Abstract
The hydrocolloids carrageenan and agar are the major fraction industrially extracted and commercialized from red seaweeds. However, this type of macroalgae also contains a variety of components with nutritional, functional and biological properties. In the context of sustainability and bioeconomy, where the integral utilization of the natural resources is incentivized, the sequential separation and valorization of seaweed components with biological properties of interest for food, nutraceuticals, cosmeceuticals and pharmaceuticals is proposed. In this work, a review of the available conventional and alternative greener and efficient extraction for obtaining red seaweed bioactives is presented. The potential of emerging technologies for the production of valuable oligomers from carrageenan and agar is also commented, and finally, the sequential extraction of the constituent fractions is discussed.
1. Introduction
Seaweeds are widespread and traditionally used in Eastern countries for food and for medicinal purposes. In Western countries, despite being included recently in the diet, direct human consumption is still unusual, mainly being used for the production of hydrocolloids with thickening and gelling properties. Among seaweeds, red algae (Rhodophyta) contain high amounts of polysaccharides (floridean starch and sulfated galactans, such as carrageenans or agarans), proteins and derived peptides (phycobiliproteins, phycolectins and mycosporine-like amino acids), minerals and other valuable compounds, such as polyphenols and lipids [,]. The whole algae of red seaweeds have been traditionally used as food, while agars and carrageenans have been extracted for multiple purposes, namely for food, pharmaceutical applications and biotechnological applications.
The reader can find compilations on the chemical and nutritional characteristics of seaweeds as a feed livestock resource [] and its health-promoting properties [], including the anticancer [] and antiviral [] features. Additionally, there have been some interesting reviews on the use of red seaweeds for carrageenans [], agar and carrageenan oligosaccharides []. The extraction technology, which influences the composition, structure and properties of the target solutes, is conventionally addressed using chemicals, in long term operation, and with high energy consumption []. The recent developments of blue biotechnology and novel extraction techniques meeting the requirements of low cost, sustainability, food-compatibility and industrial scale feasibility to obtain seaweed components [], the systematical selection of operational variables of emerging extraction technologies [] or the use of alternative solvents, such as ionic liquids [], or supercritical CO2 for macro and microalgae [] have been reviewed. Scarcer studies are focused on the extraction of red seaweed components, such as carrageenan and agar fractions [,], photoprotective substances [] and pigments []. Particular interest has been on the influence on the depolymerization of saccharidic and protein components, since both the type of extraction process and the operational conditions have to be controlled depending on the future uses []; however, further development and additional studies are still needed []. The mentioned studies are oriented to the selective extraction of some valuable fractions, but scarce information is found for the simultaneous utilization of the different components in a more rational scheme, following the philosophy of the biorefineries []. Biomass refineries, with a production scheme analogous to the petroleum refineries, are aimed at obtaining a wide range of products from renewable raw materials, including value added components for the food, cosmetic and pharmaceutical industries, as well as biofuels. These multistage multiproduct processes are based on the sequential fractionation of the biomass and on their subsequent physical, chemical or biotechnological transformation into the target final products. This sustainable approach adopts an integral utilization of resources, promoting the development of a marine bio-economy.
The present review presents an overview of the properties and potential applications of red seaweed bioactives, the specific technologies for extraction and also for the depolymerization of agar and carrageenan into oligosaccharides, as well as the potential of these techniques for the extraction of other red seaweed components. Both conventional and emerging extraction and depolymerization technologies are discussed with the aim of promoting the sustainability based on (i) the development of clean processes and (ii) the integral utilization and valorization of resources following the philosophy of biomass biorefineries.
2. Components: Properties and Extraction
2.1. Polysaccharides
Polysaccharides are the main components in marine algae according to their abundance and their current commercial value based on their technological features [,]. More recently, attention has been directed to their health benefits [,]. These polysaccharides, generally not digested by humans, are considered to be dietary fibers []. The composition, structure and rheological properties are influenced by the algal source, life-stage, growth, environment and by the extraction method []. Agars and carrageenans are major cell wall polysaccharides in red macroalgae, also known as galactans, accounting for up to 40–50% of the dry weight. They are highly anionic homopolysaccharides, composed of a backbone built from disaccharide blocks of d-galactose and 3,6-anhydrogalactose (l-AHG in agar and d-AHG in carrageenan) with different sulfation, methylation and pyruvation patterns that vary among species [,]. The high electronegative charge density from their sulfated esters favors the electrostatic interactions with specific proteins, determining their biological effects, which are also closely related to the structural features [,,,]. Proteins, minerals and lipids also confer red seaweed important structural value [].
2.1.1. Agar
- Composition, structure, occurrence and properties
Agar is a linear polysaccharide composed of alternating (1,3) linked d-galactose and (1,4) linked 3,6-anhydro-l-galactose [] and substituted in some degree by sulfate, methyl or pyruvate groups [,,]. The molecular structure of agar polysaccharides, particularly the type and location of sulfate esters, appears to be species-specific []. Agar has two different constituents: agarose and agaropectin (Figure 1). Agarose is a neutral linear polysaccharide composed of three linked β-d-galactose and four linked 3,6-anhydro-α-l-galactose. Agaropectin is an acid polysaccharide containing sulfate groups, pyruvic acid, and d-glucuronic acid conjugated to agarobiose. Agarose accounts for up to 70% of the mixture and is responsible for gelling, whereas agaropectin is responsible for thickening characteristics. Different derived agarose molecules can be obtained from chemical or enzymatic degradation. Most of the corresponding hydrolysis products such as agarooligosaccharides (AOSs), neoagarooligosaccharides (NAOSs), neoagarobiose (NAB) and 3,6-anhydro-l-galactose (l-AHG) exhibit biological activities [].
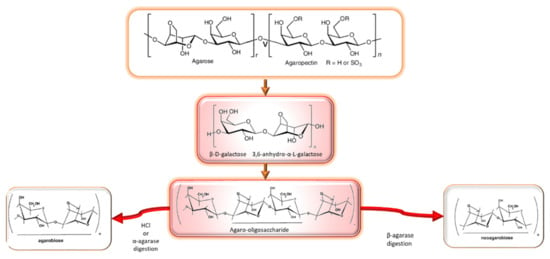
Figure 1.
Scheme of the agar constituents (agarose and agaropectine) and different derived molecules with biological activities, adapted from [,].
Agar is mainly found in the cell matrix of seaweeds of the order Gelidiales (Gelidium and Pterocladia) and Gracilariales (Gracilaria and Hydropuntia), which have become the major worldwide source. Its abundance and easier exploitation made G. tenuistipitata an economically important raw material for agar production []. In comparison with agars from Gelidium and Pterocladia, agars from Gracilaria can have higher degrees of sulfation, methoxylation and pyruvylation [].
The agar properties are dependent on the species and environmental characteristics of the collection or cultivation area, such as season, life cycle and geographical features [,] and the storage, extraction processes and postharvest storage [,,,,,]. The quality of agar is determined by the type, pattern and degree of substitution as well as molecular weight, chemical composition (pyruvate, methyoxyl and sulfate) and physical properties (gel strength, gel syneresis, viscosity, gelling and melting temperatures) that determine its market value [,,]. The agar gel strength, in terms of elastic modulus (G′), of systems formulated at 1.5% agar in milli-Q water is around 238 Pa at 25 °C, with gelling temperatures of 48 °C, and those agars with gel strengths greater than 6.9 × 104 Pa are referred to as high quality agars [].
Agar is a generally recognized as safe (GRAS) food additive in the United States and a food additive approved in Europe (E406). Agar cannot be digested in the gastrointestinal tract because humans lack α/β-agarases, but can be metabolized by intestinal bacteria to d-galactose []. Agar is demanded as gelling agent and stabilizing agent, and as cryoprotectants in the pharmaceutical, cosmetics and food industries [,,,,]. The human food industry demands for 80% production, and biotechnological applications for the remaining 20% []. The importance of these products is based on high market demand for agar and the higher price compared to alginates and carrageenans [,,]. It is used as a gelling, thickening and stabilizing agent in food formulations and it has also been used in microbiological media and in chromatographic techniques. Most native agars from Gracilaria are not bacteriological grade agar due to their high content of methoxyls, but they can be food and reactive grade [].
- Extraction processes: conventional and emerging technologies
The storage conditions and duration before extraction affects the agar quality from Gracilaria, since seaweeds are susceptible to degradation by agarolytic enzymes and bacteria. Some species from temperate and cold water could be more resistant to hydrolysis during storage. Postharvest treatment with acid, alkali or formaldehyde is necessary to prevent enzymatic and microbial degradation [,,,,,]. Another factor requiring attention after harvesting algae is correct drying under 20% moisture and packing, and avoid wetting during the transporting and storage period, but dewatering pre-treatments have to be defined according to the species and to collection season [].
Although Gelidium agar has better quality and is easily extracted with boiling water, the gelling ability of agars from Gracilaria sp can be enhanced by an alkali pretreatment to convert α-l-galactose 6 sulfate into 3,6-anhydro-α-l-galactose. This treatment reduces the sulfate content and improves the gelling properties as evidenced by higher gel strength, gelling, melting temperatures and viscosity []. Generally, the alkali-treatment was most effective for obtaining more galactose-rich hydrocolloids []. However, agar degradation and diffusion towards the aqueous medium could occur, reducing the extraction yield [,], although in some cases, no reduction was observed []. Alkaline pretreatment variables, such as alkaly type and concentration or heating time and temperature affected the yield and quality of the agar. Regardless the alkaline concentration, NaOH rendered agar with a higher quality than KOH [,]. Compilations of conditions are also found in [], being the optimal in the range 5–7% NaOH, up to 80–100 °C for 0.5–3 h [,,,,], but higher alkali concentrations (10%) [], shorter times [] and the application of several stages [] have also been reported. An alternative pretreatment was proposed by Roleda [], which consisted of soaking the Gelidiella acerosa air dried sample in 0.5% acetic acid for 1 h at 16–20 °C, then 1 h steam pressure at 15–20 psi and boiling at 100 °C. Freile-Pelegrín [] proposed the cultivation of Gracilaria cornes under dark and salinity treatments (50 and 25% salinity) to replace the alkali treatment. Pigments, such as chlorophyll, carotenoids and phycoerythrobilin, can be leached out during the alkaline pretreatment and an alternative environmentally friendly scalable photobleaching process for Gracilaria asiatica and Gracilaria lemaneiformis with 3–5% NaOH and photobleaching for 5 h was proposed []. The pigments and the agar sulfate contents decreased during the photobleaching agar extraction process, and the gel strength increased during the photolysis.
The industrial agar extraction process is based on using hot water during several hours under conventional heating, a time-consuming process requiring high solvent consumption and generating large amounts of waste disposal. Therefore, water recycling has been suggested []. Compilations on agar yield (10–43.4%) can be found in []. Cold extraction with distilled water at room temperature was reported for Gracilaria birdiae [], but agar extracts prepared at 20 °C showed a wider size distribution (1–30,000 kDa) []. For the chemical liquefaction of agarose, acid prehydrolysis has been commonly employed [,,]. Mild conditions, such as low acid concentrations, low temperatures or short reaction times, result in even-numbered oligosaccharides due to the preferential cleavage of α-1,3-glycosidic linkages and the release of the acid-labile l-AHG at the reducing end converts even-numbered AOSs into odd-numbered ones. The released l-AHG is readily degraded into 5-hydroxymethylfurfural []. Table 1 summarizes some representative examples of the conventional and emerging technologies proposed for agar extraction. Another disadvantage of alkali treatment is the generation of effluents with environmental impact if not properly treated []. Enzymatic treatment could be a more ecofriendly alternative to improve the gel strength of agar. Shukla [] proposed the use of sulfatase/sulfohydrolase to decrease the sulfate content and increase both the 3,6-anhydrogalactose content and gel strength of agar. However, the cost could not make the process commercially competitive with this sulfatase/sulfohydrolase compared to alkaline treatment [].
Processes based on combined heat and ultrasound treatments would enable reducing the amount of time and energy needed. Martínez-Sanz et al., [] observed a four-fold reduction in time without affecting the yields and properties of Gelidium sesquipedale agar-based extracts. The extracts also contained proteins, polyphenols and minerals, conferring antioxidant capacity to the browned softer gels. In contrast, an alkali pre-treatment could yield almost pure agars with higher molecular weights and crystallinities and resulted in stiffer gels, but lower extraction yields.
Microwave assisted extraction (MAE) allowed reducing the required 2–4 h for agar extraction in conventional processes to a very short period, consuming less energy and solvent volume and reducing waste disposal requirements [,]. Navarro and Stortz [] used microwave assisted alkaline modification to improve the gelling properties of carrageenans from Iridaea undulosa and porphyran from Porphyra columbina. Substantial depolymerization of Gracilaria vermiculophylla agar was observed in microwave assisted extraction with lower values of viscosity and molecular weight (54 kDa against 111 kDa) and methylation degree than those obtained in conventional extraction []. Intermittent microwave treatment was proposed for the extraction of sulfated porphyran from Porphyra dentata with ethanol and the gelling capacity of extracted porphyran was not affected [].
Other technologies have been proposed, such as ionic liquid-based extraction [], radiation to increase the agar yield from Gelidiella acerosa, at 15 kGy yield increased, but the gel strength decreased and the sulfate level did not vary significantly [].

Table 1.
Some examples of technologies proposed for agar extraction.
Table 1.
Some examples of technologies proposed for agar extraction.
| Pretreatment/Extraction | Seaweed | Gel Properties | Reference |
|---|---|---|---|
| P: - E: Distilled water; pH 6.3–6.4; 100 °C, 1.5 h; ethanol precipitation | Gracilaria cornes | GS: (1.2–2.5) × 104; Tg: 39.2–41.8; Tm: 74.3–82.6; Mw: ND | [] |
| P: 1–15% NaOH, 90 °C, 1 h, 0.025% HCl, 1 h E: Water, 100 °C, 2 h, ethanol precipitation | Gracilaria verrucosa | GS: (1.6–1.8, 2.6–2.7) × 104; Tg: 32–43; Tm: 49–80.5; Mw: ND | [] |
| P: - E: Distilled water, 20–28 °C, 15 h, ethanol precipitation | Gracilaria birdiae | GS: ND; Tg: ND; Tm: ND; Mw: 1–30,000 | [] |
| P: - E: Water, 80–100 °C, 2–4 h; ethanol precipitation | Hydropuntia cornea | GS: (0.7–1.3) × 104; Tg: 25–32.1; Tm: 65–79; Mw: 342–371 kDa | [] |
| 1 P: 5–7% NaOH, 80–100 °C, 0.5–3 h E: Water, 80 °C, pH 6.2, 90 min, ethanol precipitation | Gracilaria vermiculophylla | GS: (0.9–1.2) × 105; Tg: 52–68; Tm: 92–95; Mw: ND | [] |
| 1 P: 1–5% NaOH, 30–85 °C, 1–2 h E: Water, 700–115 °C, 2–3 h, 1–2 stages, ethanol precipitation | Gracilaria corticata, Gracilaria eucheumoides, Gracilaria cliftonii, Gracilaria lemaneiformis | GS: (1.2–4.2) × 104; Tg: ~32; Tm: ~78; Mw: ND | [,,,] |
| P: 5% NaOH, 1–48 h, room temperature. Dil. H2SO4, 15 min E: Water, 100 °C, 1 h 30 min, ethanol precipitation | Gracilaria manilaensis | GS: (1–4.9) × 104; Tg: ND; Tm: ND; Mw: ND | [] |
| P: - E: Pressurized water extraction, 120 °C, 15 min, ethanol precipitation | Gracilaria vermiculophylla | GS: 1.3 × 105; Tg: 40.7; Tm: 93.1; Mw: ND | [] |
| P: Acetic acid, 16–20 °C, 1 h E: Steam pressure, 15–20 psi; ethanol precipitation | Gelidiella acerosa | GS: (4.9–6.9) × 104; Tg: 42–47; Tm: 90–98; Mw: ND | [,] |
| 1 P: 2.5 M NaOH, 90 °C, 2 h E: Water, 90 °C, 2 h; ultrasound assisted, 30 min, 400 w, 24 kHz; ethanol precipitation | Gelidium sesquipedale | GS: (0.2–1.2) × 105; Tg: ND; Tm: ND; Mw: (2.5–11) × 105 | [] |
| 1 P: 0.1 M NaOH, 22 °C E: Enzyme (60 °C, 12 h, pH 8) and ultrasound assisted extraction (60 °C, 30 min, 60 W); ethanol precipitation | Gracilaria birdiae | GS: ND; Tg: ND; Tm: ND; Mw: 20–45 | [] |
| P: - E: Protease digestion, 60 °C, 6 h, pH 5 | Gracilaria cornea | GS: ND; Tg: ND; Tm: ND; Mw: ND | [] |
| P: Radiation, at 5–15 kGy E: Water, 95–100 °C or pressure cooking 121 °C, 15 psi, 1 h; ethanol precipitation | Gelidiella acerosa | GS: (2.5–6.0) × 104; Tg: ND; Tm: ND; Mw: ND | [] |
1 Optional pretreatment; P: pretreatment conditions; E: extraction conditions; GS: gel strength (G′, elastic modulus at 25 °C, Pa); Tg: gelling temperature (°C); Tm: Melting temperature (°C); MW: Molecular weight (kDa); ND: not determined.
- Agarooligosaccharides: properties and production strategies
Two oligosaccharides can be formed depending on the moiety of end sugar, namely, agaro-oligosaccharides and neoagaro-oligosaccharides []. Neoagarobiose, α-l-3,6-anhydro-l-galactosyl-(1→3)-β-d-galactopyranose, is the basic unit of neoagarooligosaccharides. Neoagarooligosaccha-rides were found to be safe up to 5000 mg/kg body weight in acute oral toxicity tests with rat and beagle dog models [].
The biological activities of agar oligosaccharides include anti-microbial, antiviral [], prebiotic [], anti-tumoral, immunomodulatory, anti-inflammatory [,,,,,,,,], glucosidase inhibitory [], anticariogenic [], hepatoprotective [], antioxidant [,] and other properties of interest for skin care [,,,] (Figure 2). Liu et al. [] summarized research progress on biological activities of agaro-oligosaccharide. Agaro-oligosaccharides display antioxidant effects which differ according to their degree of polymerization []; additionally, Kazłowski et al. [] summarized the influence of the degree of polymerization (DP) of agar oligomers on their physiological activities. Agarose is biocompatible and has been used for neural and cartilage tissue repair [] and for the preparation of biomaterials [,]. Due to its low cell adhesiveness and slow degradation rate, agarose was composited with fast degradable biomaterials for drug delivery, tissue engineering and wound healing [].

Figure 2.
Influence of the depolymerization degree (DP) of agar oligomers on their biological properties [,,,,,].
Agaro-oligosaccharides (AOS) are conventionally prepared by acid hydrolysis of agars; however, this method produces substantial pollution and wastes. Alternative strategies have also been proposed using the same subsequent stages for purification, usually based on ultrafiltration, ethanolic precipitation, purification by chromatography and further in activated carbon [,,].
Several acids have been used to hydrolyze agar. Chen [] compared the use of hydrochloric acid, citric acid and cationic exchange resin; the latter avoided the neutralization step and offered higher yield of agaro-oligosaccharides with high DP (octaose and decaose) and low content of agarobiose. Hydrochloric acid hydrolysis produced DP lower than 6, whereas citric acid yielded small amount of oligosaccharides, mainly agarooctaose and agarodecaose.
Alternative methods have been used to hydrolyze agar, such as enzymatic, physical and chemical degradation. Enzymatic hydrolysis, which can be performed by agarases [], show disadvantages such as the low activity, low stability and productivity, which limit their wide application in industry. However, chemical degradation, especially acid hydrolysis, is available for industrial preparation because of its simplicity, rapidity, low cost and high yield []. Different bacteria have been used as a source of agarolytic enzymes, i.e., Flammeovirga pacifica [], Streptomyces coelicolor [] or Agarivorans sp. JA-1 []. Agar oligosaccharides can be produced by hydrolysis using chemicals or agarolytic enzymes. Since agarose comprises alternating l-AHG and d-galactose units linked by α-1,3- and β-1,4-glycosidic bonds, two types of agarases exist: α-agarases cleave the α-1,3 linkages of agarose endolytically and produce agaro-oligosaccharides AOSs as the reaction products. Neoagarooligosaccharides are prepared from agar by β-agarase hydrolysis, by cleaving the β-1,4-glycosidic linkages of agarose endolytically or exolytically, and also releases neoagarooligosaccharides with neoagarobiose or neogarobiose alone, respectively. Agaro-oligosaccharides obtained by enzymatic degradation exhibited high solubility percentages, water and oil absorption capacities, as well as considerable 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid (ABTS+) radical scavenging and ferric reducing antioxidant activities, depending on the degree hydrolysis []. Kazłowski et al. [,] prepared neoagaro-oligosaccharides by β-agarase digestion and agaro-oligosaccharides by HCl hydrolysis from agarose and observed that the enzymatically prepared oligosaccharides usually show a low DP and a broad range of bioactivities, whereas those from acid hydrolysis contain only oligosaccharides with odd numbers of sugar unit [].
The agaro-oligosaccharides, obtained from commercial agarose through enzymatic hydrolysis, did not show improvement on its oil and water absorption capacities. Furthermore, a higher degree hydrolysis could lead to increase the reducing capacity and antiradical properties (Table 2).

Table 2.
Examples of techniques for depolymerization of agar.
Zou et al. [] prepared low molecular weight polysaccharides (3.2, 10.5, 29.0, and 48.8 kDa) from Pyropia yezoensis using microwave-assisted acid hydrolysis. The lower molecular weight (Mw) product (3.2 kDa) was the most efficient protecting wheat seedlings against salt stress. These authors also indicated that microwave irradiation accelerated the reaction rate. Stronger gels were also obtained using microwave assisted extraction when compared with gels produced applying the traditional method. The average sulfate content was similar to the obtained by the traditional method from G. vermiculophylla produced in the selected integrated multitrophic aquaculture (IMTA) system. However, radical depolymerization assisted by ultrasounds induced a loss of sulfate functions in addition to the shortening of the polysaccharides chain length []. It should be indicated that the microwave assisted extraction approach requires less energy and solvent than conventional processes, while generating fewer wastes.
Free-radical depolymerization of polysaccharides, based on the formation of free radicals ·OH by the Fenton reaction using a metallic catalyst, was proposed as a reproducible scalable technique for the degradation of polysaccharides without changes in structural features, producing an average molar mass of 1500 kDa [].
High-pressure homogenization performed on the polysaccharide of Halymenia durvillei showed their feasibility and effectiveness and showed that an advantage of the degradation at high pressure was the ease and speed of the preparation [].
It has been suggested that ultrasound promotes the extraction of other non-sulfated polysaccharides []. One of the fractions obtained by ultrasonic degradation of Porphyra yezoensis polysaccharides did not change the main structure of polysaccharides and enhanced the antioxidant properties of the agar fractions [].
Combination of techniques was also useful, enzyme and ultrasound assisted extraction led to the same sulfated polysaccharides from Gracilaria birdiae [], but the yield was higher when both techniques were jointly applied to alkaline treated seaweeds. Combined techniques were used in the extraction of pigments and sulfated polysaccharides from the red alga G. verrucosa. The method is easy to use, allows the extraction of pigments and agar highly quantitative in one step. Sulfated polysaccharides obtained were similar to agar extracted directly from dried material without any treatment. Compared to the common agar extraction method, enzyme mixtures tested for R-phycoerythrin can be proposed as pretreatment for agar extraction. However, Öğretmen and Duyar [] observed that autoclave provided lower agar yields than water bath from Gelidium latifolium.
Agaro-oligosaccharide dried products show high thermal and pH stability; however, the drying step is highly relevant to other product properties, both functional (water solubility index, water absorption capacity and oil absorption capacity) and antioxidant (radical scavenger and reducing capacity). Kang et al. [] observed the highest solubility index in spray dried products and the highest water and oil absorption capacities in freeze dried products, which showed minimal color deterioration and higher oxidative stability, whereas the oven drying could be more deleterious. Antiradical properties were high in freeze-dried and spray-dried oligosaccharide powders.
2.1.2. Carrageenan
- Composition, structure, occurrence and properties
Carrageenans are high-molecular-weight linear hydrophilic, sulfated galactans formed by alternate units of d-galactose and 3,6-anhydrogalactose alternately linked by α-1,3 and β-1,4 glycosidic linkages. They can be classified according to differences in their average molecular mass and to the number and the position of the sulfate ester groups and the occurrence of a 3,6 anhydro-ring in the α-linked galactose. The three types with higher commercial importance, namely κ-, ι- and λ-carrageenan, are presented in Figure 3.
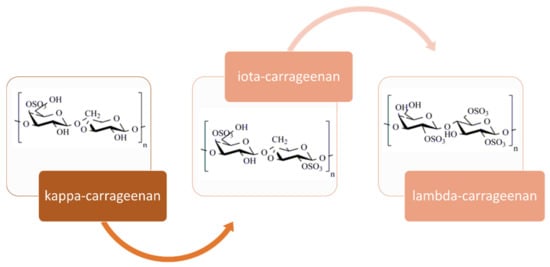
Figure 3.
Repeating units in the main three types of carrageenan with commercial interest, adapted from Pereira [].
Carrageenans are mainly obtained from the genus Chondrus, Eucheuma, Gigartina, Iridae, Furcellaria and Hypnea, and the expansion and increased demand led to the introduction of the cultivation of Kappaphycus alvarezii and Eucheuma denticulatum, with a predominant content of k- and i-carrageenan, respectively, available all year []. Chondrus crispus, the original source, which contains a mixture of k- and λ- carrageenan, could be a model organism [] and there is a renewed interest in the cultivation for cold water carrageenophytes, although the economics need to be carefully considered []. The differences in composition and molecular conformation determines the rheological profiles, gel properties and textures of carrageenans as well as the interactions with other gelling agents and food ingredients [].
The three most commercially exploited carrageenans are kappa (κ-), iota (ι-) and lambda (λ-) carrageenans, which can be separately provided or as a well-defined mixture, since most of the seaweeds contain hybrid carrageenans []. Mu and nu carrageenans are the precursors in carrageenan, which are converted to kappa and iota, respectively, by means of alkaline modification. In the natural state, unmodified kappa and iota carrageenans account for around 30% mu and nu-carrageenans, remaining less than 5% after alkaline modification, randomly distributed within the repeating structures []. Origin, species or extraction processing conditions notably affect the type and quality of carrageenan isolated. The carrageenan types vary among species from the Cystocloniaceae family predominantly produce iota-carrageenans; the Gigartinaceae family lead to hybrid kappa-iota carrageenans and lambda-family carrageenans (sporophytic plants); the Phyllophoraceae family produces kappa-iota hybrid-carrageenans []. Commonly, κ-carrageenan is commercially isolated from Kappaphycus alvarezii red seaweed through a hot extraction process, whereas λ-carrageenan is more hygroscopic and it is usually extracted from red seaweeds of the genera Gigartina or Chondrus by drum dryer or alcohol precipitation process []. Concerning ι-carrageenan, it is commercially extracted from Eucheuma denticulatum by the freeze thaw or gel process []. The highest carrageenan yields can exceed 70% (dry basis) for some species such as B. gelatinum, K. alvarezii or K. striatum. Other species have values close to 30%, such as E. denticulatum or C. crispus. Sulfate content in carrageenans varies from 20% in κ-carrageenan, to 33% in ι-carrageenan and to 41% in λ-carrageenan []. It is well known that the carrageenans gelation process is affected by the biopolymer content, temperature, ionic strength of the solution and the cation type and content, being the most effective K+ and Rb+. In general, iota carrageenans form soft and elastic gels with higher gelling temperatures than κ-carrageenans []. In contrast, λ-carrageenan did not form gels, being used solely as thickening agent []. Gelling temperatures ranged from 32 to 36 °C for the κ-carrageenans, whereas ι-carrageenans exhibited values of 70–74 °C. Gel strengths, elastic modulus at 25 °C, varied between 4000–6500 Pa for alkali pre-treated samples [].
Carrageenan is a natural ingredient, used for decades in food applications and generally recognized as safe (GRAS) by the Food and Drug Administration; furthermore, carrageenan and semi-refined carrageenan are food additives (E-407 and E407a, respectively) approved by European Food Safety Authority. The food viscosity specification is equivalent to an average molecular weight greater than 100 kDa, and commercial food carrageenan have Mw in the range 200–800 kDa [,]. Carrageenan is not degraded nor absorbed in the gastrointestinal tract [,,] and should not contain low Mw fractions, poligeenan or degraded carrageenan, since it exhibits toxicological properties at high doses [] and can induce gastrointestinal irritation and cancer in animal models [] or can lead to cell death and inflammatory responses on human colonic epithelial cells [,], bacterial dysbiosis and shifted community composition [] and decreased anti-inflammatory bacteria []. Further studies of its impact on digestive proteolysis, the colon microbiome and inflammation [,,] and the effects on predisposed populations [,] are required. Carrageenans are also used for the pharmaceutical sector [,], based on anti-inflammatory [], antiviral [,,,,,], anticoagulant [,], immunomodulatory [], antitumoral [], antioxidant [,,], anti-angiogenic [] and neuroprotective [] activities. The biological properties of carrageenans have been reviewed in several publications [,,,,].
Carrageenans are widely used as an inflammatory inducing agent in experimental animals [], and have also been proposed for the immobilization and encapsulation of biocatalysts [], for entrapping lactic bacteria and enzymes [], for microencapsulation of probiotics in mixtures of k-carrageenan with carboxymethylcellulose []. Recent pharmaceutical applications are found in drug delivery and for tissue regeneration []. Carrageenans are highly biocompatible and are used as ingredients for films, beads, microparticles, nanoparticles, hydrogels, inhalable and injectable systems [,,] used alone or in combination with other polymers, and its mucoadhesive properties have been exploited in the preparation of aerogel microparticles for mucosal drug delivery [].
The role of carrageenans in agriculture has also been confirmed [,,,,,,,], since it stimulates growth [] and increases defense responses against viruses [,] and abiotic stresses [].
- Extraction processes: conventional and emerging technologies
Specific details of the commercial extraction processes are trade secrets for the manufacturers of carrageenan. The seaweed is usually dried quickly to prevent degradation during transportation to the processing facilities. The original method to produce the commercial carrageenans is based on washing to remove impurities, such as sand, epiphytes and salt and the carrageenan extraction in a hot aqueous solution, neutral or alkaline, filtration, recovery from the solution by alcohol precipitation, separation of the precipitate, drying and milling. However, this method is time and energy consuming, and has a low extraction efficiency. Alternatively, extraction of minerals, protein and lipids can be proposed, leaving in the raffinate the carrageenan and cellulose as a semi-refined low purity carrageenan. The main variables during extraction, namely temperature, pH, time and alkaline pre-treatment (alkaline agent, concentration and time), have to be optimized for each seaweed to maximize the structural and gelling properties [,]. The alkali pretreatment of red seaweed increases the ratio of ι- versus κ-hybrid [], but usually, an excessive alkali content and prolonged treatment time can lower molecular mass and depressed gel properties []. Table 3 shows some representative examples of carrageenan extraction procedures.

Table 3.
Carrageenan yields and extraction procedures.
Alternatively, low temperature has been proposed to extract low-molecular-weight carrageenans [] and to maintain reducing, antiradical and anticoagulant activities, probably due to the higher sulfate content, which would be lost after hot-water, acid and alkali treatments []. Other options can lower time, energy demand and the consumption of water, chemicals and solvents. Among the novel extraction techniques to enhance the extraction efficiency are pressurized solvent extraction, microwave-, ultrasonic- and enzyme-assisted extractions [,,].
Microwave-assisted extraction offers a reduction in time and energy consumption, thus enhancing the process efficiency []. Operating in closed vessel, more efficient desulfation was observed and the κ/ι hybrid carrageenan obtained was comparable to that extracted by the conventional technique []. Boulho et al. [] did not observe significant differences in the carrageenan (predominantly iota-) yield from Solieria chordalis, were observed between MAE and conventional method under alkaline conditions, and the product showed antiviral activity against Herpes simplex virus type 1. Almutairi et al. [] reported λ-carrageenan discoloration occurring during microwave irradiation for the aqueous solutions exposed to microwave heating.
The ultrasound assisted processes both alkaline and aqueous, shortened extraction times compared to the conventional method, avoiding degradation of labile compounds, showing a slight variation in sulfate, AG and Gal contents and viscosity []. Youssof et al. [] reported that they doubled the yields attained in four–eight longer times with conventional extraction without affecting the chemical structure and molar mass distribution of carrageenans.
- Carraoligosaccharides: properties and production strategies
Oligocarrageenans are oligomers of sulfated galactose, usually DP 2–20 [,], prepared by depolymerization by acid hydrolysis. Despite the degraded carrageenan caused significant mucosal ulceration of the colon, associated to histopathological changes, epithelial thinning, slight erosion, cellular infiltration and other negative changes in animal organisms [], carrageenan oligosaccharides exhibit several biological activities, influenced by their molecular weight and sulfation degree.
Carrageenan oligosaccharides show scavenging properties against reactive oxygen species [], hydroxyl radicals, superoxide anion, nitric oxide and hydrogen peroxide []. They also present anti-inflammatory and immunomodulatory [,,,], anticoagulant [], antimicrobial and antiviral [,,,,,] and healing [] properties. They also showed anticarcinogenic action [,,,,], with low cytotoxicity [] and synergistic effects with conventional drugs, improving the immunocompetence damaged by these drugs. The oligocarrageenans promote plant growth by enhancing photosynthesis, nitrogen assimilation, embryogenesis, basal metabolism, cell division, regulation of phytohormone synthesis [,,,] and by increasing protection against viral, fungal and bacterial infections [,,,], partly due to the accumulation of compounds with antimicrobial activity [].
Partial depolymerization by chemical or enzymatic hydrolysis to obtain a range of oligosaccharides is a common strategy for structural analysis and for characterization of activity [,]. The biological profile of the products may be influenced by the depolymerization method, since it affects their size and molecular weight. In addition, carrageenan or their derived oligosaccharides may also be chemically modified by oversulfation, desulfation, acetylation or phosphorylation to achieve better physicochemical and biological properties [,,,], i.e., the antioxidant [] and antiviral [] activities.
Acid hydrolysis has been considered as a common and rapid method. The presence of acid and oxidizing agents may induce carrageenan depolymerization through cleavage of glycosidic linkages, a process accelerated by dissolved oxygen, high temperature and low pH. In order to limit undesirable degradation, high temperature and short time mild acid hydrolysis is preferred []. Karlsson and Singh [] reported that carrageenans were stable to desulfation during acid (pH 2) hydrolysis at 35 and 55 °C. Kalitnik et al. [] used mild acid hydrolysis of Chondrus armatus κ-carrageenan under conditions avoiding excess destruction of 3,6-AGal and observed that mild and acid hydrolysis cause breakage of inside α-1,3 links, producing mainly odd-numbered oligosaccharides. Mild acid hydrolysis at 37 ºC increased yields of even fractions in comparison with those obtained at 60 °C [].
Enzymatic hydrolysis offers advantages due to its high efficiency under mild conditions, avoids the use of polluting chemicals, and the resulting oligosaccharides generated show higher homogeneity and lower polydispersity, thus providing compounds with improved and reproducible biological properties []. In addition, it avoids side reactions leading to undesired modifications of the native structure and the release of high amounts of monosaccharides and undesirable toxic products []. The enzymatic method, either using non-specific commercial enzymes or carragenases, is a relatively costly alternative. Carrageenases, produced only by marine gram negative bacterial species, are endohydrolases that hydrolyze the internal β-1,4 linkages in carrageenans [,,]. Table 4 summarizes some representative examples of depolymerized carrageenans.
Microwave assisted degradation allowed a reduction in operation times and almost did not change the structure and constitutions of the λ-carrageenan [], and κ-carrageenan [] with antiviral properties. However, the special high-pressure equipment needed could be difficult to operate []. Operation in closed vessels and in open vessel was reported; additionally, operation in domestic devices could be proposed for acid hydrolysis [].
During ozonation the depolymerization of polysaccharides causes chemical changes, as well as physicochemical and rheological modifications, since ozonation of κ-carrageenan leads to the formation of carbonyl, carboxyl or double bonds; however, the sulfate groups in k-carrageenans were maintained [].
The ultrasound-assisted depolymerization of κ-carrageenan is simple, suitable for food applications and energy saving, since it is faster than thermal depolymerization at lower temperatures []. The susceptibility to ultrasound assisted degradation differs among the carrageenan types, being higher for κ- than for τ-carrageenans [] and possibly occurs due to the ultrasonically induced breakage of non-covalent bonds in κ-carrageenan molecules [].

Table 4.
Examples of technologies used for extraction of carrageenan oligomers.
Table 4.
Examples of technologies used for extraction of carrageenan oligomers.
| Depolymerization | Seaweed or Polyssaccharide | Properties | References |
|---|---|---|---|
| Acid hydrolysis | Carrageenan (C) | Mw: κ-, 510–4000; ι-, 110–3300; λ-, 660–5800 | [] |
| Acid hydrolysis | Eucheuma cottonii | DP: κ-, 6–20 | [] |
| Enzymatic | Chondrus armatus, Kappaphycus alvarezii, Tichocarpus crinitus | Mw: ĸ-, 2.2–4.3 | [,] |
| Enzymatic | Carrageenan (C) | Mw: ĸ-, 681–798 | [] |
| High-Pressure | Halymenia durvillei | Mw: λ-, 260–1100 | [] |
| Irradiation | Carrageenan (C) | Mw: κ-, 8.5–32.1; ι-, 3.1–6.9; λ-, 2.7–6.5 | [] |
| Microwave assisted | Solieria chordalis, Chondrus ocellatus | Mw: λ-, 3–240 Mw: λ-, 650 | [,] |
| Ozonization | Carrageenan (C) | Mw: ĸ-, 10–200 | [] |
| Radical depolymerization | Halymenia durvillei | Mw: λ-, 3.3–890 | [] |
| Subcritical water extraction ionic liquids as catalyst | Kappaphycus alvarezii | Mw: ĸ-, 10–60 | [] |
| Ultrasound assisted | Kappaphycus alvarezii, Eucheuma cottonii | Mw: ĸ-, 545 Mw: ĸ-, 160–240 | [,] |
C: commercial; Mw: Molecular weight (kDa); DP: degree of polymerization.
Carrageenan can be degraded by gamma irradiation, operating in different systems (solid, gel or solution) at ambient temperature and the molecular weights can be lowered to 8–100 kDa with a narrow distribution, but different yield and susceptibility to degradation occur for the different carrageenan types. Abad [] reported the use of irradiation with gamma rays at room temperature to depolymerize polysaccharides with enhanced antioxidant properties []. Irradiated κ-carrageenan as incorporated as antioxidants in many food systems, but the toxicity of radiolytic products from irradiated κ-carrageenan have to be studied further [].
Comparative studies have revealed that the method of depolymerization strongly influences the properties of carrageenan oligomers []. The chemical depolymerization (free radical or mild acid hydrolysis), produced oligomers with lower Mw (1.2–3.5 kDa) than the enzymatic depolymerization using a recombinant kappa-carrageenase from Pseudoalteromonas carrageenovora, yielding 2.2 kDa oligomers from Chondrus armatus and Kappaphycus alvarezii k-carrageenans and 4.3 kDa oligomers from Tichocarpus crinitus κ/β-carrageenans. Low molecular weight derivatives obtained by mild acid hydrolysis showed higher antiviral activity than those obtained by free radical depolymerization, which were more active than those enzymatically prepared. Sun et al. [] observed that mild acid hydrolysis caused higher saccharide degradation than H2O2 depolymerization and κ-carrageenase digestion; the original sulfate content was substantially retained and all the hydrolysates had stronger reducing power than the polysaccharide, with H2O2 hydrolysates being the most potent. After free radical treatment at 40 °C for 4 h, the low-molecular weight oligosaccharides from κ-carrageenan ranged from disaccharide to octasaccharide. The degradation with a κ-carrageenase hydrolyzing the β-1,4 linkages to a series of homologous, even-numbered oligosaccharides (An-G4S)n, yielding 2, 4, 6, 8 and 10 DP, being dominant the tetra- and hexasaccharides []. Whereas for H2O2 treatment, the scavenging ability increased with time as a result from the increment of –COOH groups, the scavenging ability of HCl hydrolysates and enzymatic hydrolysates decreased when the molecular weight decreased. Other combinations have been suggested, i.e., radical depolymerization, and high-pressure homogenization led to several samples of various and controlled molar masses of Halymenia durvillei [].
2.2. Protein
The protein content in red algae, higher than in brown and green groups, accounts for 10–50% of the dry weight, being comparable or higher than in some foods [,] and the essential aminoacid content, accounting for 25–50% of the total amino acids, is similar as in other protein sources such as casein, ovalbumin and leguminous [,,,]. The protein contents differ according to the species and seasonal conditions [,], being the highest in Porphyra, followed by Palmaria sp. Nitrogen-to-protein conversion factors of 4.92, lower than for brown and green algae have been proposed [,] and algae may contain non-protein nitrogen, resulting in an overestimation of their protein content. Although the digestibility of proteins seems to be limited by the algae non-proteic fraction [,] they have been proposed for inclusion in diets of ruminants, hens, rabbit, poultry and pigs [].
Red algae have a characteristic bright pink color caused by phycobiliproteins. Phycobiliproteins are covalently bound via cysteine amino acids to pigmented phycobilins [,]. They are classified into phycoerythrin (red) and phycocyanin (blue). The two types of phycoerythrin (PE) were named after the taxa of the organism form which they were first isolated: R-PE from Rhodophyta and B-PE from Bangiales. Phycocyanins are further subdivided into C-phycocyanin, R-phycocyanin, allophycocyanin or phycoerythrocyanin. Examples of phycobiliproteins found in red seaweeds are shown in Figure 4.

Figure 4.
Structure of phycobiliproteins and micosporine-like-aminoacids, adapted from [,].
Phycobiliproteins are commercially used in foods, nutraceuticals, cosmetics as a colorant and for their therapeutic value, namely their antimicrobial, antioxidant, anti-inflammatory, neuroprotective, hepatoprotective, immunomodulating and anticarcinogenic properties [,,,,,,,,]. They can improve the efficacy of standard anticancer drugs, lower their side effects [] and act as photosensitizers for the treatment of tumoral cells []. They are also used as fluorescent markers in clinical diagnostics and immunological analysis.
The storage conditions influence the preservation of phycoerythrin (R-PE) and freezing was reported as the best preservation method []. Significant changes in phycoerythrin and phycocyanin were also observed after different culinary treatments []. Whereas drying and hydration did not affect the content of phycoerythrin, boiling and steaming caused lowered values.
- Extraction processes: conventional and emerging technologies
Since the extraction of proteins from seaweeds is complicated by the presence of cell wall polysaccharides, the classical procedures are based on the use of buffer, osmotic shock, detergents or the application of alkali treatment, some examples are summarized in Table 5. Different physico-chemical and enzymatic pretreatments have been suggested to enhance the yields, such as repeated freeze-thaw cycles [,], or grinding in liquid nitrogen of the freeze-dried seaweeds [] aided in the release of R-phycoerythrin. Their further purification from the crude extract has been usually addressed through ammonium sulfate precipitation [] or also by sucrose step-gradient ultracentrifugation [] followed by purification by gel filtration and by ion exchange chromatography [,,,].
The use of enzymes degrading the cell wall polysaccharides as an alternative method to improve the extraction and the solubilization of algal proteins [], since firstly reported by Amano and Noda [] suggested the use of a mixture of enzymes from the gut of abalone Haliotis discus and a commercial one to enhance the extraction of proteins from Porphyra yezoensis. Enzyme assisted extraction could improve the physicochemical characteristics, volatile compounds and organoleptic quality of plant proteins producing peptides and amino acids with less salt and carcinogenic compounds than acid hydrolysis []. Both the extraction efficiency and the composition of the extracts depended on the seaweed [], but the influence of the type of enzyme is also determinant on yields, composition and properties. Whereas some cellulases enhanced the protein extraction yields when used alone [,], in other studies, polysaccharidases alone or in mixtures caused only a partial digestion of seaweed cell walls and did not improve the yields [,] and mixtures of cellulase with carrageenase or agarase were more favorable [,]. Proteolytic hydrolysis is usually proposed to obtain bioactive peptides; however, the protease treatments also enhanced the extraction of antioxidants from Palmaria palmata compared to carbohydrases and cold water extraction [].

Table 5.
Red seaweed protein extraction.
Table 5.
Red seaweed protein extraction.
| Technologies | Seaweed | Product | Properties | Reference |
|---|---|---|---|---|
| Accelerated solvent extraction (acetone or methanol) | Porphyra umbilicalis | Carbohydrate/Phlorotannin extraction | Antioxidant | [] |
| Carbohydrase hydrolysis under high hydrostatic pressure | Palmaria palmata, Solieria chordalis | Antioxidant peptides | Antioxidant | [] |
| Enzyme hydrolysis with: protease, agarase, carrageenase, xylanase, cellulase | Gelidium pusillum Chondrus crispus, Gracilaria verrucosa, Palmaria palmata Osmundea pinnatifida, Codium tomentosum, Solieria chordalis | Antioxidant peptides, protein, phycobiliproteins, R-phycoerythrin | Antioxidant, α-glucosidase inhibition anti-inflammatory | [,,,,,,,,,,] |
| Freezing and thawing | Porphyra haitanensis, Gelidium pusillum | Phycobiliproteins (R-PE and R-PC) | Antioxidant | [,] |
| Grinding freeze-dried seaweed in liquid nitrogen | Mastocarpus stellatus | R-phycoerythrin | Antioxidant | [] |
| Homogenization in water or buffer | Chondrus crispus, Palmaria palmata, Heterosiphonia japonica, Gelidium pusillum | Phycobiliproteins (R-PE and R-PC) | Antioxidant, antidiabetic, antitumor | [,,,] |
| Osmotic shock | Palmaria palmata, Polysiphonia urceolata | Bioactive peptides, R-phycoerythrin | Antioxidant, prevention of atherosclerosis | [,] |
| Subcritical water, optionally catalyst | Hypnea musciformis, Kappaphycus alvarezii | Protein, antioxidants, emulsifyiers | Antioxidant, emulsifyier | [,] |
| Ultrasound-assisted extraction | Palmaria palmata, Porphyra umbilicalis | Bioactive peptides R-PE and R-PC | Antioxidant | [,,,] |
| Ultrasound-assisted extraction | Gelidium pusillum, Porphyra yezoensis | R-PE, R-PC, taurine | Antioxidant | [,] |
| Ultrasound and enzyme-assisted extraction | Osmundea pinnatifida, Codium tomentosum | Protein | Antioxidant, prebiotic effect | [] |
Additionally, it can be useful in combination with other intensification technologies. Le Guillard et al. [] reported ultrasound-assisted extraction and ultrasound-assisted enzymatic hydrolysis with an enzymatic cocktail for the extraction of R-phycoerythrin from Grateloupia turuturu. They recommended the use of 22 °C to avoid R-PE destruction, and 40 °C when the objective was liquefaction. Enzymatic hydrolysis was combined with mechanical methods, namely, ultrasonication []. Suwal [] reported on the use of a non-thermal high hydrostatic pressure (400 MPa, 20 min) processing combined with polysaccharidases to improve the extraction of proteins, polyphenols and polysaccharides from Palmaria palmata and Solieria chordalis; the effect of this technique being dependent on the seaweed species and the enzyme used. Mittal et al. [] compared different pre-treatments for extraction of phycobiliproteins from Gelidium pusillum and observed a synergistic effect of ultrasonication when employed in combination with other conventional extraction methods, although ultrasonication alone was not efficient. However, Harrysson et al. [] observed that the pH-shift protein extraction provided the highest protein yields and concentration in the extracts from Porphyra umbilicalis, compared to sonication. Fitzgerald et al. [] used a papain digestion of crude Palmaria palmate protein obtained by osmotic shock and ultrasound assisted extraction, with the aim of obtaining bioactive peptides for the prevention of atherosclerosis and the hydrolysate was nontoxic.
Gereniu et al. [] extracted protein from Kappaphycus alvarezii processed by pressurized hot water extraction. Whereas the hydrolysis efficiency increased from 150 °C to 270 °C, and decreased at 300 °C due to decomposition and protein denaturation, the highest foaming properties were attained at 150 °C, whereas the best emulsifying properties were found at 300 °C. Pangestuti et al. [] proposed the hydrolysis of Hypnea musciformis using subcritical water extraction (120–270 °C) to obtain antioxidant and functional material. They found increased protein and sugar content at 120–150 °C, more marked at higher temperatures (180–210 °C), showing the highest antioxidant activity and thermostable emulsifying properties, which could be related to the increased solubility of protein, to the hydrolysis of oligosaccharides and the degradation of monosaccharides.
Wang et al. [] reported on the use of ultrasound-assisted extraction during the purification of taurine from Porphyra yezoensis. This sulfur-containing amino acid can enhance seafood profile flavour. Homotaurine, an aminosulfonate compound present in different species, has shown in vitro and in vivo neuroprotective effect and could be a promising drug for both prevention of Alzheimer’s disease []. Operating at 40 °C and 300 W, the ultrasonic process lowered the extraction time by nine compared to the conventional extraction.
2.3. Lipids and Fatty acids
In red seaweed, lipids and fatty acids are present in low amounts, generally 1–5% of the dry weight [,]; however, they contain significantly higher levels or polyunsaturated fatty acids than vegetables and have been proposed as a chemotaxonomic tool to differentiate macroalgae []. Macroalgae also contain various other lipids and lipid like compounds such as sterols, phospholipids and glycolipids, but red seaweeds have a high ω-3 fatty acids content, being a rich source of α-linolenic acid (ALA) [18:3(ω3)], AA, eicosapentaenoic acid (EPA) [20:5(n-3)], and docosahexaenoic acid (DHA) [22:6(ω3)]), and most species showed a nutritionally beneficial ω6/ω3 ratio [,,] (Table 6). Some macroalgae present a low ω6/ω3 ratio, the ω3 polysunsaturated fatty acids (PUFAs) cannot be synthesized by humans and are thus obtained only through dietary sources. Their therapeutic, especially eicosapentaenoic acid (EPA), has been shown in the reduction of blood cholesterol, and in the protection against cardiovascular and coronary heart diseases [], and they have anti-inflammatory, anti-thrombotic and anti-arrhythmic properties [].
Total fatty acid concentrations vary among species, accounting for 1–8 in % of dry weight, showing significant differences in the fatty acid profiles [], which can also be depending on the storage conditions (time and temperature) and the solvent also influences the yields and composition of the lipid extracts [].
Kumari et al. [] compiled the total lipid content and fatty acid distribution of different seaweeds and suggested that the variations observed between different species of the same genus was more likely to be due to the inter-specific/intra-generic variations rather than to geographical and environmental conditions as apparent from the minor variations found with the environmental parameters for the studied collection sites.
- Extraction processes: conventional and emerging technologies
The growing interest in PUFA-rich lipids from seaweeds for incorporation into foods has led to an increasing demand for novel extraction techniques with food grade solvents providing high extraction yields. Supercritical CO2 extraction of bioactives (neutral lipids and antioxidants) from microalgae and seaweeds [] is performed in a non-oxidizing atmosphere, which can prevent degradation. Drying and crushing are required stages despite the high energy consumption of the first stage. Chen and Chou [] reported similar fatty acid profiles of different red seaweeds extracted by supercritical fluids extraction method; however, Cheung [] observed increased proportions of Hypnea charoides PUFAs with operation pressure. The total fatty acid content and the EPA content in the extract produced by pH-shift was slightly reduced compared to that in the crude seaweed from Porphyra umbilicalis [].
Patra et al. [] reported the use of microwave assisted hydrodistillation to extract the volatile oil from Porphyra tenera, which showed radical scavenging properties comparable with BHT and α-tocopherol. Kumari et al. [] reported on the application of sonication and buffer individually on the lipid extraction from Gracilaria corticata with analytical purposes. Table 6 shows the extraction yields and the PUFA ratio for different red seaweed genus obtained with conventional and alternative extraction technologies.

Table 6.
Total lipid (TL) content, polysunsaturated fatty acids PUFA ratio and distribution in red seaweed extracts.
Table 6.
Total lipid (TL) content, polysunsaturated fatty acids PUFA ratio and distribution in red seaweed extracts.
| Seaweed Genus | Extraction | TL (mg/g fr. wt.) | PUFA/SFA | ω6/ω3 | Reference |
|---|---|---|---|---|---|
| Acanthophora | CHF/M/PB | 6.8–10.4 | 0.79–0.94 | 0.9–1.8 | [] |
| Asparagopsis | CSE (H) | 3.0 | 0.06 | 0.62 | [] |
| Bangia | SFE | 13.3 dw | 2.8 | 2.22 | [] |
| Bornetia | CSE (H) | 5.3 | 0.76 | 0.29 | [] |
| Botryocladia | CHF/M/PB | 2.3–5.2 | 0.49–0.54 | 1.7–3.6 | [] |
| Coelarthrum | CHF/M/PB | 7.7 | 0.67 | 5.7 | [] |
| Delisea | CSE (Et; DCM:M) | 2.2 | 1.35 | 0.4 | [] |
| Galaxaura | SFE | 19.8 dw | 0.98 | 0.71 | [] |
| Gastroclonium | CHF/M/PB | 4.3 | 0.59 | 5.1 | [] |
| Gelidiopsis | CHF/M/PB | 5.5 | 0.84 | 0.8 | [] |
| Gelidiella | CHF/M/PB | 6.7 | 0.98 | 0.6 | [] |
| Gracilaria | CHF/M/PB | 2.9–9.7 | 0.15–2.13 | 0.6–1.9 | [] |
| Grateloupia | CHF/M/PB; SFE | 5.0–6.4, 13.6 dw | 0.74–1.4 | 0.5–1.9 | [,] |
| Griffithsia | CHF/M/PB | 4.2 | [] | ||
| Halymenia | CHF/M/PB; SFE | 10–18.8 dw | 1.37–1.8 | 1.7–5 | [,] |
| Helmintocladia | SFE | 19.7 dw | 1.05 | 1 | [] |
| Hypnea | SCF: 50 °C, 37.9 MPa | 5.8–7.8 | 0.31–0.43 | 0.8–16 | [,] |
| Jania | CSE (H) | 2 | 0.79 | 0.60 | [] |
| Jania | CHF/M/PB | 12.2 | 0.32 | 2.9 | [] |
| Laurencia | CSE (Et; DCM:M) | 5.4–16.0 | 0.41–1.08 | 0.4–1.7 | [,] |
| Liagora | SFE | 17.6–21.5 dw | 0.94–1.43 | 0.42 | [] |
| Peyssonelia | CSE (H) | 4.8 | 1.33 | 1.9 | [] |
| Porphyra | MAHD: 40 W, water | 11.2–12.4 dw | 2.4–2.5 | 1.2–9.1 | [,] |
| Pterocladiella | CSE (H) | 5.5 | 0.51 | 0.9 | [] |
| Pyropia | CHF/M/PB | 7.0–7.7 | 1.23–1.76 | 0.7–1.4 | [] |
| Rhodymenia | CHF/M/PB | 7.1 | 0.87 | 88.2 | [] |
| Sarconema | CHF/M/PB | 4.3–9.8 | 0.27–1.04 | 2.4–2.5 | [] |
| Solieria | CHF/M/PB | 9.0 | 0.35 | 0.8 | [] |
| Cryptonemia | CHF/M/PB | 11.3 | 0.86–1.28 | 0.9–18.8 | [] |
| Odonthalia | CHF/M/PB | 11.4 | 0.72 | 0.6 | [] |
| Polysiphonia | CHF/M/PB | 9.6 | 0.53 | 1.1 | [] |
| Scinaia | CHF/M/PB | 5.2–17 dw | 0.23–1.86 | 1.1–5.3 | [,] |
| Palmaria | CSE (Et; DCM:M) | 14–46 dw | 0.49–1.1 | 0.21–0.41 | [,] |
| Vertebrata | CSE (Et; DCM:M) | 13–18 dw | 0.79 | 0.4 | [] |
CSE: Conventional solvent extraction; CHF/M/PB: chloroform–methanol–phosphate buffer; Et: ether extraction; DCM:M: dichloromethane/methanol; H: hexane; MAHD: Microwave assisted hydrodistillation; SFE: Supercritical fluid extraction.
2.4. Extractives
The solvent influences the composition and activity as well as the mechanism of action of extracts []. Many studies aimed at the solvent extraction of polyphenols, flavonoids and carotenoids []. Organic solvents, such as ethanol, methanol, acetone or their mixtures such as chloroform:methanol, have been used for the extraction of antioxidant components, some illustrative examples are shown in Table 7. The choice of extracting solvents with different polarities can have a significant effect due to the different nature of compounds present in the seaweeds and also species–species differences. Intensification with ultrasound was suggested to enhance the solvent extraction process [].
Supercritical fluid extraction with pure carbon dioxide can be favorable for the extraction of apolar compounds [], the addition of a small amount of polar modifiers may increase the affinity of this solvent for relatively polar compounds. Zheng et al. [] obtained extracts, mainly composed by sesquiterpenes, ketones, fatty acids, phenols and sterols from Gloiopeltis tenax by supercritical carbon dioxide extraction with ethanol as modifier, and reported remarkable antioxidant and antimicrobial activity. Ospina et al. [] reported the extraction of Gracilaria mammillaris extracts compounds with antioxidant activity using supercritical CO2 modified with ethanol.
When the simultaneous extraction of different components is addressed, the selection of the enzyme activities could be relevant, i.e., for the extraction of phenolics from P. palmate, protease provided higher contents than water extract, whereas some carbohydrases showed lower contents, an effect ascribed to their ability of proteases to liberate LMW peptides and amino acids by proteases, which could also enhance the scavenging activities of the extracts []. Combination of enzyme digestion with cellulase and hemicellulose, which disrupted or weakened the structural integrity of the seaweed cell wall and high hydrostatic pressure (HHP) increased the accessibility of enzymes, accelerating the release of intracellular polyphenols from P. palmata, and from S. chordalis []. In some cases, organic solvent extraction was more efficient than emerging techniques, i.e., for phenolics from Osmundea pinnatifida, and Codium tomentosum and was more efficient than hot water extraction or than enzyme or ultrasound assisted extraction []; however, the benefits of using greener solvents have to be considered.

Table 7.
Examples of extraction of bioactives from red seaweeds.
Table 7.
Examples of extraction of bioactives from red seaweeds.
| Solvent | Seaweed | Activity | Reference |
|---|---|---|---|
| Ethanol (70–80%), methanol (80%), Acetone, ethyl acetate, chloroform:methanol (2:1) (80%), dimethyl sulfoxide (80%) | Gracilaria changii, Gelidium amansii, Kappaphycus alvarezii, Osmundea pinnatifida, Codium tomentosum, Gracilaria lemaneiformis | Antioxidant, glucose uptake regulation, anti-diabetic, neuroprotective, gastroprotective | [,,,,] |
| Enzyme (proteases, carbohydrases) assisted | Parmaria palmate | Antioxidant | [] |
| Phosphate buffer | G. amansii | Antitumoral | [] |
| Ultrasound-assisted | Laurencia obtusa | Antioxidant | [] |
| Supercritical CO2 | Gloiopeltis tenax, Gracilaria mammillaris | Antioxidant, antimicrobial | [,] |
| Enzyme and high hydrostatic pressure | Palmaria palmate, Solieria chordalis | Antioxidant | [] |
Mycosporine-like amino acids are low-molecular-weight, water-soluble components with antioxidant and photoprotective properties found in red seaweeds. Since they have been reported as the strongest UVA-absorbing compounds in nature, they have been proposed as photoprotective materials for skin care products. The antioxidant and antiproliferative activities and mycosporine-like amino acid depended on locations varying in UV-exposure [], with higher values summer and in shallow waters than in deeper waters [,]. Conventional extraction with organic solvents has been reported, i.e., methanol [], but the ultrasound assistance was also proposed to obtain UV-absorbing compounds [].
2.5. Minerals
Seaweeds are particularly rich in minerals and trace elements, showing ash contents, in the range 20–40% w/w, and could be a good source of K, Ca, Fe, Mg and other trace elements essential for human nutrition [,,]. Seaweeds concentrate minerals due to their capacity to retain inorganic marine substances from seawater based on the characteristics of their cell surface polysaccharides [], and contain 10–20 times the minerals of land plants. The Na/K ratios were below 1.5 and can be proposed for low sodium diets, since diets with a high Na/K ratio have been related to the incidence of hypertension.
Jaballi et al. [] reported the ability of a mineral and antioxidant-rich extract from Chondrus canaliculatus to improve the toxicity caused by a fungicide in adult rat, being effective against hematotoxicity, genotoxicity and oxidative stress in the blood and bone and maintained osteomineral metabolism and bone histo-architecture.
3. Combined Extraction
Most of the proposed extraction processes are not selective and apart from the target compound, others can also be obtained. This could be illustrated with some examples. Extraction by enzymatic hydrolysis of R-phycoerythrin from Gracilaria verrucosa causes the release of a small amount of polysaccharides, which could be recovered in the coproduct []. Some intensification technologies also favor the simultaneous extraction of different components. During protein extraction, the mineral content in the extracts could be enhanced using accelerated solvent extraction produced extracts compared to that of conventional extracts, whereas the pH-shift-produced extracts had lower ash content than the whole biomass. Although the co-extraction of other compounds different from the target ones could difficult and make more expensive the purification stages, the presence of other high-value food components could confer additional value and synergistic functional and biological properties to the final product. This could occur, as fatty acids together with proteins could be of interest for producing multi-functional protein extracts [].
Some authors proposed the use of more than one fraction, such as the sequential extraction of R-phycoerythrin and agar from Gracilaria verrucosa [], Yuan et al. [] proposed an initial extraction of the pigments for decolorization of Gracilaria lemaneiformis before agar production, allowing also to recover the removed fractions as natural antioxidants. Niu et al. [] observed that after water extraction of proteins from Gracilaria lemaneiformis and further purification of R-phycoerythrin the remaining biomass was used for agar extraction. The yield of agar and its properties showed no significant difference from those obtained from the direct agar extraction from the dried algae. However, the R-phycoerythin recovery and purity were lower than when it was extracted from fresh algae. The most frequent approach consists on the valorization of the waste fractions after phycocolloid extraction as a source of protein. Cian et al. [,] reported the use of Porphyra columbina wastes to obtain proteins that after proteolystic digestion to produce fractions with immunosuppressive, antihypertensive and antioxidant actions.
Particularly interesting is the integral utilization of the raw material, following a biorefinery approach. In order to make use of all seaweed components requires a rational processing of the whole material, and the algal processing by-products according to the biorefinery concept to allow a complete utilization of biomass [,,]. This alternative processing approach would provide different products and applications, favoring the economics of a process that would not rely exclusively on one product and could be adapted to the demand and needs of different sectors.
Figure 5 represents a general flow diagram for a multistage multipurpose biorefinery processing of red seaweeds. The suggested scheme is based on the initial production of food or feed products, with the final ones being destined to energetic and soil applications. It is desirable that biorefineries are designed in a flexible way allowing the possibility of processing different seaweeds, obtaining different products including those of high volume/low quality and those of high quality/low volume ones. If possible, it is also recommended to integrate food and non-food sectors. In the extraction stages, the utilization of more efficient greener technologies is recommended to enhance the yields and productivities, keeping the products quality and lowering energetic and operation costs.
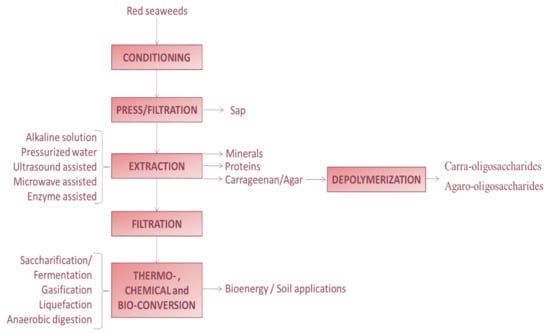
Figure 5.
Simplified flow diagram of a red seaweed biorefinery.
Different authors proposed the utilization of agarophytic biomass for biorefinery including the energetic uses [,,,,] and for the production of chemicals, such as 5-hydroxymethyl furfural, levulinic acid and formic acid from K. alvarezii []. However, in the present review, the valorization of bioactives is emphasized, and this type of seaweed is highly interesting, since they contain a high proportion of proteins, some being colorants (R-phycoerythrin, R-phycocyanin), fatty acids and minerals. Therefore, biotechnological, nutraceutical and pharmaceutical applications have been highlighted.
Even when the ethanol production was also considered in their approach, Baghel et al. [], have designed a complete valorization of Gracilaria corticata bioactives, including phycobiliproteins, lipids and agar. The solid residue after phycocolloid extraction is still a good source of bioactives and has been explored in a number of studies. Based on the ingent amounts generated during industrial processing, their valorization would also report environmental benefits. Cian et al. [] used this waste from Porphyra columbina to obtain low molecular weight peptides with angiotensin-converting-enzyme (ACE) inhibitory action, as well as antioxidant properties, which could also be due to some phenolic compounds. Laohakunjit [] proposed the hydrolysis of Gracilaria fisheri residue after agar extraction and the protein hydrolysate was used to obtain free amino acids and odorant compounds valuable as an umami conferring tasting product.
Despite the fact that seaweed biorefineries have started to develop later than terrestrial ones, they offer environmental and economic advantages and show higher potential as a source of nutrients, hydrocolloids, pigments, bioactives and energy, and, based on their complex and exclusive composition, red seaweeds are particularly interesting for their cascading valorization in food, cosmetic and therapeutic applications.
4. Conclusions
The integral utilization of the valuable components from red seaweeds is a technologically feasible approach with environmental and economic advantages. Apart from gelling biopolymers, a number of bioactive compounds with nutritional, functional or biological features can be recovered from red macroalgae using conventional and greener technologies. The challenge is the sequential extraction of these components using emerging technologies for the integral valorization of this type of macroalgae. This opens new attractive alternatives to fulfill the growing market’s demand for natural bioactive compounds of interest in the food, cosmetic, personal care, biomedical or pharmaceutical field.
Author Contributions
All the authors have read, approved, and made substantial contributions to the manuscript. H.D. conceived the study, built the database, made the drafting of the manuscript and the critical revision. M.D.T. made the drafting of the manuscript and the critical revision. N.F.-F. supported the drafting of the manuscript.
Funding
This research was funded by the Ministry of Science, Innovation and Universities of Spain (RTI2018-096376-B-I00). M.D.T. thanks the Spanish Ministry of Science, Innovation and Universities for her postdoctoral grant (IJCI-2016-27535), and N.F.-F. thanks Xunta de Galicia for her postdoctoral grant (ED481B 2018/071).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Cian, R.E.; Drago, S.R.; De Medina, F.S.; Martínez-Augustin, O. Proteins and carbohydrates from red seaweeds: Evidence for beneficial effects on gut function and microbiota. Mar. Drugs 2015, 13, 5358–5383. [Google Scholar] [CrossRef]
- Dhanalakshmi, S.; Jayakumari, S.A. Perspective studies on marine red algae—Hypnea valentiae. Drug Invent. Today 2018, 10, 266–267. [Google Scholar]
- Makkar, H.P.S.; Tran, G.; Heuzé, V.; Giger-Reverdin, S.; Lessire, M.; Lebas, F.; Ankers, P. Seaweeds for livestock diets: A review. Anim. Feed Sci. Technol. 2016, 212, 1–17. [Google Scholar] [CrossRef]
- Sanjeewa, K.K.A.; Lee, W.W.; Jeon, Y.J. Nutrients and bioactive potentials of edible green and red seaweed in Korea. Fish. Aquat. Sci. 2018, 21, 19. [Google Scholar] [CrossRef]
- Ruan, B.F.; Ge, W.W.; Lin, M.X.; Li, Q.S. A review of the components of seaweeds as potential candidates in cancer therapy. Anti-Cancer Agents Med. Chem. 2018, 18, 354–366. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Wang, A.; Lu, Z.; Qin, C.; Hu, J.; Yin, J. Overview on the antiviral activities and mechanisms of marine polysaccharides from seaweeds. Carbohydr. Res. 2017, 453, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Youssouf, L.; Lallemand, L.; Giraud, P.; Soulé, F.; Bhaw-Luximon, A.; Meilhac, O.; D’Hellencourt, C.L.; Jhurry, D.; Couprie, J. Ultrasound-assisted extraction and structural characterization by NMR of alginates and carrageenans from seaweeds. Carbohydr. Polym. 2017, 166, 55–63. [Google Scholar] [CrossRef]
- Cheong, K.L.; Qiu, H.M.; Du, H.; Liu, Y.; Khan, B.M. Oligosaccharides derived from red seaweed: Production, properties, and potential health and cosmetic applications. Molecules 2018, 23, 2451. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Lai, T.K.; Tye, Y.Y.; Rizal, S.; Chong, E.W.N.; Yap, S.W.; Hamzah, A.A.; Nurul Fazita, M.R.; Paridah, M.T. A review of extractions of seaweed hydrocolloids: Properties and applications. Express Polym. Lett. 2018, 12, 296–317. [Google Scholar] [CrossRef]
- Grosso, C.; Valentão, P.; Ferreres, F.; Andrade, P.B. Alternative and efficient extraction methods for marine-derived compounds. Mar. Drugs 2015, 13, 3182–3230. [Google Scholar] [CrossRef] [PubMed]
- Ciko, A.M.; Jokić, S.; Šubarić, D.; Jerković, I. Overview on the application of modern methods for the extraction of bioactive compounds from marine macroalgae. Mar. Drugs 2018, 16, 348. [Google Scholar] [CrossRef] [PubMed]
- Chew, K.W.; Juan, J.C.; Phang, S.M.; Ling, T.C.; Show, P.L. An overview on the development of conventional and alternative extractive methods for the purification of agarose from seaweed. Sep. Sci. Technol. 2018, 53, 467–480. [Google Scholar] [CrossRef]
- Machmudah, S.; Wahyudiono Kanda, H.; Goto, M. Supercritical fluids extraction of valuable compounds from algae: Future perspectives and challenges. Eng. J. 2018, 22, 13–30. [Google Scholar] [CrossRef]
- Xu, S.Y.; Huang, X.; Cheong, K.L. Recent advances in marine algae polysaccharides: Isolation, structure, and activities. Mar. Drugs 2017, 15, 388. [Google Scholar] [CrossRef] [PubMed]
- Pangestuti, R.; Siahaan, E.A.; Kim, S.K. Photoprotective substances derived from marine algae. Mar. Drugs 2018, 16, 399. [Google Scholar] [CrossRef]
- Aryee, A.N.; Agyei, D.; Akanbi, T.O. Recovery and utilization of seaweed pigments in food processing. Curr. Opin. Food Sci. 2018, 19, 113–119. [Google Scholar] [CrossRef]
- Gómez-Ordóñez, E.; Jiménez-Escrig, A.; Rupérez, P. Bioactivity of sulfated polysaccharides from the edible red seaweed Mastocarpus stellatus. Bioact. Carbohydr. Diet. Fibre 2014, 3, 29–40. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Suk, W.Y.; Owolabi, F.A.T.; Haafiz, M.K.M.; Fazita, M.; Deepu, G.; Hasan, M.; Samsul, R. Techno-functional properties of edible packaging films at different polysaccharide blends. J. Phys. Sci. 2019, 30, 23–41. [Google Scholar]
- Torres, M.D.; Kraan, S.; Domínguez, H. Seaweed biorefinery. Rev. Environ. Sci. Bio/Technol. 2019, 18, 335–388. [Google Scholar] [CrossRef]
- Meinita, M.D.N.; Kang, J.Y.; Jeong, G.T.; Koo, H.M.; Park, S.M.; Hong, Y.K. Bioethanol production from the acid hydrolysate of the carrageenophyte Kappaphycus alvarezii (cottonii). J. Appl. Phycol. 2012, 24, 857–862. [Google Scholar] [CrossRef]
- Pomin, V.H. Structural and functional insights into sulfated galactans: A systematic review. Glycoconj. J. 2010, 27, 1–12. [Google Scholar] [CrossRef]
- Schultz-Johansen, M.; Bech, P.K.; Hennessy, R.C.; Glaring, M.A.; Barbeyron, T.; Czjzek, M.; Stougaard, P.A. Novel enzyme portfolio for red algal polysaccharide degradation in the marine bacterium Paraglaciecola hydrolytica S66T encoded in a sizeable polysaccharide utilization locus. Front. Microbiol. 2018, 9, 839. [Google Scholar] [CrossRef]
- Mabeau, S.; Fleurence, J. Seaweed in Food Products: Biochemical and Nutritional Aspects. Trends Food Sci. Technol. 1993, 4, 103–107. [Google Scholar] [CrossRef]
- Rhein-Knudsen, N.; Ale, M.T.; Ajalloueian, F.; Yu, L.; Meyer, A.S. Rheological properties of agar and carrageenan from Ghanaian red seaweeds. Food Hydrocoll. 2017, 63, 50–58. [Google Scholar] [CrossRef]
- Usov, A.I. Polysaccharides of the red algae. Adv. Carbohydr. Chem. Biochem. 2011, 65, 115–217. [Google Scholar]
- Usov, A.I. Structural analysis of red seaweed galactans of agar and carrageenan groups. Food Hydrocoll. 1998, 12, 301–308. [Google Scholar] [CrossRef]
- Lahaye, M. Developments on gelling algal galactans, their structure and physico-chemistry. J. Appl. Phycol. 2001, 13, 173–184. [Google Scholar] [CrossRef]
- Campo, V.L.; Kawano, D.F.; Silva, D.B., Jr.; Carvalho, I. Carrageenans: Biological properties, chemical modifications and structural analysis—A review. Carbohydr. Polym. 2009, 77, 167–180. [Google Scholar] [CrossRef]
- Martínez-Sanz, M.; Gómez-Mascaraque, L.G.; Ballester, A.R.; Martínez-Abad, A.; Brodkorb, A.; López-Rubio, A. Production of unpurified agar-based extracts from red seaweed Gelidium sesquipedale by means of simplified extraction protocols. Algal Res. 2019, 38, 101420. [Google Scholar] [CrossRef]
- Lahaye, M.; Yaphe, W. The Chemical Structure of Agar from Gracilaria compressa (C. Agardh) Greville, G. cervicornis (Turner) J. Agardh, G. damaecornis J. Agardh and G. domingensis Sonder ex Kützing (Gigartinales, Rhodophyta). Botanica Marina 1989, 32, 369–378. [Google Scholar] [CrossRef]
- Armisén, R. World-wide use and importance of Gracilaria. J. Appl. Phycol. 1995, 7, 231–243. [Google Scholar] [CrossRef]
- Lee, W.K.; Lim, Y.Y.; Leow, A.T.C.; Namasivayam, P.; Abdullah, J.O.; Ho, C.L. Factors affecting yield and gelling properties of agar. J. Appl. Phycol. 2017, 29, 1527–1540. [Google Scholar] [CrossRef]
- Craigie, J. Seaweed extract stimuli in plant science and agriculture. J. Appl. Phycol. 2011, 23, 371–393. [Google Scholar] [CrossRef]
- Yun, E.J.; Yu, S.; Kim, K.H. Current knowledge on agarolytic enzymes and the industrial potential of agar-derived sugars. Appl. Microbiol. Biotechnol. 2017, 101, 5581–5589. [Google Scholar] [CrossRef]
- Yarnpakdee, S.; Benjakul, S.; Kingwascharapong, P. Physico-chemical and gel properties of agar from Gracilaria tenuistipitata from the lake of Songkhla, Thailand. Food Hydrocoll. 2015, 51, 217–226. [Google Scholar] [CrossRef]
- Sousa, A.M.M.; Morais, S.; Abreu, M.H.; Pereira, R.; Sousa-Pinto, I.; Cabrita, E.J.; Delerue-Matos, C.; Gonçalves, M.P. Structural, physical, and chemical modifications induced by microwave heating on native agar-like galactans. J. Agric. Food Chem. 2012, 60, 4977–4985. [Google Scholar] [CrossRef]
- Vergara-Rodarte, M.A.; Hernández-Carmona, G.; Rodríguez-Montesinos, Y.E.; Arvizu-Higuera, D.L.; Riosmena-Rodríguez, R.; Murillo-Álvarez, J.I. Seasonal variation of agar from Gracilaria vermiculophylla, effect of alkali treatment time, and stability of its Colagar. J. Appl. Phycol. 2010, 22, 753–759. [Google Scholar] [CrossRef]
- Marinho-Soriano, E.; Bourret, E. Effects of season on the yield and quality of agar from Gracilaria species (Gracilariaceae Rhodophyta). Bioresour. Technol. 2003, 90, 329–333. [Google Scholar] [CrossRef]
- Marinho-Soriano, E.; Bourret, E. Polysaccharides from the red seaweed Gracilaria dura (Gracilariales, Rhodophyta). Bioresour. Technol. 2005, 96, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Romero, J.B.; Villanueva, R.D.; Montaño, M.N.E. Stability of agar in the seaweed Gracilaria eucheumatoides (Gracilariales, Rhodophyta) during postharvest storage. Bioresour. Technol. 2008, 99, 8151–8155. [Google Scholar] [CrossRef]
- Chirapart, A.; Munkit, J.; Lewmanomont, K. Changes in yield and quality of agar from the agarophytes, Gracilaria fisheri and G. tenuistipitata var. liui cultivated in earthen ponds. Kasetsart J. Nat. Sci. 2006, 40, 529–540. [Google Scholar]
- Arvizu-Higuera, D.L.; Rodríguez-Montesinos, Y.E.; Murillo-Alvarez, J.I.; Muñoz-Ochoa, M.; Hernández-Carmona, G. Effect of alkali treatment time and extraction time on agar from Gracilaria vermiculophylla. J. Appl. Phycol. 2008, 20, 515–519. [Google Scholar] [CrossRef]
- Kohajdová, Z.; Karovičová, J.; Gajdošová, Ž. Importance of hydrocolloids in bakery industry. Potravinarstvo 2008, 2, 9–18. [Google Scholar]
- Kazłowski, B.; Pan, C.L.; Ko, Y.T. Separation and quantification of neoagaro- and agaro-oligosaccharide products generated from agarose digestion by β-agarase and HCl in liquid chromatography systems. Carbohydr. Res. 2008, 343, 2443–2450. [Google Scholar] [CrossRef]
- Hong, S.J.; Lee, J.H.; Kim, E.J.; Yang, H.J.; Park, J.S.; Hong, S.K. Toxicological evaluation of neoagarooligosaccharides prepared by enzymatic hydrolysis of agar. Regul. Toxicol. Pharm. 2017, 90, 9–21. [Google Scholar] [CrossRef]
- Freile-Pelegrín, Y.; Robledo, D. Influence of alkali treatment on agar from Gracilaria cornea from Yucatán, México. J. Appl. Phycol. 1997, 9, 533–539. [Google Scholar]
- Freile-Pelegrın, Y.; Murano, E. Agars from three species of Gracilaria (Rhodophyta) from Yucatan Peninsula. Bioresour. Technol. 2005, 96, 295–302. [Google Scholar] [CrossRef]
- Sousa, A.M.M.; Alves, V.D.; Morais, S.; Delerue-Matos, C.; Gonçalves, M.P. Agar extraction from integrated multitrophic aquacultured Gracilaria vermiculophylla: Evaluation of a microwave-assisted process using response surface methodology. Bioresour. Technol. 2010, 101, 3258–3267. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, R.D.; Sousa, A.M.M.; Gonçalves, M.P.; Nilsson, M.; Hilliou, L. Production and properties of agar from the invasive marine alga, Gracilaria vermiculophylla (Gracilariales, Rhodophyta). J. Appl. Phycol. 2010, 22, 211–220. [Google Scholar] [CrossRef]
- Kumar, V.; Fotedar, R. Agar extraction process for Gracilaria cliftonii (Withell, Millar, & Kraft, 1994). Carbohydr. Polym. 2009, 78, 813–819. [Google Scholar]
- Saha, D.; Bhattacharya, S. Hydrocolloids as thickening and gelling agents in food: A critical review. J. Food Sci. Technol. 2010, 47, 587–590. [Google Scholar] [CrossRef]
- Murano, E. Chemical structure and quality of agars from Gracilaria. J. Appl. Phycol. 1995, 7, 245–254. [Google Scholar] [CrossRef]
- Yousefi, M.K.; Islami, H.R.; Filizadeh, Y. Effect of extraction process on agar properties of Gracilaria corticata (Rhodophyta) collected from the persian gulf. Phycologia 2013, 52, 481–487. [Google Scholar] [CrossRef]
- Gallagher, J.A.; Turner, L.B.; Adams, J.M.M.; Barrento, S.; Dyer, P.W.; Theodorou, M.K. Species variation in the effects of dewatering treatment on macroalgae. J. Appl. Phycol. 2018, 30, 2305–2316. [Google Scholar] [CrossRef]
- Rath, J.; Adhikary, S.P. Effect of alkali treatment on the yield and quality of agar from red alga Gracilaria verrucosa (Rhodophyta, Gracilariales) occurring at different salinity gradient of Chilika lake. Indian J. Mar. Sci. 2004, 33, 202–205. [Google Scholar]
- Ahmad, R.; Surif, M.; Ramli, N.; Yahya, N.; Nor, A.R.M.; Bekbayeva, L.A. Preliminary study on the agar content and agar gel strength of Gracilaria manilaensis using different agar extraction processes. World Appl. Sci. J. 2011, 15, 184–188. [Google Scholar]
- González-Leija, J.A.; Hernández-Garibay, E.; Pacheco-Ruíz, I.; Guardado-Puentes, J.; Espinoza-Avalos, J.; López-Vivas, J.M.; Bautista-Alcantar, J. Optimization of the yield and quality of agar from Gracilariopsis lemaneiformis (Gracilariales) from the Gulf of California using an alkaline treatment. J. Appl. Phycol. 2009, 21, 321–326. [Google Scholar] [CrossRef]
- Roleda, M.Y.; Montaño, N.E.; Ganzon-Fortes, E.T.; Villanueva, R.D. Acetic acid pretreatment in agar extraction of Philippine Gelidiella acerosa (Forsskaal) Feldmann et Hamel (Rhodophyta, Gelidiales). Bot. Mar. 1997, 40, 63–69. [Google Scholar] [CrossRef][Green Version]
- Freile-Pelegrín, Y.; Robledo, D.; Pedersén, M.; Bruno, E.; Rönnqvist, J. Effect of dark and salinity treatment in the yield and quality of agar from Gracilaria cornea (Rhodophyceae). Cienc. Mar. 2002, 28, 289–296. [Google Scholar] [CrossRef]
- Li, H.; Huang, J.; Xin, Y.; Zhang, B.; Jin, Y.; Zhang, W. Optimization and scale-up of a new photobleaching agar extraction process from Gracilaria lemaneiformis. J. Appl. Phycol. 2009, 21, 247–254. [Google Scholar] [CrossRef]
- Maciel, J.S.; Chaves, L.S.; Souza, B.W.S.; Teixeira, D.I.A.; Freitas, A.L.P.; Feitosa, J.P.A.; de Paula, R.C.M. Structural characterization of cold extracted fraction of soluble sulfated polysaccharide from red seaweed Gracilaria birdiae. Carbohydr. Polym. 2008, 71, 559–565. [Google Scholar] [CrossRef]
- Al-Alawi, A.; Chitra, P.; Al-Mamun, A.; Al-Marhubi, I.; Rahman, M.S. Characterization of red seaweed extracts treated by water, acid and alkaline solutions. Int. J. Food Eng. 2018, 14, 20170353. [Google Scholar] [CrossRef]
- Kim, M.; Yim, J.H.; Kim, S.-Y.; Kim, H.S.; Lee, W.G.; Kim, S.J.; Kang, P.S.; Lee, C.-K. In vitro inhibition of influenza A virus infection by marine microalga-derived sulfated polysaccharide p-KG03. Antivir. Res. 2012, 93, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Boo, S.M.; Mansilla, A.; Yoon, H.S. Unique repeat and plasmid sequences in the mitochondrial genome of Gracilaria chilensis (Gracilariales, Rhodophyta). Phycologia 2015, 54, 20–23. [Google Scholar] [CrossRef]
- Park, S.Y.; Lee, E.S.; Han, S.H.; Lee, H.Y.; Lee, S. Antioxidative effects of two native berry species, Empetrum nigrum var. japonicum K. Koch and Rubus buergeri Miq., from the Jeju Island of Korea. J. Food Biochem. 2012, 36, 675–682. [Google Scholar]
- Villanueva, R.; Montaño, M.N. Enhancement of carrageenan gel quality in the commercially important tropical seaweed Eucheuma denticulatum (Rhodophyta), with postharvest treatment in low-nutrient conditions. Bot. Mar. 2014, 57, 217–223. [Google Scholar] [CrossRef]
- Shukla, M.K.; Kumar, M.; Prasad, K.; Reddy, C.R.K.; Jha, B. Partial characterization of sulfohydrolase from Gracilaria dura and evaluation of its potential application in improvement of the agar quality. Carbohydr. Polym. 2011, 85, 157–163. [Google Scholar] [CrossRef]
- Navarro, D.A.; Stortz, C.A. Microwave-assisted alkaline modification of red seaweed galactans. Carbohydr. Polym. 2005, 62, 187–191. [Google Scholar] [CrossRef]
- Wu, S.C.; Lin, Y.P.; King, V.A.E. Optimization of intermittent microwave-assisted extraction of sulfated porphyran from Porphyra dentate. Trans. ASABE 2014, 57, 103–110. [Google Scholar]
- Villanueva, R.D.; Rumbaoa, R.O.; Gomez, A.V.; Loquias, M.M.; De La Rosa, A.M.; Montaño, N.E. γ-Irradiation in the extraction of agar from Gelidiella acerosa (Forsskaal) Feldmann et Hamel. Bot. Mar. 1998, 41, 199–202. [Google Scholar] [CrossRef]
- Pereira-Pacheco, F.; Robledo, D.; Rodríguez-Carvajal, L.; Freile-Pelegrín, Y. Optimization of native agar extraction from Hydropuntia cornea from Yucatán, México. Bioresour. Technol. 2007, 98, 1278–1284. [Google Scholar] [CrossRef]
- Villanueva, R.D.; Pagba, C.V.; Montaño, N.E. Optimized agar extraction from Gracilaria eucheumoides Harvey. Bot. Mar. 1997, 40, 369–372. [Google Scholar] [CrossRef]
- Li, H.; Yu, X.; Jin, Y.; Zhang, W.; Liu, Y. Development of an eco-friendly agar extraction technique from the red seaweed Gracilaria lemaneiformis. Bioresour. Technol. 2008, 99, 3301–3305. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.; Siddhanta, A.K.; Ganesan, M.; Ramavat, B.K.; Jha, B.; Ghosh, P.K. Agars of Gelidiella acerosa of west and southeast coasts of India. Bioresour. Technol. 2007, 98, 1907–1915. [Google Scholar] [CrossRef]
- Fidelis, G.P.; Camara, R.B.G.; Queiroz, M.F.; Costa, M.S.S.P.; Santos, P.C.; Rocha, H.A.O.; Costa, L.S. Proteolysis, NaOH and ultrasound-enhanced extraction of anticoagulant and antioxidant sulfated polysaccharides from the edible seaweed, Gracilaria birdiae. Molecules 2014, 19, 18511–18526. [Google Scholar] [CrossRef] [PubMed]
- Coura, C.O.; Souza, R.B.; Rodrigues, J.A.G.; Vanderlei, E.D.S.O.; De Araújo, I.W.F.; Ribeiro, N.A.; Frota, A.F.; Ribeiro, K.A.; Chaves, H.V.; Pereira, K.M.A.; et al. Mechanisms involved in the anti-inflammatory action of a polysulfated fraction from Gracilaria cornea in rats. PLoS ONE 2015, 10, e0119319. [Google Scholar] [CrossRef]
- Chen, H.M.; Zheng, L.; Yan Agaro, X.J. Bioactivity research of oligosaccharides. Food Technol. Biotechnol. 2005, 43, 29–36. [Google Scholar]
- Mazumder, S.; Ghosal, P.K.; Pujol, C.A.; Carlucci, M.J.; Damonte, E.B.; Ray, B. Isolation, chemical investigation and antiviral activity of polysaccharides from Gracilaria corticata (Gracilariaceae, Rhodophyta). Int. J. Biol. Macromol. 2002, 31, 87–95. [Google Scholar] [CrossRef]
- Bhattarai, Y.; Kashyap, P.C. Agaro-oligosaccharides: A new frontier in the fight against coloncancer? Am. J. Physiol.- Gastrointest. Liver Physiol. 2016, 310, G335–G336. [Google Scholar] [CrossRef]
- Enoki, T.; Okuda, S.; Kudo, Y.; Takashima, F.; Sagawa, H.; Kato, I. Oligosaccharides from agar inhibit pro-inflammatory mediator release by inducing heme oxygenase 1. Biosci. Biotechnol. Biochem. 2010, 74, 766–770. [Google Scholar] [CrossRef]
- Enoki, T.; Tominaga, T.; Takashima, F.; Ohnogi, H.; Sagawa, H.; Kato, I. Anti-tumor-promoting activities of agaro-oligosaccharides on two-stage mouse skin carcinogenesis. Biol. Pharm. Bull. 2012, 35, 1145–1149. [Google Scholar] [CrossRef]
- Higashimura, Y.; Naito, Y.; Takagi, T.; Mizushima, K.; Hirai, Y.; Harusato, A.; Ohnogi, H.; Yamaji, R.; Inui, H.; Nakano, Y.; et al. Oligosaccharides from agar inhibit murine intestinal inflammation through the induction of heme oxygenase-1 expression. J. Gastroenterol. 2013, 48, 897–909. [Google Scholar] [CrossRef]
- Jin, M.; Liu, H.; Hou, Y.; Chan, Z.; Di, W.; Li, L.; Zeng, R. Preparation, characterization and alcoholic liver injury protective effects of algal oligosaccharides from Gracilaria lemaneiformis. Food Res. Int. 2017, 100, 186–195. [Google Scholar] [CrossRef]
- Jang, M.K.; Lee, D.G.; Kim, N.Y.; Yu, K.H.; Jang, H.J.; Lee, S.W.; Jang, H.J.; Lee, Y.H. Purification and characterization of neoagarotetraose from hydrolyzed agar. J. Microbiol. Biotechnol. 2009, 19, 1197–1200. [Google Scholar]
- Hehemann, J.H.; Correc, G.; Thomas, F.; Bernard, T.; Barbeyron, T.; Jam, M.; Helbert, W.; Michel, G.; Czjzek, M. Biochemical and structural characterization of the complex agarolytic enzyme system from the marine bacterium Zobellia galactanivorans. J. Biol. Chem. 2012, 28, 30571–30584. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.Y.; Mei, J.F.; Yi, Y.; Chen, J.S.; Ying, G.Q. Advances in study on biological activities of agaro-oligosaccharide. Pharm. Biotechnol. 2008, 15, 493–496. [Google Scholar]
- Kazłowski, B.; Liang, C.; Yuan, P.; Ko, P. Monitoring and preparation of neoagaro- and agaro-oligosaccharide products by high performance anion exchange chromatography systems. Carbohydr. Polym. 2015, 122, 351–358. [Google Scholar] [CrossRef]
- Tripathi, A.; Kathuria, N.; Kumar, A. Elastic and macroporous agarose–599 gelatin cryogels with isotropic and anisotropic porosity for tissue engineering. J. Biomed. Mater. Res. Part A 2009, 90, 680–694. [Google Scholar] [CrossRef]
- Ramana Ramya, J.; Thanigai Arul, K.; Sathiamurthi, P.; Asokan, K.; Narayana Kalkura, S. Novel gamma irradiated agarose-gelatin-hydroxyapatite nanocomposite scaffolds for skin tissue regeneration. Ceram. Int. 2016, 42, 11045–11054. [Google Scholar] [CrossRef]
- Gao, M.; Lu, P.; Bednark, B.; Lynam, D.; Conner, J.M.; Sakamoto, J.; Tuszynski, M.H. Templated agarose scaffolds for the support of motor axon regeneration into sites of complete spinal cord transection. Biomaterials 2013, 34, 1529–1536. [Google Scholar] [CrossRef]
- Zhang, L.M.; Wu, C.X.; Huang, J.Y.; Peng, X.H.; Chen, P.; Tang, S.Q. Synthesis and characterization of a degradable composite agarose/HA hydrogel. Carbohydr. Polym. 2012, 88, 1445–1452. [Google Scholar] [CrossRef]
- Hu, J.; Zhu, Y.; Tong, H.; Shen, X.; Chen, L.; Ran, J. A detailed study of homogeneous agarose/hydroxyapatite nanocomposites for load-bearing bone tissue. Int. J. Biol. Macromol. 2016, 82, 134–143. [Google Scholar] [CrossRef]
- Zou, P.; Lu, X.; Jing, C.; Yuan, Y.; Lu, Y.; Zhang, C.; Meng, L.; Zhao, H.; Li, Y. Low-molecular-weightt polysaccharides from Pyropia yezoensis enhance tolerance of wheat seedlings (Triticum aestivum L.) to salt stress. Front. Plant Sci. 2018, 9, 427. [Google Scholar] [CrossRef] [PubMed]
- Kang, O.L.; Ghani, M.; Hassan, O.; Rahmati, S.; Ramli, N. Novel agaro-oligosaccharide production through enzymatic hydrolysis: Physicochemical properties and antioxidant activities. Food Hydrocoll. 2014, 42, 304–308. [Google Scholar] [CrossRef]
- Fenoradosoa, T.A.; Laroche, C.; Delattre, C.; Dulong, V.; Cerf, D.L.; Picton, L.; Michaud, P. Rheological behavior and non-enzymatic degradation of a sulfated galactan from Halymenia durvillei (Halymeniales, Rhodophyta). Appl. Biochem. Biotechnol. 2012, 167, 1303–1313. [Google Scholar] [CrossRef]
- Zhou, C.; Yu, X.; Zhang, Y.; He, R.; Ma, H. Ultrasonic degradation, purification and analysis of structure and antioxidant activity of polysaccharide from Porphyra yezoensis Udea. Carbohydr. Polym. 2012, 87, 2046–2051. [Google Scholar] [CrossRef]
- Poupard, N.; Badarou, P.; Fasani, F.; Groult, H.; Bridiau, N.; Sannier, F.; Bordenave-Juchereau, S.; Kieda, C.; Piot, J.M.; Grillon, C.; et al. Assessment of heparanase-mediated angiogenesis using microvascular endothelial cells: Identification of λ-Carrageenan derivative as a potent anti angiogenic agent. Mar. Drugs 2017, 15, 134. [Google Scholar] [CrossRef]
- Öğretmen, Ö.Y.; Duyar, H.A. The effect of different extraction methods and pre-treatments on agar yield and physico-chemical properties of Gelidium latifolium (Gelidiaceae, Rhodophyta) from Sinop Peninsula Coast of Black Sea, Turkey. J. Appl. Phycol. 2018, 30, 1355–1360. [Google Scholar] [CrossRef]
- Pereira, L.; Gheda, S.; Ribeiro-Claro, P. Analysis by vibrational spectroscopy of seaweed with potential use in food, pharmaceutical and cosmetic industries. Int. J. Carbohydr. Chem. 2013, 2013, 537202. [Google Scholar] [CrossRef]
- Knudsen, N.R.; Ale, M.T.; Meyer, A.S. Seaweed hydrocolloid production: An update on enzyme assisted extraction and modification technologies. Mar. Drugs 2015, 13, 3340–3359. [Google Scholar] [CrossRef]
- Collén, J.; Cornish, M.L.; Craigie, J.; Ficko-Blean, E.; Hervé, C.; Krueger-Hadfield, S.A.; Leblanc, C.; Michel, G.; Potin, P.; Tonon, T.; et al. Chondrus crispus—A present and historical model organism for red seaweeds. Adv. Bot. Res. 2014, 71, 53–89. [Google Scholar]
- Pereira, L.; Critchley, A.T.; Amado, A.M.; Ribeiro-Claro, P.J.A. A comparative analysis of phycocolloids produced by underutilized versus industrially utilized carrageenophytes (Gigartinales, Rhodophyta). J. Appl. Phycol. 2009, 21, 599–605. [Google Scholar] [CrossRef]
- Blakemore, W.R.; Harpell, A.R. Carrageenan. In Food Stabilisers, Thickeners and Gelling Agents; Imeson, A., Ed.; Blackwell Publishing Ltd.: Oxford, UK, 2009; pp. 73–94. [Google Scholar]
- Pereira, L.; Meireles, F.; Gaspar, R. Population studies and carrageenan properties in eight gigartinales (Rhodophyta) from Iberian Peninsula. In Seaweeds: Agricultural Uses, Biological and Antioxidant Agents; Nova Science Publishers, Inc.: New York, NY, USA, 2014; pp. 115–134. [Google Scholar]
- Vera, J.; Castro, J.; González, A.; Moenne, A. Seaweed polysaccharides and derived oligosaccharides stimulate defense responses and protection against pathogens in plants. Mar. Drugs 2011, 9, 2514–2525. [Google Scholar] [CrossRef]
- Necas, J.; Bartosikova, L. Carrageenan: A review. Vet. Med. 2013, 58, 187–205. [Google Scholar] [CrossRef]
- Ghanbarzadeh, M.; Golmoradizadeh, A.; Homaei, A. Carrageenans and carrageenases: Versatile polysaccharides and promising marine enzymes. Phytochem. Rev. 2018, 17, 535–571. [Google Scholar] [CrossRef]
- Cunha, L.; Grenha, A. Sulfated seaweed polysaccharides as multifunctional materials in drug delivery applications. Mar. Drugs 2016, 14, 42. [Google Scholar] [CrossRef]
- Van de Vijver, M.J.; He, Y.D.; van’t Veer, L.J.; Dai, H.; Hart, A.A.; Voskuil, D.W.; Schreiber, G.J.; Peterse, J.L.; Roberts, C.; Marton, M.J.; et al. A gene-expression signature as a predictor of survival in breast cancer. N. Engl. J. Med. 2002, 347, 1999–2009. [Google Scholar] [CrossRef]
- Cohen, S.M.; Ito, N. A critical review of the toxicological effects of carrageenan and processed Eucheuma seaweed on the gastrointestinal tract. Crit. Rev. Toxicol. 2002, 32, 413–444. [Google Scholar] [CrossRef]
- Weiner, M.L. Food additive carrageenan: Part II: A critical review of carrageenan in vivo safety studies. Crit. Rev. Toxicol. 2014, 44, 244–269. [Google Scholar] [CrossRef]
- Weiner, M.L. Parameters and pitfalls to consider in the conduct of food additive research, Carrageenan as a case study. Crit. Rev. Toxicol. 2016, 87, 31–44. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Borthakur, A.; Dudeja, P.K.; Tobacman, J.K. Carrageenan induces cell cycle arrest in human intestinal epithelial cells in vitro. J. Nutr. 2008, 138, 469–475. [Google Scholar] [CrossRef]
- Chen, M.; Schliep, M.; Willows, R.D.; Cai, Z.L.; Neilan, B.A.; Scheer, H. A red-shifted chlorophyll. Science 2010, 329, 1318–1319. [Google Scholar] [CrossRef]
- Munyaka, P.M.; Sepehri, S.; Ghia, J.E.; Khafipour, E. Carrageenan gum and adherent invasive Escherichia coli in a piglet model of inflammatory bowel disease: Impact on intestinal mucosa-associated microbiota. Front. Microbiol. 2016, 7, 462. [Google Scholar] [CrossRef]
- Shang, Q.; Sun, W.; Shan, X.; Jiang, H.; Cai, C.; Hao, J.; Li, G.; Yu, G. Carrageenan-induced colitis is associated with decreased population of anti-inflammatory bacterium, Akkermansia muciniphila, in the gut microbiota of C57BL/6J mice. Toxicol. Lett. 2017, 279, 87–95. [Google Scholar] [CrossRef]
- Wei, W.; Feng, W.; Xin, G.; Tingting, N.; Zhanghe, Z.; Haimin, C.; Xiaojun, Y. Enhanced effect of κ-carrageenan on TNBS-induced inflammation in mice. Int. Immunopharmacol. 2016, 39, 218–228. [Google Scholar] [CrossRef]
- Wu, W.; Wang, F.; Gao, X.; Niu, T.; Zhu, X.; Yan, X.; Chen, H. Synergistic effect of κ-carrageenan on oxazolone-induced inflammation in BALB/c mice. BMC Gastroenterol. 2016, 16, 41. [Google Scholar] [CrossRef]
- David, S.; Shani Levi, C.; Fahoum, L.; Ungar, Y.; Meyron-Holtz, E.G.; Shpigelman, A.; Lesmes, U. Revisiting the carrageenan controversy: Do we really understand the digestive fate and safety of carrageenan in our foods? Food Funct. 2018, 9, 1344–1352. [Google Scholar] [CrossRef]
- David, S.; Fahoum, L.; Rozen, G.; Shaoul, R.; Shpigelman, A.; Meyron-Holtz, E.G.; Lesmes, U. Reply to the Comment on “Revisiting the carrageenan controversy: Do we really understand the digestive fate and safety of carrageenan in our foods?”. Food Funct. 2019, 10, 1763–1766. [Google Scholar] [CrossRef]
- De Sousa Oliveira Vanderlei, E.; De Araújo, I.W.F.; Quinderé, A.L.G.; Fontes, B.P.; Eloy, Y.R.G.; Rodrigues, J.A.G.; Silva, A.A.R.E.; Chaves, H.V.; Jorge, R.J.B.; De Menezes, D.B.; et al. The involvement of the HO-1 pathway in the anti-inflammatory action of a sulfated polysaccharide isolated from the red seaweed Gracilaria birdiae. Inflamm. Res. 2011, 60, 1121–1130. [Google Scholar] [CrossRef]
- Inic-Kanada, A.; Stein, E.; Stojanovic, M.; Schuerer, N.; Ghasemian, E.; Filipovic, A.; Marinkovic, E.; Kosanovic, D.; Barisani-Asenbauer, T. Effects of iota-carrageenan on ocular Chlamydia trachomatis infection in vitro and in vivo. J. Appl. Phycol. 2018, 30, 2601–2610. [Google Scholar] [CrossRef]
- Talarico, L.B.; Zibetti, R.G.; Faria, P.C.; Scolaro, L.A.; Duarte, M.E.; Noseda, M.D.; Pujol, C.A.; Damonte, E.B. Anti-herpes simplex virus activity of sulfated galactans from the red seaweeds Gymnogongrus griffithsiae and Cryptonemia crenulata. Int. J. Biol. Macromol. 2004, 34, 63–71. [Google Scholar] [CrossRef]
- Talarico, L.B.; Pujol, C.A.; Zibetti, R.G.; Faría, P.C.; Noseda, M.D.; Duarte, M.E.; Damonte, E.B. The antiviral activity of sulfated polysaccharides against dengue virus is dependent on virus serotype and host cell. Antivir. Res. 2005, 66, 103–110. [Google Scholar] [CrossRef]
- Cáceres, P.J.; Carlucci, M.J.; Damonte, E.B.; Matsuhiro, B.; Zúñiga, E.A. Carrageenans from Chilean samples of Stenogramme interrupta (Phyllophoraceae): Structural analysis and biological activity. Phytochemistry 2000, 53, 81–86. [Google Scholar] [CrossRef]
- Chattopadhyay, K.; Ghosh, T.; Pujol, C.A.; Carlucci, M.J.; Damonte, E.B.; Ray, B. Polysaccharides from Gracilaria corticata: Sulfation, chemical characterization and anti-HSV activities. Int. J. Biol. Macromol. 2008, 43, 346–351. [Google Scholar] [CrossRef]
- Yuan, H.; Song, J.; Li, X.; Li, N.; Dai, J. Immunomodulation and antitumor activity of kappa-carrageenan oligosaccharides. Cancer Lett. 2006, 243, 228–234. [Google Scholar] [CrossRef]
- Liu, J.; Hafting, J.; Critchley, A.T.; Banskota, A.H.; Prithiviraj, B. Components of the cultivated red seaweed Chondrus crispus enhance the immune response of Caenorhabditis elegans to Pseudomonas aeruginosa through the pmk-1, daf-2/daf-16, and skn-1 pathways. Appl. Environ. Microbiol. 2013, 79, 7343–7350. [Google Scholar] [CrossRef]
- Souza, M.P.; Vaz, A.F.M.; Costa, T.B.; Cerqueira, M.A.; De Castro, C.M.M.B.; Vicente, A.A.; Carneiro-da-Cunha, M.G. Construction of a Biocompatible and Antioxidant Multilayer Coating by Layer-by-Layer Assembly of κ-Carrageenan and Quercetin Nanoparticles. Food Bioprocess Technol. 2018, 11, 1050–1060. [Google Scholar] [CrossRef]
- Sun, L.; Wang, S.; Gong, X.; Zhao, M.; Fu, X.; Wang, L. Isolation, purification and characteristics of R-phycoerythrin from a marine macroalga Heterosiphonia japonica. Protein Expr. Purif. 2010, 64, 146–154. [Google Scholar] [CrossRef]
- De Jesus Raposo, M.F.; De Morais, A.M.B.; De Morais, R.M.S.C. Marine polysaccharides from algae with potential biomedical applications. Mar. Drugs 2015, 13, 2967–3028. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, L.; Reis, R.P. Cultivation of the red algae Kappaphycus alvarezii in Brazil and its pharmacological potential. Braz. J. Pharmacogn. 2012, 22, 748–752. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Saurabh, C.K.; Tye, Y.Y.; Lai, T.K.; Easa, A.M.; Rosamah, E.; Fazita, M.R.N.; Syakir, M.I.; Adnan, A.S.; Fizree, H.M.; et al. Seaweed based sustainable films and composites for food and pharmaceutical applications: A review. Renew. Sustain. Energy Rev. 2017, 77, 353–362. [Google Scholar] [CrossRef]
- Morris, C.E. How does fertility of the substrate affect intra-specific competition? Evidence and synthesis from self-thinning. Ecol. Res. 2003, 18, 287–305. [Google Scholar] [CrossRef]
- Briones, A.V.; Sato, T. Encapsulation of glucose oxidase (GOD) in polyelectrolyte complexes of chitosan-carrageenan. React. Funct. Polym. 2010, 70, 19–27. [Google Scholar] [CrossRef]
- Zhang, W.T.; Yue, C.; Huang, Q.W.; Yuan, K.; Yan, A.J.; Shi, S. Contents of eight saccharides in unprocessed and processed Rehmannia glutinosa and content changes at different processing time points. Chin. Tradit. Herb. Drug. 2016, 47, 1132–1136. [Google Scholar]
- Dafe, A.; Etemadi, H.; Zarredar, H.; Mahdavinia, G.R. Development of novel carboxymethyl cellulose/k-carrageenan blends as an enteric delivery vehicle for probiotic bacteria. Int. J. Biol. Macromol. 2017, 97, 299–307. [Google Scholar] [CrossRef]
- Li, L.; Ni, R.; Shao, Y.; Mao, S. Carrageenan and its applications in drug delivery. Carbohydr. Polym. 2014, 103, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sahiner, N.; Sagbas, S.; Yllmaz, S. Microgels derived from different forms of carrageenans, kappa, iota, and lambda for biomedical applications. MRS Adv. 2017, 2, 2521–2527. [Google Scholar] [CrossRef]
- Gonçalves, A.L.; Pires, J.C.M.; Simões, M. Biotechnological potential of Synechocystis salina co-cultures with selected microalgae and cyanobacteria: Nutrients removal, biomass and lipid production. Bioresour. Technol. 2016, 200, 279–286. [Google Scholar] [CrossRef]
- Ghannam, A.; Abbas, A.; Alek, H.; Al-Waari, Z.; Al-Ktaifani, M. Enhancement of local plant immunity against tobacco mosaic virus infection after treatment with sulphated-carrageenan from redalga (Hypnea musciformis). Physiol. Mol. Plant Pathol. 2013, 84, 19–27. [Google Scholar] [CrossRef]
- Mercier, L.; Lafitte, C.; Borderies, G.; Briand, X.; Esquerré-Tugayé, M.T.; Fournier, J. The algal polysaccharide carrageenans can act as an elicitor of plant defence. New Phytol. 2001, 149, 43–51. [Google Scholar] [CrossRef]
- Nagorskaya, V.P.; Reunov, A.V.; Lapshina, L.A.; Ermak, I.M.; Barabanova, A.O. Inhibitory effect of κ/β-carrageenan from red alga Tichocarpus crinitus on the development of a potato virus X infection in leaves of Datura stramonium L. Biol. Bull. 2010, 37, 653–658. [Google Scholar] [CrossRef]
- Sangha, J.S.; Ravichandran, S.; Prithiviraj, K.; Critchley, A.T.; Prithiviraj, B. Sulfated macroalgal polysaccharides λ-carrageenan and ι-carrageenan differentially alter Arabidopsis thaliana resistance to Sclerotinia sclerotiorum. Physiol. Mol. Plant Pathol. 2010, 75, 38–45. [Google Scholar] [CrossRef]
- Sangha, J.S.; Khan, W.; Ji, X.; Zhang, J.; Mills, A.A.S.; Critchley, A.T.; Prithiviraj, B. Carrageenans, sulphated polysaccharides of red seaweeds, differentially affect Arabidopsis thaliana resistance to Trichoplusia ni (Cabbage looper). PLoS ONE 2011, 6, e26834. [Google Scholar] [CrossRef]
- Sangha, J.S.; Kandasamy, S.; Khan, W.; Bahia, N.S.; Singh, R.P.; Critchley, A.T.; Prithiviraj, B. λ-Carrageenan suppresses tomato chlorotic dwarf viroid (TCDVd) replication and symptom expression in tomatoes. Mar. Drugs 2015, 13, 2875–2889. [Google Scholar] [CrossRef] [PubMed]
- Shukla, P.S.; Borza, T.; Critchley, A.T.; Prithiviraj, B. Carrageenans from red seaweeds as promoters of growth and elicitors of defense response in plants. Front. Mar. Sci. 2016, 3, 81. [Google Scholar] [CrossRef]
- Vera, J.; Castro, J.; Contreras, R.A.; González, A.; Moenne, A. Oligocarrageenans induce a long-term and broad-range protection against pathogens in tobacco plants (var. Xanthi). Physiol. Mol. Plant Pathol. 2012, 79, 31–39. [Google Scholar] [CrossRef]
- Bi, Y.; Hu, Y.; Zhou, Z.G. Genetic variation of Laminaria japonica (Phaeophyta) populations in China as revealed by RAPD markers. Acta Oceanol. Sin. 2011, 30, 103–112. [Google Scholar] [CrossRef]
- Azevedo, G.; Torres, M.D.; Sousa-Pinto, I.; Hilliou, L. Effect of pre-extraction alkali treatment on the chemical structure and gelling properties of extracted hybrid carrageenan from Chondrus crispus and Ahnfeltiopsis devoniensis. Food Hydrocoll. 2015, 50, 150–158. [Google Scholar] [CrossRef]
- Hilliou, L.; Larotonda, F.D.S.; Abreu, P.; Ramos, A.M.; Sereno, A.M.; Goncalves, M.P. Effect of extraction parameters on the chemical structure and gel properties of κ/ι-hybrid carrageenans obtained from Mastocarpus stellatus. Biomol. Eng. 2006, 23, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Boulho, R.; Marty, C.; Freile-Pelegrín, Y.; Robledo, D.; Bourgougnon, N.; Bedoux, G. Antiherpetic (HSV-1) activity of carrageenans from the red seaweed Solieria chordalis (Rhodophyta, Gigartinales) extracted by microwave-assisted extraction (MAE). J. Appl. Phycol. 2017, 29, 2219–2228. [Google Scholar] [CrossRef]
- Rafiquzzaman, S.M.; Ahmed, R.; Lee, J.M.; Noh, G.; Jo, G.A.; Kong, I.S. Improved methods for isolation of carrageenan from Hypnea musciformis and its antioxidant activity. J. Appl. Phycol. 2016, 28, 1265–1274. [Google Scholar] [CrossRef]
- Vázquez-Delfín, E.; Robledo, D.; Freile-Pelegrín, Y. Microwave-assisted extraction of the Carrageenan from Hypnea musciformis (Cystocloniaceae, Rhodophyta). J. Appl. Phycol. 2014, 26, 901–907. [Google Scholar] [CrossRef]
- Estevez, J.M.; Ciancia, M.; Cerezo, A.S. The system of low-molecular-weight carrageenans and agaroids from the room-temperature-extracted fraction of Kappaphycus alvarezii. Carbohydr. Res. 2000, 325, 287–299. [Google Scholar] [CrossRef]
- Almutairi, F.M.; Adams, G.G.; Kök, M.S.; Lawson, C.J.; Gahler, R.; Wood, S.; Foster, T.J.; Rowe, A.J.; Harding, S.E. An analytical ultracentrifugation based study on the conformation of lambda carrageenan in aqueous solution. Carbohydr. Polym. 2013, 97, 203–209. [Google Scholar] [CrossRef]
- Tang, F.; Chen, F.; Li, F. Preparation and potential in vivo anti-influenza virus activity of low molecular-weight k-carrageenans and their derivatives. J. Appl. Polym. Sci. 2013, 127, 2110–2115. [Google Scholar] [CrossRef]
- Ratnawati, R.; Prasetyaningrum, A.; Wardhani, D.H. Kinetics and thermodynamics of ultrasound-assisted depolymerization of κ-carrageenan. Bull. Chem. React. Eng. Catal. 2016, 11, 48–58. [Google Scholar] [CrossRef]
- Sokolova, R.V.; Ermakova, S.P.; Awada, S.M.; Zvyagintseva, T.N.; Kanaan, H.M. Composition, structural characteristics, and antitumor properties of polysaccharides from the brown algae Dictyopteris polypodioides and Sargassum sp. Chem. Nat. Compd. 2011, 47, 329–334. [Google Scholar] [CrossRef]
- Hamias, R.; Wolak, T.; Huleihel, M.; Paran, E.; Levy-Ontman, O. Red alga polysaccharides attenuate angiotensin II-induced inflammation in coronary endothelial cells. Biochem. Biophys. Res. Commun. 2018, 500, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Kalitnik, A.A.; Marcov, P.A.; Anastyuk, S.D.; Barabanova, A.O.B.; Glazunov, V.P.; Popov, S.V.; Ovodov, Y.S.; Yermak, I.M. Gelling polysaccharide from Chondrus armatus and its oligosaccharides: The structural peculiarities and anti-inflammatory activity. Carbohydr. Polym. 2015, 115, 768–775. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.-X.; Guan, H.-S. The antiviral activities and mechanisms of marine polysaccharides: An overview. Marine Drugs 2012, 10, 2795–2816. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Ogamo, A.; Saito, T.; Watanabe, J.; Uchiyama, H.; Nakagawa, Y. Preparation and anti-HIV activity of low-molecular-weight carrageenans and their sulfated derivatives. Carbohydr. Polym. 1997, 32, 51–55. [Google Scholar] [CrossRef]
- Yamada, S.; Kosugi, I.; Katano, H.; Fukui, Y.; Kawasaki, H.; Arai, Y.; Kurane, I.; Inoue, N. In vivo imaging assay for the convenient evaluation of antiviral compounds against cytomegalovirus in mice. Antivir. Res. 2010, 88, 45–52. [Google Scholar] [CrossRef]
- Abad, L.V.; Kudo, H.; Saiki, S.; Nagasawa, N.; Tamada, M.; Katsumura, Y.; Aranilla, C.T.; Relleve, L.S.; De La Rosa, A.M. Radiation degradation studies of carrageenans. Carbohydr. Polym. 2009, 78, 100–106. [Google Scholar] [CrossRef]
- Zhou, G.; Sun, Y.; Xin, H.; Zhang, Y.; Li, Z.; Xu, Z. In vivo antitumor and immunomodulation activities of different molecular weight lambda-carrageenans from Chondrus ocellatus. Pharmacol. Res. 2004, 50, 47–53. [Google Scholar] [CrossRef]
- Raman, M.; Doble, M. κ-Carrageenan from marine red algae, Kappaphycus alvarezii—A functional food to prevent colon carcinogenesis. J. Funct. Foods 2015, 15, 354–364. [Google Scholar] [CrossRef]
- Zhou, G.; Xin, H.; Sheng, W.; Sun, Y.; Li, Z.; Xu, Z. In vivo growth-inhibition of S180 tumor by mixture of 5-Fu and low molecular lambda-carrageenan from Chondrus ocellatus. Pharmacol. Res. 2005, 51, 153–157. [Google Scholar] [CrossRef]
- Zhou, G.; Sheng, W.; Yao, W.; Wang, C. Effect of low molecular λ-carrageenan from Chondrus ocellatus on antitumor H-22 activity of 5-Fu. Pharmacol. Res. 2006, 53, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Lemonnier-Le Penhuizic, C.; Chatelet, C.; Kloareg, B.; Potin, P. Carrageenan oligosaccharides enhance stress-induced microspore embryogenesis in Brassica oleracea var. italica. Plant Sci. 2001, 160, 1211–1220. [Google Scholar] [CrossRef]
- Muñoz, A.M.; Ponce, J.C.; Araya, J.V. Method to Stimulate Carbon Fixation in Plants with an Aqueous Solution of Oligo-Carrageenans Selected from Kappa1, Kappa2, Lambda or Iota. U.S. Patent 12/911,790, 5 May 2011. [Google Scholar]
- Castro, J.; Vera, J.; González, A.; Moenne, A. Oligo-carrageenans stimulate growth by enhancing photosynthesis, basal metabolism, and cell cycle in tobacco plants (var. Burley). J. Plant Growth Regul. 2012, 31, 173–185. [Google Scholar] [CrossRef]
- Saucedo, S.; Contreras, R.A.; Moenne, A. Oligo-carrageenan kappa increases C, N and S assimilation, auxin and gibberellin contents, and growth in Pinus radiata trees. J. For. Res. 2015, 26, 635–640. [Google Scholar] [CrossRef]
- Abad, L.V.; Nasimova, I.R.; Relleve, L.S.; Aranilla, C.T.; De La Rosa, A.M.; Shibayama, M. Dynamic light scattering studies of irradiated kappa carrageenan. Int. J. Biol. Macromol. 2004, 34, 81–88. [Google Scholar] [CrossRef]
- González, A.; Castro, J.; Vera, J.; Moenne, A. Seaweed oligosaccharides stimulate plant growth by enhancing carbon and nitrogen assimilation, basal metabolism, and cell division. J. Plant Growth Regul. 2013, 32, 443–448. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, B.; Wu, Y.; Liu, Y.; Gu, X.; Zhang, H.; Wang, C.; Cao, H.; Huang, L.; Wang, Z. Structural characterization and antioxidant activities of κ-carrageenan oligosaccharides degraded by different methods. Food Chem. 2015, 178, 311–318. [Google Scholar] [CrossRef]
- Prasetyaningrum, A.; Jos, B.; Dharmawan, Y.; Octaviani, R.V.; Ratnawati, R. Chemical and spectral characterization of the ozonation products of κ-carrageenan. MATEC Web Conf. 2018, 156, 05006. [Google Scholar] [CrossRef][Green Version]
- Kalitnik, A.A.; Byankina Barabanova, A.O.; Nagorskaya, V.P.; Reunov, A.V.; Glazunov, V.P.; Solov’eva, T.F.; Yermak, I.M. Low molecular weight derivatives of different carrageenan types and their antiviral activity. J. Appl. Phycol. 2013, 25, 65–72. [Google Scholar] [CrossRef]
- Karlsson, A.; Singh, S.K. Acid hydrolysis of sulphated polysaccharides. Desulphation and the effect on molecular mass. Carbohydr. Polym. 1999, 38, 7–15. [Google Scholar] [CrossRef]
- Yang, B.; Yu, G.; Zhao, X.; Jiao, G.; Ren, S.; Chai, W. Mechanism of mild acid hydrolysis of galactan polysaccharides with highly ordered disaccharide repeats leading to a complete series of exclusively odd-numbered oligosaccharides. FEBS J. 2009, 276, 2125–2137. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, P.S.; Saxena, A. Bacterial carrageenases: An overview of production and biotechnological applications. 3 Biotech 2016, 6, 146. [Google Scholar] [CrossRef]
- Lii, C.Y.; Chen, C.H.; Yeh, A.I.; Lai, V.M.F. Preliminary study on the degradation kinetics of agarose and carrageenans by ultrasound. Food Hydrocoll. 1999, 13, 477–481. [Google Scholar] [CrossRef]
- Hu, X.; Jiang, X.; Aubree, E.; Boulenguer, P.; Critchley, A.T. Preparation and in vivo antitumor activity of κ-carrageenan oligosaccharides. Pharm. Biol. 2006, 44, 646–650. [Google Scholar] [CrossRef]
- Abad, L.V.; Relleve, L.S.; Racadio, C.D.T.; Aranilla, C.T.; De la Rosa, A.M. Antioxidant activity potential of gamma irradiated carrageenan. Appl. Radiat. Isot. 2013, 79, 73–79. [Google Scholar] [CrossRef]
- Gereniu, C.R.N.; Saravana, P.S.; Chun, B.S. Recovery of carrageenan from Solomon Islands red seaweed using ionic liquid-assisted subcritical water extraction. Sep. Purif. Technol. 2018, 196, 309–317. [Google Scholar] [CrossRef]
- Choi, E.M.; Kim, G.H.; Lee, Y.S. Atractylodes japonica root extract protects osteoblastic MC3T3-E1 cells against hydrogen peroxide-induced inhibition of osteoblastic differentiation. Phytother. Res. 2009, 23, 1537–1542. [Google Scholar] [CrossRef]
- Fleurence, J. The enzymatic degradation of algal cell walls: A useful approach for improving protein accessibility? J. Appl. Phycol. 1999, 11, 313–314. [Google Scholar] [CrossRef]
- Vieira, E.F.; Soares, C.; Machado, S.; Correia, M.; Ramalhosa, M.J.; Oliva-Teles, M.T.; Paula Carvalho, A.; Domingues, V.F.; Antunes, F.; Oliveira, T.A.C.; et al. Seaweeds from the Portuguese coast as a source of proteinaceous material: Total and free amino acid composition profile. Food Chem. 2018, 269, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Galland-Irmouli, A.V.; Fleurence, J.; Lamghari, R.; Luçon, M.; Rouxel, C.; Barbaroux, O.; Bronowicki, J.P.; Villaume, C.; Guéant, J.L. Nutritional value of proteins from edible seaweed Palmaria palmata (Dulse). J. Nutr. Biochem. 1999, 10, 353–359. [Google Scholar] [CrossRef]
- Wang, T.; Jónsdóttir, R.; Kristinsson, H.G.; Hreggvidsson, G.O.; Jónsson, J.Ó.; Thorkelsson, G.; Ólafsdóttire, G. Enzyme-enhanced extraction of antioxidant ingredients from red algae Palmaria palmata. LWT Food Sci. Technol. 2010, 43, 1387–1393. [Google Scholar] [CrossRef]
- Paiva, L.; Lima, E.; Patarra, R.F.; Neto, A.I.; Baptista, J. Edible Azorean macroalgae as source of rich nutrients with impact on human health. Food Chem. 2014, 164, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Guiry, M.D. The Seaweed Site: Information on Marine Algae. 2014. Available online: http://www.seaweed.ie/ (accessed on 26 April 2019).
- Misurcová, L. Chemical Composition of Seaweeds. In Handbook of Marine Macroalgae: Biotechnology and Applied Phycology; Kim, S.K., Ed.; John Wiley & Sons: New York, NY, USA, 2012; p. 567. [Google Scholar]
- Roman, B.L.; Pham, V.; Lawson, N.D.; Kulik, M.; Childs, S.; Lekven, A.C.; Garrity, D.M.; Moon, R.T.; Fishman, M.C.; Lechleider, R.J.; et al. Disruption of acvrl1 increases endothelial cell number in zebrafish cranial vessels. Development 2002, 129, 3009–3019. [Google Scholar]
- Sekar, S.; Chandramohan, M. Phycobiliproteins as a commodity: Trends in applied research, patents and commercialization. J. Appl. Phycol. 2008, 20, 113–136. [Google Scholar] [CrossRef]
- Fernández-Rojas, B.; Hernández-Juárez, J.; Pedraza-Chaverri, J. Nutraceutical properties of phycocyanin. J. Funct. Foods 2014, 11, 375–392. [Google Scholar] [CrossRef]
- Chandra, R.; Parra-Saldivar, R.; Hafiz, M.N.I. Phycobiliproteins: A novel green tool from marine origin blue-green algae and red algae—A review. Pept. Lett. 2016, 23, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Manirafasha, E.; Ndikubwimana, T.; Zeng, X.; Lu, Y.; Jing, K. Phycobiliprotein: Potential microalgae derived pharmaceutical and biological reagent. Biochem. Eng. J. 2016, 109, 282–296. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, Y.; Yin, Q.; Liu, G.; Liu, H.; Huang, Y.; Li, B. Phycocyanin: A potential drug for cancer treatment. J. Cancer 2017, 8, 3416–3429. [Google Scholar] [CrossRef]
- Hao, S.; Yan, Y.; Li, S.; Zhao, L.; Zhang, C.; Liu, L.; Wang, C. The in vitro anti-tumor activity of phycocyanin against non-small cell lung cancer cells. Mar. Drugs 2018, 16, 178. [Google Scholar] [CrossRef] [PubMed]
- Mittal, R.; Raghavarao, K.S.M.S. Extraction of R-Phycoerythrin from marine macro-algae, Gelidium pusillum, employing consortia of enzymes. Algal Res. 2018, 34, 1–11. [Google Scholar] [CrossRef]
- Gantar, M.; Dhandayuthapani, S.; Rathinavelu, A. Phycocyanin induces apoptosis and enhances the effect of topotecan on prostate cell line LNCaP. J. Med. Food 2012, 15, 1091–1095. [Google Scholar] [CrossRef]
- Huang, B.; Wang, G.C.; Zeng, C.K.; Li, Z.G. The experimental research of R- phycoerythrin subunits on cancer treatment—A new photosensitizer in PDT. Cancer Biother. Radiopharm. 2002, 17, 35–42. [Google Scholar]
- Munier, M.; Dumay, J.; Morançais, M.; Jaouen, P.; Fleurence, J. Variation in the biochemical composition of the edible seaweed Grateloupia turuturu Yamada harvested from two sampling sites on the brittany coast (France): The influence of storage method on the extraction of the seaweed pigment r-phycoerythrin. J. Chem. 2013, 2013, 568548. [Google Scholar] [CrossRef]
- Pina, A.L.; Costa, A.R.; Lage-Yusty, M.A.; López-Hernández, J. An evaluation of edible red seaweed (Chondrus crispus) components and their modification during the cooking process. LWT Food Sci. Technol. 2014, 56, 175–180. [Google Scholar] [CrossRef]
- Niu, J.F.; Wang, G.C.; Zhou, B.C.; Lin, X.Z.; Chen, C.S. Purification of R-phycoerythrin from Porphyra haitanensis (Bangiales, Rhodophyta) using expanded-bed absorption. J. Phycol. 2007, 43, 1339–1347. [Google Scholar] [CrossRef]
- Nguyen, H.P.T.; Morançais, M.; Fleurence, J.; Dumay, J. Mastocarpus stellatus as a source of R-phycoerythrin: Optimization of enzyme assisted extraction using response surface methodology. J. Appl. Phycol. 2017, 29, 1563–1570. [Google Scholar] [CrossRef]
- Wang, C.; Kim, J.H.; Kim, S.W. Synthetic biol-ogy and metabolic engineering for marine carotenoids: New opportunities and future prospects. Mar. Drugs 2014, 12, 4810–4832. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, N.; Suresh, V.; Thangam, R.; Kurinjimalar, C.; Kavitha, G.; Murugan, P.; Rengasamy, R. Isolation and characterization of macromolecular protein R-Phycoerythrin from Portieria hornemannii. Int. J. Biol. Macromol. 2013, 55, 150–160. [Google Scholar] [CrossRef]
- Niu, J.F.; Wang, G.C.; Tseng, C.K. Method for large-scale isolation and purification of R-phycoerythrin from red alga Polysiphonia urceolata Grev. Protein Expr. Purif. 2006, 49, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.-F.; Wang, G.-C.; Lin, X.-Z.; Cheng, Z. Large-scale recovery of C-phycocyanin from Spirulina platensis using expanded bed adsorption chromatography. J. Chromatogr. B 2007, 850, 267–276. [Google Scholar] [CrossRef]
- Galland-Irmouli, A.V.; Pons, L.; Luçon, M.; Villaume, C.; Mrabet, N.T.; Guéant, J.L.; Fleurence, J. One-step purification of R-phycoerythrin from the red macroalga Palmaria palmata using preparative polyacrylamide gel electrophoresis. J. Chromatogr. B Biomed. Sci. Appl. 2000, 739, 117–123. [Google Scholar] [CrossRef]
- Amano, H.; Noda, H. Proteins of protoplasts from red alga Porphyra yezoensis. Nippon Suisan Gakkaishi 1990, 56, 1859–1864. [Google Scholar] [CrossRef]
- Laohakunjit, N.; Selamassakul, O.; Kerdchoechuen, O. Seafood-like flavour obtained from the enzymatic hydrolysis of the protein by-products of seaweed Gracilaria sp. Food Chem. 2014, 158, 162–170. [Google Scholar] [CrossRef]
- Rodrigues, D.; Sousa, S.; Silva, A.; Amorim, M.; Pereira, L.; Rocha-Santos, T.A.P.; Gomes, A.M.P.; Duarte, A.C.; Freitas, A.C. Impact of enzyme- and ultrasound-assisted extraction methods on biological properties of red, brown, and green seaweeds from the Central West Coast of Portugal. J. Agric. Food Chem. 2015, 63, 3177–3188. [Google Scholar] [CrossRef]
- Hardouin, K.; Burlot, A.S.; Umami, A.; Tanniou, A.; Stiger-Pouvreau, V.; Widowati, I.; Bedoux, G.; Bourgougnon, N. Biochemical and antiviral activities of enzymatic hydrolysates from different invasive French seaweeds. J. Appl. Phycol. 2014, 26, 1029–1042. [Google Scholar] [CrossRef]
- Fleurence, J.; Massiani, L.; Guyader, O.; Mabeau, S. Use of enzymatic cell wall degradation for improvement of protein extraction from Chondrus crispus, Gracilaria verrucosa and Palmaria palmata. J. Appl. Phycol. 1995, 7, 393–397. [Google Scholar] [CrossRef]
- Denis, C.; Le Jeune, H.; Gaudin, P.; Fleurence, J. An evaluation of methods for quantifying the enzymatic degradation of red seaweed Grateloupia turuturu. J. Appl. Phycol. 2009, 21, 153–159. [Google Scholar] [CrossRef]
- Harrysson, H.; Hayes, M.; Eimer, F.; Carlsson, N.G.; Toth, G.B.; Undeland, I. Production of protein extracts from Swedish red, green, and brown seaweeds, Porphyra umbilicalis Kützing, Ulva lactuca Linnaeus, and Saccharina latissima (Linnaeus) J. V. Lamouroux using three different methods. J. Appl. Phycol. 2018, 30, 3565–3580. [Google Scholar] [CrossRef]
- Suwal, S.; Perreault, V.; Marciniak, A.; Tamigneaux, É.; Deslandes, É.; Bazinet, L.; Jacques, H.; Beaulieu, L.; Doyen, A. Effects of high hydrostatic pressure and polysaccharidases on the extraction of antioxidant compounds from red macroalgae, Palmaria palmata and Solieria chordalis. J. Food Eng. 2019, 252, 53–59. [Google Scholar] [CrossRef]
- Fleurence, R.L.; Iglesias, C.P.; Torgerson, D.J. Economic evaluations of interventions for the prevention and treatment of osteoporosis: A structured review of the literature. Osteoporos Int. 2005, 17, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Dumay, J.; Clément, N.; Morançais, M.; Fleurence, J. Optimization of hydrolysis conditions of Palmaria palmata to enhance R-phycoerythrin extraction. Bioresour. Technol. 2013, 131, 21–27. [Google Scholar] [CrossRef]
- Fitzgerald, C.; Gallagher, E.; O’Connor, P.; Prieto, J.; Mora-Soler, L.; Grealy, M.; Hayes, M. Development of a seaweed derived platelet activating factor acetylhydrolase (PAF-AH) inhibitory hydrolysate, synthesis of inhibitory peptides and assessment of their toxicity using the Zebrafish larvae assay. Peptides 2013, 50, 119–124. [Google Scholar] [CrossRef]
- Harnedy, P.A.; O’Keeffe, M.B.; FitzGerald, R.J. Fractionation and identification of antioxidant peptides from an enzymatically hydrolysed Palmaria palmata protein isolate. Food Res. Int. 2017, 100, 416–422. [Google Scholar] [CrossRef]
- Mensi, F.; Ksouri, J.; Seale, E. A statistical approach for optimization of R-phycoerythrin extraction from the red algae Gracilaria verrucosa by enzymatic hydrolysis using central composite design and desirability function. J. Appl. Phycol. 2012, 24, 915–926. [Google Scholar] [CrossRef]
- Mensi, F. Agar yield from R-phycoerythrin extraction by-product of the red alga Gracilaria verrucosa. J. Appl. Phycol. 2019, 31, 741–751. [Google Scholar] [CrossRef]
- Lee, D.; Nishizawa, M.; Shimizu, Y.; Saeki, H. Anti-inflammatory effects of dulse Palmaria palmata resulting from the simultaneous water-extraction of phycobiliproteins and chlorophyll a. Food Res. Int. 2017, 100, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Mittal, R.; Tavanandi, H.A.; Mantri, V.A.; Raghavarao, K.S.M.S. Ultrasound assisted methods for enhanced extraction of phycobiliproteins from marine macro-algae, Gelidium pusillum. Ultrason. Sonochem. 2017, 38, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Corey, P.; Kim, J.K.; Garbary, D.J.; Prithiviraj, B.; Duston, J. Bioremediation potential of Chondrus crispus (Basin Head) and Palmaria palmata: Effect of temperature and high nitrate on nutrient removal. J. Appl. Phycol. 2012, 24, 441–448. [Google Scholar] [CrossRef]
- Gereniu, C.R.N.; Saravana, P.S.; Getachew, A.T.; Chun, B.S. Characteristics of functional materials recovered from Solomon Islands red seaweed (Kappaphycus alvarezii) using pressurized hot water extraction. J. Appl. Phycol. 2017, 29, 1609–1621. [Google Scholar] [CrossRef]
- Pangestuti, R.; Getachew, A.T.; Siahaan, E.A.; Chun, B.-S. Characterization of functional materials derived from tropical red seaweed Hypnea musciformis produced by subcritical water extraction systems. J. Appl. Phycol. 2019. [Google Scholar] [CrossRef]
- Wang, F.; Guo, X.Y.; Zhang, D.N.; Wu, Y.; Wu, T.; Chen, Z.G. Ultrasound-assisted extraction and purification of taurine from the red algae Porphyra yezoensis. Ultrason. Sonochem. 2015, 24, 36–42. [Google Scholar] [CrossRef]
- Le Guillard, C.; Dumay, J.; Donnay-Moreno, C.; Bruzac, S.; Ragon, J.-Y.; Fleurence, J.; Bergé, J.-P. Ultrasound-assisted extraction of R-phycoerythrin from Grateloupia turuturu with and without enzyme addition. Algal Res. 2015, 12, 522–528. [Google Scholar] [CrossRef]
- Caltagirone, C.; Ferrannini, L.; Marchionni, N.; Nappi, G.; Scapagnini, G.; Trabucchi, M. The potential protective effect of tramiprosate (homotaurine) against Alzheimer’s disease: A review. Aging Clin. Exp. Res. 2012, 24, 580–587. [Google Scholar]
- Khotimchenko, S.V. Fatty acids of species in the genus Codium. Botanica Marina 2003, 46, 456–460. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed; functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Kumari, S.; Vardhana, S.; Cammer, M.; Curado, S.; Santos, L.; Sheetz, M.P.; Dustin, M.L. T lymphocyte myosin IIA is required for Maturation of the immunological synapse. Front. Immunol. 2012, 3, 230. [Google Scholar] [CrossRef]
- Pereira, L.C.C.; da Silva, N.I.S.; da Costa, R.M.; Asp, N.E.; da Costa, K.G.; Vila Concejo, A. Seasonal changes in oceanographic processes at an equatorial macrotidal beach in northen Brazil. Cont. Shelf Res. 2012, 43, 95–106. [Google Scholar] [CrossRef]
- Kumari, P.; Bijo, A.J.; Mantri, V.A.; Reddy, C.R.K.; Jha, B. Fatty acid profiling of tropical marine macroalgae: An analysis from chemotaxonomic and nutritional perspectives. Phytochemistry 2013, 86, 44–56. [Google Scholar] [CrossRef]
- Chen, C.Y.; Chou, H.N. Screening of red algae filaments as a potential alternative source of eicosapentaenoic acid. Mar. Biotechnol. 2002, 4, 189–192. [Google Scholar] [CrossRef]
- Kumari, P.; Kumar, M.; Gupta, V.; Reddy, C.R.K.; Jha, B. Tropical marine macroalgae as potential sources of nutritionally important PUFAs. Food Chem. 2010, 120, 749–757. [Google Scholar] [CrossRef]
- Schmid, M.; Kraft, L.G.K.; van der Loos, L.M.; Kraft, G.T.; Virtue, P.; Nichols, P.D.; Hurd, C.L. Southern Australian seaweeds: A promising resource for omega-3 fatty acids. Food Chem. 2018, 265, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Crampon, C.; Boutin, O.; Badens, E. Supercritical carbon dioxide extraction of molecules of interest from microalgae and seaweeds. Ind. Eng. Chem. Res. 2011, 50, 8941–8953. [Google Scholar] [CrossRef]
- Cheung, P.C.K. Temperature and pressure effects onsupercriticalcarbon dioxide extraction of n-3 fatty acids from red seaweed. Food Chem. 1999, 65, 399–403. [Google Scholar] [CrossRef]
- Patra, J.K.; Lee, S.W.; Kwon, Y.S.; Park, J.G.; Baek, K.H. Chemical characterization and antioxidant potential of volatile oil from an edible seaweed Porphyra tenera (Kjellman, 1897). Chem. Cent. J. 2017, 11, 34. [Google Scholar] [CrossRef]
- Kumari, P.; Reddy, C.R.K.; Jha, B. Comparative evaluation and selection of a method for lipid and fatty acid extraction from macroalgae. Anal. Biochem. 2011, 415, 134–144. [Google Scholar] [CrossRef]
- Mæhre, H.K.; Malde, M.K.; Eilertsen, K.E.; Elvevoll, E.O. Characterization of protein, lipid and mineral contents in common Norwegian seaweeds and evaluation of their potential as food and feed. J. Sci. Food Agric. 2014, 94, 3281–3290. [Google Scholar] [CrossRef]
- Chen, Y.H.; Tu, C.J.; Wu, H.T. Growth-inhibitory effects of the red alga Gelidium amansii on cultured cells. Biol. Pharm. Bull. 2004, 27, 180–184. [Google Scholar] [CrossRef]
- Chan, P.T.; Matanjun, P.; Yasir, S.M.; Tan, T.S. Antioxidant activities and polyphenolics of various solvent extracts of red seaweed, Gracilaria changii. J. Appl. Phycol. 2015, 27, 2377–2386. [Google Scholar] [CrossRef]
- Topuz, O.K.; Gokoglu, N.; Yerlikaya, P.; Ucak, I.; Gumus, B. Optimization of antioxidant activity and phenolic compound extraction conditions from red seaweed (Laurencia obtusa). J. Aquat. Food Prod. Technol. 2016, 25, 414–422. [Google Scholar] [CrossRef]
- Zheng, J.; Chen, Y.; Yao, F.; Chen, W.; Shi, G. Chemical composition and antioxidant/antimicrobial activities in supercritical carbon dioxide fluid extract of Gloiopeltis tenax. Mar. Drugs 2012, 10, 2634–2647. [Google Scholar]
- Ospina, M.; Castro-Vargas, H.I.; Parada-Alfonso, F. Antioxidant capacity of Colombian seaweeds: 1. Extracts obtained from Gracilaria mammillaris by means ofsupercriticalfluid extraction. J. Supercrit. Fluids 2017, 128, 314–322. [Google Scholar] [CrossRef]
- Kang, J.Y.; Chun, B.S.; Lee, M.C.; Choi, J.S.; Choi, I.S.; Hong, Y.K. Anti-inflammatory Activity and Chemical Composition of Essential Oil Extracted with Supercritical CO2 from the Brown Seaweed Undaria pinnatifida. J. Essent. Oil Bear. Pl. 2016, 19, 46–51. [Google Scholar] [CrossRef]
- Yuan, S.; Duan, Z.; Lu, Y.; Ma, X.; Wang, S. Optimization of decolorization process in agar production from Gracilaria lemaneiformis and evaluation of antioxidant activities of the extract rich in natural pigments. 3 Biotech 2018, 8, 8. [Google Scholar] [CrossRef]
- Yuan, Y.V.; Westcott, N.D.; Hu, C.; Kitts, D.D. Mycosporine-like amino acid composition of the edible red alga, Palmaria palmata (Dulse) harvested from the west and east coasts of Grand Manan Island, New Brunswick. Food Chem. 2009, 112, 321–328. [Google Scholar] [CrossRef]
- Aguilera, J.; Bischof, K.; Karsten, U.; Hanelt, D.; Wiencke, C. Seasonal variation in ecophysiological patterns in macroalgae from an Arctic fjord. II. Pigment accumulation and biochemical defense systems against high light stress. Mar. Biol. 2002, 140, 1087–1095. [Google Scholar]
- Bedoux, G.; Hardouin, K.; Marty, C.; Taupin, L.; Vandanjon, L.; Bourgougnon, N. Chemical characterization and photoprotective activity measurement of extracts from the red macroalga Solieria chordalis. Bot. Mar. 2014, 57, 291–301. [Google Scholar] [CrossRef]
- Rupérez, P. Mineral content of edible marine seaweeds. Food Chem. 2002, 79, 23–26. [Google Scholar] [CrossRef]
- Kraan, S. Mass-cultivation of carbohydrate rich macroalgae, a possible solution for sustainable biofuel production. Mitig. Adapt. Strateg. Glob. Chang. 2013, 18, 27–46. [Google Scholar] [CrossRef]
- Jaballi, I.; Saad, H.B.; Bkhairia, I.; Cherif, B.; Kallel, C.; Boudawara, O.; Droguet, M.; Magné, C.; Hakim, A.; Amara, I.B. Cytoprotective effects of the red marine alga Chondrus canaliculatus against Maneb-Induced hematotoxicity and bone oxidative damages in adult rats. Biol. Trace Elem. Res. 2018, 184, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.F.; Zhang, M.H.; Li, D.W.; Yang, W.D.; Liu, J.S.; Bai, W.B. Improvement of neutral lipid and polyunsaturated fatty acid biosynthesis by overexpressing a type 2 diacylglycerol acyltransferase in marine diatom Phaeodactylum tricornutum. Mar. Drugs 2013, 11, 4558–4569. [Google Scholar] [CrossRef] [PubMed]
- Cian, R.; Martínez, O.; Drago, S. Bioactive properties of peptides obtained by enzymatic hydrolysis from protein byproducts of Porphyra columbina. Food Res. Int. 2012, 49, 364–372. [Google Scholar] [CrossRef]
- Cian, R.E.; Vioque, J.; Drago, S.R. Enzyme proteolysis enhanced extraction of ACE inhibitory and antioxidant compounds (peptides and polyphenols) from Porphyra columbiana residual cake. J. Appl. Phycol. 2013, 25, 1197–1206. [Google Scholar] [CrossRef]
- Baghel, R.S.; Trivedi, N.; Reddy, C.R. A simple process for recovery of a stream of products from marine macroalgal biomass. Bioresour. Technol. 2016, 203, 160–165. [Google Scholar] [CrossRef]
- Gajaria, T.K.; Suthar, P.; Baghel, R.S.; Balar, N.B.; Sharnagat, P.; Mantri, V.A.; Reddy, C.R.K. Integration of protein extraction with a stream of byproducts from marine macroalgae: A model forms the basis for marine bioeconomy. Bioresour. Technol. 2017, 243, 867–873. [Google Scholar] [CrossRef]
- Kumar, S.; Gupta, R.; Kumar, G.; Sahoo, D.; Kuhad, R.C. Bioethanol production from Gracilaria verricosa, a red alga, in a biorefinery approach. Bioresour. Technol. 2013, 135, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Baghel, R.R.S.; Trivedi, N.; Gupta, V.; Neori, A.; Reddy, C.R.K.; Lali, A.; Jha, B. Biorefining of marine macroalgal biomass for production of biofuel and commodity chemicals. Green Chem. 2015, 17, 2436–2443. [Google Scholar] [CrossRef]
- Tan, I.S.; Lee, K.T. Enzymatic hydrolysis and fermentation of seaweed solid wastes for bioethanol production: An optimization study. Energy 2014, 78, 53–62. [Google Scholar] [CrossRef]
- Francavilla, M.P.; Manara, P.; Kamaterou, M.; Monteleone, M.; Zabanioutou, A. Cascade approach of red macroalgae Gracilaria gracilis sustainable valorization by extraction of phycobiliproteins and pyrolysis of residue. Bioresour. Technol. 2015, 184, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Ingle, K.; Vitkin, E.; Robin, A.; Yakhini, Z.; Mishori, D.; Golberg, A. Macroalgae biorefinery from Kappaphycus alvarezii: Conversion, modeling and performance prediction for India and Philippines as examples. BioEnergy Res. 2018, 11, 22–32. [Google Scholar] [CrossRef]
- Mondal, D.; Sharma, M.; Prasad, K.; Meena, R.; Siddhanta, A.; Ghosh, P. Fuel intermediates, agricultural nutrients and pure water from Kappaphycus alvarezii seaweed. RSC Adv. 2013, 3, 17989–17997. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).