Ultrasound-Based Morphological and Functional Assessment in Male CrossFit Athletes with Unilateral Subacromial Shoulder Pain: An Observational Study
Abstract
1. Introduction
2. Methods and Methods
2.1. Study Design
2.2. Sample Size Calculations
2.3. Participants
2.4. Descriptive Data
2.5. USI Assessment
2.6. Statistical Analysis
3. Results
3.1. Demographics Data
3.2. USI Differences
4. Discussion
Limitations and Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Szeles, P.R.d.Q.; Costa, T.S.d.; Cunha, R.A.d.; Hespanhol, L.; Pochini Ade, C.; Ramos, L.A.; Cohen, M. CrossFit and the Epidemiology of Musculoskeletal Injuries: A Prospective 12-Week Cohort Study. Orthop. J. Sports Med. 2020, 8, 2325967120908884. Available online: https://pubmed.ncbi.nlm.nih.gov/32284938/ (accessed on 20 December 2022). [CrossRef] [PubMed]
- Lenz, J.E.; Szymski, D.; Krueckel, J.; Weber, J.; Krieger, F.; Karius, T.; Meffert, R.; Alt, V.; Fehske, K. From Sweat to Strain: An Epidemiological Analysis of Training-Related Injuries in CrossFit®. Open Access J. Sports Med. 2024, 15, 91. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, D.; Zwicker, R.L.; Quinn, B.J.; Myer, G.D.; Stracciolini, A. Part II: Comparison of Crossfit-Related Injury Presenting to Sports Medicine Clinic by Sex and Age. Clin. J. Sport. Med. 2020, 30, 251–256. Available online: https://pubmed.ncbi.nlm.nih.gov/31842052/ (accessed on 3 September 2024). [CrossRef] [PubMed]
- Bolia, I.K.; Collon, K.; Bogdanov, J.; Lan, R.; Petrigliano, F.A. Management Options for Shoulder Impingement Syndrome in Athletes: Insights and Future Directions. Open Access J. Sports Med. 2021, 12, 43. [Google Scholar] [CrossRef] [PubMed]
- Cools, A.M.; Michener, L.A. Shoulder pain: Can one label satisfy everyone and everything? Br. J. Sports Med. 2017, 51, 416–417. Available online: https://pubmed.ncbi.nlm.nih.gov/27806952/ (accessed on 14 July 2025). [CrossRef] [PubMed]
- Lewis, J. Rotator cuff related shoulder pain: Assessment, management and uncertainties. Man. Ther. 2016, 23, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Ludewig, P.M.; Lawrence, R.L.; Braman, J.P. What’s in a Name? Using Movement System Diagnoses Versus Pathoanatomic Diagnoses. J. Orthop. Sports Phys. Ther. 2013, 43, 280–283. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J. The End of an Era? J. Orthop. Sports Phys. Ther. 2018, 48, 127–129. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, E.H.; Aibinder, W.R. Shoulder Impingement Syndrome. Phys. Med. Rehabil. Clin. N. Am. 2023, 34, 311–334. [Google Scholar] [CrossRef] [PubMed]
- Schwank, A.; Blazey, P.; Asker, M.; Møller, M.; Hägglund, M.; Gard, S.; Skazalski, C.; Andersson, S.H.; Horsley, I.; Whiteley, R.; et al. 2022 Bern Consensus Statement on Shoulder Injury Prevention, Rehabilitation, and Return to Sport for Athletes at All Participation Levels. J. Orthop. Sports Phys. Ther. 2022, 52, 11–28. Available online: https://pubmed.ncbi.nlm.nih.gov/34972489/ (accessed on 18 July 2024). [CrossRef] [PubMed]
- Millett, P.J.; Warth, R.J. Physical Examination of the Shoulder: An Evidence-Based Approach. Springer: New York, NY, USA, 2015; Print. [Google Scholar]
- Ambegaonkar, J.P.; Jordan, M.; Wiese, K.R.; Caswell, S.V. Kinesiophobia in Injured Athletes: A Systematic Review. J. Funct. Morphol. Kinesiol. 2024, 9, 78. [Google Scholar] [CrossRef] [PubMed]
- Wijntjes, J.; van Alfen, N. Muscle ultrasound: Present state and future opportunities. Muscle Nerve. 2021, 63, 455–466. Available online: https://pubmed.ncbi.nlm.nih.gov/33051891/ (accessed on 12 October 2022). [CrossRef] [PubMed]
- Bernstorff, M.A.; Schumann, N.; Schwake, L.; Somberg, O.; Balke, M.; Schildhauer, T.; Königshausen, M. Shoulder pathologies in CrossFit: A magnetic resonance imaging study of 51 cases. J. Sports Med. Phys. Fit. 2024, 64, 475–482. Available online: https://pubmed.ncbi.nlm.nih.gov/38445843/ (accessed on 14 July 2025). [CrossRef] [PubMed]
- Purim, K.S.M.; Zilli, A.; de Almeida Leite, G.F.; Susuki, G.K.; Hapner, L.M.S.; Zangari, M.A.C. Musculoskeletal Injuries in Competitive CrossFit Athletes. Rev. Bras. Ortop. 2024, 59, e976–e980. Available online: https://pubmed.ncbi.nlm.nih.gov/39711647/ (accessed on 14 July 2025).
- Lastra-Rodríguez, L.; Llamas-Ramos, I.; Rodríguez-Pérez, V.; Llamas-Ramos, R.; López-Rodríguez, A.F. Musculoskeletal Injuries and Risk Factors in Spanish CrossFit® Practitioners. Healthcare 2023, 11, 1346. Available online: https://pmc.ncbi.nlm.nih.gov/articles/PMC10178070/ (accessed on 14 July 2025). [CrossRef] [PubMed]
- Levine, B.D.; Motamedi, K.; Seeger, L.L. Imaging of the shoulder: A comparison of MRI and ultrasound. Curr. Sports Med. Rep. 2012, 11, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Cools, A.M.; Cambier, D.; Witvrouw, E.E. Screening the athlete’s shoulder for impingement symptoms: A clinical reasoning algorithm for early detection of shoulder pathology. Br. J. Sports Med. 2008, 42, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Eliason, A.; Harringe, M.; Engström, B.; Sunding, K.; Werner, S. Bilateral ultrasound findings in patients with unilateral subacromial pain syndrome. Physiother. Theory Pract. 2022, 38, 2568–2579. Available online: https://pubmed.ncbi.nlm.nih.gov/34402715/ (accessed on 15 July 2025). [CrossRef] [PubMed]
- Hunter, D.J.; Rivett, D.A.; McKiernan, S.; Snodgrass, S.J. Acromiohumeral distance and supraspinatus tendon thickness in people with shoulder impingement syndrome compared to asymptomatic age and gender-matched participants: A case control study. BMC Musculoskelet. Disord. 2021, 22, 1004. Available online: https://pubmed.ncbi.nlm.nih.gov/34852803/ (accessed on 29 December 2022). [CrossRef] [PubMed]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M.; STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. Int. J. Surg. 2014, 12, 1500–1524. Available online: https://pubmed.ncbi.nlm.nih.gov/25046751/ (accessed on 11 July 2023). [CrossRef] [PubMed]
- Shrestha, B.; Dunn, L. The Declaration of Helsinki on Medical Research involving Human Subjects: A Review of Seventh Revision. J. Nepal. Health Res. Counc. 2020, 17, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Michener, L.A.; Walsworth, M.K.; Doukas, W.C.; Murphy, K.P. Reliability and diagnostic accuracy of 5 physical examination tests and combination of tests for subacromial impingement. Arch. Phys. Med. Rehabil. 2009, 90, 1898–1903. [Google Scholar] [CrossRef] [PubMed]
- Garrow, J.S. Quetelet index as indicator of obesity. Lancet 1986, 1, 1219. Available online: https://pubmed.ncbi.nlm.nih.gov/2871462/ (accessed on 11 July 2023). [CrossRef] [PubMed]
- Hougs Kjær, B.; Ellegaard, K.; Wieland, I.; Warming, S.; Juul-Kristensen, B. Intra-rater and inter-rater reliability of the standardized ultrasound protocol for assessing subacromial structures. Physiother. Theory Pract. 2017, 33, 398–409. Available online: https://pubmed.ncbi.nlm.nih.gov/28481725/ (accessed on 14 July 2025). [CrossRef] [PubMed]
- Kjær, B.H.; de Wandele, I.; Spanhove, V.; Juul-Kristensen, B.; Cools, A.M. Subacromial space outlet in female patients with multidirectional instability based on hypermobile Ehlers-Danlos syndrome and hypermobility spectrum disorder measured by ultrasound. J. Shoulder. Elbow. Surg. 2020, 29, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, T.J.; Jonczy, M.; Buck, F.M.; Sutter, R.; Puskas, G.J.; Pfirrmann, C.W.A. Ultrasound of the coracoacromial ligament in asymptomatic volunteers and patients with shoulder impingement. Acta Radiol. 2016, 57, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Miyake, S.; Tamai, M.; Takeuchi, Y.; Izaki, T.; Arashiro, Y.; Shibata, Y.; Shibata, T.; Yamamoto, T. Alteration of coracoacromial ligament thickness at the acromial undersurface in patients with rotator cuff tears. JSES Int. 2022, 6, 468. [Google Scholar] [CrossRef] [PubMed]
- Mazaleyrat, M.; Barthélémy, R.; Bouilleau, L.; Charousset, C.; Berhouet, J. Inter- and intra-observer reproducibility of ultrasound analysis of the long head of the biceps. Orthop. Traumatol. Surg. Res. 2020, 106, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, A.; Freire, G.; Alonso, R.; Afonso, P.D.; Pires, L. Bicipital groove cross-sectional area on ultrasonography: Does a correlation to intra-articular tendon pathology exist? Orthop. Traumatol. Surg. Res. 2021, 107, 102747. Available online: https://pubmed.ncbi.nlm.nih.gov/33333282/ (accessed on 16 July 2025). [CrossRef] [PubMed]
- Day, J.M.; Uhl, T. Thickness of the lower trapezius and serratus anterior using ultrasound imaging during a repeated arm lifting task. Man. Ther. 2013, 18, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Guermazi, A.; Roemer, F.W.; Robinson, P.; Tol, J.L.; Regatte, R.R.; Crema, M.D. Imaging of muscle injuries in sports medicine: Sports imaging series. Radiology 2017, 282, 646–663. [Google Scholar] [CrossRef] [PubMed]
- Bley, B.; Abid, W. Imaging of Tendinopathy: A Physician’s Perspective. J. Orthop. Sports Phys. Ther. 2015, 45, 826–828. [Google Scholar] [CrossRef] [PubMed]
- Barreto, R.P.G.; Braman, J.P.; Ludewig, P.M.; Ribeiro, L.P.; Camargo, P.R. Bilateral magnetic resonance imaging findings in individuals with unilateral shoulder pain. J. Shoulder Elbow Surg. 2019, 28, 1699–1706. Available online: https://pubmed.ncbi.nlm.nih.gov/31279721/ (accessed on 22 December 2023). [CrossRef] [PubMed]
- Couanis, G.; Breidahl, W.; Burnham, S. The relationship between subacromial bursa thickness on ultrasound and shoulder pain in open water endurance swimmers over time. J. Sci. Med. Sport 2015, 18, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Soker, G.; Gulek, B.; Soker, E.; Kaya, O.; Inan, I.; Arslan, M.; Esen, K.; Memis, D.; Yilmaz, C. Sonographic assessment of subacromial bursa distension during arm abduction: Establishing a threshold value in the diagnosis of subacromial impingement syndrome. J. Med. Ultrason. 2018, 45, 287–294. Available online: https://pubmed.ncbi.nlm.nih.gov/29075914/ (accessed on 21 March 2024). [CrossRef] [PubMed]
- Navarro-Ledesma, S.; Struyf, F.; Labajos-Manzanares, M.T.; Fernandez-Sanchez, M.; Morales-Asencio, J.M.; Luque-Suarez, A. Does the acromiohumeral distance matter in chronic rotator cuff related shoulder pain? Musculoskelet. Sci. Pract. 2017, 29, 38–42. Available online: https://pubmed.ncbi.nlm.nih.gov/28315580/ (accessed on 28 July 2024). [CrossRef] [PubMed]
- Ting, D.S.; Yang Jlan Lin, K.H.; Wang, T.G.; Lin, J.J. Alteration in coracohumeral ligament and distance in people with symptoms of subcoracoid impingement. BMC Musculoskelet. Disord. 2023, 24, 58. [Google Scholar] [CrossRef] [PubMed]
- Mc Auliffe, S.; Whiteley, R.; Malliaras, P.; O’Sullivan, K. Central sensitisation in different tendinopathies: Are we comparing apples and oranges? Br. J. Sports Med. 2019, 53, 142–143. [Google Scholar] [CrossRef] [PubMed]
- Márquez, G.; Romero-Arenas, S.; Marín-Pagán, C.; Vera-Ibañez, A.; FernáNdez Del Olmo, M.; Taube, W. Peripheral and central fatigue after high intensity resistance circuit training. Muscle Nerve. 2017, 56, 152–159. Available online: https://pubmed.ncbi.nlm.nih.gov/28346689/ (accessed on 26 December 2023). [CrossRef] [PubMed]
- van der Vlist, A.C.; Veen, J.M.; van Oosterom, R.F.; van Veldhoven, P.L.J.; Verhaar, J.A.N.; de Vos, R.J. Ultrasound Doppler Flow in Patients With Chronic Midportion Achilles Tendinopathy: Is Surface Area Quantification a Reliable Method? J. Ultrasound Med. 2020, 39, 731–739. Available online: https://pubmed.ncbi.nlm.nih.gov/31724758/ (accessed on 26 January 2024). [CrossRef] [PubMed]
- Noble, J.A. Ultrasound image segmentation and tissue characterization. Proc. Inst. Mech. Eng. H. 2010, 224, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.; Kim, H.; Han, S.B.; Song, H.S. Ultrasound Findings Aid Decisions to Repair Partial Articular Supraspinatus Tendon Avulsion. J. Ultrasound Med. 2020, 39, 2005–2011. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.; Barker-Davies, R.M.; Bennett, A.N.; Fong, D.T.P.; Wheeler, P.C.; Lewis, M.; Ranson, C. Sport and exercise medicine consultants are reliable in assessing tendon neovascularity using ultrasound Doppler. BMJ Open Sport—Exerc. Med. 2018, 4, 298. [Google Scholar] [CrossRef] [PubMed]
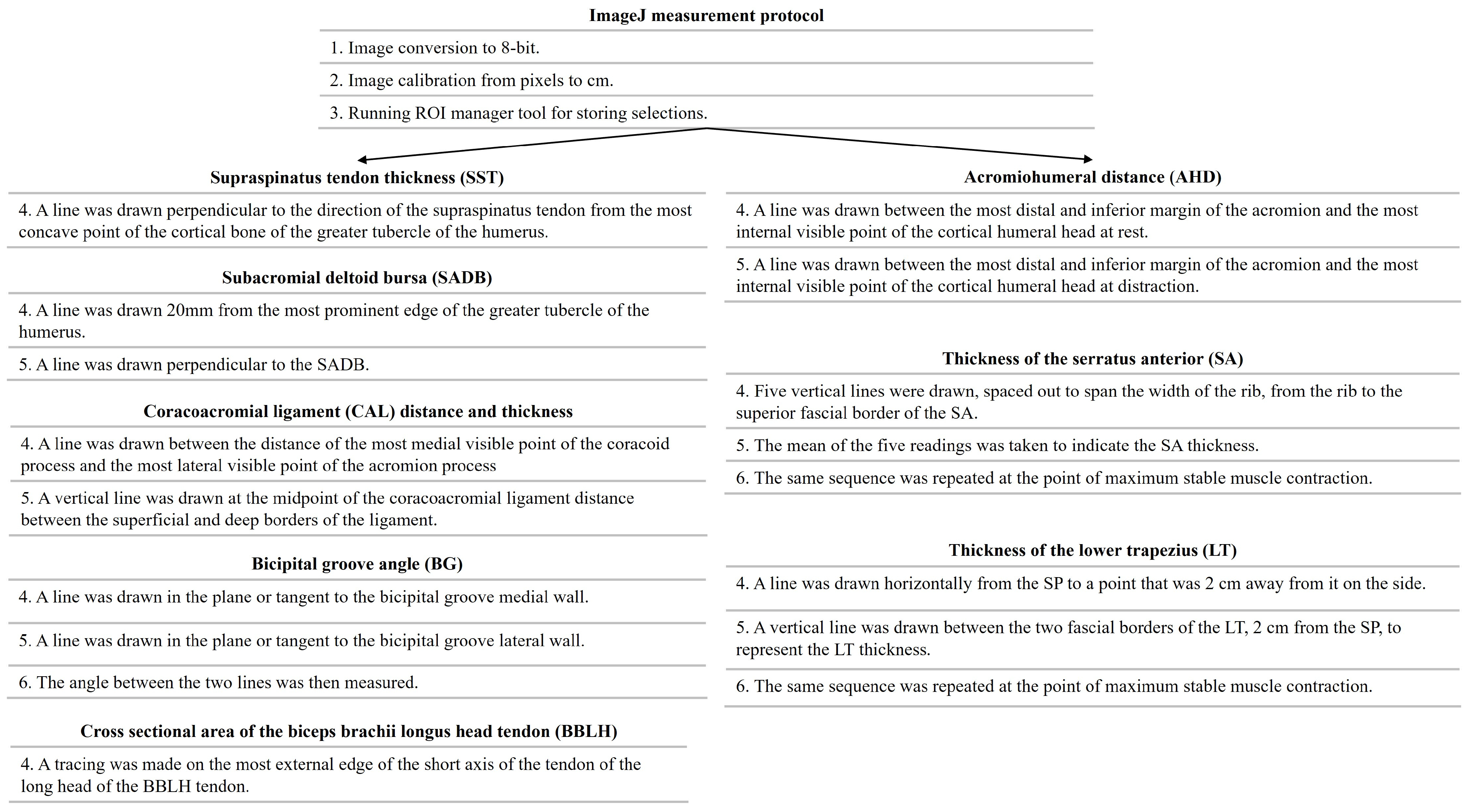
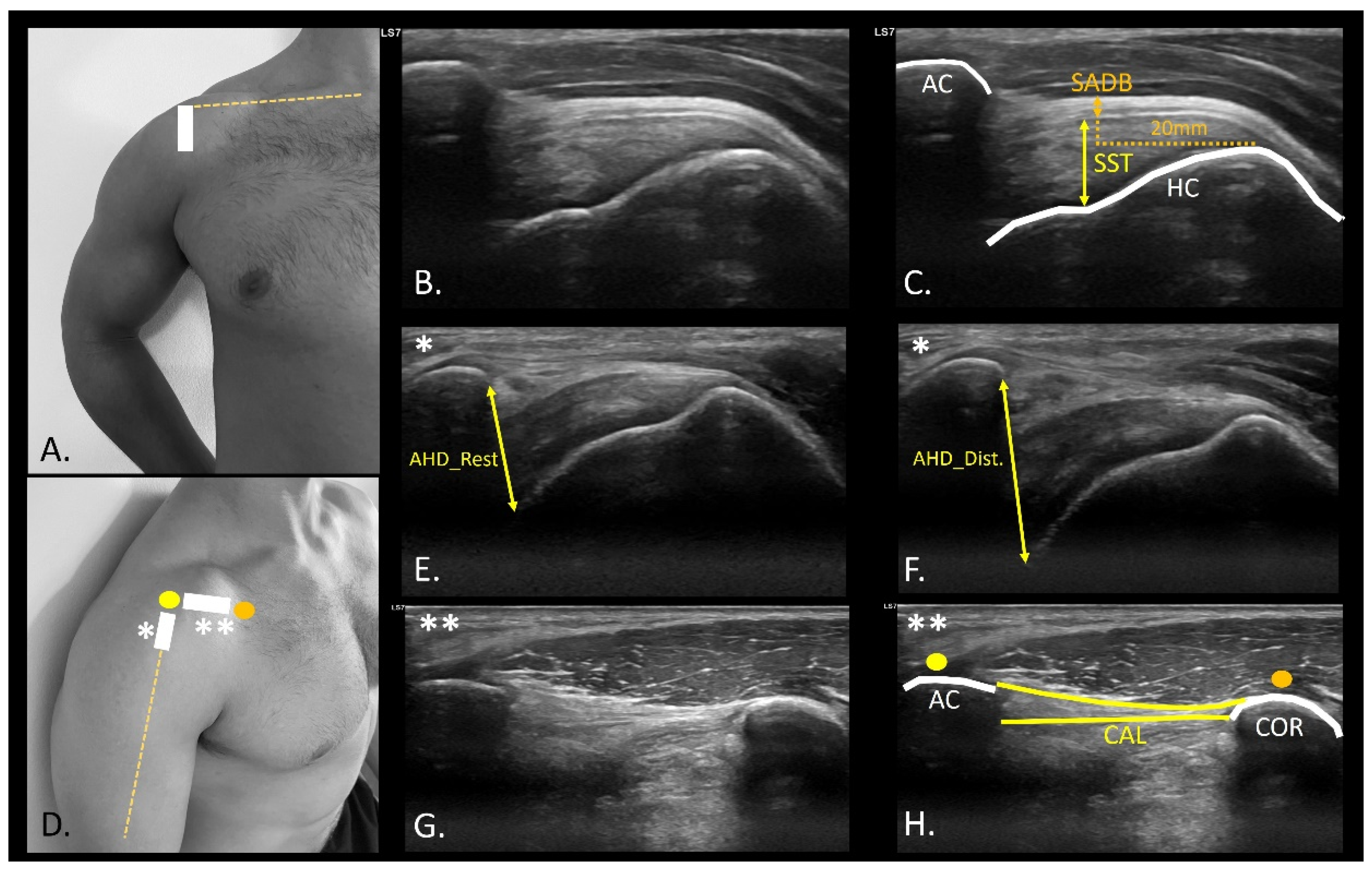
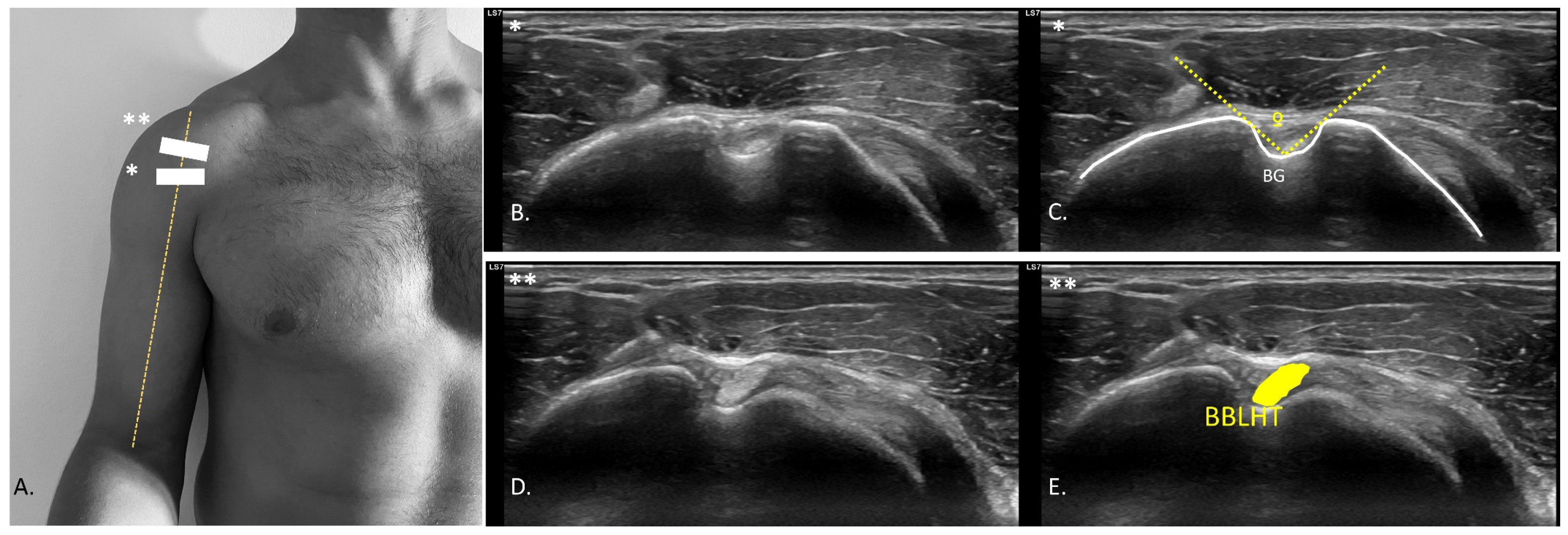
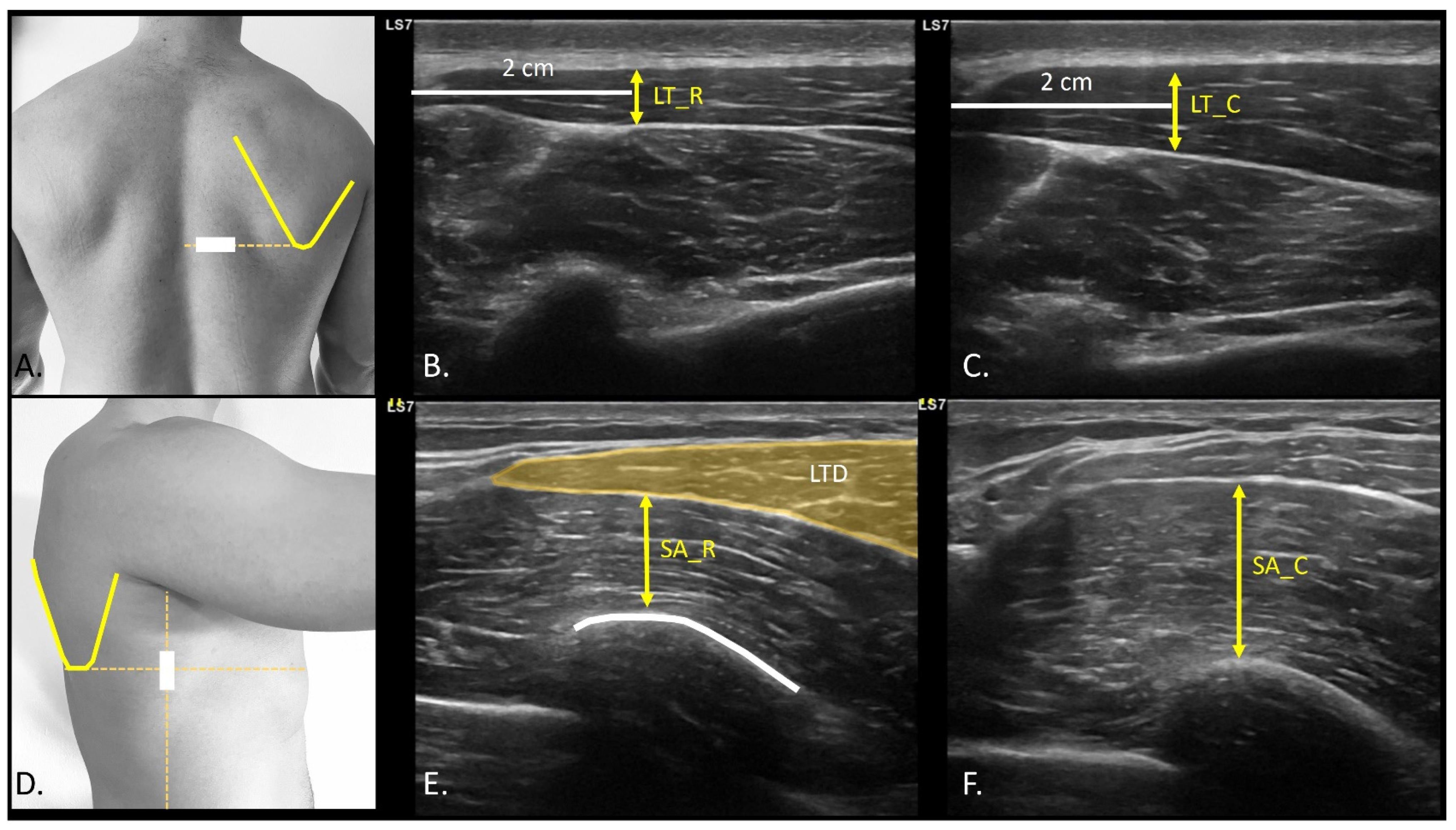
| Descriptive Variables | Total Sample (n = 20) |
|---|---|
| Age (years) | 25.70 ± 4.03 (20.00–32.00) |
| Weight (kg) | 86.37 ± 8.86 (75.00–110.00) |
| Height (m) | 1.79 ± 0.07 (1.70–1.91) |
| BMI (kg/m2) | 26.84 ± 2.62 (23.77–34.72) |
| Dominant Side (R/L) (%) | 15/5 (75%/25%) |
| Painful Side (R/L) (%) | 12/8 (66.67%/33.33%) |
| USI Variables | PS (n = 20) | NPS (n = 20) | Mean Differences (95%CI) | PS vs. NPS p-Value (ES) |
|---|---|---|---|---|
| SST (cm) | 0.62 ± 0.07 (0.45–0.73) | 0.60 ± 0.06 (0.44–0.70) | 0.02 (−0.01–0.04) | 0.136 (0.34) |
| AHD_Rest (cm) | 0.90 ± 0.9 (0.75–1.06) | 0.92 ± 0.12 (0.73–1.24) | −0.02 (−0.07–0.02) | 0.285 (0.24) |
| AHD_Dist. (cm) | 1.13 ± 0.21 (0.80–1.50) | 1.13 ± 0.24 (0.78–1.75) | −0.01 (−0.6–0.05) | 0.944 (0.02) |
| AHD_Dif. (cm) | 0.23 ± 0.17 (0.04–0.60) | 0.21 ± 0.16 (0.02–0.56) | 0.02 (−0.02–0.07) | 0.341 (0.21) |
| BBLHT CSA (cm2) | 0.11 ± 0.02 (0.08–0.16) | 0.12 ± 0.02 (0.08–0.16) | −0.01 (−0.2–0.01) | 0.177 (0.31) |
| BG (°) | 118.26 ± 8.17 (95.00–130.80) | 117.55 ± 10.66 (95.40–132.00) | 0.71 (−3.31–4.74) | 0.714 (0.08) |
| CAL distance (cm) | 2.89 ± 0.29 (2.16–3.36) | 2.82 ± 0.37 (2.05–3.77) | 0.002 (−0.04–0.16) | 0.231 (0.27) |
| CAL thickness (mm) | 1.01 ± 0.03 (0.26–1.64) | 1.02 ± 0.03 (0.59–1.69) | −0.0002 (−0.018–0.017) | 0.977 (0.006) |
| SADB thickness (mm) | 0.9 ± 0.53 (0.4–2.5) | 0.7 ± 0.43 (0.2–1.7) | 0.2 (0.07–0.32) | 0.004 (0.74) * |
| SA_Rest (cm) | 0.75 ± 0.22 (0.40–1.26) | 0.77 ± 0.30 (0.26–1.58) | −0.01 (−0.12–0.09) | 0.758 (0.07) |
| SA_Cont. (cm) | 1.10 ± 0.30 (0.64–1.59) | 1.18 ± 0.39 (0.46–2.05) | −0.08 (−0.24–0.08) | 0.326 (0.23) |
| SA_Dif. (cm) | 0.35 ± 0.16 (0.07–0.78) | 0.41 ± 0.26 (0.02–0.99) | −0.06 (−0.19–0.07) | 0.338 (0.22) |
| LT_Rest (cm) | 0.46 ± 0.12 (0.25–0.72) | 0.49 ± 0.21 (0.20–1.21) | −0.03 (−0.11–0.05) | 0.477 (0.16) |
| LT_Cont. (cm) | 0.62 ± 0.17 (0.30–0.94) | 0.66 ± 0.28 (0.24–1.52) | −0.04 (−0.16–0.07) | 0.450 (0.17) |
| LT_Dif. (cm) | 0.15 ± 0.13 (0.01–0.57) | 0.17 ± 0.14 (0.02–0.67) | −0.01 (−0.06–0.03) | 0.543 (0.13) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerineau, F.; Cools, A.; Almazán-Polo, J.; Sosa-Reina, M.D.; Abuín-Porras, V.; Baroa-Fernández, C.; García-Ginés, P.; Román-Franganillo, A.; González-de-la-Flor, Á. Ultrasound-Based Morphological and Functional Assessment in Male CrossFit Athletes with Unilateral Subacromial Shoulder Pain: An Observational Study. Medicina 2025, 61, 1304. https://doi.org/10.3390/medicina61071304
Guerineau F, Cools A, Almazán-Polo J, Sosa-Reina MD, Abuín-Porras V, Baroa-Fernández C, García-Ginés P, Román-Franganillo A, González-de-la-Flor Á. Ultrasound-Based Morphological and Functional Assessment in Male CrossFit Athletes with Unilateral Subacromial Shoulder Pain: An Observational Study. Medicina. 2025; 61(7):1304. https://doi.org/10.3390/medicina61071304
Chicago/Turabian StyleGuerineau, Fabien, Ann Cools, Jaime Almazán-Polo, María Dolores Sosa-Reina, Vanesa Abuín-Porras, Cristian Baroa-Fernández, Pablo García-Ginés, Ana Román-Franganillo, and Ángel González-de-la-Flor. 2025. "Ultrasound-Based Morphological and Functional Assessment in Male CrossFit Athletes with Unilateral Subacromial Shoulder Pain: An Observational Study" Medicina 61, no. 7: 1304. https://doi.org/10.3390/medicina61071304
APA StyleGuerineau, F., Cools, A., Almazán-Polo, J., Sosa-Reina, M. D., Abuín-Porras, V., Baroa-Fernández, C., García-Ginés, P., Román-Franganillo, A., & González-de-la-Flor, Á. (2025). Ultrasound-Based Morphological and Functional Assessment in Male CrossFit Athletes with Unilateral Subacromial Shoulder Pain: An Observational Study. Medicina, 61(7), 1304. https://doi.org/10.3390/medicina61071304







