Feasibility of Ductus Venosus Doppler Screening During First Trimester Ultrasound: Prospective Multicenter Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
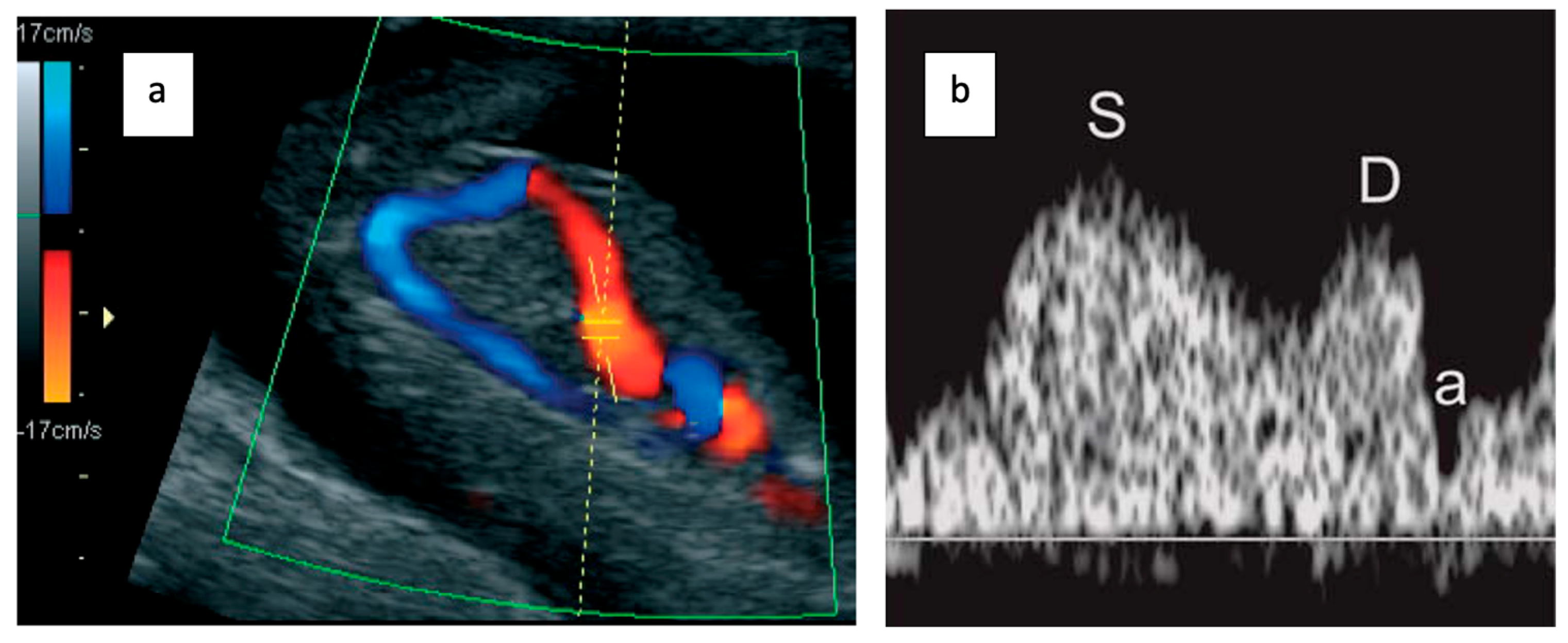
2.2. Ductus Venosus Doppler Assessment
- Examination during fetal quiescence.
- Image magnification to include the fetal thorax and abdomen.
- Right ventral mid-sagittal view with color flow mapping showing the umbilical vein, DV, and heart.
- Small pulsed Doppler sample volume (0.5–1.0 mm) positioned in the aliasing area above the umbilical sinus.
- Insonation angle < 30°.
- Low filter setting (50–70 Hz).
- High sweep speed (2–3 cm/s) for waveform analysis.
- Qualitative assessment of the a-wave (positive, absent, or reversed).
- Manual tracing for DV pulsatility index (PIV) measurement.
2.3. Image Acquisition and Quality Assessment
2.4. Data Collection
2.5. Ethics and Data Protection
2.6. Statistical Analysis
3. Results
3.1. Patient Inclusion and Characteristics
3.2. Feasibility of Ductus Venosus Assessment
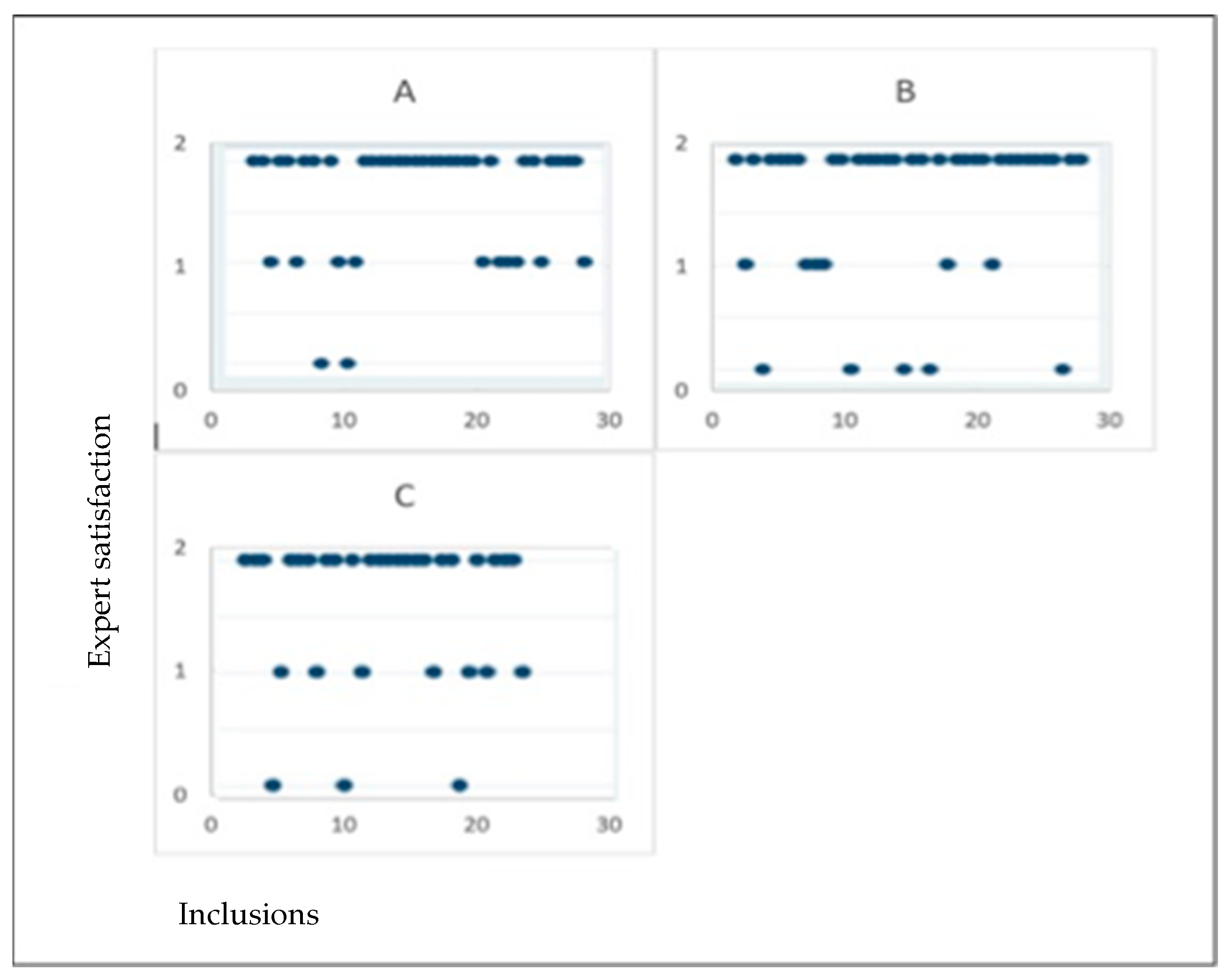
3.3. Agreement Between Expert and Sonographers
3.4. Correlation with Herman Score
4. Discussion
4.1. Principal Findings
4.2. Comparison with Previous Studies
4.3. Clinical Implications
4.4. Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | Body Mass Index |
| CHDs | Congenital Heart Defects |
| CRL | Crown–Rump Length |
| DV | Ductus Venosus |
| FMF | Fetal Medicine Foundation |
| GDPR | General Data Protection Regulation |
| HAS | Haute Autorité de Santé |
| NT | Nuchal Translucency |
| OR | Odds Ratio |
| PIV | Pulsatility Index for Veins |
References
- Khoshnood, B.; Lelong, N.; Houyel, L.; Thieulin, A.C.; Jouannic, J.M.; Magnier, S.; Delezoide, A.-L.; Magny, J.-F.; Rambaud, C.; Bonnet, D. Prevalence, timing of diagnosis and mortality of newborns with congenital heart defects: A population-based study. Heart 2012, 98, 1667–1673. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Andrade, E.; Patwardhan, M.; Cruz-Lemini, M.; Luewan, S. Early Evaluation of the Fetal Heart. Fetal Diagn. Ther. 2017, 42, 161–173. [Google Scholar] [CrossRef]
- Huhta, J.C. First-trimester screening for congenital heart disease. Curr. Opin. Cardiol. 2016, 31, 72–77. [Google Scholar] [CrossRef]
- Eleftheriades, M.; Tsapakis, E.; Sotiriadis, A.; Manolakos, E.; Hassiakos, D.; Botsis, D. Detection of congenital heart defects throughout pregnancy; impact of first trimester ultrasound screening for cardiac abnormalities. J. Matern.-Fetal Neonatal Med. Eur. Assoc. Perinat. Med. Fed. Asia Ocean. Perinat. Soc. Int. Soc. Perinat. Obstet. 2012, 25, 2546–2550. [Google Scholar] [CrossRef]
- Makrydimas, G.; Sotiriadis, A.; Ioannidis, J.P.A. Screening performance of first-trimester nuchal translucency for major cardiac defects: A meta-analysis. Am. J. Obstet. Gynecol. 2003, 189, 1330–1335. [Google Scholar] [CrossRef] [PubMed]
- Zoppi, M.A.; Putzolu, M.; Ibba, R.M.; Floris, M.; Monni, G. First-trimester ductus venosus velocimetry in relation to nuchal translucency thickness and fetal karyotype. Fetal Diagn. Ther. 2002, 17, 52–57. [Google Scholar] [CrossRef]
- Papatheodorou, S.I.; Evangelou, E.; Makrydimas, G.; Ioannidis, J.P.A. First-trimester ductus venosus screening for cardiac defects: A meta-analysis. BJOG Int. J. Obstet. Gynaecol. 2011, 118, 1438–1445. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, S.C.; Korones, S.B.; Berendes, H.W. Congenital heart disease in 56,109 births incidence and natural history. Circulation 1971, 43, 323–332. [Google Scholar] [CrossRef]
- Czuba, B.; Zarotyński, D.; Dubiel, M.; Borowski, D.; Węgrzyn, P.; Cnota, W.; Reska-Nycz, M.; Mączka, M.; Wielgoś, M.; Sodowski, K. Screening for trisomy 21 based on maternal age, nuchal translucency measurement, first trimester biochemistry and quantitative and qualitative assessment of the flow in the DV—The assessment of efficacy. Ginekol. Pol. 2017, 88, 481–485. [Google Scholar] [CrossRef]
- Dmitrovic, A.; Jeremic, K.; Babic, U.M.; Perovic, M.; Mihailovic, T.; Opric, D.; Zečevic, N.; Gojnić-Dugalić, M. Early fetal heart ultrasonography as additional indicator for chromosomopathies. Clin. Exp. Obs. Gynecol. 2016, 43, 245–249. [Google Scholar] [CrossRef]
- Wiechec, M.; Knafel, A.; Nocun, A.; Wiercinska, E.; Ludwin, A.; Ludwin, I. What are the most common first-trimester ultrasound findings in cases of Turner syndrome? J. Matern.-Fetal Neonatal Med. Off. J. Eur. Assoc. Perinat. Med. Fed. Asia Ocean. Perinat. Soc. Int. Soc. Perinat. Obstet. 2017, 30, 1632–1636. [Google Scholar] [CrossRef]
- Murta, C.G.V.; Moron, A.F.; Avila, M.A.P.; Weiner, C.P. Application of ductus venosus Doppler velocimetry for the detection of fetal aneuploidy in the first trimester of pregnancy. Fetal Diagn. Ther. 2002, 17, 308–314. [Google Scholar] [CrossRef]
- Baś-Budecka, E.; Perenc, M.; Sieroszewski, P. The role of fetal nuchal translucency (NT) and ductus venosus blood flow (DV) in the detection of congenital heart defects. Ginekol. Pol. 2010, 81, 272–276. [Google Scholar]
- Favre, R.; Cherif, Y.; Kohler, M.; Kohler, A.; Hunsinger, M.-C.; Bouffet, N.; Tanghe, M.; Cancellier, M.; Nisand, I. The role of fetal nuchal translucency and ductus venosus Doppler at 11–14 weeks of gestation in the detection of major congenital heart defects. Ultrasound Obstet. Gynecol. 2003, 21, 239–243. [Google Scholar] [CrossRef]
- Wagner, P.; Eberle, K.; Sonek, J.; Berg, C.; Gembruch, U.; Hoopmann, M.; Prodan, N.; Kagan, K.O. First-trimester ductus venosus velocity ratio as a marker of major cardiac defects. Ultrasound Obstet. Gynecol. 2019, 53, 663–668. [Google Scholar] [CrossRef]
- Seckin, K.D.; Karslı, M.F.; Baser, E.; Yeral, M.I.; Tasin, C.; Ozgu Erdinc, A.S.; Danisman, N. Obstetric outcomes in pregnancies with normal nuchal translucency and abnormal ductus venosus Doppler in the first trimester ultrasonography. J. Obstet. Gynaecol. J. Inst. Obstet. Gynaecol. 2016, 36, 440–443. [Google Scholar] [CrossRef] [PubMed]
- Stagnati, V.; Zanardini, C.; Fichera, A.; Pagani, G.; Quintero, R.A.; Bellocco, R.; Prefumo, F. Early prediction of twin-to-twin transfusion syndrome: Systematic review and meta-analysis. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2017, 49, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Savoia, F.; Scala, C.; Coppola, M.; Riemma, G.; Vitale, S.G.; Mikuš, M.; Schiattarella, A.; La Verde, M.; Colacurci, N.; De Franciscis, P. The diagnostic performance of the ductus venosus for the detection of cardiac defects in the first trimester: A systematic review and diagnostic test accuracy meta-analysis. Arch. Gynecol. Obstet. 2022, 308, 435–451. [Google Scholar] [CrossRef]
- Maiz, N.; Plasencia, W.; Dagklis, T.; Faros, E.; Nicolaides, K. Ductus venosus Doppler in fetuses with cardiac defects and increased nuchal translucency thickness. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2008, 31, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Maiz, N.; Kagan, K.O.; Milovanovic, Z.; Celik, E.; Nicolaides, K.H. Learning curve for Doppler assessment of ductus venosus flow at 11 + 0 to 13 + 6 weeks’ gestation. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2008, 31, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Karadzov Orlic, N.; Egic, A.; Damnjanovic-Pazin, B.; Lukic, R.; Joksic, I.; Mikovic, Z. Screening performance of congenital heart defects in first trimester using simple cardiac scan, nuchal translucency, abnormal ductus venosus blood flow and tricuspid regurgitation. Congenit. Heart Dis. 2019, 14, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Minnella, G.P.; Crupano, F.M.; Syngelaki, A.; Zidere, V.; Akolekar, R.; Nicolaides, K.H. Diagnosis of major heart defects by routine first-trimester ultrasound examination: Association with increased nuchal translucency, tricuspid regurgitation and abnormal flow in ductus venosus. Ultrasound Obstet. Gynecol. 2020, 55, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Oh, C.; Harman, C.; Baschat, A.A. Abnormal first-trimester ductus venosus blood flow: A risk factor for adverse outcome in fetuses with normal nuchal translucency. Ultrasound Obstet. Gynecol. 2007, 30, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Chelemen, T.; Syngelaki, A.; Maiz, N.; Allan, L.; Nicolaides, K.H. Contribution of Ductus Venosus Doppler in First-Trimester Screening for Major Cardiac Defects. Fetal Diagn. Ther. 2011, 29, 127–134. [Google Scholar] [CrossRef]
- Berg, C.; Kremer, C.; Geipel, A.; Kohl, T.; Germer, U.; Gembruch, U. Ductus venosus blood flow alterations in fetuses with obstructive lesions of the right heart. Ultrasound Obstet. Gynecol. 2006, 28, 137–142. [Google Scholar] [CrossRef]
- Martínez, J.M.; Comas, M.; Borrell, A.; Bennasar, M.; Gómez, O.; Puerto, B.; Gratacós, E. Abnormal first-trimester ductus venosus blood flow: A marker of cardiac defects in fetuses with normal karyotype and nuchal translucency. Ultrasound Obstet. Gynecol. 2010, 35, 267–272. [Google Scholar] [CrossRef]
- Timmerman, E.; Clur, S.A.; Pajkrt, E.; Bilardo, C.M. First-trimester measurement of the ductus venosus pulsatility index and the prediction of congenital heart defects. Ultrasound Obstet. Gynecol. 2010, 36, 668–675. [Google Scholar] [CrossRef]
| Average (SD ‡) | Total | Percentage (%) | |
|---|---|---|---|
| Body mass index (BMI, kg/m2) | 25.3 (6.3) | ||
| Normal BMI (18–24.9) | 45 | 51.8 | |
| Overweight (25–29.9) | 28 | 32.2 | |
| Obesity (>30) | 13 | 14.9 | |
| Missing values | 1 | 1.1 | |
| Gravidity | |||
| Primigravida | 42 | 48.3 | |
| Multigravida | 44 | 50.6 | |
| Missing values | 1 | 1.1 | |
| Antecedent of uterine surgery | |||
| Cesarian | 5 | 5.7 | |
| Curettage | 11 | 12.6 | |
| Gestational age | 12.4 (0.58) | ||
| ≤ 12WG † | 22 | 25.3 | |
| >12WG † | 65 | 74.7 | |
| Echogenicity | |||
| Good | 68 | 78.2 | |
| Medium | 13 | 14.9 | |
| Poor | 6 | 6.9 | |
| Difficulties in obtaining the shot | |||
| Easy | 62 | 72.3 | |
| Medium | 19 | 21.8 | |
| Difficult | 6 | 6.9 | |
| Additional examination time | |||
| None | 66 | 75.9 | |
| Medium | 18 | 20.7 | |
| Important | 3 | 3.4 | |
| Satisfaction of the sonographer | |||
| No | 7 | 1.1 | |
| Medium | 21 | 24.1 | |
| Yes | 59 | 67.8 |
| Expert Judgement | Total | “Good” | “Medium” or “Unsatisfactory” | p-Value |
|---|---|---|---|---|
| Total | 87 | 51 (58.6%) | 36 (41.4%) | |
| Sonographer | 0.4 | |||
| A | 30 | 18 (60%) | 12 (40%) | |
| B | 30 | 15 (50%) | 15 (50%) | |
| C | 27 | 18 (66.7%) | 9 (33.3%) | |
| Body mass index (BMI, kg/m2) | 0.6 | |||
| Normal (18–24.9) | 45 | 28 (62.2%) | 17 (37.8%) | |
| Overweight (25–29.9) | 28 | 14 (50%) | 14 (50%) | |
| Obesity (>30) | 13 | 8 (61.5%) | 5 (38.5%) | |
| Gravidity | 0.5 | |||
| Primigravida | 42 | 27 (64.3%) | 15 (35.7%) | |
| Multigravida | 44 | 23 (52.3%) | 21 (47.7%) | |
| Gestational age | 0.2 | |||
| ≤12WG † | 22 | 18 (81.8%) | 4 (18.2%) | |
| >12WG † | 65 | 41 (63.1%) | 24 (36.9%) | |
| Echogenicity | 0.1 | |||
| Good | 68 | 41 (60.2%) | 26 (38.8%) | |
| Medium | 13 | 8 (61.5%) | 5 (38.5%) | |
| Poor | 6 | 1 (16.7%) | 5 (83%) | |
| Fetal presentation | 0.03 | |||
| Difficult | 4 | 0 | 4 (100%) | |
| Not mentioned | 83 | 50 (60.2%) | 32 (38.5%) | |
| Additional examination time | <0.01 | |||
| None | 66 | 57 (86.4%) | 9 (13.6%) | |
| Medium | 18 | 2 (11.1%) | 16 (88.9%) | |
| Important | 3 | 0 | 3 (100%) | |
| Satisfaction of the sonographer | <0.01 | |||
| No | 7 | 1 (14.3%) | 6 (85.7%) | |
| Medium | 22 | 4 (18.2%) | 17 (77.3%) | |
| Yes | 58 | 45 (77.5%) | 13 (22.4%) |
| Herman Score | ≤7 | >7 | Total | p-Value |
|---|---|---|---|---|
| Expert | 0.01 | |||
| Unsatisfied | 28 | 8 | 36 | |
| Satisfied | 23 | 28 | 51 | |
| Feasibility | <0.05 | |||
| “Unsatisfactory” | 3 | 0 | 3 | |
| “Medium” | 19 | 14 | 33 | |
| “Good” | 19 | 32 | 51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joinau-Zoulovits, F.; Bouzidi, A.; Etienne, F.; Levêque, C. Feasibility of Ductus Venosus Doppler Screening During First Trimester Ultrasound: Prospective Multicenter Study. Medicina 2025, 61, 1391. https://doi.org/10.3390/medicina61081391
Joinau-Zoulovits F, Bouzidi A, Etienne F, Levêque C. Feasibility of Ductus Venosus Doppler Screening During First Trimester Ultrasound: Prospective Multicenter Study. Medicina. 2025; 61(8):1391. https://doi.org/10.3390/medicina61081391
Chicago/Turabian StyleJoinau-Zoulovits, Félicia, Anissa Bouzidi, Françoise Etienne, and Christine Levêque. 2025. "Feasibility of Ductus Venosus Doppler Screening During First Trimester Ultrasound: Prospective Multicenter Study" Medicina 61, no. 8: 1391. https://doi.org/10.3390/medicina61081391
APA StyleJoinau-Zoulovits, F., Bouzidi, A., Etienne, F., & Levêque, C. (2025). Feasibility of Ductus Venosus Doppler Screening During First Trimester Ultrasound: Prospective Multicenter Study. Medicina, 61(8), 1391. https://doi.org/10.3390/medicina61081391






