A Place to Call Home: An Analysis of the Bacterial Communities in Two Tethya rubra Samaai and Gibbons 2005 Populations in Algoa Bay, South Africa
Abstract
:1. Introduction
2. Results
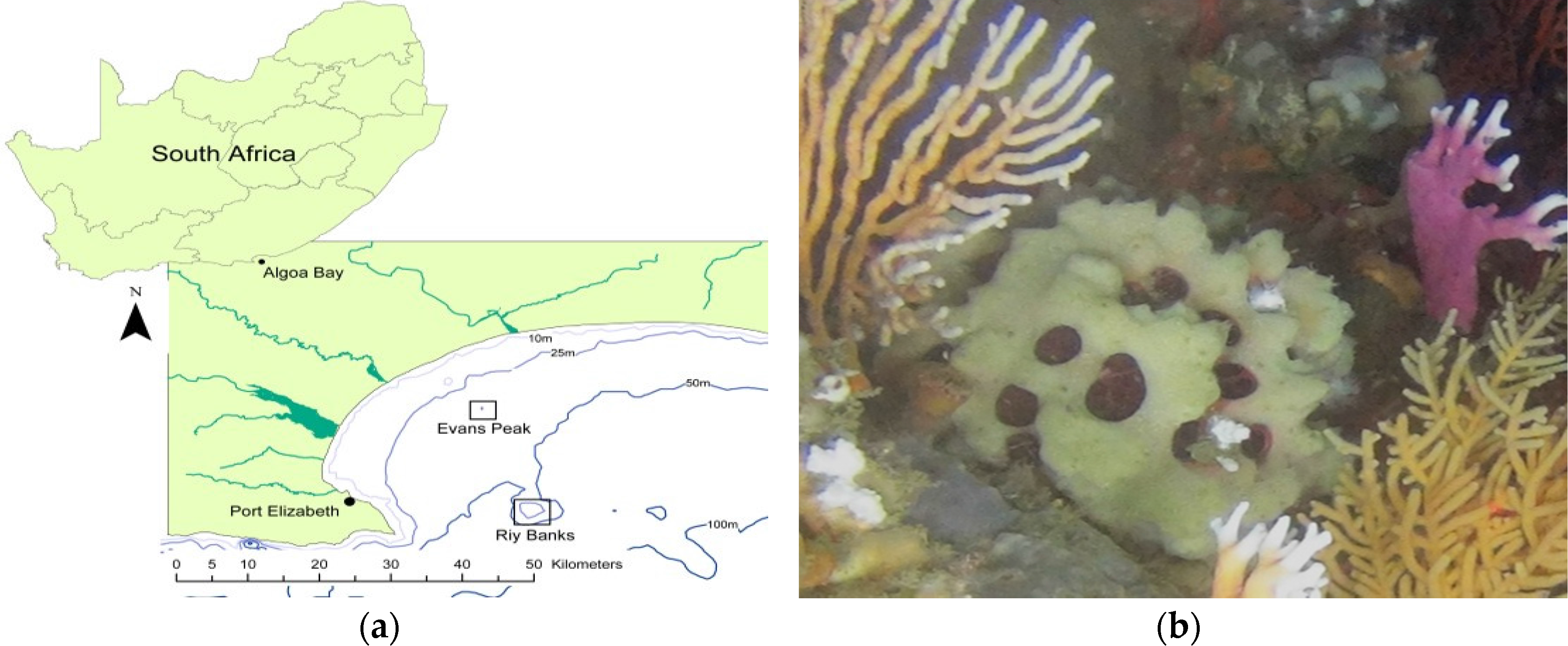
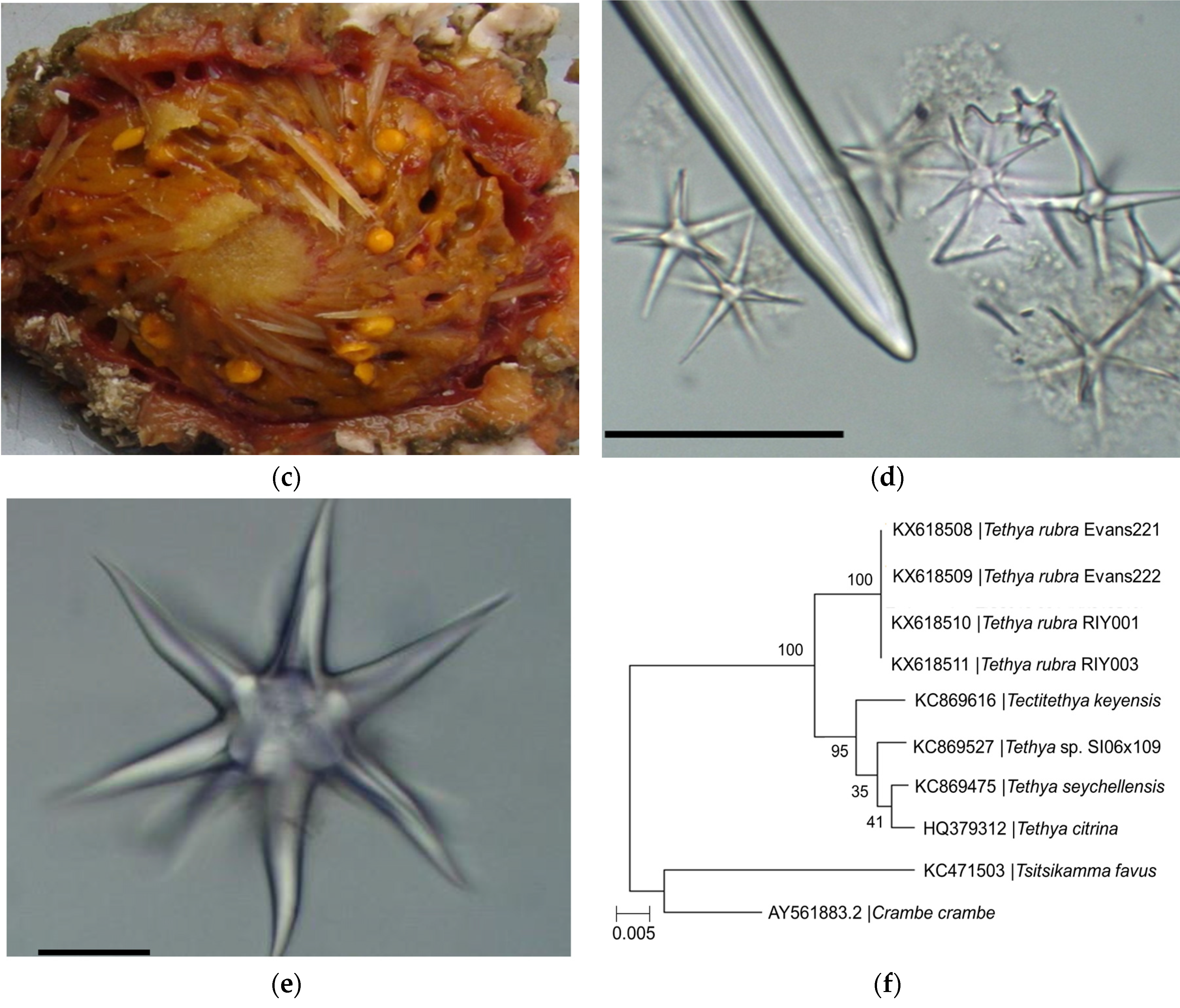
2.1. Identification of Sponge Specimens
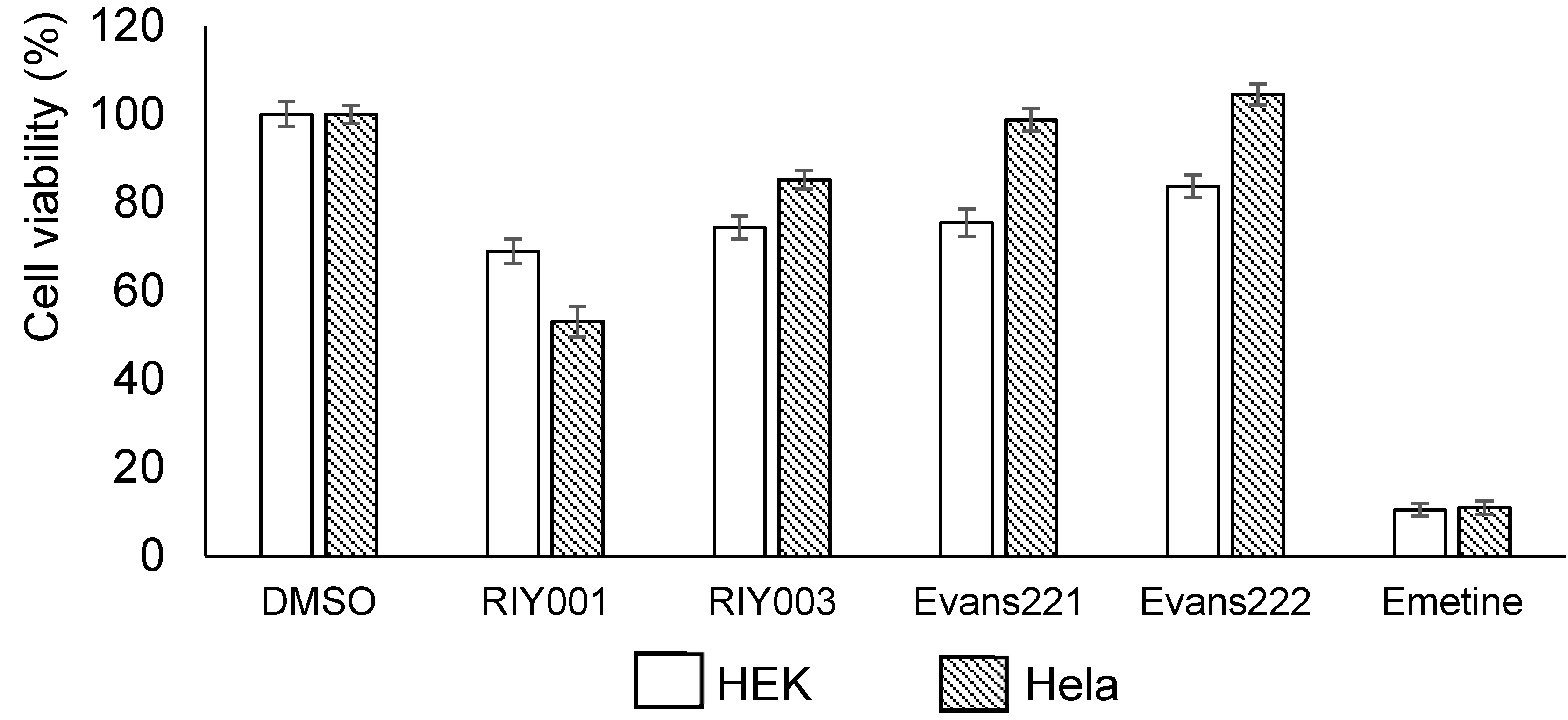
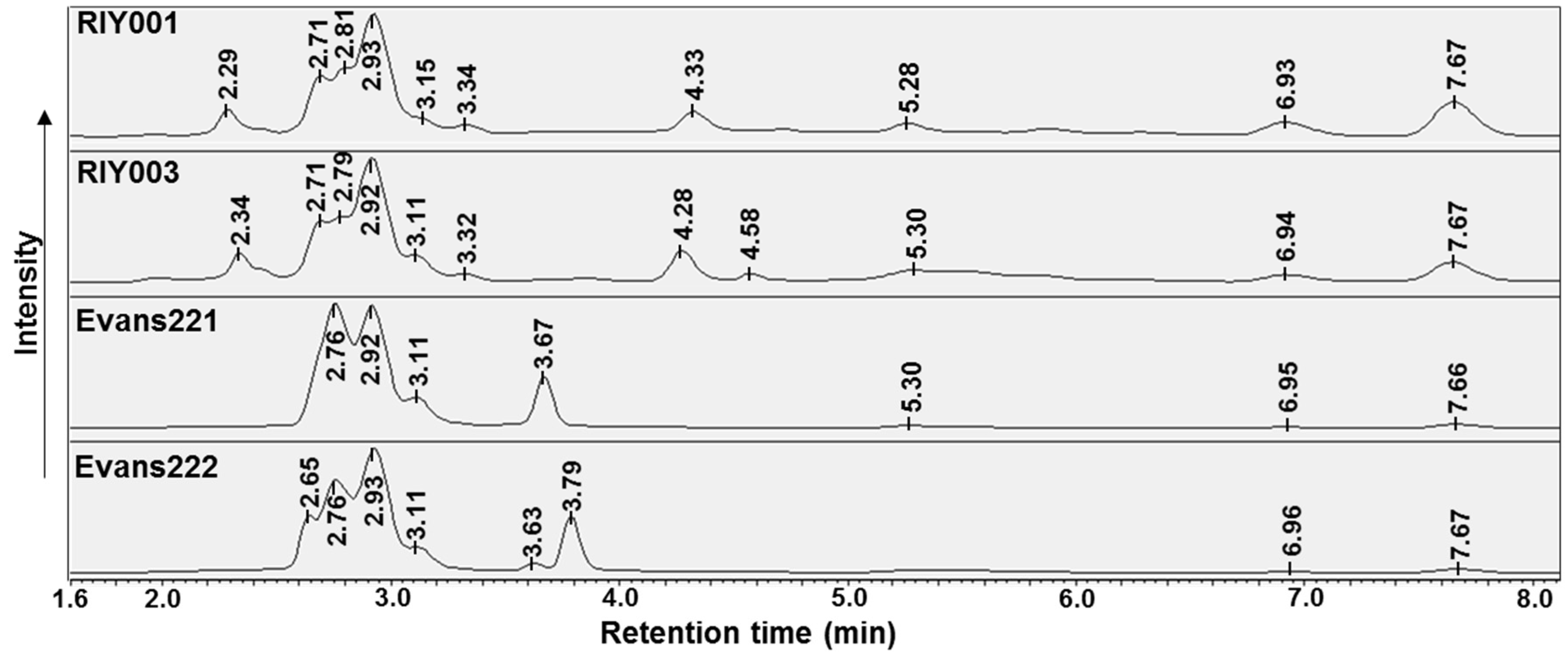
2.2. Biological Activity Assays
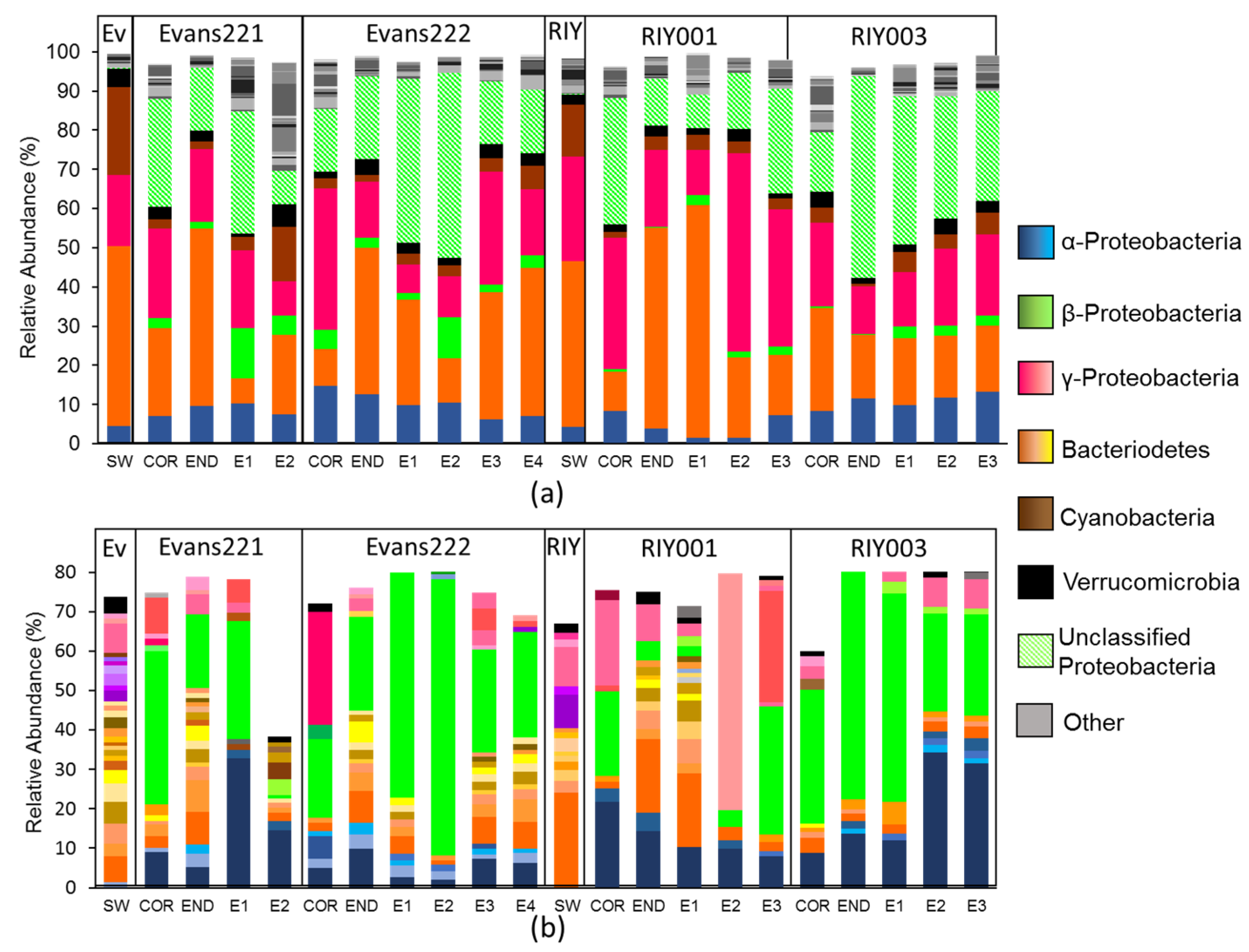
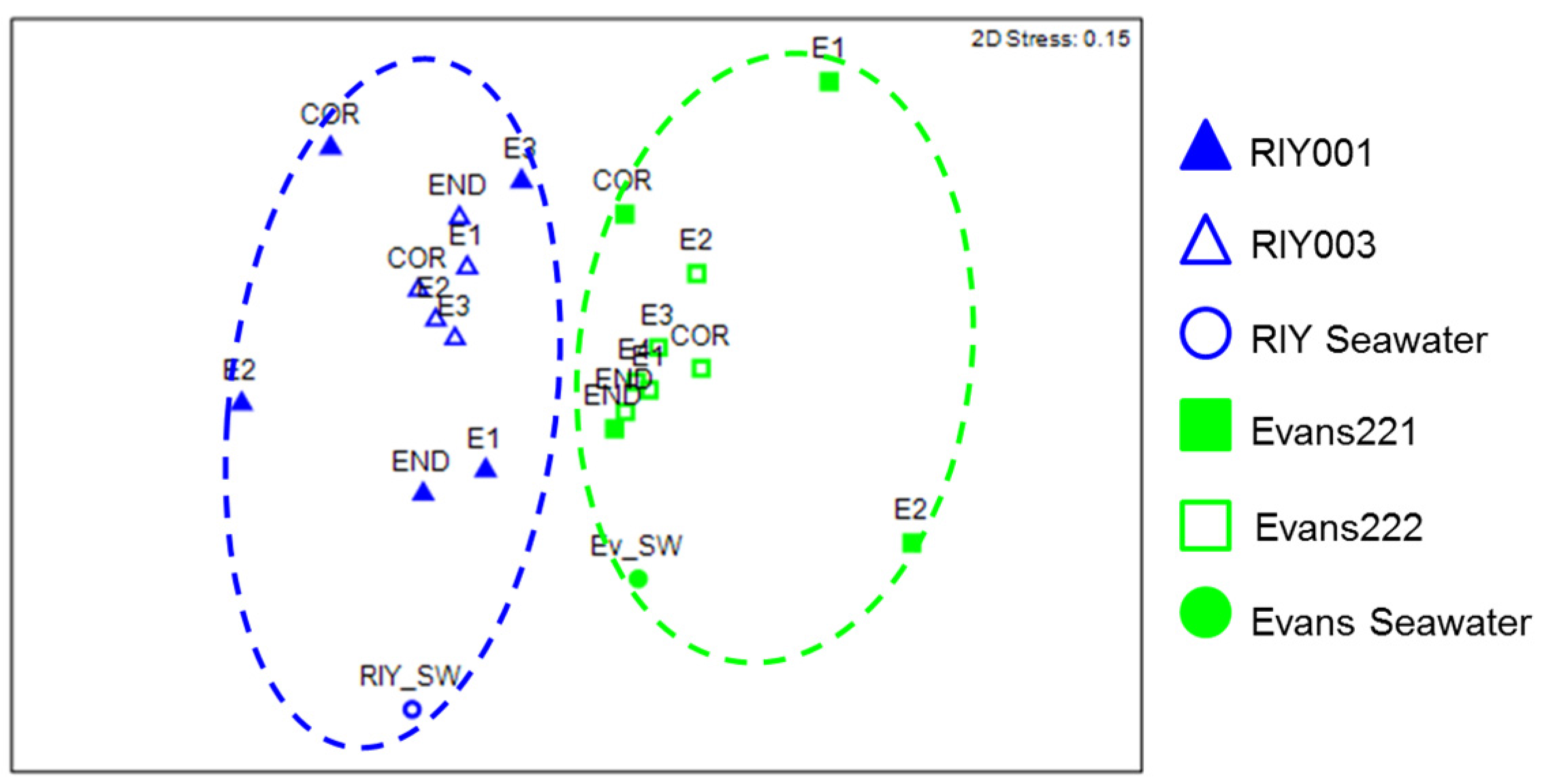
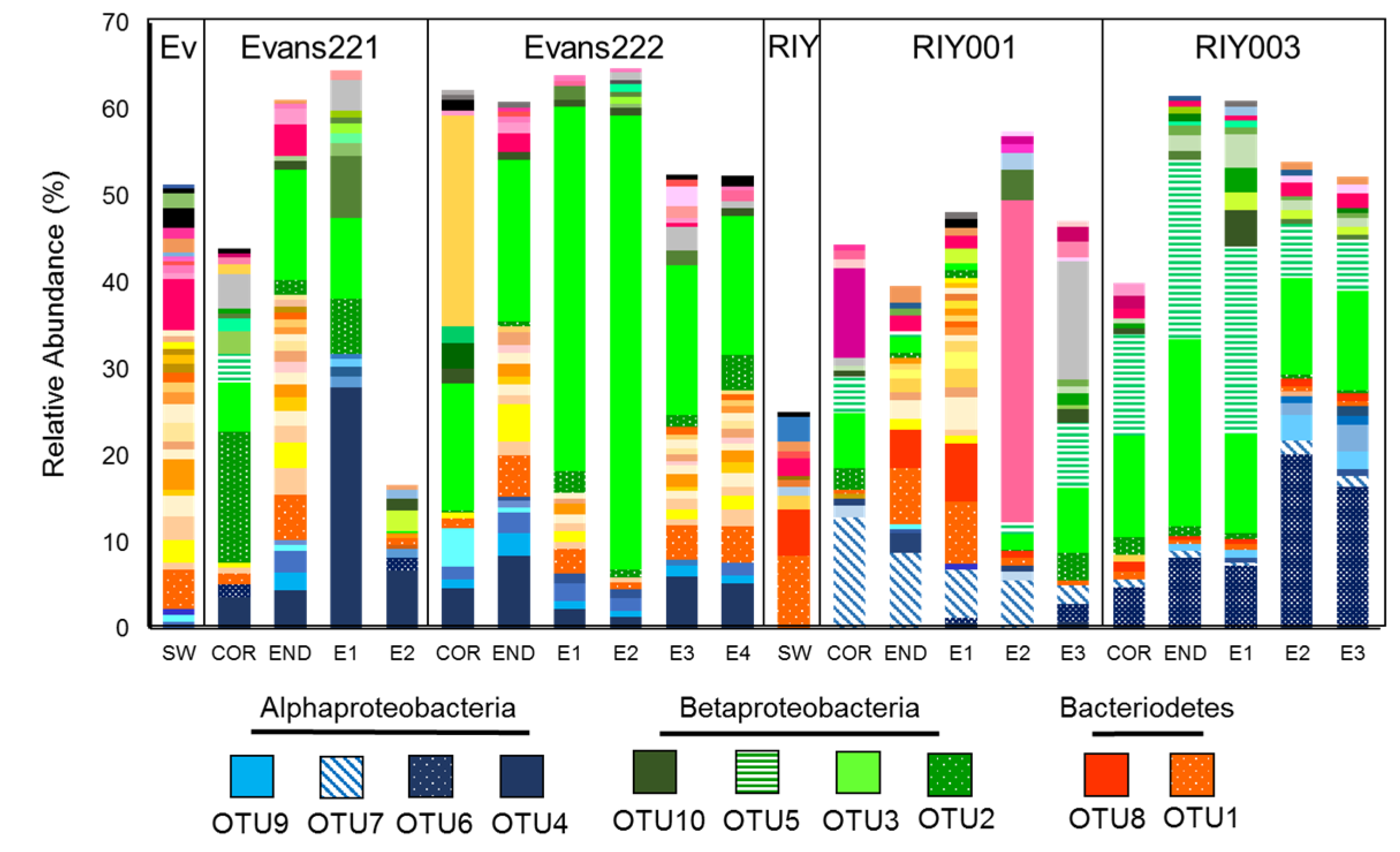
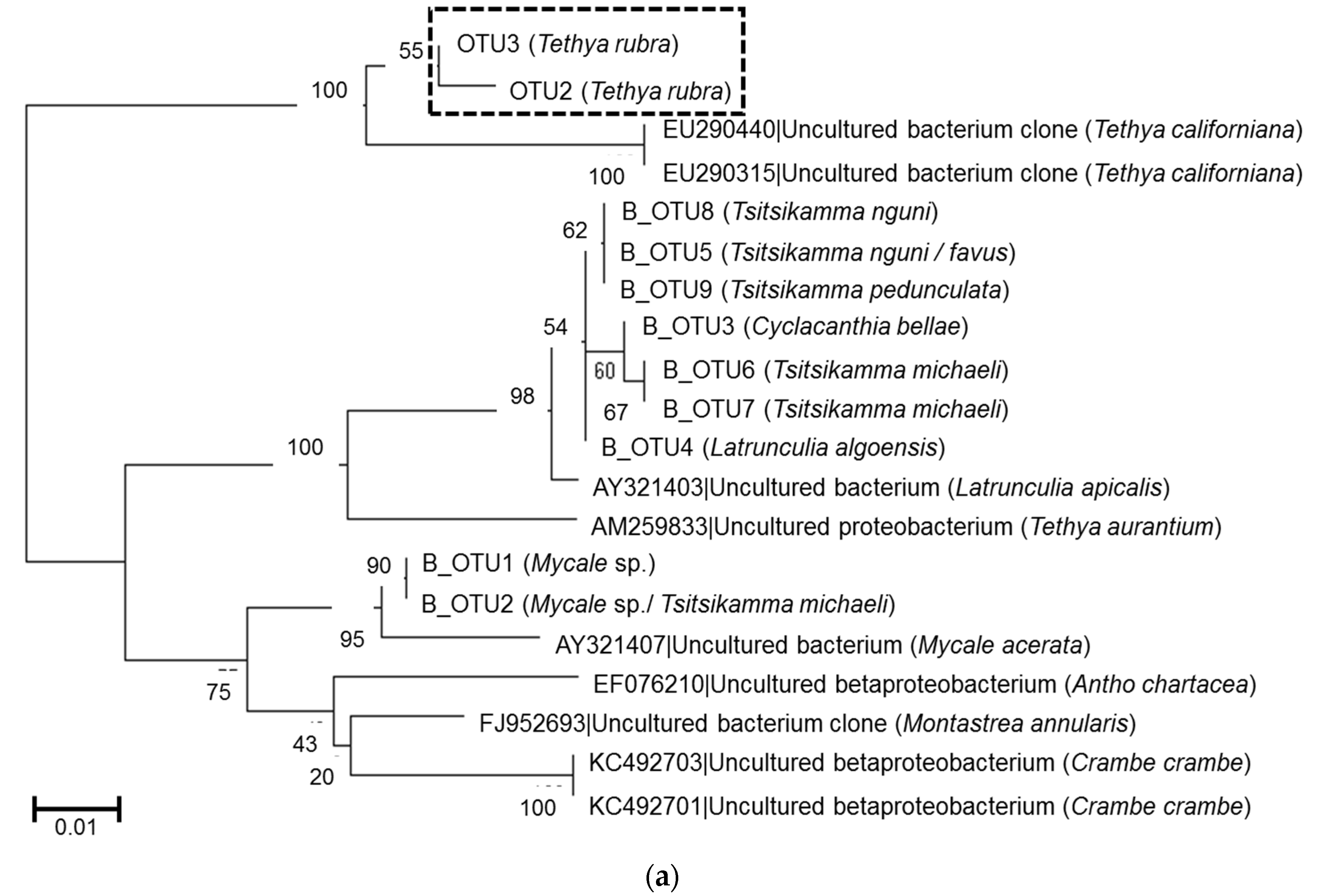
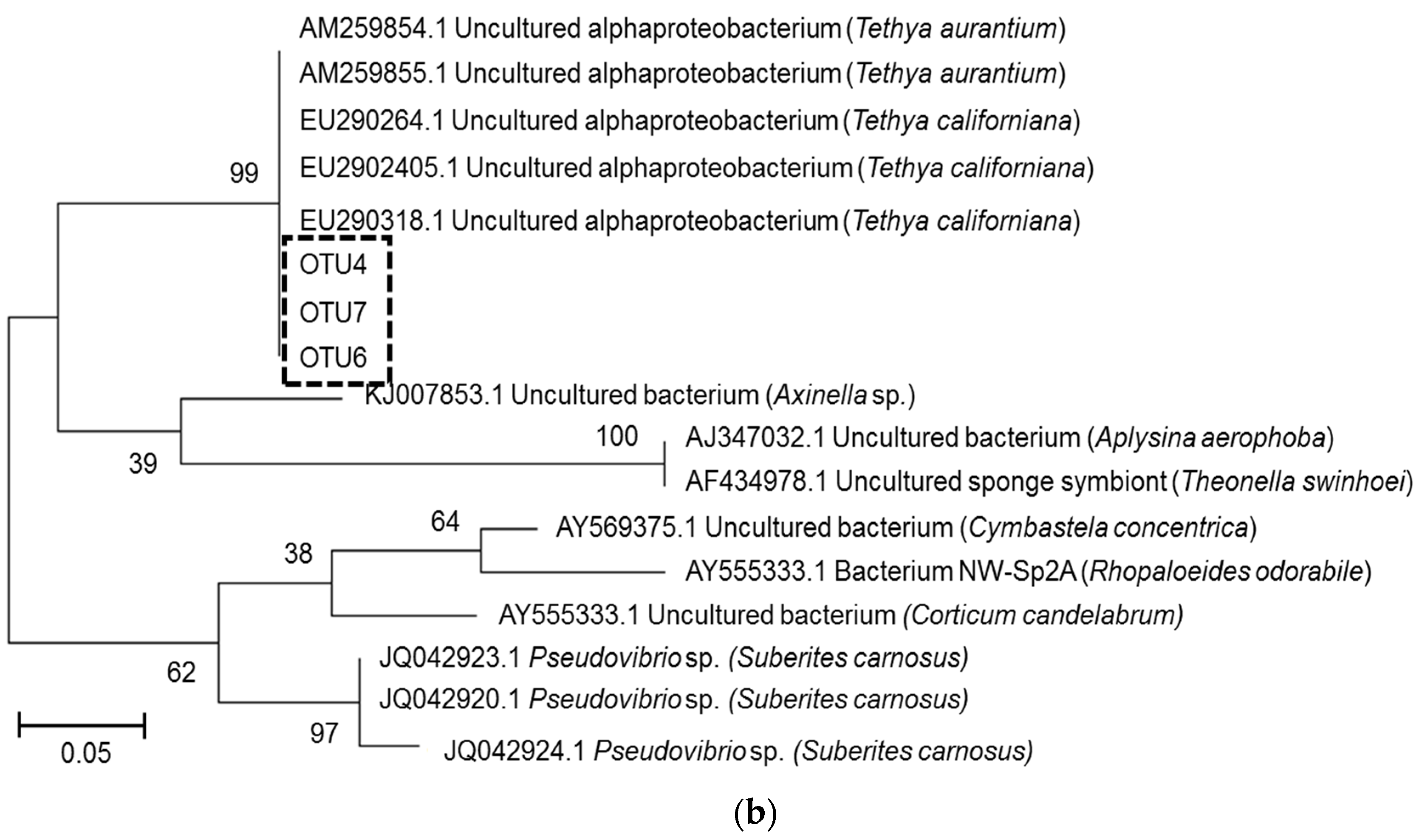
2.3. Microbial Community Analysis
3. Discussion
4. Materials and Methods
4.1. Sample Collection
4.2. Sample Processing and DNA Extraction
4.3. Identification of Sponge Specimens
4.4. Generation of Crude Extracts
4.5. Bioactivity Assays
4.6. UV-HPLC Analysis
4.7. Amplicon Library Preparation and Pyrosequencing
4.8. Sequence Data Curation and Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Vogel, S. Current-induced flow through living sponges in nature. Proc. Natl. Acad. Sci. USA 1977, 74, 2069–2071. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.W.; Radax, R.; Steger, D.; Wagner, M. Sponge-Associated Microorganisms: Evolution, Ecology, and Biotechnological Potential. Microbiol. Mol. Biol. Rev. 2007, 71, 295–347. [Google Scholar] [CrossRef] [PubMed]
- Bright, M.; Bulgheresi, S. A complex journey: Transmission of microbial symbionts. Nat. Rev. Microbiol. 2010, 8, 218–230. [Google Scholar] [CrossRef] [PubMed]
- De Voogd, N.J.; Cleary, D.F.R.; Polónia, A.R.M.; Gomes, N.C.M. Bacterial community composition and predicted functional ecology of sponges, sediment and seawater from the thousand islands reef complex, West Java, Indonesia. FEMS Microbiol. Ecol. 2015, 91, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hentschel, U.; Usher, K.M.; Taylor, M.W. Marine sponges as microbial fermenters. FEMS Microbiol. Ecol. 2006, 55, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Ribes, M.; Jimenez, E.; Yahel, G.; Lopez-Sendino, P.; Diez, B.; Massana, R.; Sharop, J.H.; Coma, R. Functional convergence of microbes associated with temperate marine sponges. Environ. Microbiol. 2012, 14, 1224–1239. [Google Scholar] [CrossRef] [PubMed]
- Freeman, M.F.; Vagstad, A.L.; Piel, J. Polytheonamide biosynthesis showcasing the metabolic potential of sponge-associated uncultivated ‘Entotheonella’ bacteria. Curr. Opin. Chem. Biol. 2016, 31, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Keren, R.; Lavy, A.; Mayzel, B.; Ilan, M. Culturable associated-bacteria of the sponge Theonella swinhoei show tolerance to high arsenic concentrations. Front. Microbiol. 2015, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Webster, N.S.; Taylor, M.W. Marine sponges and their microbial symbionts: Love and other relationships. Environ. Microbiol. 2012, 14, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Wakimoto, T.; Egami, Y.; Nakashima, Y.; Wakimoto, Y.; Mori, T.; Awakawa, T.; Ito, T.; Kenmoku, H.; Asakawa, Y.; Piel, J.; et al. Calyculin biogenesis from a pyrophosphate protoxin produced by a sponge symbiont. Nat. Chem. Biol. 2014, 10, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Van Soest, R. Latrunculiidae Topsent. World Porifera Database. van Soest, R.W.M., Boury-Esnault, N., Hooper, J.N.A., Rützler, K., de Voogd, N.J., de Alvarez Glasby, B., Hajdu, E., Pisera, A.B., Manconi, R., Schoenberg, C., et al., Eds.; 1922. Available online: http://www.marinespecies.org/aphia.php?p=taxdetails&id=131671 (accessed on 11 January 2016).
- Sarà, M. Family Tethyidae Gray, 1848. In Systema Porifera: A Guide to the Classification of Sponges; Hooper, J., van Soest, R.W.M., Eds.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2002; Volume 2, pp. 245–265. [Google Scholar]
- Sarà, M.; Bavestrello, G. Tethya omanensis, a remarkable new species from an Oman cave (Porifera, Demospongiae). B Zool. 1995, 62, 23–27. [Google Scholar] [CrossRef]
- Bowerbank, J.S. Contributions to a General History of the Spongiadae. Part I. Proc. Zool. Soc. 1872, 142–143, 115–129. [Google Scholar]
- De Paula, J.C.; Desoti, V.C.; Sampiron, E.G.; Martins, S.C.; Ueda-Nakamura, T.; Ribiero, S.M.; Bianco, E.M.; Silva, S.O.; de Oliveira, G.G.; Nakamura, C.V. Trypanocidal activity of organic extracts from the Brazilian and Spanish marine sponges. Braz. J. Pharmacog. 2015, 25, 651–656. [Google Scholar] [CrossRef]
- Ribiero, S.M.; Cassiano, K.M.; Cavalcanti, D.N.; Teixeira, V.L.; Pereira, R.C. Isolated and synergistic effects of chemical and structural defenses of two species of Tethya (Porifera: Demospongiae). J. Sea Res. 2012, 68, 57–62. [Google Scholar] [CrossRef]
- Bergmann, W.; Feeney, R.J. The Isolation of a New Thyine Pentoside from Sponges1. J. Am. Chem. Soc. 1950, 72, 2809–2810. [Google Scholar] [CrossRef]
- Bergmann, W.; Feeney, R.J. Contributions to the study of Marine Products. XXXII. The Nucleosides of Sponges1. J. Org. Chem. 1951, 16, 981–987. [Google Scholar] [CrossRef]
- Walwick, E.A.; Dekker, C.A.; Roberts, W.K. Cyclization during Phosphorylation of Uridine and Cytidine by Polyphosphoric Acid—A New Route to the 0-2-2’ Cyclonucleosides. Proc. Chem. Soc. 1959, 3, 84. [Google Scholar]
- Lee, W.W.; Benitez, A.; Goodman, L.; Baker, B.R. Potential anticancer agents. 1 xl. synthesis of the β-anomer of 9-(d-arabinofuranosyl)-adenine. J. Am. Chem. Soc. 1960, 82, 2648–2649. [Google Scholar]
- Bertin, M.J.; Schwartz, S.L.; Lee, J.; Korobeynikov, A.; Dorrestein, P.C.; Gerwick, L.; Gerwick, W.H. Spongosine Production by a Vibrio harveyi Strain Associated with the Sponge Tectitethya crypta. J. Nat. Prod. 2015, 78, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y. Isolation and Structure Elucidation of Bioactive Secondary Metabolites from the Sponge-Associated Fungus Aspergillus sp.; Heinrich-Heine-Universität Düsseldorf: Düsseldorf, Germany, 2012. [Google Scholar]
- Wiese, J.; Ohelendorf, B.; Blumel, M.; Schmaljohann, R.; Imhoff, J.F. Phylogenetic Identification of Fungi Isolated from the Marine Sponge Tethya aurantium and Identification of Their Secondary Metabolites. Mar. Drugs 2011, 9, 561–585. [Google Scholar] [CrossRef] [PubMed]
- Samaai, T.; Gibbons, M.J. Demospongiae taxonomy and biodiversity of the Benguela region on the west coast of South Africa. Afr. Nat. Hist. 2005, 1, 1–96. [Google Scholar]
- Thiel, V.; Neulinger, S.C.; Staufenberger, T.; Schmaljohann, R.; Imhoff, J.F. Spatial distribution of sponge-associated bacteria in the Mediterranean sponge Tethya aurantium. FEMS Microbiol. Ecol. 2007, 59, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0. Molecular Biology and Evolution. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Matcher, G.F.; Waterworth, S.C.; Walmsley, T.A.; Matsatsa, T.; Parker-Nance, S.; Davies Coleman, M.T.; Dorrington, R.A. Keeping it in the family: Co-evolution of latrunculid sponges and their dominant bacterial symbionts. Microbiologyopen 2016. [Google Scholar] [CrossRef] [PubMed]
- Croue, J.; West, N.J.; Ecande, M.L.; Intertaglia, L.; Lebaron, P.; Suzuki, M.T. A single betaproteobacterium dominates the microbial community of the crambescidine-containing sponge Crambe crambe. Sci. Rep. 2013, 3, 2583. [Google Scholar] [CrossRef] [PubMed]
- Webster, N.S.; Wilson, K.J.; Blackall, L.L.; Hill, R.T. Phylogenetic Diversity of Bacteria Associated with the Marine Sponge Rhopaloeides odorabile. Appl. Environ. Microbiol. 2001, 67, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Webster, N.S.; Negri, A.P.; Munro, M.M.H.G.; Battershill, C.N. Diverse microbial communities inhabit Antarctic sponges. Environ. Microbiol. 2004, 6, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, M.A.; Watson, J.R.; Degnan, S.M. Draft Genomes Shed Light on the Dual Bacterial Symbiosis that Dominates the Microbiome of the Coral Reef Sponge Amphimedon queenslandica. Front. Mar. Sci. 2016. [Google Scholar] [CrossRef]
- Sipkema, D.; Blanch, H.W. Spatial distribution of bacteria associated with the marine sponge Tethya californiana. Mar. Biol. 2010, 157, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Simister, R.L.; Deines, P.; Botté, E.S.; Webster, N.S.; Taylor, M.W. Sponge-specific clusters revisited: A comprehensive phylogeny of sponge-associated microorganisms. Environ. Microbiol. 2012, 14, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Webster, N.S.; Hill, R.T. The culturable microbial community of the great barrier reef sponge Rhopaloeides odorabile is dominated by an Alpha-Proteobacterium. Mar. Biol. 2001, 138, 843–851. [Google Scholar] [CrossRef]
- Enticknap, J.J.; Kelly, M.; Peraud, O.; Hill, R.T. Characterization of a Culturable Alphaproteobacterial Symbiont Common to Many Marine Sponges and Evidence for Vertical Transmission via Sponge Larvae. Appl. Environ. Microbiol. 2006, 72, 3724–3732. [Google Scholar] [CrossRef] [PubMed]
- Peneseyen, A.; Tebben, J.; Lee, M.; Thomas, T.; Kjelleberg, S.; Harder, T.; Egan, S. Identification of the Antibacterial Compound Produced by the Marine Epiphytic Bacterium Pseudovibrio sp. D323 and Related Sponge-Associated Bacteria. Mar. Drugs 2011, 9, 1391–1402. [Google Scholar] [CrossRef] [PubMed]
- McCormack, G.P.; Kelly, M. New indications of the phylogenetic affinity of Spongosorites suberitoides Diaz et al. 1993 (Porifera Demospongiae) as revealed by 28s ribosomal DNA. J. Nat. Hist. 2002, 36, 1009–1021. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qian, P.Y. Conservative fragments in bacterial 16S rRNA genes and primer design for 16S ribosomal DNA amplicons in metagenomic studies. PLoS ONE 2009, 4, e7401. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2008. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2.4-1. Available online: https://CRAN.R-project.org/package=vegan (accessed on 11 January 2016).
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waterworth, S.C.; Jiwaji, M.; Kalinski, J.-C.J.; Parker-Nance, S.; Dorrington, R.A. A Place to Call Home: An Analysis of the Bacterial Communities in Two Tethya rubra Samaai and Gibbons 2005 Populations in Algoa Bay, South Africa. Mar. Drugs 2017, 15, 95. https://doi.org/10.3390/md15040095
Waterworth SC, Jiwaji M, Kalinski J-CJ, Parker-Nance S, Dorrington RA. A Place to Call Home: An Analysis of the Bacterial Communities in Two Tethya rubra Samaai and Gibbons 2005 Populations in Algoa Bay, South Africa. Marine Drugs. 2017; 15(4):95. https://doi.org/10.3390/md15040095
Chicago/Turabian StyleWaterworth, Samantha C., Meesbah Jiwaji, Jarmo-Charles J. Kalinski, Shirley Parker-Nance, and Rosemary A. Dorrington. 2017. "A Place to Call Home: An Analysis of the Bacterial Communities in Two Tethya rubra Samaai and Gibbons 2005 Populations in Algoa Bay, South Africa" Marine Drugs 15, no. 4: 95. https://doi.org/10.3390/md15040095
APA StyleWaterworth, S. C., Jiwaji, M., Kalinski, J.-C. J., Parker-Nance, S., & Dorrington, R. A. (2017). A Place to Call Home: An Analysis of the Bacterial Communities in Two Tethya rubra Samaai and Gibbons 2005 Populations in Algoa Bay, South Africa. Marine Drugs, 15(4), 95. https://doi.org/10.3390/md15040095





