Acute Coronary Syndrome Management in Older Patients: A Dual-Center Retrospective Cohort Study
Abstract
1. Introduction
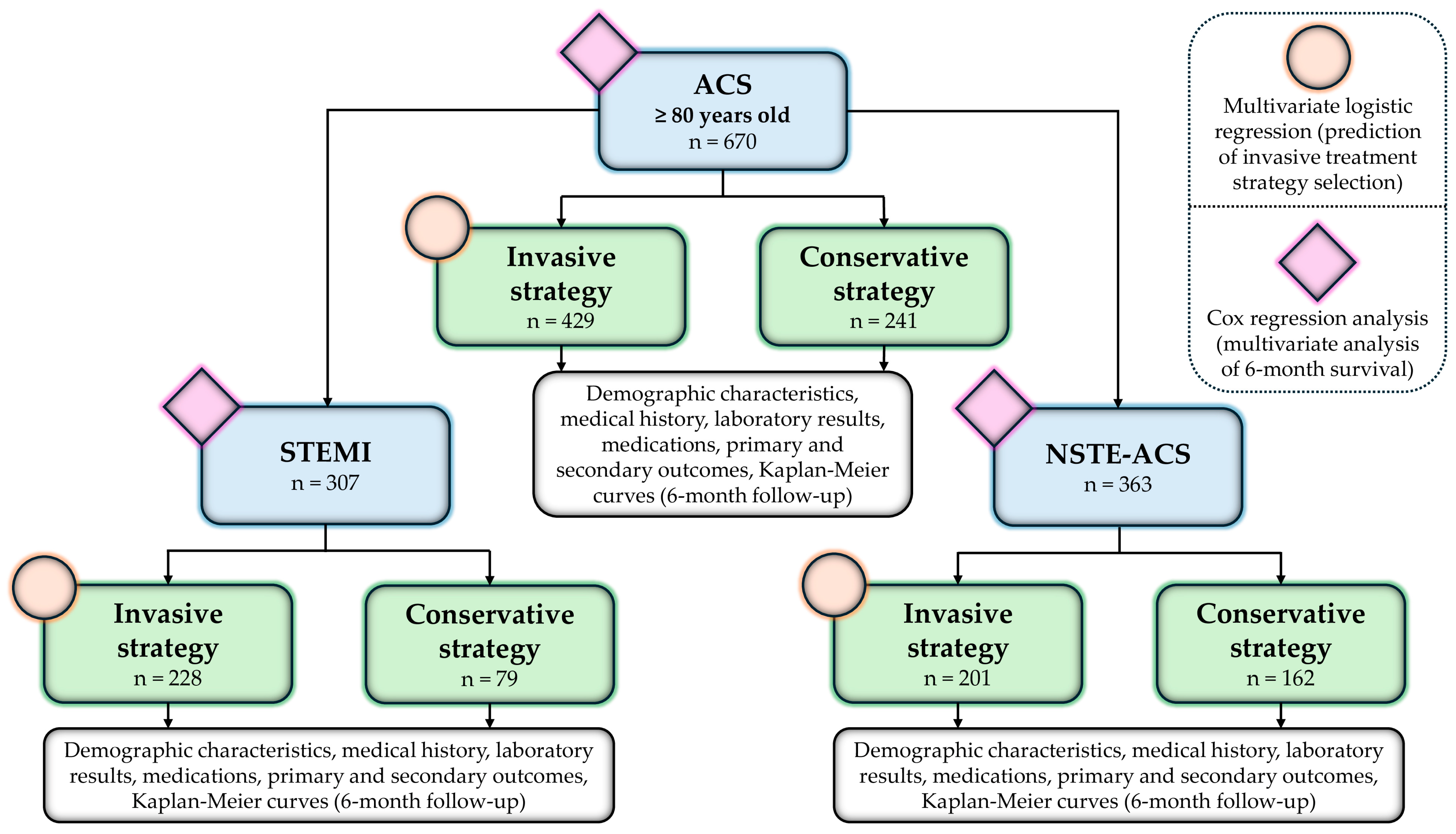
2. Materials and Methods
2.1. Patients and Data Collection
2.2. Treatment Strategies and Outcomes
2.3. Statistical Analysis
3. Results
3.1. Demographic, Clinical, and Laboratory Characteristics
3.1.1. Acute Coronary Syndrome
3.1.2. ST-Elevation Myocardial Infarction
3.1.3. Non-ST-Elevation Acute Coronary Syndrome
3.2. Treatment Strategies
3.2.1. Predictors of Invasive Strategy
3.2.2. Discharge Medications
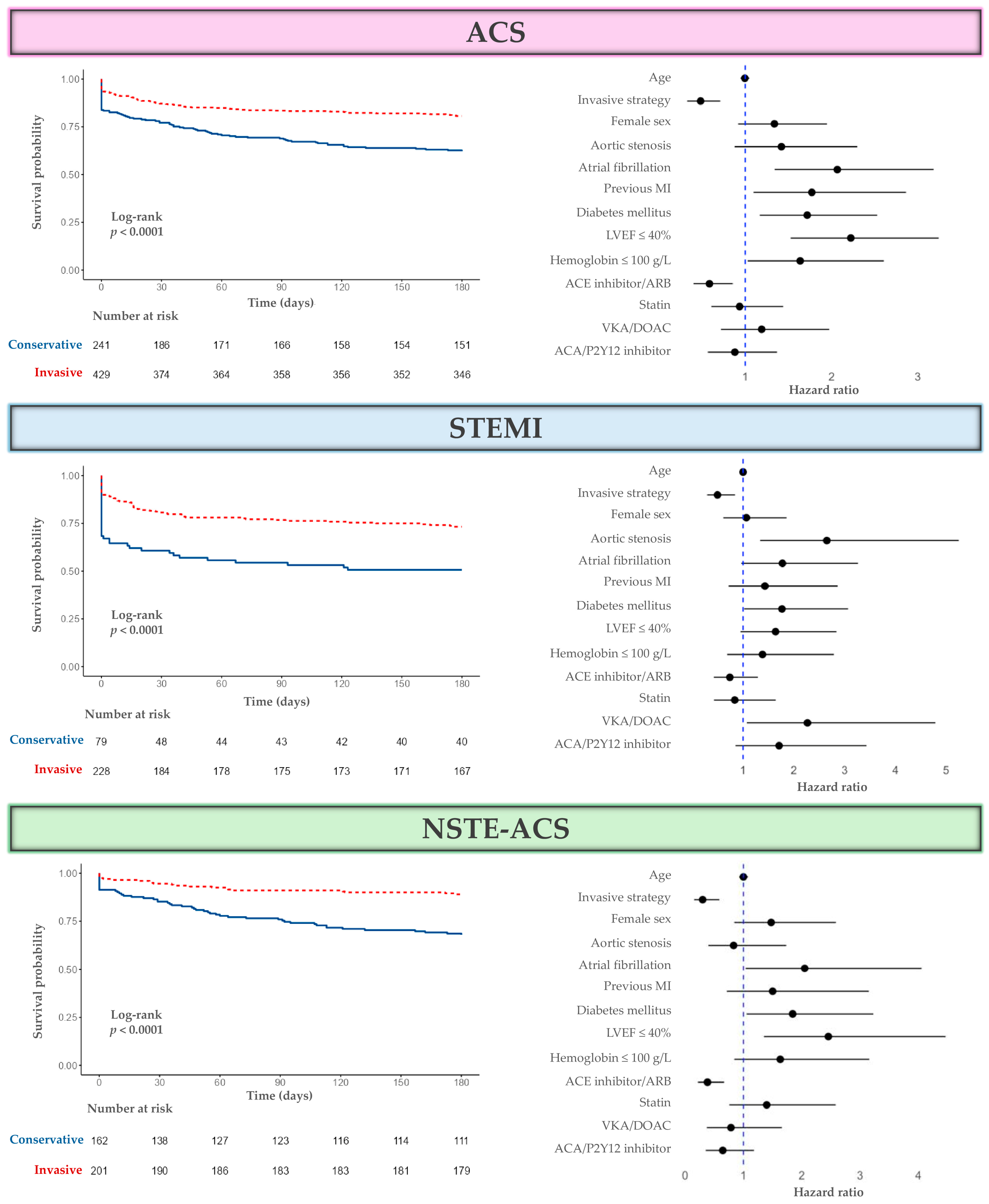
3.3. Outcomes and Survival
3.3.1. Primary and Secondary Outcomes
3.3.2. Survival Analysis
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE | Angiotensin-converting enzyme |
| ACS | Acute coronary syndrome |
| ARB | Angiotensin receptor blocker |
| CI | Confidence interval |
| COPD | Chronic obstructive pulmonary disease |
| CRP | C-reactive protein |
| CVI | Cerebrovascular insult |
| ESC | European Society of Cardiology |
| HR | Hazard ratio |
| IQR | Interquartile range |
| LDL | Low-density lipoprotein |
| LVEF | Left ventricular ejection fraction |
| MACE | Major adverse cardiovascular event |
| MI | Myocardial infarction |
| NSTE-ACS | Non-ST-elevation acute coronary syndrome |
| NSTEMI | Non-ST-elevation myocardial infarction |
| NT-proBNP | N-terminal pro–B-type natriuretic peptide |
| OR | Odds ratio |
| PSM | Propensity score matching |
| RCT | Randomized controlled trial |
| SD | Standard deviation |
| STEMI | ST-elevation myocardial infarction |
References
- Damluji, A.A.; Forman, D.E.; Wang, T.Y.; Chikwe, J.; Kunadian, V.; Rich, M.W.; Young, B.A.; Page, R.L.; DeVon, H.A.; Alexander, K.P.; et al. Management of Acute Coronary Syndrome in the Older Adult Population: A Scientific Statement from the American Heart Association. Circulation 2023, 147, e32–e62. [Google Scholar] [CrossRef]
- Andreotti, F.; Rocca, B.; Husted, S.; Ajjan, R.A.; Berg, J.T.; Cattaneo, M.; Collet, J.P.; De Caterina, R.; Fox, K.A.; Halvorsen, S.; et al. Antithrombotic therapy in the elderly: Expert position paper of the European Society of Cardiology Working Group on Thrombosis. Eur. Heart J. 2015, 36, 3238–3249. [Google Scholar] [CrossRef] [PubMed]
- Rosengren, A.; Wallentin, L.; Simoons, M.; Gitt, A.K.; Behar, S.; Battler, A.; Hasdai, D. Age, clinical presentation, and outcome of acute coronary syndromes in the Euroheart acute coronary syndrome survey. Eur. Heart J. 2006, 27, 789–795. [Google Scholar] [CrossRef]
- Singam, N.S.V.; Fine, C.; Fleg, J.L. Cardiac changes associated with vascular aging. Clin. Cardiol. 2020, 43, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Ya, J.; Bayraktutan, U. Vascular Ageing: Mechanisms, Risk Factors, and Treatment Strategies. Int. J. Mol. Sci. 2023, 24, 11538. [Google Scholar] [CrossRef] [PubMed]
- Marschall, A.; Rivero, F.; del Val, D.; Bastante, T.; López Soberón, E.; Gómez Sánchez, I.; Basabe Velasco, E.; Alfonso, F.; de la Torre Hernández, J.M.; Martí Sánchez, D. Bleeding Risk in Elderly Patients Undergoing Percutaneous Coronary Intervention: A Comprehensive Review. J. Clin. Med. 2025, 14, 1194. [Google Scholar] [CrossRef] [PubMed]
- Inouye, S.K.; Studenski, S.; Tinetti, M.E.; Kuchel, G.A. Geriatric syndromes: Clinical, research, and policy implications of a core geriatric concept. J. Am. Geriatr. Soc. 2007, 55, 780–791. [Google Scholar] [CrossRef]
- Goyal, P.; Kwak, M.J.; Al Malouf, C.; Kumar, M.; Rohant, N.; Damluji, A.A.; Denfeld, Q.E.; Bircher, K.K.; Krishnaswami, A.; Alexander, K.P.; et al. Geriatric Cardiology: Coming of Age. JACC Adv. 2022, 1, 100070. [Google Scholar] [CrossRef]
- Nanna, M.G.; Hajduk, A.M.; Krumholz, H.M.; Murphy, T.E.; Dreyer, R.P.; Alexander, K.P.; Geda, M.; Tsang, S.; Welty, F.K.; Safdar, B.; et al. Sex-Based Differences in Presentation, Treatment, and Complications Among Older Adults Hospitalized for Acute Myocardial Infarction: The SILVER-AMI Study. Circ. Cardiovasc. Qual. Outcomes 2019, 12, e005691. [Google Scholar] [CrossRef]
- Friedman, A.; Chudow, J.; Merritt, Z.; Shulman, E.; Fisher, J.D.; Ferrick, K.J.; Krumerman, A. Electrocardiogram abnormalities in older individuals by race and ethnicity. J. Electrocardiol. 2020, 63, 91–93. [Google Scholar] [CrossRef]
- Gore, M.O.; Seliger, S.L.; Defilippi, C.R.; Nambi, V.; Christenson, R.H.; Hashim, I.A.; Hoogeveen, R.C.; Ayers, C.R.; Sun, W.; McGuire, D.K.; et al. Age- and sex-dependent upper reference limits for the high-sensitivity cardiac troponin T assay. J. Am. Coll. Cardiol. 2014, 63, 1441–1448. [Google Scholar] [CrossRef]
- Lowry, M.T.H.; Doudesis, D.; Wereski, R.; Kimenai, D.M.; Tuck, C.; Ferry, A.V.; Bularga, A.; Taggart, C.; Lee, K.K.; Chapman, A.R.; et al. Influence of Age on the Diagnosis of Myocardial Infarction. Circulation 2022, 146, 1135–1148. [Google Scholar] [CrossRef]
- Bourgeois, F.T.; Orenstein, L.; Ballakur, S.; Mandl, K.D.; Ioannidis, J.P.A. Exclusion of Elderly People from Randomized Clinical Trials of Drugs for Ischemic Heart Disease. J. Am. Geriatr. Soc. 2017, 65, 2354–2361. [Google Scholar] [CrossRef]
- Pitkala, K.H.; Strandberg, T.E. Clinical trials in older people. Age Ageing 2022, 51, afab282. [Google Scholar] [CrossRef] [PubMed]
- Tegn, N.; Abdelnoor, M.; Aaberge, L.; Endresen, K.; Smith, P.; Aakhus, S.; Gjertsen, E.; Dahl-Hofseth, O.; Ranhoff, A.H.; Gullestad, L.; et al. Invasive versus Conservative Strategy in Patients Aged 80 Years or Older with Non-ST-Elevation Myocardial Infarction or Unstable Angina Pectoris (After Eighty Study): An Open-Label Randomised Controlled Trial. Lancet Lond. Engl. 2016, 387, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.-A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the Management of Acute Coronary Syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef]
- Savonitto, S.; Cavallini, C.; Petronio, A.S.; Murena, E.; Antonicelli, R.; Sacco, A.; Steffenino, G.; Bonechi, F.; Mossuti, E.; Manari, A.; et al. Early aggressive versus initially conservative treatment in elderly patients with non-ST-segment elevation acute coronary syndrome: A randomized controlled trial. JACC Cardiovasc. Interv. 2012, 5, 906–916. [Google Scholar] [CrossRef]
- Sanchis, J.; Núñez, E.; Barrabés, J.A.; Marín, F.; Consuegra-Sánchez, L.; Ventura, S.; Valero, E.; Roqué, M.; Bayés-Genís, A.; Del Blanco, B.G.; et al. Randomized comparison between the invasive and conservative strategies in comorbid elderly patients with non-ST elevation myocardial infarction. Eur. J. Intern. Med. 2016, 35, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Hirlekar, G.; Libungan, B.; Karlsson, T.; Bäck, M.; Herlitz, J.; Albertsson, P. Percutaneous coronary intervention in the very elderly with NSTE-ACS: The randomized 80+ study. Scand. Cardiovasc. J. 2020, 54, 315–321. [Google Scholar] [CrossRef] [PubMed]
- De Belder, A.; Myat, A.; Blaxill, J.; Haworth, P.; O’Kane, P.D.; Hatrick, R.; Aggarwal, R.K.; Davie, A.; Smith, W.; Gerber, R.; et al. Revascularisation or medical therapy in elderly patients with acute anginal syndromes: The RINCAL randomised trial. EuroIntervention 2021, 17, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Sanchis, J.; Bueno, H.; Miñana, G.; Guerrero, C.; Martí, D.; Martínez-Sellés, M.; Domínguez-Pérez, L.; Díez-Villanueva, P.; Barrabés, J.A.; Marín, F.; et al. Effect of Routine Invasive vs Conservative Strategy in Older Adults with Frailty and Non–ST-Segment Elevation Acute Myocardial Infarction: A Randomized Clinical Trial. JAMA Intern. Med. 2023, 183, 407–415. [Google Scholar] [CrossRef]
- Kotanidis, C.P.; Mills, G.B.; Bendz, B.; Berg, E.S.; Hildick-Smith, D.; Hirlekar, G.; Milasinovic, D.; Morici, N.; Myat, A.; Tegn, N.; et al. Invasive vs. Conservative Management of Older Patients with Non-ST-Elevation Acute Coronary Syndrome: Individual Patient Data Meta-Analysis. Eur. Heart J. 2024, 45, 2052–2062. [Google Scholar] [CrossRef] [PubMed]
- Kunadian, V.; Mossop, H.; Shields, C.; Bardgett, M.; Watts, P.; Teare, M.D.; Pritchard, J.; Adams-Hall, J.; Runnett, C.; Ripley, D.P.; et al. Invasive Treatment Strategy for Older Patients with Myocardial Infarction. N. Engl. J. Med. 2024, 391, 1673–1684. [Google Scholar] [CrossRef]
- Sui, Y.G.; Teng, S.Y.; Qian, J.; Wu, Y.; Dou, K.F.; Tang, Y.D.; Qiao, S.B.; Wu, Y.J. Invasive versus conservative strategy in consecutive patients aged 80 years or older with non-ST-segment elevation myocardial infarction: A retrospective study in China. J. Geriatr. Cardiol. 2019, 16, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Xing, Y.L.; Wang, H.; Wang, S.; Miao, Y.; Huang, W.; Zhang, K.; Li, H.W.; Sun, Y.; Chen, H. Invasive treatment strategy in patients aged 80 years or older with non-ST-elevation acute coronary syndromes: A retrospective cohort study. Cardiovasc. Diagn. Ther. 2022, 12, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Kunniardy, P.; Koshy, A.N.; Meehan, G.; Murphy, A.C.; Ramchand, J.; Clark, D.J.; Farouque, O.; Yudi, M.B. Invasive versus conservative management in patients aged ≥85 years presenting with non-ST-elevation myocardial infarction. Intern. Med. J. 2022, 52, 1167–1173. [Google Scholar] [CrossRef]
- Sui, Y.G.; Teng, S.Y.; Qian, J.; Wu, Y.; Dou, K.F.; Tang, Y.D.; Qiao, S.B.; Wu, Y.J. A retrospective study of an invasive versus conservative strategy in patients aged ≥80 years with acute ST-segment elevation myocardial infarction. J. Int. Med. Res. 2019, 47, 4431–4441. [Google Scholar] [CrossRef]
- Rozenfeld, K.L.; Lupu, L.; Merdler, I.; Morgan, S.; Banai, S.; Shacham, Y. Invasive versus Conservative Treatment Approach among Older Adult Patients Admitted with Acute ST-Segment Elevation Myocardial Infarction. Ann. Geriatr. Med. Res. 2022, 26, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Alkhushail, A.; Kohli, S.; Mitchel, A.; Smith, R.; Ilsely, C. Prognosis of primary percutaneous coronary intervention in elderly patients with ST-elevation myocardial infarction. J. Saudi. Heart Assoc. 2015, 27, 85–90. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yudi, M.B.; Jones, N.; Fernando, D.; Clark, D.J.; Ramchand, J.; Jones, E.; Dakis, R.; Johnson, D.; Chan, R.; Islam, A.; et al. Management of Patients Aged ≥85 Years with ST-Elevation Myocardial Infarction. Am. J. Cardiol. 2016, 118, 44–48. [Google Scholar] [CrossRef]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef] [PubMed]
- Collet, J.P.; Thiele, H.; Barbato, E.; Barthélémy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef] [PubMed]
- Damman, P.; Clayton, T.; Wallentin, L.; Lagerqvist, B.; Fox, K.A.; Hirsch, A.; Windhausen, F.; Swahn, E.; Pocock, S.J.; Tijssen, J.G.; et al. Effects of age on long-term outcomes after a routine invasive or selective invasive strategy in patients presenting with non-ST segment elevation acute coronary syndromes: A collaborative analysis of individual data from the FRISC II-ICTUS-RITA-3 (FIR) trials. Heart 2012, 98, 207–213. [Google Scholar] [CrossRef]
- Flather, M.D.; Yusuf, S.; Køber, L.; Pfeffer, M.; Hall, A.; Murray, G.; Torp-Pedersen, C.; Ball, S.; Pogue, J.; Moyé, L.; et al. Long-term ACE-inhibitor therapy in patients with heart failure or left-ventricular dysfunction: A systematic overview of data from individual patients. ACE-Inhibitor Myocardial Infarction Collaborative Group. Lancet 2000, 355, 1575–1581. [Google Scholar] [CrossRef]
- Montoy, J.C.C.; Shen, Y.C.; Hsia, R.Y. Trends in Inequities in the Treatment of and Outcomes for Women and Minorities with Myocardial Infarction. Ann. Emerg. Med. 2022, 80, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Liu, K.T.; Lu, H.T.; Mohd Ali, R.; Fong, A.Y.Y.; Wan Ahmad, W.A. Sex and gender differences in presentation, treatment and outcomes in acute coronary syndrome, a 10 year study from a multi-ethnic Asian population: The Malaysian National Cardiovascular Disease Database-Acute Coronary Syndrome (NCVD-ACS) registry. PLoS ONE 2021, 16, e0246474. [Google Scholar] [CrossRef]
- Spadafora, L.; Pastena, P.; Cacciatore, S.; Betti, M.; Biondi-Zoccai, G.; D’Ascenzo, F.; De Ferrari, G.M.; De Filippo, O.; Versaci, F.; Sciarretta, S.; et al. One-Year Prognostic Differences and Management Strategies between ST-Elevation and Non-ST-Elevation Myocardial Infarction: Insights from the PRAISE Registry. Am. J. Cardiovasc. Drugs 2025, 1–11. [Google Scholar] [CrossRef] [PubMed]
| Parameter | ACS | p | ||
|---|---|---|---|---|
| All (n = 670) | Invasive Strategy (n = 429) | Conservative Strategy (n = 241) | ||
| No. (%) Mean ± SD Median (IQR) | No. (%) Mean ± SD Median (IQR) | No. (%) Mean ± SD Median (IQR) | ||
| Age | 83 (81–86) | 83 (81–86) | 84 (82–87) | <0.001 * |
| Sex | 0.035 * | |||
| Woman | 339 (50.6%) | 204 (47.6%) | 135 (56.0%) | |
| Men | 331 (49.4%) | 225 (52.4%) | 106 (44.0%) | |
| ACS type | <0.001 * | |||
| STEMI | 307 (45.8%) | 228 (53.1%) | 79 (32.8%) | |
| NSTE-ACS | 363 (54.2%) | 201 (46.9%) | 162 (67.2%) | |
| BMI (kg/m2) | 26.5 (23.9–29.4) | 26.5 (23.9–29.4) | 26.4 (23.6–29.6) | 0.594 |
| SBP (mmHg) | 135 (120–150) | 135 (120–150) | 137 ± 25 | 0.391 |
| DBP (mmHg) | 80 (70–88) | 78 (70–86) | 80 (70–90) | 0.118 |
| Pulse (beats/min) | 80 (67–90) | 78 (66–88) | 80 (70–95) | 0.003 * |
| Smoking history | 100 (14.9%) | 69 (16.1%) | 31 (12.9) | 0.262 |
| Previous MI | 117 (17.5%) | 70 (16.3%) | 47 (19.5%) | 0.297 |
| Previous revascularization | 135 (20.1%) | 79 (18.4%) | 56 (23.2%) | 0.135 |
| Previous CVI | 77 (11.5%) | 45 (10.5%) | 32 (13.3%) | 0.277 |
| Arterial hypertension | 586 (87.5%) | 380 (88.6%) | 206 (85.5%) | 0.245 |
| Diabetes mellitus | 214 (31.9%) | 145 (33.8%) | 69 (28.6%) | 0.168 |
| Hyperlipidemia | 311 (46.4%) | 214 (49.9%) | 97 (40.2%) | 0.016 * |
| COPD | 27 (4.0%) | 15 (3.5%) | 12 (5.0%) | 0.349 |
| Malignant disease | 129 (19.3%) | 78 (18.2%) | 51 (21.2%) | 0.348 |
| Atrial fibrillation | 189 (28.2%) | 107 (24.9%) | 82 (34.0%) | 0.012 * |
| Aortic stenosis | 77 (11.5%) | 33 (7.7%) | 44 (18.3%) | <0.001 * |
| Mitral regurgitation | 85 (12.7%) | 52 (12.1%) | 33 (13.7%) | 0.557 |
| Peripheral artery disease | 44 (6.6%) | 34 (7.9%) | 10 (4.1%) | 0.058 |
| Chronic medications 1 | ||||
| ACA/P2Y12 inhibitor | 217 (33.1%) | 125 (29.9%) | 92 (38.7%) | 0.022 * |
| ACE inhibitor/ARB | 412 (62.8%) | 266 (63.6%) | 146 (61.3%) | 0.559 |
| Beta blocker | 305 (46.5%) | 173 (41.4%) | 132 (55.5%) | <0.001 * |
| Statin | 193 (29.4%) | 111 (26.6%) | 82 (34.5%) | 0.033 * |
| Blood parameters | ||||
| Hemoglobin (g/L) | 128 (118–140) | 131 (119–141) | 124 (112–137) | <0.001 * |
| Platelets (×109/L) | 218 (177–266) | 213 (180–260) | 223 (173–281) | 0.297 |
| Total cholesterol (mmol/L) | 4.4 (3.6–5.3) | 4.6 (3.8–5.4) | 4.1 (3.2–5.1) | <0.001 * |
| LDL cholesterol (mmol/L) | 2.7 (2.0–3.4) | 2.9 (2.0–3.6) | 2.4 (1.7–3.3) | 0.001 * |
| Triglycerides (mmol/L) | 1.2 (1.0–1.6) | 1.3 (1.0–1.7) | 1.2 (1.0–1.5) | 0.051 |
| NT-proBNP (pg/mL) | 4923 (1967–12,796) | 3979 (1631–10,482) | 6296 (2853–16,813) | 0.004 * |
| Creatinine (μmol/L) | 103 (82–131) | 99 (82–127) | 109 (80–144) | 0.023 * |
| eGFR (mL/min/1.73 m2) | 51 (37–66) | 53 (40–66) | 46 (31–64) | 0.004 * |
| C-reactive protein (mg/L) | 11.3 (3.4–51.7) | 8.8 (2.8–33.7) | 21.1 (4.9–73.8) | <0.001 * |
| In-hospital | ||||
| Major bleeding | 16 (2.4%) | 12 (2.8%) | 4 (1.7%) | 0.355 |
| LVEF ≤ 40% | 200 (29.9%) | 115 (26.8%) | 85 (35.3%) | 0.022 * |
| Cardiogenic shock | 37 (5.5%) | 21 (4.9%) | 16 (6.6%) | 0.343 |
| Parameter | ACS | p | ||
|---|---|---|---|---|
| All (n = 436) | Invasive Strategy (n = 218) | Conservative Strategy (n = 218) | ||
| No. (%) Median (IQR) | No. (%) Median (IQR) | No. (%) Median (IQR) | ||
| Age | 84 (81–87) | 83 (81–87) | 84 (82–87) | 0.147 |
| Sex | 0.700 | |||
| Woman | 240 (55.0%) | 122 (56.0%) | 118 (54.1%) | |
| Men | 196 (45.0%) | 96 (44.0%) | 100 (45.9%) | |
| ACS type | <0.001 * | |||
| STEMI | 200 (45.9%) | 129 (59.2%) | 71 (32.6%) | |
| NSTE-ACS | 236 (54.1%) | 89 (40.8%) | 147 (67.4%) | |
| BMI (kg/m2) | 26.1 (23.6–29.4) | 26 (23.9–29.0) | 26.1 (23.4–29.5) | 0.805 |
| SBP (mmHg) | 135 (120–150) | 130 (118–145) | 137 (120–150) | 0.030 * |
| DBP (mmHg) | 80 (69–87) | 77 (65–85) | 80 (70–90) | 0.009 * |
| Pulse (beats/min) | 80 (68–90) | 80 (66–88) | 80 (70–95) | 0.076 |
| Smoking history | 60 (13.8%) | 31 (14.2%) | 29 (13.3%) | 0.781 |
| Previous MI | 79 (18.1%) | 32 (14.7%) | 47 (21.6%) | 0.062 |
| Previous revascularization | 89 (20.4%) | 35 (16.1%) | 54 (24.8%) | 0.024 * |
| Previous CVI | 55 (12.6%) | 29 (13.3%) | 26 (11.9%) | 0.665 |
| Arterial hypertension | 381 (87.4%) | 192 (88.1%) | 189 (86.7%) | 0.665 |
| Diabetes mellitus | 143 (32.8%) | 77 (35.3%) | 66 (30.3%) | 0.262 |
| Hyperlipidemia | 189 (43.3%) | 98 (45.0%) | 91 (41.7%) | 0.499 |
| COPD | 20 (4.6%) | 9 (4.1%) | 11 (5.0%) | 0.647 |
| Malignant disease | 87 (20.0%) | 42 (19.3%) | 45 (20.6%) | 0.719 |
| Atrial fibrillation | 143 (32.8%) | 72 (33.0%) | 71 (32.6%) | 0.919 |
| Aortic stenosis | 65 (14.9%) | 31 (14.2%) | 34 (15.6%) | 0.687 |
| Mitral regurgitation | 62 (14.2%) | 35 (16.1%) | 27 (12.4%) | 0.273 |
| Peripheral artery disease | 23 (5.3%) | 13 (6.0%) | 10 (4.6%) | 0.520 |
| Chronic medications 1 | ||||
| ACA/P2Y12 inhibitor | 143 (32.8%) | 58 (26.6%) | 85 (39.0%) | 0.006 * |
| ACE inhibitor/ARB | 263 (60.3%) | 130 (59.6%) | 133 (61.0%) | 0.769 |
| Beta blocker | 214 (49.1%) | 91 (41.7%) | 123 (56.4%) | 0.002 * |
| Statin | 125 (28.7%) | 49 (22.5%) | 76 (34.9%) | 0.004 * |
| Blood parameters | ||||
| Hemoglobin (g/L) | 127 (115–139) | 128 (117–139) | 125 (114–138) | 0.250 |
| Platelets (×109/L) | 220 (175–273) | 218 (177–267) | 224 (174–281) | 0.435 |
| Total cholesterol (mmol/L) | 4.3 (3.5–5.2) | 4.5 (3.7–5.2) | 4.1 (3.2–5.1) | 0.012 * |
| LDL cholesterol (mmol/L) | 2.5 (1.9–3.3) | 2.7 (2.1–3.4) | 2.4 (1.7–3.3) | 0.029 * |
| Triglycerides (mmol/L) | 1.3 (1.0–1.6) | 1.3 (1.0–1.8) | 1.2 (1.0–1.5) | 0.065 |
| NT-proBNP (pg/mL) | 5299 (2463–14,353) | 5031 (2350–14,353) | 5463 (2655–15,103) | 0.549 |
| Creatinine (μmol/L) | 106 (80–137) | 99 (81–133) | 108 (80–144) | 0.259 |
| eGFR (mL/min/1.73 m2) | 49 (34–66) | 51 (35–66) | 47 (32–66) | 0.368 |
| C-reactive protein (mg/L) | 14.3 (4.0–61.7) | 11.8 (3.6–48.7) | 18.0 (4.6–75.6) | 0.029 * |
| In-hospital | ||||
| Major bleeding | 8 (1.8%) | 4 (1.8%) | 4 (1.8%) | 1.000 |
| LVEF ≤ 40% | 150 (34.4%) | 78 (35.8%) | 72 (33.0%) | 0.545 |
| Cardiogenic shock | 29 (6.7%) | 14 (6.4%) | 15 (6.9%) | 0.848 |
| Parameter | ACS | p | ||
|---|---|---|---|---|
| All (n = 670) | STEMI (n = 307) | NSTE-ACS (n = 363) | ||
| No. (%) Median (IQR) | No. (%) Median (IQR) | No. (%) Median (IQR) | ||
| Age | 83 (81–86) | 83 (81–87) | 83 (81–86) | 0.842 |
| Sex | <0.001 * | |||
| Woman | 339 (50.6%) | 188 (61.2%) | 151(41.6%) | |
| Men | 331 (49.4%) | 119 (38.8%) | 212 (58.4%) | |
| Smoking history | 100 (14.9%) | 44 (14.3%) | 56 (15.4%) | 0.692 |
| Previous MI | 117 (17.5%) | 34 (11.1%) | 83 (22.9%) | <0.001 * |
| Previous revascularization | 135 (20.1%) | 33 (10.7%) | 102 (28.1%) | <0.001 * |
| Previous CVI | 77 (11.5%) | 37 (12.1%) | 40 (11.0%) | 0.676 |
| Arterial hypertension | 586 (87.6%) | 265 (86.3%) | 321 (88.4%) | 0.411 |
| Diabetes mellitus | 214 (31.9%) | 95 (30.9%) | 119 (32.8%) | 0.611 |
| Hyperlipidemia | 311 (46.4%) | 130 (42.3%) | 181 (49.9%) | 0.052 |
| COPD | 27 (4.0%) | 10 (3.3%) | 17 (4.7%) | 0.350 |
| Malignant disease | 129 (19.3%) | 51 (16.6%) | 78 (21.5%) | 0.111 |
| Atrial fibrillation | 189 (28.2%) | 94 (30.6%) | 95 (26.2%) | 0.202 |
| Aortic stenosis | 77 (11.5%) | 21 (6.8%) | 56 (15.4%) | <0.001 * |
| Mitral regurgitation | 85 (12.7%) | 36 (11.7%) | 49 (13.5%) | 0.492 |
| Peripheral artery disease | 44 (6.6%) | 17 (5.5%) | 27 (7.4%) | 0.322 |
| In-hospital | ||||
| Major bleeding | 16 (2.4%) | 8 (2.6%) | 8 (2.2%) | 0.734 |
| LVEF ≤ 40% | 200 (29.9%) | 114 (37.1%) | 86 (23.7%) | <0.001 * |
| Cardiogenic shock | 37 (5.5%) | 34 (11.1%) | 3 (0.8%) | <0.001 * |
| Treatment strategy | <0.001 * | |||
| Invasive strategy | 429 (64.0%) | 228 (74.3%) | 201 (55.4%) | |
| Conservative strategy | 241 (36.0%) | 79 (25.7%) | 162 (44.6%) | |
| Primary outcomes All-cause mortality | ||||
| In-hospital mortality | 67 (10.0%) | 48 (15.6%) | 19 (5.2%) | <0.001 * |
| Thirty-day mortality | 110 (16.4%) | 75 (24.4%) | 35 (9.6%) | <0.001 * |
| Six-month mortality | 174 (26.0%) | 100 (32.6%) | 74 (20.4%) | <0.001 * |
| Secondary outcomes | ||||
| Recurrent MI | 17 (2.5%) | 6 (2.0%) | 11 (3.0%) | 0.378 |
| CVI | 8 (1.2%) | 3 (1.0%) | 5 (1.4%) | 0.635 |
| Parameter | STEMI | p | ||
|---|---|---|---|---|
| All (n = 307) | Invasive Strategy (n = 228) | Conservative Strategy (n = 79) | ||
| No. (%) Mean ± SD Median (IQR) | No. (%) Mean ± SD Median (IQR) | No. (%) Mean ± SD Median (IQR) | ||
| Age | 83 (81–87) | 83 (81–86) | 84 (82–87) | 0.038 * |
| Sex | 0.073 | |||
| Woman | 188 (61.2%) | 133 (58.3%) | 55 (69.6%) | |
| Men | 119 (38.8%) | 95 (41.7%) | 24 (30.4%) | |
| BMI (kg/m2) | 26.7 (23.9–29.4) | 26.7 (23.9–29.4) | 26.7 (23.8–29.6) | 0.768 |
| SBP (mmHg) | 130 ± 25 | 129 ± 25 | 132 ± 25 | 0.350 |
| DBP (mmHg) | 77 (68–85) | 75 (65–85) | 80 ± 14 | 0.019 * |
| Pulse (beats/min) | 80 (68–90) | 80 (66–90) | 85 ± 20 | 0.069 |
| Smoking history | 44 (14.3%) | 36 (15.8%) | 8 (10.1%) | 0.216 |
| Previous MI | 34 (11.1%) | 26 (11.4%) | 8 (10.1%) | 0.755 |
| Previous revascularization | 33 (10.7%) | 24 (10.5%) | 9 (11.4%) | 0.830 |
| Previous CVI | 37 (12.1%) | 26 (11.4%) | 11 (13.9%) | 0.553 |
| Arterial hypertension | 265 (86.3%) | 199 (87.3%) | 66 (83.5%) | 0.405 |
| Diabetes mellitus | 95 (30.9%) | 72 (31.6%) | 23 (29.1%) | 0.683 |
| Hyperlipidemia | 130 (42.3%) | 100 (43.9%) | 30 (38.0%) | 0.362 |
| COPD | 10 (3.3%) | 10 (4.4%) | 0 (0.0%) | 0.058 |
| Malignant disease | 51 (16.6%) | 39 (17.1%) | 12 (15.2) | 0.693 |
| Atrial fibrillation | 94 (30.6%) | 60 (26.3%) | 34 (43.0%) | 0.005 * |
| Aortic stenosis | 21 (6.8%) | 13 (5.7%) | 8 (10.1%) | 0.179 |
| Mitral regurgitation | 36 (11.7%) | 26 (11.4%) | 10 (12.7%) | 0.765 |
| Peripheral artery disease | 17 (5.5%) | 14 (6.1%) | 3 (3.8%) | 0.433 |
| Chronic medications 1 | ||||
| ACA/P2Y12 inhibitor | 69 (23.2%) | 47 (21.4%) | 22 (28.6%) | 0.197 |
| ACE inhibitor/ARB | 162 (54.5%) | 117 (53.2%) | 45 (58.4%) | 0.425 |
| Beta blocker | 115 (38.7%) | 76 (34.5%) | 39 (50.6%) | 0.013 * |
| Statin | 61 (20.5%) | 43 (19.5%) | 18 (23.4%) | 0.474 |
| Blood parameters | ||||
| Hemoglobin (g/L) | 128 (119–140) | 128 (119–140) | 125 (118–137) | 0.302 |
| Platelets (×109/L) | 229 (187–277) | 224 (187–269) | 240 (190–294) | 0.170 |
| Total cholesterol (mmol/L) | 4.7 ± 1.3 | 4.9 ± 1.3 | 4.2 ± 1.3 | 0.003 * |
| LDL cholesterol (mmol/L) | 2.9 (2.2–3.5) | 3.0 (2.3–3.8) | 2.6 ± 1.1 | 0.010 * |
| Triglycerides (mmol/L) | 1.2 (1.0–1.6) | (1.0–1.6) | 1.1 (1.0–1.4) | 0.266 |
| NT-proBNP (pg/mL) | 5887 (3225–15,106) | 5473 (2853–13,447) | 9969 (5113–19,216) | 0.006 * |
| Creatinine (μmol/L) | 102 (81–130) | 99 (81–127) | 110 (82–145) | 0.073 |
| eGFR (mL/min/1.73 m2) | 49 (36–64) | 53 (39–66) | 44 (30–59) | 0.007 * |
| C-reactive protein (mg/L) | 15.5 (5.0–68.1) | 11.7 (4.0–48.7) | 46.3 (9.2–99.8) | <0.001 * |
| In-hospital | ||||
| Major bleeding | 8 (2.6%) | 7 (3.1%) | 1 (1.3%) | 0.386 |
| LVEF ≤ 40% | 114 (37.1%) | 76 (33.3%) | 38 (48.1%) | 0.019 * |
| Cardiogenic shock | 34 (11.1%) | 20 (8.8%) | 14 (17.7%) | 0.029 * |
| Parameter | NSTE-ACS | p | ||
|---|---|---|---|---|
| All (n = 363) | Invasive Strategy (n = 201) | Conservative Strategy (n = 162) | ||
| No. (%) Mean ± SD Median (IQR) | No. (%) Mean ± SD Median (IQR) | No. (%) Mean ± SD Median (IQR) | ||
| Age | 83 (81–86) | 83 (81–85) | 84 (82–87) | <0.001 * |
| Sex | 0.007 * | |||
| Woman | 151 (41.6%) | 71 (35.3%) | 80 (49.4%) | |
| Men | 212 (58.4%) | 130 (64.7%) | 82 (50.6%) | |
| BMI (kg/m2) | 26.4 (23.8–29.6) | 26.4 (24.0–29.5) | 26.4 ± 4.2 | 0.419 |
| SBP (mmHg) | 140 (125–152) | 140 (127–153) | 140 (120–151) | 0.293 |
| DBP (mmHg) | 80 (70–90) | 80 (70–90) | 79 ± 13 | 0.598 |
| Pulse (beats/min) | 77 (67–90) | 75 (65–87) | 80 (70–94) | 0.004 * |
| Smoking history | 56 (15.4%) | 33 (16.4%) | 23 (14.2%) | 0.560 |
| Previous MI | 83 (22.9%) | 44 (21.9%) | 39 (24.1%) | 0.622 |
| Previous revascularization | 102 (28.1%) | 55 (27.4%) | 47 (29.0%) | 0.728 |
| Previous CVI | 40 (11.0%) | 19 (9.5%) | 21 (13.0%) | 0.288 |
| Arterial hypertension | 321 (88.4%) | 181 (90.0%) | 140 (86.4%) | 0.282 |
| Diabetes mellitus | 119 (32.8%) | 73 (36.3%) | 46 (28.4%) | 0.110 |
| Hyperlipidemia | 181 (49.9%) | 114 (56.7%) | 67 (41.4%) | 0.004 * |
| COPD | 17 (4.7%) | 5 (2.5%) | 12 (7.4%) | 0.027 * |
| Malignant disease | 78 (21.5%) | 39 (19.4%) | 39 (24.1%) | 0.281 |
| Atrial fibrillation | 95 (26.2%) | 47 (23.4%) | 48 (29.6%) | 0.178 |
| Aortic stenosis | 56 (15.4%) | 20 (10.0%) | 36 (22.2%) | 0.001 * |
| Mitral regurgitation | 49 (13.5%) | 26 (12.9%) | 23 (14.2%) | 0.726 |
| Peripheral artery disease | 27 (7.4%) | 20 (10.0%) | 7 (4.3%) | 0.042 * |
| Chronic medications 1 | ||||
| ACA/P2Y12 inhibitor | 148 (41.2%) | 78 (39.4%) | 70 (43.5%) | 0.434 |
| ACE inhibitor/ARB | 250 (69.9%) | 149 (75.3%) | 101 (62.7%) | 0.010 * |
| Beta blocker | 190 (52.9%) | 97 (49.0%) | 93 (57.8%) | 0.098 |
| Statin | 132 (36.8%) | 68 (34.3%) | 64 (39.8%) | 0.291 |
| Blood parameters | ||||
| Hemoglobin (g/L) | 129 (115–140) | 132 (120–141) | 122 ± 22 | <0.001 * |
| Platelets (×109/L) | 205 (169–257) | 202 (167–249) | 213 (169–271) | 0.206 |
| Total cholesterol (mmol/L) | 4.3 (3.4–5.2) | 4.4 (3.6–5.2) | 4.1 (3.2–5.2) | 0.135 |
| LDL cholesterol (mmol/L) | 2.5 (1.8–3.4) | 2.6 (1.8–3.4) | 2.4 (1.7–3.3) | 0.185 |
| Triglycerides (mmol/L) | 1.3 (1.0–1.7) | 1.3 (1.0–1.8) | 1.2 (1.0–1.6) | 0.051 |
| NT-proBNP (pg/mL) | 3407 (1246–11,120) | 2350 (799–5806) | 5029 (1794–13,393) | 0.002 * |
| Creatinine (μmol/L) | 105 (82–133) | 97 (84–126) | 108 (79–144) | 0.190 |
| eGFR (mL/min/1.73 m2) | 52 (37–67) | 54 ± 19 | 50 (32–67) | 0.093 |
| C-reactive protein (mg/L) | 8.8 (2.4–35.9) | 5.2 (1.9–22.6) | 15.1 (3.9–58.4) | <0.001 * |
| In-hospital | ||||
| Major bleeding | 8 (2.2%) | 5 (2.5%) | 3 (1.9%) | 0.682 |
| LVEF ≤ 40% | 86 (23.7%) | 39 (19.4%) | 47 (29.0%) | 0.032 * |
| Cardiogenic shock | 3 (0.8%) | 1 (0.5%) | 2 (1.2%) | 0.441 |
| Variable | OR | 95% CI | p |
|---|---|---|---|
| Age | 1.10 | 1.03–1.18 | 0.005 * |
| Pulse 1 | 1.00 | 0.99–1.02 | 0.641 |
| NT-proBNP 1 | 1.00 | 1.00–1.00 | 0.980 |
| Creatinine 1 | 1.00 | 1.00–1.00 | 0.770 |
| C-reactive protein 1 | 1.01 | 1.00–1.01 | 0.012 * |
| Female sex | 0.74 | 0.44–1.23 | 0.242 |
| Hyperlipidemia | 1.38 | 0.80–2.39 | 0.245 |
| ACA/P2Y12 inhibitor 2 | 0.69 | 0.40–1.19 | 0.183 |
| Beta blocker 2 | 0.64 | 0.38–1.06 | 0.084 |
| Statin 2 | 0.86 | 0.48–1.57 | 0.632 |
| Atrial fibrillation | 0.83 | 0.49–1.40 | 0.475 |
| Aortic stenosis | 0.59 | 0.28–1.22 | 0.153 |
| Peripheral artery disease | 4.35 | 0.73–25.76 | 0.106 |
| Hemoglobin ≤ 100 g/L 1 | 0.70 | 0.31–1.57 | 0.391 |
| LVEF ≤ 40% 3 | 0.69 | 0.40–1.19 | 0.186 |
| Variable | OR | 95% CI | p |
|---|---|---|---|
| Age | 1.11 | 1.03–1.19 | 0.004 * |
| Pulse 1 | 1.01 | 1.00–1.03 | 0.085 |
| C-reactive protein 1 | 1.00 | 1.00–1.01 | 0.087 |
| Female sex | 0.64 | 0.39–1.04 | 0.072 |
| Hyperlipidemia | 1.29 | 0.78–2.14 | 0.320 |
| ACE inhibitor/ARB 2 | 1.32 | 0.77–2.27 | 0.317 |
| Beta blocker 2 | 0.68 | 0.41–1.11 | 0.121 |
| Aortic stenosis | 0.45 | 0.23–0.87 | 0.018 * |
| COPD | 0.29 | 0.09–0.92 | 0.035 * |
| Peripheral artery disease | 1.68 | 0.58–4.89 | 0.340 |
| Hemoglobin ≤ 100 g/L 1 | 0.44 | 0.20–0.98 | 0.043 * |
| LVEF ≤ 40% 3 | 0.62 | 0.35–1.10 | 0.102 |
| Variable | OR | 95% CI | p |
|---|---|---|---|
| Age | 1.13 | 1.00–1.28 | 0.057 |
| DBP 1 | 1.03 | 1.00–1.07 | 0.135 |
| Pulse 1 | 1.00 | 0.97–1.02 | 0.740 |
| LDL cholesterol 1 | 0.76 | 0.47–1.22 | 0.253 |
| NT-proBNP 1 | 1.00 | 1.00–1.00 | 0.320 |
| Creatinine 1 | 1.00 | 0.99–1.01 | 0.449 |
| C-reactive protein 1 | 1.01 | 1.00–1.02 | 0.096 |
| Female sex | 0.66 | 0.23–1.91 | 0.441 |
| Beta blocker 2 | 1.04 | 0.39–2.81 | 0.934 |
| Atrial fibrillation | 0.58 | 0.20–1.65 | 0.309 |
| LVEF ≤ 40% 3 | 1.30 | 0.41–4.10 | 0.665 |
| Parameter | ACS | p | ||
|---|---|---|---|---|
| All 1 (n = 603) | Invasive Strategy (n = 401) | Conservative Strategy (n = 202) | ||
| No. (%) | No. (%) | No. (%) | ||
| Discharge medication | ||||
| ACA/P2Y12 inhibitor | 559 (92.7%) | 395 (98.5%) | 164 (81.2%) | <0.001 * |
| ACE inhibitor/ARB | 485 (80.4%) | 334 (83.3%) | 151 (74.8%) | 0.013 * |
| Beta blocker | 497 (82.4%) | 329 (82.0%) | 168 (83.2%) | 0.732 |
| Statin | 574 (95.2%) | 391 (97.5%) | 183 (90.6%) | <0.001 * |
| VKA/DOAC | 151 (25.0%) | 83 (20.7%) | 68 (33.7%) | <0.001 * |
| Parameter | STEMI | p | ||
|---|---|---|---|---|
| All 1 (n = 259) | Invasive Strategy (n = 205) | Conservative Strategy (n = 54) | ||
| No. (%) | No. (%) | No. (%) | ||
| Discharge medication | ||||
| ACA/P2Y12 inhibitor | 244 (94.2%) | 202 (98.5%) | 42 (77.8%) | <0.001 * |
| ACE inhibitor/ARB | 203 (78.4%) | 165 (80.5%) | 38 (70.4%) | 0.108 |
| Beta blocker | 207 (79.9%) | 164 (80.0%) | 43 (79.6%) | 0.952 |
| Statin | 255 (98.5%) | 205 (100.0%) | 50 (92.6%) | <0.001 * |
| VKA/DOAC | 70 (27.0%) | 44 (21.5%) | 26 (48.1%) | <0.001 * |
| Parameter | NSTE-ACS | p | ||
|---|---|---|---|---|
| All 1 (n = 344) | Invasive Strategy (n = 196) | Conservative Strategy (n = 148) | ||
| No. (%) | No. (%) | No. (%) | ||
| Discharge medication | ||||
| ACA/P2Y12 inhibitor | 315 (91.6%) | 193 (98.5%) | 122 (82.4%) | <0.001 * |
| ACE inhibitor/ARB | 282 (82.0%) | 169 (86.2%) | 113 (76.4%) | 0.018 * |
| Beta blocker | 290 (84.3%) | 165 (84.2%) | 125 (84.5%) | 0.944 |
| Statin | 319 (92.7%) | 186 (94.9%) | 133 (89.9%) | 0.075 |
| VKA/DOAC | 81 (23.5%) | 39 (19.9%) | 42 (28.4%) | 0.066 |
| Parameter | ACS | p | ||
|---|---|---|---|---|
| All (n = 670) | Invasive Strategy (n = 429) | Conservative Strategy (n = 241) | ||
| No. (%) | No. (%) | No. (%) | ||
| Primary outcomes All-cause mortality | ||||
| In-hospital mortality | 67 (10.0%) | 28 (6.5%) | 39 (16.2%) | <0.001 * |
| Thirty-day mortality | 110 (16.4%) | 55 (12.8%) | 55 (22.8%) | <0.001 * |
| Six-month mortality | 174 (26.0%) | 83 (19.3%) | 91 (37.8%) | <0.001 * |
| Secondary outcomes | ||||
| Recurrent MI | 17 (2.5%) | 12 (2.8%) | 5 (2.1%) | 0.586 |
| CVI | 8 (1.2%) | 5 (1.2%) | 3 (1.2%) | 0.928 |
| MACE | 184 (27.5%) | 89 (20.7%) | 95 (39.4%) | <0.001 * |
| Parameter | ACS | p | ||
|---|---|---|---|---|
| All (n = 436) | Invasive Strategy (n = 218) | Conservative Strategy (n = 218) | ||
| No. (%) | No. (%) | No. (%) | ||
| Primary outcomes All-cause mortality | ||||
| In-hospital mortality | 56 (12.8%) | 21 (9.6%) | 35 (16.1%) | 0.045 * |
| Thirty-day mortality | 88 (20.2%) | 39 (17.9%) | 49 (22.9%) | 0.233 |
| Six-month mortality | 138 (31.7%) | 57 (26.1%) | 81 (37.2%) | 0.013 * |
| Secondary outcomes | ||||
| Recurrent MI | 14 (3.2%) | 9 (4.1%) | 5 (2.3%) | 0.277 |
| CVI | 4 (0.9%) | 1 (0.5%) | 3 (1.4%) | 0.315 |
| MACE | 145 (33.3%) | 60 (27.5%) | 85 (39.0%) | 0.011 * |
| Parameter | STEMI | p | ||
|---|---|---|---|---|
| All (n = 307) | Invasive Strategy (n = 228) | Conservative Strategy (n = 79) | ||
| No. (%) | No. (%) | No. (%) | ||
| Primary outcomes All-cause mortality | ||||
| In-hospital mortality | 48 (15.6%) | 23 (10.1%) | 25 (31.6%) | <0.001 * |
| Thirty-day mortality | 75 (24.4%) | 44 (19.3%) | 31 (39.2%) | <0.001 * |
| Six-month mortality | 100 (32.6%) | 61 (26.8%) | 39 (49.4%) | <0.001 * |
| Secondary outcomes | ||||
| Recurrent MI | 6 (2.0%) | 4 (1.8%) | 2 (2.5%) | 0.667 |
| CVI | 3 (1.0%) | 3 (1.3%) | 0 (0.0%) | 0.306 |
| MACE | 102 (33.2%) | 63 (27.6%) | 39 (49.4%) | <0.001 * |
| Parameter | NSTE-ACS | p | ||
|---|---|---|---|---|
| All (n = 363) | Invasive Strategy (n = 201) | Conservative Strategy (n = 162) | ||
| No. (%) | No. (%) | No. (%) | ||
| Primary outcomes All-cause mortality | ||||
| In-hospital mortality | 19 (2.5%) | 5 (2.5%) | 14 (8.6%) | 0.009 * |
| Thirty-day mortality | 35 (9.6%) | 11 (5.5%) | 24 (14.8%) | 0.003 * |
| Six-month mortality | 74 (20.4%) | 22 (10.9%) | 52 (32.1%) | <0.001 * |
| Secondary outcomes | ||||
| Recurrent MI | 11 (3.0%) | 8 (4.0%) | 3 (1.9%) | 0.240 |
| CVI | 5 (1.4%) | 2 (1.0%) | 3 (1.9%) | 0.486 |
| MACE | 82 (22.6%) | 26 (12.9%) | 56 (34.6%) | <0.001 * |
| Parameter | ACS | |||||
|---|---|---|---|---|---|---|
| Univariate | Multivariate | |||||
| HR | 95% CI | p | HR | 95% CI | p | |
| Age | 1.02 | 0.97–1.07 | 0.442 | 1.00 | 0.95–1.06 | 0.961 |
| Invasive strategy | 0.59 | 0.39–0.90 | 0.013 * | 0.53 | 0.35–0.81 | 0.003 * |
| Female sex | 1.15 | 0.77–1.72 | 0.483 | 1.30 | 0.86–1.97 | 0.217 |
| Aortic stenosis | 1.30 | 0.78–2.17 | 0.307 | 1.45 | 0.85–2.48 | 0.173 |
| Atrial fibrillation | 1.33 | 0.89–1.99 | 0.162 | 1.59 | 0.99–2.55 | 0.054 |
| Previous MI | 0.84 | 0.50–1.39 | 0.495 | 0.82 | 0.46–1.46 | 0.492 |
| Diabetes mellitus | 1.83 | 1.22–2.72 | 0.003 * | 1.88 | 1.23–2.87 | 0.003 * |
| LVEF ≤ 40% | 1.92 | 1.29–2.85 | 0.001 * | 2.13 | 1.40–3.23 | <0.001 * |
| Hemoglobin ≤ 100 g/L | 1.84 | 1.13–3.01 | 0.015 * | 1.46 | 0.87–2.46 | 0.154 |
| ACE inhibitor/ARB 1 | 0.61 | 0.41–0.91 | 0.015 * | 0.58 | 0.38–0.89 | 0.013 * |
| Statin 1 | 0.87 | 0.56–1.33 | 0.512 | 0.92 | 0.55–1.53 | 0.742 |
| VKA/DOAC 1 | 1.41 | 0.88–2.27 | 0.155 | 1.10 | 0.63–1.93 | 0.738 |
| ACA/P2Y12 inhibitor 1 | 0.88 | 0.58–1.35 | 0.570 | 0.97 | 0.59–1.58 | 0.892 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gjuras, K.; Jurin, I.; Jurin, H.; Margetić, E.; Skorić, B.; Bulum, J.; Hadžibegović, I.; Zeljković, I.; Pavlov, M.; Manola, Š.; et al. Acute Coronary Syndrome Management in Older Patients: A Dual-Center Retrospective Cohort Study. Medicina 2025, 61, 1436. https://doi.org/10.3390/medicina61081436
Gjuras K, Jurin I, Jurin H, Margetić E, Skorić B, Bulum J, Hadžibegović I, Zeljković I, Pavlov M, Manola Š, et al. Acute Coronary Syndrome Management in Older Patients: A Dual-Center Retrospective Cohort Study. Medicina. 2025; 61(8):1436. https://doi.org/10.3390/medicina61081436
Chicago/Turabian StyleGjuras, Karlo, Ivana Jurin, Hrvoje Jurin, Eduard Margetić, Boško Skorić, Joško Bulum, Irzal Hadžibegović, Ivan Zeljković, Marin Pavlov, Šime Manola, and et al. 2025. "Acute Coronary Syndrome Management in Older Patients: A Dual-Center Retrospective Cohort Study" Medicina 61, no. 8: 1436. https://doi.org/10.3390/medicina61081436
APA StyleGjuras, K., Jurin, I., Jurin, H., Margetić, E., Skorić, B., Bulum, J., Hadžibegović, I., Zeljković, I., Pavlov, M., Manola, Š., & Marić Bešić, K. (2025). Acute Coronary Syndrome Management in Older Patients: A Dual-Center Retrospective Cohort Study. Medicina, 61(8), 1436. https://doi.org/10.3390/medicina61081436








