Management and Outcomes of Urinary Tract Involvement in Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy (CRS/HIPEC): A Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Statistical Analysis
2.2. Use of Artificial Intelligence (AI) Tools
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sugarbaker, P.H.; Jablonski, K.A. Prognostic features of 51 colorectal and 130 appendiceal cancer patients with peritoneal carcinomatosis treated by cytoreductive surgery and intraperitoneal chemotherapy. Ann. Surg. 1995, 221, 124–132. [Google Scholar] [CrossRef]
- Spratt, J.S.; Adcock, R.A.; Muskovin, M.; Sherrill, W.; McKeown, J. Clinical delivery system for intraperitoneal hyperthermic chemotherapy. Cancer Res. 1980, 40, 256–260. [Google Scholar]
- Sugarbaker, P.H. Intraperitoneal chemotherapy for treatment and prevention of peritoneal carcinomatosis and sarcomatosis. Dis. Colon Rectum 1994, 37 (Suppl. S2), S115–S122. [Google Scholar] [CrossRef]
- Sugarbaker, P.H. Prevention and treatment of peritoneal metastases: A comprehensive review. Indian J. Surg. Oncol. 2019, 10, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Smeenk, R.M.; Bex, A.; Verwaal, V.J.; Horenblas, S.; Zoetmulder, F.A. Pseudomyxoma peritonei and the urinary tract: Involvement and treatment related complications. J. Surg. Oncol. 2006, 93, 20–23. [Google Scholar] [CrossRef] [PubMed]
- Braam, H.J.; Van Oudheusden, T.R.; De Hingh, I.H.; Nienhuijs, S.W.; Boerma, D.; Wiezer, M.J.; Van Ramshorst, B. Urological procedures in patients with peritoneal carcinomatosis of colorectal cancer treated with HIPEC: Morbidity and survival analysis. Anticancer Res. 2015, 35, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Sugarbaker, P.H. Management of peritoneal-surface malignancy: The surgeon’s role. Langenbeck’s Arch. Surg. 1999, 384, 576–587. [Google Scholar] [CrossRef]
- Parikh, M.S.; Johnson, P.; Romanes, J.P.; Freitag, H.E.; Spring, M.E.; Garcia-Henriquez, N.; Monson, J.R. Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Colorectal Peritoneal Metastases: A Systematic Review. Dis. Colon Rectum 2022, 65, 16–26. [Google Scholar] [CrossRef]
- Yurttas, C.; Hoffmann, G.; Tolios, A.; Haen, S.P.; Schwab, M.; Koenigsrainer, I.; Koenigsrainer, A.; Beckert, S.; Loeffler, M.W. Systematic review of variations in hyperthermic intraperitoneal chemotherapy (HIPEC) for peritoneal metastasis from colorectal cancer. J. Clin. Med. 2018, 7, 567. [Google Scholar] [CrossRef]
- Verwaal, V.J.; van Ruth, S.; de Bree, E.; van Slooten, G.W.; van Tinteren, H.; Boot, H.; Zoetmulder, F.A. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J. Clin. Oncol. 2003, 21, 3737–3743. [Google Scholar] [CrossRef]
- Franko, J.; Ibrahim, Z.; Gusani, N.J.; Holtzman, M.P.; Bartlett, D.L.; Zeh, H.J., III. Cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion versus systemic chemotherapy alone for colorectal peritoneal carcinomatosis. Cancer 2010, 116, 3756–3762. [Google Scholar] [CrossRef]
- Verwaal, V.J. Long-term results of cytoreduction and HIPEC followed by systemic chemotherapy. Cancer J. 2009, 15, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Yan, T.D.; Black, D.; Morris, D.L. A systematic review and metaanalysis of cytoreductive surgery with perioperative intraperitoneal chemotherapy for peritoneal carcinomatosis of colorectal origin. Ann. Surg. Oncol. 2009, 16, 2152–2165. [Google Scholar] [CrossRef] [PubMed]
- Glehen, O.; Kwiatkowski, F.; Sugarbaker, P.H.; Elias, D.; Levine, E.A.; De Simone, M.; Barone, R.; Yonemura, Y.; Cavaliere, F.; Quenet, F.; et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: A multi-institutional study. J. Clin. Oncol. 2004, 22, 3284–3292. [Google Scholar] [CrossRef] [PubMed]
- Baratti, D.; Kusamura, S.; Iusco, D.; Bonomi, S.; Grassi, A.; Virzì, S.; Leo, E.; Deraco, M. Postoperative complications after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy affect long-term outcome of patients with peritoneal metastases from colorectal cancer: A two-center study of 101 patients. Dis. Colon Rectum 2014, 57, 858–868. [Google Scholar] [CrossRef]
- Cavaliere, F.; De Simone, M.; Virzi, S.; Deraco, M.; Rossi, C.R.; Garofalo, A.; Di Filippo, F.; Giannarelli, D.; Vaira, M.; Valle, M.; et al. Prognostic factors and oncologic outcome in 146 patients with colorectal peritoneal carcinomatosis treated with cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy: Italian multicenter study S.I.T.I.L.O. Eur. J. Surg. Oncol. 2011, 37, 148–154. [Google Scholar] [CrossRef]
- Quenet, F.; Goéré, D.; Mehta, S.S.; Roca, L.; Dumont, F.; Hessissen, M.; Saint-Aubert, B.; Elias, D. Results of two bi-institutional prospective studies using intraperitoneal oxaliplatin with or without irinotecan during HIPEC after cytoreductive surgery for colorectal carcinomatosis. Ann. Surg. 2011, 254, 294–301. [Google Scholar] [CrossRef]
- Sluiter, N.R.; Rovers, K.P.; Salhi, Y.; Vlek, S.L.; Coupé, V.M.; Verheul, H.M.; Kazemier, G.; de Hingh, I.H.; Tuynman, J.B. Metachronous peritoneal metastases after adjuvant chemotherapy are associated with poor outcome after cytoreduction and HIPEC. Ann. Surg. Oncol. 2018, 25, 2347–2356. [Google Scholar] [CrossRef]
- Passot, G.; Vaudoyer, D.; Cotte, E.; You, B.; Isaac, S.; Gilly, F.N.; Mohamed, F.; Glehen, O. Progression following neoadjuvant systemic chemotherapy may not be a contraindication to a curative approach for colorectal carcinomatosis. Ann. Surg. 2012, 256, 125–129. [Google Scholar] [CrossRef]
- Elias, D.; Gilly, F.; Boutitie, F.; Quenet, F.; Bereder, J.M.; Mansvelt, B.; Lorimier, G.; Dube, P.; Glehen, O. Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: Retrospective analysis of 523 patients from a multicentric french study. J. Clin. Oncol. 2010, 28, 63–68. [Google Scholar] [CrossRef]
- Honoré, C.; Souadka, A.; Goéré, D.; Dumont, F.; Deschamps, F.; Elias, D. HIPEC for peritoneal carcinomatosis: Does an associated urologic procedure increase morbidity? Ann. Surg. Oncol. 2012, 19, 104–109. [Google Scholar] [CrossRef]
- Votanopoulos, K.I.; Randle, R.W.; Craven, B.; Swett, K.R.; Levine, E.A.; Shen, P.; Stewart, J.H.; Mirzazadeh, M. Significance of urinary tract involvement in patients treated with cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC). Ann. Surg. Oncol. 2014, 21, 868–874. [Google Scholar] [CrossRef]
- Gilmour, D.T.; Das, S.; Flowerdew, G. Rates of urinary tract injury from gynecologic surgery and the role of intraoperative cystoscopy. Obstet. Gynecol. 2006, 107, 1366–1372. [Google Scholar] [CrossRef]
- Chou, M.-T.; Wang, C.-J.; Lien, R.C. Prophylactic ureteral catheterization in gynecologic surgery: A 12-year randomized trial in a community hospital. Int. Urogynecol. J. Pelvic Floor Dysfunct. 2009, 20, 689–693. [Google Scholar] [CrossRef] [PubMed]
- Coccolini, F.; Lotti, M.; Manfredi, R.; Catena, F.; Vallicelli, C.; De Iaco, P.A.; Da Pozzo, L.; Frigerio, L.; Ansaloni, L. Ureteral stenting in cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy as a routine procedure: Evidence and necessity. Urol. Int. 2012, 89, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Jacquet, P.; Sugarbaker, P.H. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer. Treat. Res. 1996, 82, 359–374. [Google Scholar] [CrossRef] [PubMed]
- Elias, D.; Blot, F.; El Otmany, A.; Antoun, S.; Lasser, P.; Boige, V.; Rougier, P.; Ducreux, M. Curative treatment of peritoneal carcinomatosis arising from colorectal cancer by complete resection and intraperitoneal chemotherapy. Cancer 2001, 92, 71–76. [Google Scholar] [CrossRef]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef]
- Sugarbaker, P.H. Peritoneal carcinomatosis: Natural history and rational therapeutic interventions using intraperitoneal chemotherapy. Cancer Treat. Res. 1996, 81, 149–168. [Google Scholar] [CrossRef]
- Berger, Y.; Aycart, S.; Tabrizian, P.; Agmon, Y.; Mandeli, J.; Heskel, M.; Hiotis, S.; Sarpel, U.; Labow, D.M. Cytoreductive surgery and hyperthermic intraper-itoneal chemotherapy in patients with liver involvement. J. Surg. Oncol. 2016, 113, 432–437. [Google Scholar] [CrossRef]
- Lyon, T.D.; Turner, I.I.R.M.; Nikonow, T.N.; Wang, L.; Uy, J.; Ramalingam, L.; Holtzman, M.P.; Pingpank, J.F.; Bartlett, D.L.; Davies, B.J. Effect of a concomitant urologic procedure on outcomes following cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. J. Surg. Oncol. 2016, 113, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Leapman, M.S.; Jibara, G.; Tabrizian, P.; Franssen, B.; Yang, M.J.; Romanoff, A.; Hall, S.J.; Palese, M.; Sarpel, U.; Hiotis, S.; et al. Genitourinary resection at the time of cytoreductive surgery and heated intraperitoneal chemotherapy for peritoneal carcinomatosis is not associated with increased morbidity or worsened oncologic outcomes: A case-matched study. Ann. Surg. Oncol. 2014, 21, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Pinar, U.; Tremblay, J.F.; Passot, G.; Dazza, M.; Glehen, O.; Tuech, J.J.; Pocard, M.; BIG-RENAPE working group. Reconstruction after ureteral resection during HIPEC surgery: Re-implantation with uretero-neocystostomy seems safer than end-to-end anastomosis. J. Visc. Surg. 2017, 154, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Quénet, F.; Elias, D.; Roca, L.; Goéré, D.; Ghouti, L.; Pocard, M.; Facy, O.; Arvieux, C.; Lorimier, G.; Pezet, D.; et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 256–266. [Google Scholar] [CrossRef]
- Pereira, F.; Serrano, A.; Manzanedo, I.; Pérez-Viejo, E.; González-Moreno, S.; González-Bayón, L.; Arjona-Sánchez, A.; Torres, J.; Ramos, I.; Barrios, M.E.; et al. GECOP-MMC: Phase IV randomized clinical trial to evaluate the efficacy of hyperthermic intraperitoneal chemotherapy (HIPEC) with mytomicin-C after complete surgical cytoreduction in patients with colon cancer peritoneal metastases. BMC Cancer 2022, 22, 536. [Google Scholar] [CrossRef]
- Prada-Villaverde, A.; Esquivel, J.; Lowy, A.M.; Markman, M.; Chua, T.; Pelz, J.; Baratti, D.; Baumgartner, J.M.; Berri, R.; Bretcha-Boix, P.; et al. The American Society of Peritoneal Surface Malignancies evaluation of HIPEC with Mitomycin C versus Oxaliplatin in 539 patients with colon cancer undergoing a complete cytoreductive surgery. J. Surg. Oncol. 2014, 110, 779–785. [Google Scholar] [CrossRef]
- Kitaguchi, D.; Park, E.J.; Baik, S.H.; Sasaki, S.; Tsukada, Y.; Ito, M. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus R0 resection for resectable colorectal cancer with peritoneal metastases and low peritoneal cancer index scores: A collaborative observational study from Korea and Japan. Int. J. Surg. 2023, 110, 45–52. [Google Scholar] [CrossRef]
- Ji, Z.H.; Fu, Y.B.; Liu, G.; Yu, Y.; Li, B.; Su, Y.D.; Yang, R.; Liang, X.L.; Li, Y. Intraoperative urinary tract resection and construction in CRS + HIPEC procedures: A single center retrospective analysis. World J. Surg. Oncol. 2024, 22, 171. [Google Scholar] [CrossRef]

| Characteristic | Value n (%) |
|---|---|
| Gender | |
| Female | 32 (64%) |
| Male | 18 (36%) |
| Age (year) mean ± SD (range) | 51 ± 12.7 (19–72) |
| BMI (kg/m2) mean ± SD (range) | 26.2 ± 5.1 (20–43) |
| Severe malnutrition | 5 (10%) |
| ASA score | |
| ASA 1 | 8 (16%) |
| ASA 2 | 26 (52%) |
| ASA 3 | 16 (32%) |
| Origin of carcinomatosis | |
| Colorectal cancer | 19 (38%) |
| Ovarian cancer | 19 (38%) |
| PMP | 9 (18%) |
| Others | 3 (6%) |
| Neoadjuvant chemotherapy | 32 (64%) |
| Liver metastasis | 2 (4%) |
| Prior stoma | 2 (4%) |
| Prophylactic ureteral stent | 8 (16%) |
| Prior surgery | 28 (56%) |
| Hysterectomy/salpingo-oophorectomy | 9 (18%) |
| Appendectomy | 4 (8%) |
| Colectomy | 10 (20%) |
| Cholecystectomy | 5 (10%) |
| Operative Data | Value n (%) |
|---|---|
| PCI (median) (range) | 19 (5–25) |
| PCI < 20 | 26 (52%) |
| PCI ≥ 20 | 24 (48%) |
| Surgical resection (n) | 262 |
| Pelvic peritonectomy | 40 (15.3%) |
| Omentectomy | 44 (16.8%) |
| Cholecystectomy | 25 (9.5%) |
| Small bowel resection | 34 (13%) |
| Splenectomy | 25 (9.5%) |
| Hepatic metastasectomy | 5 (2%) |
| Gastrectomy | 5 (2%) |
| Diaphragmatic stripping | 22 (8.4%) |
| Colorectal resection | 47 (18%) |
| Hysterectomy/salpingo-oophorectomy | 8 (3.1%) |
| Distal pancreatectomy | 6 (2.3%) |
| Partial resection of psoas muscle | 1 (0.4%) |
| Postoperative stoma creation | 24 (48%) |
| Completeness of cytoreduction score | |
| CC-0 | 25 (50%) |
| CC-1 | 20 (40%) |
| CC-2 | 5 (10%) |
| Number of anastomosis (median) (range) | 1 (0–4) |
| Operative time(min), median (range) | 480 (180–710) |
| Estimated blood loss (mL), median (range) | 400 (100–3200) |
| Peritonectomy (median) (range) | 4 (2–4) |
| Surgical procedures (median) (range) | 5 (1–9) |
| Surgical procedures ≥ 5 | 27 (54%) |
| (A) | ||
| Organ/Procedure | Resection n (%) | Reconstruction n (%) |
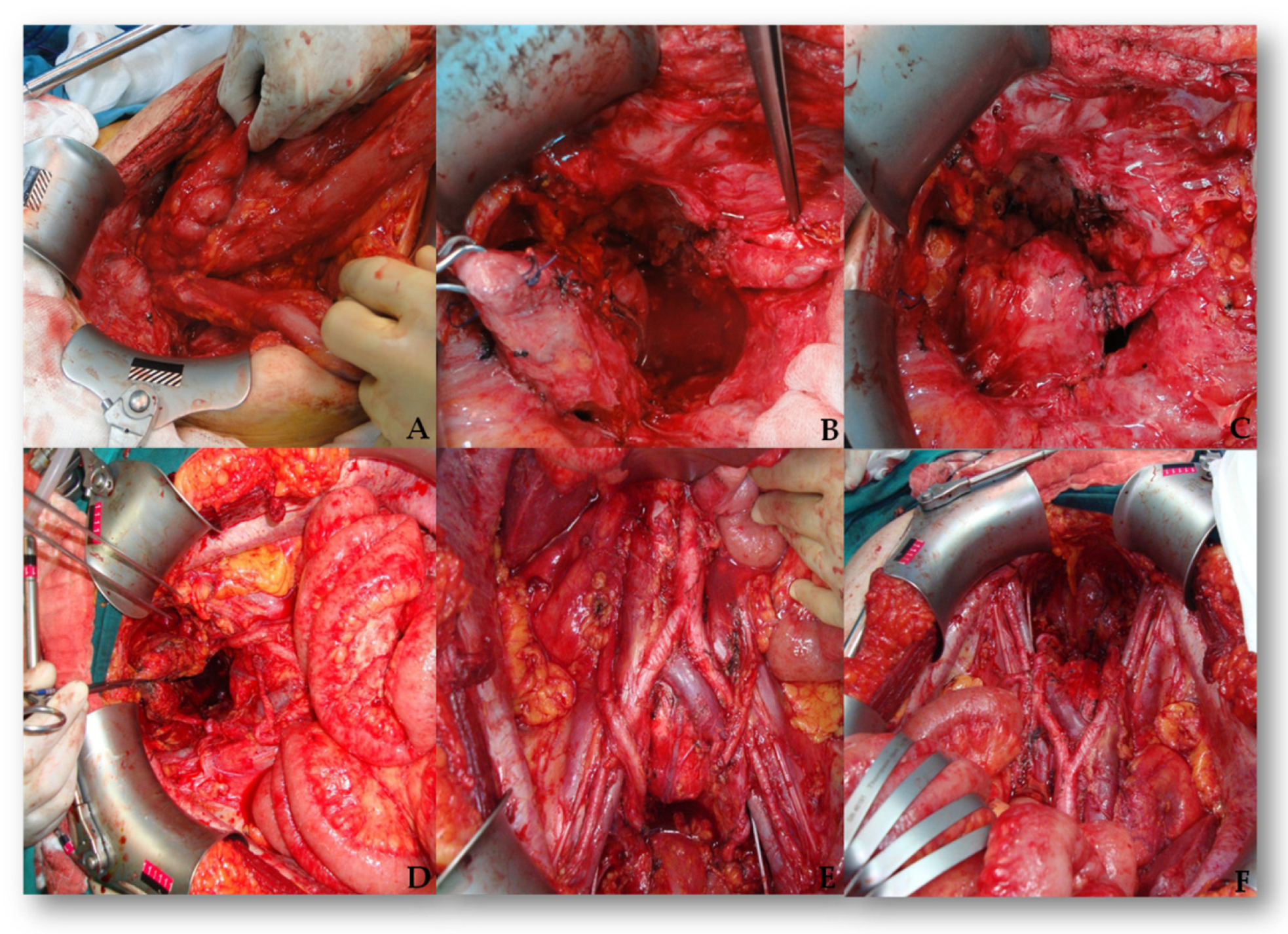
| Kidney | 3 (5.4%) | - |
| Ureter | 21 (37.5%) (18 unilateral and 3 bilateral) | - |
| Bladder | 15 (partial) (26.8%) | 17 (30.4%) |
| (B) | ||
| Organ/Procedure Reason | Tumor Invasion n (%) | Iatrogenic Injury n (%) |
| Kidney | 3 (5.4%) | - |
| Ureter | 10 (17.9%) | 11 (19.6%) |
| Bladder | 15 (26.8%) | 17 (30.4%) |
| Urinary Complications | |||
|---|---|---|---|
| Yes (n = 9) | No (n = 41) | p Value | |
| Age (year), mean ± SD | 51.3 ± 15.6 | 50.7 ± 11.6 | 0.655 |
| BMI (kg/m2), mean ± SD | 26 ± 2.2 | 25.4 ± 4.4 | 0.766 |
| Number of anastomosis, median (range) | 2 (0–3) | 1 (0–4) | 0.426 |
| Estimated blood loss (mL), median (range) | 800 (0–1600) | 400 (0–3200) | 0.619 |
| Peritonectomy, median (range) | 4 (2–4) | 4 (2–4) | 0.661 |
| Operation duration, median (range) | 450 (300–600) | 480 (180–710) | 0.766 |
| Postoperative stoma presence, n (%) | 5 (55%) | 19 (46%) | 0.721 |
| Urology consultation, n (%) | 0 | 6 (14%) | 0.576 |
| Gender (F/M), n | 6/3 | 26/15 | 1 |
| PCI ≥ 20, n (%) | 8 (89%) | 16 (39%) | 0.009 |
| Number of surgery ≥ 5, n (%) | 6 (66%) | 21 (51%) | 0.479 |
| Etiology, n (%) | |||
| Colorectal cancer | 6 (66%) | 13 (31%) | 0.114 |
| Ovarian cancer | 2 (22%) | 17 (41%) | 0.485 |
| PMP | 0 | 9 (22%) | 0.283 |
| Others | 1 (11%) | 2 (4.9%) | 0.949 |
| CC-0, n (%) | 7 (77.8%) | 18 (43.9%) | 0.138 |
| ASA ≥ 3, n (%) | 2 (22.2%) | 13 (31.7%) | 0.705 |
| Severe malnutrition, n (%) | 1 (11.1%) | 4 (9.8%) | 1 |
| Prior surgery, n (%) | 4 (44.4%) | 15 (36.5%) | 0.713 |
| Prophylactic ureteral stent, n (%) | 2 (22.2%) | 6 (14.6%) | 0.623 |
| Hospital stay (days), median (range) | 24 (15–66) | 21(10–50) | 0.112 |
| Survival Time (Months) | Survival Rate (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Estimate Median | Std. Error | 95% CI | 1-Year Survival | 2-Year Survival | 3-Year Survival | p Value | ||
| LB | UB | |||||||
| Total | 23 | 4.1 | 14.9 | 31.1 | 79.8 | 42.9 | 0.0 | - |
| Urinary complications | ||||||||
| Yes | 23 | 3.8 | 15.6 | 30.5 | 77.8 | 29.2 | 0.0 | 0.683 |
| No | 27 | 4.0 | 5.5 | 48.5 | 83.9 | 40.5 | 16.9 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karakayali, F.; Aktas, M.K.; Aytac, E.; Sungurtekin, U.; Demirbas, S.; Oncel, M.; Ozturk, E.; Colak, T.; Ince, M.; Haksal, M.; et al. Management and Outcomes of Urinary Tract Involvement in Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy (CRS/HIPEC): A Retrospective Cohort Study. Medicina 2025, 61, 1331. https://doi.org/10.3390/medicina61081331
Karakayali F, Aktas MK, Aytac E, Sungurtekin U, Demirbas S, Oncel M, Ozturk E, Colak T, Ince M, Haksal M, et al. Management and Outcomes of Urinary Tract Involvement in Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy (CRS/HIPEC): A Retrospective Cohort Study. Medicina. 2025; 61(8):1331. https://doi.org/10.3390/medicina61081331
Chicago/Turabian StyleKarakayali, Feza, Melik Kagan Aktas, Erman Aytac, Ugur Sungurtekin, Sezai Demirbas, Mustafa Oncel, Ersin Ozturk, Tahsin Colak, Mehmet Ince, Mustafa Haksal, and et al. 2025. "Management and Outcomes of Urinary Tract Involvement in Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy (CRS/HIPEC): A Retrospective Cohort Study" Medicina 61, no. 8: 1331. https://doi.org/10.3390/medicina61081331
APA StyleKarakayali, F., Aktas, M. K., Aytac, E., Sungurtekin, U., Demirbas, S., Oncel, M., Ozturk, E., Colak, T., Ince, M., Haksal, M., Coskun, S., & Sokmen, S. (2025). Management and Outcomes of Urinary Tract Involvement in Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy (CRS/HIPEC): A Retrospective Cohort Study. Medicina, 61(8), 1331. https://doi.org/10.3390/medicina61081331






