Seasonal Patterns of Preterm Birth During the COVID-19 Pandemic: A Retrospective Cohort Study in Romania
Abstract
1. Introduction
2. Material and Methods
2.1. Study Setting
2.2. Data Sources and Study Population
2.3. Definitions and Measurements
2.4. Statistical Analysis
2.5. Ethical Approval
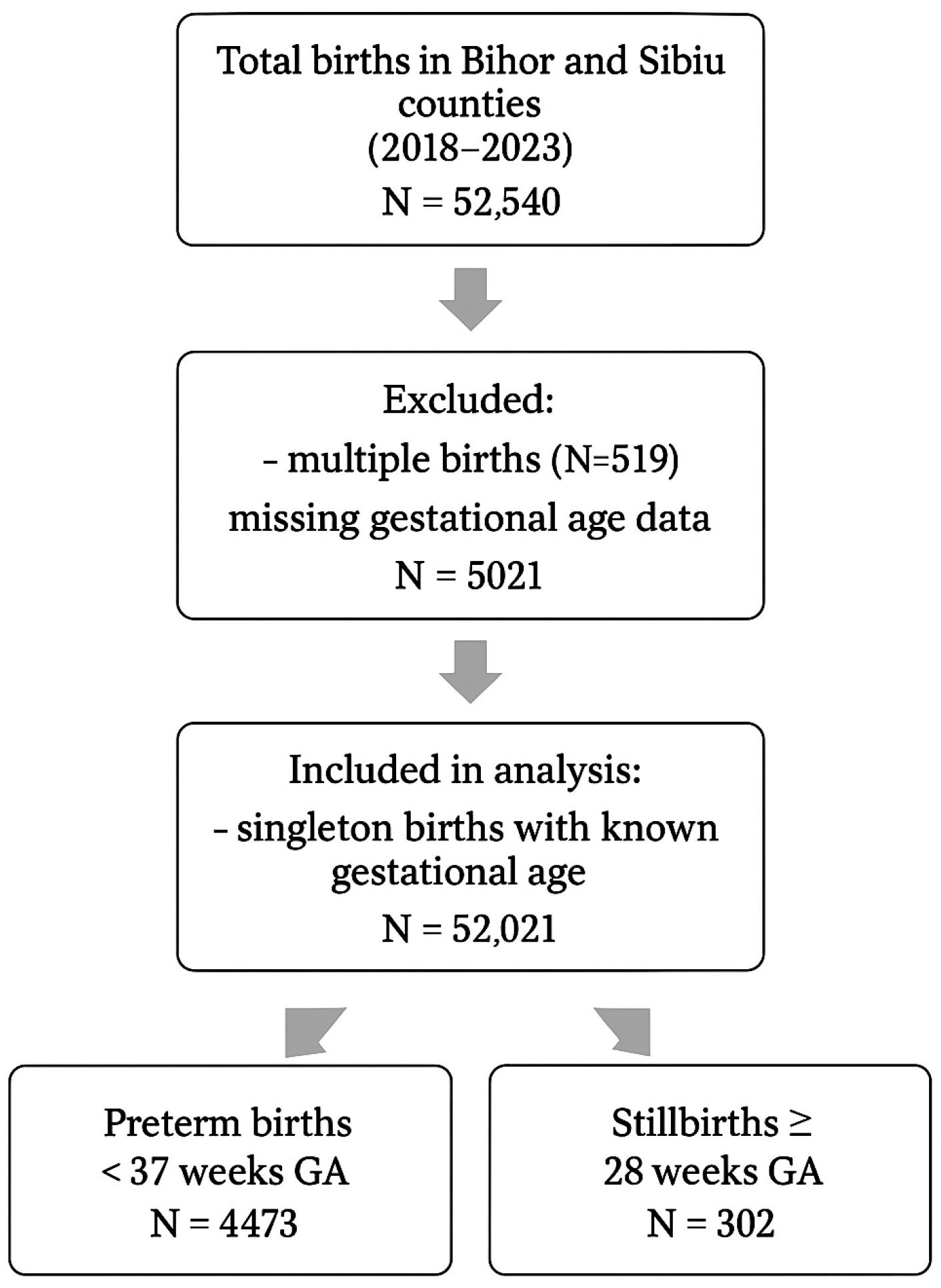
3. Results
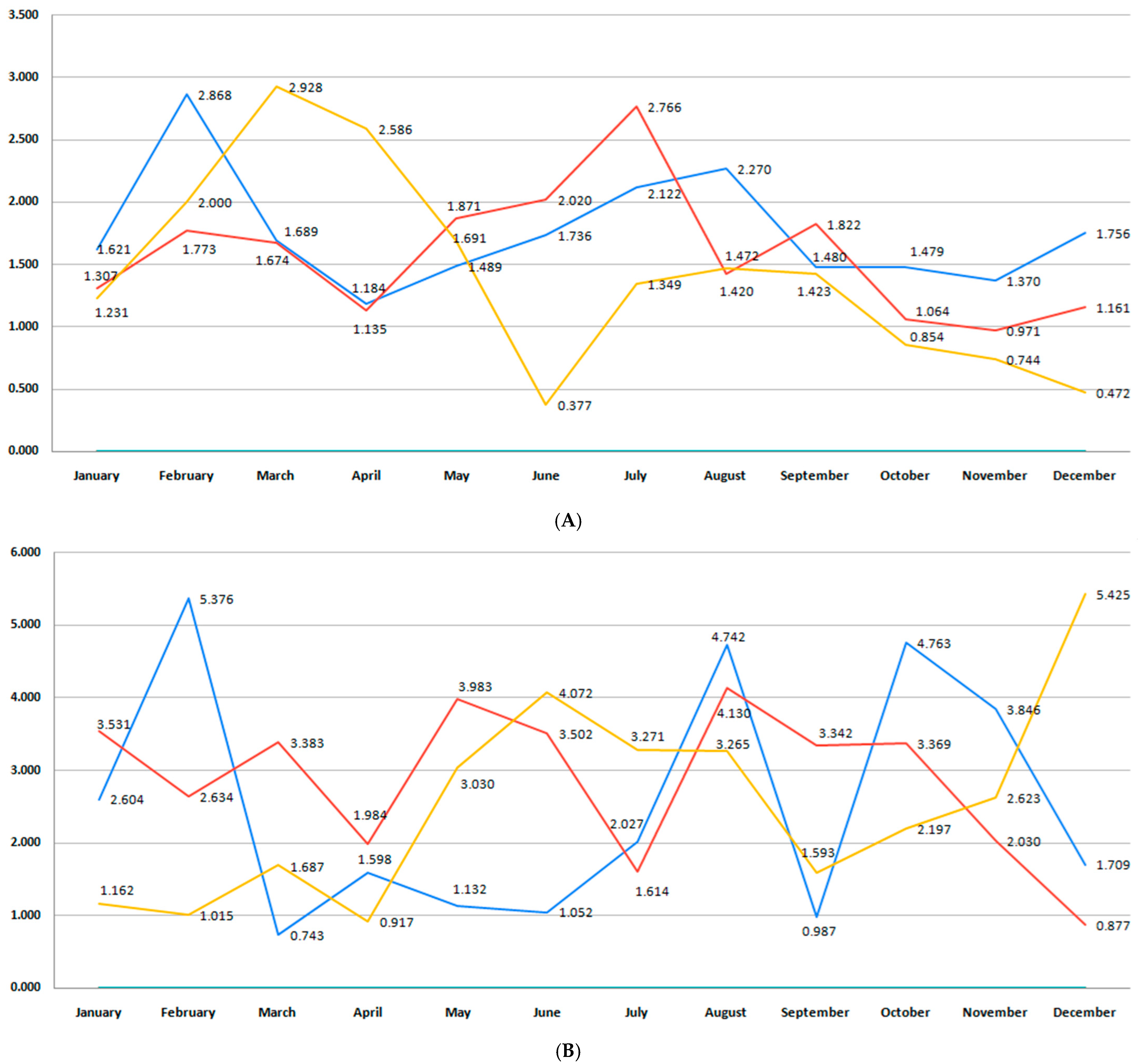
3.1. Birth Rate
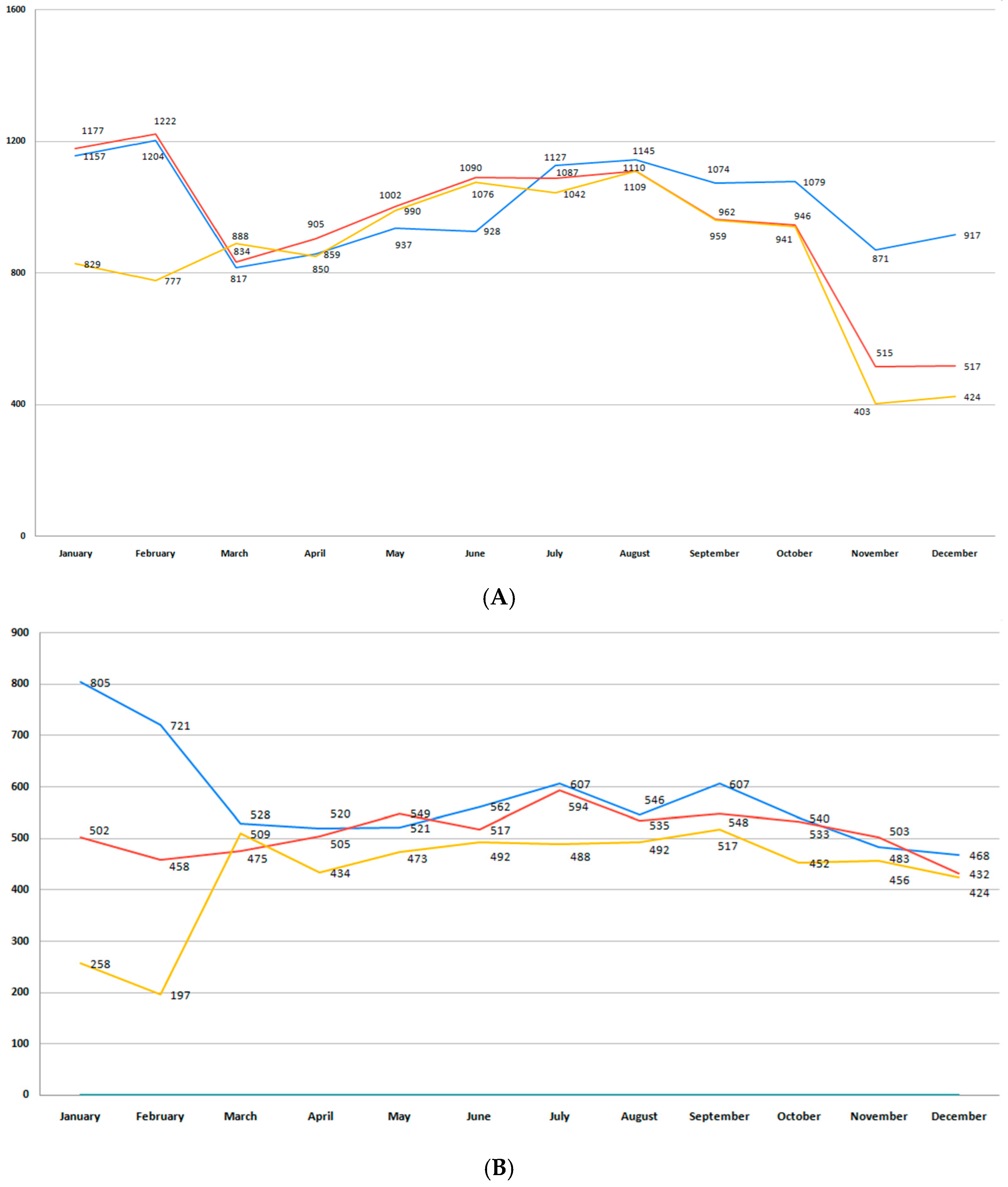
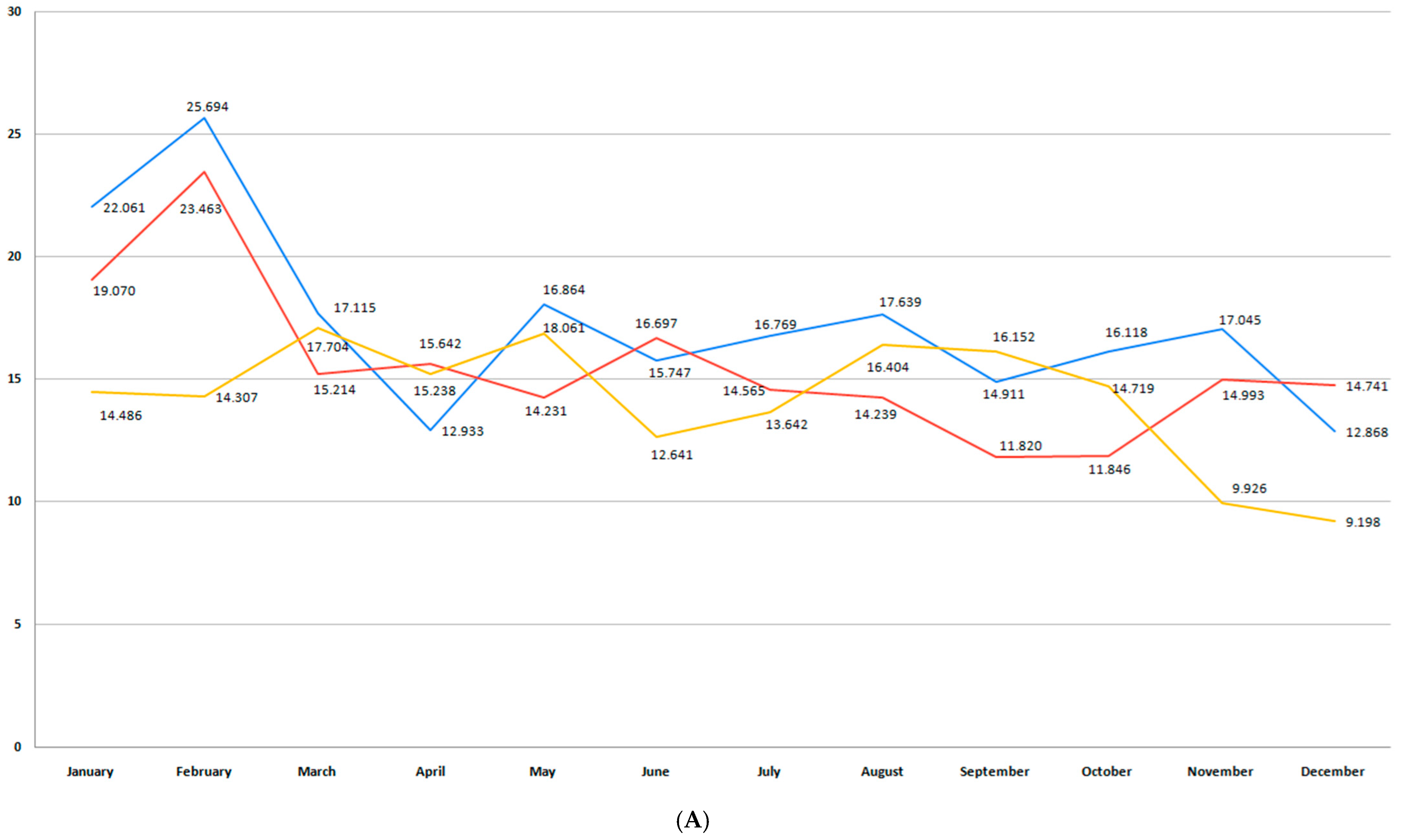
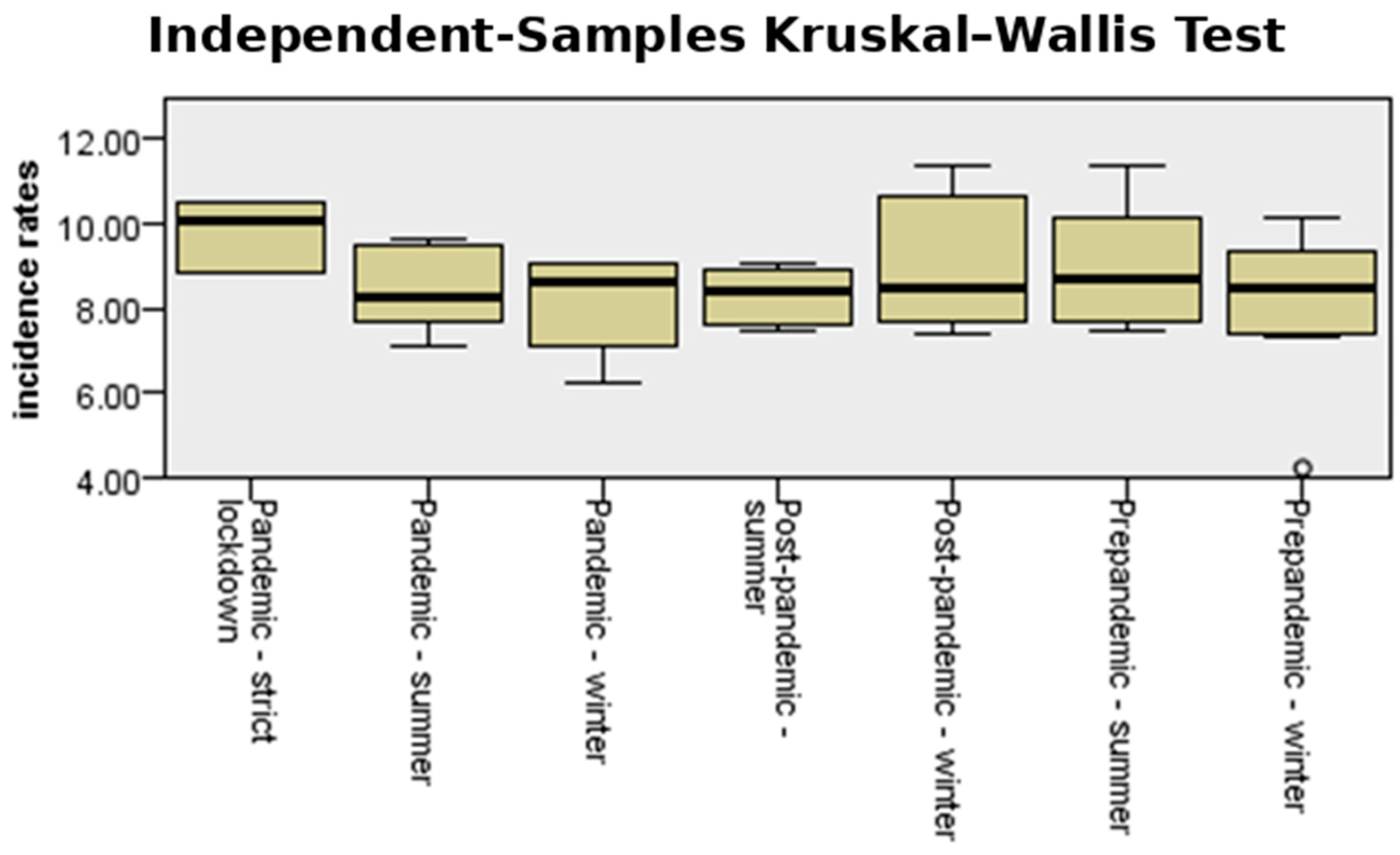
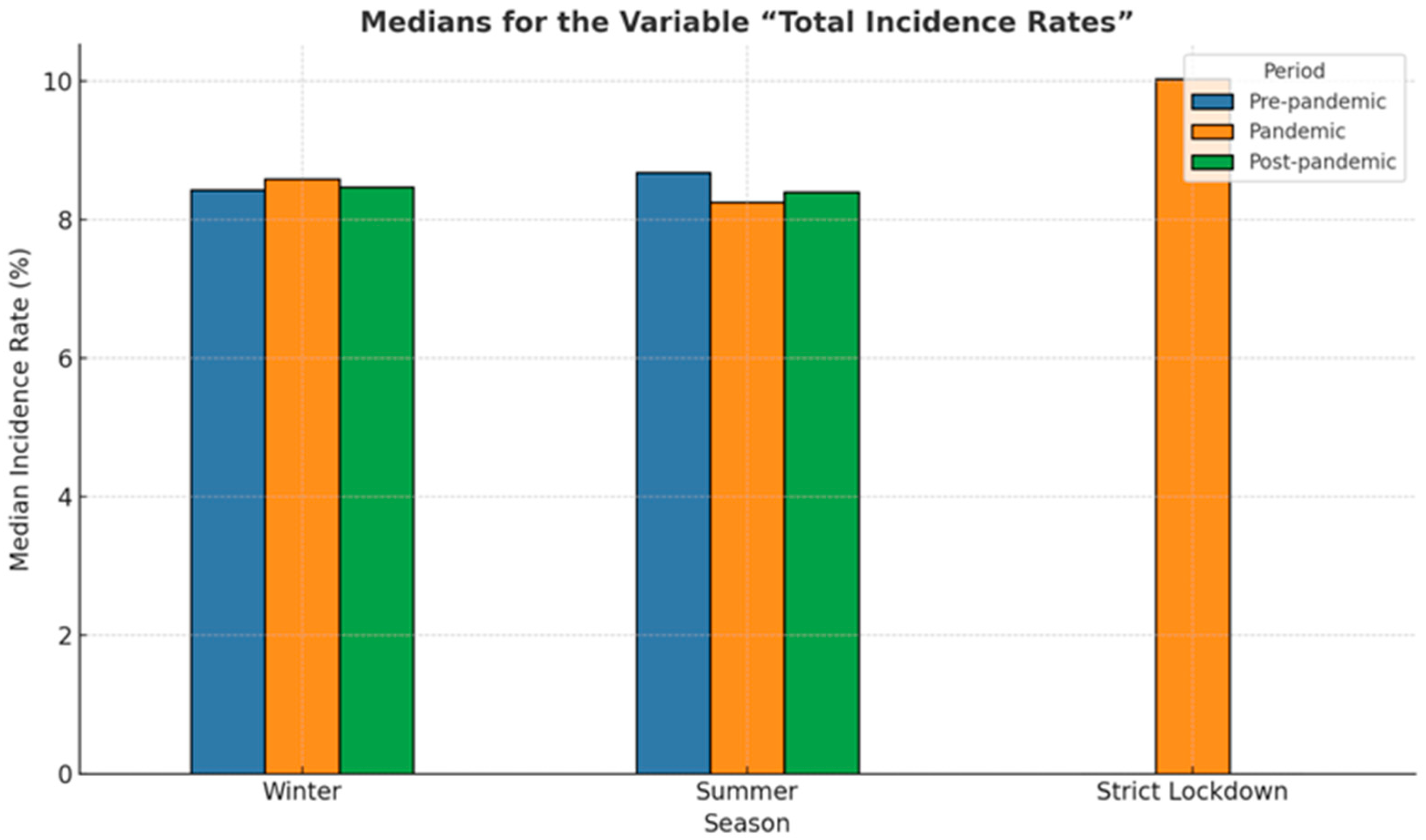
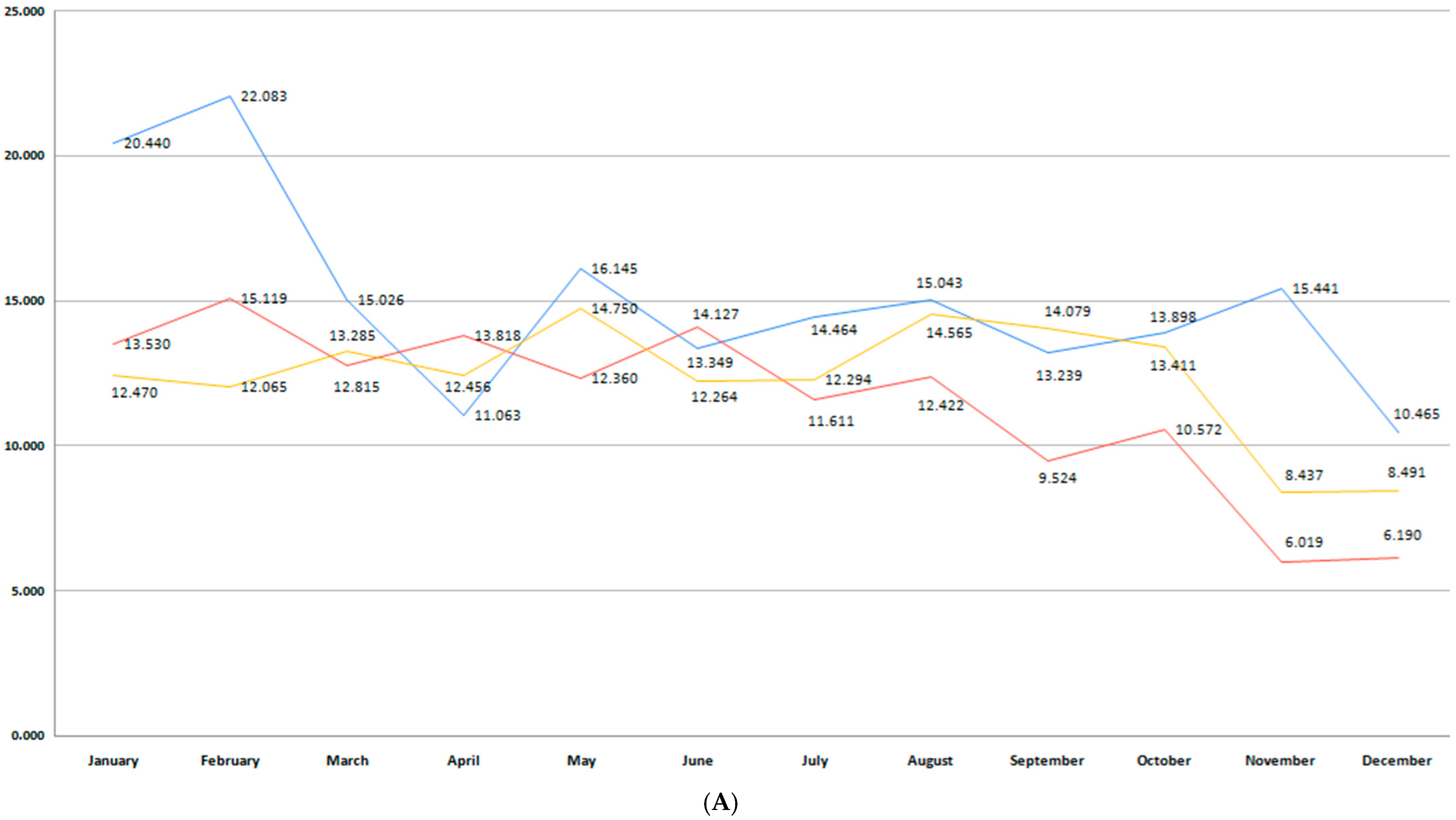
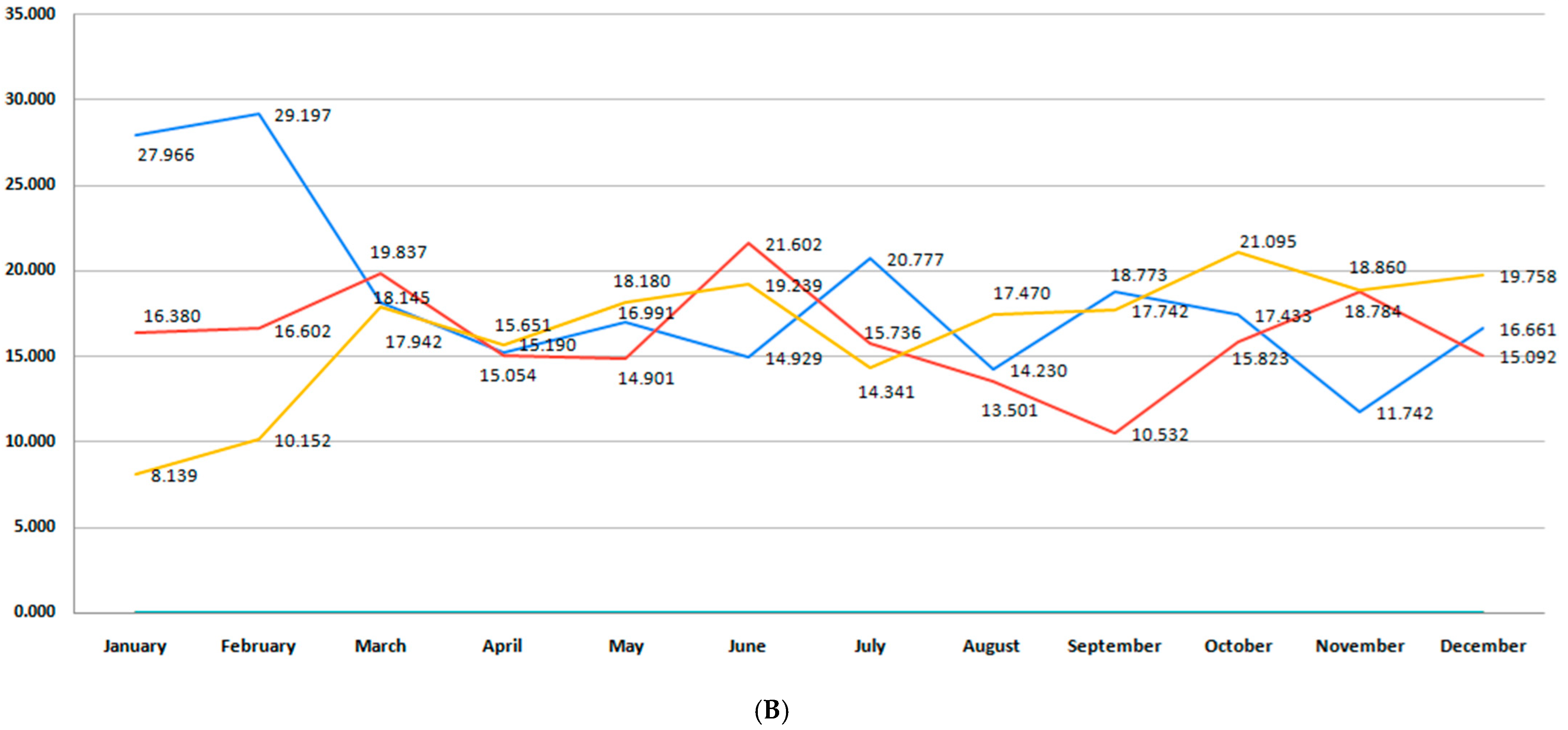
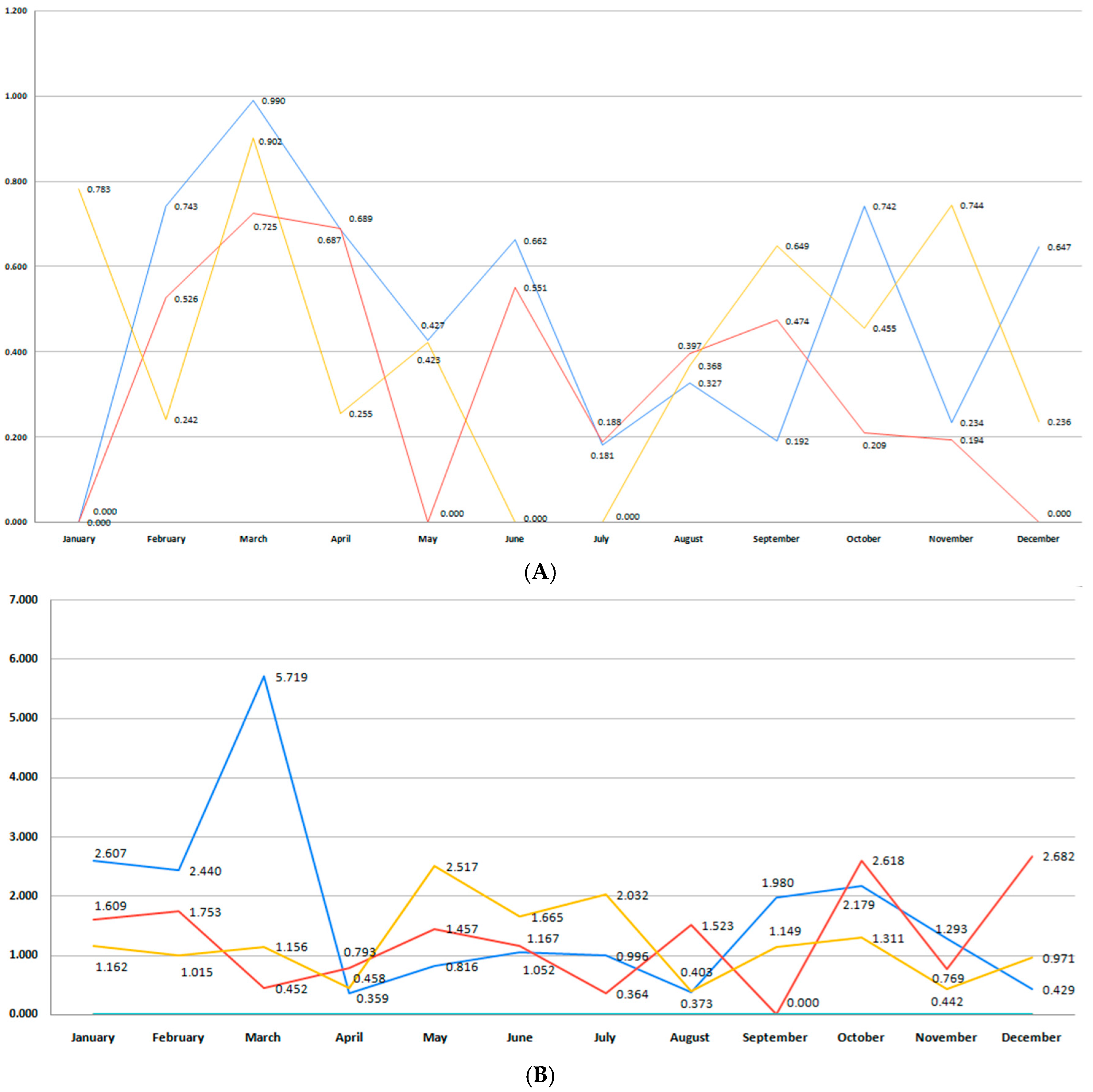
3.2. Incidence of Premature Birth
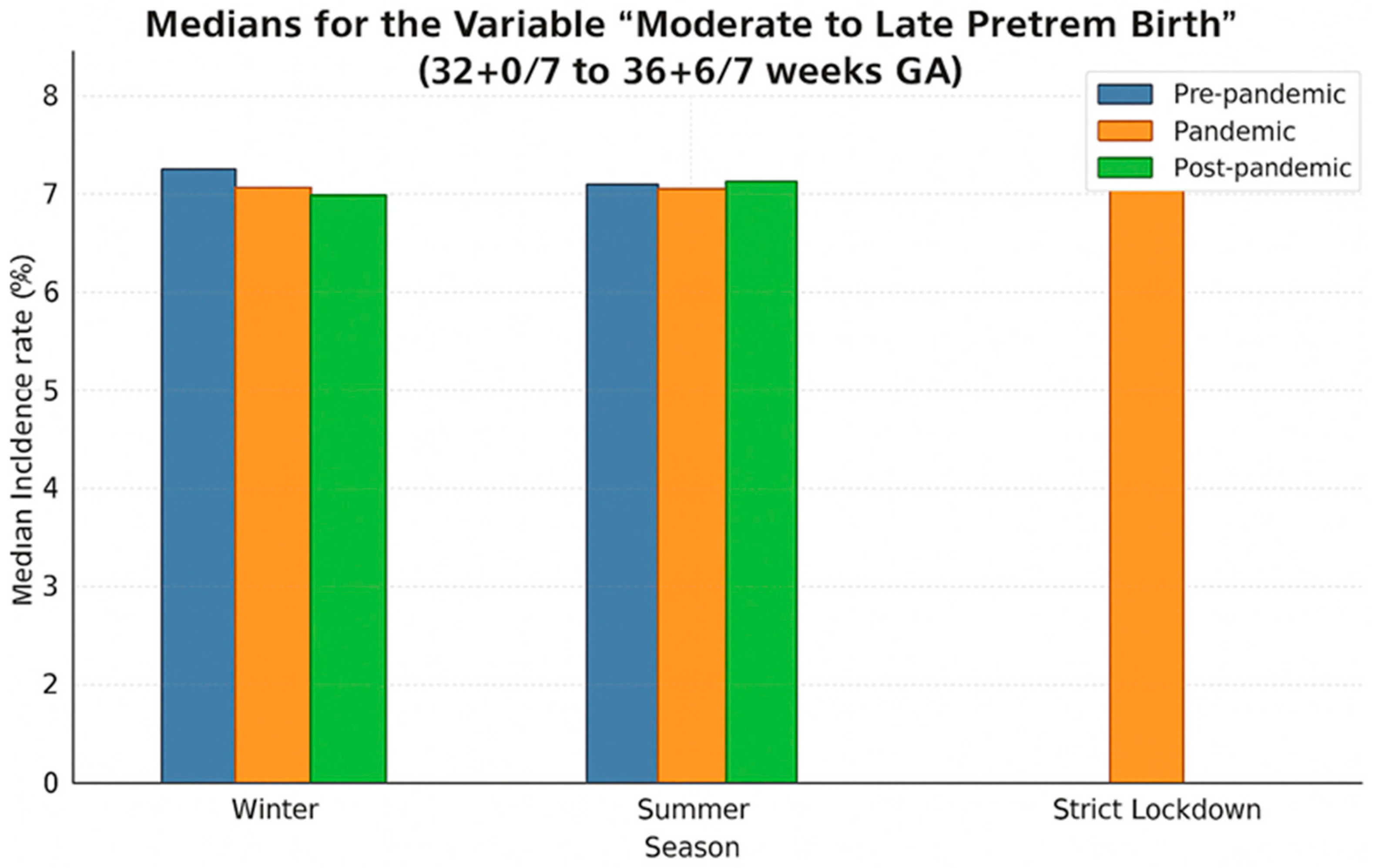
3.3. Moderate and Late Preterm Birth (32+0/7–36+6/7 Weeks GA)
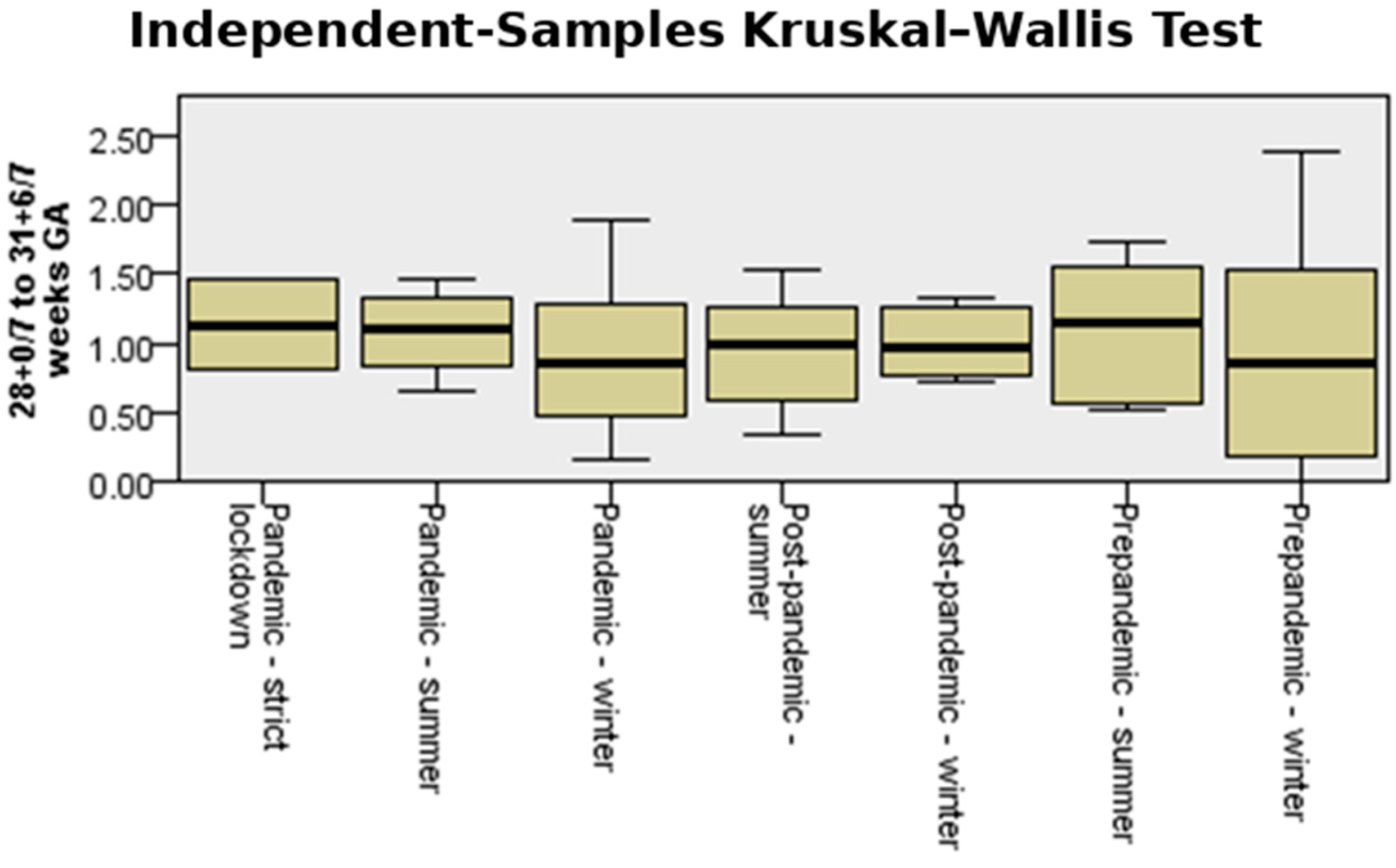
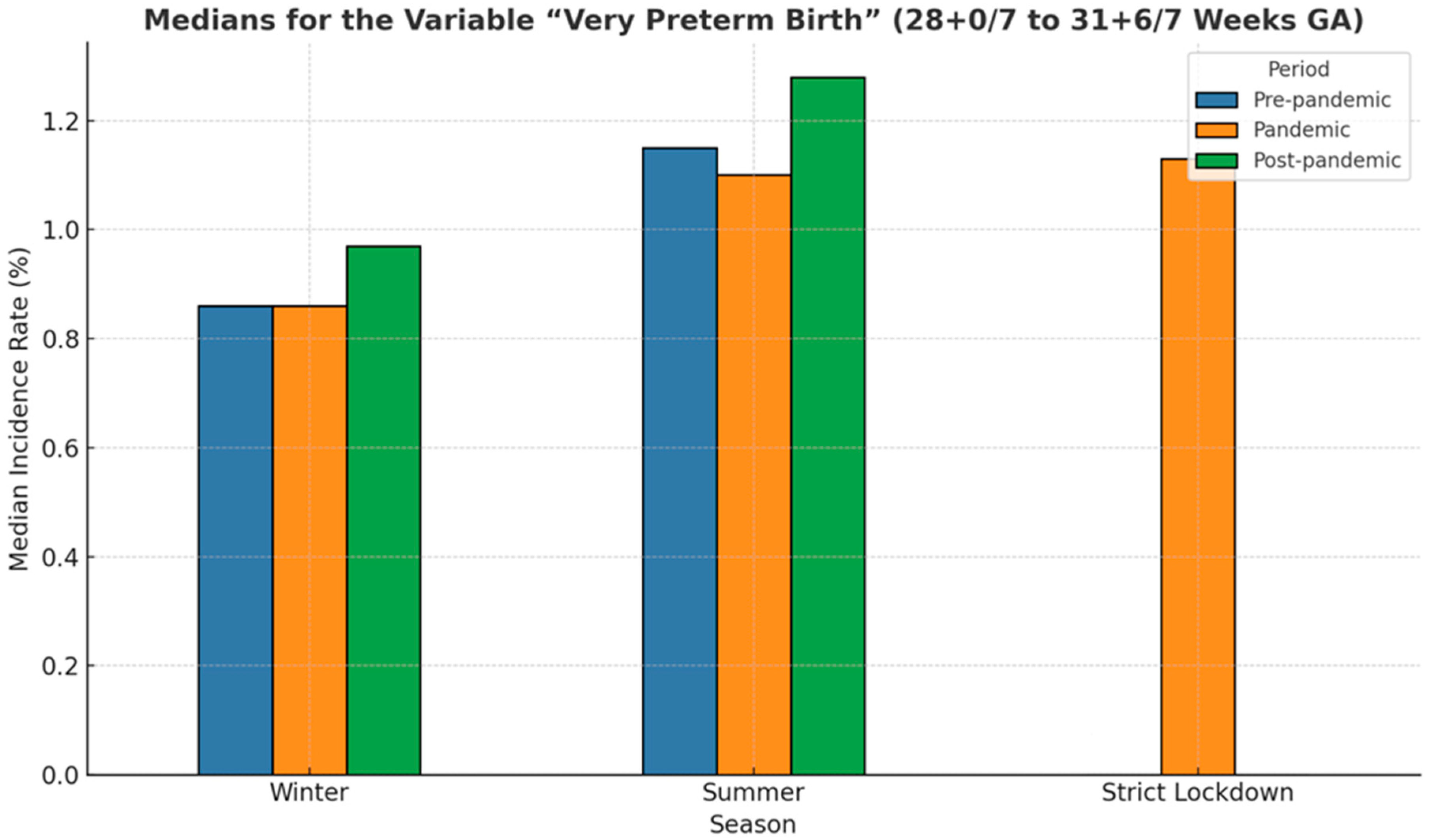
3.4. Very Preterm Birth (28 0/7–31 6/7 Weeks GA)
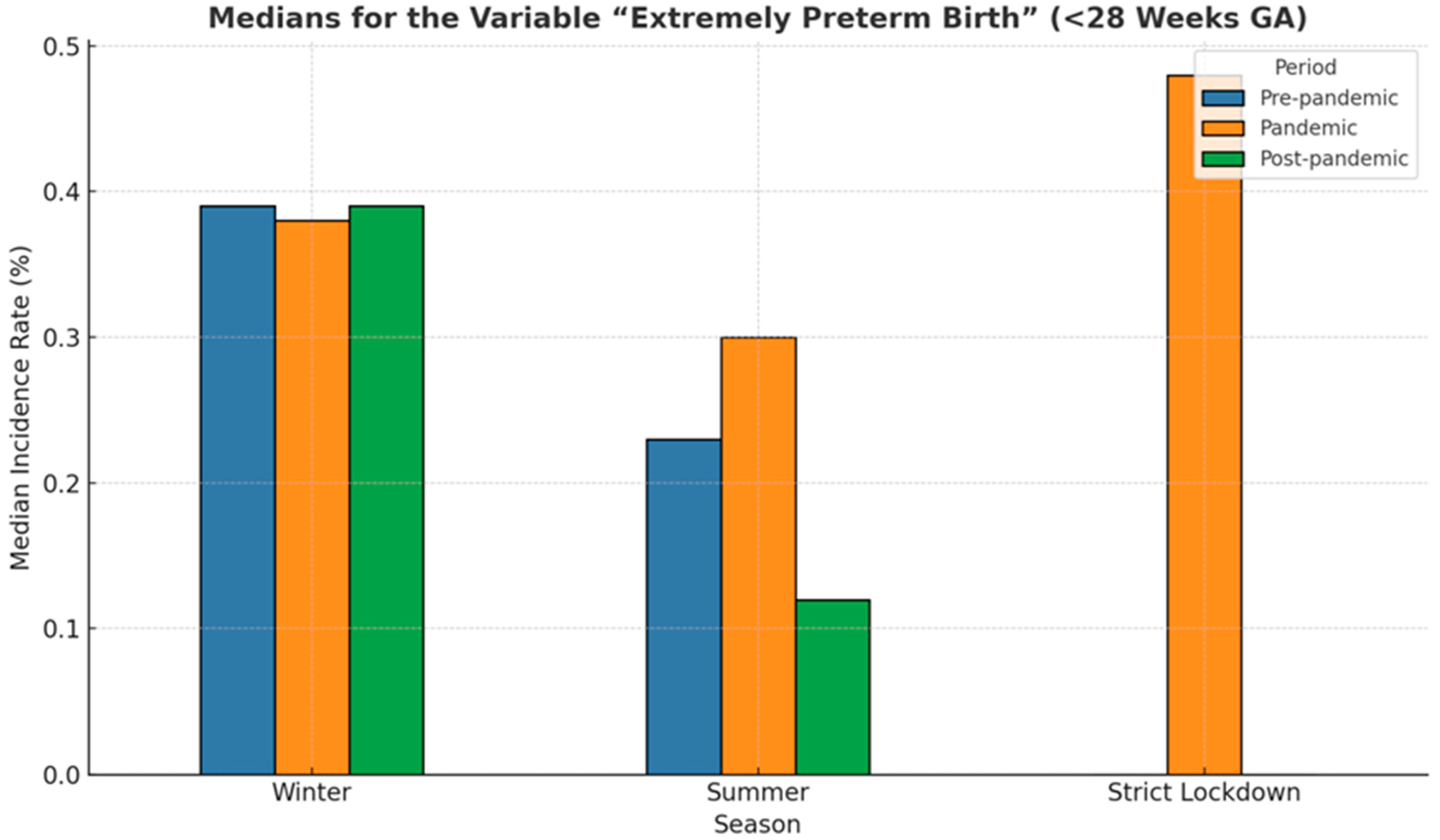
3.5. Extremely Preterm Birth (<28 Weeks GA)
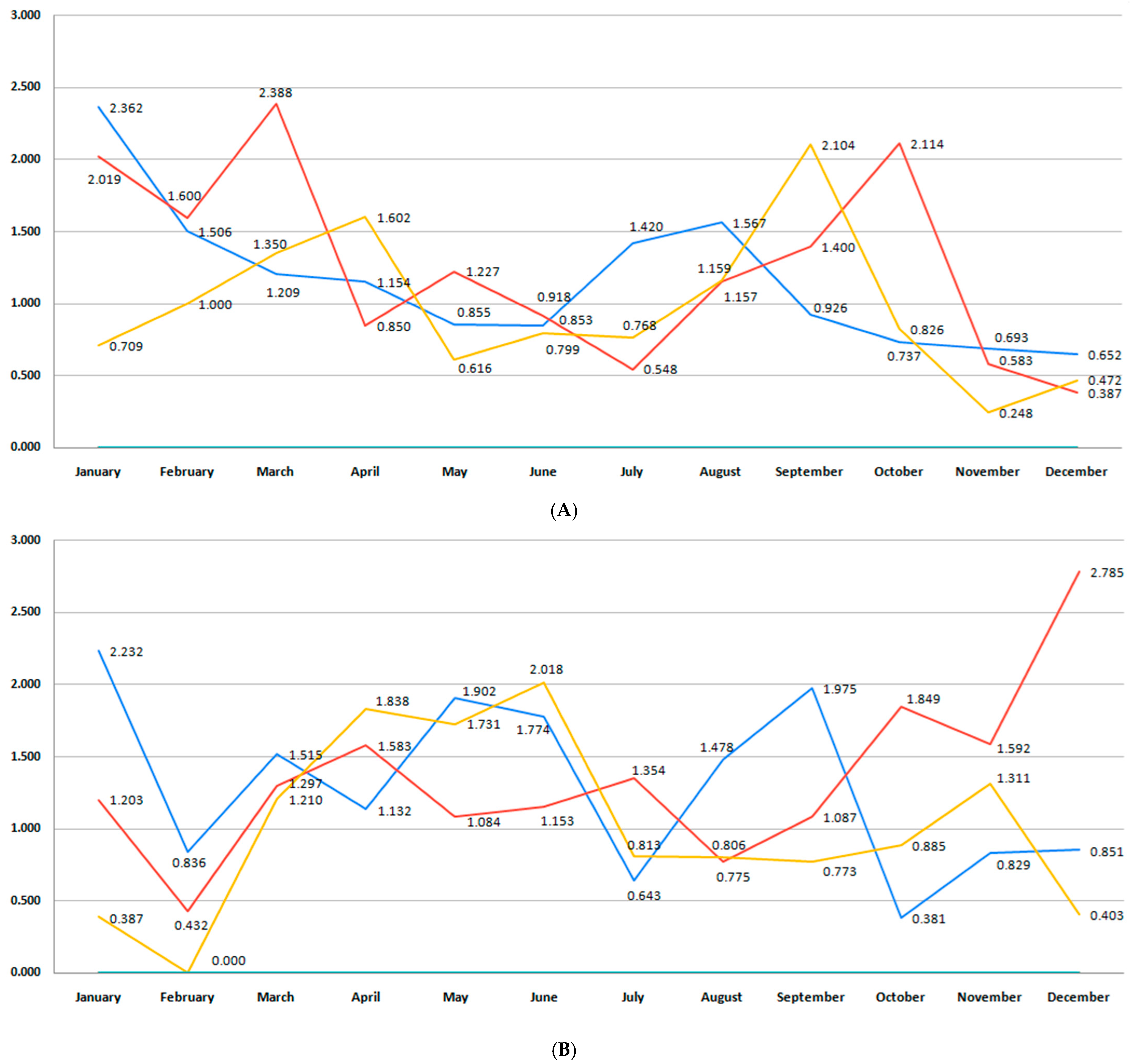
3.6. Incidence of Stillbirth
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perin, J.; Mulick, A.; Yeung, D.; Villavicencio, F.; Lopez, G.; Strong, K.L.; Prieto-Merino, D.; Cousens, S.; Black, R.E.; Liu, L. Global, regional, and national causes of under-5 mortality in 2000–19: An updated systematic analysis with implications for the Sustainable Development Goals. Lancet Child Adolesc. Health 2022, 6, 106–115. [Google Scholar] [CrossRef]
- Manuck, T.A.; Rice, M.M.; Bailit, J.L.; Grobman, W.A.; Reddy, U.M.; Wapner, R.J.; Thorp, J.M.; Caritis, S.N.; Prasad, M.; Tita, A.T.N.; et al. Preterm neonatal morbidity and mortality by gestational age: A contemporary cohort. Am. J. Obstet. Gynecol. 2016, 215, 103.e101–103.e114. [Google Scholar] [CrossRef] [PubMed]
- Toma, A.I.; Dima, V.; Alexe, A.; Bojan, C.; Nemeș, A.F.; Gonț, B.F.; Arghirescu, A.; Necula, A.I.; Fieraru, A.; Stoiciu, R.; et al. Early Intervention Guided by the General Movements Examination at Term Corrected Age—Short Term Outcomes. Life 2024, 14, 480. [Google Scholar] [CrossRef] [PubMed]
- Escobar, G.J.; McCormick, M.C.; Zupancic, J.A.F.; Coleman-Phox, K.; Armstrong, M.A.; Greene, J.D.; Eichenwald, E.C.; Richardson, D.K. Unstudied infants: Outcomes of moderately premature infants in the neonatal intensive care unit. Arch. Dis. Child. Fetal Neonatal Ed. 2006, 91, F238. [Google Scholar] [CrossRef]
- Kinney, H.C. The Near-Term (Late Preterm) Human Brain and Risk for Periventricular Leukomalacia: A Review. Semin. Perinatol. 2006, 30, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Ruys, C.A.; van de Lagemaat, M.; Rotteveel, J.; Finken, M.J.J.; Lafeber, H.N. Improving long-term health outcomes of preterm infants: How to implement the findings of nutritional intervention studies into daily clinical practice. Eur. J. Pediatr. 2021, 180, 1665–1673. [Google Scholar] [CrossRef]
- Baroutis, G.; Athanasios, M.; Derek, H.; Antsaklis, A. Preterm birth seasonality in Greece: An epidemiological study. J. Matern.-Fetal Neonatal Med. 2012, 25, 1406–1412. [Google Scholar] [CrossRef]
- Becker, S. Seasonal Patterns of Births and Conception Throughout the World. In Temperature and Environmental Effects on the Testis; Zorgniotti, A.W., Ed.; Springer: Boston, MA, USA, 1991; pp. 59–72. [Google Scholar]
- Rogowski, J.A.; Staiger, D.; Patrick, T.; Horbar, J.; Kenny, M.; Lake, E.T. Nurse Staffing and NICU Infection Rates. JAMA Pediatr. 2013, 167, 444–450. [Google Scholar] [CrossRef]
- Watson, S.I.; Arulampalam, W.; Petrou, S.; Marlow, N.; Morgan, A.S.; Draper, E.; Modi, N. The effects of a one-to-one nurse-to-patient ratio on the mortality rate in neonatal intensive care: A retrospective, longitudinal, population-based study. Arch. Dis. Child. Fetal Neonatal Ed. 2016, 101, F195–F200. [Google Scholar] [CrossRef]
- StC Hamilton, K.E.; Redshaw, M.E.; Tarnow-Mordi, W. Nurse staffing in relation to risk-adjusted mortality in neonatal care. Arch. Dis. Child. Fetal Neonatal Ed. 2007, 92, F99. [Google Scholar] [CrossRef]
- Choi, A.; Hwang, H.S.; Sung Kwon, H.; Sohn, I.S.; Yang, S.-W.; Kwak, D.W. 966 Seasonality and the causes of spontaneous preterm birth. Am. J. Obstet. Gynecol. 2024, 230, S510–S511. [Google Scholar] [CrossRef]
- Cooperstock, M.; Wolfe, R.A. Seasonality of preterm birth in the collaborative project: Demographic factors. Am. J. Epidemiol. 1986, 124, 234–241. [Google Scholar] [CrossRef]
- Strand, L.B.; Barnett, A.G.; Tong, S. The influence of season and ambient temperature on birth outcomes: A review of the epidemiological literature. Environ. Res. 2011, 111, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Bekkar, B.; Pacheco, S.; Basu, R.; DeNicola, N. Association of Air Pollution and Heat Exposure With Preterm Birth, Low Birth Weight, and Stillbirth in the US: A Systematic Review. JAMA Netw. Open 2020, 3, e208243. [Google Scholar] [CrossRef]
- Matsuda, S.; Kahyo, H. Seasonality of Preterm Births in Japan. Int. J. Epidemiol. 1992, 21, 91–100. [Google Scholar] [CrossRef]
- Rojansky, N.; Brzezinski, A.; Schenker, J.G. Seasonality in human reproduction: An update. Hum. Reprod. 1992, 7, 735–745. [Google Scholar] [CrossRef]
- Krombholz, H. Global Patterns of Seasonal Variation in Human Birth Rates—Influence of Climate and Economic and Social Factors. PsychArchives 2023. [Google Scholar] [CrossRef]
- Nagel, L.E.; Reisch, B.; Schwenk, U.; Kimmig, K.R.; Darkwah Oppong, M.; Dzietko, M.; Gellhaus, A.; Iannaccone, A. Impact of 2 years of COVID-19 pandemic on preterm birth: Experience from a tertiary center of obstetrics in western Germany. Int. J. Gynecol. Obstet. 2024, 166, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Hedermann, G.; Hedley, P.L.; Bækvad-Hansen, M.; Hjalgrim, H.; Rostgaard, K.; Poorisrisak, P.; Breindahl, M.; Melbye, M.; Hougaard, D.M.; Christiansen, M.; et al. Danish premature birth rates during the COVID-19 lockdown. Arch. Dis. Child. Fetal Neonatal Ed. 2021, 106, 93. [Google Scholar] [CrossRef]
- Berghella, V.; Boelig, R.; Roman, A.; Burd, J.; Anderson, K. Decreased incidence of preterm birth during coronavirus disease 2019 pandemic. Am. J. Obstet. Gynecol. MFM 2020, 2, 100258. [Google Scholar] [CrossRef] [PubMed]
- Matheson, A.; McGannon, C.J.; Malhotra, A.; Palmer, K.R.; Stewart, A.E.; Wallace, E.M.; Mol, B.W.; Hodges, R.J.; Rolnik, D.L. Prematurity Rates During the Coronavirus Disease 2019 (COVID-19) Pandemic Lockdown in Melbourne, Australia. Obstet. Gynecol. 2021, 137, 405–407. [Google Scholar] [CrossRef]
- Galis, R.; Trif, P.; Mudura, D.; Murvai, R.; Daina, L.G.; Szasz, F.; Negrini, R.; Hatos, A.; Gyarmati, B.F.; Daly, M.C.; et al. Preterm birth and stillbirth during COVID-19 pandemic in Bihor County/Romania. Front. Reprod. Health 2024, 6, 1286496. [Google Scholar] [CrossRef]
- Roy, K.P.; Helen, P.; Elizabeth, R.; Mandy, D.; Mendinaro, I.; Deirdre, M.; Nuala, H.O.C.; Colum, P.D. Unprecedented reduction in births of very low birthweight (VLBW) and extremely low birthweight (ELBW) infants during the COVID-19 lockdown in Ireland: A ‘natural experiment’ allowing analysis of data from the prior two decades. BMJ Glob. Health 2020, 5, e003075. [Google Scholar] [CrossRef]
- Dong, M.; Qian, R.; Wang, J.; Fan, J.; Ye, Y.; Zhou, H.; Win, B.; Reid, E.; Zheng, S.; Lv, Y.; et al. Associations of COVID-19 lockdown with gestational length and preterm birth in China. BMC Pregnancy Childbirth 2021, 21, 795. [Google Scholar] [CrossRef] [PubMed]
- Klumper, J.; Kazemier, B.M.; Been, J.V.; Bloemenkamp, K.W.M.; de Boer, M.A.; Erwich, J.J.H.M.; Heidema, W.; Klumper, F.J.C.M.; Bijvank, S.W.A.N.; Oei, S.G.; et al. Association between COVID-19 lockdown measures and the incidence of iatrogenic versus spontaneous very preterm births in the Netherlands: A retrospective study. BMC Pregnancy Childbirth 2021, 21, 767. [Google Scholar] [CrossRef] [PubMed]
- Arnaez, J.; Ochoa-Sangrador, C.; Caserío, S.; Gutiérrez, E.P.; Jiménez, M.d.P.; Castañón, L.; Benito, M.; Peña, A.; Hernández, N.; Hortelano, M.; et al. Lack of changes in preterm delivery and stillbirths during COVID-19 lockdown in a European region. Eur. J. Pediatr. 2021, 180, 1997–2002. [Google Scholar] [CrossRef]
- Calvert, C.; Brockway, M.; Zoega, H.; Miller, J.E.; Been, J.V.; Amegah, A.K.; Racine-Poon, A.; Oskoui, S.E.; Abok, I.I.; Aghaeepour, N.; et al. Changes in preterm birth and stillbirth during COVID-19 lockdowns in 26 countries. Nat. Hum. Behav. 2023, 7, 529–544. [Google Scholar] [CrossRef] [PubMed]
- Chmielewska, B.; Barratt, I.; Townsend, R.; Kalafat, E.; van der Meulen, J.; Gurol-Urganci, I.; O’Brien, P.; Morris, E.; Draycott, T.; Thangaratinam, S.; et al. Effects of the COVID-19 pandemic on maternal and perinatal outcomes: A systematic review and meta-analysis. Lancet Glob. Health 2021, 9, e759–e772. [Google Scholar] [CrossRef]
- Zeitlin, J.; Philibert, M.; Barros, H.; Broeders, L.; Cap, J.; Draušnik, Ž.; Engjom, H.; Farr, A.; Fresson, J.; Gatt, M.; et al. Socioeconomic disparities in changes to preterm birth and stillbirth rates during the first year of the COVID-19 pandemic: A study of 21 European countries. Eur. J. Public Health 2024, 34, i58–i66. [Google Scholar] [CrossRef]
- Bobei, T.-I.; Haj Hamoud, B.; Sima, R.-M.; Gorecki, G.-P.; Poenaru, M.-O.; Olaru, O.-G.; Ples, L. The Impact of SARS-CoV-2 Infection on Premature Birth—Our Experience as COVID Center. Medicina 2022, 58, 587. [Google Scholar] [CrossRef]
- Nemes, A.F.; Toma, A.I.; Dima, V.; Serboiu, S.C.; Necula, A.I.; Stoiciu, R.; Ulmeanu, A.I.; Marinescu, A.; Ulmeanu, C. Use of Lung Ultrasound in Reducing Radiation Exposure in Neonates with Respiratory Distress: A Quality Management Project. Medicina 2024, 60, 308. [Google Scholar] [CrossRef]
- Chicea, R.; Neagu, A.D.; Chicea, E.D.; Grindeanu, A.S.; Bratu, D.G.; Boicean, A.G.; Roman, M.D.; Fleacă, S.R.; Chicea, L.M.; Teacoe, D.A.; et al. Impact of SARS-CoV-2 Infection on Maternal and Neonatal Outcome in Correlation with Sociodemographic Aspects: A Retrospective Case-Control Study. J. Clin. Med. 2023, 12, 6322. [Google Scholar] [CrossRef] [PubMed]
- Cucerea, M.; Simon, M.; Stoicescu, S.M.; Blaga, L.D.; Galiș, R.; Stamatin, M.; Olariu, G.; Ognean, M.L. Neonatal Resuscitation Practices in Romania: A Survey of the Romanian Association of Neonatology (ANR) and the Union of European Neonatal and Perinatal Societies (UENPS). J. Crit. Care Med. 2024, 10, 19–29. [Google Scholar] [CrossRef] [PubMed]
- The Weather Channel. Available online: https://weather.com/weather/tenday/l/6bd9f7b1ac9946cd9944ef844989e1a4641dac9a960e32af74885094d561d50f (accessed on 11 May 2025).
- Wikipedia. Sibiu County. Available online: https://en.wikipedia.org/wiki/Sibiu_County (accessed on 11 May 2025).
- Wikipedia. Bihor County. Available online: https://en.wikipedia.org/wiki/Bihor_County (accessed on 11 May 2025).
- Ministerul Sănătăţii. Ordinul Ministerului Sănătății nr. 359/2012; Ministerul Sănătăţii: Bucharest, Romania, 2012. [Google Scholar]
- Towers, C.V.; Bonebrake, R.; Padilla, G.; Rumney, P. The effect of transport on the rate of severe intraventricular hemorrhage in very low birth weight infants. Obstet. Gynecol. 2000, 95, 291–295. [Google Scholar] [CrossRef]
- Shlossman, P.A.; Manley, J.S.; Sciscione, A.C.; Colmorgen, G.H.C. An Analysis of Neonatal Morbidity and Mortality in Maternal (In Utero) and Neonatal Transports at 24–34 Weeks’ Gestation. Am. J. Perinatol. 1997, 14, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.T.C.; Ratnavel, N. A prospective review of adverse events during interhospital transfers of neonates by a dedicated neonatal transfer service. Pediatr. Crit. Care Med. 2008, 9, 289–293. [Google Scholar] [CrossRef]
- Institutul National de Statistica. Evenimente Demografice Anul 2020; Institutul National de Statistica: Bucharest, Romania, 2021; p. 78. [Google Scholar]
- Ministerul Sănătăţii. Buletin Infromativ—Miscarea Naturala a Populatiei nr. 10/2021; Institutul National de Sanatate Publica, Centrul Naţional de Statistică şi Informatică în Sănătate Publică: Bucharest, Romania, 2021; p. 15. [Google Scholar]
- Jurca, C.; Bembea, M.; Pallag, A.; Mureşan, M.; Szilagyi, A.; Balmoș, A.; Pop, O.; Jurca, A.; Dobjanschi, L. Pharmacotherapeutical considerations in the treatment and management of neonatal hyperammonaemia. Farmacia 2018, 66, 216–222. [Google Scholar]
- Winkler-Dworak, M.; Zeman, K.; Sobotka, T. Birth rate decline in the later phase of the COVID-19 pandemic: The role of policy interventions, vaccination programmes, and economic uncertainty. Hum. Reprod. Open 2024, 2024, hoae052. [Google Scholar] [CrossRef]
- Pomar, L.; Favre, G.; de Labrusse, C.; Contier, A.; Boulvain, M.; Baud, D. Impact of the first wave of the COVID-19 pandemic on birth rates in Europe: A time series analysis in 24 countries. Hum. Reprod. 2022, 37, 2921–2931. [Google Scholar] [CrossRef]
- Mooi-Reci, I.; Wooden, M.; Zilio, F. Baby Bump or Baby Slump? COVID-19, Lockdowns, and their Effects on Births in Australia. SSM—Popul. Health 2024, 25, 101604. [Google Scholar] [CrossRef]
- Sobotka, T.; Zeman, K.; Jasilioniene, A.; Winkler-Dworak, M.; Brzozowska, Z.; Alustiza-Galarza, A.; Németh, L.; Jdanov, D. Pandemic Roller-Coaster? Birth Trends in Higher-Income Countries During the COVID-19 Pandemic. Popul. Dev. Rev. 2024, 50, 23–58. [Google Scholar] [CrossRef]
- Murvai, V.R.; Galiș, R.; Panaitescu, A.; Radu, C.M.; Ghitea, T.C.; Trif, P.; Onița-Avram, M.; Vesa, A.A.; Huniadi, A. Antiphospholipid syndrome in pregnancy: A comprehensive literature review. BMC Pregnancy Childbirth 2025, 25, 337. [Google Scholar] [CrossRef]
- Vaccaro, C.; Mahmoud, F.; Aboulatta, L.; Aloud, B.; Eltonsy, S. The impact of COVID-19 first wave national lockdowns on perinatal outcomes: A rapid review and meta-analysis. BMC Pregnancy Childbirth 2021, 21, 676. [Google Scholar] [CrossRef]
- Yang, J.; D’Souza, R.; Kharrat, A.; Fell, D.B.; Snelgrove, J.W.; Shah, P.S. COVID-19 pandemic and population-level pregnancy and neonatal outcomes in general population: A living systematic review and meta-analysis (Update#2: November 20, 2021). Acta Obstet. Gynecol. Scand. 2022, 101, 273–292. [Google Scholar] [CrossRef]
- Basu, R.; Malig, B.; Ostro, B. High Ambient Temperature and the Risk of Preterm Delivery. Am. J. Epidemiol. 2010, 172, 1108–1117. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Steer, P.J.; Filippi, V. Seasonal patterns and preterm birth: A systematic review of the literature and an analysis in a London-based cohort. BJOG Int. J. Obstet. Gynaecol. 2006, 113, 1280–1288. [Google Scholar] [CrossRef] [PubMed]
- Friger, M.; Shoham-Vardi, I.; Abu-Saad, K. Trends and seasonality in birth frequency: A comparison of Muslim and Jewish populations in southern Israel: Daily time series analysis of 200 009 births, 1988–2005. Hum. Reprod. 2009, 24, 1492–1500. [Google Scholar] [CrossRef] [PubMed]
- Giorgis-Allemand, L.; Pedersen, M.; Bernard, C.; Aguilera, I.; Beelen, R.M.J.; Chatzi, L.; Cirach, M.; Danileviciute, A.; Dedele, A.; van Eijsden, M.; et al. The Influence of Meteorological Factors and Atmospheric Pollutants on the Risk of Preterm Birth. Am. J. Epidemiol. 2017, 185, 247–258. [Google Scholar] [CrossRef]
- Hviid, A.; Laksafoss, A.; Hedley, P.; Lausten-Thomsen, U.; Hjalgrim, H.; Christiansen, M.; Olsen, S.F. Assessment of Seasonality and Extremely Preterm Birth in Denmark. JAMA Netw. Open 2022, 5, e2145800. [Google Scholar] [CrossRef]
- Ministerul Sănătăţii. Mortalitate Perinatala; Institutul National de Sanatate Publica, Centrul Naţional de Statistică şi Informatică în Sănătate Publică: Bucharest, Romania, 2020; p. 40. [Google Scholar]
- Ministerul Sănătăţii. Mortalitate Perinatala 2021; Institutul National de Sanatate Publica: Bucharest, Romania, 2021; p. 40. [Google Scholar]
- Diggikar, S.; Galis, R.; Nagesh, K.; Pandita, A.; Ognean, M.L.; Rüdiger, M.; Mazela, J.; Kramer, B.W. Surfactant therapy—The conundrum of which infant should be given, when, which drug in what dose via which route of administration? Semin. Fetal Neonatal Med. 2024, 29, 101568. [Google Scholar] [CrossRef]
- Okeke, E.N.; Abubakar, I.S.; De Guttry, R. In Nigeria, Stillbirths and Newborn Deaths Increased During The COVID-19 Pandemic. Health Aff. 2021, 40, 1797–1805. [Google Scholar] [CrossRef] [PubMed]
- Dandona, R.A.-O.X.; Kumar, G.A.; Akbar, M.; Dora, S.S.P.; Dandona, L. Substantial increase in stillbirth rate during the COVID-19 pandemic: Results from a population-based study in the Indian state of Bihar. BMJ Glob. Health 2023, 8, e013021. [Google Scholar] [CrossRef]
- Kc, A.; Gurung, R.; Kinney, M.V.; Sunny, A.K.; Moinuddin, M.; Basnet, O.; Paudel, P.; Bhattarai, P.; Subedi, K.; Shrestha, M.P.; et al. Effect of the COVID-19 pandemic response on intrapartum care, stillbirth, and neonatal mortality outcomes in Nepal: A prospective observational study. Lancet Glob. Health 2020, 8, e1273–e1281. [Google Scholar] [CrossRef] [PubMed]
- Libretti, A.; Troìa, L.; Cappello, A.M.; Casarotti, C.; D’Amato, A.T.; Dallarda, G.; Ghio, M.; Nicosia, A.; Ricci, D.; Savasta, F.; et al. Pregnancy and neonatal outcomes of SARS-CoV-2 infection discovered at the time of delivery: A tertiary center experience in North Italy. J. Perinat. Med. 2024, 52, 215–221. [Google Scholar] [CrossRef]
- Farhadi, R.; Noori, H.; GhaffariSaravi, V.; Moosazadeh, M. Stillbirth and Preterm Birth During Lockdown Periods in 5 Waves of COVID-19 Pandemic in Northern Iran: A Region-Wide Cohort Study in Mazandaran Province. Health Serv. Res. Manag. Epidemiol. 2023, 10, 23333928231180561. [Google Scholar] [CrossRef]
- Hui, L.; Marzan, M.B.; Potenza, S.; Rolnik, D.L.; Pritchard, N.; Said, J.M.; Palmer, K.R.; Whitehead, C.L.; Sheehan, P.M.; Ford, J.; et al. Increase in preterm stillbirths in association with reduction in iatrogenic preterm births during COVID-19 lockdown in Australia: A multicenter cohort study. Am. J. Obstet. Gynecol. 2022, 227, 491.e1–491.e17. [Google Scholar] [CrossRef]
- Padmajam, M.; Behera, D.K. Disruptions in Accessing Women’s Health Care Services: Evidence Using COVID-19 Health Services Disruption Survey. Matern. Child Health J. 2023, 27, 395–406. [Google Scholar] [CrossRef]
- Ilea, C.D.N.; Daina, M.D.; Venter, A.C.; Șuteu, C.L.; Sabău, M.; Badau, D.; Daina, L.G. The Motivation of Medical Staff and the Work Interestedness in the Context of the COVID-19 Pandemic, in a Tertiary Hospital in Romania. Healthcare 2023, 11, 813. [Google Scholar] [CrossRef]
- Simon, S.; John, S.; Lisonkova, S.; Razaz, N.; Muraca, G.M.; Boutin, A.; Bedaiwy, M.A.; Brandt, J.S.; Ananth, C.V.; Joseph, K.S. Obstetric Intervention and Perinatal Outcomes During the Coronavirus Disease 2019 (COVID-19) Pandemic. Obstet. Gynecol. 2023, 142, 1405–1415. [Google Scholar] [CrossRef]
- Militaru, A.; Armean, P.; Ghita, N.; Andrei, D.P. Barriers to Healthcare Access During the Coronavirus Disease 2019 (COVID-19) Pandemic: A Cross-Sectional Study Among Romanian Patients with Chronic Illnesses and Confirmed SARS-CoV-2 Infection. Healthcare 2025, 13, 1333. [Google Scholar] [CrossRef] [PubMed]
- Volintiru, C.; Gherghina, S. We are in this together: Stakeholder cooperation during COVID-19 in Romania. Eur. Political Sci. 2023, 22, 384–394. [Google Scholar] [CrossRef]
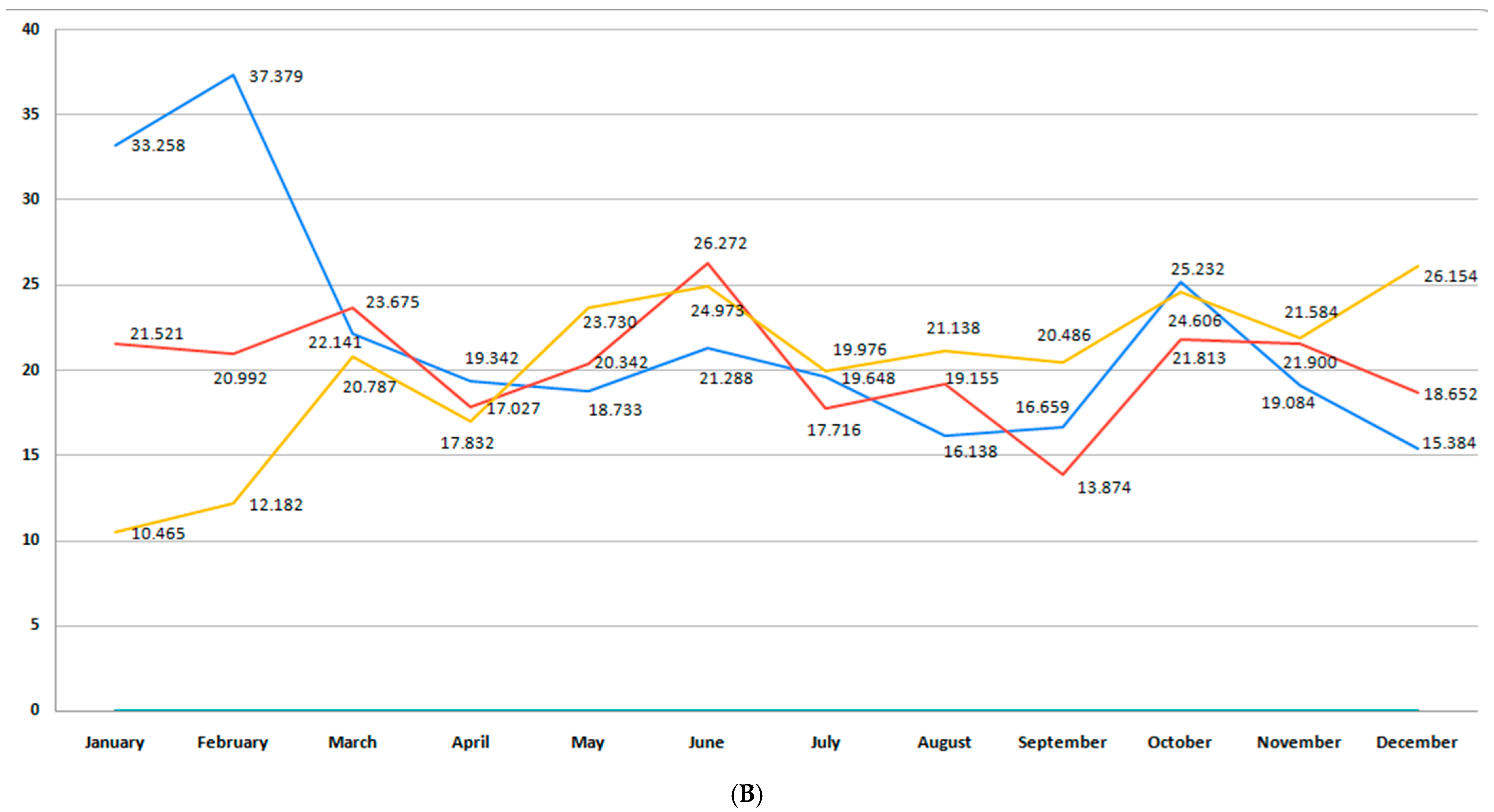


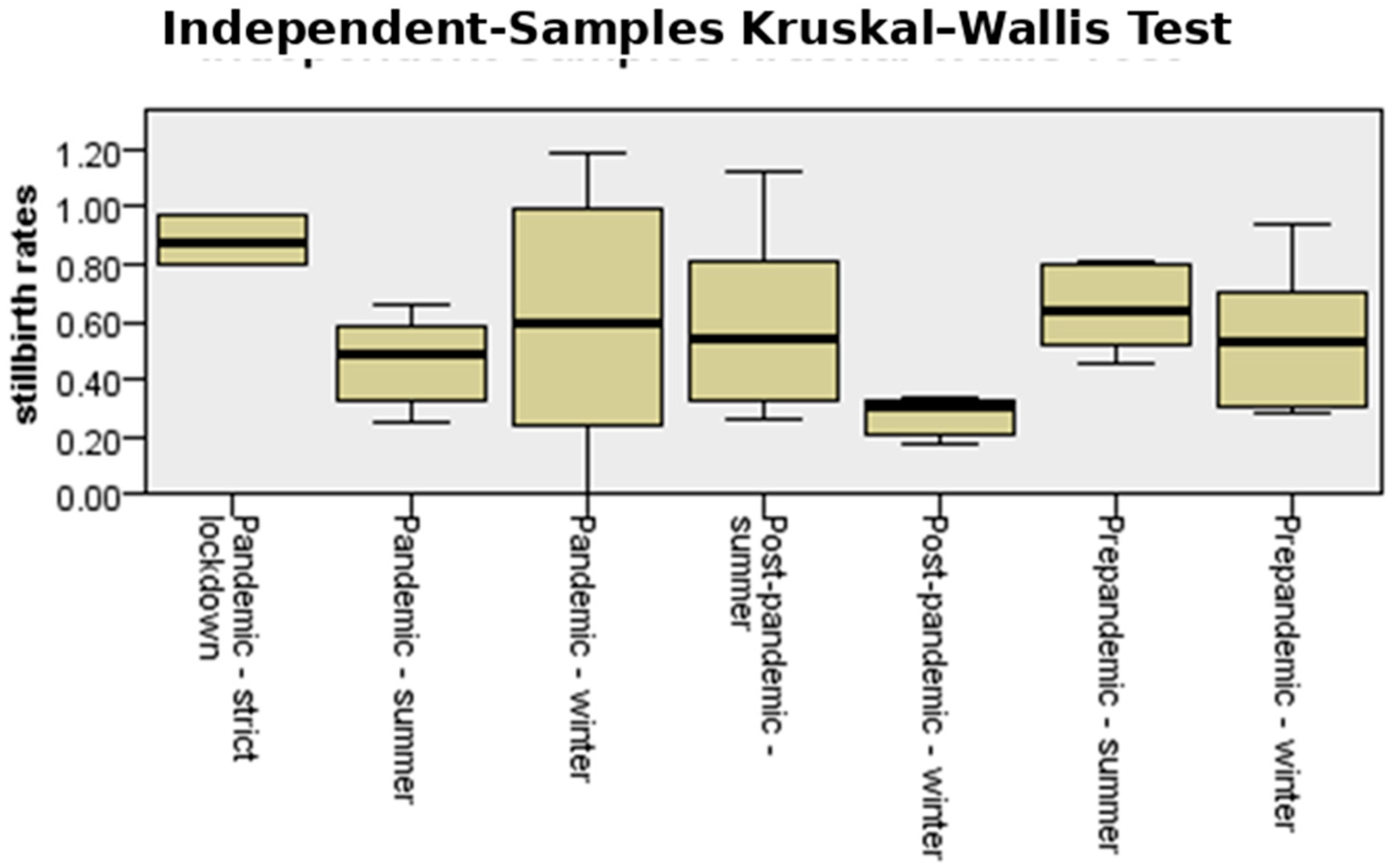
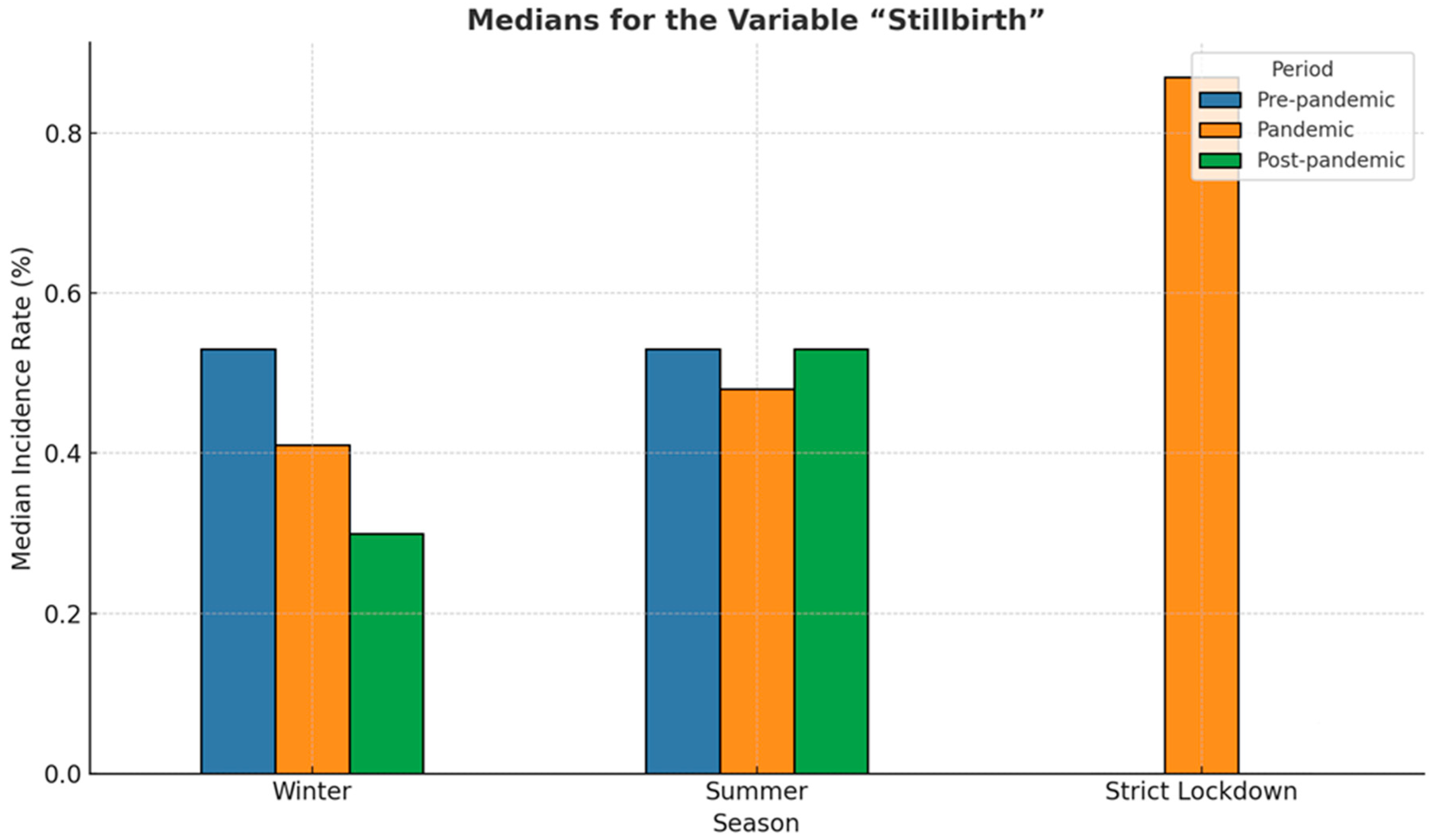
| Stillbirths | Minimum | Maximum | Mean | Std. Deviation |
|---|---|---|---|---|
| Strict lockdown | 0.803 | 0.972 | 0.88237 | 0.085405 |
| Pre-pandemicwinter | 0.287 | 0.936 | 0.53387 | 0.236241 |
| Pandemicwinter | 0.000 | 1.119 | 0.52128 | 0.427681 |
| Post-pandemicwinter | 0.179 | 0.333 | 0.27865 | 0.068955 |
| Pre-pandemic summer | 0.287 | 0.812 | 0.56765 | 0.210876 |
| Pandemicsummer | 0.250 | 0.661 | 0.46394 | 0.146640 |
| Post-pandemicsummer | 0.263 | 1.119 | 0.58597 | 0.317756 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trif, P.; Sava, C.; Mudura, D.; Kramer, B.W.; Galiș, R.; Ognean, M.L.; Iuhas, A.; Jurca, C.M. Seasonal Patterns of Preterm Birth During the COVID-19 Pandemic: A Retrospective Cohort Study in Romania. Medicina 2025, 61, 1398. https://doi.org/10.3390/medicina61081398
Trif P, Sava C, Mudura D, Kramer BW, Galiș R, Ognean ML, Iuhas A, Jurca CM. Seasonal Patterns of Preterm Birth During the COVID-19 Pandemic: A Retrospective Cohort Study in Romania. Medicina. 2025; 61(8):1398. https://doi.org/10.3390/medicina61081398
Chicago/Turabian StyleTrif, Paula, Cristian Sava, Diana Mudura, Boris W. Kramer, Radu Galiș, Maria Livia Ognean, Alin Iuhas, and Claudia Maria Jurca. 2025. "Seasonal Patterns of Preterm Birth During the COVID-19 Pandemic: A Retrospective Cohort Study in Romania" Medicina 61, no. 8: 1398. https://doi.org/10.3390/medicina61081398
APA StyleTrif, P., Sava, C., Mudura, D., Kramer, B. W., Galiș, R., Ognean, M. L., Iuhas, A., & Jurca, C. M. (2025). Seasonal Patterns of Preterm Birth During the COVID-19 Pandemic: A Retrospective Cohort Study in Romania. Medicina, 61(8), 1398. https://doi.org/10.3390/medicina61081398








