Integral Utilization of Red Seaweed for Bioactive Production
Abstract
1. Introduction
2. Components: Properties and Extraction
2.1. Polysaccharides
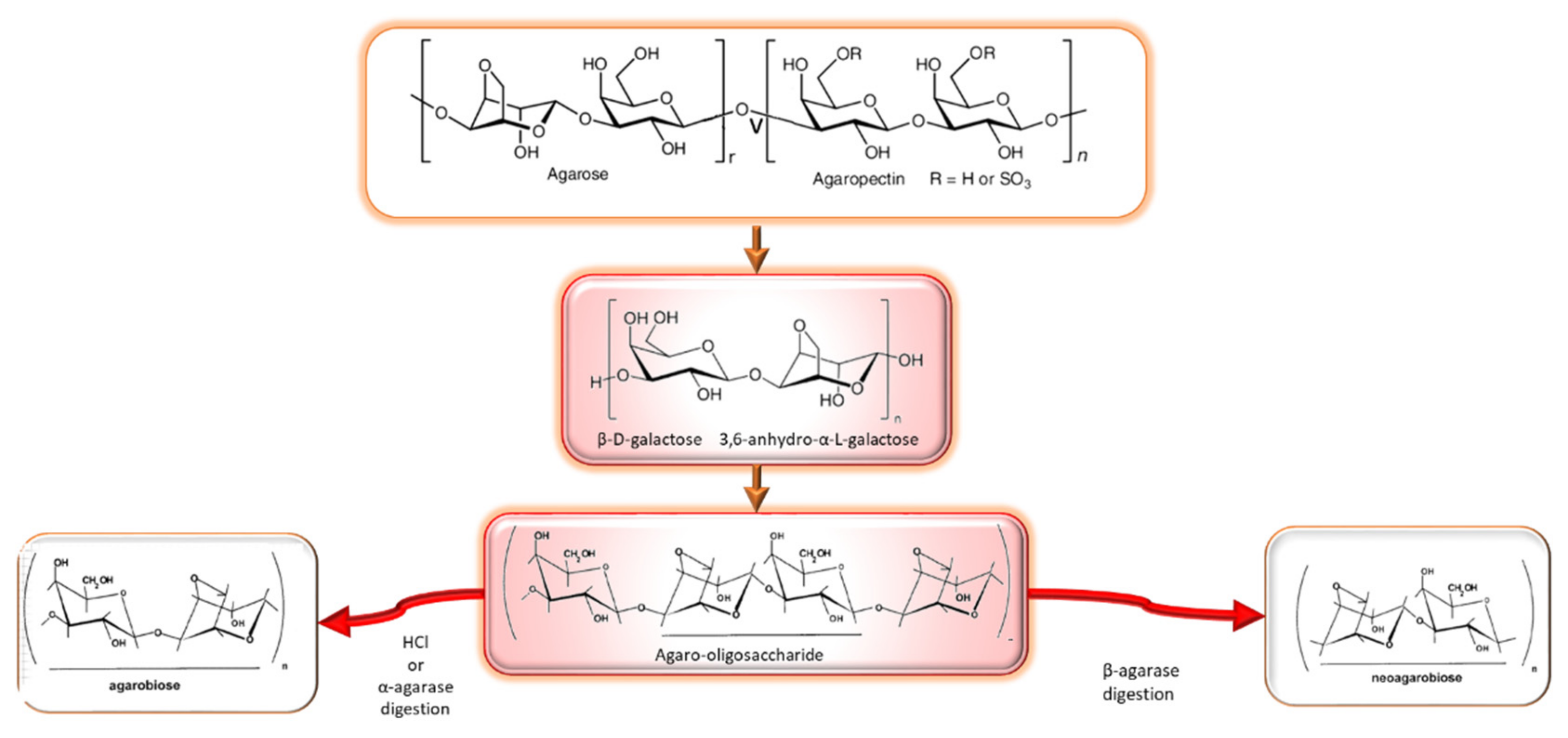
2.1.1. Agar
- Composition, structure, occurrence and properties
- Extraction processes: conventional and emerging technologies
| Pretreatment/Extraction | Seaweed | Gel Properties | Reference |
|---|---|---|---|
| P: - E: Distilled water; pH 6.3–6.4; 100 °C, 1.5 h; ethanol precipitation | Gracilaria cornes | GS: (1.2–2.5) × 104; Tg: 39.2–41.8; Tm: 74.3–82.6; Mw: ND | [59] |
| P: 1–15% NaOH, 90 °C, 1 h, 0.025% HCl, 1 h E: Water, 100 °C, 2 h, ethanol precipitation | Gracilaria verrucosa | GS: (1.6–1.8, 2.6–2.7) × 104; Tg: 32–43; Tm: 49–80.5; Mw: ND | [55] |
| P: - E: Distilled water, 20–28 °C, 15 h, ethanol precipitation | Gracilaria birdiae | GS: ND; Tg: ND; Tm: ND; Mw: 1–30,000 | [61] |
| P: - E: Water, 80–100 °C, 2–4 h; ethanol precipitation | Hydropuntia cornea | GS: (0.7–1.3) × 104; Tg: 25–32.1; Tm: 65–79; Mw: 342–371 kDa | [71] |
| 1 P: 5–7% NaOH, 80–100 °C, 0.5–3 h E: Water, 80 °C, pH 6.2, 90 min, ethanol precipitation | Gracilaria vermiculophylla | GS: (0.9–1.2) × 105; Tg: 52–68; Tm: 92–95; Mw: ND | [37] |
| 1 P: 1–5% NaOH, 30–85 °C, 1–2 h E: Water, 700–115 °C, 2–3 h, 1–2 stages, ethanol precipitation | Gracilaria corticata, Gracilaria eucheumoides, Gracilaria cliftonii, Gracilaria lemaneiformis | GS: (1.2–4.2) × 104; Tg: ~32; Tm: ~78; Mw: ND | [50,53,72,73] |
| P: 5% NaOH, 1–48 h, room temperature. Dil. H2SO4, 15 min E: Water, 100 °C, 1 h 30 min, ethanol precipitation | Gracilaria manilaensis | GS: (1–4.9) × 104; Tg: ND; Tm: ND; Mw: ND | [56] |
| P: - E: Pressurized water extraction, 120 °C, 15 min, ethanol precipitation | Gracilaria vermiculophylla | GS: 1.3 × 105; Tg: 40.7; Tm: 93.1; Mw: ND | [48] |
| P: Acetic acid, 16–20 °C, 1 h E: Steam pressure, 15–20 psi; ethanol precipitation | Gelidiella acerosa | GS: (4.9–6.9) × 104; Tg: 42–47; Tm: 90–98; Mw: ND | [58,74] |
| 1 P: 2.5 M NaOH, 90 °C, 2 h E: Water, 90 °C, 2 h; ultrasound assisted, 30 min, 400 w, 24 kHz; ethanol precipitation | Gelidium sesquipedale | GS: (0.2–1.2) × 105; Tg: ND; Tm: ND; Mw: (2.5–11) × 105 | [29] |
| 1 P: 0.1 M NaOH, 22 °C E: Enzyme (60 °C, 12 h, pH 8) and ultrasound assisted extraction (60 °C, 30 min, 60 W); ethanol precipitation | Gracilaria birdiae | GS: ND; Tg: ND; Tm: ND; Mw: 20–45 | [75] |
| P: - E: Protease digestion, 60 °C, 6 h, pH 5 | Gracilaria cornea | GS: ND; Tg: ND; Tm: ND; Mw: ND | [76] |
| P: Radiation, at 5–15 kGy E: Water, 95–100 °C or pressure cooking 121 °C, 15 psi, 1 h; ethanol precipitation | Gelidiella acerosa | GS: (2.5–6.0) × 104; Tg: ND; Tm: ND; Mw: ND | [70] |
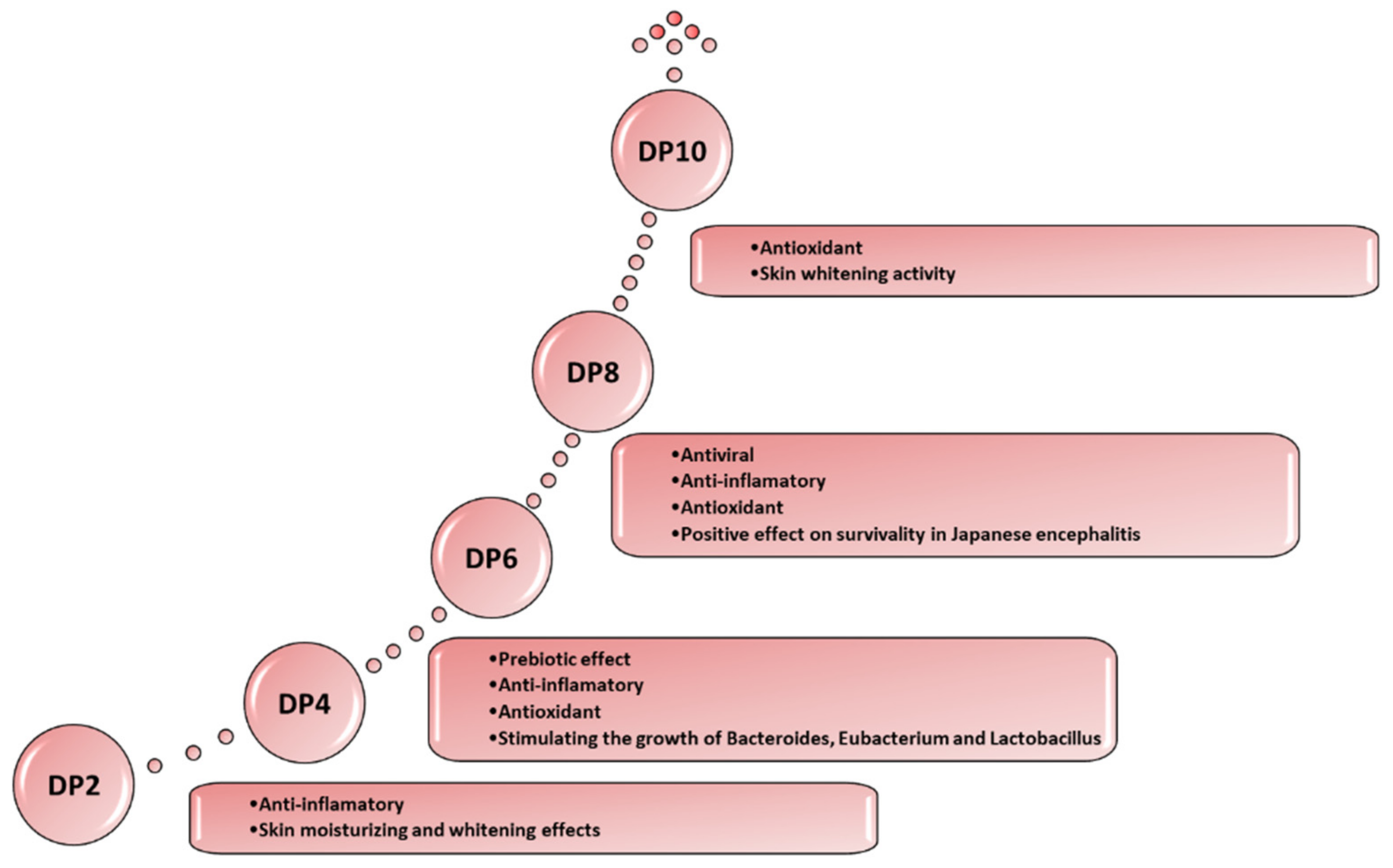
- Agarooligosaccharides: properties and production strategies
2.1.2. Carrageenan
- Composition, structure, occurrence and properties
- Extraction processes: conventional and emerging technologies
- Carraoligosaccharides: properties and production strategies
| Depolymerization | Seaweed or Polyssaccharide | Properties | References |
|---|---|---|---|
| Acid hydrolysis | Carrageenan (C) | Mw: κ-, 510–4000; ι-, 110–3300; λ-, 660–5800 | [179] |
| Acid hydrolysis | Eucheuma cottonii | DP: κ-, 6–20 | [180] |
| Enzymatic | Chondrus armatus, Kappaphycus alvarezii, Tichocarpus crinitus | Mw: ĸ-, 2.2–4.3 | [176,178] |
| Enzymatic | Carrageenan (C) | Mw: ĸ-, 681–798 | [183] |
| High-Pressure | Halymenia durvillei | Mw: λ-, 260–1100 | [95] |
| Irradiation | Carrageenan (C) | Mw: κ-, 8.5–32.1; ι-, 3.1–6.9; λ-, 2.7–6.5 | [184] |
| Microwave assisted | Solieria chordalis, Chondrus ocellatus | Mw: λ-, 3–240 Mw: λ-, 650 | [152,162] |
| Ozonization | Carrageenan (C) | Mw: ĸ-, 10–200 | [177] |
| Radical depolymerization | Halymenia durvillei | Mw: λ-, 3.3–890 | [95] |
| Subcritical water extraction ionic liquids as catalyst | Kappaphycus alvarezii | Mw: ĸ-, 10–60 | [185] |
| Ultrasound assisted | Kappaphycus alvarezii, Eucheuma cottonii | Mw: ĸ-, 545 Mw: ĸ-, 160–240 | [158,182] |
2.2. Protein
- Extraction processes: conventional and emerging technologies
| Technologies | Seaweed | Product | Properties | Reference |
|---|---|---|---|---|
| Accelerated solvent extraction (acetone or methanol) | Porphyra umbilicalis | Carbohydrate/Phlorotannin extraction | Antioxidant | [219] |
| Carbohydrase hydrolysis under high hydrostatic pressure | Palmaria palmata, Solieria chordalis | Antioxidant peptides | Antioxidant | [220] |
| Enzyme hydrolysis with: protease, agarase, carrageenase, xylanase, cellulase | Gelidium pusillum Chondrus crispus, Gracilaria verrucosa, Palmaria palmata Osmundea pinnatifida, Codium tomentosum, Solieria chordalis | Antioxidant peptides, protein, phycobiliproteins, R-phycoerythrin | Antioxidant, α-glucosidase inhibition anti-inflammatory | [197,201,215,216,221,222,223,224,225,226,227] |
| Freezing and thawing | Porphyra haitanensis, Gelidium pusillum | Phycobiliproteins (R-PE and R-PC) | Antioxidant | [210,228] |
| Grinding freeze-dried seaweed in liquid nitrogen | Mastocarpus stellatus | R-phycoerythrin | Antioxidant | [207] |
| Homogenization in water or buffer | Chondrus crispus, Palmaria palmata, Heterosiphonia japonica, Gelidium pusillum | Phycobiliproteins (R-PE and R-PC) | Antioxidant, antidiabetic, antitumor | [130,189,228,229] |
| Osmotic shock | Palmaria palmata, Polysiphonia urceolata | Bioactive peptides, R-phycoerythrin | Antioxidant, prevention of atherosclerosis | [210,223] |
| Subcritical water, optionally catalyst | Hypnea musciformis, Kappaphycus alvarezii | Protein, antioxidants, emulsifyiers | Antioxidant, emulsifyier | [230,231] |
| Ultrasound-assisted extraction | Palmaria palmata, Porphyra umbilicalis | Bioactive peptides R-PE and R-PC | Antioxidant | [215,219,223,228] |
| Ultrasound-assisted extraction | Gelidium pusillum, Porphyra yezoensis | R-PE, R-PC, taurine | Antioxidant | [228,232] |
| Ultrasound and enzyme-assisted extraction | Osmundea pinnatifida, Codium tomentosum | Protein | Antioxidant, prebiotic effect | [215] |
2.3. Lipids and Fatty acids
- Extraction processes: conventional and emerging technologies
| Seaweed Genus | Extraction | TL (mg/g fr. wt.) | PUFA/SFA | ω6/ω3 | Reference |
|---|---|---|---|---|---|
| Acanthophora | CHF/M/PB | 6.8–10.4 | 0.79–0.94 | 0.9–1.8 | [237] |
| Asparagopsis | CSE (H) | 3.0 | 0.06 | 0.62 | [238] |
| Bangia | SFE | 13.3 dw | 2.8 | 2.22 | [240] |
| Bornetia | CSE (H) | 5.3 | 0.76 | 0.29 | [238] |
| Botryocladia | CHF/M/PB | 2.3–5.2 | 0.49–0.54 | 1.7–3.6 | [237] |
| Coelarthrum | CHF/M/PB | 7.7 | 0.67 | 5.7 | [237] |
| Delisea | CSE (Et; DCM:M) | 2.2 | 1.35 | 0.4 | [242] |
| Galaxaura | SFE | 19.8 dw | 0.98 | 0.71 | [240] |
| Gastroclonium | CHF/M/PB | 4.3 | 0.59 | 5.1 | [237] |
| Gelidiopsis | CHF/M/PB | 5.5 | 0.84 | 0.8 | [237] |
| Gelidiella | CHF/M/PB | 6.7 | 0.98 | 0.6 | [237] |
| Gracilaria | CHF/M/PB | 2.9–9.7 | 0.15–2.13 | 0.6–1.9 | [237] |
| Grateloupia | CHF/M/PB; SFE | 5.0–6.4, 13.6 dw | 0.74–1.4 | 0.5–1.9 | [237,240] |
| Griffithsia | CHF/M/PB | 4.2 | [237] | ||
| Halymenia | CHF/M/PB; SFE | 10–18.8 dw | 1.37–1.8 | 1.7–5 | [237,240] |
| Helmintocladia | SFE | 19.7 dw | 1.05 | 1 | [240] |
| Hypnea | SCF: 50 °C, 37.9 MPa | 5.8–7.8 | 0.31–0.43 | 0.8–16 | [237,243] |
| Jania | CSE (H) | 2 | 0.79 | 0.60 | [238] |
| Jania | CHF/M/PB | 12.2 | 0.32 | 2.9 | [237] |
| Laurencia | CSE (Et; DCM:M) | 5.4–16.0 | 0.41–1.08 | 0.4–1.7 | [237,242] |
| Liagora | SFE | 17.6–21.5 dw | 0.94–1.43 | 0.42 | [240] |
| Peyssonelia | CSE (H) | 4.8 | 1.33 | 1.9 | [238] |
| Porphyra | MAHD: 40 W, water | 11.2–12.4 dw | 2.4–2.5 | 1.2–9.1 | [240,245] |
| Pterocladiella | CSE (H) | 5.5 | 0.51 | 0.9 | [238] |
| Pyropia | CHF/M/PB | 7.0–7.7 | 1.23–1.76 | 0.7–1.4 | [237] |
| Rhodymenia | CHF/M/PB | 7.1 | 0.87 | 88.2 | [237] |
| Sarconema | CHF/M/PB | 4.3–9.8 | 0.27–1.04 | 2.4–2.5 | [237] |
| Solieria | CHF/M/PB | 9.0 | 0.35 | 0.8 | [237] |
| Cryptonemia | CHF/M/PB | 11.3 | 0.86–1.28 | 0.9–18.8 | [237] |
| Odonthalia | CHF/M/PB | 11.4 | 0.72 | 0.6 | [237] |
| Polysiphonia | CHF/M/PB | 9.6 | 0.53 | 1.1 | [237] |
| Scinaia | CHF/M/PB | 5.2–17 dw | 0.23–1.86 | 1.1–5.3 | [237,240] |
| Palmaria | CSE (Et; DCM:M) | 14–46 dw | 0.49–1.1 | 0.21–0.41 | [241,247] |
| Vertebrata | CSE (Et; DCM:M) | 13–18 dw | 0.79 | 0.4 | [247] |
2.4. Extractives
| Solvent | Seaweed | Activity | Reference |
|---|---|---|---|
| Ethanol (70–80%), methanol (80%), Acetone, ethyl acetate, chloroform:methanol (2:1) (80%), dimethyl sulfoxide (80%) | Gracilaria changii, Gelidium amansii, Kappaphycus alvarezii, Osmundea pinnatifida, Codium tomentosum, Gracilaria lemaneiformis | Antioxidant, glucose uptake regulation, anti-diabetic, neuroprotective, gastroprotective | [215,248,249,253,254] |
| Enzyme (proteases, carbohydrases) assisted | Parmaria palmate | Antioxidant | [190] |
| Phosphate buffer | G. amansii | Antitumoral | [248] |
| Ultrasound-assisted | Laurencia obtusa | Antioxidant | [250] |
| Supercritical CO2 | Gloiopeltis tenax, Gracilaria mammillaris | Antioxidant, antimicrobial | [251,252] |
| Enzyme and high hydrostatic pressure | Palmaria palmate, Solieria chordalis | Antioxidant | [220] |
2.5. Minerals
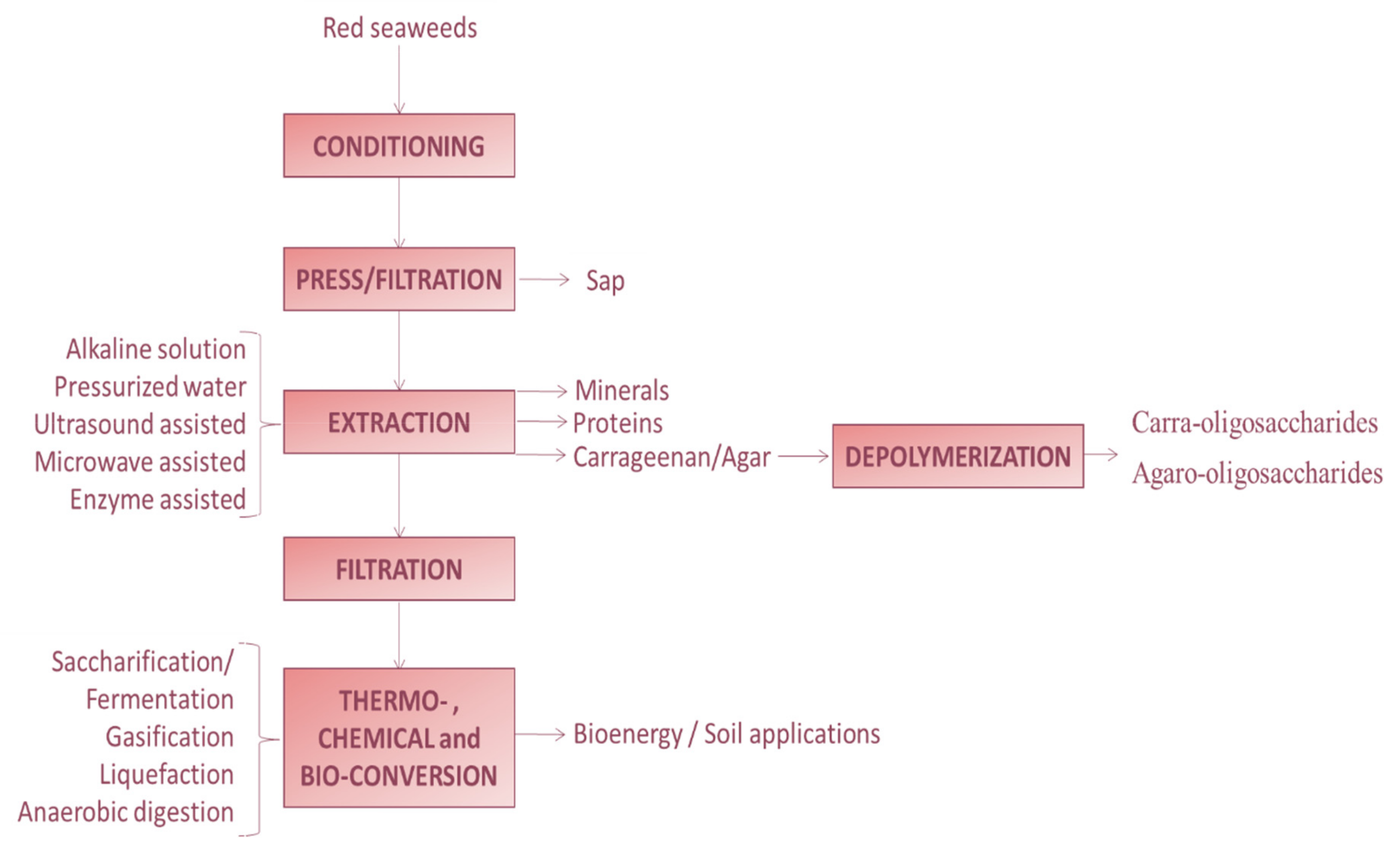
3. Combined Extraction
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cian, R.E.; Drago, S.R.; De Medina, F.S.; Martínez-Augustin, O. Proteins and carbohydrates from red seaweeds: Evidence for beneficial effects on gut function and microbiota. Mar. Drugs 2015, 13, 5358–5383. [Google Scholar] [CrossRef]
- Dhanalakshmi, S.; Jayakumari, S.A. Perspective studies on marine red algae—Hypnea valentiae. Drug Invent. Today 2018, 10, 266–267. [Google Scholar]
- Makkar, H.P.S.; Tran, G.; Heuzé, V.; Giger-Reverdin, S.; Lessire, M.; Lebas, F.; Ankers, P. Seaweeds for livestock diets: A review. Anim. Feed Sci. Technol. 2016, 212, 1–17. [Google Scholar] [CrossRef]
- Sanjeewa, K.K.A.; Lee, W.W.; Jeon, Y.J. Nutrients and bioactive potentials of edible green and red seaweed in Korea. Fish. Aquat. Sci. 2018, 21, 19. [Google Scholar] [CrossRef]
- Ruan, B.F.; Ge, W.W.; Lin, M.X.; Li, Q.S. A review of the components of seaweeds as potential candidates in cancer therapy. Anti-Cancer Agents Med. Chem. 2018, 18, 354–366. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Wang, A.; Lu, Z.; Qin, C.; Hu, J.; Yin, J. Overview on the antiviral activities and mechanisms of marine polysaccharides from seaweeds. Carbohydr. Res. 2017, 453, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Youssouf, L.; Lallemand, L.; Giraud, P.; Soulé, F.; Bhaw-Luximon, A.; Meilhac, O.; D’Hellencourt, C.L.; Jhurry, D.; Couprie, J. Ultrasound-assisted extraction and structural characterization by NMR of alginates and carrageenans from seaweeds. Carbohydr. Polym. 2017, 166, 55–63. [Google Scholar] [CrossRef]
- Cheong, K.L.; Qiu, H.M.; Du, H.; Liu, Y.; Khan, B.M. Oligosaccharides derived from red seaweed: Production, properties, and potential health and cosmetic applications. Molecules 2018, 23, 2451. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Lai, T.K.; Tye, Y.Y.; Rizal, S.; Chong, E.W.N.; Yap, S.W.; Hamzah, A.A.; Nurul Fazita, M.R.; Paridah, M.T. A review of extractions of seaweed hydrocolloids: Properties and applications. Express Polym. Lett. 2018, 12, 296–317. [Google Scholar] [CrossRef]
- Grosso, C.; Valentão, P.; Ferreres, F.; Andrade, P.B. Alternative and efficient extraction methods for marine-derived compounds. Mar. Drugs 2015, 13, 3182–3230. [Google Scholar] [CrossRef] [PubMed]
- Ciko, A.M.; Jokić, S.; Šubarić, D.; Jerković, I. Overview on the application of modern methods for the extraction of bioactive compounds from marine macroalgae. Mar. Drugs 2018, 16, 348. [Google Scholar] [CrossRef] [PubMed]
- Chew, K.W.; Juan, J.C.; Phang, S.M.; Ling, T.C.; Show, P.L. An overview on the development of conventional and alternative extractive methods for the purification of agarose from seaweed. Sep. Sci. Technol. 2018, 53, 467–480. [Google Scholar] [CrossRef]
- Machmudah, S.; Wahyudiono Kanda, H.; Goto, M. Supercritical fluids extraction of valuable compounds from algae: Future perspectives and challenges. Eng. J. 2018, 22, 13–30. [Google Scholar] [CrossRef]
- Xu, S.Y.; Huang, X.; Cheong, K.L. Recent advances in marine algae polysaccharides: Isolation, structure, and activities. Mar. Drugs 2017, 15, 388. [Google Scholar] [CrossRef] [PubMed]
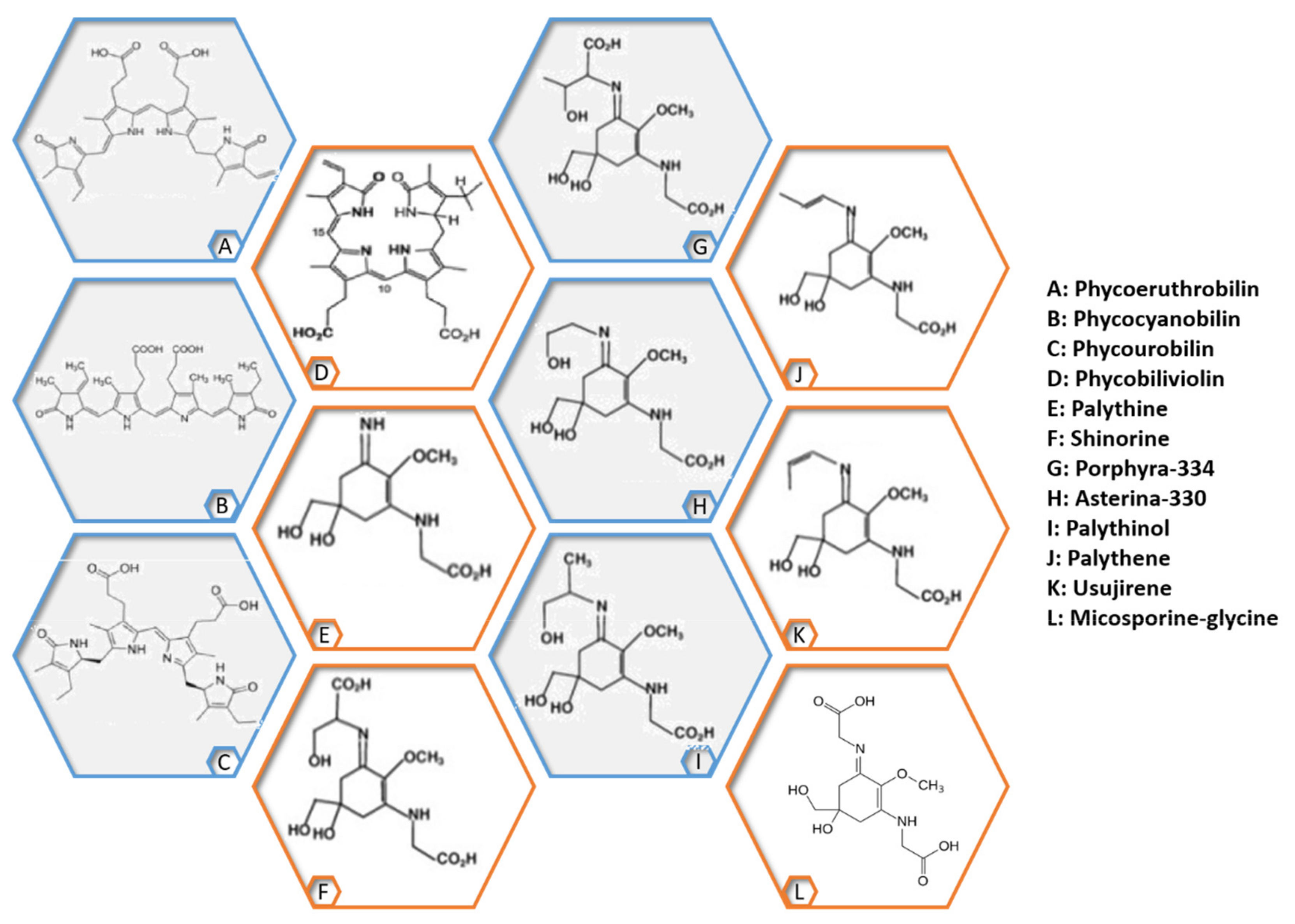
- Pangestuti, R.; Siahaan, E.A.; Kim, S.K. Photoprotective substances derived from marine algae. Mar. Drugs 2018, 16, 399. [Google Scholar] [CrossRef]
- Aryee, A.N.; Agyei, D.; Akanbi, T.O. Recovery and utilization of seaweed pigments in food processing. Curr. Opin. Food Sci. 2018, 19, 113–119. [Google Scholar] [CrossRef]
- Gómez-Ordóñez, E.; Jiménez-Escrig, A.; Rupérez, P. Bioactivity of sulfated polysaccharides from the edible red seaweed Mastocarpus stellatus. Bioact. Carbohydr. Diet. Fibre 2014, 3, 29–40. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Suk, W.Y.; Owolabi, F.A.T.; Haafiz, M.K.M.; Fazita, M.; Deepu, G.; Hasan, M.; Samsul, R. Techno-functional properties of edible packaging films at different polysaccharide blends. J. Phys. Sci. 2019, 30, 23–41. [Google Scholar]
- Torres, M.D.; Kraan, S.; Domínguez, H. Seaweed biorefinery. Rev. Environ. Sci. Bio/Technol. 2019, 18, 335–388. [Google Scholar] [CrossRef]
- Meinita, M.D.N.; Kang, J.Y.; Jeong, G.T.; Koo, H.M.; Park, S.M.; Hong, Y.K. Bioethanol production from the acid hydrolysate of the carrageenophyte Kappaphycus alvarezii (cottonii). J. Appl. Phycol. 2012, 24, 857–862. [Google Scholar] [CrossRef]
- Pomin, V.H. Structural and functional insights into sulfated galactans: A systematic review. Glycoconj. J. 2010, 27, 1–12. [Google Scholar] [CrossRef]
- Schultz-Johansen, M.; Bech, P.K.; Hennessy, R.C.; Glaring, M.A.; Barbeyron, T.; Czjzek, M.; Stougaard, P.A. Novel enzyme portfolio for red algal polysaccharide degradation in the marine bacterium Paraglaciecola hydrolytica S66T encoded in a sizeable polysaccharide utilization locus. Front. Microbiol. 2018, 9, 839. [Google Scholar] [CrossRef]
- Mabeau, S.; Fleurence, J. Seaweed in Food Products: Biochemical and Nutritional Aspects. Trends Food Sci. Technol. 1993, 4, 103–107. [Google Scholar] [CrossRef]
- Rhein-Knudsen, N.; Ale, M.T.; Ajalloueian, F.; Yu, L.; Meyer, A.S. Rheological properties of agar and carrageenan from Ghanaian red seaweeds. Food Hydrocoll. 2017, 63, 50–58. [Google Scholar] [CrossRef]
- Usov, A.I. Polysaccharides of the red algae. Adv. Carbohydr. Chem. Biochem. 2011, 65, 115–217. [Google Scholar]
- Usov, A.I. Structural analysis of red seaweed galactans of agar and carrageenan groups. Food Hydrocoll. 1998, 12, 301–308. [Google Scholar] [CrossRef]
- Lahaye, M. Developments on gelling algal galactans, their structure and physico-chemistry. J. Appl. Phycol. 2001, 13, 173–184. [Google Scholar] [CrossRef]
- Campo, V.L.; Kawano, D.F.; Silva, D.B., Jr.; Carvalho, I. Carrageenans: Biological properties, chemical modifications and structural analysis—A review. Carbohydr. Polym. 2009, 77, 167–180. [Google Scholar] [CrossRef]
- Martínez-Sanz, M.; Gómez-Mascaraque, L.G.; Ballester, A.R.; Martínez-Abad, A.; Brodkorb, A.; López-Rubio, A. Production of unpurified agar-based extracts from red seaweed Gelidium sesquipedale by means of simplified extraction protocols. Algal Res. 2019, 38, 101420. [Google Scholar] [CrossRef]
- Lahaye, M.; Yaphe, W. The Chemical Structure of Agar from Gracilaria compressa (C. Agardh) Greville, G. cervicornis (Turner) J. Agardh, G. damaecornis J. Agardh and G. domingensis Sonder ex Kützing (Gigartinales, Rhodophyta). Botanica Marina 1989, 32, 369–378. [Google Scholar] [CrossRef]
- Armisén, R. World-wide use and importance of Gracilaria. J. Appl. Phycol. 1995, 7, 231–243. [Google Scholar] [CrossRef]
- Lee, W.K.; Lim, Y.Y.; Leow, A.T.C.; Namasivayam, P.; Abdullah, J.O.; Ho, C.L. Factors affecting yield and gelling properties of agar. J. Appl. Phycol. 2017, 29, 1527–1540. [Google Scholar] [CrossRef]
- Craigie, J. Seaweed extract stimuli in plant science and agriculture. J. Appl. Phycol. 2011, 23, 371–393. [Google Scholar] [CrossRef]
- Yun, E.J.; Yu, S.; Kim, K.H. Current knowledge on agarolytic enzymes and the industrial potential of agar-derived sugars. Appl. Microbiol. Biotechnol. 2017, 101, 5581–5589. [Google Scholar] [CrossRef]
- Yarnpakdee, S.; Benjakul, S.; Kingwascharapong, P. Physico-chemical and gel properties of agar from Gracilaria tenuistipitata from the lake of Songkhla, Thailand. Food Hydrocoll. 2015, 51, 217–226. [Google Scholar] [CrossRef]
- Sousa, A.M.M.; Morais, S.; Abreu, M.H.; Pereira, R.; Sousa-Pinto, I.; Cabrita, E.J.; Delerue-Matos, C.; Gonçalves, M.P. Structural, physical, and chemical modifications induced by microwave heating on native agar-like galactans. J. Agric. Food Chem. 2012, 60, 4977–4985. [Google Scholar] [CrossRef]
- Vergara-Rodarte, M.A.; Hernández-Carmona, G.; Rodríguez-Montesinos, Y.E.; Arvizu-Higuera, D.L.; Riosmena-Rodríguez, R.; Murillo-Álvarez, J.I. Seasonal variation of agar from Gracilaria vermiculophylla, effect of alkali treatment time, and stability of its Colagar. J. Appl. Phycol. 2010, 22, 753–759. [Google Scholar] [CrossRef]
- Marinho-Soriano, E.; Bourret, E. Effects of season on the yield and quality of agar from Gracilaria species (Gracilariaceae Rhodophyta). Bioresour. Technol. 2003, 90, 329–333. [Google Scholar] [CrossRef]
- Marinho-Soriano, E.; Bourret, E. Polysaccharides from the red seaweed Gracilaria dura (Gracilariales, Rhodophyta). Bioresour. Technol. 2005, 96, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Romero, J.B.; Villanueva, R.D.; Montaño, M.N.E. Stability of agar in the seaweed Gracilaria eucheumatoides (Gracilariales, Rhodophyta) during postharvest storage. Bioresour. Technol. 2008, 99, 8151–8155. [Google Scholar] [CrossRef]
- Chirapart, A.; Munkit, J.; Lewmanomont, K. Changes in yield and quality of agar from the agarophytes, Gracilaria fisheri and G. tenuistipitata var. liui cultivated in earthen ponds. Kasetsart J. Nat. Sci. 2006, 40, 529–540. [Google Scholar]
- Arvizu-Higuera, D.L.; Rodríguez-Montesinos, Y.E.; Murillo-Alvarez, J.I.; Muñoz-Ochoa, M.; Hernández-Carmona, G. Effect of alkali treatment time and extraction time on agar from Gracilaria vermiculophylla. J. Appl. Phycol. 2008, 20, 515–519. [Google Scholar] [CrossRef]
- Kohajdová, Z.; Karovičová, J.; Gajdošová, Ž. Importance of hydrocolloids in bakery industry. Potravinarstvo 2008, 2, 9–18. [Google Scholar]
- Kazłowski, B.; Pan, C.L.; Ko, Y.T. Separation and quantification of neoagaro- and agaro-oligosaccharide products generated from agarose digestion by β-agarase and HCl in liquid chromatography systems. Carbohydr. Res. 2008, 343, 2443–2450. [Google Scholar] [CrossRef]
- Hong, S.J.; Lee, J.H.; Kim, E.J.; Yang, H.J.; Park, J.S.; Hong, S.K. Toxicological evaluation of neoagarooligosaccharides prepared by enzymatic hydrolysis of agar. Regul. Toxicol. Pharm. 2017, 90, 9–21. [Google Scholar] [CrossRef]
- Freile-Pelegrín, Y.; Robledo, D. Influence of alkali treatment on agar from Gracilaria cornea from Yucatán, México. J. Appl. Phycol. 1997, 9, 533–539. [Google Scholar]
- Freile-Pelegrın, Y.; Murano, E. Agars from three species of Gracilaria (Rhodophyta) from Yucatan Peninsula. Bioresour. Technol. 2005, 96, 295–302. [Google Scholar] [CrossRef]
- Sousa, A.M.M.; Alves, V.D.; Morais, S.; Delerue-Matos, C.; Gonçalves, M.P. Agar extraction from integrated multitrophic aquacultured Gracilaria vermiculophylla: Evaluation of a microwave-assisted process using response surface methodology. Bioresour. Technol. 2010, 101, 3258–3267. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, R.D.; Sousa, A.M.M.; Gonçalves, M.P.; Nilsson, M.; Hilliou, L. Production and properties of agar from the invasive marine alga, Gracilaria vermiculophylla (Gracilariales, Rhodophyta). J. Appl. Phycol. 2010, 22, 211–220. [Google Scholar] [CrossRef]
- Kumar, V.; Fotedar, R. Agar extraction process for Gracilaria cliftonii (Withell, Millar, & Kraft, 1994). Carbohydr. Polym. 2009, 78, 813–819. [Google Scholar]
- Saha, D.; Bhattacharya, S. Hydrocolloids as thickening and gelling agents in food: A critical review. J. Food Sci. Technol. 2010, 47, 587–590. [Google Scholar] [CrossRef]
- Murano, E. Chemical structure and quality of agars from Gracilaria. J. Appl. Phycol. 1995, 7, 245–254. [Google Scholar] [CrossRef]
- Yousefi, M.K.; Islami, H.R.; Filizadeh, Y. Effect of extraction process on agar properties of Gracilaria corticata (Rhodophyta) collected from the persian gulf. Phycologia 2013, 52, 481–487. [Google Scholar] [CrossRef]
- Gallagher, J.A.; Turner, L.B.; Adams, J.M.M.; Barrento, S.; Dyer, P.W.; Theodorou, M.K. Species variation in the effects of dewatering treatment on macroalgae. J. Appl. Phycol. 2018, 30, 2305–2316. [Google Scholar] [CrossRef]
- Rath, J.; Adhikary, S.P. Effect of alkali treatment on the yield and quality of agar from red alga Gracilaria verrucosa (Rhodophyta, Gracilariales) occurring at different salinity gradient of Chilika lake. Indian J. Mar. Sci. 2004, 33, 202–205. [Google Scholar]
- Ahmad, R.; Surif, M.; Ramli, N.; Yahya, N.; Nor, A.R.M.; Bekbayeva, L.A. Preliminary study on the agar content and agar gel strength of Gracilaria manilaensis using different agar extraction processes. World Appl. Sci. J. 2011, 15, 184–188. [Google Scholar]
- González-Leija, J.A.; Hernández-Garibay, E.; Pacheco-Ruíz, I.; Guardado-Puentes, J.; Espinoza-Avalos, J.; López-Vivas, J.M.; Bautista-Alcantar, J. Optimization of the yield and quality of agar from Gracilariopsis lemaneiformis (Gracilariales) from the Gulf of California using an alkaline treatment. J. Appl. Phycol. 2009, 21, 321–326. [Google Scholar] [CrossRef]
- Roleda, M.Y.; Montaño, N.E.; Ganzon-Fortes, E.T.; Villanueva, R.D. Acetic acid pretreatment in agar extraction of Philippine Gelidiella acerosa (Forsskaal) Feldmann et Hamel (Rhodophyta, Gelidiales). Bot. Mar. 1997, 40, 63–69. [Google Scholar] [CrossRef][Green Version]
- Freile-Pelegrín, Y.; Robledo, D.; Pedersén, M.; Bruno, E.; Rönnqvist, J. Effect of dark and salinity treatment in the yield and quality of agar from Gracilaria cornea (Rhodophyceae). Cienc. Mar. 2002, 28, 289–296. [Google Scholar] [CrossRef]
- Li, H.; Huang, J.; Xin, Y.; Zhang, B.; Jin, Y.; Zhang, W. Optimization and scale-up of a new photobleaching agar extraction process from Gracilaria lemaneiformis. J. Appl. Phycol. 2009, 21, 247–254. [Google Scholar] [CrossRef]
- Maciel, J.S.; Chaves, L.S.; Souza, B.W.S.; Teixeira, D.I.A.; Freitas, A.L.P.; Feitosa, J.P.A.; de Paula, R.C.M. Structural characterization of cold extracted fraction of soluble sulfated polysaccharide from red seaweed Gracilaria birdiae. Carbohydr. Polym. 2008, 71, 559–565. [Google Scholar] [CrossRef]
- Al-Alawi, A.; Chitra, P.; Al-Mamun, A.; Al-Marhubi, I.; Rahman, M.S. Characterization of red seaweed extracts treated by water, acid and alkaline solutions. Int. J. Food Eng. 2018, 14, 20170353. [Google Scholar] [CrossRef]
- Kim, M.; Yim, J.H.; Kim, S.-Y.; Kim, H.S.; Lee, W.G.; Kim, S.J.; Kang, P.S.; Lee, C.-K. In vitro inhibition of influenza A virus infection by marine microalga-derived sulfated polysaccharide p-KG03. Antivir. Res. 2012, 93, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Boo, S.M.; Mansilla, A.; Yoon, H.S. Unique repeat and plasmid sequences in the mitochondrial genome of Gracilaria chilensis (Gracilariales, Rhodophyta). Phycologia 2015, 54, 20–23. [Google Scholar] [CrossRef]
- Park, S.Y.; Lee, E.S.; Han, S.H.; Lee, H.Y.; Lee, S. Antioxidative effects of two native berry species, Empetrum nigrum var. japonicum K. Koch and Rubus buergeri Miq., from the Jeju Island of Korea. J. Food Biochem. 2012, 36, 675–682. [Google Scholar]
- Villanueva, R.; Montaño, M.N. Enhancement of carrageenan gel quality in the commercially important tropical seaweed Eucheuma denticulatum (Rhodophyta), with postharvest treatment in low-nutrient conditions. Bot. Mar. 2014, 57, 217–223. [Google Scholar] [CrossRef]
- Shukla, M.K.; Kumar, M.; Prasad, K.; Reddy, C.R.K.; Jha, B. Partial characterization of sulfohydrolase from Gracilaria dura and evaluation of its potential application in improvement of the agar quality. Carbohydr. Polym. 2011, 85, 157–163. [Google Scholar] [CrossRef]
- Navarro, D.A.; Stortz, C.A. Microwave-assisted alkaline modification of red seaweed galactans. Carbohydr. Polym. 2005, 62, 187–191. [Google Scholar] [CrossRef]
- Wu, S.C.; Lin, Y.P.; King, V.A.E. Optimization of intermittent microwave-assisted extraction of sulfated porphyran from Porphyra dentate. Trans. ASABE 2014, 57, 103–110. [Google Scholar]
- Villanueva, R.D.; Rumbaoa, R.O.; Gomez, A.V.; Loquias, M.M.; De La Rosa, A.M.; Montaño, N.E. γ-Irradiation in the extraction of agar from Gelidiella acerosa (Forsskaal) Feldmann et Hamel. Bot. Mar. 1998, 41, 199–202. [Google Scholar] [CrossRef]
- Pereira-Pacheco, F.; Robledo, D.; Rodríguez-Carvajal, L.; Freile-Pelegrín, Y. Optimization of native agar extraction from Hydropuntia cornea from Yucatán, México. Bioresour. Technol. 2007, 98, 1278–1284. [Google Scholar] [CrossRef]
- Villanueva, R.D.; Pagba, C.V.; Montaño, N.E. Optimized agar extraction from Gracilaria eucheumoides Harvey. Bot. Mar. 1997, 40, 369–372. [Google Scholar] [CrossRef]
- Li, H.; Yu, X.; Jin, Y.; Zhang, W.; Liu, Y. Development of an eco-friendly agar extraction technique from the red seaweed Gracilaria lemaneiformis. Bioresour. Technol. 2008, 99, 3301–3305. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.; Siddhanta, A.K.; Ganesan, M.; Ramavat, B.K.; Jha, B.; Ghosh, P.K. Agars of Gelidiella acerosa of west and southeast coasts of India. Bioresour. Technol. 2007, 98, 1907–1915. [Google Scholar] [CrossRef]
- Fidelis, G.P.; Camara, R.B.G.; Queiroz, M.F.; Costa, M.S.S.P.; Santos, P.C.; Rocha, H.A.O.; Costa, L.S. Proteolysis, NaOH and ultrasound-enhanced extraction of anticoagulant and antioxidant sulfated polysaccharides from the edible seaweed, Gracilaria birdiae. Molecules 2014, 19, 18511–18526. [Google Scholar] [CrossRef] [PubMed]
- Coura, C.O.; Souza, R.B.; Rodrigues, J.A.G.; Vanderlei, E.D.S.O.; De Araújo, I.W.F.; Ribeiro, N.A.; Frota, A.F.; Ribeiro, K.A.; Chaves, H.V.; Pereira, K.M.A.; et al. Mechanisms involved in the anti-inflammatory action of a polysulfated fraction from Gracilaria cornea in rats. PLoS ONE 2015, 10, e0119319. [Google Scholar] [CrossRef]
- Chen, H.M.; Zheng, L.; Yan Agaro, X.J. Bioactivity research of oligosaccharides. Food Technol. Biotechnol. 2005, 43, 29–36. [Google Scholar]
- Mazumder, S.; Ghosal, P.K.; Pujol, C.A.; Carlucci, M.J.; Damonte, E.B.; Ray, B. Isolation, chemical investigation and antiviral activity of polysaccharides from Gracilaria corticata (Gracilariaceae, Rhodophyta). Int. J. Biol. Macromol. 2002, 31, 87–95. [Google Scholar] [CrossRef]
- Bhattarai, Y.; Kashyap, P.C. Agaro-oligosaccharides: A new frontier in the fight against coloncancer? Am. J. Physiol.- Gastrointest. Liver Physiol. 2016, 310, G335–G336. [Google Scholar] [CrossRef]
- Enoki, T.; Okuda, S.; Kudo, Y.; Takashima, F.; Sagawa, H.; Kato, I. Oligosaccharides from agar inhibit pro-inflammatory mediator release by inducing heme oxygenase 1. Biosci. Biotechnol. Biochem. 2010, 74, 766–770. [Google Scholar] [CrossRef]
- Enoki, T.; Tominaga, T.; Takashima, F.; Ohnogi, H.; Sagawa, H.; Kato, I. Anti-tumor-promoting activities of agaro-oligosaccharides on two-stage mouse skin carcinogenesis. Biol. Pharm. Bull. 2012, 35, 1145–1149. [Google Scholar] [CrossRef]
- Higashimura, Y.; Naito, Y.; Takagi, T.; Mizushima, K.; Hirai, Y.; Harusato, A.; Ohnogi, H.; Yamaji, R.; Inui, H.; Nakano, Y.; et al. Oligosaccharides from agar inhibit murine intestinal inflammation through the induction of heme oxygenase-1 expression. J. Gastroenterol. 2013, 48, 897–909. [Google Scholar] [CrossRef]
- Jin, M.; Liu, H.; Hou, Y.; Chan, Z.; Di, W.; Li, L.; Zeng, R. Preparation, characterization and alcoholic liver injury protective effects of algal oligosaccharides from Gracilaria lemaneiformis. Food Res. Int. 2017, 100, 186–195. [Google Scholar] [CrossRef]
- Jang, M.K.; Lee, D.G.; Kim, N.Y.; Yu, K.H.; Jang, H.J.; Lee, S.W.; Jang, H.J.; Lee, Y.H. Purification and characterization of neoagarotetraose from hydrolyzed agar. J. Microbiol. Biotechnol. 2009, 19, 1197–1200. [Google Scholar]
- Hehemann, J.H.; Correc, G.; Thomas, F.; Bernard, T.; Barbeyron, T.; Jam, M.; Helbert, W.; Michel, G.; Czjzek, M. Biochemical and structural characterization of the complex agarolytic enzyme system from the marine bacterium Zobellia galactanivorans. J. Biol. Chem. 2012, 28, 30571–30584. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.Y.; Mei, J.F.; Yi, Y.; Chen, J.S.; Ying, G.Q. Advances in study on biological activities of agaro-oligosaccharide. Pharm. Biotechnol. 2008, 15, 493–496. [Google Scholar]
- Kazłowski, B.; Liang, C.; Yuan, P.; Ko, P. Monitoring and preparation of neoagaro- and agaro-oligosaccharide products by high performance anion exchange chromatography systems. Carbohydr. Polym. 2015, 122, 351–358. [Google Scholar] [CrossRef]
- Tripathi, A.; Kathuria, N.; Kumar, A. Elastic and macroporous agarose–599 gelatin cryogels with isotropic and anisotropic porosity for tissue engineering. J. Biomed. Mater. Res. Part A 2009, 90, 680–694. [Google Scholar] [CrossRef]
- Ramana Ramya, J.; Thanigai Arul, K.; Sathiamurthi, P.; Asokan, K.; Narayana Kalkura, S. Novel gamma irradiated agarose-gelatin-hydroxyapatite nanocomposite scaffolds for skin tissue regeneration. Ceram. Int. 2016, 42, 11045–11054. [Google Scholar] [CrossRef]
- Gao, M.; Lu, P.; Bednark, B.; Lynam, D.; Conner, J.M.; Sakamoto, J.; Tuszynski, M.H. Templated agarose scaffolds for the support of motor axon regeneration into sites of complete spinal cord transection. Biomaterials 2013, 34, 1529–1536. [Google Scholar] [CrossRef]
- Zhang, L.M.; Wu, C.X.; Huang, J.Y.; Peng, X.H.; Chen, P.; Tang, S.Q. Synthesis and characterization of a degradable composite agarose/HA hydrogel. Carbohydr. Polym. 2012, 88, 1445–1452. [Google Scholar] [CrossRef]
- Hu, J.; Zhu, Y.; Tong, H.; Shen, X.; Chen, L.; Ran, J. A detailed study of homogeneous agarose/hydroxyapatite nanocomposites for load-bearing bone tissue. Int. J. Biol. Macromol. 2016, 82, 134–143. [Google Scholar] [CrossRef]
- Zou, P.; Lu, X.; Jing, C.; Yuan, Y.; Lu, Y.; Zhang, C.; Meng, L.; Zhao, H.; Li, Y. Low-molecular-weightt polysaccharides from Pyropia yezoensis enhance tolerance of wheat seedlings (Triticum aestivum L.) to salt stress. Front. Plant Sci. 2018, 9, 427. [Google Scholar] [CrossRef] [PubMed]
- Kang, O.L.; Ghani, M.; Hassan, O.; Rahmati, S.; Ramli, N. Novel agaro-oligosaccharide production through enzymatic hydrolysis: Physicochemical properties and antioxidant activities. Food Hydrocoll. 2014, 42, 304–308. [Google Scholar] [CrossRef]
- Fenoradosoa, T.A.; Laroche, C.; Delattre, C.; Dulong, V.; Cerf, D.L.; Picton, L.; Michaud, P. Rheological behavior and non-enzymatic degradation of a sulfated galactan from Halymenia durvillei (Halymeniales, Rhodophyta). Appl. Biochem. Biotechnol. 2012, 167, 1303–1313. [Google Scholar] [CrossRef]
- Zhou, C.; Yu, X.; Zhang, Y.; He, R.; Ma, H. Ultrasonic degradation, purification and analysis of structure and antioxidant activity of polysaccharide from Porphyra yezoensis Udea. Carbohydr. Polym. 2012, 87, 2046–2051. [Google Scholar] [CrossRef]
- Poupard, N.; Badarou, P.; Fasani, F.; Groult, H.; Bridiau, N.; Sannier, F.; Bordenave-Juchereau, S.; Kieda, C.; Piot, J.M.; Grillon, C.; et al. Assessment of heparanase-mediated angiogenesis using microvascular endothelial cells: Identification of λ-Carrageenan derivative as a potent anti angiogenic agent. Mar. Drugs 2017, 15, 134. [Google Scholar] [CrossRef]
- Öğretmen, Ö.Y.; Duyar, H.A. The effect of different extraction methods and pre-treatments on agar yield and physico-chemical properties of Gelidium latifolium (Gelidiaceae, Rhodophyta) from Sinop Peninsula Coast of Black Sea, Turkey. J. Appl. Phycol. 2018, 30, 1355–1360. [Google Scholar] [CrossRef]
- Pereira, L.; Gheda, S.; Ribeiro-Claro, P. Analysis by vibrational spectroscopy of seaweed with potential use in food, pharmaceutical and cosmetic industries. Int. J. Carbohydr. Chem. 2013, 2013, 537202. [Google Scholar] [CrossRef]
- Knudsen, N.R.; Ale, M.T.; Meyer, A.S. Seaweed hydrocolloid production: An update on enzyme assisted extraction and modification technologies. Mar. Drugs 2015, 13, 3340–3359. [Google Scholar] [CrossRef]
- Collén, J.; Cornish, M.L.; Craigie, J.; Ficko-Blean, E.; Hervé, C.; Krueger-Hadfield, S.A.; Leblanc, C.; Michel, G.; Potin, P.; Tonon, T.; et al. Chondrus crispus—A present and historical model organism for red seaweeds. Adv. Bot. Res. 2014, 71, 53–89. [Google Scholar]
- Pereira, L.; Critchley, A.T.; Amado, A.M.; Ribeiro-Claro, P.J.A. A comparative analysis of phycocolloids produced by underutilized versus industrially utilized carrageenophytes (Gigartinales, Rhodophyta). J. Appl. Phycol. 2009, 21, 599–605. [Google Scholar] [CrossRef]
- Blakemore, W.R.; Harpell, A.R. Carrageenan. In Food Stabilisers, Thickeners and Gelling Agents; Imeson, A., Ed.; Blackwell Publishing Ltd.: Oxford, UK, 2009; pp. 73–94. [Google Scholar]
- Pereira, L.; Meireles, F.; Gaspar, R. Population studies and carrageenan properties in eight gigartinales (Rhodophyta) from Iberian Peninsula. In Seaweeds: Agricultural Uses, Biological and Antioxidant Agents; Nova Science Publishers, Inc.: New York, NY, USA, 2014; pp. 115–134. [Google Scholar]
- Vera, J.; Castro, J.; González, A.; Moenne, A. Seaweed polysaccharides and derived oligosaccharides stimulate defense responses and protection against pathogens in plants. Mar. Drugs 2011, 9, 2514–2525. [Google Scholar] [CrossRef]
- Necas, J.; Bartosikova, L. Carrageenan: A review. Vet. Med. 2013, 58, 187–205. [Google Scholar] [CrossRef]
- Ghanbarzadeh, M.; Golmoradizadeh, A.; Homaei, A. Carrageenans and carrageenases: Versatile polysaccharides and promising marine enzymes. Phytochem. Rev. 2018, 17, 535–571. [Google Scholar] [CrossRef]
- Cunha, L.; Grenha, A. Sulfated seaweed polysaccharides as multifunctional materials in drug delivery applications. Mar. Drugs 2016, 14, 42. [Google Scholar] [CrossRef]
- Van de Vijver, M.J.; He, Y.D.; van’t Veer, L.J.; Dai, H.; Hart, A.A.; Voskuil, D.W.; Schreiber, G.J.; Peterse, J.L.; Roberts, C.; Marton, M.J.; et al. A gene-expression signature as a predictor of survival in breast cancer. N. Engl. J. Med. 2002, 347, 1999–2009. [Google Scholar] [CrossRef]
- Cohen, S.M.; Ito, N. A critical review of the toxicological effects of carrageenan and processed Eucheuma seaweed on the gastrointestinal tract. Crit. Rev. Toxicol. 2002, 32, 413–444. [Google Scholar] [CrossRef]
- Weiner, M.L. Food additive carrageenan: Part II: A critical review of carrageenan in vivo safety studies. Crit. Rev. Toxicol. 2014, 44, 244–269. [Google Scholar] [CrossRef]
- Weiner, M.L. Parameters and pitfalls to consider in the conduct of food additive research, Carrageenan as a case study. Crit. Rev. Toxicol. 2016, 87, 31–44. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Borthakur, A.; Dudeja, P.K.; Tobacman, J.K. Carrageenan induces cell cycle arrest in human intestinal epithelial cells in vitro. J. Nutr. 2008, 138, 469–475. [Google Scholar] [CrossRef]
- Chen, M.; Schliep, M.; Willows, R.D.; Cai, Z.L.; Neilan, B.A.; Scheer, H. A red-shifted chlorophyll. Science 2010, 329, 1318–1319. [Google Scholar] [CrossRef]
- Munyaka, P.M.; Sepehri, S.; Ghia, J.E.; Khafipour, E. Carrageenan gum and adherent invasive Escherichia coli in a piglet model of inflammatory bowel disease: Impact on intestinal mucosa-associated microbiota. Front. Microbiol. 2016, 7, 462. [Google Scholar] [CrossRef]
- Shang, Q.; Sun, W.; Shan, X.; Jiang, H.; Cai, C.; Hao, J.; Li, G.; Yu, G. Carrageenan-induced colitis is associated with decreased population of anti-inflammatory bacterium, Akkermansia muciniphila, in the gut microbiota of C57BL/6J mice. Toxicol. Lett. 2017, 279, 87–95. [Google Scholar] [CrossRef]
- Wei, W.; Feng, W.; Xin, G.; Tingting, N.; Zhanghe, Z.; Haimin, C.; Xiaojun, Y. Enhanced effect of κ-carrageenan on TNBS-induced inflammation in mice. Int. Immunopharmacol. 2016, 39, 218–228. [Google Scholar] [CrossRef]
- Wu, W.; Wang, F.; Gao, X.; Niu, T.; Zhu, X.; Yan, X.; Chen, H. Synergistic effect of κ-carrageenan on oxazolone-induced inflammation in BALB/c mice. BMC Gastroenterol. 2016, 16, 41. [Google Scholar] [CrossRef]
- David, S.; Shani Levi, C.; Fahoum, L.; Ungar, Y.; Meyron-Holtz, E.G.; Shpigelman, A.; Lesmes, U. Revisiting the carrageenan controversy: Do we really understand the digestive fate and safety of carrageenan in our foods? Food Funct. 2018, 9, 1344–1352. [Google Scholar] [CrossRef]
- David, S.; Fahoum, L.; Rozen, G.; Shaoul, R.; Shpigelman, A.; Meyron-Holtz, E.G.; Lesmes, U. Reply to the Comment on “Revisiting the carrageenan controversy: Do we really understand the digestive fate and safety of carrageenan in our foods?”. Food Funct. 2019, 10, 1763–1766. [Google Scholar] [CrossRef]
- De Sousa Oliveira Vanderlei, E.; De Araújo, I.W.F.; Quinderé, A.L.G.; Fontes, B.P.; Eloy, Y.R.G.; Rodrigues, J.A.G.; Silva, A.A.R.E.; Chaves, H.V.; Jorge, R.J.B.; De Menezes, D.B.; et al. The involvement of the HO-1 pathway in the anti-inflammatory action of a sulfated polysaccharide isolated from the red seaweed Gracilaria birdiae. Inflamm. Res. 2011, 60, 1121–1130. [Google Scholar] [CrossRef]
- Inic-Kanada, A.; Stein, E.; Stojanovic, M.; Schuerer, N.; Ghasemian, E.; Filipovic, A.; Marinkovic, E.; Kosanovic, D.; Barisani-Asenbauer, T. Effects of iota-carrageenan on ocular Chlamydia trachomatis infection in vitro and in vivo. J. Appl. Phycol. 2018, 30, 2601–2610. [Google Scholar] [CrossRef]
- Talarico, L.B.; Zibetti, R.G.; Faria, P.C.; Scolaro, L.A.; Duarte, M.E.; Noseda, M.D.; Pujol, C.A.; Damonte, E.B. Anti-herpes simplex virus activity of sulfated galactans from the red seaweeds Gymnogongrus griffithsiae and Cryptonemia crenulata. Int. J. Biol. Macromol. 2004, 34, 63–71. [Google Scholar] [CrossRef]
- Talarico, L.B.; Pujol, C.A.; Zibetti, R.G.; Faría, P.C.; Noseda, M.D.; Duarte, M.E.; Damonte, E.B. The antiviral activity of sulfated polysaccharides against dengue virus is dependent on virus serotype and host cell. Antivir. Res. 2005, 66, 103–110. [Google Scholar] [CrossRef]
- Cáceres, P.J.; Carlucci, M.J.; Damonte, E.B.; Matsuhiro, B.; Zúñiga, E.A. Carrageenans from Chilean samples of Stenogramme interrupta (Phyllophoraceae): Structural analysis and biological activity. Phytochemistry 2000, 53, 81–86. [Google Scholar] [CrossRef]
- Chattopadhyay, K.; Ghosh, T.; Pujol, C.A.; Carlucci, M.J.; Damonte, E.B.; Ray, B. Polysaccharides from Gracilaria corticata: Sulfation, chemical characterization and anti-HSV activities. Int. J. Biol. Macromol. 2008, 43, 346–351. [Google Scholar] [CrossRef]
- Yuan, H.; Song, J.; Li, X.; Li, N.; Dai, J. Immunomodulation and antitumor activity of kappa-carrageenan oligosaccharides. Cancer Lett. 2006, 243, 228–234. [Google Scholar] [CrossRef]
- Liu, J.; Hafting, J.; Critchley, A.T.; Banskota, A.H.; Prithiviraj, B. Components of the cultivated red seaweed Chondrus crispus enhance the immune response of Caenorhabditis elegans to Pseudomonas aeruginosa through the pmk-1, daf-2/daf-16, and skn-1 pathways. Appl. Environ. Microbiol. 2013, 79, 7343–7350. [Google Scholar] [CrossRef]
- Souza, M.P.; Vaz, A.F.M.; Costa, T.B.; Cerqueira, M.A.; De Castro, C.M.M.B.; Vicente, A.A.; Carneiro-da-Cunha, M.G. Construction of a Biocompatible and Antioxidant Multilayer Coating by Layer-by-Layer Assembly of κ-Carrageenan and Quercetin Nanoparticles. Food Bioprocess Technol. 2018, 11, 1050–1060. [Google Scholar] [CrossRef]
- Sun, L.; Wang, S.; Gong, X.; Zhao, M.; Fu, X.; Wang, L. Isolation, purification and characteristics of R-phycoerythrin from a marine macroalga Heterosiphonia japonica. Protein Expr. Purif. 2010, 64, 146–154. [Google Scholar] [CrossRef]
- De Jesus Raposo, M.F.; De Morais, A.M.B.; De Morais, R.M.S.C. Marine polysaccharides from algae with potential biomedical applications. Mar. Drugs 2015, 13, 2967–3028. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, L.; Reis, R.P. Cultivation of the red algae Kappaphycus alvarezii in Brazil and its pharmacological potential. Braz. J. Pharmacogn. 2012, 22, 748–752. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Saurabh, C.K.; Tye, Y.Y.; Lai, T.K.; Easa, A.M.; Rosamah, E.; Fazita, M.R.N.; Syakir, M.I.; Adnan, A.S.; Fizree, H.M.; et al. Seaweed based sustainable films and composites for food and pharmaceutical applications: A review. Renew. Sustain. Energy Rev. 2017, 77, 353–362. [Google Scholar] [CrossRef]
- Morris, C.E. How does fertility of the substrate affect intra-specific competition? Evidence and synthesis from self-thinning. Ecol. Res. 2003, 18, 287–305. [Google Scholar] [CrossRef]
- Briones, A.V.; Sato, T. Encapsulation of glucose oxidase (GOD) in polyelectrolyte complexes of chitosan-carrageenan. React. Funct. Polym. 2010, 70, 19–27. [Google Scholar] [CrossRef]
- Zhang, W.T.; Yue, C.; Huang, Q.W.; Yuan, K.; Yan, A.J.; Shi, S. Contents of eight saccharides in unprocessed and processed Rehmannia glutinosa and content changes at different processing time points. Chin. Tradit. Herb. Drug. 2016, 47, 1132–1136. [Google Scholar]
- Dafe, A.; Etemadi, H.; Zarredar, H.; Mahdavinia, G.R. Development of novel carboxymethyl cellulose/k-carrageenan blends as an enteric delivery vehicle for probiotic bacteria. Int. J. Biol. Macromol. 2017, 97, 299–307. [Google Scholar] [CrossRef]
- Li, L.; Ni, R.; Shao, Y.; Mao, S. Carrageenan and its applications in drug delivery. Carbohydr. Polym. 2014, 103, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sahiner, N.; Sagbas, S.; Yllmaz, S. Microgels derived from different forms of carrageenans, kappa, iota, and lambda for biomedical applications. MRS Adv. 2017, 2, 2521–2527. [Google Scholar] [CrossRef]
- Gonçalves, A.L.; Pires, J.C.M.; Simões, M. Biotechnological potential of Synechocystis salina co-cultures with selected microalgae and cyanobacteria: Nutrients removal, biomass and lipid production. Bioresour. Technol. 2016, 200, 279–286. [Google Scholar] [CrossRef]
- Ghannam, A.; Abbas, A.; Alek, H.; Al-Waari, Z.; Al-Ktaifani, M. Enhancement of local plant immunity against tobacco mosaic virus infection after treatment with sulphated-carrageenan from redalga (Hypnea musciformis). Physiol. Mol. Plant Pathol. 2013, 84, 19–27. [Google Scholar] [CrossRef]
- Mercier, L.; Lafitte, C.; Borderies, G.; Briand, X.; Esquerré-Tugayé, M.T.; Fournier, J. The algal polysaccharide carrageenans can act as an elicitor of plant defence. New Phytol. 2001, 149, 43–51. [Google Scholar] [CrossRef]
- Nagorskaya, V.P.; Reunov, A.V.; Lapshina, L.A.; Ermak, I.M.; Barabanova, A.O. Inhibitory effect of κ/β-carrageenan from red alga Tichocarpus crinitus on the development of a potato virus X infection in leaves of Datura stramonium L. Biol. Bull. 2010, 37, 653–658. [Google Scholar] [CrossRef]
- Sangha, J.S.; Ravichandran, S.; Prithiviraj, K.; Critchley, A.T.; Prithiviraj, B. Sulfated macroalgal polysaccharides λ-carrageenan and ι-carrageenan differentially alter Arabidopsis thaliana resistance to Sclerotinia sclerotiorum. Physiol. Mol. Plant Pathol. 2010, 75, 38–45. [Google Scholar] [CrossRef]
- Sangha, J.S.; Khan, W.; Ji, X.; Zhang, J.; Mills, A.A.S.; Critchley, A.T.; Prithiviraj, B. Carrageenans, sulphated polysaccharides of red seaweeds, differentially affect Arabidopsis thaliana resistance to Trichoplusia ni (Cabbage looper). PLoS ONE 2011, 6, e26834. [Google Scholar] [CrossRef]
- Sangha, J.S.; Kandasamy, S.; Khan, W.; Bahia, N.S.; Singh, R.P.; Critchley, A.T.; Prithiviraj, B. λ-Carrageenan suppresses tomato chlorotic dwarf viroid (TCDVd) replication and symptom expression in tomatoes. Mar. Drugs 2015, 13, 2875–2889. [Google Scholar] [CrossRef] [PubMed]
- Shukla, P.S.; Borza, T.; Critchley, A.T.; Prithiviraj, B. Carrageenans from red seaweeds as promoters of growth and elicitors of defense response in plants. Front. Mar. Sci. 2016, 3, 81. [Google Scholar] [CrossRef]
- Vera, J.; Castro, J.; Contreras, R.A.; González, A.; Moenne, A. Oligocarrageenans induce a long-term and broad-range protection against pathogens in tobacco plants (var. Xanthi). Physiol. Mol. Plant Pathol. 2012, 79, 31–39. [Google Scholar] [CrossRef]
- Bi, Y.; Hu, Y.; Zhou, Z.G. Genetic variation of Laminaria japonica (Phaeophyta) populations in China as revealed by RAPD markers. Acta Oceanol. Sin. 2011, 30, 103–112. [Google Scholar] [CrossRef]
- Azevedo, G.; Torres, M.D.; Sousa-Pinto, I.; Hilliou, L. Effect of pre-extraction alkali treatment on the chemical structure and gelling properties of extracted hybrid carrageenan from Chondrus crispus and Ahnfeltiopsis devoniensis. Food Hydrocoll. 2015, 50, 150–158. [Google Scholar] [CrossRef]
- Hilliou, L.; Larotonda, F.D.S.; Abreu, P.; Ramos, A.M.; Sereno, A.M.; Goncalves, M.P. Effect of extraction parameters on the chemical structure and gel properties of κ/ι-hybrid carrageenans obtained from Mastocarpus stellatus. Biomol. Eng. 2006, 23, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Boulho, R.; Marty, C.; Freile-Pelegrín, Y.; Robledo, D.; Bourgougnon, N.; Bedoux, G. Antiherpetic (HSV-1) activity of carrageenans from the red seaweed Solieria chordalis (Rhodophyta, Gigartinales) extracted by microwave-assisted extraction (MAE). J. Appl. Phycol. 2017, 29, 2219–2228. [Google Scholar] [CrossRef]
- Rafiquzzaman, S.M.; Ahmed, R.; Lee, J.M.; Noh, G.; Jo, G.A.; Kong, I.S. Improved methods for isolation of carrageenan from Hypnea musciformis and its antioxidant activity. J. Appl. Phycol. 2016, 28, 1265–1274. [Google Scholar] [CrossRef]
- Vázquez-Delfín, E.; Robledo, D.; Freile-Pelegrín, Y. Microwave-assisted extraction of the Carrageenan from Hypnea musciformis (Cystocloniaceae, Rhodophyta). J. Appl. Phycol. 2014, 26, 901–907. [Google Scholar] [CrossRef]
- Estevez, J.M.; Ciancia, M.; Cerezo, A.S. The system of low-molecular-weight carrageenans and agaroids from the room-temperature-extracted fraction of Kappaphycus alvarezii. Carbohydr. Res. 2000, 325, 287–299. [Google Scholar] [CrossRef]
- Almutairi, F.M.; Adams, G.G.; Kök, M.S.; Lawson, C.J.; Gahler, R.; Wood, S.; Foster, T.J.; Rowe, A.J.; Harding, S.E. An analytical ultracentrifugation based study on the conformation of lambda carrageenan in aqueous solution. Carbohydr. Polym. 2013, 97, 203–209. [Google Scholar] [CrossRef]
- Tang, F.; Chen, F.; Li, F. Preparation and potential in vivo anti-influenza virus activity of low molecular-weight k-carrageenans and their derivatives. J. Appl. Polym. Sci. 2013, 127, 2110–2115. [Google Scholar] [CrossRef]
- Ratnawati, R.; Prasetyaningrum, A.; Wardhani, D.H. Kinetics and thermodynamics of ultrasound-assisted depolymerization of κ-carrageenan. Bull. Chem. React. Eng. Catal. 2016, 11, 48–58. [Google Scholar] [CrossRef]
- Sokolova, R.V.; Ermakova, S.P.; Awada, S.M.; Zvyagintseva, T.N.; Kanaan, H.M. Composition, structural characteristics, and antitumor properties of polysaccharides from the brown algae Dictyopteris polypodioides and Sargassum sp. Chem. Nat. Compd. 2011, 47, 329–334. [Google Scholar] [CrossRef]
- Hamias, R.; Wolak, T.; Huleihel, M.; Paran, E.; Levy-Ontman, O. Red alga polysaccharides attenuate angiotensin II-induced inflammation in coronary endothelial cells. Biochem. Biophys. Res. Commun. 2018, 500, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Kalitnik, A.A.; Marcov, P.A.; Anastyuk, S.D.; Barabanova, A.O.B.; Glazunov, V.P.; Popov, S.V.; Ovodov, Y.S.; Yermak, I.M. Gelling polysaccharide from Chondrus armatus and its oligosaccharides: The structural peculiarities and anti-inflammatory activity. Carbohydr. Polym. 2015, 115, 768–775. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.-X.; Guan, H.-S. The antiviral activities and mechanisms of marine polysaccharides: An overview. Marine Drugs 2012, 10, 2795–2816. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Ogamo, A.; Saito, T.; Watanabe, J.; Uchiyama, H.; Nakagawa, Y. Preparation and anti-HIV activity of low-molecular-weight carrageenans and their sulfated derivatives. Carbohydr. Polym. 1997, 32, 51–55. [Google Scholar] [CrossRef]
- Yamada, S.; Kosugi, I.; Katano, H.; Fukui, Y.; Kawasaki, H.; Arai, Y.; Kurane, I.; Inoue, N. In vivo imaging assay for the convenient evaluation of antiviral compounds against cytomegalovirus in mice. Antivir. Res. 2010, 88, 45–52. [Google Scholar] [CrossRef]
- Abad, L.V.; Kudo, H.; Saiki, S.; Nagasawa, N.; Tamada, M.; Katsumura, Y.; Aranilla, C.T.; Relleve, L.S.; De La Rosa, A.M. Radiation degradation studies of carrageenans. Carbohydr. Polym. 2009, 78, 100–106. [Google Scholar] [CrossRef]
- Zhou, G.; Sun, Y.; Xin, H.; Zhang, Y.; Li, Z.; Xu, Z. In vivo antitumor and immunomodulation activities of different molecular weight lambda-carrageenans from Chondrus ocellatus. Pharmacol. Res. 2004, 50, 47–53. [Google Scholar] [CrossRef]
- Raman, M.; Doble, M. κ-Carrageenan from marine red algae, Kappaphycus alvarezii—A functional food to prevent colon carcinogenesis. J. Funct. Foods 2015, 15, 354–364. [Google Scholar] [CrossRef]
- Zhou, G.; Xin, H.; Sheng, W.; Sun, Y.; Li, Z.; Xu, Z. In vivo growth-inhibition of S180 tumor by mixture of 5-Fu and low molecular lambda-carrageenan from Chondrus ocellatus. Pharmacol. Res. 2005, 51, 153–157. [Google Scholar] [CrossRef]
- Zhou, G.; Sheng, W.; Yao, W.; Wang, C. Effect of low molecular λ-carrageenan from Chondrus ocellatus on antitumor H-22 activity of 5-Fu. Pharmacol. Res. 2006, 53, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Lemonnier-Le Penhuizic, C.; Chatelet, C.; Kloareg, B.; Potin, P. Carrageenan oligosaccharides enhance stress-induced microspore embryogenesis in Brassica oleracea var. italica. Plant Sci. 2001, 160, 1211–1220. [Google Scholar] [CrossRef]
- Muñoz, A.M.; Ponce, J.C.; Araya, J.V. Method to Stimulate Carbon Fixation in Plants with an Aqueous Solution of Oligo-Carrageenans Selected from Kappa1, Kappa2, Lambda or Iota. U.S. Patent 12/911,790, 5 May 2011. [Google Scholar]
- Castro, J.; Vera, J.; González, A.; Moenne, A. Oligo-carrageenans stimulate growth by enhancing photosynthesis, basal metabolism, and cell cycle in tobacco plants (var. Burley). J. Plant Growth Regul. 2012, 31, 173–185. [Google Scholar] [CrossRef]
- Saucedo, S.; Contreras, R.A.; Moenne, A. Oligo-carrageenan kappa increases C, N and S assimilation, auxin and gibberellin contents, and growth in Pinus radiata trees. J. For. Res. 2015, 26, 635–640. [Google Scholar] [CrossRef]
- Abad, L.V.; Nasimova, I.R.; Relleve, L.S.; Aranilla, C.T.; De La Rosa, A.M.; Shibayama, M. Dynamic light scattering studies of irradiated kappa carrageenan. Int. J. Biol. Macromol. 2004, 34, 81–88. [Google Scholar] [CrossRef]
- González, A.; Castro, J.; Vera, J.; Moenne, A. Seaweed oligosaccharides stimulate plant growth by enhancing carbon and nitrogen assimilation, basal metabolism, and cell division. J. Plant Growth Regul. 2013, 32, 443–448. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, B.; Wu, Y.; Liu, Y.; Gu, X.; Zhang, H.; Wang, C.; Cao, H.; Huang, L.; Wang, Z. Structural characterization and antioxidant activities of κ-carrageenan oligosaccharides degraded by different methods. Food Chem. 2015, 178, 311–318. [Google Scholar] [CrossRef]
- Prasetyaningrum, A.; Jos, B.; Dharmawan, Y.; Octaviani, R.V.; Ratnawati, R. Chemical and spectral characterization of the ozonation products of κ-carrageenan. MATEC Web Conf. 2018, 156, 05006. [Google Scholar] [CrossRef][Green Version]
- Kalitnik, A.A.; Byankina Barabanova, A.O.; Nagorskaya, V.P.; Reunov, A.V.; Glazunov, V.P.; Solov’eva, T.F.; Yermak, I.M. Low molecular weight derivatives of different carrageenan types and their antiviral activity. J. Appl. Phycol. 2013, 25, 65–72. [Google Scholar] [CrossRef]
- Karlsson, A.; Singh, S.K. Acid hydrolysis of sulphated polysaccharides. Desulphation and the effect on molecular mass. Carbohydr. Polym. 1999, 38, 7–15. [Google Scholar] [CrossRef]
- Yang, B.; Yu, G.; Zhao, X.; Jiao, G.; Ren, S.; Chai, W. Mechanism of mild acid hydrolysis of galactan polysaccharides with highly ordered disaccharide repeats leading to a complete series of exclusively odd-numbered oligosaccharides. FEBS J. 2009, 276, 2125–2137. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, P.S.; Saxena, A. Bacterial carrageenases: An overview of production and biotechnological applications. 3 Biotech 2016, 6, 146. [Google Scholar] [CrossRef]
- Lii, C.Y.; Chen, C.H.; Yeh, A.I.; Lai, V.M.F. Preliminary study on the degradation kinetics of agarose and carrageenans by ultrasound. Food Hydrocoll. 1999, 13, 477–481. [Google Scholar] [CrossRef]
- Hu, X.; Jiang, X.; Aubree, E.; Boulenguer, P.; Critchley, A.T. Preparation and in vivo antitumor activity of κ-carrageenan oligosaccharides. Pharm. Biol. 2006, 44, 646–650. [Google Scholar] [CrossRef]
- Abad, L.V.; Relleve, L.S.; Racadio, C.D.T.; Aranilla, C.T.; De la Rosa, A.M. Antioxidant activity potential of gamma irradiated carrageenan. Appl. Radiat. Isot. 2013, 79, 73–79. [Google Scholar] [CrossRef]
- Gereniu, C.R.N.; Saravana, P.S.; Chun, B.S. Recovery of carrageenan from Solomon Islands red seaweed using ionic liquid-assisted subcritical water extraction. Sep. Purif. Technol. 2018, 196, 309–317. [Google Scholar] [CrossRef]
- Choi, E.M.; Kim, G.H.; Lee, Y.S. Atractylodes japonica root extract protects osteoblastic MC3T3-E1 cells against hydrogen peroxide-induced inhibition of osteoblastic differentiation. Phytother. Res. 2009, 23, 1537–1542. [Google Scholar] [CrossRef]
- Fleurence, J. The enzymatic degradation of algal cell walls: A useful approach for improving protein accessibility? J. Appl. Phycol. 1999, 11, 313–314. [Google Scholar] [CrossRef]
- Vieira, E.F.; Soares, C.; Machado, S.; Correia, M.; Ramalhosa, M.J.; Oliva-Teles, M.T.; Paula Carvalho, A.; Domingues, V.F.; Antunes, F.; Oliveira, T.A.C.; et al. Seaweeds from the Portuguese coast as a source of proteinaceous material: Total and free amino acid composition profile. Food Chem. 2018, 269, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Galland-Irmouli, A.V.; Fleurence, J.; Lamghari, R.; Luçon, M.; Rouxel, C.; Barbaroux, O.; Bronowicki, J.P.; Villaume, C.; Guéant, J.L. Nutritional value of proteins from edible seaweed Palmaria palmata (Dulse). J. Nutr. Biochem. 1999, 10, 353–359. [Google Scholar] [CrossRef]
- Wang, T.; Jónsdóttir, R.; Kristinsson, H.G.; Hreggvidsson, G.O.; Jónsson, J.Ó.; Thorkelsson, G.; Ólafsdóttire, G. Enzyme-enhanced extraction of antioxidant ingredients from red algae Palmaria palmata. LWT Food Sci. Technol. 2010, 43, 1387–1393. [Google Scholar] [CrossRef]
- Paiva, L.; Lima, E.; Patarra, R.F.; Neto, A.I.; Baptista, J. Edible Azorean macroalgae as source of rich nutrients with impact on human health. Food Chem. 2014, 164, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Guiry, M.D. The Seaweed Site: Information on Marine Algae. 2014. Available online: http://www.seaweed.ie/ (accessed on 26 April 2019).
- Misurcová, L. Chemical Composition of Seaweeds. In Handbook of Marine Macroalgae: Biotechnology and Applied Phycology; Kim, S.K., Ed.; John Wiley & Sons: New York, NY, USA, 2012; p. 567. [Google Scholar]
- Roman, B.L.; Pham, V.; Lawson, N.D.; Kulik, M.; Childs, S.; Lekven, A.C.; Garrity, D.M.; Moon, R.T.; Fishman, M.C.; Lechleider, R.J.; et al. Disruption of acvrl1 increases endothelial cell number in zebrafish cranial vessels. Development 2002, 129, 3009–3019. [Google Scholar]
- Sekar, S.; Chandramohan, M. Phycobiliproteins as a commodity: Trends in applied research, patents and commercialization. J. Appl. Phycol. 2008, 20, 113–136. [Google Scholar] [CrossRef]
- Fernández-Rojas, B.; Hernández-Juárez, J.; Pedraza-Chaverri, J. Nutraceutical properties of phycocyanin. J. Funct. Foods 2014, 11, 375–392. [Google Scholar] [CrossRef]
- Chandra, R.; Parra-Saldivar, R.; Hafiz, M.N.I. Phycobiliproteins: A novel green tool from marine origin blue-green algae and red algae—A review. Pept. Lett. 2016, 23, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Manirafasha, E.; Ndikubwimana, T.; Zeng, X.; Lu, Y.; Jing, K. Phycobiliprotein: Potential microalgae derived pharmaceutical and biological reagent. Biochem. Eng. J. 2016, 109, 282–296. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, Y.; Yin, Q.; Liu, G.; Liu, H.; Huang, Y.; Li, B. Phycocyanin: A potential drug for cancer treatment. J. Cancer 2017, 8, 3416–3429. [Google Scholar] [CrossRef]
- Hao, S.; Yan, Y.; Li, S.; Zhao, L.; Zhang, C.; Liu, L.; Wang, C. The in vitro anti-tumor activity of phycocyanin against non-small cell lung cancer cells. Mar. Drugs 2018, 16, 178. [Google Scholar] [CrossRef] [PubMed]
- Mittal, R.; Raghavarao, K.S.M.S. Extraction of R-Phycoerythrin from marine macro-algae, Gelidium pusillum, employing consortia of enzymes. Algal Res. 2018, 34, 1–11. [Google Scholar] [CrossRef]
- Gantar, M.; Dhandayuthapani, S.; Rathinavelu, A. Phycocyanin induces apoptosis and enhances the effect of topotecan on prostate cell line LNCaP. J. Med. Food 2012, 15, 1091–1095. [Google Scholar] [CrossRef]
- Huang, B.; Wang, G.C.; Zeng, C.K.; Li, Z.G. The experimental research of R- phycoerythrin subunits on cancer treatment—A new photosensitizer in PDT. Cancer Biother. Radiopharm. 2002, 17, 35–42. [Google Scholar]
- Munier, M.; Dumay, J.; Morançais, M.; Jaouen, P.; Fleurence, J. Variation in the biochemical composition of the edible seaweed Grateloupia turuturu Yamada harvested from two sampling sites on the brittany coast (France): The influence of storage method on the extraction of the seaweed pigment r-phycoerythrin. J. Chem. 2013, 2013, 568548. [Google Scholar] [CrossRef]
- Pina, A.L.; Costa, A.R.; Lage-Yusty, M.A.; López-Hernández, J. An evaluation of edible red seaweed (Chondrus crispus) components and their modification during the cooking process. LWT Food Sci. Technol. 2014, 56, 175–180. [Google Scholar] [CrossRef]
- Niu, J.F.; Wang, G.C.; Zhou, B.C.; Lin, X.Z.; Chen, C.S. Purification of R-phycoerythrin from Porphyra haitanensis (Bangiales, Rhodophyta) using expanded-bed absorption. J. Phycol. 2007, 43, 1339–1347. [Google Scholar] [CrossRef]
- Nguyen, H.P.T.; Morançais, M.; Fleurence, J.; Dumay, J. Mastocarpus stellatus as a source of R-phycoerythrin: Optimization of enzyme assisted extraction using response surface methodology. J. Appl. Phycol. 2017, 29, 1563–1570. [Google Scholar] [CrossRef]
- Wang, C.; Kim, J.H.; Kim, S.W. Synthetic biol-ogy and metabolic engineering for marine carotenoids: New opportunities and future prospects. Mar. Drugs 2014, 12, 4810–4832. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, N.; Suresh, V.; Thangam, R.; Kurinjimalar, C.; Kavitha, G.; Murugan, P.; Rengasamy, R. Isolation and characterization of macromolecular protein R-Phycoerythrin from Portieria hornemannii. Int. J. Biol. Macromol. 2013, 55, 150–160. [Google Scholar] [CrossRef]
- Niu, J.F.; Wang, G.C.; Tseng, C.K. Method for large-scale isolation and purification of R-phycoerythrin from red alga Polysiphonia urceolata Grev. Protein Expr. Purif. 2006, 49, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.-F.; Wang, G.-C.; Lin, X.-Z.; Cheng, Z. Large-scale recovery of C-phycocyanin from Spirulina platensis using expanded bed adsorption chromatography. J. Chromatogr. B 2007, 850, 267–276. [Google Scholar] [CrossRef]
- Galland-Irmouli, A.V.; Pons, L.; Luçon, M.; Villaume, C.; Mrabet, N.T.; Guéant, J.L.; Fleurence, J. One-step purification of R-phycoerythrin from the red macroalga Palmaria palmata using preparative polyacrylamide gel electrophoresis. J. Chromatogr. B Biomed. Sci. Appl. 2000, 739, 117–123. [Google Scholar] [CrossRef]
- Amano, H.; Noda, H. Proteins of protoplasts from red alga Porphyra yezoensis. Nippon Suisan Gakkaishi 1990, 56, 1859–1864. [Google Scholar] [CrossRef]
- Laohakunjit, N.; Selamassakul, O.; Kerdchoechuen, O. Seafood-like flavour obtained from the enzymatic hydrolysis of the protein by-products of seaweed Gracilaria sp. Food Chem. 2014, 158, 162–170. [Google Scholar] [CrossRef]
- Rodrigues, D.; Sousa, S.; Silva, A.; Amorim, M.; Pereira, L.; Rocha-Santos, T.A.P.; Gomes, A.M.P.; Duarte, A.C.; Freitas, A.C. Impact of enzyme- and ultrasound-assisted extraction methods on biological properties of red, brown, and green seaweeds from the Central West Coast of Portugal. J. Agric. Food Chem. 2015, 63, 3177–3188. [Google Scholar] [CrossRef]
- Hardouin, K.; Burlot, A.S.; Umami, A.; Tanniou, A.; Stiger-Pouvreau, V.; Widowati, I.; Bedoux, G.; Bourgougnon, N. Biochemical and antiviral activities of enzymatic hydrolysates from different invasive French seaweeds. J. Appl. Phycol. 2014, 26, 1029–1042. [Google Scholar] [CrossRef]
- Fleurence, J.; Massiani, L.; Guyader, O.; Mabeau, S. Use of enzymatic cell wall degradation for improvement of protein extraction from Chondrus crispus, Gracilaria verrucosa and Palmaria palmata. J. Appl. Phycol. 1995, 7, 393–397. [Google Scholar] [CrossRef]
- Denis, C.; Le Jeune, H.; Gaudin, P.; Fleurence, J. An evaluation of methods for quantifying the enzymatic degradation of red seaweed Grateloupia turuturu. J. Appl. Phycol. 2009, 21, 153–159. [Google Scholar] [CrossRef]
- Harrysson, H.; Hayes, M.; Eimer, F.; Carlsson, N.G.; Toth, G.B.; Undeland, I. Production of protein extracts from Swedish red, green, and brown seaweeds, Porphyra umbilicalis Kützing, Ulva lactuca Linnaeus, and Saccharina latissima (Linnaeus) J. V. Lamouroux using three different methods. J. Appl. Phycol. 2018, 30, 3565–3580. [Google Scholar] [CrossRef]
- Suwal, S.; Perreault, V.; Marciniak, A.; Tamigneaux, É.; Deslandes, É.; Bazinet, L.; Jacques, H.; Beaulieu, L.; Doyen, A. Effects of high hydrostatic pressure and polysaccharidases on the extraction of antioxidant compounds from red macroalgae, Palmaria palmata and Solieria chordalis. J. Food Eng. 2019, 252, 53–59. [Google Scholar] [CrossRef]
- Fleurence, R.L.; Iglesias, C.P.; Torgerson, D.J. Economic evaluations of interventions for the prevention and treatment of osteoporosis: A structured review of the literature. Osteoporos Int. 2005, 17, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Dumay, J.; Clément, N.; Morançais, M.; Fleurence, J. Optimization of hydrolysis conditions of Palmaria palmata to enhance R-phycoerythrin extraction. Bioresour. Technol. 2013, 131, 21–27. [Google Scholar] [CrossRef]
- Fitzgerald, C.; Gallagher, E.; O’Connor, P.; Prieto, J.; Mora-Soler, L.; Grealy, M.; Hayes, M. Development of a seaweed derived platelet activating factor acetylhydrolase (PAF-AH) inhibitory hydrolysate, synthesis of inhibitory peptides and assessment of their toxicity using the Zebrafish larvae assay. Peptides 2013, 50, 119–124. [Google Scholar] [CrossRef]
- Harnedy, P.A.; O’Keeffe, M.B.; FitzGerald, R.J. Fractionation and identification of antioxidant peptides from an enzymatically hydrolysed Palmaria palmata protein isolate. Food Res. Int. 2017, 100, 416–422. [Google Scholar] [CrossRef]
- Mensi, F.; Ksouri, J.; Seale, E. A statistical approach for optimization of R-phycoerythrin extraction from the red algae Gracilaria verrucosa by enzymatic hydrolysis using central composite design and desirability function. J. Appl. Phycol. 2012, 24, 915–926. [Google Scholar] [CrossRef]
- Mensi, F. Agar yield from R-phycoerythrin extraction by-product of the red alga Gracilaria verrucosa. J. Appl. Phycol. 2019, 31, 741–751. [Google Scholar] [CrossRef]
- Lee, D.; Nishizawa, M.; Shimizu, Y.; Saeki, H. Anti-inflammatory effects of dulse Palmaria palmata resulting from the simultaneous water-extraction of phycobiliproteins and chlorophyll a. Food Res. Int. 2017, 100, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Mittal, R.; Tavanandi, H.A.; Mantri, V.A.; Raghavarao, K.S.M.S. Ultrasound assisted methods for enhanced extraction of phycobiliproteins from marine macro-algae, Gelidium pusillum. Ultrason. Sonochem. 2017, 38, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Corey, P.; Kim, J.K.; Garbary, D.J.; Prithiviraj, B.; Duston, J. Bioremediation potential of Chondrus crispus (Basin Head) and Palmaria palmata: Effect of temperature and high nitrate on nutrient removal. J. Appl. Phycol. 2012, 24, 441–448. [Google Scholar] [CrossRef]
- Gereniu, C.R.N.; Saravana, P.S.; Getachew, A.T.; Chun, B.S. Characteristics of functional materials recovered from Solomon Islands red seaweed (Kappaphycus alvarezii) using pressurized hot water extraction. J. Appl. Phycol. 2017, 29, 1609–1621. [Google Scholar] [CrossRef]
- Pangestuti, R.; Getachew, A.T.; Siahaan, E.A.; Chun, B.-S. Characterization of functional materials derived from tropical red seaweed Hypnea musciformis produced by subcritical water extraction systems. J. Appl. Phycol. 2019. [Google Scholar] [CrossRef]
- Wang, F.; Guo, X.Y.; Zhang, D.N.; Wu, Y.; Wu, T.; Chen, Z.G. Ultrasound-assisted extraction and purification of taurine from the red algae Porphyra yezoensis. Ultrason. Sonochem. 2015, 24, 36–42. [Google Scholar] [CrossRef]
- Le Guillard, C.; Dumay, J.; Donnay-Moreno, C.; Bruzac, S.; Ragon, J.-Y.; Fleurence, J.; Bergé, J.-P. Ultrasound-assisted extraction of R-phycoerythrin from Grateloupia turuturu with and without enzyme addition. Algal Res. 2015, 12, 522–528. [Google Scholar] [CrossRef]
- Caltagirone, C.; Ferrannini, L.; Marchionni, N.; Nappi, G.; Scapagnini, G.; Trabucchi, M. The potential protective effect of tramiprosate (homotaurine) against Alzheimer’s disease: A review. Aging Clin. Exp. Res. 2012, 24, 580–587. [Google Scholar]
- Khotimchenko, S.V. Fatty acids of species in the genus Codium. Botanica Marina 2003, 46, 456–460. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed; functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Kumari, S.; Vardhana, S.; Cammer, M.; Curado, S.; Santos, L.; Sheetz, M.P.; Dustin, M.L. T lymphocyte myosin IIA is required for Maturation of the immunological synapse. Front. Immunol. 2012, 3, 230. [Google Scholar] [CrossRef]
- Pereira, L.C.C.; da Silva, N.I.S.; da Costa, R.M.; Asp, N.E.; da Costa, K.G.; Vila Concejo, A. Seasonal changes in oceanographic processes at an equatorial macrotidal beach in northen Brazil. Cont. Shelf Res. 2012, 43, 95–106. [Google Scholar] [CrossRef]
- Kumari, P.; Bijo, A.J.; Mantri, V.A.; Reddy, C.R.K.; Jha, B. Fatty acid profiling of tropical marine macroalgae: An analysis from chemotaxonomic and nutritional perspectives. Phytochemistry 2013, 86, 44–56. [Google Scholar] [CrossRef]
- Chen, C.Y.; Chou, H.N. Screening of red algae filaments as a potential alternative source of eicosapentaenoic acid. Mar. Biotechnol. 2002, 4, 189–192. [Google Scholar] [CrossRef]
- Kumari, P.; Kumar, M.; Gupta, V.; Reddy, C.R.K.; Jha, B. Tropical marine macroalgae as potential sources of nutritionally important PUFAs. Food Chem. 2010, 120, 749–757. [Google Scholar] [CrossRef]
- Schmid, M.; Kraft, L.G.K.; van der Loos, L.M.; Kraft, G.T.; Virtue, P.; Nichols, P.D.; Hurd, C.L. Southern Australian seaweeds: A promising resource for omega-3 fatty acids. Food Chem. 2018, 265, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Crampon, C.; Boutin, O.; Badens, E. Supercritical carbon dioxide extraction of molecules of interest from microalgae and seaweeds. Ind. Eng. Chem. Res. 2011, 50, 8941–8953. [Google Scholar] [CrossRef]
- Cheung, P.C.K. Temperature and pressure effects onsupercriticalcarbon dioxide extraction of n-3 fatty acids from red seaweed. Food Chem. 1999, 65, 399–403. [Google Scholar] [CrossRef]
- Patra, J.K.; Lee, S.W.; Kwon, Y.S.; Park, J.G.; Baek, K.H. Chemical characterization and antioxidant potential of volatile oil from an edible seaweed Porphyra tenera (Kjellman, 1897). Chem. Cent. J. 2017, 11, 34. [Google Scholar] [CrossRef]
- Kumari, P.; Reddy, C.R.K.; Jha, B. Comparative evaluation and selection of a method for lipid and fatty acid extraction from macroalgae. Anal. Biochem. 2011, 415, 134–144. [Google Scholar] [CrossRef]
- Mæhre, H.K.; Malde, M.K.; Eilertsen, K.E.; Elvevoll, E.O. Characterization of protein, lipid and mineral contents in common Norwegian seaweeds and evaluation of their potential as food and feed. J. Sci. Food Agric. 2014, 94, 3281–3290. [Google Scholar] [CrossRef]
- Chen, Y.H.; Tu, C.J.; Wu, H.T. Growth-inhibitory effects of the red alga Gelidium amansii on cultured cells. Biol. Pharm. Bull. 2004, 27, 180–184. [Google Scholar] [CrossRef]
- Chan, P.T.; Matanjun, P.; Yasir, S.M.; Tan, T.S. Antioxidant activities and polyphenolics of various solvent extracts of red seaweed, Gracilaria changii. J. Appl. Phycol. 2015, 27, 2377–2386. [Google Scholar] [CrossRef]
- Topuz, O.K.; Gokoglu, N.; Yerlikaya, P.; Ucak, I.; Gumus, B. Optimization of antioxidant activity and phenolic compound extraction conditions from red seaweed (Laurencia obtusa). J. Aquat. Food Prod. Technol. 2016, 25, 414–422. [Google Scholar] [CrossRef]
- Zheng, J.; Chen, Y.; Yao, F.; Chen, W.; Shi, G. Chemical composition and antioxidant/antimicrobial activities in supercritical carbon dioxide fluid extract of Gloiopeltis tenax. Mar. Drugs 2012, 10, 2634–2647. [Google Scholar]
- Ospina, M.; Castro-Vargas, H.I.; Parada-Alfonso, F. Antioxidant capacity of Colombian seaweeds: 1. Extracts obtained from Gracilaria mammillaris by means ofsupercriticalfluid extraction. J. Supercrit. Fluids 2017, 128, 314–322. [Google Scholar] [CrossRef]
- Kang, J.Y.; Chun, B.S.; Lee, M.C.; Choi, J.S.; Choi, I.S.; Hong, Y.K. Anti-inflammatory Activity and Chemical Composition of Essential Oil Extracted with Supercritical CO2 from the Brown Seaweed Undaria pinnatifida. J. Essent. Oil Bear. Pl. 2016, 19, 46–51. [Google Scholar] [CrossRef]
- Yuan, S.; Duan, Z.; Lu, Y.; Ma, X.; Wang, S. Optimization of decolorization process in agar production from Gracilaria lemaneiformis and evaluation of antioxidant activities of the extract rich in natural pigments. 3 Biotech 2018, 8, 8. [Google Scholar] [CrossRef]
- Yuan, Y.V.; Westcott, N.D.; Hu, C.; Kitts, D.D. Mycosporine-like amino acid composition of the edible red alga, Palmaria palmata (Dulse) harvested from the west and east coasts of Grand Manan Island, New Brunswick. Food Chem. 2009, 112, 321–328. [Google Scholar] [CrossRef]
- Aguilera, J.; Bischof, K.; Karsten, U.; Hanelt, D.; Wiencke, C. Seasonal variation in ecophysiological patterns in macroalgae from an Arctic fjord. II. Pigment accumulation and biochemical defense systems against high light stress. Mar. Biol. 2002, 140, 1087–1095. [Google Scholar]
- Bedoux, G.; Hardouin, K.; Marty, C.; Taupin, L.; Vandanjon, L.; Bourgougnon, N. Chemical characterization and photoprotective activity measurement of extracts from the red macroalga Solieria chordalis. Bot. Mar. 2014, 57, 291–301. [Google Scholar] [CrossRef]
- Rupérez, P. Mineral content of edible marine seaweeds. Food Chem. 2002, 79, 23–26. [Google Scholar] [CrossRef]
- Kraan, S. Mass-cultivation of carbohydrate rich macroalgae, a possible solution for sustainable biofuel production. Mitig. Adapt. Strateg. Glob. Chang. 2013, 18, 27–46. [Google Scholar] [CrossRef]
- Jaballi, I.; Saad, H.B.; Bkhairia, I.; Cherif, B.; Kallel, C.; Boudawara, O.; Droguet, M.; Magné, C.; Hakim, A.; Amara, I.B. Cytoprotective effects of the red marine alga Chondrus canaliculatus against Maneb-Induced hematotoxicity and bone oxidative damages in adult rats. Biol. Trace Elem. Res. 2018, 184, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.F.; Zhang, M.H.; Li, D.W.; Yang, W.D.; Liu, J.S.; Bai, W.B. Improvement of neutral lipid and polyunsaturated fatty acid biosynthesis by overexpressing a type 2 diacylglycerol acyltransferase in marine diatom Phaeodactylum tricornutum. Mar. Drugs 2013, 11, 4558–4569. [Google Scholar] [CrossRef] [PubMed]
- Cian, R.; Martínez, O.; Drago, S. Bioactive properties of peptides obtained by enzymatic hydrolysis from protein byproducts of Porphyra columbina. Food Res. Int. 2012, 49, 364–372. [Google Scholar] [CrossRef]
- Cian, R.E.; Vioque, J.; Drago, S.R. Enzyme proteolysis enhanced extraction of ACE inhibitory and antioxidant compounds (peptides and polyphenols) from Porphyra columbiana residual cake. J. Appl. Phycol. 2013, 25, 1197–1206. [Google Scholar] [CrossRef]
- Baghel, R.S.; Trivedi, N.; Reddy, C.R. A simple process for recovery of a stream of products from marine macroalgal biomass. Bioresour. Technol. 2016, 203, 160–165. [Google Scholar] [CrossRef]
- Gajaria, T.K.; Suthar, P.; Baghel, R.S.; Balar, N.B.; Sharnagat, P.; Mantri, V.A.; Reddy, C.R.K. Integration of protein extraction with a stream of byproducts from marine macroalgae: A model forms the basis for marine bioeconomy. Bioresour. Technol. 2017, 243, 867–873. [Google Scholar] [CrossRef]
- Kumar, S.; Gupta, R.; Kumar, G.; Sahoo, D.; Kuhad, R.C. Bioethanol production from Gracilaria verricosa, a red alga, in a biorefinery approach. Bioresour. Technol. 2013, 135, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Baghel, R.R.S.; Trivedi, N.; Gupta, V.; Neori, A.; Reddy, C.R.K.; Lali, A.; Jha, B. Biorefining of marine macroalgal biomass for production of biofuel and commodity chemicals. Green Chem. 2015, 17, 2436–2443. [Google Scholar] [CrossRef]
- Tan, I.S.; Lee, K.T. Enzymatic hydrolysis and fermentation of seaweed solid wastes for bioethanol production: An optimization study. Energy 2014, 78, 53–62. [Google Scholar] [CrossRef]
- Francavilla, M.P.; Manara, P.; Kamaterou, M.; Monteleone, M.; Zabanioutou, A. Cascade approach of red macroalgae Gracilaria gracilis sustainable valorization by extraction of phycobiliproteins and pyrolysis of residue. Bioresour. Technol. 2015, 184, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Ingle, K.; Vitkin, E.; Robin, A.; Yakhini, Z.; Mishori, D.; Golberg, A. Macroalgae biorefinery from Kappaphycus alvarezii: Conversion, modeling and performance prediction for India and Philippines as examples. BioEnergy Res. 2018, 11, 22–32. [Google Scholar] [CrossRef]
- Mondal, D.; Sharma, M.; Prasad, K.; Meena, R.; Siddhanta, A.; Ghosh, P. Fuel intermediates, agricultural nutrients and pure water from Kappaphycus alvarezii seaweed. RSC Adv. 2013, 3, 17989–17997. [Google Scholar] [CrossRef]

| Depolymerization Technique | Seaweed or Polysaccharide | Activity | Reference |
|---|---|---|---|
| Acid (HCl, citric acid, and cationic exchange resin (solid acid)) | Agar (C) | Antioxidant and -glucosidase inhibition | [77] |
| Enzymatic | Agarose (C) Gracilaria cornea Gracilaria lemaneiformis | Functional, antioxidant, skin whitening | [76,83,84,94] |
| Free-radical induced | Halymenia durvillei | ND | [95] |
| High-pressure homogenization | Halymenia durvillei | ND | [95] |
| Microwave assisted | Pyropia yezoensis | Antioxidant | [93] |
| Ultrasound assisted | Porphyra yezoensis Gracilaria birdiae | Anticoagulant, antioxidant | [75,96] |
| Process 1 | Seaweed | Properties | Reference |
|---|---|---|---|
| 1 P: 6% KOH, 80 °C, 3 h E: Water, 90–105 °C, 1.5 h, ethanol precipitation | Hypnea musciformis, Kappaphycus alvarezii, Solieria chordalis | Y: 19–27; GS: (4–6.5) × 103; Tg: 32–36, 70–74 | [24,152] |
| P: - E: Water, room temperature, 24 h, ethanol precipitation | Mastocarpus stellatus | Y: 15–30 BP: antioxidant, anti-coagulant activities | [17] |
| 1 P1,2: 3% KOH, 90 °C, 4 h E1: Water, room temp, 12 h, ethanol precipitation E2: Ultrasound assisted extraction, 15–30 min, 400–500 W, ethanol precipitation | Kappaphycus alvarezii, Euchema denticulatum, Hypnea musciformis | E1: Y: 30–40 E2: Y: 32–49, higher yield with shorter times BP: No differences in antioxidant features | [7,153] |
| P1: 3% KOH, 85 °C, 3.5 h E1: Water, 85 °C, 12 h, ethanol precipitation P2:- E2: Microwave assisted closed vessels, 85–105 °C, 10–20 min, ethanol precipitation | Hypnea musciformis, Solieria chordalis | E1: Y: 20–40 E2: Y: 15–25; higher desulfation degree; BP: antiviral | [152,154] |
| P, E: Alkali extraction, ethanol precipitation | Chondracanthus acicularis, Chondracanthus teedei, Gigartina pistillata, Chondrus crispus | Y: 15–45% | [104] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres, M.D.; Flórez-Fernández, N.; Domínguez, H. Integral Utilization of Red Seaweed for Bioactive Production. Mar. Drugs 2019, 17, 314. https://doi.org/10.3390/md17060314
Torres MD, Flórez-Fernández N, Domínguez H. Integral Utilization of Red Seaweed for Bioactive Production. Marine Drugs. 2019; 17(6):314. https://doi.org/10.3390/md17060314
Chicago/Turabian StyleTorres, Maria Dolores, Noelia Flórez-Fernández, and Herminia Domínguez. 2019. "Integral Utilization of Red Seaweed for Bioactive Production" Marine Drugs 17, no. 6: 314. https://doi.org/10.3390/md17060314
APA StyleTorres, M. D., Flórez-Fernández, N., & Domínguez, H. (2019). Integral Utilization of Red Seaweed for Bioactive Production. Marine Drugs, 17(6), 314. https://doi.org/10.3390/md17060314