Type 2 Diabetes Mellitus in Patients with Different Types of Thyroid Nodular Lesions Among Western Romanian Patients: A Comprehensive Clinical, Biochemical, and Hormonal Analysis
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, F.; Li, Y.; Yu, X.; Wang, X.; Lin, Z.; Song, B.; Tian, L.; Feng, C.; Shan, Z.; Teng, W. The Relationship and Gender Disparity Between Thyroid Nodules and Metabolic Syndrome Components Based on a Recent Nationwide Cross-Sectional Study and Meta-Analysis. Front. Endocrinol. 2021, 12, 736972. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Li, C.; Li, X.; Sun, W.; Wang, Y. Association of systemic immune-inflammation index (SII) and aggregate index of systemic inflammation (AISI) with thyroid nodules in patients with type 2 diabetes mellitus: A retrospective study. BMC Endocr. Disord. 2023, 23, 251. [Google Scholar] [CrossRef]
- Tamez-Pérez, H.-E.; Martínez, E.; Quintanilla-Flores, D.L.; Tamez-Peña, A.L.; Gutiérrez-Hermosillo, H.; Díaz de León-González, E. The rate of primary hypothyroidism in diabetic patients is greater than in the non-diabetic population: An observational study. Med. Clin. 2012, 138, 475–477. [Google Scholar] [CrossRef]
- Miao, S.; Jing, M.; Sheng, R.; Cui, D.; Lu, S.; Zhang, X.; Jing, S.; Zhang, X.; Shan, T.; Shan, H.; et al. The analysis of differential diagnosis of benign and malignant thyroid nodules based on ultrasound reports. Gland Surg. 2020, 9, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Zhang, L.; Huang, X.; Xu, F.; Chen, G.; Zheng, W. Different types of diabetes mellitus and risk of thyroid cancer: A meta-analysis of cohort studies. Front. Endocrinol. 2022, 13, 971213. [Google Scholar] [CrossRef]
- Kitahara, C.M.; Schneider, A.B. Epidemiology of thyroid cancer. Cancer Epidemiol. Biomark. Prev. 2022, 31, 1284–1297. [Google Scholar] [CrossRef]
- Zhang, H.-M.; Feng, Q.-W.; Niu, Y.-X.; Su, Q.; Wang, X. Thyroid nodules in type 2 diabetes mellitus. Curr. Med. Sci. 2019, 39, 576–581. [Google Scholar] [CrossRef]
- Chauhan, A.; Patel, S.S. Thyroid Hormone and Diabetes Mellitus Interplay: Making Management of Comorbid Disorders Complicated. Horm. Metab. Res. 2024, 56, 845–858. [Google Scholar] [CrossRef]
- Onitilo, A.A.; Engel, J.M.; Glurich, I.; Stankowski, R.V.; Williams, G.M.; Doi, S.A. Diabetes and cancer II: Role of diabetes medications and influence of shared risk factors. Cancer Causes Control. 2012, 23, 991–1008. [Google Scholar] [CrossRef]
- Yeo, Y.; Ma, S.H.; Hwang, Y.; Horn-Ross, P.L.; Hsing, A.; Lee, K.E.; Park, Y.J.; Park, D.J.; Yoo, K.Y.; Park, S.K. Diabetes mellitus and risk of thyroid cancer: A meta-analysis. PLoS ONE 2014, 9, e98135. [Google Scholar] [CrossRef]
- Hussein, S.M.M.; AbdElmageed, R.M. The relationship between type 2 diabetes mellitus and related thyroid diseases. Cureus 2021, 13, e20697. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Soriguer, F.; Gutiérrez-Repiso, C.; Rubio-Martin, E.; Linares, F.; Cardona, I.; López-Ojeda, J.; Pacheco, M.; González-Romero, S.; Garriga, M.J.; Velasco, I.; et al. Iodine intakes of 100-300 μg/d do not modify thyroid function and have modest anti-inflammatory effects. Br. J. Nutr. 2011, 105, 1783–1790. [Google Scholar] [CrossRef] [PubMed]
- Macvanin, M.T.; Gluvic, Z.; Zafirovic, S.; Gao, X.; Essack, M.; Isenovic, E.R. The protective role of nutritional antioxidants against oxidative stress in thyroid disorders. Front. Endocrinol. 2023, 13, 1092837. [Google Scholar] [CrossRef]
- Moura Neto, A.; Parisi, M.C.; Tambascia, M.A.; Pavin, E.J.; Alegre, S.M.; Zantut-Wittmann, D.E. Relationship of thyroid hormone levels and cardiovascular events in patients with type 2 diabetes. Endocrine 2014, 45, 84–91. [Google Scholar] [CrossRef]
- Xing, M. Oxidative stress: A new risk factor for thyroid cancer. Endocr.-Relat. Cancer 2012, 19, C7–C11. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, C.; Gao, X.; Han, Y.; Teng, W.; Shan, Z. Correlation Between Thyroid Nodules and Metabolic Syndrome: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2021, 12, 730279. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hadgu, R.; Worede, A.; Ambachew, S. Prevalence of thyroid dysfunction and associated factors among adult type 2 diabetes mellitus patients, 2000–2022: A systematic review and meta-analysis. Syst. Rev. 2024, 13, 119. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tiwari, A.; Balasundaram, P. Public Health Considerations Regarding Obesity. [Updated 5 June 2023]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK572122/ (accessed on 29 January 2025).
- Li, L.; Liu, S.; Yu, J. Autoimmune thyroid disease and type 1 diabetes mellitus: Same pathogenesis; new perspective? Ther. Adv. Endocrinol. Metab. 2020, 11, 2042018820958329. [Google Scholar] [CrossRef]
- Nederstigt, C.; Corssmit, E.P.; de Koning, E.J.; Dekkers, O.M. Incidence and prevalence of thyroid dysfunction in type 1 diabetes. J. Diabetes Complicat. 2016, 30, 420–425. [Google Scholar] [CrossRef]
- Orzan, A.; Novac, C.; Mihu, M.; Tirgoviste, C.I.; Balgradean, M. Type 1 Diabetes and Thyroid Autoimmunity in Children. Maedica 2016, 11, 308–312. [Google Scholar] [PubMed] [PubMed Central]
- Giovannucci, E.L.; Harlan, D.M.; Archer, M.C.; Bergenstal, R.M.; Gapstur, S.M.; Habel, L.A.; Pollak, M.; Regensteiner, J.G.; Yee, D. Diabetes and cancer: A consensus report. Diabetes Care 2010, 33, 1674–1685. [Google Scholar] [CrossRef]
- Zhu, B.; Qu, S. The Relationship Between Diabetes Mellitus and Cancers and Its Underlying Mechanisms. Front. Endocrinol. 2022, 13, 800995. [Google Scholar] [CrossRef]
- Kadiyala, R.; Peter, R.; Okosieme, O.E. Thyroid dysfunction in patients with diabetes: Clinical implications and screening strategies. Int. J. Clin. Pract. 2010, 64, 1130–1139. [Google Scholar] [CrossRef]
- Baltzis, D.; Eleftheriadou, I.; Veves, A. Pathogenesis and Treatment of Impaired Wound Healing in Diabetes Mellitus: New Insights. Adv. Ther. 2014, 31, 817–836. [Google Scholar] [CrossRef]
- Palma, C.C.S.S.V.; Pavesi, M.; Nogueira, V.G.; Clemente, E.L.S.; Vasconcellos, M.d.F.B.M.P.; Pereira, L.C.; Pacheco, F.F.; Braga, T.G.; Bello, L.d.F.; Soares, J.O.; et al. Prevalence of thyroid dysfunction in patients with diabetes mellitus. Diabetol. Metab. Syndr. 2013, 5, 58. [Google Scholar] [CrossRef]
- Vondra, K.; Vrbikova, J.; Dvorakova, K. Thyroid gland diseases in adult patients with diabetes mellitus. Minerva Endocrinol. 2005, 30, 217–236. [Google Scholar]
- Biondi, B.; Kahaly, G.J.; Robertson, R.P. Thyroid Dysfunction and Diabetes Mellitus: Two Closely Associated Disorders. Endocr. Rev. 2019, 40, 789–824. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gierach, M.; Gierach, J.; Junik, R. Insulin resistance and thyroid disorders. Endokrynol. Pol. 2014, 65, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Spira, D.; Buchmann, N.; Dörr, M.; Markus, M.R.P.; Nauck, M.; Schipf, S.; Spranger, J.; Demuth, I.; Steinhagen-Thiessen, E.; Völzke, H.; et al. Association of thyroid function with insulin resistance: Data from two population-based studies. Eur. Thyroid J. 2022, 11, e210063. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rong, F.; Dai, H.; Wu, Y.; Li, J.; Liu, G.; Chen, H.; Zhang, X. Association between thyroid dysfunction and type 2 diabetes: A meta-analysis of prospective observational studies. BMC Med. 2021, 19, 257. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yin, D.T.; He, H.; Yu, K.; Xie, J.; Lei, M.; Ma, R.; Li, H.; Wang, Y.; Liu, Z. The association between thyroid cancer and insulin resistance, metabolic syndrome and its components: A systematic review and meta-analysis. Int. J. Surg. 2018, 57, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, Q.; Yang, Y.; Yao, J.; Liao, L.; Dong, J. High prevalence of thyroid carcinoma in patients with insulin resistance: A meta-analysis of case-control studies. Aging 2021, 13, 22232–22241. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, W.; Sun, Y.; Zhang, Y.; Yin, D. The causal relationships between inflammatory cytokines, blood metabolites, and thyroid cancer: A two-step Mendelian randomization analysis. Discov. Oncol. 2025, 16, 301. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Le, T.N.; Bright, R.; Truong, V.K.; Li, J.; Juneja, R.; Vasilev, K. Key biomarkers in type 2 diabetes patients: A systematic review. Diabetes Obes. Metab. 2025, 27, 7–22. [Google Scholar] [CrossRef]
- Crnčić, T.B.; Tomaš, M.I.; Girotto, N.; Ivanković, S.G. Risk Factors for Thyroid Cancer: What Do We Know So Far? Acta Clin. Croat. 2020, 59 (Suppl. S1), 66–72. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Matei, M.; Vlad, M.M.; Golu, I.; Dumitru, C.Ș.; De Scisciolo, G.; Matei, S.-C. Can Routine Laboratory Tests Be Suggestive in Determining Suspicions of Malignancy in the Case of Thyroid Nodules? Medicina 2023, 59, 1488. [Google Scholar] [CrossRef]
- Zhao, J.; Tian, Y.; Jia, Z.; Yao, J.; Liao, L.; Dong, J. Abnormal Glucose Metabolism Parameters and the Aggressiveness of Differentiated Thyroid Carcinoma: A Hospital-Based Cross-Section Study in China. Front. Endocrinol. 2022, 13, 806349. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, R.; Zhang, J.; Zou, G.; Li, S.; Wang, J.; Li, X.; Xu, J. Diabetes Mellitus and Thyroid Cancers: Risky Correlation, Underlying Mechanisms and Clinical Prevention. Diabetes Metab. Syndr. Obes. 2024, 17, 809–823. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bhatti, J.S.; Sehrawat, A.; Mishra, J.; Sidhu, I.S.; Navik, U.; Khullar, N.; Kumar, S.; Bhatti, G.K.; Reddy, P.H. Oxidative stress in the pathophysiology of type 2 diabetes and related complications: Current therapeutics strategies and future perspectives. Free Radic. Biol. Med. 2022, 184, 114–134. [Google Scholar] [CrossRef] [PubMed]
- Kochman, J.; Jakubczyk, K.; Bargiel, P.; Janda-Milczarek, K. The Influence of Oxidative Stress on Thyroid Diseases. Antioxidants 2021, 10, 1442. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Elumalai, S.; Karunakaran, U.; Moon, J.S.; Won, K.C. NADPH Oxidase (NOX) Targeting in Diabetes: A Special Emphasis on Pancreatic β-Cell Dysfunction. Cells 2021, 10, 1573. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dang, H.; Sheng, J.; Tang, P.; Peng, X.; Zhang, R.; Zhao, X.; Hu, J.; Xu, T. The role and mechanism of NADPH oxidase in the development and progression of thyroid carcinoma. Am. J. Cancer Res. 2023, 13, 4366–4375. [Google Scholar] [PubMed] [PubMed Central]
- Kościuszko, M.; Buczyńska, A.; Krętowski, A.J.; Popławska-Kita, A. Could Oxidative Stress Play a Role in the Development and Clinical Management of Differentiated Thyroid Cancer? Cancers 2023, 15, 3182. [Google Scholar] [CrossRef]
- Wang, D.; Feng, J.F.; Zeng, P.; Yang, Y.H.; Luo, J.; Yang, Y.W. Total oxidant/antioxidant status in sera of patients with thyroid cancers. Endocr.-Relat. Cancer 2011, 18, 773–782. [Google Scholar] [CrossRef]
- Gao, J.; Liu, J. Correlation of serum thyrotropin and thyroid hormone levels with diabetic kidney disease: A cross-sectional study. BMC Endocr. Disord. 2024, 24, 170. [Google Scholar] [CrossRef]
- Tang, Y.; Yan, T.; Wang, G.; Chen, Y.; Zhu, Y.; Jiang, Z.; Yang, M.; Li, C.; Li, Z.; Yu, P.; et al. Correlation between Insulin Resistance and Thyroid Nodule in Type 2 Diabetes Mellitus. Int. J. Endocrinol. 2017, 2017, 1617458. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gu, Y.; Li, H.; Bao, X.; Zhang, Q.; Liu, L.; Meng, G.; Wu, H.; Du, H.; Shi, H.; Xia, Y.; et al. The Relationship Between Thyroid Function and the Prevalence of Type 2 Diabetes Mellitus in Euthyroid Subjects. J. Clin. Endocrinol. Metab. 2017, 102, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Zheng, Y.; Zhang, M.; Li, M.; Qiang, W.; Sui, J.; Guo, H.; Shi, B.; He, M. Lower Free Thyroxine Levels Are Associated with Diabetic Kidney Disease in Males with Type 2 Diabetes Mellitus: An Observational Cross-Sectional Study. Biomedicines 2024, 12, 2370. [Google Scholar] [CrossRef]
- Zhao, X.; Sun, J.; Xin, S.; Zhang, X. Predictive Effects of FT3/FT4 on Diabetic Kidney Disease: An Exploratory Study on Hospitalized Euthyroid Patients with T2DM in China. Biomedicines 2023, 11, 2211. [Google Scholar] [CrossRef]
- Nanu, M.; Delia, C.E.; Toma, G.M.; Ardeleanu, I.; Nanu, I.; Stemate, M.; Nuta, D.; Gheorghiu, M.L. Iodine Status in Romania After 20 Years of Mandatory Salt Iodization: Discordant Results in Schoolchildren and Neonates. Acta Endocrinol. 2024, 20, 80–89. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Karakaya, R.E.; Saka, M.; Ozdemir, D. Determining the relationship between dietary iodine intake, urinary iodine excretion and thyroid functions in people with type 2 diabetes mellitus. Arch Endocrinol. Metab. 2020, 64, 383–389. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, D.W.; Lang, B.H.H.; McLeod, D.S.A.; Newbold, K.; Haymart, M.R. Thyroid cancer. Lancet 2023, 401, 1531–1544. [Google Scholar] [CrossRef]
- Borlea, A.; Moisa-Luca, L.; Popescu, A.; Bende, F.; Stoian, D. Combining CEUS and ultrasound parameters in thyroid nodule and cancer diagnosis: A TIRADS-based evaluation. Front. Endocrinol. 2024, 15, 1417449. [Google Scholar] [CrossRef]
- Borlea, A.; Borcan, F.; Sporea, I.; Dehelean, C.A.; Negrea, R.; Cotoi, L.; Stoian, D. TI-RADS Diagnostic Performance: Which Algorithm Is Superior and How Elastography and 4D Vascularity Improve the Malignancy Risk Assessment. Diagnostics 2020, 10, 180. [Google Scholar] [CrossRef]
- Streinu, D.R.; Neagoe, O.C.; Borlea, A.; Icma, I.; Derban, M.; Stoian, D. Enhancing diagnostic precision in thyroid nodule assessment: Evaluating the efficacy of a novel cell preservation technique in fine-needle aspiration cytology. Front. Endocrinol. 2024, 15, 1438063. [Google Scholar] [CrossRef]
- Dumitru, C.S.; Ceausu, A.R.; Comsa, S.; Raica, M. Loss of E-Cadherin Expression Correlates With Ki-67 in Head and Neck Squamous Cell Carcinoma. In Vivo 2022, 36, 1150–1154. [Google Scholar] [CrossRef]
- Dumitru, C.S.; Raica, M. Vascular Endothelial Growth Factor Family and Head and Neck Squamous Cell Carcinoma. Anticancer Res. 2023, 43, 4315–4326. [Google Scholar] [CrossRef]
- Cosoroabă, R.M.; Gaje, N.P.; Ceauşu, A.R.; Dumitru, C.Ş.; Todor, L.; Popovici, R.A.; Porumb, A.; Domocoş, D.; Miron, M.I. The mast cell reaction in premalignant and malignant lesions of the head and neck. Rom. J. Morphol. Embryol. 2022, 63, 407–411. [Google Scholar] [CrossRef]
- Dumitru, C.S.; Raica, M. A Splice Form of VEGF, a Potential Anti-Angiogenetic Form of Head and Neck Squamous Cell Cancer Inhibition. Int. J. Mol. Sci. 2024, 25, 8855. [Google Scholar] [CrossRef]
- Dumitru, C.S.; Ceausu, A.R.; Gaje, N.P.; Suciu, C.S.; Raica, M. Proliferating Lymphatic Endothelial Cells as a Prognostic Marker in Head and Neck Squamous Cell Carcinoma. Int. J. Mol. Sci. 2022, 23, 9793. [Google Scholar] [CrossRef] [PubMed]
- Mehran, L.; Delbari, N.; Amouzegar, A.; Hasheminia, M.; Tohidi, M.; Azizi, F. Reduced Sensitivity to Thyroid Hormone Is Associated with Diabetes and Hypertension. J. Clin. Endocrinol. Metab. 2022, 107, 167–176. [Google Scholar] [CrossRef]
- Schiller, A.; Gadalean, F.; Schiller, O.; Timar, R.; Bob, F.; Munteanu, M.; Stoian, D.; Mihaescu, A.; Timar, B.; Seguro, A.C. Vitamin D deficiency—prognostic marker or mortality risk factor in end stage renal disease patients with diabetes mellitus treated with hemodialysis—A prospective multicenter study. PLoS ONE 2015, 10, e0126586. [Google Scholar] [CrossRef]
- Ginoudis, A.; Ioannidou, S.; Tsakiroglou, G.; Kazeli, K.; Vagdatli, E.; Lymperaki, E. Correlation of Albumin, Red Cell Distribution Width and Other Biochemical and Hematological Parameters with Glycated Hemoglobin in Diabetic, Prediabetic and Non-Diabetic Patients. Int. J. Mol. Sci. 2024, 25, 8037. [Google Scholar] [CrossRef]
- National Guideline Centre (UK). Thyroid Function tests: Thyroid Disease: Assessment and Management; National Institute for Health and Care Excellence (NICE): London, UK, 2019. [Google Scholar]
- Bosma, M.; Du Puy, R.S.; Ballieux, B.E.P.B. Screening for thyroid dysfunction with free T4 instead of thyroid stimulating hormone (TSH) improves efficiency in older adults in primary care. Age Ageing 2022, 51, afac215. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
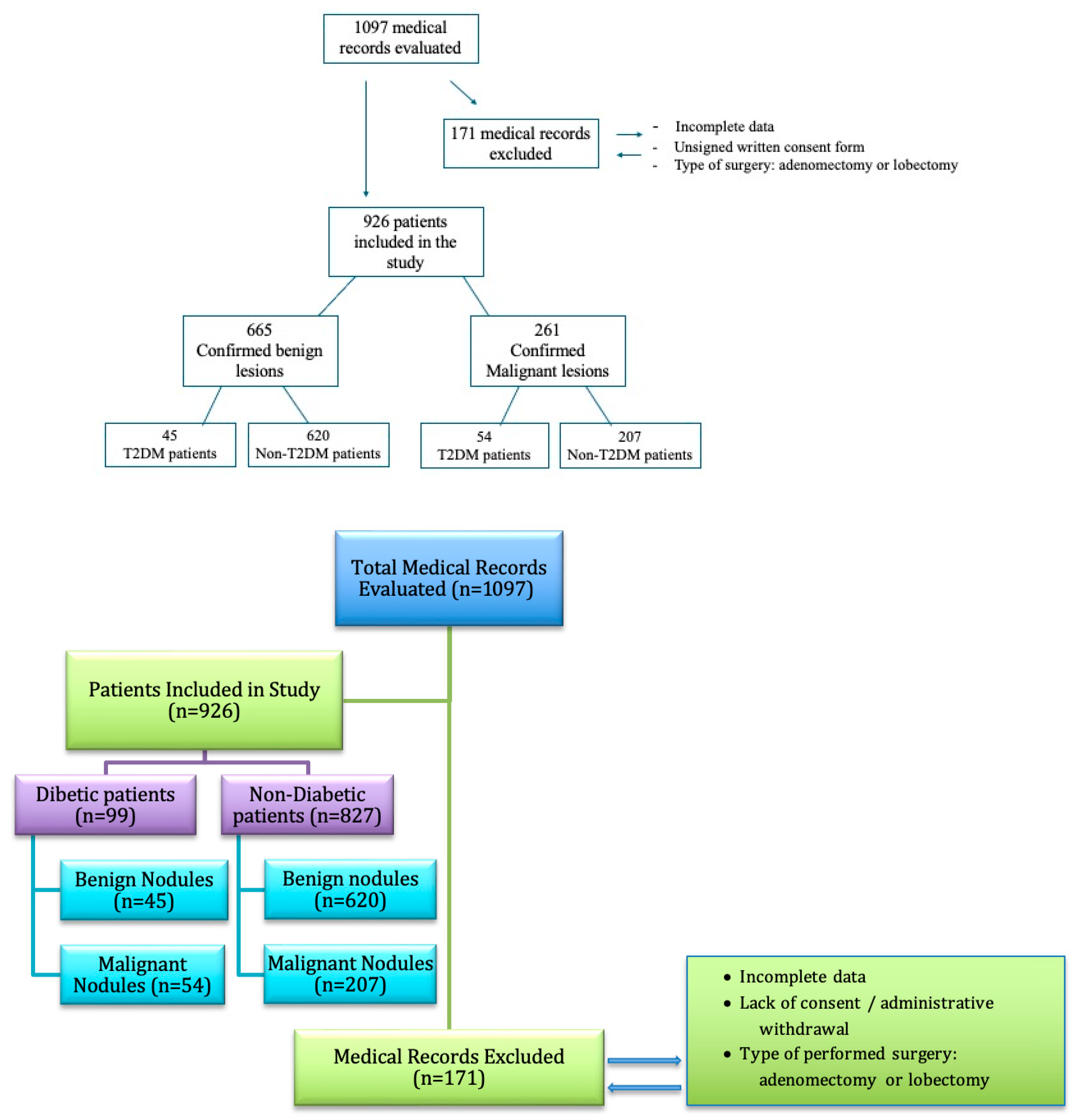
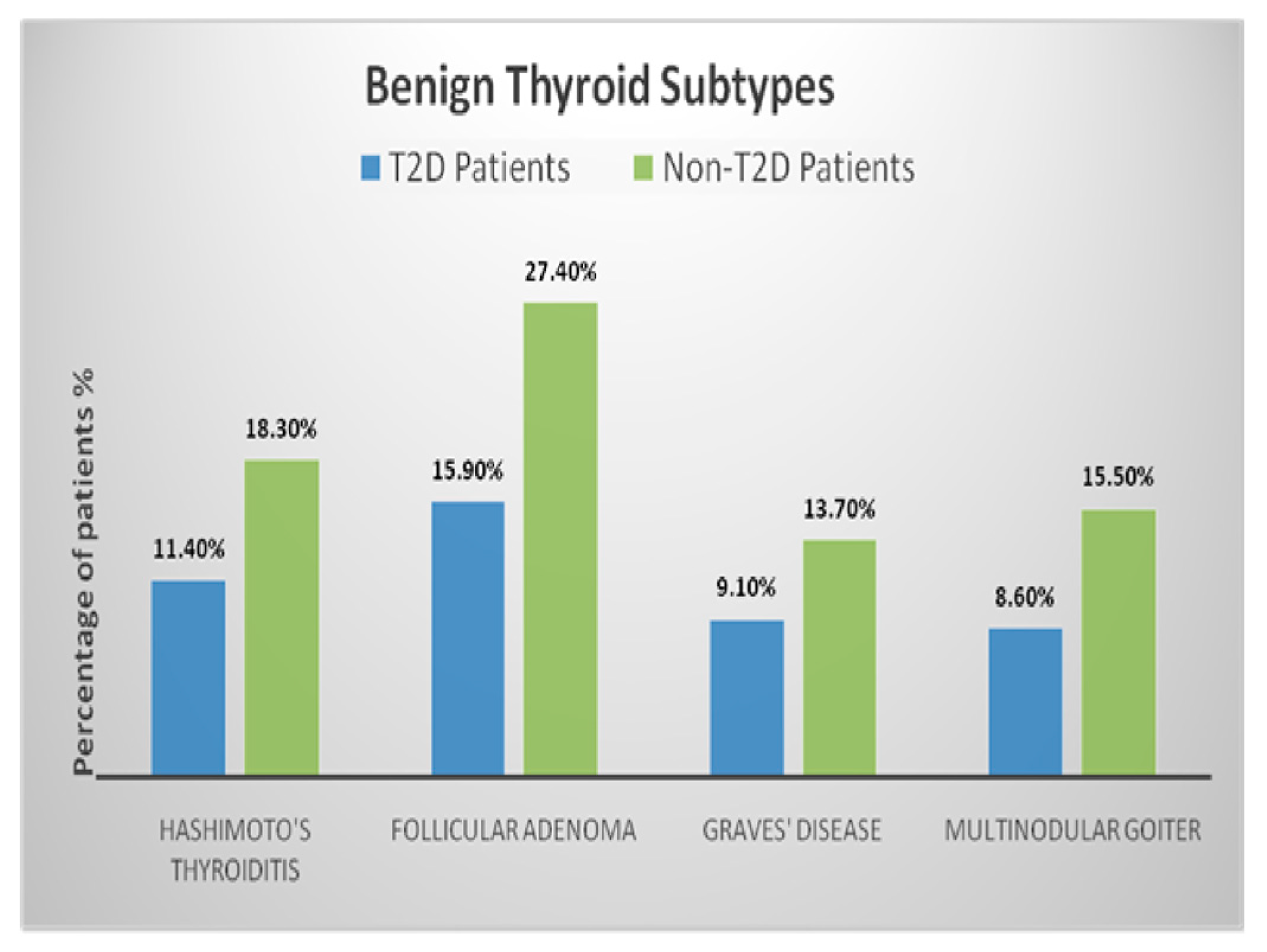
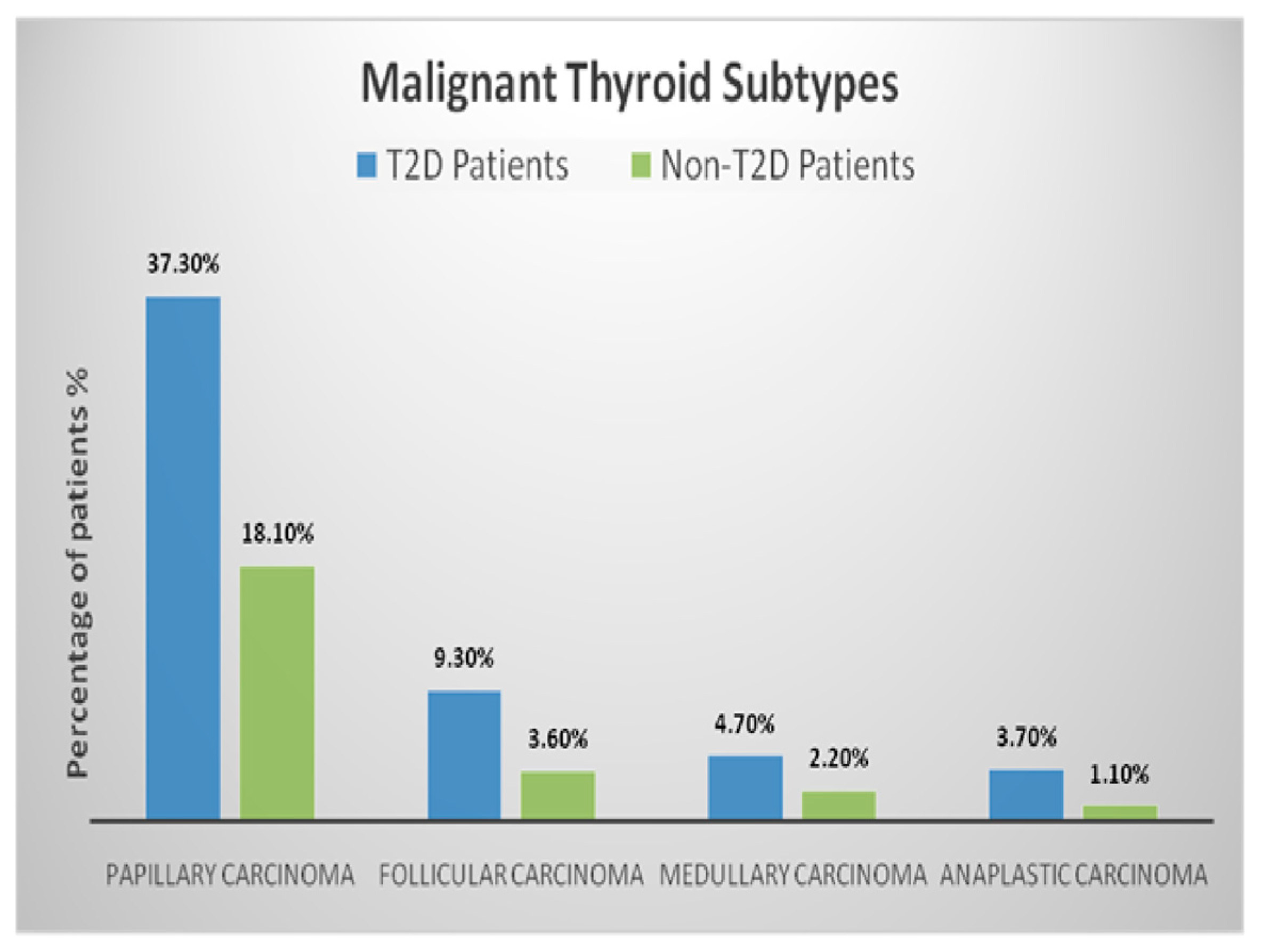
| Characteristic | T2D Patients n = 99 (Men ± SD/%) | Non-T2D Patients n = 827 (Men ± SD/%) | p-Value |
|---|---|---|---|
| Age (years) | 62.01 ± 9.04 | 54.31 ± 12.31 | <0.01 |
| Gender (F %) | 91.91 | 91.01 | 0.900 |
| Living Environment (Urban%) | 62.62% | 67.47 | 0.47 |
| BMI (kg/m2) | 31 ± 5 | 25 ± 4 | <0.01 |
| Normal Weight % | 10 | 40 | 0.01 |
| Overweight % | 30 | 35 | |
| Obesity Class I % | 25 | 15 | |
| Obesity Class II % | 20 | 8 | |
| Obesity Class III % | 15 | 2 | |
| Glycemia (mg/dL) | 140 ± 30 | 95 ± 10 | <0.01 |
| HbA1c % | 8.5 ± 1.2 | 5.5 ± 0.5 | <0.01 |
| Creatinine (mg/dL) | 1.2 ± 0.3 | 1.0 ± 0.2 | 0.05 |
| FT3 (pmol/L) | 4.93 ± 0.92 | 5.04 ± 0.900 | 0.238 |
| FT4 (pmol/L) | 16.05 ± 2.71 | 14.78 ± 3.868 | <0.001 |
| TSH (mIU/L) | 1.62 ± 1.24 | 1.95 ± 2.69 | 0.032 |
| Parameter | T2D Patients n = 99 (Men ± SD) | Non-T2D Patients n = 827 (Men ± SD) | p-Value |
|---|---|---|---|
| RBC (×106/µL) | 4.65 ± 0.43 | 4.64 ± 0.40 | 0.862 |
| WBC (×103/µL) | 8.53 ± 1.98 | 7.57 ± 1.88 | 9.869 |
| Neutrophils (×103/µL) | 5.56 ± 1.65 | 4.78 ± 1.61 | 1.655 |
| Lymphocytes (×103/µL) | 2.25 ± 0.74 | 2.14 ± 0.73 | 0.146 |
| PLT (×103/µL) | 281.22 ± 69.37 | 269.36 ± 65.72 | 0.108 |
| ESR (mm/h) | 28.94 ± 10.89 | 20.96 ± 6.71 | 4.528 |
| Fibrinogen (mg/dL) | 377.61 ± 87.99 | 354.78 ± 88.15 | 0.016 |
| Characteristic | T2D Patients (%) n = 99 | Non-T2D Patients (%) n = 827 | Odds Ratio (95% CI) | p-Value |
|---|---|---|---|---|
| Malignant Nodules | 55% (n = 54) | 25% (n = 207) | 3.59 (2.35–5.50) | 0.001 |
| Test | Original p-Value | Holm–Bonferroni-Adjusted p-Value |
|---|---|---|
| Age (years) | <0.01 | 0.05 |
| BMI (kg/m2) | <0.01 | 0.05 |
| HbA1c % | <0.01 | 0.05 |
| Glycemia | <0.01 | 0.05 |
| Creatinine (mg/dL) | 0.05 | 0.25 |
| FT4 | <0.001 | 0.01 |
| TSH | 0.032 | 0.16 |
| Fibrinogen (mg/dL) | 0.016 | 0.08 |
| Benign Nodules | 0.03 | 0.15 |
| Malignant Nodules | 0.001 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matei, M.; Matei, S.-C.; Petrașcu, F.-M.; Golu, I.; Balaş, M.; Amzăr, D.; Ungureanu, A.-M.; Natarâş, B.R.; Vlad, M.M. Type 2 Diabetes Mellitus in Patients with Different Types of Thyroid Nodular Lesions Among Western Romanian Patients: A Comprehensive Clinical, Biochemical, and Hormonal Analysis. Medicina 2025, 61, 1270. https://doi.org/10.3390/medicina61071270
Matei M, Matei S-C, Petrașcu F-M, Golu I, Balaş M, Amzăr D, Ungureanu A-M, Natarâş BR, Vlad MM. Type 2 Diabetes Mellitus in Patients with Different Types of Thyroid Nodular Lesions Among Western Romanian Patients: A Comprehensive Clinical, Biochemical, and Hormonal Analysis. Medicina. 2025; 61(7):1270. https://doi.org/10.3390/medicina61071270
Chicago/Turabian StyleMatei, Mervat, Sergiu-Ciprian Matei, Flavia-Medana Petrașcu, Ioana Golu, Melania Balaş, Daniela Amzăr, Ana-Maria Ungureanu, Bianca Roxana Natarâş, and Mihaela Maria Vlad. 2025. "Type 2 Diabetes Mellitus in Patients with Different Types of Thyroid Nodular Lesions Among Western Romanian Patients: A Comprehensive Clinical, Biochemical, and Hormonal Analysis" Medicina 61, no. 7: 1270. https://doi.org/10.3390/medicina61071270
APA StyleMatei, M., Matei, S.-C., Petrașcu, F.-M., Golu, I., Balaş, M., Amzăr, D., Ungureanu, A.-M., Natarâş, B. R., & Vlad, M. M. (2025). Type 2 Diabetes Mellitus in Patients with Different Types of Thyroid Nodular Lesions Among Western Romanian Patients: A Comprehensive Clinical, Biochemical, and Hormonal Analysis. Medicina, 61(7), 1270. https://doi.org/10.3390/medicina61071270







