Effect of Fermented Fish Oil on Fine Particulate Matter-Induced Skin Aging
Abstract
1. Introduction
2. Results
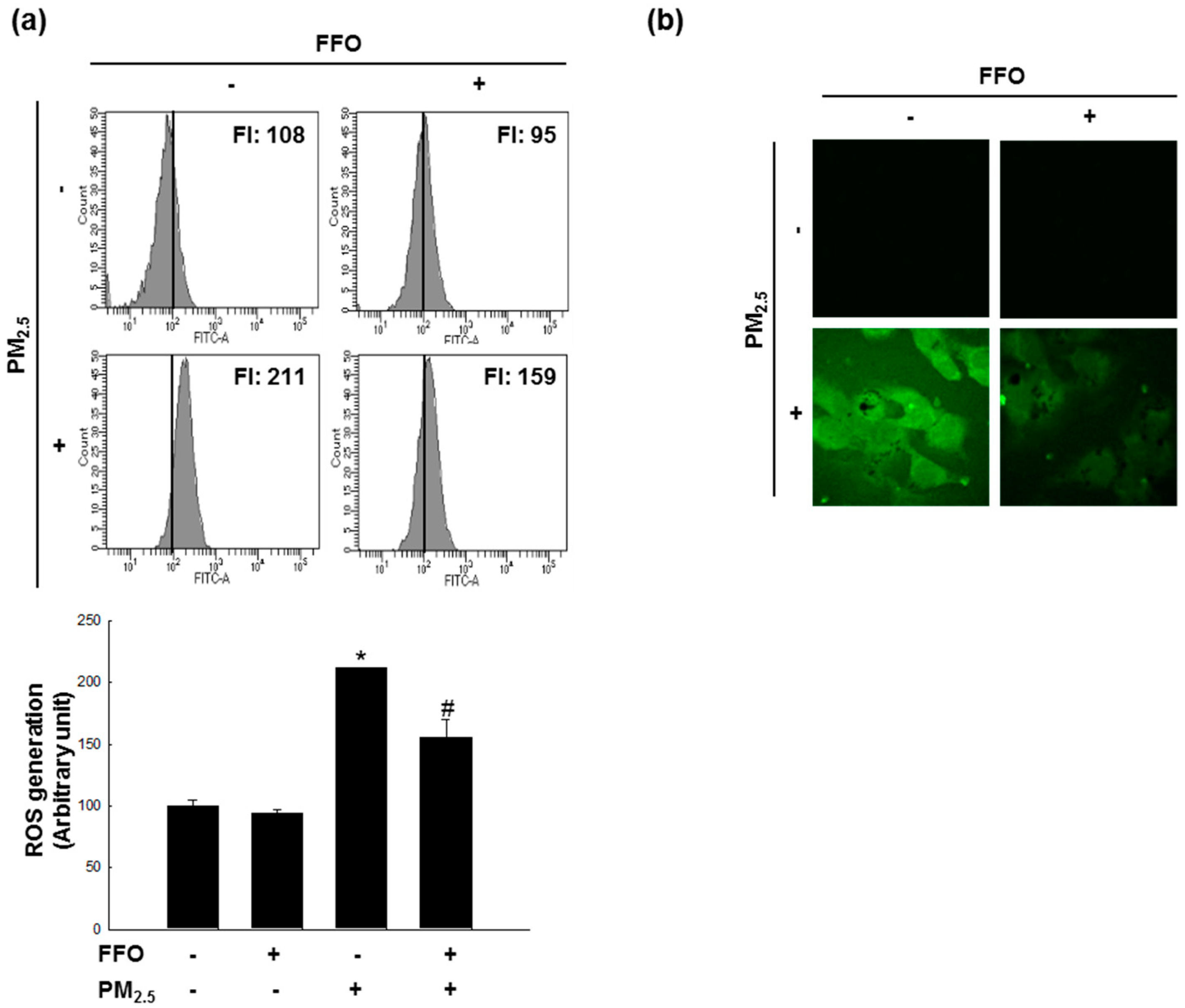
2.1. Effect of FFO on PM2.5-Induced Intracellular ROS
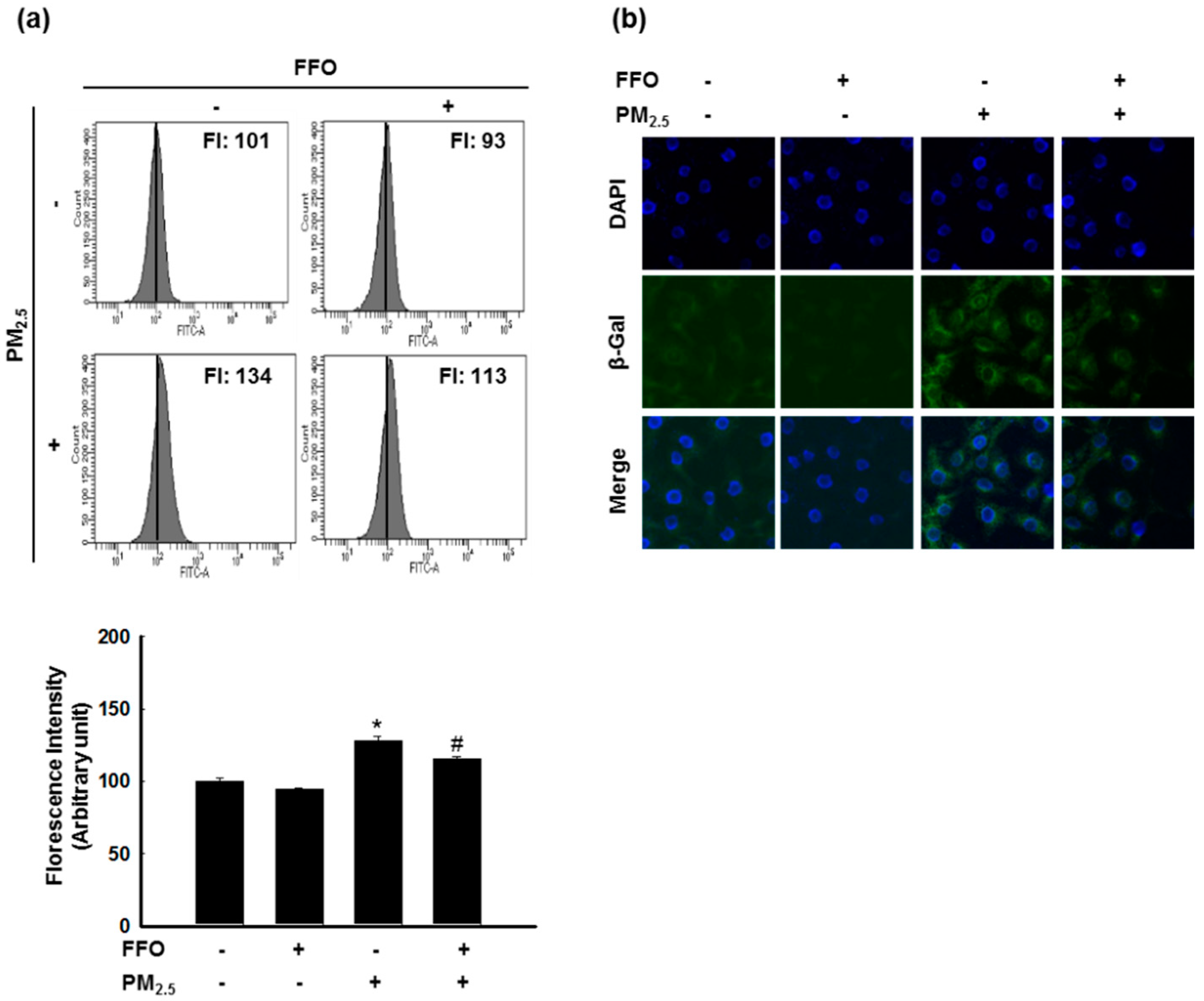
2.2. PM2.5-Induced Keratinocyte Senescence
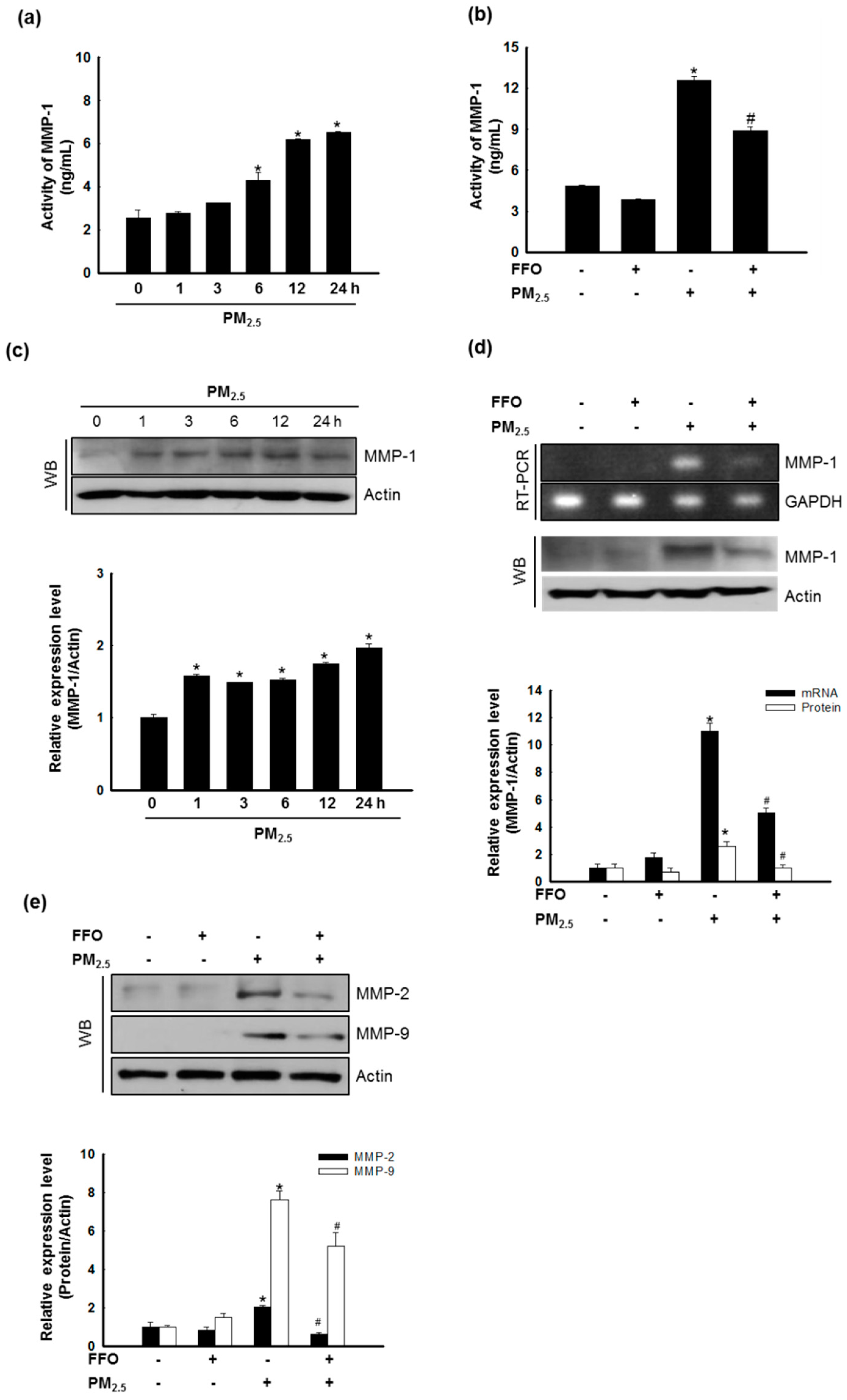
2.3. Effect of FFO on PM2.5-Induced MMP-1 Activation and MMP Expression
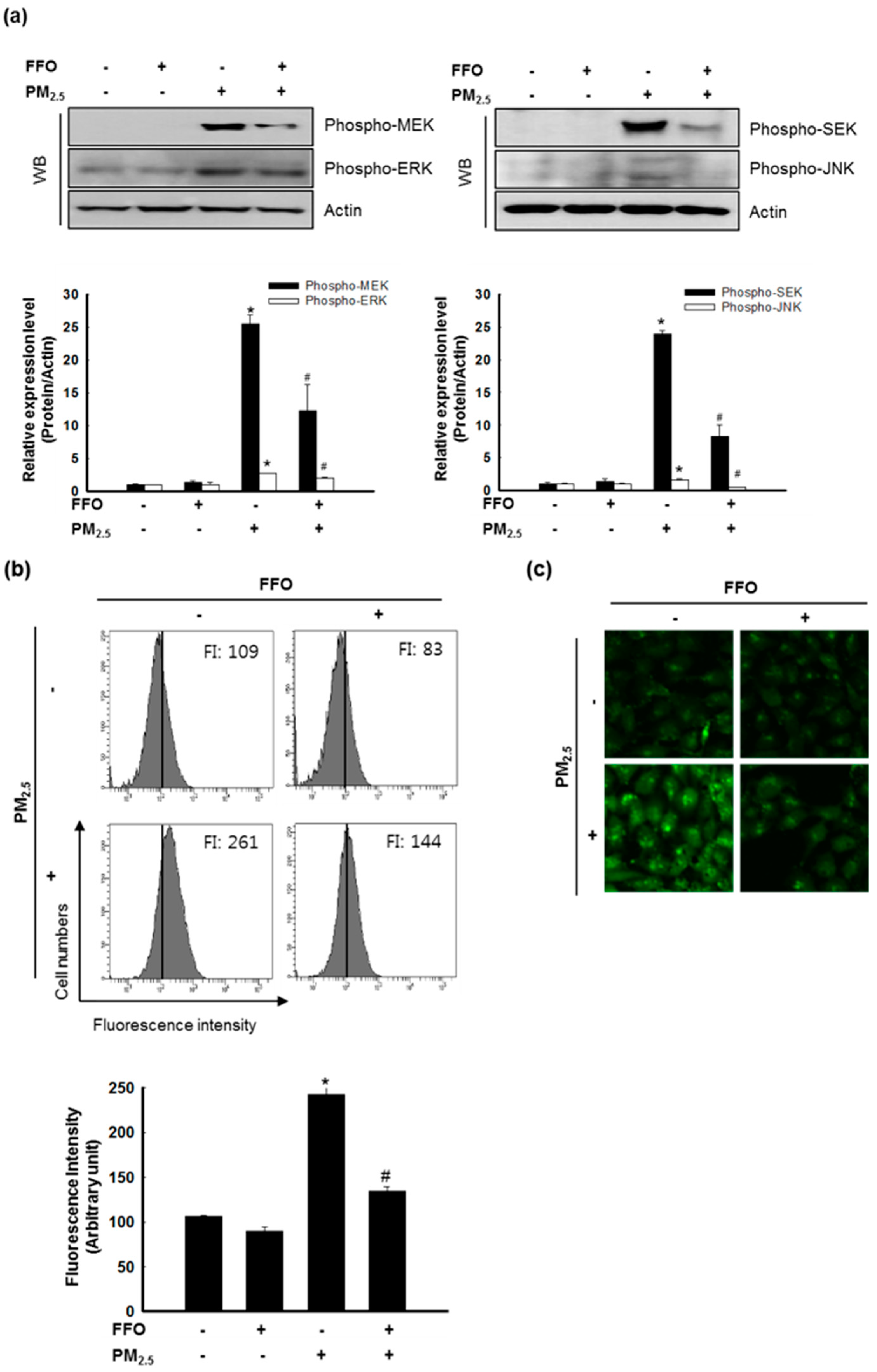
2.4. Effect of FFO on PM2.5-Induced MAPKs and Intracellular Ca2+ Levels
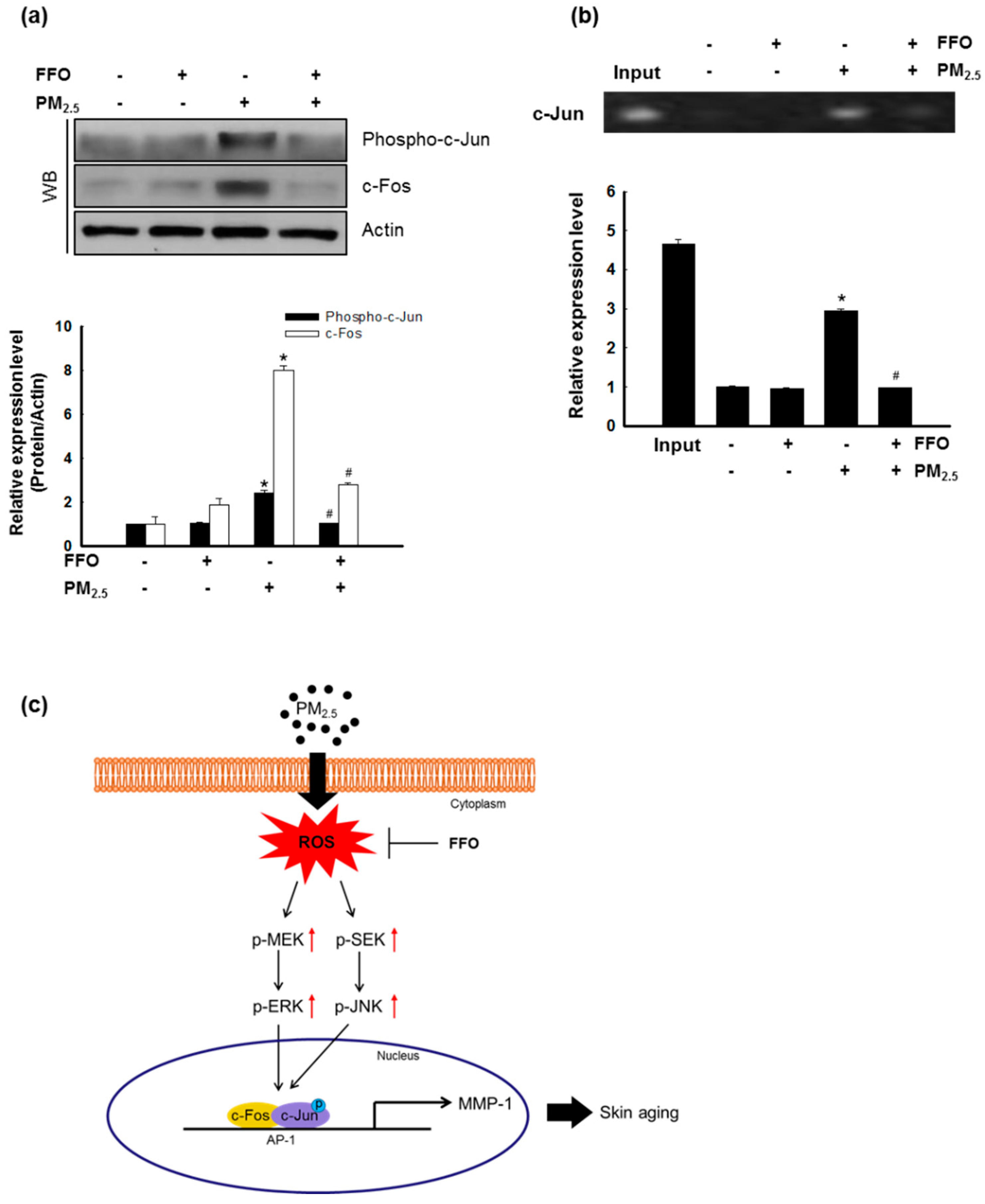
2.5. Effect of FFO on PM2.5-Induced Transcription Factor Activator Protein 1 (AP-1) Expression
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Treatment
4.2. Detection of Intracellular ROS
4.3. Detection of β-Galactosidase Activity
4.4. MMP-1 Activity
4.5. Reverse Transcription–PCR (RT-PCR)
4.6. Western Blot Analysis
4.7. Measurement of Ca2+ Levels
4.8. Chromatin Immunoprecipitation (ChIP) Assay
4.9. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- De Jager, T.L.; Cockrell, A.E.; Du Plessis, S.S. Ultraviolet Light Induced Generation of Reactive Oxygen Species. Adv. Exp. Med. Biol. 2017, 996, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Ryu, O.; Park, B.K.; Bang, M.; Cho, K.S.; Lee, S.H.; Gonzales, E.L.T.; Yang, S.M.; Kim, S.; Eun, P.H.; Lee, J.Y.; et al. Effects of several cosmetic preservatives on ROS-dependent apoptosis of rat neural progenitor cells. Biomol. Ther. 2018, 26, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Abbas, S.; Alam, S.; Pal, A.; Kumar, M.; Singh, D.; Ansari, K.M. UVB exposure enhanced benzanthrone-induced inflammatory responses in SKH-1 mouse skin by activating the expression of COX-2 and iNOS through MAP kinases/NF-κB/AP-1 signalling pathways. Food Chem. Toxicol. 2016, 96, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Ranneh, Y.; Ali, F.; Akim, A.M.; Hamid, H.A.; Khazaai, H.; Fadel, A. Crosstalk between reactive oxygen species and pro-inflammatory markers in developing various chronic diseases: A review. Appl. Biol. Chem. 2017, 60, 327–338. [Google Scholar] [CrossRef]
- Kim, K.H.; Kabir, E.; Kabir, S. A review on the human health impact of airborne particulate matter. Environ. Int. 2015, 74, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.E.; Cho, D.; Park, H.J. Air pollution and skin diseases: Adverse effects of airborne particulate matter on various skin diseases. Life Sci. 2016, 152, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.C.; Lee, C.W.; Tsai, M.H.; Ko, H.H.; Fang, J.Y.; Chiang, Y.C.; Liang, C.J.; Hsu, L.F.; Hu, S.C.S.; Yen, F.L. Eupafolin nanoparticles protect HaCaT keratinocytes from particulate matter-induced inflammation and oxidative stress. Int. J. Nanomed. 2016, 11, 3907–3926. [Google Scholar] [CrossRef]
- Seok, J.K.; Lee, J.W.; Kim, Y.M.; Boo, Y.C. Punicalagin and (-)-Epigallocatechin-3-gallate rescue cell viability and attenuate inflammatory responses of human epidermal keratinocytes exposed to airborne particulate matter PM10. Skin Pharmacol. Physiol. 2018, 31, 134–143. [Google Scholar] [CrossRef]
- Chaiprasongsuk, A.; Lohakul, J.; Soontrapa, K.; Sampattavanich, S.; Akarasereenont, P.; Panich, U. Activation of Nrf2 reduces UVA-mediated MMP-1 upregulation via MAPK/AP-1 signaling cascades: The photoprotective effects of sulforaphane and hispidulin. J. Pharmacol. Exp. Ther. 2017, 360, 388–398. [Google Scholar] [CrossRef]
- Kim, J.; Kim, M.B.; Yun, J.G.; Hwang, J.K. Protective effects of standardized siegesbeckia glabrescens extract and its active compound kirenol against UVB-induced photoaging through inhibition of MAPK/NF-κB pathways. J. Microbiol. Biotechnol. 2017, 27, 242–250. [Google Scholar] [CrossRef]
- Leirós, G.J.; Kusinsky, A.G.; Balañá, M.E.; Hagelin, K. Triolein reduces MMP-1 upregulation in dermal fibroblasts generated by ROS production in UVB-irradiated keratinocytes. J. Dermatol. Sci. 2017, 85, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Kim, S.Y.; Noh, E.M.; Song, H.K.; Lee, G.S.; Kwon, K.B.; Lee, Y.R. Reversine inhibits MMP-1 and MMP-3 expressions by suppressing of ROS/MAPK/AP-1 activation in UV-stimulated human keratinocytes and dermal fibroblasts. Exp. Dermatol. 2018, 27, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Xuan, S.H.; Park, Y.M.; Ha, J.H.; Jeong, Y.J.; Park, S.N. The effect of dehydroglyasperin C on UVB-mediated MMPs expression in human HaCaT cells. Pharmacol. Rep. 2017, 69, 1224–1231. [Google Scholar] [CrossRef] [PubMed]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of matrix metalloproteinases in photoaging and photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef]
- Lu, J.; Guo, J.H.; Tu, X.L.; Zhang, C.; Zhao, M.; Zhang, Q.W.; Gao, F.H. Tiron inhibits UVB-induced AP-1 binding sites transcriptional activation on MMP-1 and MMP-3 promoters by MAPK signaling pathway in human dermal fibroblasts. PLoS ONE 2016, 11, e0159998. [Google Scholar] [CrossRef]
- Park, J.E.; Hyun, Y.J.; Piao, M.J.; Kang, K.A.; Ryu, Y.S.; Shilnikova, K.; Zhen, A.X.; Ahn, M.J.; Ahn, Y.S.; Koh, Y.S.; et al. Mackerel-derived fermented fish oil protects skin against UVB-induced cellular damage by inhibiting oxidative stress. J. Funct. Foods 2018, 46, 147–158. [Google Scholar] [CrossRef]
- Gerber, P.A.; Buhren, B.A.; Schrumpf, H.; Hevezi, P.; Bölke, E.; Sohn, D.; Jänicke, R.; Belum, V.R.; Robert, C.; Lacouture, M.E.; et al. Mechanisms of skin aging induced by EGFR inhibitors. Support Care Cancer 2016, 24, 4241–4248. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.H.; Rhie, S.J.; Kim, Y.C. Antioxidant and skin anti-aging effects of marigold methanol extract. Toxicol. Res. 2018, 34, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Avadhani, K.S.; Manikkath, J.; Tiwari, M.; Chandrasekhar, M.; Godavarthi, A.; Vidya, S.M.; Hariharapura, R.C.; Kalthur, G.; Udupa, N.; Mutalik, S. Skin delivery of epigallocatechin-3-gallate (EGCG) and hyaluronic acid loaded nano-transfersomes for antioxidant and anti-aging effects in UV radiation induced skin damage. Drug Deliv. 2017, 24, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.H.; Chang, L.S. Caffeine induces matrix metalloproteinase-2 (MMP-2) and MMP-9 down-regulation in human leukemia U937 cells via Ca2+/ROS-mediated suppression of ERK/c-fos pathway and activation of p38 MAPK/c-jun pathway. J. Cell. Physiol. 2010, 224, 775–785. [Google Scholar] [CrossRef]
- Kim, M.; Park, Y.G.; Lee, H.J.; Lim, S.J.; Nho, C.W. Youngiasides A and C isolated from Youngia denticulatum inhibit UVB-induced MMP Expression and promote type I procollagen production via repression of MAPK/AP-1/NF-κB and activation of AMPK/Nrf2 in HaCaT cells and human dermal fibroblasts. J. Agric. Food Chem. 2015, 63, 5428–5438. [Google Scholar] [CrossRef] [PubMed]
- Romani, A.; Cervellati, C.; Muresan, X.M.; Belmonte, G.; Pecorelli, A.; Cervellati, F.; Benedusi, M.; Evelson, P.; Valacchi, G. Keratinocytes oxidative damage mechanisms related to airbone particle matter exposure. Mech. Ageing Dev. 2018, 172, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.F.; Xu, Y.H.; Shi, M.H.; Lian, Y.X. The impact of PM2.5 on the human respiratory system. J. Thorac. Dis. 2016, 8, 69–74. [Google Scholar] [CrossRef]
- Subedi, L.; Lee, T.H.; Wahedi, H.M.; Baek, S.H.; Kim, S.Y. Resveratrol-enriched rice attenuates UVB-ROS-induced skin aging via downregulation of inflammatory cascades. Oxid. Med. Cell. Longev. 2017, 2017, 8379539. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, S.; Yoshida, M.; Ando, H.; Ichihashi, M. Establishment of photoaging in vitro by repetitive UVA irradiation: Induction of characteristic markers of senescence and its prevention by PAPLAL with potent catalase activity. Photochem. Photobiol. 2018, 94, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Park, S.Y.; Hwang, E.; Zhang, M.; Seo, S.A.; Lin, P.; Yi, T.H. Thymus vulgaris alleviates UVB irradiation induced skin damage via inhibition of MAPK/AP-1 and activation of Nrf2-ARE antioxidant system. J. Cell. Mol. Med. 2017, 21, 336–348. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.P.; Oh, K.N.; Yun, H.J.; Jeong, H.G. The flavonoids apigenin and luteolin suppress ultraviolet A-induced matrix metalloproteinase-1 expression via MAPKs and AP-1-dependent signaling in HaCaT cells. J. Dermatol. Sci. 2011, 61, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Oh, G.H.; Kim, M.J.; Hwang, J.K. Fucosterol inhibits matrix metalloproteinase expression and promotes type-1 procollagen production in UVB-induced HaCaT cells. Photochem. Photobiol. 2013, 89, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Piao, M.J.; Ahn, M.J.; Kang, K.A.; Ryu, Y.S.; Hyun, Y.J.; Shilnikova, K.; Zhen, A.X.; Jeong, J.W.; Choi, Y.H.; Kang, H.K.; et al. Particulate matter 2.5 damages skin cells by inducing oxidative stress, subcellular organelle dysfunction, and apoptosis. Arch. Toxicol. 2018, 92, 2077–2091. [Google Scholar] [CrossRef] [PubMed]
- Zorin, V.; Zorina, A.; Smetanina, N.; Kopnin, P.; Ozerov, I.V.; Leonov, S.; Isaev, A.; Klokov, D.; Osipov, A.N. Diffuse colonies of human skin fibroblasts in relation to cellular senescence and proliferation. Aging 2017, 9, 1404–1413. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hyun, Y.J.; Piao, M.J.; Kang, K.A.; Zhen, A.X.; Madushan Fernando, P.D.S.; Kang, H.K.; Ahn, Y.S.; Hyun, J.W. Effect of Fermented Fish Oil on Fine Particulate Matter-Induced Skin Aging. Mar. Drugs 2019, 17, 61. https://doi.org/10.3390/md17010061
Hyun YJ, Piao MJ, Kang KA, Zhen AX, Madushan Fernando PDS, Kang HK, Ahn YS, Hyun JW. Effect of Fermented Fish Oil on Fine Particulate Matter-Induced Skin Aging. Marine Drugs. 2019; 17(1):61. https://doi.org/10.3390/md17010061
Chicago/Turabian StyleHyun, Yu Jae, Mei Jing Piao, Kyoung Ah Kang, Ao Xuan Zhen, Pincha Devage Sameera Madushan Fernando, Hee Kyoung Kang, Yong Seok Ahn, and Jin Won Hyun. 2019. "Effect of Fermented Fish Oil on Fine Particulate Matter-Induced Skin Aging" Marine Drugs 17, no. 1: 61. https://doi.org/10.3390/md17010061
APA StyleHyun, Y. J., Piao, M. J., Kang, K. A., Zhen, A. X., Madushan Fernando, P. D. S., Kang, H. K., Ahn, Y. S., & Hyun, J. W. (2019). Effect of Fermented Fish Oil on Fine Particulate Matter-Induced Skin Aging. Marine Drugs, 17(1), 61. https://doi.org/10.3390/md17010061



