Volumetric Bone Mineral Density Assessed by Dual-Energy CT Predicts Bone Strength Suitability for Cementless Total Knee Arthroplasty
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
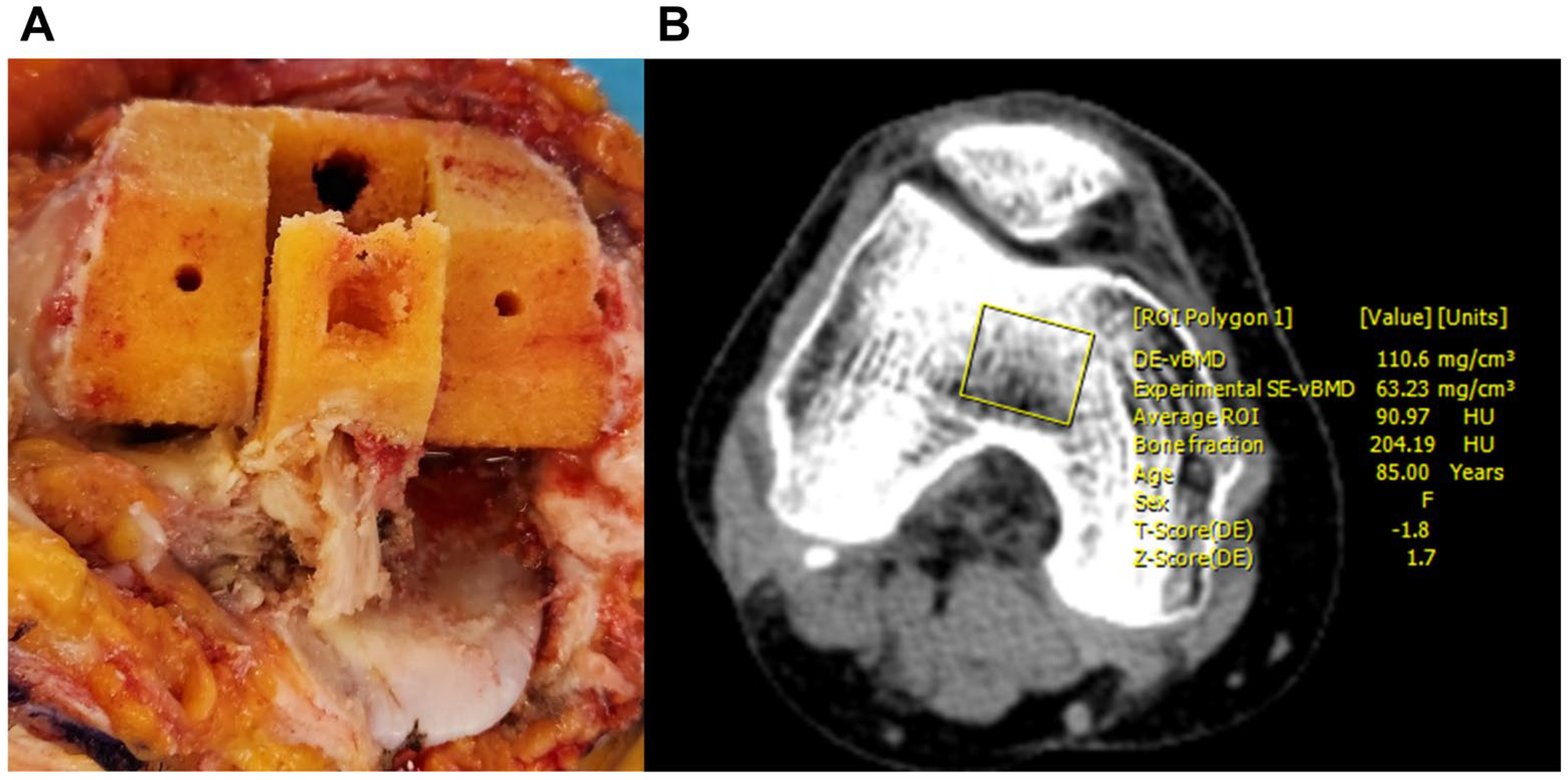
2.2. Dual-Energy CT Imaging Protocol and Analysis
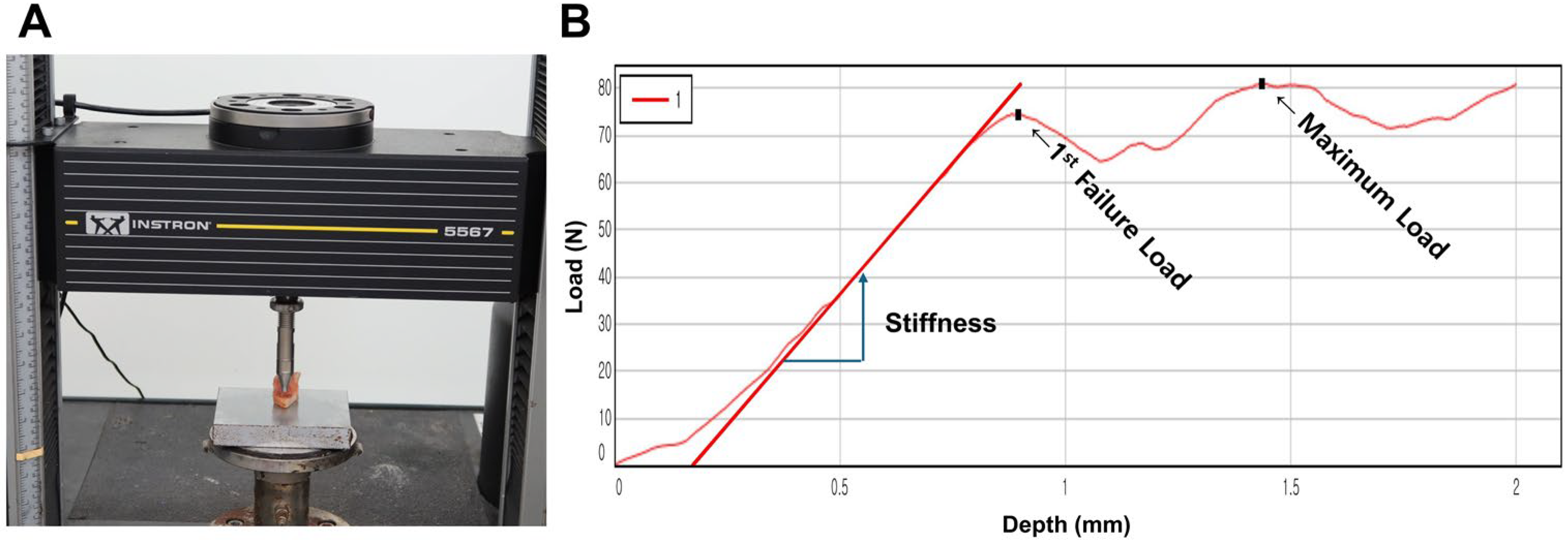
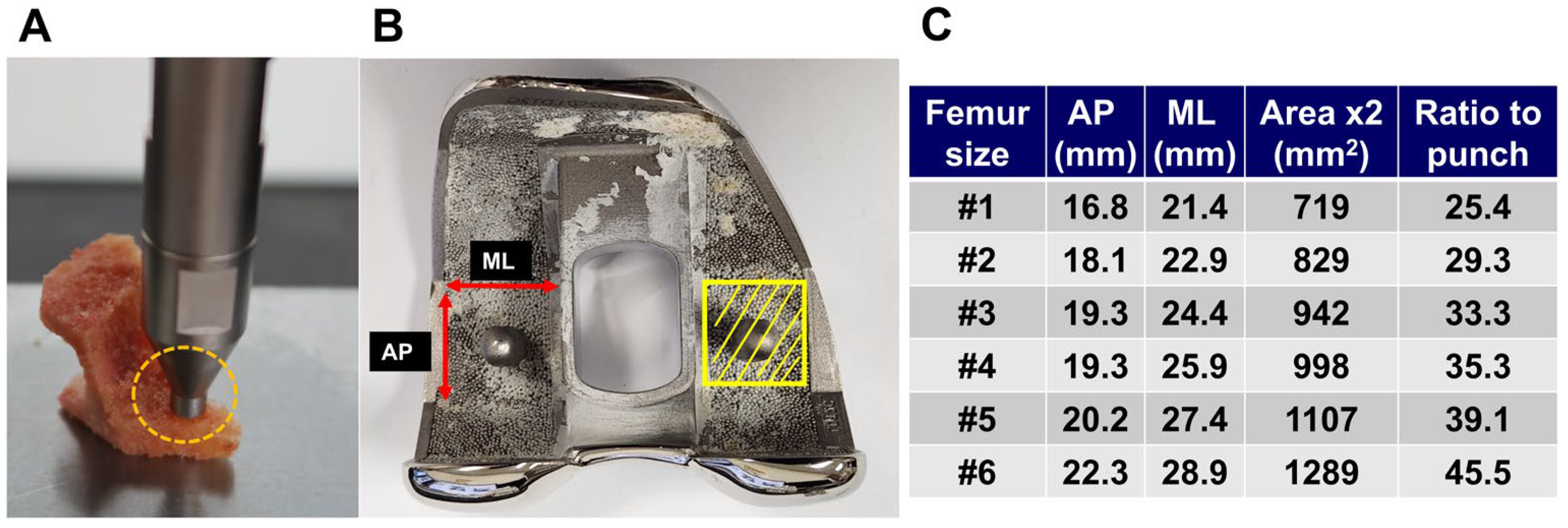
2.3. Bone Strength Assessment
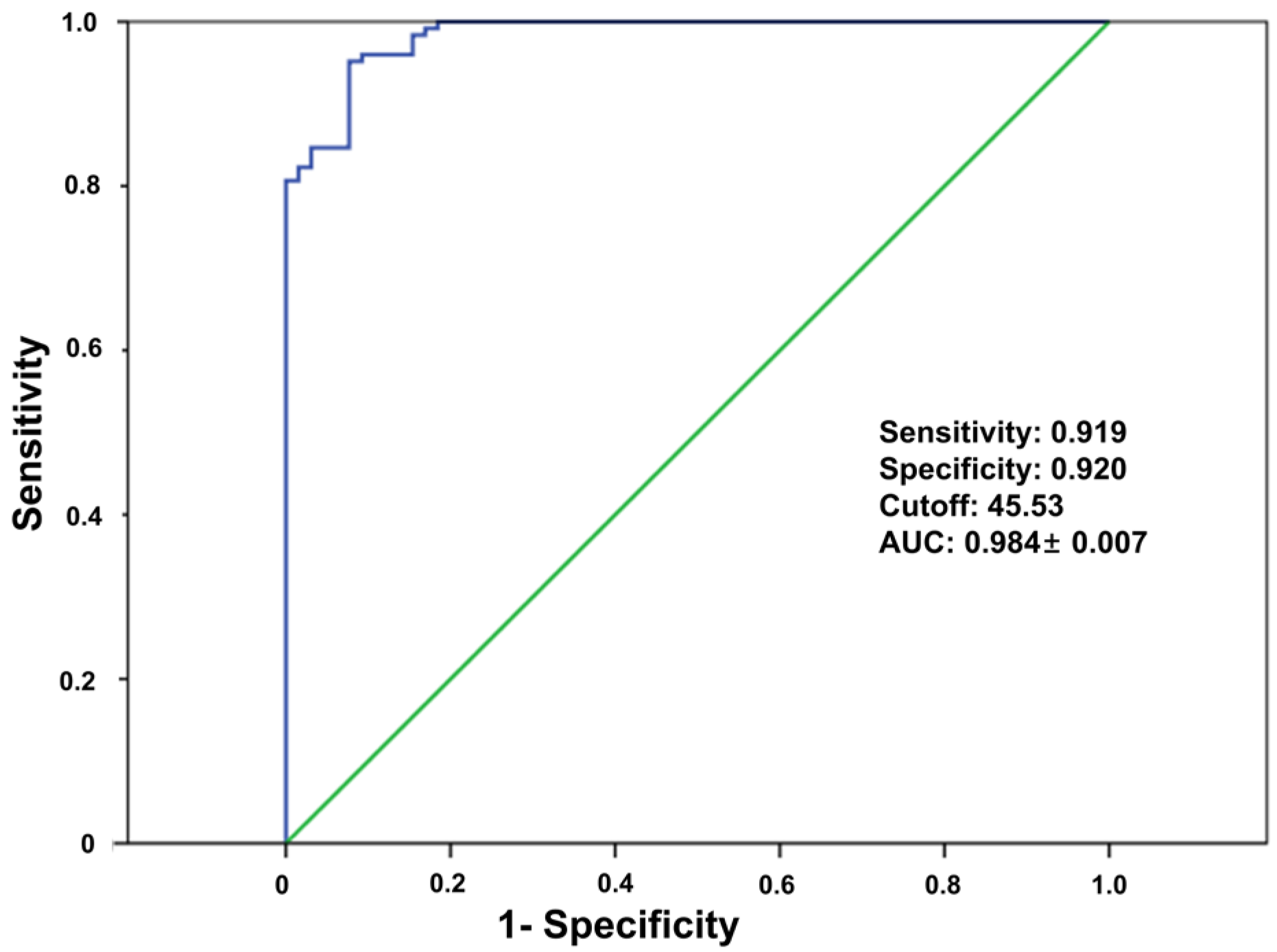
2.4. Definition of Suitability for Cementless TKA
2.5. Statistical Analysis
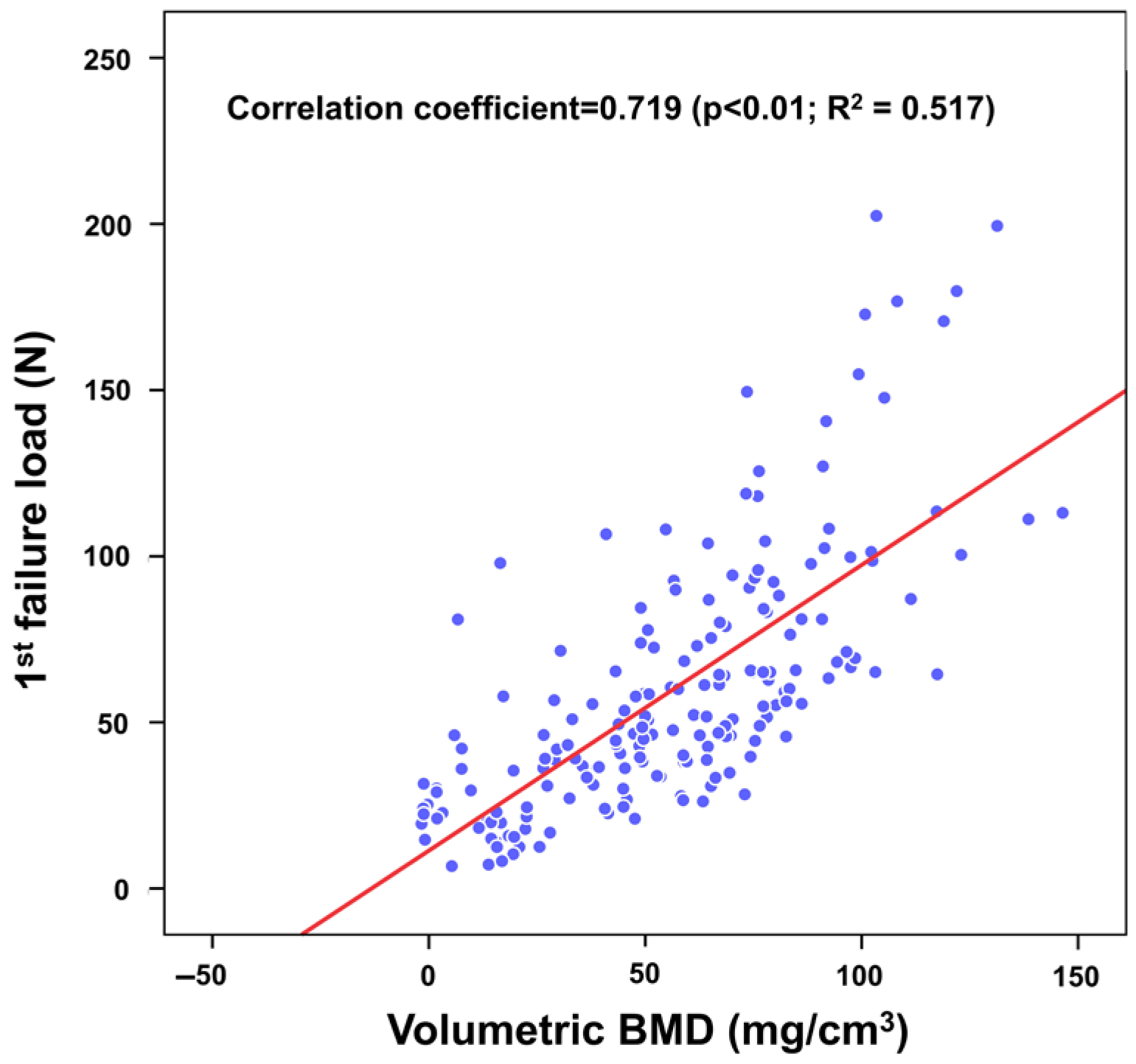
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Losina, E.; Thornhill, T.S.; Rome, B.N.; Wright, J.; Katz, J.N. The dramatic increase in total knee replacement utilization rates in the United States cannot be fully explained by growth in population size and the obesity epidemic. J. Bone Jt. Surg. Am. 2012, 94, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Park, Y.-M.; Ko, S.-H.; Hyun, K.-S.; Choi, Y.-H.; Min, D.-U.; Han, K.; Koh, H.-S. Association of general and central obesity, and their changes with risk of knee osteoarthritis: A nationwide population-based cohort study. Sci. Rep. 2023, 13, 3796. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, K.W.; Sobba, W.; Rajahraman, V.; Schwarzkopf, R.; Rozell, J.C. Does body mass index influence improvement in patient reported outcomes following total knee arthroplasty? A retrospective analysis of 3918 cases. Knee Surg. Relat. Res. 2023, 35, 21. [Google Scholar] [CrossRef] [PubMed]
- Koh, I.J.; Cho, W.S.; Choi, N.Y.; Kim, T.K. Causes, risk factors, and trends in failures after TKA in Korea over the past 5 years: A multicenter study. Clin. Orthop. Relat. Res. 2014, 472, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Roof, M.A.; Kreinces, J.B.; Schwarzkopf, R.; Rozell, J.C.; Aggarwal, V.K. Are there avoidable causes of early revision total knee arthroplasty? Knee Surg. Relat. Res. 2022, 34, 29. [Google Scholar] [CrossRef] [PubMed]
- Kamath, A.F.; Siddiqi, A.; Malkani, A.L.; Krebs, V.E. Cementless Fixation in Primary Total Knee Arthroplasty: Historical Perspective to Contemporary Application. J. Am. Acad. Orthop. Surg. 2021, 29, e363–e379. [Google Scholar] [CrossRef] [PubMed]
- Haslhofer, D.J.; Kraml, N.; Stadler, C.; Gotterbarm, T.; Klotz, M.C.; Klasan, A. Cementless fixation in total knee arthroplasty: Current evidence and future perspective. Arch. Orthop. Trauma Surg. 2024, 145, 101. [Google Scholar] [CrossRef] [PubMed]
- Bagsby, D.T.; Issa, K.; Smith, L.S.; Elmallah, R.K.; Mast, L.E.; Harwin, S.F.; Mont, M.A.; Bhimani, S.J.; Malkani, A.L. Cemented vs Cementless Total Knee Arthroplasty in Morbidly Obese Patients. J. Arthroplast. 2016, 31, 1727–1731. [Google Scholar] [CrossRef] [PubMed]
- Nakama, G.Y.; Peccin, M.S.; Almeida, G.J.; Lira Neto Ode, A.; Queiroz, A.A.; Navarro, R.D. Cemented, cementless or hybrid fixation options in total knee arthroplasty for osteoarthritis and other non-traumatic diseases. Cochrane Database Syst. Rev. 2012, 10, CD006193. [Google Scholar] [CrossRef] [PubMed]
- Restrepo, S.; Smith, E.B.; Hozack, W.J. Excellent mid-term follow-up for a new 3D-printed cementless total knee arthroplasty. Bone Jt. J. 2021, 103-B, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Laende, E.K.; Richardson, C.G.; Dunbar, M.J. Predictive value of short-term migration in determining long-term stable fixation in cemented and cementless total knee arthroplasties. Bone Jt. J. 2019, 101-B, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, E.; Schilling, C.; Grupp, T.M.; Giurea, A.; Wyers, C.; Bergh, J.v.D.; Verdonschot, N.; Janssen, D. The effect of different interference fits on the primary fixation of a cementless femoral component during experimental testing. J. Mech. Behav. Biomed. Mater. 2021, 113, 104189. [Google Scholar] [CrossRef] [PubMed]
- Suh, D.; Kwak, D.S.; Kim, Y.D.; Park, S.; Cho, N.; Koh, I.J. Central Bone Mineral Density Is Not a Useful Tool to Predict Bone Strength of the Distal Femur for Cementless Total Knee Arthroplasty. Clin. Orthop. Surg. 2024, 16, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Sung, K.H.; Choi, Y.; Cho, G.H.; Chung, C.Y.; Park, M.S.; Lee, K.M. Peripheral DXA measurement around ankle joint to diagnose osteoporosis as assessed by central DXA measurement. Skelet. Radiol. 2018, 47, 1111–1117. [Google Scholar] [CrossRef] [PubMed]
- A Alavizadeh, S.; Mohajeri-Tehrani, M.R.; Rostamian, A.; Meybodi, H.R.A.; Qorbani, M.; A Keshtkar, A.; Panahi, S.S.; Rahdari, F.; Khashayar, P. Prevalence and associated factors of T-score discordance between different sites in Iranian patients with spinal cord injury. Spinal Cord. 2014, 52, 322–326. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rajiah, P.; Sundaram, M.; Subhas, N. Dual-Energy CT in Musculoskeletal Imaging: What Is the Role Beyond Gout? AJR Am. J. Roentgenol. 2019, 213, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Wesarg, S.; Kirschner, M.; Becker, M.; Erdt, M.; Kafchitsas, K.; Khan, M.F. Dual-energy CT-based assessment of the trabecular bone in vertebrae. Methods Inf. Med. 2012, 51, 398–405. [Google Scholar] [PubMed]
- Arentsen, L.; Hansen, K.E.; Yagi, M.; Takahashi, Y.; Shanley, R.; McArthur, A.; Bolan, P.; Magome, T.; Yee, D.; Froelich, J.; et al. Use of dual-energy computed tomography to measure skeletal-wide marrow composition and cancellous bone mineral density. J. Bone Miner. Metab. 2017, 35, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Koch, V.; Hokamp, N.G.; Albrecht, M.H.; Gruenewald, L.D.; Yel, I.; Borggrefe, J.; Wesarg, S.; Eichler, K.; Burck, I.; Gruber-Rouh, T.; et al. Accuracy and precision of volumetric bone mineral density assessment using dual-source dual-energy versus quantitative CT: A phantom study. Eur. Radiol. Exp. 2021, 5, 43. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.Y.; Lee, S.-W.; In, Y.; Kim, M.S.; Kim, Y.D.; Lee, S.-Y.; Lee, J.-W.; Koh, I.J. Dual-Energy CT-Based Bone Mineral Density Has Practical Value for Osteoporosis Screening around the Knee. Medicina 2022, 58, 1085. [Google Scholar] [CrossRef] [PubMed]
- Gruenewald, L.D.; Koch, V.; Martin, S.S.; Yel, I.; Eichler, K.; Gruber-Rouh, T.; Lenga, L.; Wichmann, J.L.; Alizadeh, L.S.; Albrecht, M.H.; et al. Diagnostic accuracy of quantitative dual-energy CT-based volumetric bone mineral density assessment for the prediction of osteoporosis-associated fractures. Eur. Radiol. 2022, 32, 3076–3084. [Google Scholar] [CrossRef] [PubMed]
- Dunham, C.E.; Takaki, S.E.; Johnson, J.A.; Dunning, C.E. Mechanical properties of cancellous bone of the distal humerus. Clin Biomech 2005, 20, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Gordon, K.D.; Duck, T.R.; King, G.J.; Johnson, J.A. Mechanical properties of subchondral cancellous bone of the radial head. J. Orthop. Trauma 2003, 17, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.M.; Nielsen, P.T.; Lebech, A.; Toksvig-Larsen, S.; Lund, B. Preoperative bone mineral density of the proximal tibia and migration of the tibial component after uncemented total knee arthroplasty. J. Arthroplast. 1999, 14, 77–81. [Google Scholar] [CrossRef] [PubMed]
- D’Lima, D.D.; Patil, S.; Steklov, N.; Chien, S.; Colwell, C.W., Jr. In vivo knee moments and shear after total knee arthroplasty. J. Biomech. 2007, 40 (Suppl. S1), S11–S17. [Google Scholar] [CrossRef] [PubMed]
- Kutzner, I.; Heinlein, B.; Graichen, F.; Bender, A.; Rohlmann, A.; Halder, A.; Beier, A.; Bergmann, G. Loading of the knee joint during activities of daily living measured in vivo in five subjects. J. Biomech. 2010, 43, 2164–2173. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.E.; Choi, B.S.; Ro, D.H.; Lee, M.C.; Han, H.S. Fixed-Bearing and Higher Postoperative Knee Flexion Angle as Predictors of Satisfaction in Asian Patients Undergoing Posterior-Stabilized Total Knee Arthroplasty. Clin. Orthop. Surg. 2024, 16, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Andersen, M.R.; Winther, N.S.; Lind, T.; Schroder, H.M.; Flivik, G.; Petersen, M.M. Low Preoperative BMD Is Related to High Migration of Tibia Components in Uncemented TKA-92 Patients in a Combined DEXA and RSA Study With 2-Year Follow-Up. J. Arthroplast. 2017, 32, 2141–2146. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S.; van Hamersveld, K.T.; Marang-van de Mheen, P.J.; Kaptein, B.L.; Nelissen, R.; Toksvig-Larsen, S. Migration of a novel 3D-printed cementless versus a cemented total knee arthroplasty: Two-year results of a randomized controlled trial using radiostereometric analysis. Bone Jt. J. 2020, 102-B, 1016–1024. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Huang, J.; Yu, P.; Yan, J.; Ge, Y.; Lu, Y.; Yan, F.; Wang, L.; Du, L. Accuracy, agreement, and reliability of DECT-derived vBMD measurements: An initial ex vivo study. Eur. Radiol. 2021, 31, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Foti, G.; Longo, C.; D’oNofrio, M.; Natali, S.; Piovan, G.; Oliboni, E.; Iacono, V.; Guerriero, M.; Zorzi, C. Dual-Energy CT for Detecting Painful Knee Prosthesis Loosening. Radiology 2023, 306, e211818. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, B.; Tong, X.; Fan, Y.; Wang, S.; Liu, Y.; Fang, X.; Liu, L. Diagnostic Accuracy of Dual-Energy CT Material Decomposition Technique for Assessing Bone Status Compared with Quantitative Computed Tomography. Diagn. 2023, 13, 1751. [Google Scholar] [CrossRef] [PubMed]
- Koh, I.J.; Kim, M.W.; Kim, J.H.; Han, S.Y.; In, Y. Trends in High Tibial Osteotomy and Knee Arthroplasty Utilizations and Demographics in Korea From 2009 to 2013. J. Arthroplast. 2015, 30, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Peacock, M.; Buckwalter, K.A.; Persohn, S.; Hangartner, T.N.; Econs, M.J.; Hui, S. Race and sex differences in bone mineral density and geometry at the femur. Bone 2009, 45, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Misra, M.; Ackerman, K.E.; Bredella, M.A.; Stanford, F.C.; Faje, A.T.; Nordberg, A.; Derrico, N.P.; Bouxsein, M.L. Racial Differences in Bone Microarchitecture and Estimated Strength at the Distal Radius and Distal Tibia in Older Adolescent Girls: A Cross-Sectional Study. J. Racial Ethn. Health Disparities 2017, 4, 587–598. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Chaar, O.; Narayanakurup, J.; Abdelhamead, A.S.A.; Ro, D.H.; Kim, S.E. Do knee alignment patterns differ between Middle Eastern and East Asian populations? A propensity-matched analysis using artificial intelligence. Knee Surg. Relat. Res. 2025, 37, 11. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Values (n = 190) |
|---|---|
| Demographic data | |
| Age (year) | 68.1 ± 5.4 (53~86) |
| Gender (female) | 155 (82) |
| Height (cm) | 155.5 ± 7.0 (143.1~177.2) |
| Weight (kg) | 65.7 ± 9.6 (45.4~94.3) |
| BMI (kg/m2) | 27.2 ± 3.4 (19.6~36.5) |
| Diagnosis of osteoporosis † | |
| Normal (T score > −1.0) | 67 (35) |
| Osteopenia (−1.0 ≤ T score ≤ −2.5) | 95 (50) |
| Osteoporosis (T score < −2.5) | 28 (15) |
| DXA (T-score) | |
| Mean lumbar spines | −0.5 ± 1.5 (−3.6 ~ 4.9) |
| Femur neck | −1.2 ± 1.1 (−3.5 ~ 2.8) |
| Volumetric BMD (mg/cm3) | |
| Axial image | 55.9 ± 31.9 (−1.7~146.4) |
| Sagittal image | 50.2 ± 26.9 (−1.2~115.3) |
| Coronal image | 54.6 ± (−1.6~123.0) |
| Femoral component size † | |
| 1 | 6 (3) |
| 2 | 29 (15) |
| 3 | 81 (43) |
| 4 | 48 (25) |
| 5 | 18 (10) |
| 6 | 8 (4) |
| Parameters | Values |
|---|---|
| 1st peak force (N) | 59.5 ± 38.2 (6.7~202.5) |
| Compressive displacement at 1st peak force (mm) | 0.9 ± 0.3 (0.3~1.8) |
| Maximal force (N) | 81.2 ± 41.9 (14.6~244.0) |
| Compressive displacement at maximal force (mm) | 1.8 ± 0.3 (0.4~2.0) |
| Stiffness (N/mm) | 111.1 ± 80.2 (12.0~533.8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.H.; Kwak, D.-S.; Lee, S.-W.; Kim, Y.D.; Cho, N.; Koh, I.J. Volumetric Bone Mineral Density Assessed by Dual-Energy CT Predicts Bone Strength Suitability for Cementless Total Knee Arthroplasty. Medicina 2025, 61, 1305. https://doi.org/10.3390/medicina61071305
Lee DH, Kwak D-S, Lee S-W, Kim YD, Cho N, Koh IJ. Volumetric Bone Mineral Density Assessed by Dual-Energy CT Predicts Bone Strength Suitability for Cementless Total Knee Arthroplasty. Medicina. 2025; 61(7):1305. https://doi.org/10.3390/medicina61071305
Chicago/Turabian StyleLee, Dong Hwan, Dai-Soon Kwak, Sheen-Woo Lee, Yong Deok Kim, Nicole Cho, and In Jun Koh. 2025. "Volumetric Bone Mineral Density Assessed by Dual-Energy CT Predicts Bone Strength Suitability for Cementless Total Knee Arthroplasty" Medicina 61, no. 7: 1305. https://doi.org/10.3390/medicina61071305
APA StyleLee, D. H., Kwak, D.-S., Lee, S.-W., Kim, Y. D., Cho, N., & Koh, I. J. (2025). Volumetric Bone Mineral Density Assessed by Dual-Energy CT Predicts Bone Strength Suitability for Cementless Total Knee Arthroplasty. Medicina, 61(7), 1305. https://doi.org/10.3390/medicina61071305









