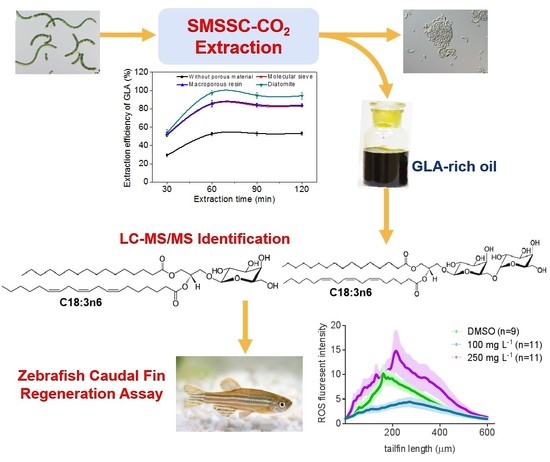
Solid Matrix-Supported Supercritical CO2 Enhances Extraction of γ-Linolenic Acid from the Cyanobacterium Arthrospira (Spirulina) platensis and Bioactivity Evaluation of the Molecule in Zebrafish
Abstract
1. Introduction
2. Results and Discussion
2.1. Quantitative Analysis of Total Lipids and Fatty Acid Composition of A. platensis
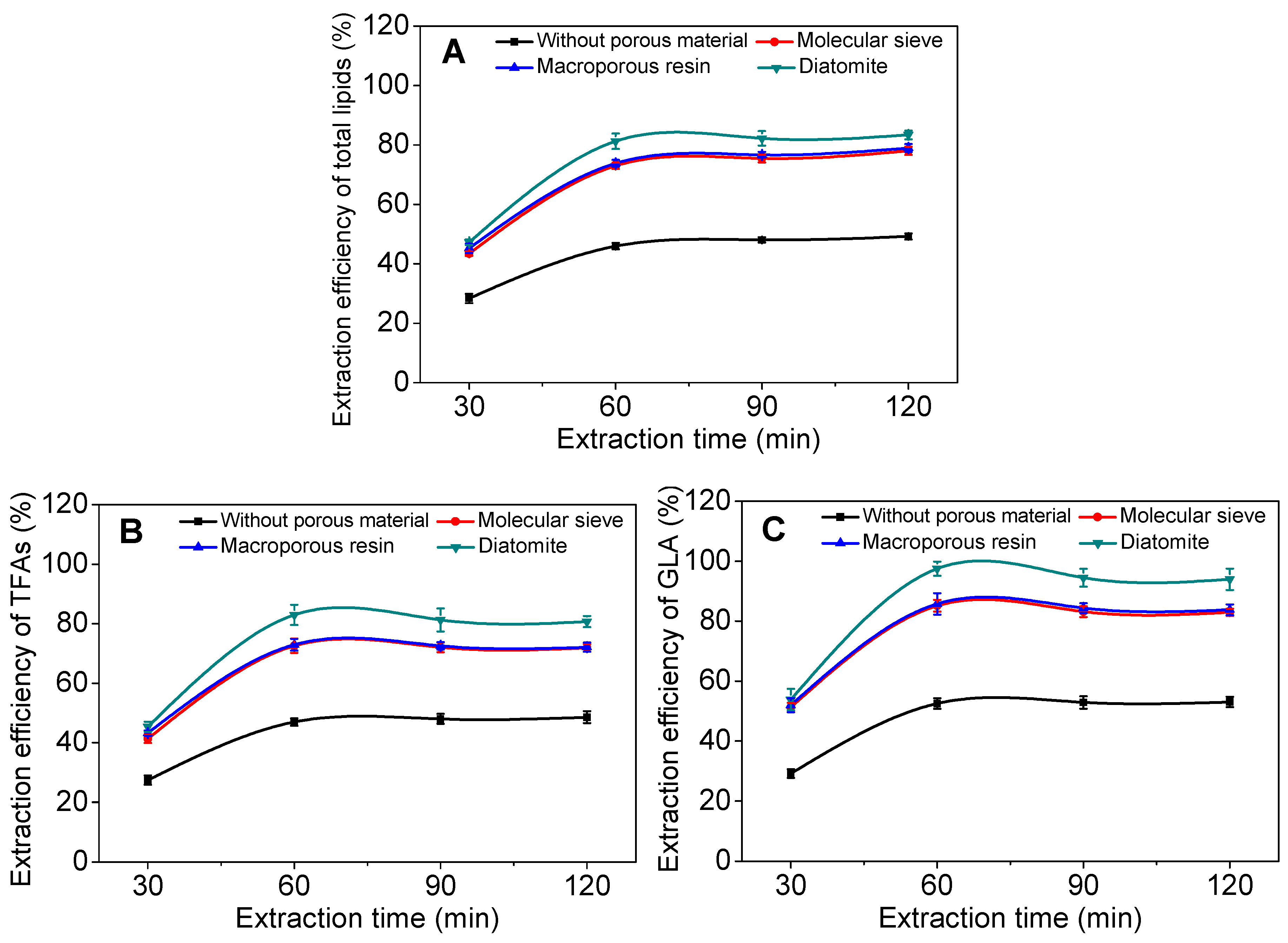
2.2. Effect of Incorporated Solid Support on GLA Extraction Efficiency
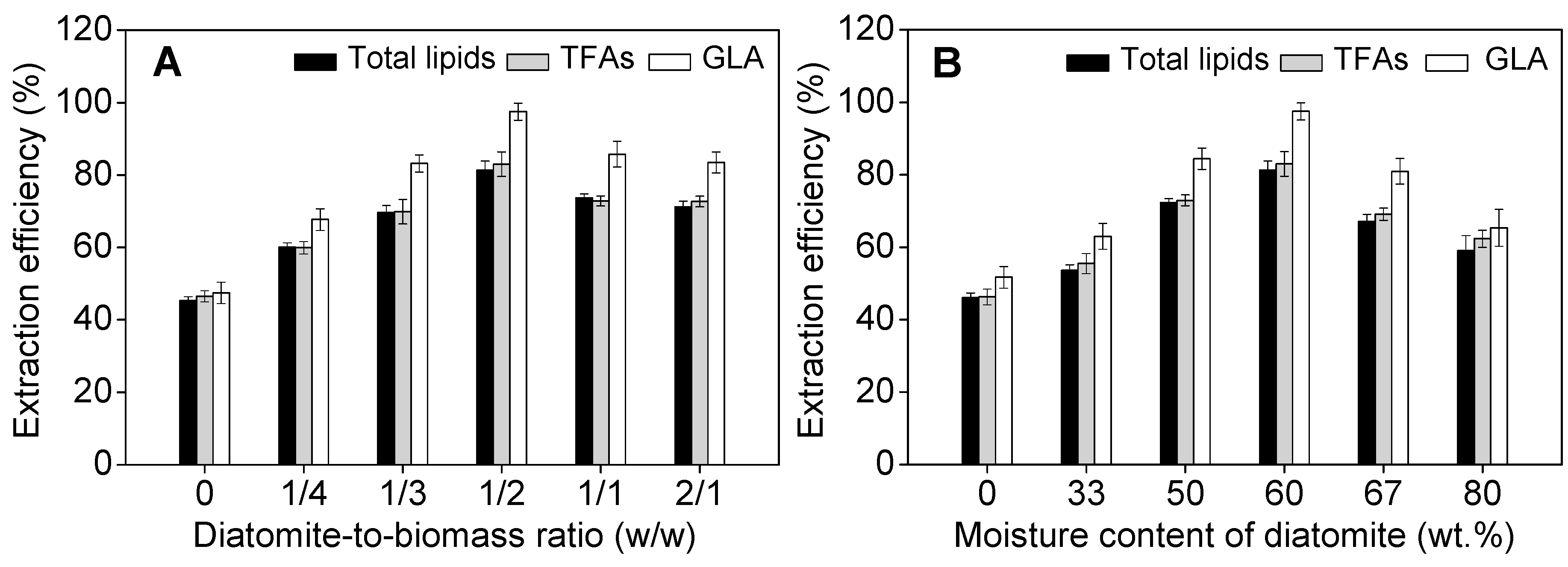
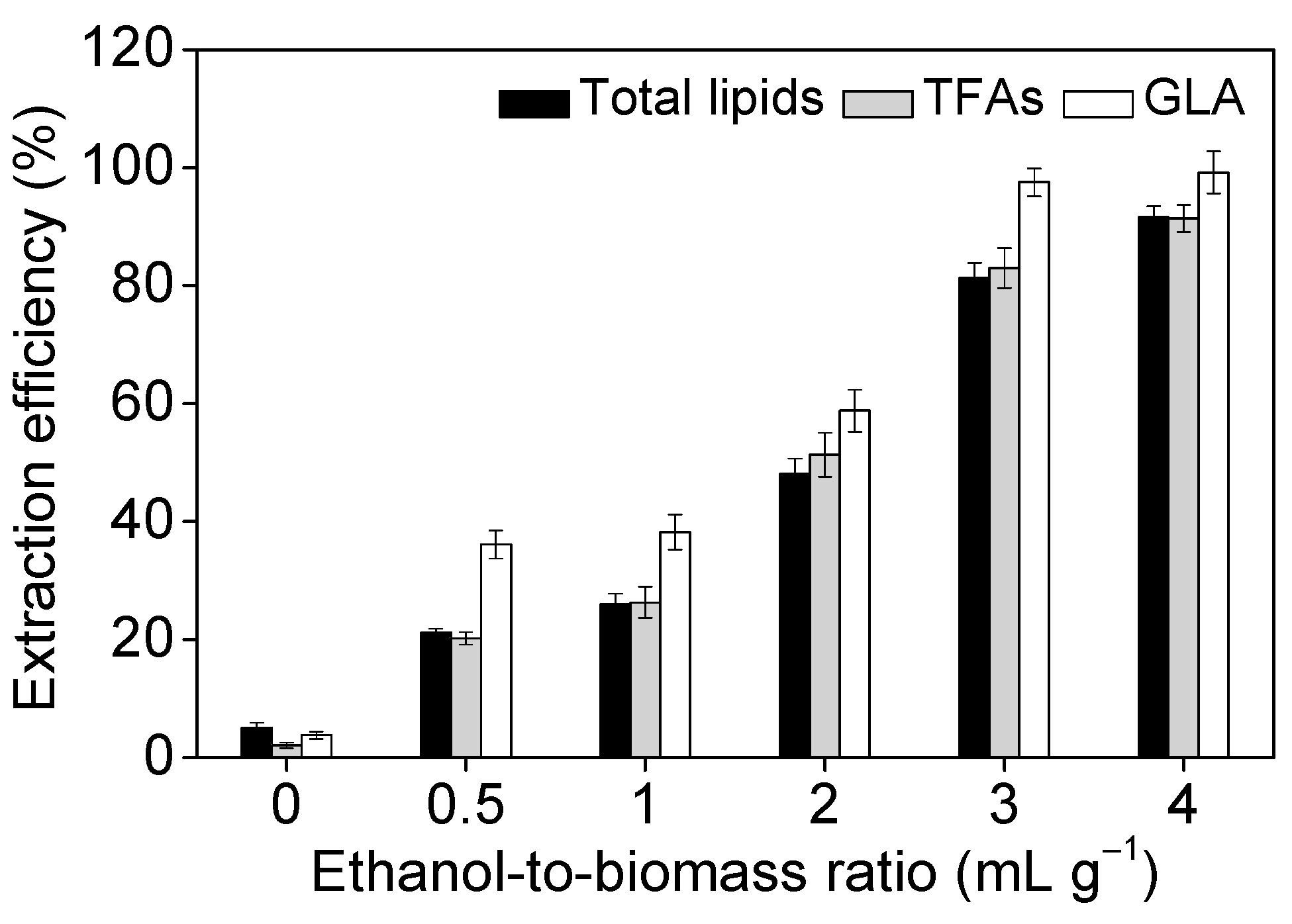
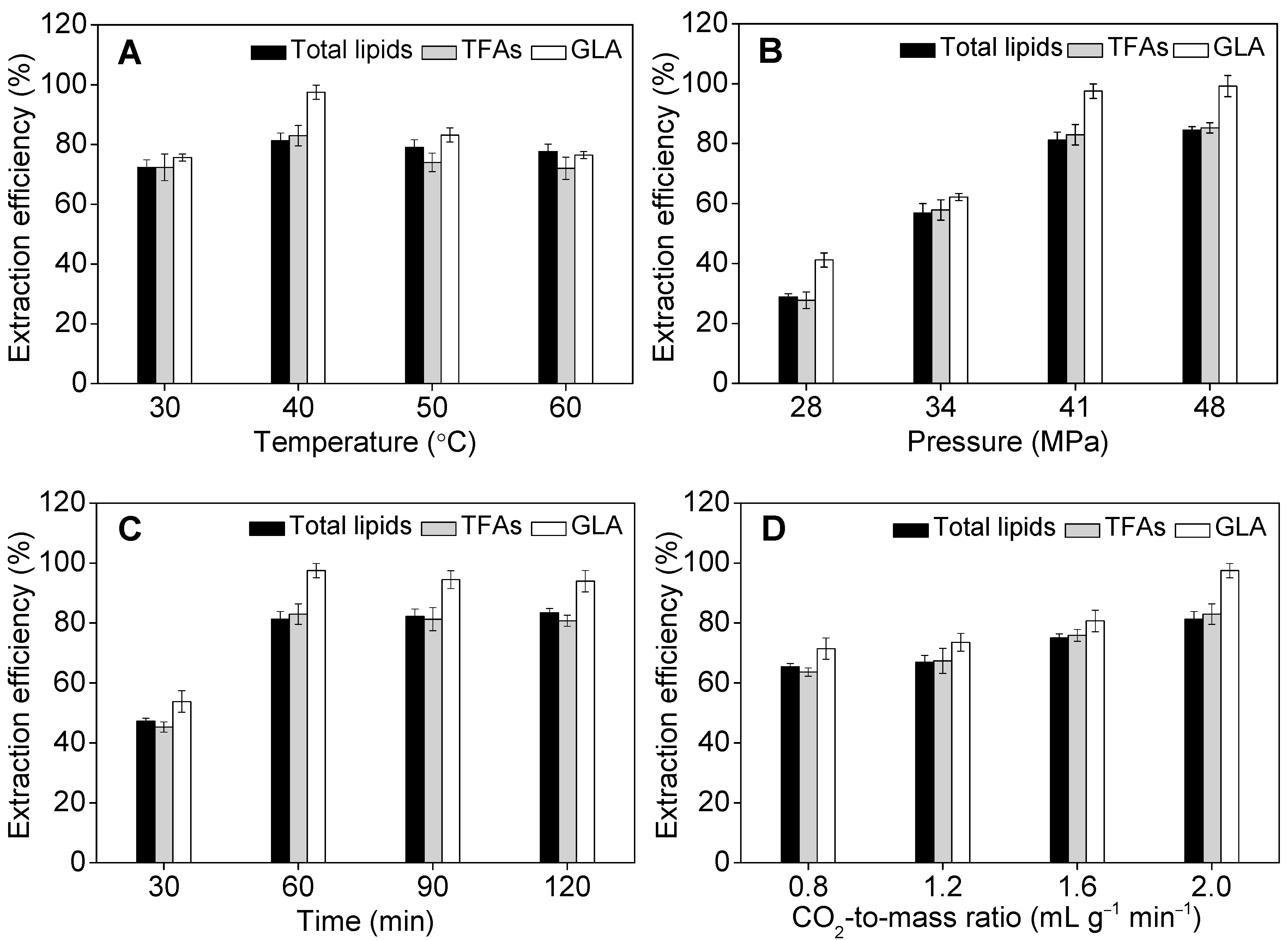
2.3. Effect of Operational Parameters on GLA Extraction Efficiency
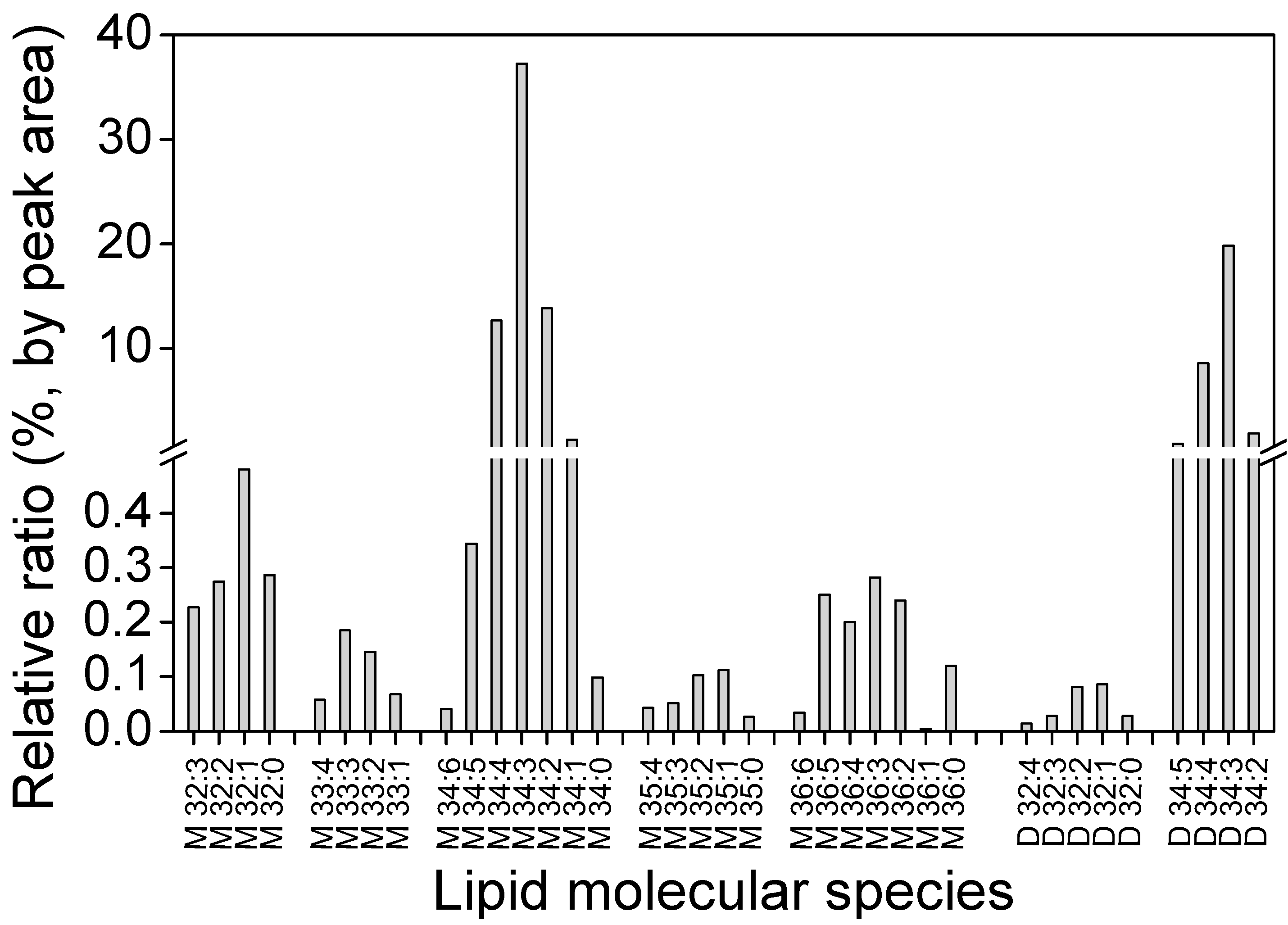
2.4. Distribution of GLA in Different Fractions
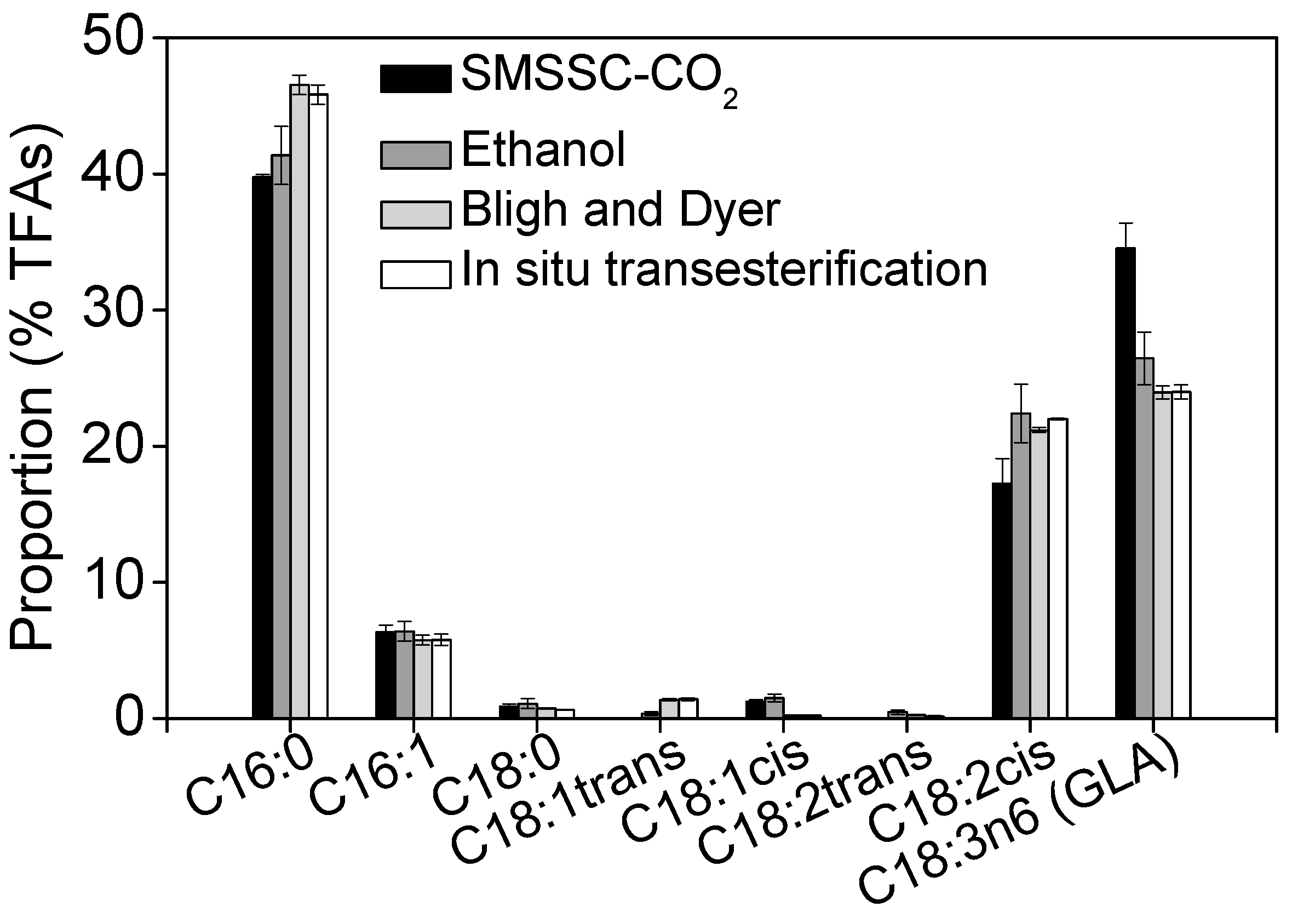
2.5. Recovery of GLA Using Different Extraction Methods
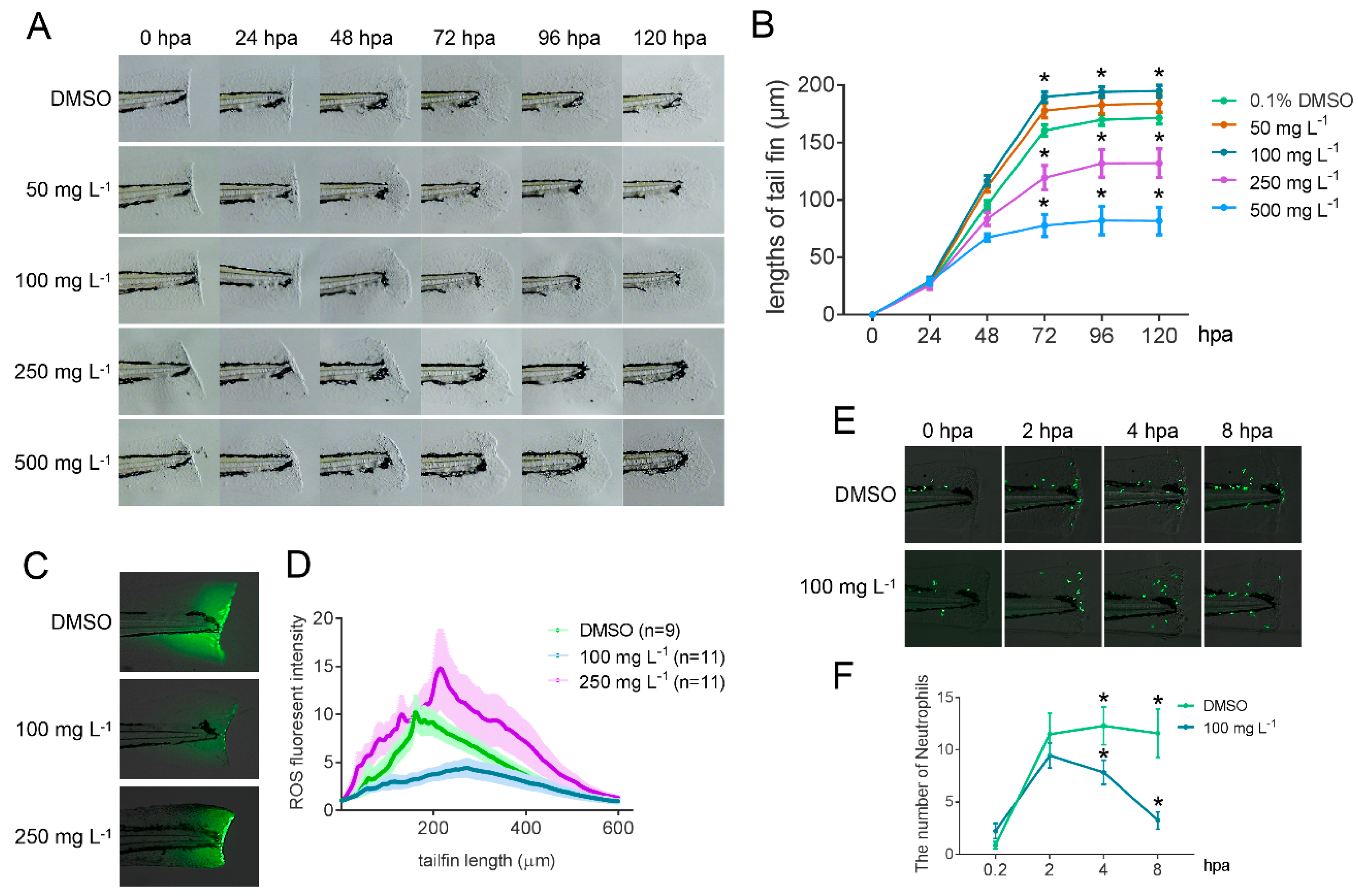
2.6. Bioactivity Test of GLA Using a Zebrafish Caudal Fin Regeneration Model
3. Materials and Methods
3.1. Materials and Reagents
3.2. Methods
3.2.1. Lipid Extraction
3.2.2. Determination of Fatty Acid Methyl Esters (FAMEs)
3.2.3. Lipids and GLA Extraction with Ethanol under Mechanical Stirring
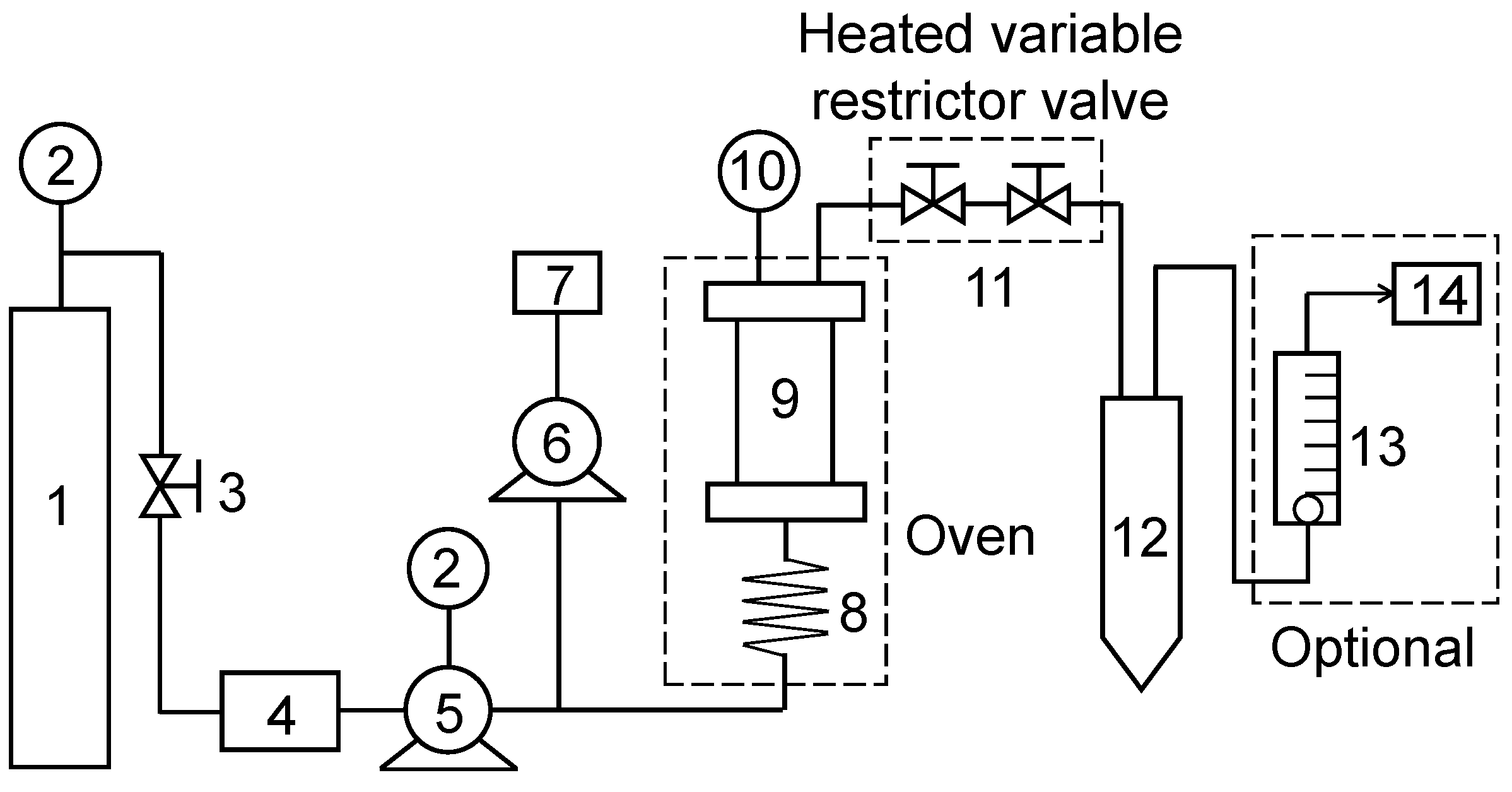
3.2.4. Supercritical Fluid Extraction
3.2.5. Determination of Yields of Total Lipids and GLA
3.2.6. Zebrafish Caudal Fin Regeneration Assay
3.3. Analytical Methods
3.3.1. Fatty Acid Analysis
3.3.2. Polar Lipids Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xiao, Y.; Gu, Y.; Purwaha, P.; Ni, K.; Law, B.; Mallik, S.; Qian, S.Y. Characterization of free radicals formed from COX-catalyzed DGLA peroxidation. Free Radic. Biol. Med. 2011, 50, 1163–1170. [Google Scholar] [CrossRef]
- Johnson, M.M.; Swan, D.D.; Surette, M.E.; Stegner, J.; Chilton, T.; Fonteh, A.N.; Chilton, F.H. Dietary supplementation with γ-linolenic acid alters fatty acid content and eicosanoid production in healthy humans. J. Nutr. 1997, 127, 1435–1444. [Google Scholar] [CrossRef]
- Zurier, R.B.; Rossetti, R.G.; Jacobson, E.W.; DeMarco, D.M.; Liu, N.Y.; Temming, J.E.; White, B.M.; Laposata, M. Gamma linolenic acid treatment of rheumatoid arthritis. A randomized, placebo-controlled trial. Arthritis Rheumatol. 1996, 39, 1808–1817. [Google Scholar] [CrossRef]
- Kast, R.E. Borage oil reduction of rheumatoid arthritis activity may be mediated by increased cAMP that suppresses tumor necrosis factor-alpha. Int. Immunopharmacol. 2001, 1, 2197–2199. [Google Scholar] [CrossRef]
- Worm, M.; Henz, B.M. Novel unconventional therapeutic approaches to atopic eczema. Dermatology 2000, 201, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Ponnala, S.; Rao, K.P.; Chaudhury, J.R.; Ahmed, J.; Rama Rao, B.; Kanjilal, S.; Hasan, Q.; Das, U.N. Effect of polyunsaturated fatty acids on diphenyl hydantoin-induced genetic damage in vitro and in vivo. Prostaglandins Leukot. Essent. Fatty Acids 2009, 80, 43–50. [Google Scholar] [CrossRef]
- Bugnariu, S. Beneficial effect of gamma-linolenic acid-enriched diet therapeutic properties of Oenothera biennis oil (evening primrose oil). Farmacia 1996, 44, 3–4. [Google Scholar]
- Wanasundara, U.N.; Wanasundara, J.P.D.; Akoh, C.C. γ-Linolenic acid: Purification and functionality. In Handbook of Functional Lipids; Akoh, C.C., Ed.; CRC Press, Taylor & Francis Group: New York, NY, USA, 2006; pp. 47–75. [Google Scholar]
- Vargas, M.A.; Rodríguez, H.; Moreno, J.; Olivares, H.; Campo, J.A.D.; Rivas, J.; Guerrero, M.G. Biochemical Composition and Fatty Acid Content of Filamentous Nitrogen-Fixing Cyanobacteria. J. Phycol. 1998, 34, 812–817. [Google Scholar] [CrossRef]
- Maslova, I.P.; Mouradyan, E.A.; Lapina, S.S.; Klyachko-Gurvich, G.L.; Los, D.A. Lipid Fatty Acid Composition and Thermophilicity of Cyanobacteria. Russ. J. Plant Physiol. 2004, 51, 353–360. [Google Scholar] [CrossRef]
- Yang, D.; Nam, S.; Hwang, S.-J.; An, K.-G.; Park, Y.-S.; Shin, K.-H.; Park, S. Fatty acid biomarkers to verify cyanobacteria feeding abilities of herbivorous consumers. J. Freshw. Ecol. 2015, 31, 77–91. [Google Scholar] [CrossRef]
- Roughan, P.G. Spirulina: A Source of Dietary Gamma-Linolenic Acid? J. Sci. Food Agric. 1989, 47, 85–93. [Google Scholar] [CrossRef]
- Leema, J.T.; Kirubagaran, R.; Vinithkumar, N.V.; Dheenan, P.S.; Karthikayulu, S. High value pigment production from Arthrospira (Spirulina) platensis cultured in seawater. Bioresour. Technol. 2010, 101, 9221–9227. [Google Scholar] [CrossRef]
- Raposo, M.F.; de Morais, R.M.; Bernardo de Morais, A.M. Bioactivity and applications of sulphated polysaccharides from marine microalgae. Mar. Drugs 2013, 11, 233–252. [Google Scholar] [CrossRef] [PubMed]
- Belay, A. Biology and Industrial Production of Arthrospira (Spirulina). In Handbook of Microalgal Culture, 2nd ed.; Richmond, A., Hu, Q., Eds.; Applied Phycology and Biotechnology, Wiley: New York, NY, USA, 2013; pp. 339–358. [Google Scholar]
- Cohen, Z.; Reungjitchachawali, M.; Siangdung, W.; Tanticharoen, M. Production and partial purification of γ-linolenic acid and some pigments from Spirulina platensis. J. Appl. Phycol. 1993, 5, 109–115. [Google Scholar] [CrossRef]
- Apdila, E.T.; Awai, K. Configuration of the sugar head of glycolipids in thylakoid membranes. Genes Genet. Syst. 2017, 92, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Sommella, E.; Conte, G.M.; Salviati, E.; Pepe, G.; Bertamino, A.; Ostacolo, C.; Sansone, F.; Prete, F.D.; Aquino, R.P.; Campiglia, P. Fast Profiling of Natural Pigments in Different Spirulina (Arthrospira platensis) Dietary Supplements by DI-FT-ICR and Evaluation of their Antioxidant Potential by Pre-Column DPPH-UHPLC Assay. Molecules 2018, 23, 1152. [Google Scholar] [CrossRef]
- Hao, S.; Li, S.; Wang, J.; Zhao, L.; Yan, Y.; Cao, Q.; Wu, T.; Liu, L.; Wang, C. Transcriptome Analysis of Phycocyanin-Mediated Inhibitory Functions on Non-Small Cell Lung Cancer A549 Cell Growth. Mar. Drugs 2018, 16, 511. [Google Scholar] [CrossRef]
- Martinez-Samano, J.; Torres-Montes de Oca, A.; Luqueno-Bocardo, O.I.; Torres-Duran, P.V.; Juarez-Oropeza, M.A. Spirulina maxima Decreases Endothelial Damage and Oxidative Stress Indicators in Patients with Systemic Arterial Hypertension: Results from Exploratory Controlled Clinical Trial. Mar. Drugs 2018, 16, 496. [Google Scholar] [CrossRef]
- Hernandez-Lepe, M.A.; Lopez-Diaz, J.A.; Juarez-Oropeza, M.A.; Hernandez-Torres, R.P.; Wall-Medrano, A.; Ramos-Jimenez, A. Effect of Arthrospira (Spirulina) maxima Supplementation and a Systematic Physical Exercise Program on the Body Composition and Cardiorespiratory Fitness of Overweight or Obese Subjects: A Double-Blind, Randomized, and Crossover Controlled Trial. Mar. Drugs 2018, 16, 364. [Google Scholar] [CrossRef] [PubMed]
- Mendiola, J.A.; Jaime, L.; Santoyo, S.; Reglero, G.; Cifuentes, A.; Ibañez, E.; Señoráns, F.J. Screening of functional compounds in supercritical fluid extracts from Spirulina platensis. Food Chem. 2007, 102, 1357–1367. [Google Scholar] [CrossRef]
- Mallikarjun Gouda, K.G.; Udaya Sankar, K.; Sarada, R.; Ravishankar, G.A. Supercritical CO2 extraction of functional compounds from Spirulina and their biological activity. J. Food Sci. Technol. 2015, 52, 3627–3633. [Google Scholar]
- Michalak, I.; Chojnacka, K.; Saeid, A. Dietary Feed Supplements and Cosmetics Formulated with Supercritical CO2 Algal Extracts. Molecules 2017, 22, 66. [Google Scholar] [CrossRef]
- Axelsson, M.; Gentili, F. A single-step method for rapid extraction of total lipids from green microalgae. PLoS ONE 2014, 9, e89643. [Google Scholar] [CrossRef]
- Sajilata, M.G.; Singhal, R.S.; Kamat, M.Y. Fractionation of lipids and purification of γ-linolenic acid (GLA) from Spirulina platensis. Food Chem. 2008, 109, 580–586. [Google Scholar] [CrossRef]
- Sajilata, M.G.; Singhal, R.S.; Kamat, M.Y. Supercritical CO2 extraction of γ-linolenic acid (GLA) from Spirulina platensis ARM 740 using response surface methodology. J. Food Eng. 2008, 84, 321–326. [Google Scholar] [CrossRef]
- Mendes, R.L.; Nobre, B.P.; Cardoso, M.T.; Pereira, A.P.; Palavra, A.F. Supercritical carbon dioxide extraction of compounds with pharmaceutical importance from microalgae. Inorg. Chim. Acta 2003, 356, 328–334. [Google Scholar] [CrossRef]
- Mendes, R.L.; Reis, A.D.; Palavra, A.F. Supercritical CO2 extraction of γ-linolenic acid and other lipids from Arthrospira (Spirulina) maxima: Comparison with organic solvent extraction. Food Chem. 2006, 99, 57–63. [Google Scholar] [CrossRef]
- Golmakani, M.-T.; Mendiola, J.A.; Rezaei, K.; Ibáñez, E. Expanded ethanol with CO2 and pressurized ethyl lactate to obtain fractions enriched in γ-Linolenic Acid from Arthrospira platensis (Spirulina). J. Supercrit. Fluids 2012, 62, 109–115. [Google Scholar] [CrossRef]
- Herrero, M.; Mendiola, J.A.; Cifuentes, A.; Ibanez, E. Supercritical fluid extraction: Recent advances and applications. J. Chromatogr. A 2010, 1217, 2495–2511. [Google Scholar] [CrossRef]
- Andrich, G.; Zinnai, A.; Nesti, U.; Venturi, F. Supercritical fluid extraction of oil from microalga Spirulina (arthrospira) platensis. Acta Aliment. 2006, 35, 195–203. [Google Scholar] [CrossRef]
- Hu, Q.H. Supercritical Carbon Dioxide Extraction of Spirulina platensis Component and Removing the Strench. J. Agric. Food Chem. 1999, 47, 2705–2706. [Google Scholar]
- Hsueh, Y.C.; Wang, B.J.; Yu, Z.R.; Wang, C.C.; Koo, M. Optimization of a Continuous Preparation Method of Arthrospira platensis γ-linolenic Acid by Supercritical Carbon Dioxide Technology using Response Surface Methodology. Sains Malays. 2015, 44, 1739–1744. [Google Scholar]
- de la Jara, A.; Ruano-Rodriguez, C.; Polifrone, M.; Assunçao, P.; Brito-Casillas, Y.; Wägner, A.M.; Serra-Majem, L. Impact of dietary Arthrospira (Spirulina) biomass consumption on human health: Main health targets and systematic review. J. Appl. Phycol. 2018, 30, 2403–2423. [Google Scholar] [CrossRef]
- Lee, J.J.; Kim, K.B.; Heo, J.; Cho, D.H.; Kim, H.S.; Han, S.H.; Ahn, K.J.; An, I.S.; An, S.; Bae, S. Protective effect of Arthrospira platensis extracts against ultraviolet B-induced cellular senescence through inhibition of DNA damage and matrix metalloproteinase-1 expression in human dermal fibroblasts. J. Photochem. Photobiol. B 2017, 173, 196–203. [Google Scholar] [CrossRef]
- Wang, F.; Miao, M.; Chen, B.; Wang, R.; Sun, B.; Ren, D.; Lu, J. Antineoplastic activity of γ-linolenic acid extract from Spirulina platensis on HepG2 cells and its inhibition effect on platelet aggregation. Food Agric. Immunol. 2014, 26, 97–108. [Google Scholar] [CrossRef]
- Esquivel-Hernandez, D.A.; Rodriguez-Rodriguez, J.; Cuellar-Bermudez, S.P.; Garcia-Perez, J.S.; Mancera-Andrade, E.I.; Nunez-Echevarria, J.E.; Ontiveros-Valencia, A.; Rostro-Alanis, M.; Garcia-Garcia, R.M.; Torres, J.A.; et al. Effect of Supercritical Carbon Dioxide Extraction Parameters on the Biological Activities and Metabolites Present in Extracts from Arthrospira platensis. Mar. Drugs 2017, 15, 174. [Google Scholar] [CrossRef] [PubMed]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef]
- Li, L.; Yan, B.; Shi, Y.Q.; Zhang, W.Q.; Wen, Z.L. Live imaging reveals differing roles of macrophages and neutrophils during zebrafish tail fin regeneration. J. Biol. Chem. 2012, 287, 25353–25360. [Google Scholar] [CrossRef]
- Limpitlaw, U.G. Ingestion of Earth materials for health by humans and animals. Int. Geol. Rev. 2010, 52, 726–744. [Google Scholar] [CrossRef]
- Holman, B.W.B.; Malau-Aduli, A.E.O. Spirulina as a livestock supplement and animal feed. J. Anim. Physiol. Anim. Nutr. 2013, 97, 615–623. [Google Scholar] [CrossRef]
- Singh, J.; Gu, S. Commercialization potential of microalgae for biofuels production. Renew. Sustain. Energy Rev. 2010, 14, 2596–2610. [Google Scholar] [CrossRef]
- Pan, J.-L.; Wang, H.-M.; Chen, C.-Y.; Chang, J.-S. Extraction of astaxanthin from Haematococcus pluvialis by supercritical carbon dioxide fluid with ethanol modifier. Eng. Life Sci. 2012, 12, 638–647. [Google Scholar] [CrossRef]
- Dong, T.; Knoshaug, E.P.; Pienkos, P.T.; Laurens, L.M.L. Lipid recovery from wet oleaginous microbial biomass for biofuel production: A critical review. Appl. Energy 2016, 177, 879–895. [Google Scholar] [CrossRef]
- Grima, E.M.; González, M.J.I.; Giménez, A.G. Solvent Extraction for Microalgae Lipids. In Algae for Biofuels and Energy; Borowitzka, M.A., Moheimani, N.R., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 187–205. [Google Scholar]
- Esquivel-Hernandez, D.A.; Lopez, V.H.; Rodriguez-Rodriguez, J.; Aleman-Nava, G.S.; Cuellar-Bermudez, S.P.; Rostro-Alanis, M.; Parra-Saldivar, R. Supercritical Carbon Dioxide and Microwave-Assisted Extraction of Functional Lipophilic Compounds from Arthrospira platensis. Int. J. Mol. Sci. 2016, 17, 658. [Google Scholar] [CrossRef]
- Thana, P.; Machmudah, S.; Goto, M.; Sasaki, M.; Pavasant, P.; Shotipruk, A. Response surface methodology to supercritical carbon dioxide extraction of astaxanthin from Haematococcus pluvialis. Bioresour. Technol. 2008, 99, 3110–3115. [Google Scholar] [CrossRef] [PubMed]
- Crampon, C.; Nikitine, C.; Zaier, M.; Lépine, O.; Tanzi, C.D.; Vian, M.A.; Chemat, F.; Badens, E. Oil extraction from enriched Spirulina platensis microalgae using supercritical carbon dioxide. J. Supercrit. Fluids 2017, 119, 289–296. [Google Scholar] [CrossRef]
- Kaufmann, B.; Christen, P. Recent extraction techniques for natural products: Microwave-assisted extraction and pressurised solvent extraction. Phytochem. Anal. 2002, 13, 105–113. [Google Scholar] [CrossRef]
- Fenn, J.B.; Mann, M.; Meng, C.K.; Wong, S.F.; Whitehouse, C.M. Electrospray ionization for mass spectrometry of large biomolecules. Science 1989, 246, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Gross, R.W. Global analyses of cellular lipidomes directly from crude extracts of biological samples by ESI mass spectrometry: A bridge to lipidomics. J. Lipid Res. 2003, 44, 1071–1079. [Google Scholar] [CrossRef]
- Marcus, D.M.; Schwarting, G.A. Immunochemical Properties of Glycolipids and Phospholipids. Adv. Immunol. 1976, 23, 203–240. [Google Scholar]
- Gunstone, F.D. Gamma linolenic acid-occurrence and physical and chemical properties. Prog. Lipid Res. 1992, 31, 145–161. [Google Scholar] [CrossRef]
- Cikos, A.M.; Jokic, S.; Subaric, D.; Jerkovic, I. Overview on the Application of Modern Methods for the Extraction of Bioactive Compounds from Marine Macroalgae. Mar. Drugs 2018, 16, 348. [Google Scholar] [CrossRef]
- He, C.; Qu, X.; Cui, L.; Wang, J.; Kang, J.X. Improved spatial learning performance of fat-1 mice is associated with enhanced neurogenesis and neuritogenesis by docosahexaenoic acid. Proc. Natl. Acad. Sci. USA 2009, 106, 11370–11375. [Google Scholar] [CrossRef] [PubMed]
- Ramprasath, V.R.; Eyal, I.; Zchut, S.; Jones, P.J. Enhanced increase of omega-3 index in healthy individuals with response to 4-week n-3 fatty acid supplementation from krill oil versus fish oil. Lipids Health Dis. 2013, 12, 178. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Bartke, N.; Van Daele, H.; Lawrence, P.; Qin, X.; Park, H.G.; Kothapalli, K.; Windust, A.; Bindels, J.; Wang, Z.; et al. Higher efficacy of dietary DHA provided as a phospholipid than as a triglyceride for brain DHA accretion in neonatal piglets. J. Lipid Res. 2014, 55, 531–539. [Google Scholar] [CrossRef]
- Wu, F.; Wang, D.D.; Wen, M.; Che, H.X.; Xue, C.H.; Yanagita, T.; Zhang, T.T.; Wang, Y.M. Comparative analyses of DHA-Phosphatidylcholine and recombination of DHA-Triglyceride with Egg-Phosphatidylcholine or Glycerylphosphorylcholine on DHA repletion in n-3 deficient mice. Lipids Health Dis. 2017, 16, 234. [Google Scholar] [CrossRef] [PubMed]
- Renshaw, S.A.; Loynes, C.A.; Trushell, D.M.; Elworthy, S.; Ingham, P.W.; Whyte, M.K. A transgenic zebrafish model of neutrophilic inflammation. Blood 2006, 108, 3976–3978. [Google Scholar] [CrossRef] [PubMed]
- Laurens, L.M.; Dempster, T.A.; Jones, H.D.; Wolfrum, E.J.; Van Wychen, S.; McAllister, J.S.; Rencenberger, M.; Parchert, K.J.; Gloe, L.M. Algal biomass constituent analysis: Method uncertainties and investigation of the underlying measuring chemistries. Anal. Chem. 2012, 84, 1879–1887. [Google Scholar] [CrossRef]
- Van Wychen, S.; Ramirez, K.; Laurens, L.M.L. Determination of Total Lipids as Fatty Acid Methyl Esters (FAME) by in Situ Transesterification Laboratory Analytical Procedure (LAP); NREL/TP-5100-60958; National Renewable Energy Laboratory: Golden, CO, USA, 2016.
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Yoon, K.; Han, D.; Li, Y.; Sommerfeld, M.; Hu, Q. Phospholipid: Diacylglycerol acyltransferase is a multifunctional enzyme involved in membrane lipid turnover and degradation while synthesizing triacylglycerol in the unicellular green microalga Chlamydomonas reinhardtii. Plant Cell 2012, 24, 3708–3724. [Google Scholar] [CrossRef]
- Xu, J.; Chen, D.; Yan, X.; Chen, J.; Zhou, C. Global characterization of the photosynthetic glycerolipids from a marine diatom Stephanodiscus sp. by ultra performance liquid chromatography coupled with electrospray ionization-quadrupole-time of flight mass spectrometry. Anal. Chim. Acta 2010, 663, 60–68. [Google Scholar] [CrossRef]
| Methods | Total Lipids Yield (% DCW) | Total Lipids Recovery (%) | GLA Yield (% DCW) | GLA Recovery (%) | GLA (% Total Lipids) | TFAs Yield (% DCW) | GLA (% TFAs) |
|---|---|---|---|---|---|---|---|
| In situ T | NA | NA | 1.26 ± 0.04 | 100 | NA | 5.25 ± 0.07 | 23.98 ± 0.52 |
| B & D | 11.52 ± 0.11 | 100 | 1.18 ±0.03 | 100 | 10.33 | 4.97 ± 0.11 | 23.95 ± 0.47 |
| Ethanol | 10.22 ± 0.17 | 88.72 | 1.13 ± 0.06 | 94.96 a | 11.06 | 4.27 ± 0.16 | 26.44 ± 1.94 |
| SMSSC-CO2 | 9.36 ± 0.30 | 81.25 | 1.16 ± 0.07 | 97.58 a | 12.39 | 3.80 ± 0.16 | 34.52 ± 1.88 |
| Strains | Biomass Forms | Solvent | Pressure (MPa) | Temp. (°C) | Time (min) | CO2-to-Mass Ratio | Total Lipids (% DCW) | GLA (% DCW) | GLA (% TFAs) | GLA Recovery of B & D (or in situ T) (%) | Reference(s) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Arthrospira platensis | 10 g spray-dried powder (0.2 mm) (with porous materials) | CO2 + ethanol | 41 | 40 | 60 | 2 mL g−1 min−1 | 9.4 | 1.16 | 34.5 | 98 (92) | This study |
| Spirulina platensis | 16 g freeze-dried (with glass wool), crushing (0.25 mm) | CO2 + 13.7 mL ethanol | 40 | 40 | 60 | 700 mL min−1 | 8.6 | 0.64 | 15.8 | 100 (78) | [27] |
| Arthrospira maxima | 5 g freeze-dried (with glass wool), crushing (0.2 mm) | CO2 + 10 mol% ethanol | 35 | 60 | NA | 2 g min−1 | NA | 0.44 | 35.5 | 45 | [28,29] |
| Arthrospira platensis | 2 g biomass (with 4 g sea sand) | 50% CO2 + ethanol | 30 | 40 | 90 | NA | 6.7 | 0.90 | 6.5–11.7 | 25 | [30] |
| Spirulina platensis | 80 g freeze-dried (with 100 g glass microspheres), crushing (≤0.37 mm) | CO2 | 70 | 55 | 90 | 10 kg h−1 | 7.8 | 0.67 | 18.5–21.1 | NA | [32] |
| Spirulina platensis | Dried powder | CO2 | 40 | 40 | 240 | 24 kg h−1 | 7.2 | 0.29 | 23.2 | NA | [33] |
| Arthrospira platensis | Dried powder | CO2 | 30 | 60 | NA | 3 mL min−1 | NA | 0.04 | NA | NA | [34] |
| Samples | Total Lipids Yield (% DCW) | TFAs Yield (% DCW) | Percentage (%) | GLA Yield (% DCW) | Percentage (%) |
|---|---|---|---|---|---|
| Extraction vessel a | 9.36 ± 0.30 | 3.80 ± 0.16 | 72.80 | 1.16 ± 0.07 | 94.31 |
| Washing b | 1.10 ± 0.04 | 0.41 ± 0.01 | 7.85 | 0.04 ± 0.00 | 3.25 |
| Biomass residues c | ND | 1.01 ± 0.02 | 19.35 | 0.03 ± 0.00 | 2.44 |
| Total | 10.46 | 5.22 | 100 | 1.23 | 100 |
| Lipids | MGDG Acyl Chain | Lipids | MGDG Acyl Chain |
|---|---|---|---|
| 32:4 | 16:3–16:1; 16:4–16:0; 14:1–18:3; 14:2–18:2 | 33:5 | 16:2–17:3 |
| 32:3 | 16:3–16:0; 16:2–16:1; 14:0–18:3 | 33:4 | 16:1–17:3; 16:0–17:4 |
| 32:2 | 16:2–16:0; 16:1–16:1; 14:0–18:2 | 33:3 | 16:0–17:3 |
| 32:1 | 16:1–16:0 | 33:2 | 16:0–17:2 |
| 32:0 | 16:0–16:0 | 33:1 | 16:0–17:1 |
| 34:5 | 16:2–18:3; 16:1–18:4 | 35:5 | 16:1–19:4; 17:2–18:3 |
| 34:4 | 16:1–18:3 | 35:4 | 16:1–19:3; 17:1–18:3 |
| 34:3 | 16:0–18:3 | 35:3 | 16:1–19:2; 16:0–19:3 |
| 34:2 | 16:0–18:2 | 35:2 | 16:0–19:2; 16:1–19:1 |
| 34:1 | 16:0–18:1 | 35:1 | 16:0–19:1 |
| 34:0 | 16:0–18:0 | 35:0 | 16:0–19:0 |
| 36:6 | 18:3–18:3 | 36:2 | 16:0–20:2; 16:1–20:1; 18:2–18:0; 18:1–18:1 |
| 36:5 | 18:2–18:3 | 36:1 | 16:0–20:1; 16:1–20:0; 18:1–18:0 |
| 36:4 | 18:3–18:1; 18:2–18:2; 16:0–20:4; 16:1–20:3 | 36:0 | 16:0–20:0 |
| 36:3 | 16:0–20:3; 16:1–20:2; 18:3–18:0; 18:2–18:1 | ||
| Lipids | DGDG acyl chain | Lipids | DGDG acyl chain |
| 34:5 | 16:2–18:3 | 32:3 | NA |
| 34:4 | 16:1–18:3 | 32:2 | 14:2–18:0; 15:2–17:0; 13:2–19:0 |
| 34:3 | 16:0–18:3 | 32:1 | 14:1–18:0; 13:1–19:0 |
| 34:2 | 16:0–18:2 | 32:0 | NA |
| Materials | Molecular Sieve a | Macroporous Resin (HP 20) a | Diatomite a |
|---|---|---|---|
| Chemical composition | 3/4CaO·1/4Na2O·Al2O3· 2SiO2·9/2H2O | Polystyrene benzene | SiO2 (>85%) |
| Particle size (µm) | 2.5 | 420–840 (20–40 mesh) | 590 (30 mesh) |
| Unit surface area (m2 g−1) | 300–1000 | 500−600 | 40–65 |
| Pore size (nm) | 2–50 | 46 | NA |
| Moisture content (%) | <2 | 45–60 | 0.11 |
| Density (g cm−3) | 0.64 | 1.05–1.09 | 0.47 |
| Functional groups | OH− | OH−/H+ | OH− |
| Hydrophobic/hydrophilic | Hydrophilic | Hydrophilic | Hydrophilic |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Li, Y.; Li, Y.; Ye, D.; Yuan, L.; Sun, Y.; Han, D.; Hu, Q. Solid Matrix-Supported Supercritical CO2 Enhances Extraction of γ-Linolenic Acid from the Cyanobacterium Arthrospira (Spirulina) platensis and Bioactivity Evaluation of the Molecule in Zebrafish. Mar. Drugs 2019, 17, 203. https://doi.org/10.3390/md17040203
Yang X, Li Y, Li Y, Ye D, Yuan L, Sun Y, Han D, Hu Q. Solid Matrix-Supported Supercritical CO2 Enhances Extraction of γ-Linolenic Acid from the Cyanobacterium Arthrospira (Spirulina) platensis and Bioactivity Evaluation of the Molecule in Zebrafish. Marine Drugs. 2019; 17(4):203. https://doi.org/10.3390/md17040203
Chicago/Turabian StyleYang, Xiaohong, Yi Li, Yanhua Li, Ding Ye, Li Yuan, Yonghua Sun, Danxiang Han, and Qiang Hu. 2019. "Solid Matrix-Supported Supercritical CO2 Enhances Extraction of γ-Linolenic Acid from the Cyanobacterium Arthrospira (Spirulina) platensis and Bioactivity Evaluation of the Molecule in Zebrafish" Marine Drugs 17, no. 4: 203. https://doi.org/10.3390/md17040203
APA StyleYang, X., Li, Y., Li, Y., Ye, D., Yuan, L., Sun, Y., Han, D., & Hu, Q. (2019). Solid Matrix-Supported Supercritical CO2 Enhances Extraction of γ-Linolenic Acid from the Cyanobacterium Arthrospira (Spirulina) platensis and Bioactivity Evaluation of the Molecule in Zebrafish. Marine Drugs, 17(4), 203. https://doi.org/10.3390/md17040203