Design, Synthesis and In Vitro Experimental Validation of Novel TRPV4 Antagonists Inspired by Labdane Diterpenes
Abstract
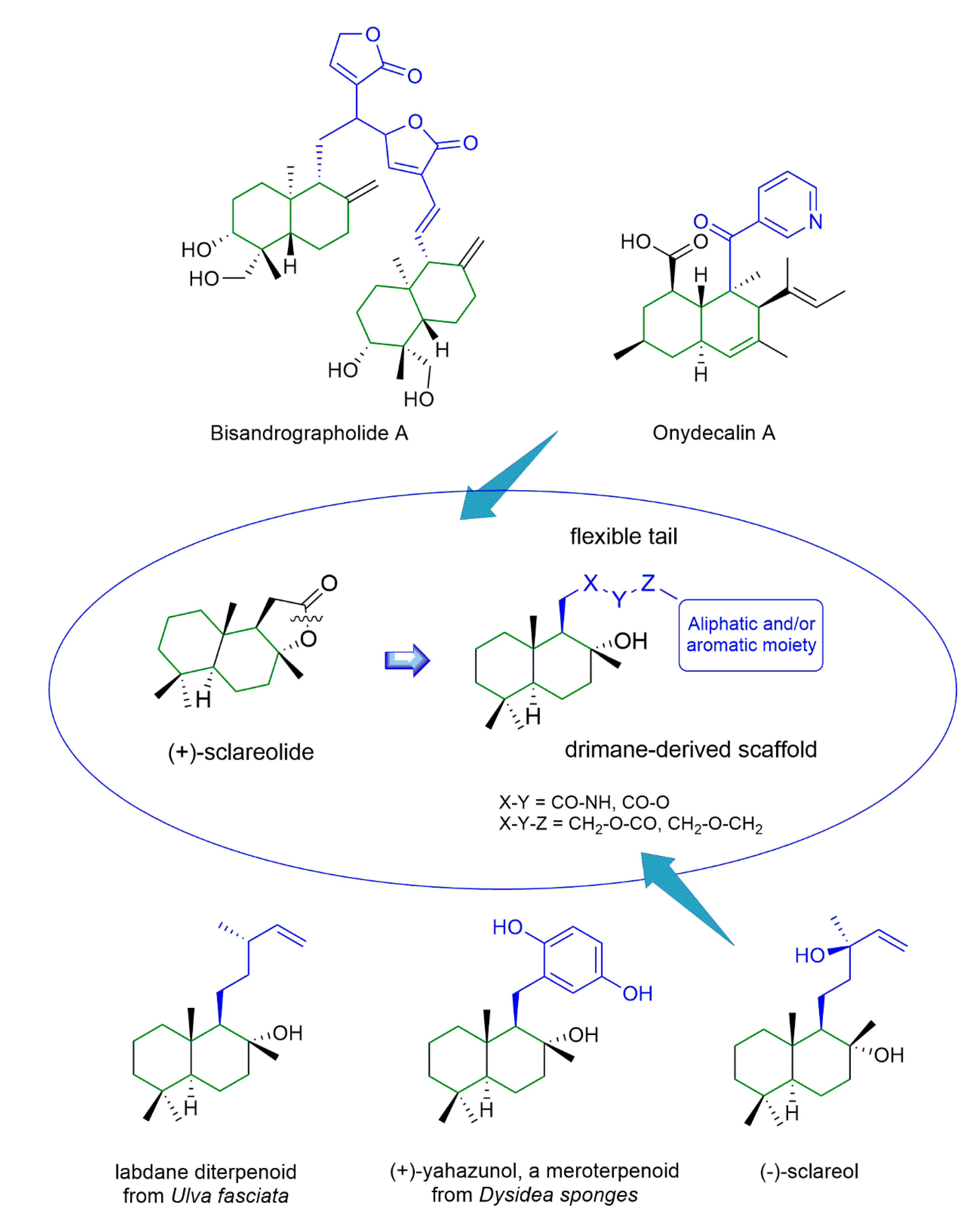
1. Introduction
2. Results
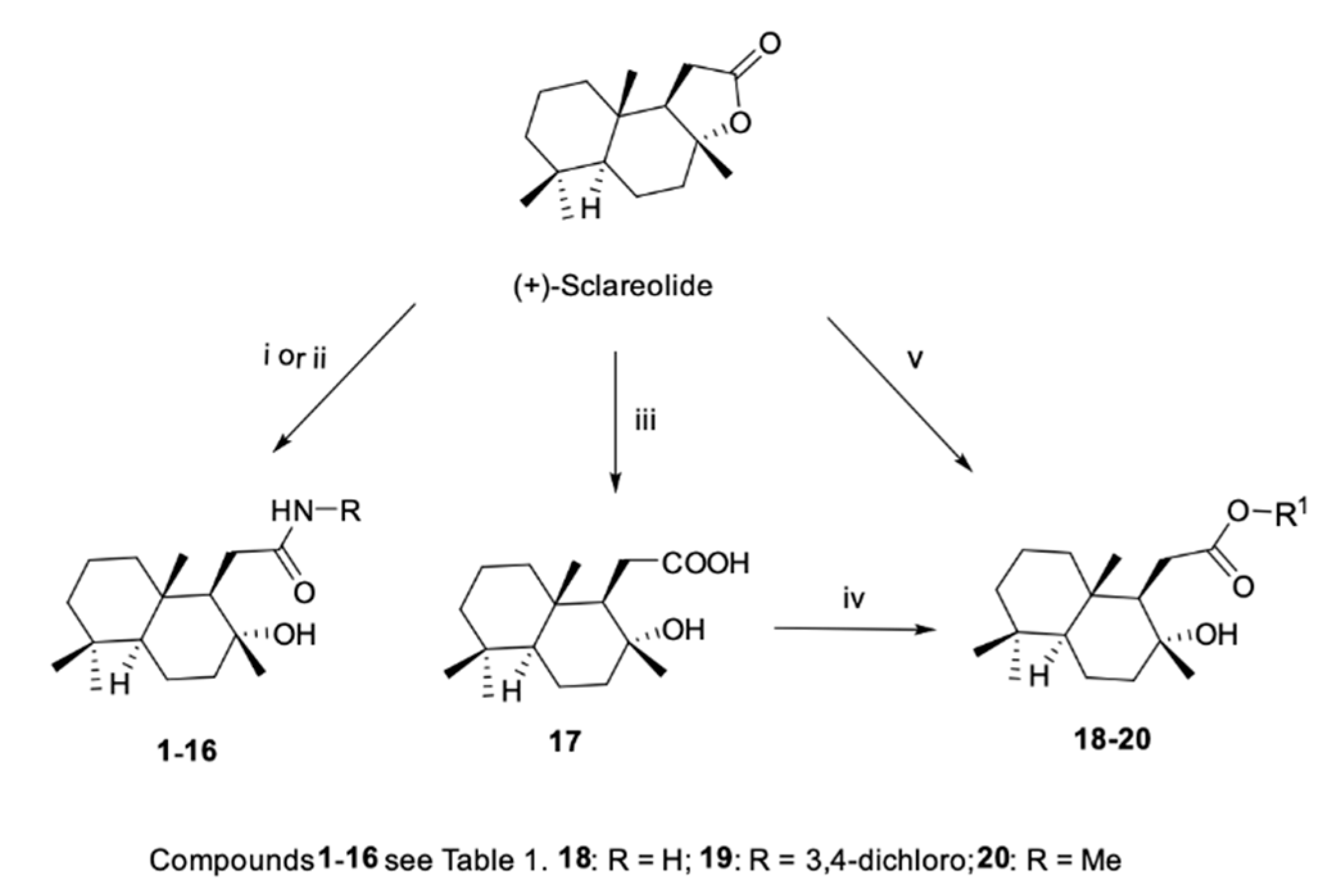
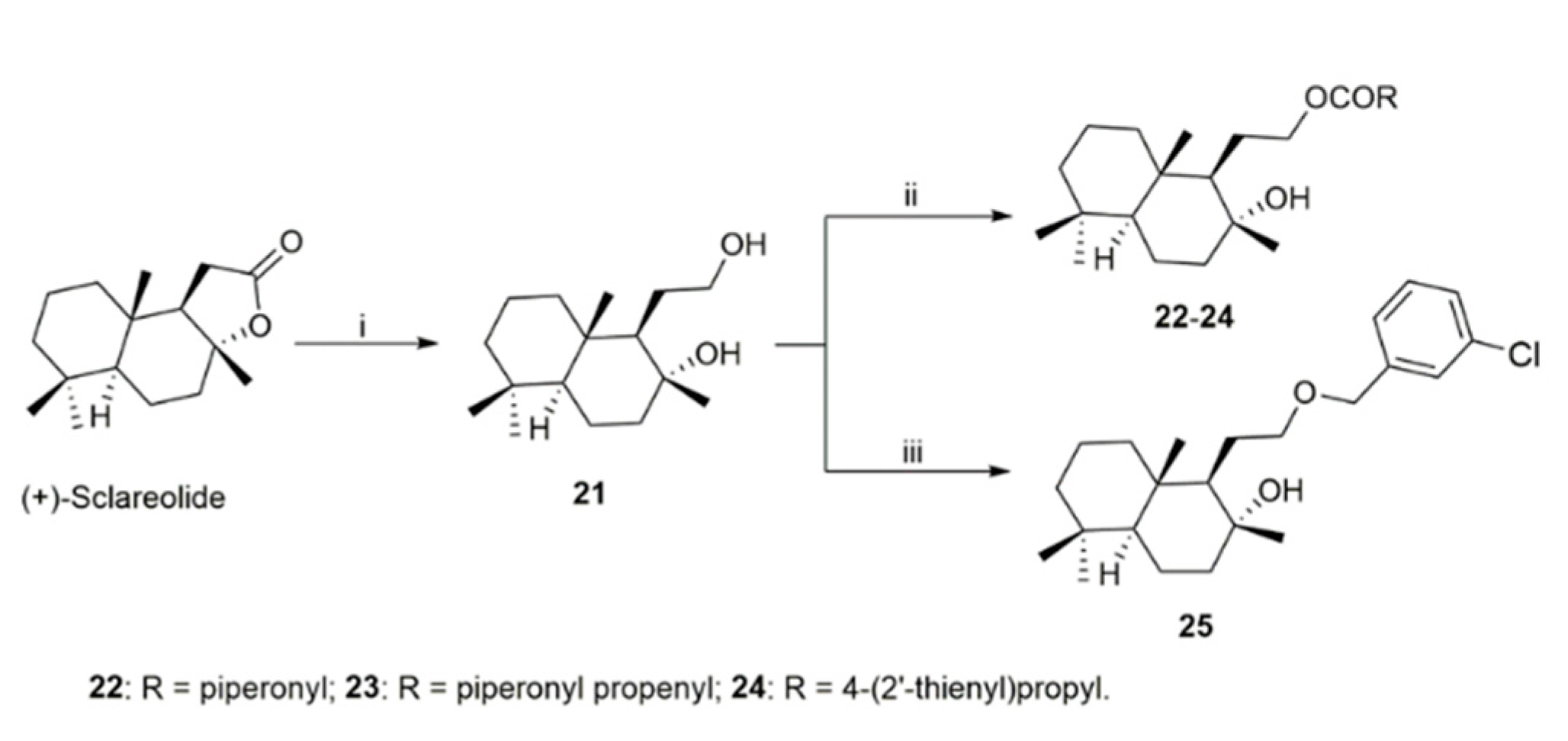
2.1. Chemistry
2.2. TRPV4 Assay
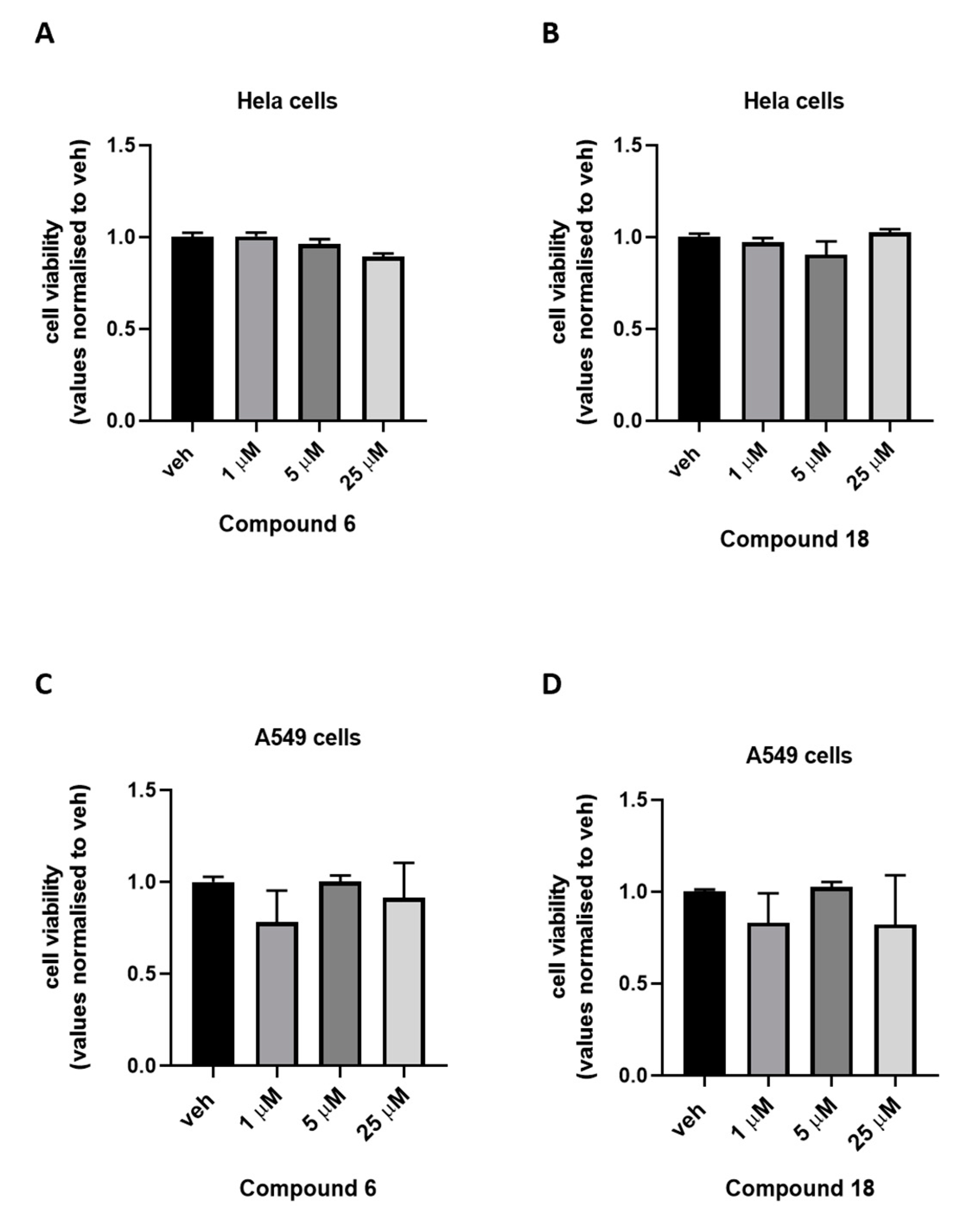
2.3. Cytotoxicity Assay
3. Discussion
Biological Activity and Structure-Activity Relationships (SARs)
4. Materials and Methods
4.1. Chemicals, Materials, and Methods
4.2. Chemistry
4.2.1. General Procedure for the Synthesis of Homodrimanyl Aryl Amides (1–3): DIBAL-H-Mediated Amidation From Anilines
2-((1R,2R,4aS,8aS)-2-Hydroxy-2,5,5,8a-tetramethyldecahydronaphthalen-1-yl)-N-phenylacetamide (1)
N-(2,5-difluorophenyl)-2-((1R,2R,4aS,8aS)-2-hydroxy-2,5,5,8a-tetramethyldecahydronaphthalen-1-yl)acetamide (2)
N-(2-(1H-pyrrol-1-yl)phenyl)-2-((1R,2R,4aS,8aS)-2-hydroxy-2,5,5,8a-tetramethyldecahydronaphthalen-1-yl)acetamide (3)
4.2.2. General Procedure for the Synthesis of Homodrimanyl Aliphatic Amides (4–16): Aminolysis Reaction from Amines
N-benzyl-2-((1R,2R,4aS,8aS)-2-hydroxy-2,5,5,8a-tetramethyldecahydronaphthalen-1-yl)acetamide (4)
N-(4-chlorobenzyl)-2-((1R,2R,4aS,8aS)-2-hydroxy-2,5,5,8a-tetramethyldecahydronaphthalen-1-yl)acetamide (5)
N-(3,4-dichlorobenzyl)-2-((1R,2R,4aS,8aS)-2-hydroxy-2,5,5,8a-tetramethyldecahydronaphthalen-1-yl)acetamide (6)
N-(4-fluorobenzyl)-2-((1R,2R,4aS,8aS)-2-hydroxy-2,5,5,8a-tetramethyldecahydronaphthalen-1-yl)acetamide (7)
2-((1R,2R,4aS,8aS)-2-hydroxy-2,5,5,8a-tetramethyldecahydronaphthalen-1-yl)-N-(4-methoxybenzyl)acetamide (8)
2-((1R,2R,4aS,8aS)-2-hydroxy-2,5,5,8a-tetramethyldecahydronaphthalen-1-yl)-N-(3 methoxybenzyl)acetamide (9)
2-((1R,2R,4aS,8aS)-2-hydroxy-2,5,5,8a-tetramethyldecahydronaphthalen-1-yl)-N-phenethylacetamide (10)
N-(4-chlorophenethyl)-2-((1R,2R,4aS,8aS)-2-hydroxy-2,5,5,8a-tetramethyldecahydronaphthalen-1-yl)acetamide (11)
N-(4-fluorophenethyl)-2-((1R,2R,4aS,8aS)-2-hydroxy-2,5,5,8a-tetramethyldecahydronaphthalen-1-yl)acetamide (12)
N-(3-fluorophenethyl)-2-((1R,2R,4aS,8aS)-2-hydroxy-2,5,5,8a-tetramethyldecahydronaphthalen-1-yl)acetamide (13)
N-(furan-2-ylmethyl)-2-((1R,2R,4aS,8aS)-2-hydroxy-2,5,5,8a-tetramethyldecahydronaphthalen-1-yl)acetamide (14)
N-((1,1′-biphenyl)-4-ylmethyl)-2-((1R,2R,4aS,8aS)-2-hydroxy-2,5,5,8a-tetramethyldecahydronaphthalen-1-yl)acetamide (15)
N-(3-(1H-imidazol-1-yl)propyl)-2-((1R,2R,4aS,8aS)-2-hydroxy-2,5,5,8a-tetramethyldecahydronaphthalen-1-yl)acetamide (16)
4.2.3. General Procedure for the Synthesis of Homodrimanyl Acid Ester (18) and (19)
Benzyl 2-((1R,2R,4aS,8aS)-2-hydroxy-2,5,5,8a-tetramethyldecahydronaphthalen-1-yl)acetate (18)
3,4-dichlorobenzyl 2-((1R,2R,4aS,8aS)-2-hydroxy-2,5,5,8a-tetramethyldecahydronaphthalen-1-yl)acetate (19)
4.2.4. General Procedure for the Synthesis of Homodrimanyl Diol Esters (22–24)
2-((1R,2R,4aS,8aS)-2-Hydroxy-2,5,5,8a-tetramethyldecahydronaphthalen-1-yl)ethyl Benzo[d][1,3]dioxole-5-carboxylate (22)
2-((1R,2R,4aS,8aS)-2-hydroxy-2,5,5,8a-tetramethyldecahydronaphthalen-1-yl)ethyl (E)-3-(benzo[d][1,3]dioxol-5-yl)acrylate (23)
2-((1R,2R,4aS,8aS)-2-hydroxy-2,5,5,8a-tetramethyldecahydronaphthalen-1-yl)ethyl 4-(thiophen-2-yl)butanoate (24)
Synthesis of (1R,2R,4aS,8aS)-1-(2-((3-Chlorobenzyl)oxy)ethyl)-2,5,5,8a-tetramethyldecahydronaphthalen-2-ol (homodrimanyl diol ether) (25)
4.3. TRPV1 and TRPV4 Channel Assays
4.4. MTT Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Singh, M.; Pal, M.; Sharma, R.P. Biological activity of the labdane diterpenes. Planta Med. 1999, 65, 2–8. [Google Scholar] [CrossRef]
- Pal, M.; Mishra, T.; Kumar, A.; Tewari, S.K. Medicinal plants: Biological evaluation of terrestrial and marine plant originated labdane diterpenes (a review). Pharm. Chem. J. 2016, 50, 558–567. [Google Scholar] [CrossRef]
- Aiello, F.; Carullo, G.; Badolato, M.; Brizzi, A. TRPV1–FAAH–COX: The Couples Game in Pain Treatment. ChemMedChem 2016, 11, 1686–1694. [Google Scholar] [CrossRef]
- Premkumar, L.S. Transient receptor potential channels as targets for phytochemicals. ACS Chem. Neurosci. 2014, 5, 1117–1130. [Google Scholar] [CrossRef] [PubMed]
- Vitale, R.M.; Schiano Moriello, A.; De Petrocellis, L. Natural compounds and synthetic drugs targeting the ionotropic cannabinoid members of transient receptor potential (TRP) channels. In New Tools to Interrogate Endocannabinoid Signalling—From Natural Compounds to Synthetic Drugs; Maccarrone, M., Ed.; RSC: London, UK, 2020; in press. [Google Scholar]
- Liedtke, W.; Choe, Y.; Martí-Renom, M.A.; Bell, A.M.; Denis, C.S.; Šali, A.; Hudspeth, A.J.; Friedman, J.M.; Heller, S. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell 2000, 103, 525–535. [Google Scholar] [CrossRef]
- Strotmann, R.; Harteneck, C.; Nunnenmacher, K.; Schultz, G.; Plant, T.D. OTRPC4, a nonselective cation channel that confers sensivity to extracellular osmolarity. Nat. Cell Biol. 2000, 2, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Paknejad, N.; Maksaev, G.; Sala-Rabanal, M.; Nichols, C.G.; Hite, R.K.; Yuan, P. Cryo-EM and X-ray structures of TRPV4 reveal insight into ion permeation and gating mechanisms. Nat. Struct. Mol. Biol. 2018, 25, 252–260. [Google Scholar] [CrossRef]
- Kuebler, W.M.; Jordt, S.E.; Liedtke, W.B. Urgent reconsideration of lung edema as a preventable outcome in COVID-19: Inhibition of TRPV4 represents a promising and feasible approach. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 318, L1239–L1243. [Google Scholar] [CrossRef]
- Jian, M.Y.; King, J.A.; Al-Mehdi, A.B.; Liedtke, W.; Townsley, M.I. High vascular pressure-induced lung injury requires P450 epoxygenase-dependent activation of TRPV4. Am. J. Respir. Cell Mol. Biol. 2008, 38, 386–392. [Google Scholar] [CrossRef]
- Vincent, F.; Duncton, M.A.J. TRPV4 Agonists and Antagonists. Curr. Top. Med. Chem. 2011, 11, 2216–2226. [Google Scholar] [CrossRef]
- Lawhorn, B.G.; Brnardic, E.J.; Behm, D.J. Recent advances in TRPV4 agonists and antagonists. Bioorg. Med. Chem. Lett. 2020, 30, 127022. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.L.; Maloney, K.N.; Pothen, R.G.; Clardy, J.; Clapham, D.E. Bisandrographolide from Andrographis paniculata activates TRPV4 channels. J. Biol. Chem. 2006, 281, 29897–29904. [Google Scholar] [CrossRef]
- Hilfiker, M.A.; Hoang, T.H.; Cornil, J.; Eidam, H.S.; Matasic, D.S.; Roethke, T.J.; Klein, M.; Thorneloe, K.S.; Cheung, M. Optimization of a novel series of TRPV4 antagonists with in vivo activity in a model of pulmonary edema. ACS Med. Chem. Lett. 2013, 4, 293–296. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cheung, M.; Bao, W.; Behm, D.J.; Brooks, C.A.; Bury, M.J.; Dowdell, S.E.; Eidam, H.S.; Fox, R.M.; Goodman, K.B.; Holt, D.A.; et al. Discovery of GSK2193874: An Orally Active, Potent, and Selective Blocker of Transient Receptor Potential Vanilloid 4. ACS Med. Chem. Lett. 2017, 8, 549–554. [Google Scholar] [CrossRef]
- Lin, Z.; Phadke, S.; Lu, Z.; Beyhan, S.; Abdel Aziz, M.H.; Reilly, C.; Schmidt, E.W. Onydecalins, Fungal Polyketides with Anti- Histoplasma and Anti-TRP Activity. J. Nat. Prod. 2018, 81, 2605–2611. [Google Scholar] [CrossRef]
- Chakraborty, K.; Lipton, A.P.; Paul Raj, R.; Vijayan, K.K. Antibacterial labdane diterpenoids of Ulva fasciata Delile from southwestern coast of the Indian Peninsula. Food Chem. 2010, 119, 1399–1408. [Google Scholar] [CrossRef]
- Pérez-García, E.; Zubía, E.; Ortega, M.J.; Carballo, J.L. Merosesquiterpenes from two sponges of the genus Dysidea. J. Nat. Prod. 2005, 68, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Caniard, A.; Zerbe, P.; Legrand, S.; Cohade, A.; Valot, N.; Magnard, J.L.; Bohlmann, J.; Legendre, L. Discovery and functional characterization of two diterpene synthases for sclareol biosynthesis in Salvia sclarea (L.) and their relevance for perfume manufacture. BMC Plant. Biol. 2012, 12, 1–13. [Google Scholar] [CrossRef]
- Coricello, A.; El-Magboub, A.; Luna, M.; Ferrario, A.; Haworth, I.S.; Gomer, C.J.; Aiello, F.; Adams, J.D. Rational drug design and synthesis of new α-Santonin derivatives as potential COX-2 inhibitors. Bioorg. Med. Chem. Lett. 2018, 28, 993–996. [Google Scholar] [CrossRef]
- Coricello, A.; Adams, J.D.; Lien, E.J.; Nguyen, C.; Perri, F.; Williams, T.J.; Aiello, F. A Walk in Nature: Sesquiterpene Lactones as Multi-Target Agents Involved in Inflammatory Pathways. Curr. Med. Chem. 2018, 27, 1501–1514. [Google Scholar] [CrossRef]
- Chen, Q.; Tang, K.; Guo, Y. Discovery of sclareol and sclareolide as filovirus entry inhibitors. J. Asian Nat. Prod. Res. 2020, 22, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Dixon, D.D.; Lockner, J.W.; Zhou, Q.; Baran, P.S. Scalable, divergent synthesis of meroterpenoids via “borono-sclareolide”. J. Am. Chem. Soc. 2012, 134, 8432–8435. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, S.; Song, Z.; Wang, G.; Li, S. Bioactivity-guided mixed synthesis accelerate the serendipity in lead optimization: Discovery of fungicidal homodrimanyl amides. Eur. J. Med. Chem. 2017, 136, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Koga, T.; Aoki, Y.; Hirose, T.; Nohira, H. Resolution of sclareolide as a key intermediate for the synthesis of Ambrox®. Tetrahedron Asymmetry 1998, 9, 3819–3823. [Google Scholar] [CrossRef]
- Aiello, F.; Badolato, M.; Pessina, F.; Sticozzi, C.; Maestrini, V.; Aldinucci, C.; Luongo, L.; Guida, F.; Ligresti, A.; Artese, A.; et al. Design and Synthesis of New Transient Receptor Potential Vanilloid Type-1 (TRPV1) Channel Modulators: Identification, Molecular Modeling Analysis, and Pharmacological Characterization of the N-(4-Hydroxy-3-methoxybenzyl)-4-(thiophen-2-yl)butanamide. ACS Chem. Neurosci. 2016, 7, 737–748. [Google Scholar] [CrossRef]
- Schiano Moriello, A.; De Petrocellis, L. Endocannabinoid Signaling: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2016; Volume 1412, ISBN 9781493935376. [Google Scholar]
- Thorneloe, K.S.; Sulpizio, A.C.; Lin, Z.; Figueroa, D.J.; Clouse, A.K.; McCafferty, G.P.; Chendrimada, T.P.; Lashinger, E.S.R.; Gordon, E.; Evans, L.; et al. N-((1S)-1-{[4-((2S)-2-{[(2,4-dichlorophenyl)sulfonyl]amino} -3-hydroxypropanoyl)-1-piperazinyl]carbonyl}-3-methylbutyl)-1-benzothiophene-2-carboxamide (GSK1016790A), a novel and potent transient receptor potential vanilloid 4 channel agonist induces urin. J. Pharmacol. Exp. Ther. 2008, 326, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Iannotti, F.A.; De Maio, F.; Panza, E.; Appendino, G.; Taglialatela-Scafati, O.; De Petrocellis, L.; Amodeo, P.; Vitale, R.M. Identification and Characterization of Cannabimovone, a Cannabinoid from Cannabis sativa, as a Novel PPAR Agonist via a Combined Computational and Functional Study. Molecules 2020, 25, 1119. [Google Scholar] [CrossRef]

| Cpd. | R | Efficacy b % | PotencyEC50 (µM) | IC50 (µM) c inh. TRPV4 | Cpd. | R | Efficacy b % | Potency EC50 (µM) | IC50 (µM)c inh. TRPV4 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 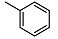 | <10 | NA d | >100 | 14 | 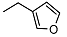 | <10 | NA | 53.5 ± 1.8 |
| 2 | 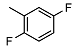 | <10 | NA | >100 | 15 | 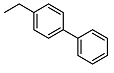 | <10 | NA | >100 |
| 3 | 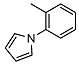 | 14.6 ± 1.5 | 1.1 ± 1.0 | 6.0 ± 0.1 | 16 | 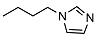 | <10 | NA | >100 |
| 4 | 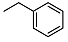 | <10 | NA | 32.0 ± 0.8 | 18 | 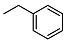 | <10 | NA | 5.41 ± 0.07 |
| 5 |  | <10 | NA | 7.7 ± 0.3 | 19 |  | <10 | NA | >100 |
| 6 |  | <10 | NA | 5.3 ± 0.3 | 20 | Me | <10 | NA | >100 |
| 7 |  | 15.8 ± 0.8 | 13.4 ± 2.6 | 16.9 ± 0.8 | 21 | H | <10 | NA | >100 |
| 8 | 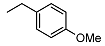 | <10 | NA | 29.7 ± 0.7 | 22 | 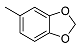 | <10 | NA | NA |
| 9 | 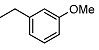 | <10 | NA | 18.1 ± 0.2 | 23 | 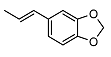 | <10 | NA | >100 |
| 10 | 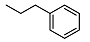 | <10 | NA | 15.6 ± 0.3 | 24 | 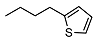 | <10 | NA | >100 |
| 11 | 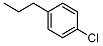 | <10 | NA | 7.0 ± 0.1 | 25 | 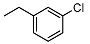 | <10 | NA | >100 |
| 12 | 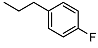 | <10 | NA | 11.4 ± 0.1 | Scde | 11.3 ± 0.7 | > 10 | >100 | |
| 13 | 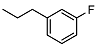 | <10 | NA | 11.9 ± 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazzotta, S.; Carullo, G.; Schiano Moriello, A.; Amodeo, P.; Di Marzo, V.; Vega-Holm, M.; Vitale, R.M.; Aiello, F.; Brizzi, A.; De Petrocellis, L. Design, Synthesis and In Vitro Experimental Validation of Novel TRPV4 Antagonists Inspired by Labdane Diterpenes. Mar. Drugs 2020, 18, 519. https://doi.org/10.3390/md18100519
Mazzotta S, Carullo G, Schiano Moriello A, Amodeo P, Di Marzo V, Vega-Holm M, Vitale RM, Aiello F, Brizzi A, De Petrocellis L. Design, Synthesis and In Vitro Experimental Validation of Novel TRPV4 Antagonists Inspired by Labdane Diterpenes. Marine Drugs. 2020; 18(10):519. https://doi.org/10.3390/md18100519
Chicago/Turabian StyleMazzotta, Sarah, Gabriele Carullo, Aniello Schiano Moriello, Pietro Amodeo, Vincenzo Di Marzo, Margarita Vega-Holm, Rosa Maria Vitale, Francesca Aiello, Antonella Brizzi, and Luciano De Petrocellis. 2020. "Design, Synthesis and In Vitro Experimental Validation of Novel TRPV4 Antagonists Inspired by Labdane Diterpenes" Marine Drugs 18, no. 10: 519. https://doi.org/10.3390/md18100519
APA StyleMazzotta, S., Carullo, G., Schiano Moriello, A., Amodeo, P., Di Marzo, V., Vega-Holm, M., Vitale, R. M., Aiello, F., Brizzi, A., & De Petrocellis, L. (2020). Design, Synthesis and In Vitro Experimental Validation of Novel TRPV4 Antagonists Inspired by Labdane Diterpenes. Marine Drugs, 18(10), 519. https://doi.org/10.3390/md18100519









