef]
- Latimer, G.E.; Horwitz, W. Official Methods of Analysis of AOAC International; AOAC International: Rockville, MD, USA, 2012. [Google Scholar]
- Janero, D.R. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic. Biol. Med. 1990, 9, 515–540. [Google Scholar] [CrossRef]
- Ōyanagui, Y. Reevaluation of assay methods and establishment of kit for superoxide dismutase activity. Anal. Biochem. 1984, 142, 290–296. [Google Scholar] [CrossRef]
- Bucolo, G.; David, H. Quantitative determination of serum triglycerides by the use of enzymes. Clin. Chem. 1973, 19, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Allain, C.C.; Poon, L.S.; Chan, C.S.; Richmond, W.; Fu, P.C. Enzymatic determination of total serum cholesterol. Clin. Chem. 1974, 20, 470–475. [Google Scholar] [CrossRef]
- Liu, K.-Z.; Shaw, R.A.; Man, A.; Dembinski, T.C.; Mantsch, H.H. Reagent-free, Simultaneous Determination of Serum Cholesterol in HDL and LDL by Infrared Spectroscopy. Clin. Chem. 2002, 48, 499–506. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Chen, N.; Jin, L.; Zhou, H.; Qiu, X. Effects of dietary arginine levels and carbohydrate-to-lipid ratios on mRNA expression of growth-related hormones in largemouth bass, Micropterus salmoides. Gen. Comp. Endocrinol. 2012, 179, 121–127. [Google Scholar] [CrossRef]
- Yu, L.; Yu, H.; Liang, X.; Li, N.; Wang, X.; Li, F.; Wu, X.; Zheng, Y.; Xue, M. Dietary butylated hydroxytoluene improves lipid metabolism, antioxidant and anti-apoptotic response of largemouth bass (Micropterus salmoides). Fish Shellfish Immunol. 2018, 72, 220–229. [Google Scholar] [CrossRef]
- Martyniuk, C.J.; Feswick, A.; Spade, D.J.; Kroll, K.J.; Barber, D.S.; Denslow, N.D. Effects of acute dieldrin exposure on neurotransmitters and global gene transcription in largemouth bass (Micropterus salmoides) hypothalamus. Neurotoxicology 2010, 31, 356–366. [Google Scholar] [CrossRef]
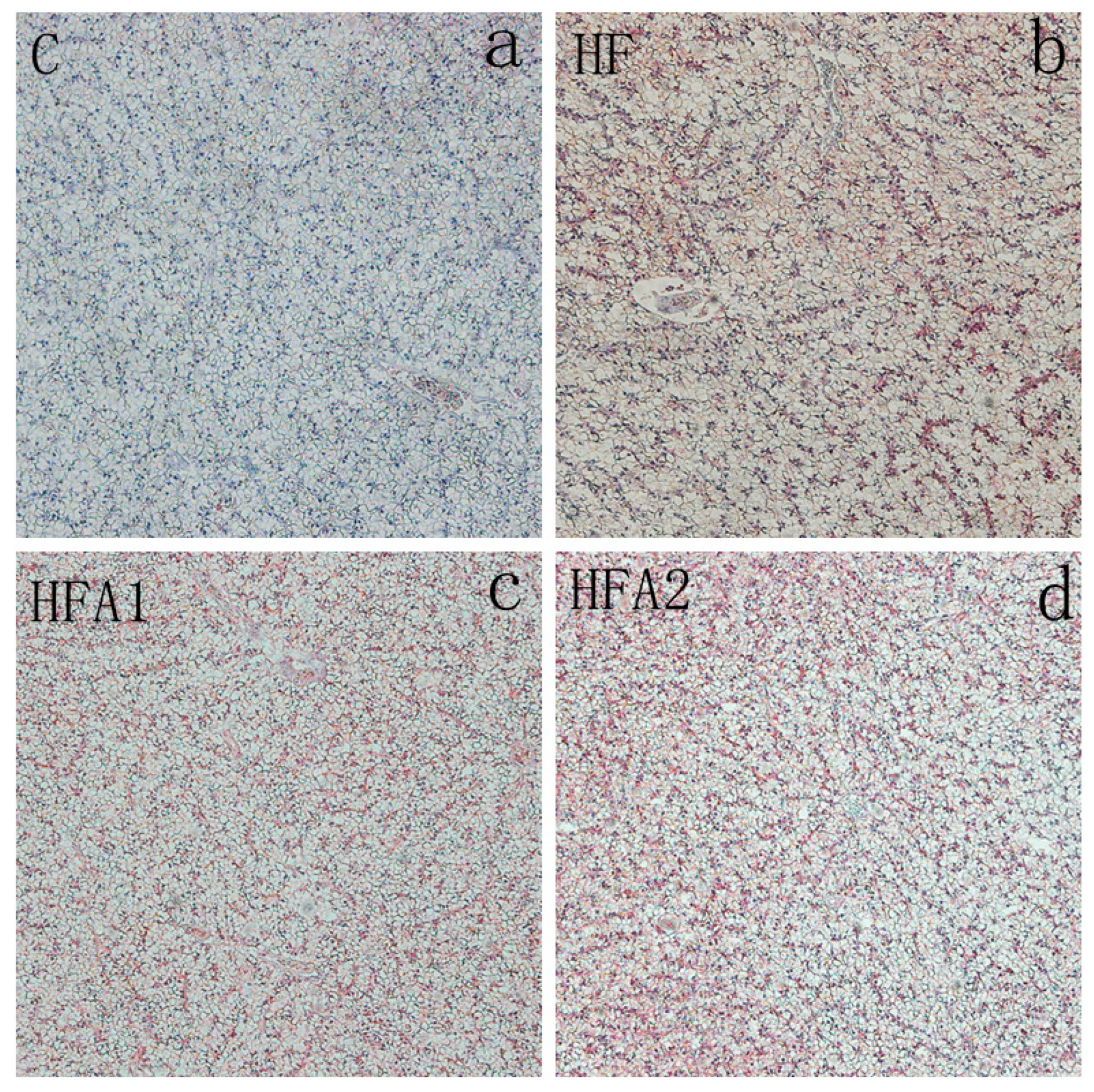
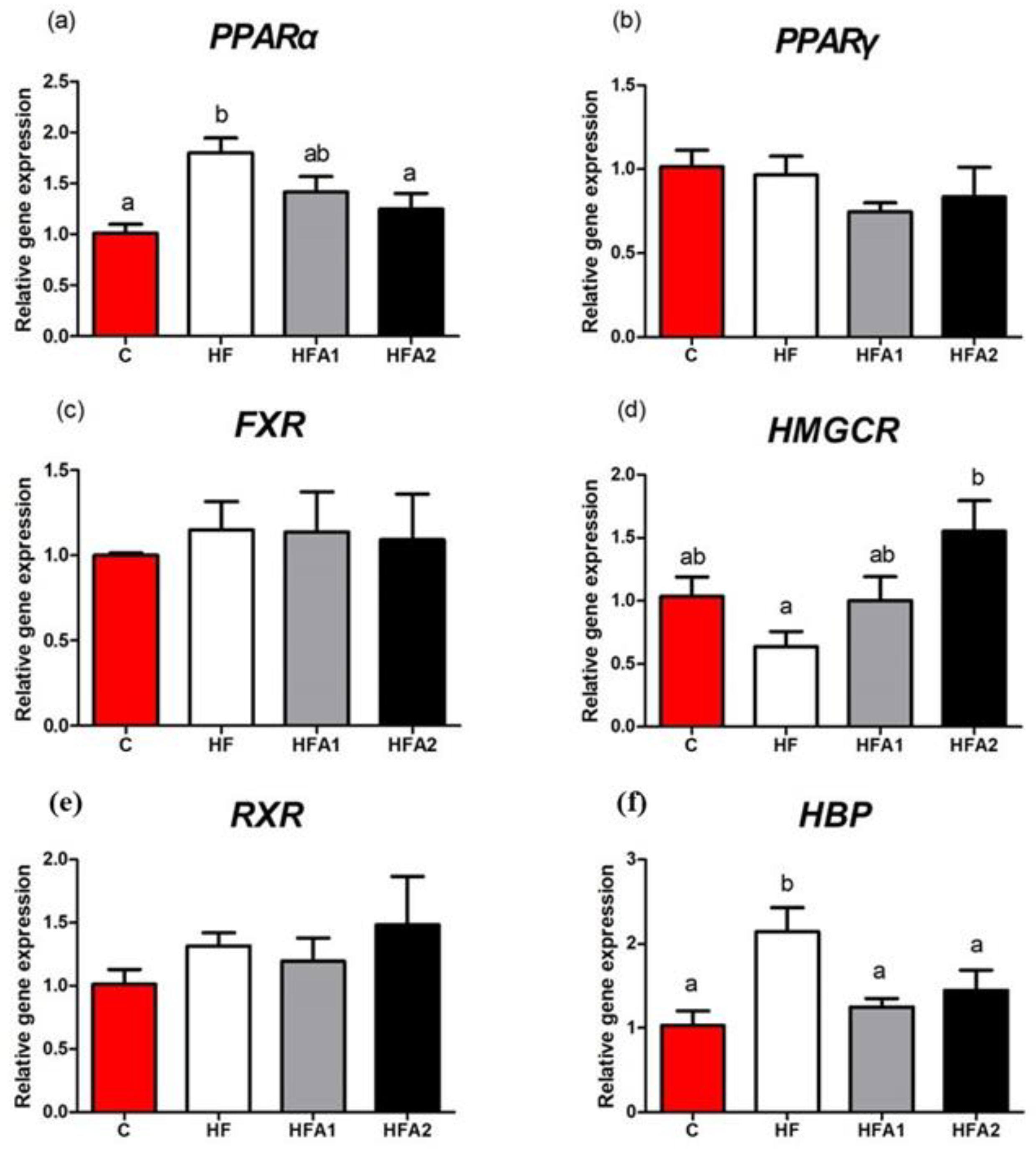

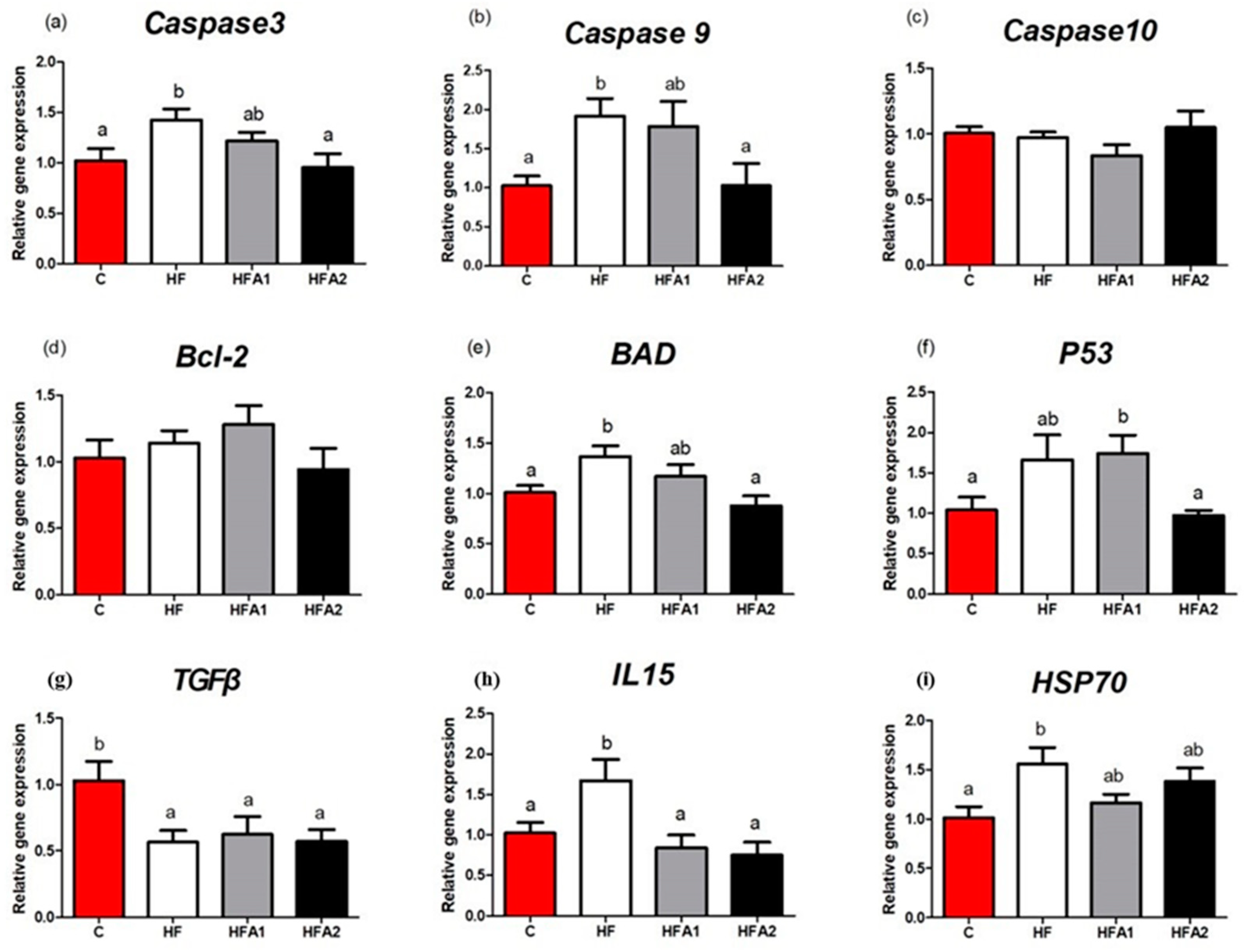
| C | HF | HFA1 | HFA2 | |
|---|---|---|---|---|
| IBW (g) | 15.26 ± 0.02 | 15.26 ± 0.01 | 15.25 ± 0.02 | 15.23 ± 0.03 |
| FBW (g) | 64.45 ± 1.21 a,b | 62.24 ± 1.17 a | 67.04 ± 1.34 b | 64.91 ± 1.13 a,b |
| WG (%) | 322.88 ± 8.05 a,b | 307.92 ± 7.43 a | 339.53 ± 8.26 b | 326.34 ± 8.42 a,b |
| SR (%) | 96.67 ± 1.67 | 96.25 ± 2.39 | 96.25 ± 3.75 | 95 ± 2.89 |
| SGR (% day−1) | 2.57 ± 0.04 ab | 2.51 ± 0.03 a | 2.64 ± 0.03 b | 2.56 ± 0.04 a,b |
| PER | 2.08 ± 0.03 a | 2.19 ± 0.06 a | 2.36 ± 0.03 b | 2.38 ± 0.04 b |
| CF (g cm−3) | 2.31 ± 0.04 | 2.3 ± 0.05 | 2.27 ± 0.03 | 2.29 ± 0.06 |
| VSI (%) | 8.06 ± 0.16 a | 8.98 ± 0.2 b | 9.12 ± 0.18 b | 8.89 ± 0.17 b |
| HSI (%) | 2.3 ± 0.06 a | 2.53 ± 0.06 b | 2.29 ± 0.07 a | 2.1 ± 0.09 a |
| IPF (%) | 1.31 ± 0.13 a | 2.09 ± 0.09 c | 1.88 ± 0.16 b,c | 1.58 ± 0.18 a,b |
| C | HF | HFA1 | HFA2 | |
|---|---|---|---|---|
| Whole-body | ||||
| Moisture | 70.38 ± 0.22 b | 69.24 ± 0.11 a | 69.01 ± 0.19 a | 68.65 ± 0.26 a |
| Crude Protein | 17.30 ± 0.15 | 16.97 ± 0.12 | 17.10 ± 0.16 | 17.32± 0.15 |
| Crude Lipid | 7.32 ± 0.81 a | 9.20± 0.21 b | 9.08 ± 0.66 b | 9.64 ± 0.31 b |
| Ash | 3.87 ± 0.11 b | 3.74 ± 0.08 a,b | 3.69 ± 0.05 a,b | 3.61 ± 0.05 a |
| Muscle | ||||
| Moisture | 78.01 ± 0.18 | 77.92 ± 0.11 | 77.72 ± 0.09 | 77.66 ± 0.10 |
| Crude Protein | 19.90 ± 0.19 | 19.73 ± 0.17 | 19.92 ± 0.06 | 19.76 ± 0.23 |
| Crude Lipid | 0.76 ± 0.11 a | 1.46 ± 0.19 b | 1.30 ± 0.05 b | 1.40 ± 0.15 b |
| Ash | 2.87 ± 0.04 b | 2.68 ± 0.06 a | 2.65 ± 0.06 a | 2.57 ± 0.03 a |
| C | HF | HFA1 | HFA2 | |
|---|---|---|---|---|
| Plasma | - | - | - | - |
| TG (mmol L−1) | 5.1 ± 0.43 a | 8.51 ± 1.07 b | 5.62± 0.38 a | 4.57± 0.82 a |
| TC (mmol L−1) | 15.63 ± 0.95 a | 19.68 ± 0.58 b | 16.17 ± 0.37 a,b | 16.45 ± 0.99 a,b |
| HDL/LDL | 0.32 ± 0.02 a,b | 0.24 ± 0.02 a | 0.27 ± 0.03 a | 0.37 ± 0.03 b |
| LDH (U L−1) | 615.37 ± 14.07 | 681.88 ± 41.96 | 626.67 ± 19.97 | 628.24 ± 23 |
| Liver | - | - | - | - |
| Lipase (U g prot−1) | 0.17 ± 0.01 | 0.16 ± 0.01 | 0.16 ± 0.02 | 0.20 ± 0.02 |
| TG (mmol g prot−1) | 0.06 ± 0.00 a | 0.07 ± 0.01 a,b | 0.08 ± 0.01 b | 0.07 ± 0.0 a,b |
| TC (mmol g prot−1) | 0.04 ± 0.01 a | 0.08 ± 0.01 b | 0.05 ± 0.01 a | 0.05 ± 0.01 a,b |
| Ingredients (%) | C | HF | HFA1 | HFA2 |
|---|---|---|---|---|
| Fish meal | 50 | 50 | 50 | 50 |
| Wheat flour | 10.9 | 10.9 | 10.825 | 10.75 |
| Chicken meal | 6.00 | 6.00 | 6.00 | 6.00 |
| Microcrystalline cellulose | 7 | 0 | 0 | 0 |
| Beer yeast | 3 | 3 | 3 | 3 |
| Shrimp meal | 3 | 3 | 3 | 3 |
| Soy protein concentrate | 1 | 1 | 1 | 1 |
| Soybean oil | 2 | 9 | 9 | 9 |
| Fish oil | 3.00 | 3.00 | 3.00 | 3.00 |
| Choline chloride (50%) | 0.5 | 0.5 | 0.5 | 0.5 |
| Monocalcium phosphate | 1.50 | 1.50 | 1.50 | 1.50 |
| Vitamin mixture a | 1 | 1 | 1 | 1 |
| Mineral mixture b | 1 | 1 | 1 | 1 |
| Vc phosphonate | 0.1 | 0.1 | 0.1 | 0.1 |
| Astaxanthin c | 0.00 | 0.00 | 0.075 | 0.15 |
| Soybean protein concentrate | 10 | 10 | 10 | 10 |
| Proximate composition (%) | - | - | - | - |
| Moisture | 6.60 | 6.54 | 6.34 | 5.68 |
| Crude protein | 49.07 | 48.48 | 49.07 | 49.18 |
| Crude lipid | 10.87 | 18.08 | 18.06 | 18.21 |
| Ash | 15.59 | 15.40 | 15.62 | 16.84 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, S.; Yin, P.; Tian, L.; Yu, Y.; Liu, Y.; Niu, J. Dietary Supplementation of Astaxanthin Improved the Growth Performance, Antioxidant Ability and Immune Response of Juvenile Largemouth Bass (Micropterus salmoides) Fed High-Fat Diet. Mar. Drugs 2020, 18, 642. https://doi.org/10.3390/md18120642
Xie S, Yin P, Tian L, Yu Y, Liu Y, Niu J. Dietary Supplementation of Astaxanthin Improved the Growth Performance, Antioxidant Ability and Immune Response of Juvenile Largemouth Bass (Micropterus salmoides) Fed High-Fat Diet. Marine Drugs. 2020; 18(12):642. https://doi.org/10.3390/md18120642
Chicago/Turabian StyleXie, Shiwei, Peng Yin, Lixia Tian, Yingying Yu, Yongjian Liu, and Jin Niu. 2020. "Dietary Supplementation of Astaxanthin Improved the Growth Performance, Antioxidant Ability and Immune Response of Juvenile Largemouth Bass (Micropterus salmoides) Fed High-Fat Diet" Marine Drugs 18, no. 12: 642. https://doi.org/10.3390/md18120642
APA StyleXie, S., Yin, P., Tian, L., Yu, Y., Liu, Y., & Niu, J. (2020). Dietary Supplementation of Astaxanthin Improved the Growth Performance, Antioxidant Ability and Immune Response of Juvenile Largemouth Bass (Micropterus salmoides) Fed High-Fat Diet. Marine Drugs, 18(12), 642. https://doi.org/10.3390/md18120642





