Cold-Adapted Glutathione S-Transferases from Antarctic Psychrophilic Bacterium Halomonas sp. ANT108: Heterologous Expression, Characterization, and Oxidative Resistance
Abstract
1. Introduction
2. Results and Discussion
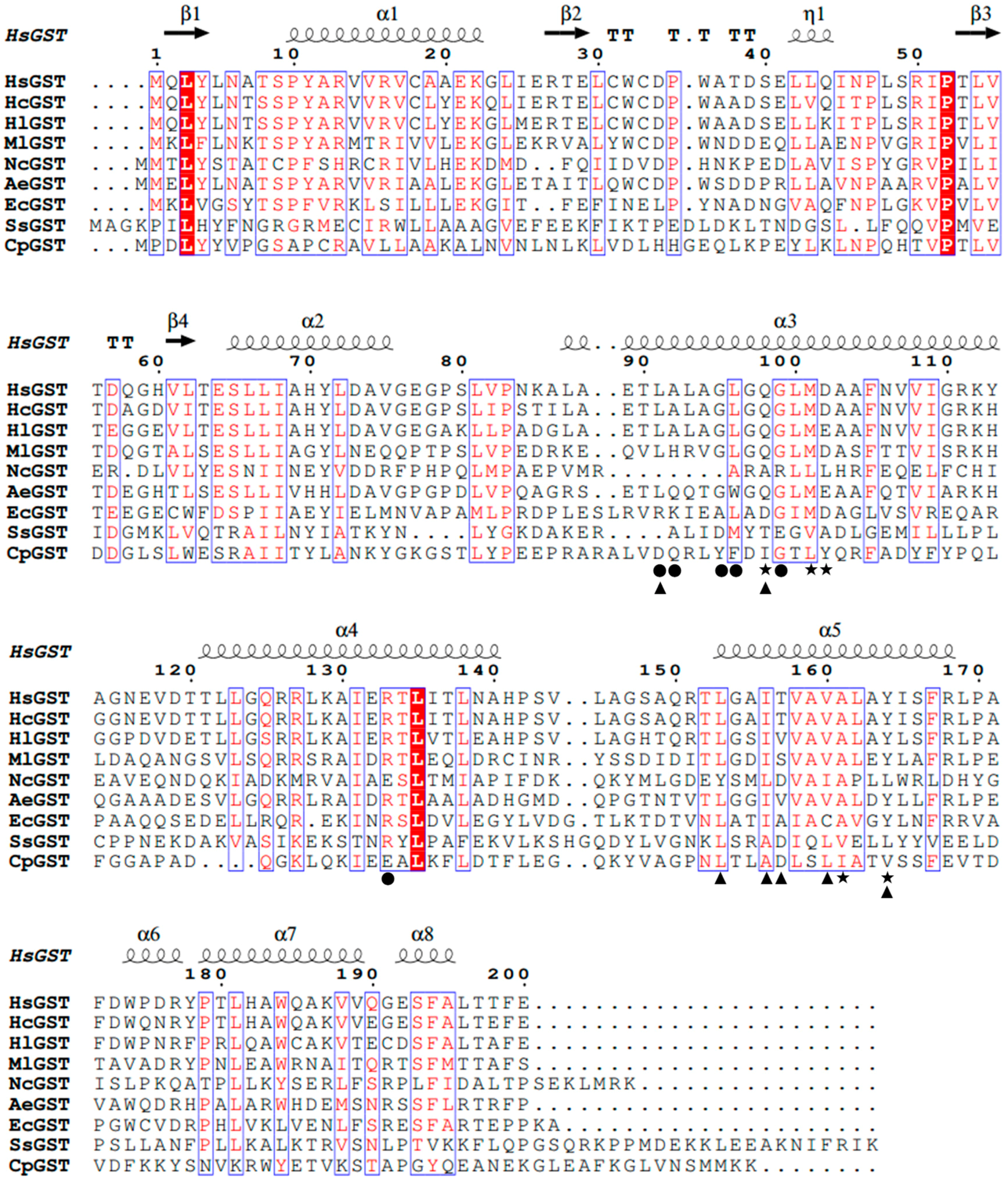
2.1. Identification of the hsgst Gene
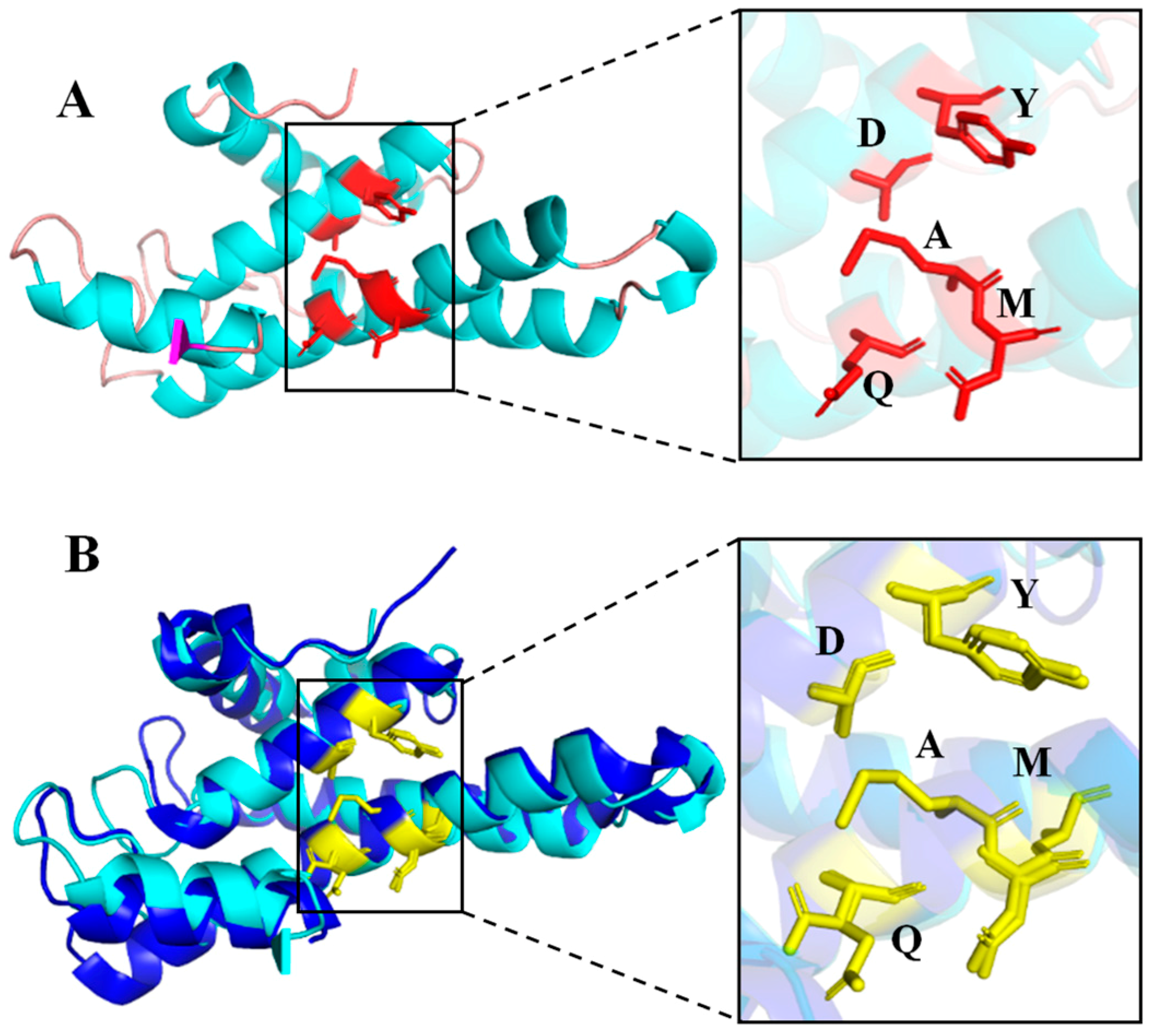
2.2. Homology Modeling Analysis
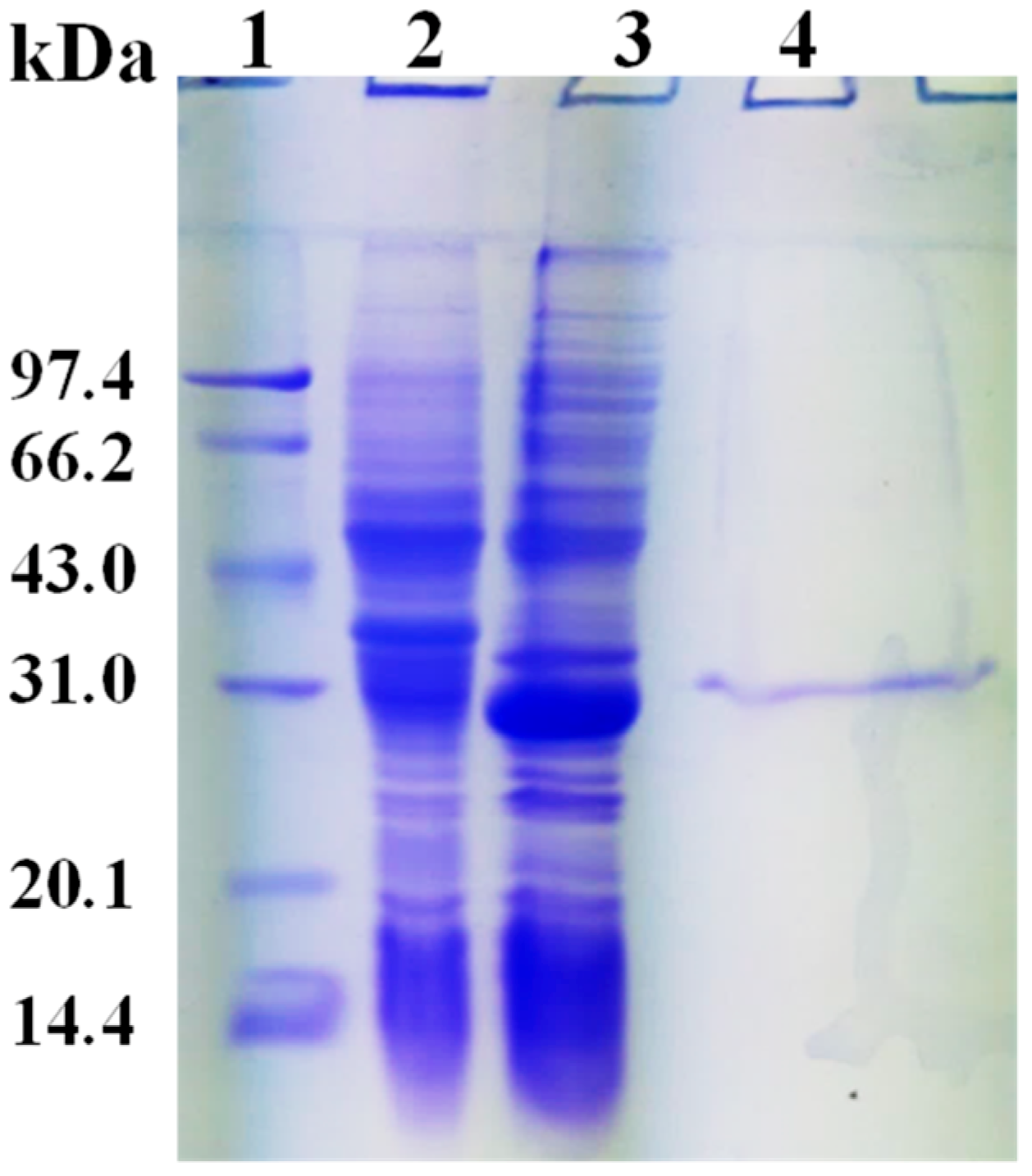
2.3. Expression and Purification of rHsGST
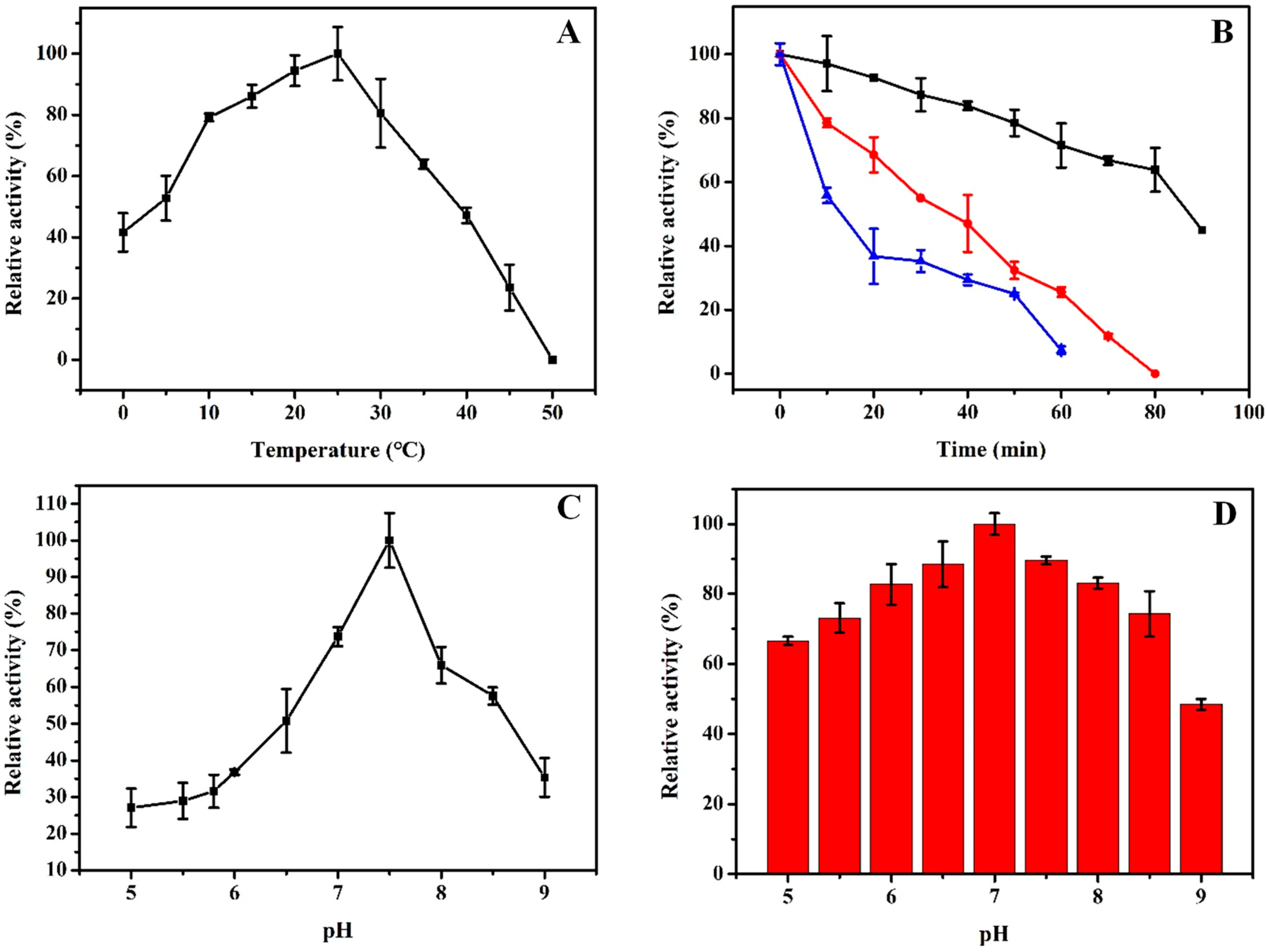
2.4. Biochemical Characterizations of rHsGST
2.5. Kinetics and Thermodynamics Parameters
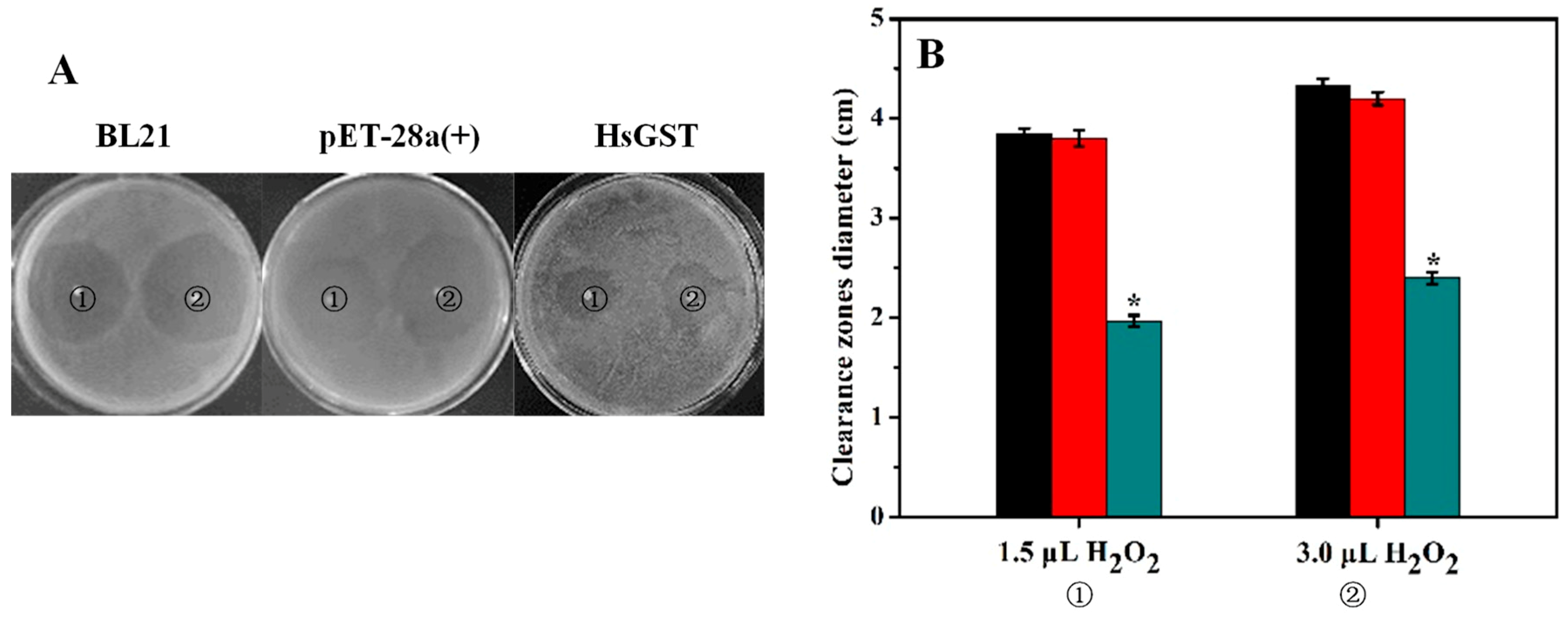
2.6. Disk Diffusion Assay
3. Materials and Methods
3.1. Strains and Materials
3.2. Identification of hsgst Gene
3.3. Analysis of HsGST
3.4. Expression and Purification of hsgst Gene in E. coli
3.5. Assay of rHsGST Activity and Protein Concentration
3.6. Biochemical Characteristics of rHsGST
3.7. Kinetics and Thermodynamics Parameters
3.8. Disk Diffusion Assay
4. Conclusions
Author Contributions
Fundings
Conflicts of Interest
References
- Kostadinova, N.; Tosi, S.; Spassova, B.; Angelova, M. Comparison of the oxidative stress response of two Antarctic fungi to different growth temperatures. Pol. Polar Res. 2017, 38, 393–408. [Google Scholar] [CrossRef]
- Chattopadhyay, M.K.; Raghu, G.; Sharma, Y.V.R.K.; Biju, A.R.; Rajasekharan, M.V.; Shivaji, S. Increase in oxidative stress at low temperature in an Antarctic bacterium. Curr. Microbiol. 2011, 62, 544–546. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, H.; Thamilarasan, S.K.; Shanmugam, A.; Natarajan, S.; Jung, H.J.; Park, J.I.; Kim, H.; Chung, M.Y.; Nou, I.S. Glutathione transferases superfamily: Cold-inducible expression of distinct GST genes in Brassica oleracea. Int. J. Mol. Sci. 2016, 17, 1211. [Google Scholar] [CrossRef] [PubMed]
- Dhar, K.; Dhar, A.; Rosazza, J.P.N. Glutathione S-transferase isoenzymes from Streptomyces griseus. Appl. Environ. Microbiol. 2003, 69, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Abunnaja, M.S.; Kurogi, K.; Mohammed, Y.I.; Sakakibara, Y.; Suiko, M.; Hassoun, E.A.; Liu, M.C. Identification and characterization of the zebrafish glutathione S-transferase Pi-1. J. Biochem. Mol. Toxicol. 2017, 31, e21948. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.Q.; Zhang, Y.L. Characterization of glutathione S-transferases from Sus scrofa, Cydia pomonella and Triticum aestivum: Their responses to cantharidin. Enzyme Microb. Technol. 2015, 69, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jung, U.; Cho, Y.S.; Seong, H.M.; Kim, S.J.; Kim, Y.C.; Chung, A.S. Characterization of a novel glutathione S-Transferase from Pseudomonas sp. DJ77. J. Biochem. Mol. Biol. 1996, 29, 111–115. [Google Scholar]
- Shi, Y.L.; Wang, Q.F.; Hou, Y.H.; Hong, Y.Y.; Han, X.; Yi, J.L.; Qu, J.J.; Lu, Y. Molecular cloning, expression and enzymatic characterization of glutathione S-transferase from Antarctic sea-ice bacteria Pseudoalteromonas sp. ANT506. Microbiol. Res. 2014, 169, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Jang, E.H.; Park, H.; Park, A.K.; Moon, J.H.; Chi, Y.M.; Ahn, I.Y. Crystallization and preliminary X-ray crystallographic studies of the q-class glutathione S-transferase from the Antarctic clam Laternula elliptica. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2008, 64, 1132–1134. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Y.; Yang, X.Q.; Zhang, Y.L. Characterization of a lambda-cyhalothrin metabolizing glutathione S-transferase CpGSTd1 from Cydia pomonella (L.). Appl. Microbiol. Biotechnol. 2014, 98, 8947–8962. [Google Scholar] [CrossRef] [PubMed]
- Kayum, M.A.; Nath, U.K.; Park, J.I.; Biswas, M.K.; Choi, E.K.; Song, J.Y.; Kim, H.T.; Nou, I.S. Genome-Wide identification, characterization, and expression profiling of glutathione S-transferase (GST) family in pumpkin reveals likely role in cold-stress tolerance. Genes 2018, 9, 84. [Google Scholar] [CrossRef] [PubMed]
- Pandey, T.; Singh, S.K.; Chhetri, G.; Tripathi, T.; Singh, A.K. Characterization of a highly pH stable chi-class glutathione S-transferase from Synechocystis PCC 6803. PLoS ONE 2015, 10, e0126811. [Google Scholar] [CrossRef] [PubMed]
- Hoarau, P.; Gnassia-Barelli, M.; Romeo, M.; Girard, J.P. Differential induction of glutathione S-transferases in the clam Ruditapes decussatus exposed to organic compounds. Environ. Toxicol. Chem. 2001, 20, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Gajewska, B.; Kazmierczak, B.; Kuzma-Kozakiewicz, M.; Jamrozik, Z.; Baranczyk-Kuzma, A. GSTP1 polymorphisms and their association with glutathione transferase and peroxidase activities in patients with motor neuron disease. CNS Neurol. Disord.-Drug Targets 2015, 14, 1328–1333. [Google Scholar] [CrossRef] [PubMed]
- Aguayo, V.; Valdes, B.; Espino, A.M. Assessment of Fasciola hepatica glutathione S-transferase as an antigen for serodiagnosis of human chronic fascioliasis. Acta Trop. 2018, 186, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.F.; Wang, Y.F.; Hou, Y.H.; Shi, Y.L.; Han, H.; Miao, M.; Wu, Y.Y.; Liu, Y.P.; Yue, X.N.; Li, Y.J. Cloning, expression and biochemical characterization of recombinant superoxide dismutase from Antarctic psychrophilic bacterium Pseudoalteromonas sp. ANT506. J. Basic Microbiol. 2016, 56, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.T.; Han, H.; Cui, B.Q.; Hou, Y.H.; Wang, Y.F.; Wang, Q.F. A glutathione peroxidase from Antarctic psychrotrophic bacterium Pseudoalteromonas sp ANT506: Cloning and heterologous expression of the gene and characterization of recombinant enzyme. Bioengineered 2017, 8, 742–749. [Google Scholar] [CrossRef] [PubMed]
- O’Dell, K.B.; Woo, H.L.; Utturkar, S.; Klingeman, D.; Brown, S.D.; Hazen, T.C. Genome sequence of Halomonas sp. Strain KO116, an ionic liquid tolerant marine bacterium isolated from a Lignin-Enriched Seawater Microcosm. Genome Announc. 2015, 3, e00402. [Google Scholar] [CrossRef]
- Gaboyer, F.; Vandenabeele-Trambouze, O.; Cao, J.; Ciobanu, M.C.; Jebbar, M.; Le Romancer, M.; Alain, K. Physiological features of Halomonas lionensis sp. nov., a novel bacterium isolated from a Mediterranean Sea sediment. Res. Microbiol. 2014, 165, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, Y.; Huang, X.; Gu, X.; Lai, W.; Peng, X.; Yang, G. Characterization of glutathione S-transferase and its immunodiagnostic potential for detecting. Taenia multiceps. Vet. Parasitol. 2017, 242, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Mannervik, B.; Danielson, U.H. Glutathione transferases—Structure and catalytic activity. CRC Crit. Rev. Biochem. 1988, 23, 283–337. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Ahn, I.Y.; Cheon, J.; Park, H. Molecular cloning and thermal stress-induced expression of a pi-class glutathione S-transferase (GST) in the Antarctic bivalve Laternula elliptica. Comp. Biochem. Phys. A. Mol. Integr. Physiol. 2009, 152, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.Q.; Pan, L.Q.; Liu, N.; Wang, L.; Miao, J.J. Cloning, characterization and tissue distribution of a pi-class glutathione S-transferase from clam (Venerupis philippinarum): Response to benzo [alpha] pyrene exposure. Comp. Biochem. Phys. C 2010, 152, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Simpson, P.J.L.; Codd, R. Cold adaptation of the mononuclear molybdoenzyme periplasmic nitrate reductase from the Antarctic bacterium Shewanella gelidimarina. Biochem. Biophys. Res. Commun. 2011, 414, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, K.S.; Cavicchioli, R. Cold-adapted enzymes. Annu. Rev. Biochem. 2006, 75, 403–433. [Google Scholar] [CrossRef] [PubMed]
- Hashim, N.H.F.; Mahadi, N.M.; Illias, R.M.; Feroz, S.R.; Abu Bakar, F.D.; Murad, A.M.A. Biochemical and structural characterization of a novel cold-active esterase-like protein from the psychrophilic yeast Glaciozyma antarctica. Extremophiles 2018, 22, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Leiros, I.; Moe, E.; Lanes, O.; Smalas, A.O.; Willassen, N.P. The structure of uracil-DNA glycosylase from Atlantic cod (Gadus morhua) reveals cold-adaptation features. Acta. Crystallogr. D 2003, 59, 1357–1365. [Google Scholar] [CrossRef] [PubMed]
- Ramli, A.N.M.; Mahadi, N.M.; Shamsir, M.S.; Rabu, A.; Joyce-Tan, K.H.; Murad, A.M.A.; Illias, R.M. Structural prediction of a novel chitinase from the psychrophilic Glaciozyma antarctica PI12 and an analysis of its structural properties and function. J. Comput. Aided Mol. Des. 2012, 26, 947–961. [Google Scholar] [CrossRef] [PubMed]
- Dou, W.; Xiao, L.S.; Niu, J.Z.; Jiang, H.B.; Wang, J.J. Characterization of the purified glutathione S-transferases from two Psocids Liposcelis bostrychophila and L. entomophila. Agric. Sci. China 2010, 9, 1008–1016. [Google Scholar] [CrossRef]
- Huang, X.L.; Fan, D.S.; Liu, L.; Feng, J.N. Identification and characterization of glutathione S-transferase genes in the Antennae of Codling Moth (Lepidoptera: Tortricidae). Ann. Entomol. Soc. Am. 2017, 110, 409–416. [Google Scholar] [CrossRef]
- Huang, Q.; Liang, L.; Wei, T.; Zhang, D.M.; Zeng, Q.Y. Purification and partial characterization of glutathione transferase from the teleost Monopterus albus. Comp. Biochem. Phys. C 2008, 147, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Zhan, S.; Xia, X.D.; Xu, P.F.; You, H.; Jin, B.R.; Li, J.H. Identification and functional characterization of an epsilon glutathione S-transferase from the beet armyworm (Spodoptera exigua). Pestic. Biochem. Phys. 2016, 132, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.H.; Jia, M.; Liu, T.; Zhang, X.Y.; Guo, Y.P.; Zhu, K.Y.; Ma, E.B.; Zhang, J.Z. Heterologous expression and characterization of a sigma glutathione S-transferase involved in carbaryl detoxification from oriental migratory locust, Locusta migratoria manilensis (Meyen). J. Insect Physiol. 2012, 58, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Jayasinghe, J.D.H.E.; Bathige, S.D.N.K.; Nam, B.H.; Noh, J.K.; Lee, J. Comprehensive characterization of three glutathione S-transferase family proteins from black rockfish (Sebastes schlegelii). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2016, 189, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Li, L.G.; Jiang, P.; Liu, R.D.; Yang, X.; Liu, L.N.; Liu, P.; Zhang, S.B.; Wang, Z.Q. Biochemical and functional characterization of the glutathione S-transferase from Trichinella spiralis. Parasitol. Res. 2015, 114, 2007–2013. [Google Scholar] [CrossRef] [PubMed]
- Qin, G.H.; Jia, M.; Liu, T.; Zhang, X.Y.; Guo, Y.P.; Zhu, K.Y.; Ma, E.B.; Zhang, J.Z. Characterization and functional analysis of four glutathione S-transferases from the Migratory Locust, Locusta migratoria. PLoS ONE 2013, 8, e58410. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, K.S.; Cavicchioli, R.; Thomas, T. Thermodynamic activation properties of elongation factor 2 (EF-2) proteins from psychrotolerant and thermophilic Archaea. Extremophiles 2002, 6, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Coyle, C.H.; Martinez, L.J.; Coleman, M.C.; Spitz, D.R.; Weintraub, N.L.; Kader, K.N. Mechanisms of H2O2-induced oxidative stress in endothelial cells. Free Radic. Biol. Med. 2006, 4, 2206–2213. [Google Scholar] [CrossRef] [PubMed]
- Sandamalika, W.M.G.; Priyathilaka, T.T.; Liyanage, D.S.; Lee, S.; Lim, H.K.; Lee, J. Molecular characterization of kappa class glutathione S-transferase from the disk abalone (Haliotis discus discus) and changes in expression following immune and stress challenges. Fish Shellfish Immunol. 2018, 77, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Yan, H.R.; Lu, W.J.; Li, Y.Z.; Guo, X.Q.; Xu, B.H. A novel Omega-class glutathione S-transferase gene in Apis cerana cerana: Molecular characterisation of GSTO2 and its protective effects in oxidative stress. Cell Stress Chaperones 2013, 18, 503–551. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Lonhienne, T.; Gerday, C.; Feller, G. Psychrophilic enzymes: Revisiting the thermodynamic parameters of activation may explain local flexibility. BBA-Protein Struct. Mol. Enzymol. 2000, 1543, 1–10. [Google Scholar] [CrossRef]
- Lee, Y.M.; Lee, K.W.; Park, H.; Park, H.G.; Raisuddin, S.; Ahn, I.Y.; Lee, J.S. Sequence, biochemical characteristics and expression of a novel Sigma-class of glutathione S-transferase from the intertidal copepod, Tigriopus japonicus with a possible role in antioxidant defense. Chemosphere 2007, 69, 893–902. [Google Scholar] [CrossRef] [PubMed]

| HsGST | EcGST | Expected Effect on HsGST | |
|---|---|---|---|
| Electrostatic interactions | Protein stability | ||
| Salt bridges | 1 | 7 | |
| Hydrogen bonds | 165 | 272 | |
| Aromatic interactions | 7 | 6 | |
| Cation-Pi interactions | 5 | 4 | |
| Hydrophobic interactions | 159 | 180 | Thermolability |
| G (Gly) | 13 | 11 | Flexibility |
| P (Pro) | 10 | 13 | |
| R (Arg) | 11 | 14 | |
| G substitution (HsGST→EcGST) | G76→N74, G78→A76, G95→A95, G97→A97, G110→Q111, G115→A116, G124→R125, G147→K147, G154→A154, G191→R191 | ||
| P substitution (EcGST→HsGST) | P64→L66, P84→A86, P114→Y113, P172→F172, P199→F199, P200→E200 | ||
| P substitution (HsGST→EcGST) | P142→V142, P170→V170, P175→C175 | Stability | |
| Reagent | Conc | Relative Activity (%) | Reagent | Conc | Relative Activity (%) |
|---|---|---|---|---|---|
| None | 100.00 | Ba2+ | 5 mM | 93.54 ± 2.90 | |
| K+ | 5 mM | 38.71 ± 3.22 | Ca2+ | 5 mM | ND |
| Ni2+ | 5 mM | ND | Mn2+ | 5 mM | 70.97 ± 3.54 |
| Fe2+ | 5 mM | 148.38 ± 6.45 | Ethanol | 25% | 29.03 ± 7.41 |
| Zn2+ | 5 mM | 41.94 ± 1.61 | H2O2 | 0.2% | 97.09 ± 2.90 |
| Mg2+ | 5 mM | 35.48 ± 7.41 | SDS | 5 mM | 54.84 ± 6.45 |
| Cu2+ | 5 mM | ND | EDTA | 5 mM | 56.45 ± 4.83 |
| Sn2+ | 5 mM | ND | DTT | 5 mM | ND |
| Substrate | CDNB | GSH |
|---|---|---|
| Vmax (nmol/min/mg) | 714.29 | 243.90 |
| Km (mM) | 2.86 | 0.27 |
| kcat (1/s) | 53.62 | 20.14 |
| kcat/Km (1/s/mM) | 18.75 | 74.59 |
| Temperature | 10 °C | 15 °C | 20 °C | 25 °C | 30 °C |
|---|---|---|---|---|---|
| ΔG (KJ/mol) | 62.85 | 63.35 | 63.82 | 64.15 | 64.59 |
| ΔH (KJ/mol) | 34.11 | 34.05 | 33.99 | 33.77 | 33.65 |
| ΔS (J/mol) | −101.48 | −101.63 | −101.77 | −101.91 | −102.05 |
| T × ΔS (KJ/mol) | −28.73 | −29.29 | −29.83 | −30.38 | −30.94 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, Y.; Qiao, C.; Wang, Y.; Wang, Y.; Ren, X.; Wei, Q.; Wang, Q. Cold-Adapted Glutathione S-Transferases from Antarctic Psychrophilic Bacterium Halomonas sp. ANT108: Heterologous Expression, Characterization, and Oxidative Resistance. Mar. Drugs 2019, 17, 147. https://doi.org/10.3390/md17030147
Hou Y, Qiao C, Wang Y, Wang Y, Ren X, Wei Q, Wang Q. Cold-Adapted Glutathione S-Transferases from Antarctic Psychrophilic Bacterium Halomonas sp. ANT108: Heterologous Expression, Characterization, and Oxidative Resistance. Marine Drugs. 2019; 17(3):147. https://doi.org/10.3390/md17030147
Chicago/Turabian StyleHou, Yanhua, Chenhui Qiao, Yifan Wang, Yatong Wang, Xiulian Ren, Qifeng Wei, and Quanfu Wang. 2019. "Cold-Adapted Glutathione S-Transferases from Antarctic Psychrophilic Bacterium Halomonas sp. ANT108: Heterologous Expression, Characterization, and Oxidative Resistance" Marine Drugs 17, no. 3: 147. https://doi.org/10.3390/md17030147
APA StyleHou, Y., Qiao, C., Wang, Y., Wang, Y., Ren, X., Wei, Q., & Wang, Q. (2019). Cold-Adapted Glutathione S-Transferases from Antarctic Psychrophilic Bacterium Halomonas sp. ANT108: Heterologous Expression, Characterization, and Oxidative Resistance. Marine Drugs, 17(3), 147. https://doi.org/10.3390/md17030147





