Real-World Sex Differences in Response to Treatment with Glucagon-like Peptide-1 Receptor Agonists: Analysis of Single-Center Outpatient Case Series
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
3.1. Participant Characteristics
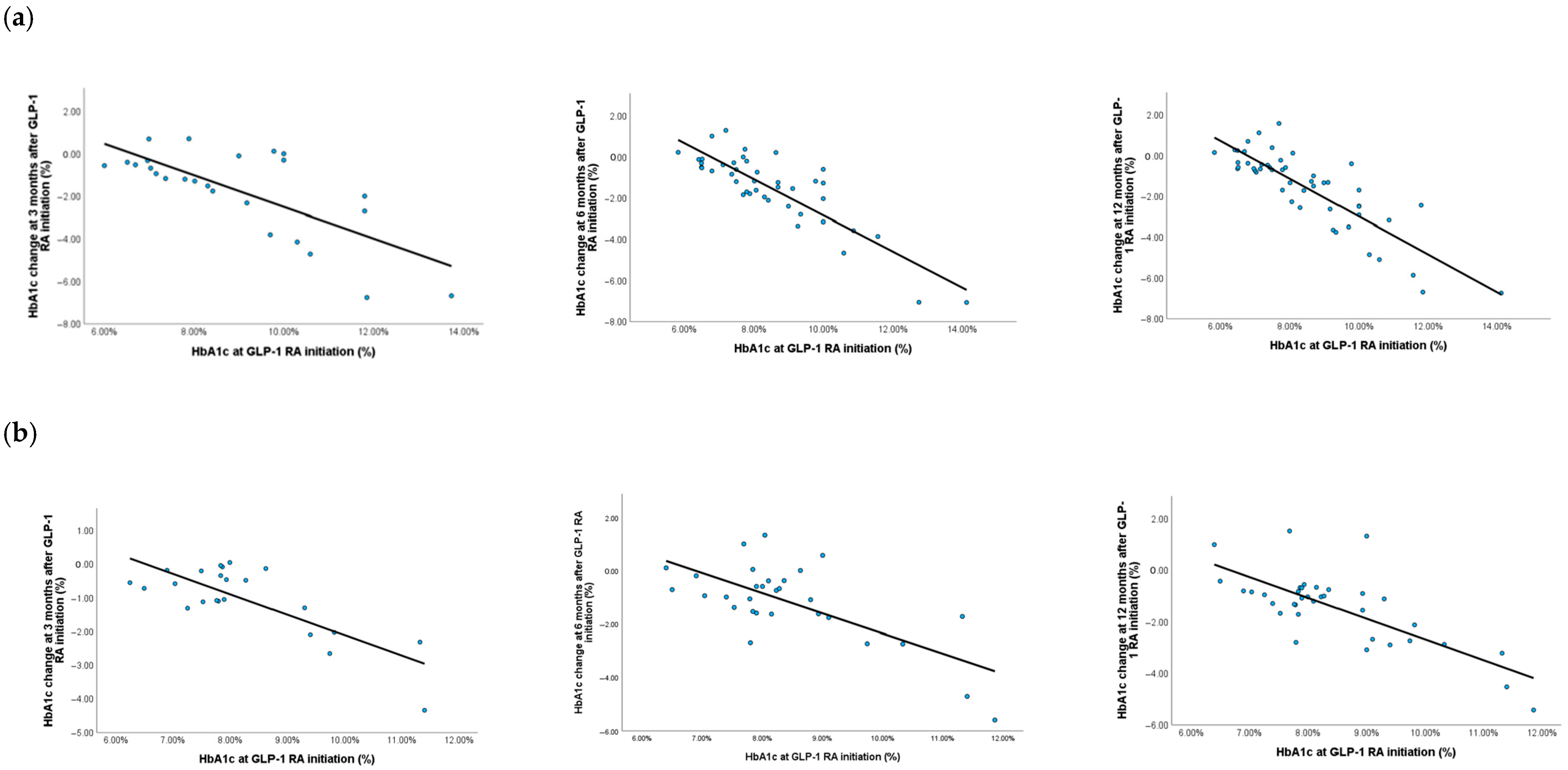
3.2. Efficacy in Improving Glycemic Control
3.3. Weight Loss Efficacy
3.4. Early Responders and Non-Responders
3.5. Adverse Drug Reactions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| T2D | Type 2 diabetes |
| GLP-1 RAs | Glucagon-like peptide-1 receptor agonists |
| SGLT2 | Sodium–glucose transporters 2 |
| MACE | Major adverse cardiovascular event |
| HbA1c | Hemoglobin A1c |
| BMI | Body mass index |
| CKD | Chronic kidney disease |
| PAD | Peripheral arterial disease |
| CAD | Coronary artery disease |
| OADs | Oral antihyperglycemic drugs |
References
- GBD 2021 Diabetes Collaborators. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee. 4. Comprehensive Medical Evaluation and Assessment of Comorbidities: Standards of Care in Diabetes-2025. Diabetes Care 2025, 48, S59–S85. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J.; Aroda, V.R.; Collins, B.S.; Gabbay, R.A.; Green, J.; Maruthur, N.M.; Rosas, S.E.; Del Prato, S.; Mathieu, C.; Mingrone, G.; et al. Management of Hyperglycemia in Type 2 Diabetes, 2022. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2022, 45, 2753–2786. [Google Scholar] [CrossRef] [PubMed]
- Buse, J.B.; Wexler, D.J.; Tsapas, A.; Rossing, P.; Mingrone, G.; Mathieu, C.; D’Alessio, D.A.; Davies, M.J. 2019 update to: Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European association for the study of diabetes (EASD). Diabetes Care 2019, 43, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Meier, J.J. Incretin hormones: Their role in health and disease. Diabetes Obes. Metab. 2018, 20, 5–21. [Google Scholar] [CrossRef] [PubMed]
- Wilding, J.P.H.; Batterham, R.L.; Calanna, S.; Davies, M.; Van Gaal, L.F.; Lingvay, I.; McGowan, B.M.; Rosenstock, J.; Tran, M.T.D.; Wadden, T.A.; et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N. Engl. J. Med. 2021, 384, 989–1002. [Google Scholar] [CrossRef] [PubMed]
- Jendle, J.; Grunberger, G.; Blevins, T.; Giorgino, F.; Hietpas, R.T.; Botros, F.T. Efficacy and safety of dulaglutide in the treatment of type 2 diabetes: A comprehensive review of the dulaglutide clinical data focusing on the AWARD phase 3 clinical trial program. Diabetes Metab. Res. Rev. 2016, 32, 776–790. [Google Scholar] [CrossRef] [PubMed]
- Rivera, F.B.; Cruz, L.L.A.; Magalong, J.V.; Ruyeras, J.M.M.J.; Aparece, J.P.; Bantayan, N.R.B.; Breitinger, K.L.; Gulati, M. Cardiovascular and renal outcomes of glucagon-like peptide 1 receptor agonists among patients with and without type 2 diabetes mellitus: A meta-analysis of randomized placebo-controlled trials. Am. J. Prev. Cardiol. 2024, 18, 100679. [Google Scholar] [CrossRef] [PubMed]
- Wysham, C.; Blevins, T.; Arakaki, R.; Colon, G.; Garcia, P.; Atisso, C.; Kuhstoss, D.; Lakshmanan, M. Efficacy and safety of dulaglutide added onto pioglitazone and metformin versus exenatide in type 2 diabetes in a randomized controlled trial (AWARD-1). Diabetes Care 2014, 3, 2159–2167. [Google Scholar] [CrossRef] [PubMed]
- Capehorn, M.S.; Catarig, A.M.; Furberg, J.K.; Janez, A.; Price, H.C.; Tadayon, S.; Vergès, B.; Marre, M. Efficacy and safety of once-weekly semaglutide 1.0mg vs once-daily liraglutide 1.2 mg as add-on to 1–3 oral antidiabetic drugs in subjects with type 2 diabetes (SUSTAIN 10). Diabetes Metab. 2020, 46, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Lombrea, A.; Romanescu, M.; Jianu, N.; Andor, M.; Suciu, M.; Man, D.E.; Danciu, C.; Dehelean, C.A.; Buda, V. Sex-Related Differences in the Pharmacological Response in, S.A.;RS-CoV-2 Infection, Dyslipidemia, and Diabetes Mellitus: A Narrative Review. Pharmaceuticals 2023, 16, 853. [Google Scholar] [CrossRef] [PubMed]
- Rentzeperi, E.; Pegiou, S.; Koufakis, T.; Grammatiki, M.; Kotsa, K. Sex Differences in Response to Treatment with Glucagon-like Peptide 1 Receptor Agonists: Opportunities for a Tailored Approach to Diabetes and Obesity Care. J. Pers. Med. 2022, 12, 454. [Google Scholar] [CrossRef] [PubMed]
- Pencek, R.; Blickensderfer, A.; Li, Y.; Brunell, S.C.; Anderson, P.W. Exenatide Twice Daily: Analysis of Effectiveness and Safety Data Stratified by Age, Sex, Race, Duration of Diabetes, and Body Mass Index. Postgrad. Med. 2012, 124, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Gallwitz, B.; Dagogo-Jack, S.; Thieu, V.; Garcia-Perez, L.E.; Pavo, I.; Yu, M.; Robertson, K.E.; Zhang, N.; Giorgino, F. Effect of once-weekly dulaglutide on glycated haemoglobin (HbA1c) and fasting blood glucose in patient subpopulations by gender, duration of diabetes and baseline HbA1c. Diabetes Obes. Metab. 2018, 20, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Hemmer, A.; Maiter, D.; Buysschaert, M.; Preumont, V. Long-term effects of, GLP-1 receptor agonists in type 2 diabetic patients: A retrospective real-life study in 131 patients. Diabetes Metab. Syndr. 2019, 13, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Durden, E.; Lenhart, G.; Lopez-Gonzalez, L.; Hammer, M.; Langer, J. Predictors of glycemic control and diabetes-related costs among type 2 diabetes patients initiating therapy with liraglutide in the United States. J. Med. Econ. 2016, 19, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Onishi, Y.; Oura, T.; Matsui, A.; Matsuura, J.; Iwamoto, N. Analysis of efficacy and safety of dulaglutide 0.75 mg stratified by sex in patients with type 2 diabetes in 2 randomized, controlled phase 3 studies in Japan. Endocr. J. 2017, 64, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Gorgojo-Martínez, J.J.; Mezquita-Raya, P.; Carretero-Gómez, J.; Castro, A.; Cebrián-Cuenca, A.; de Torres-Sánchez, A.; García-de-Lucas, M.D.; Núñez, J.; Obaya, J.C.; Soler, M.J.; et al. Clinical Recommendations to Manage Gastrointestinal Adverse Events in Patients Treated with Glp-1 Receptor Agonists: A Multidisciplinary Expert Consensus. J. Clin. Med. 2022, 12, 145. [Google Scholar] [CrossRef] [PubMed]
- Joung, K.I.; Jung, G.W.; Park, H.H.; Lee, H.; Park, S.H.; Shin, J.Y. Gender differences in adverse event reports associated with antidiabetic drugs. Sci. Rep. 2020, 10, 17545. [Google Scholar] [CrossRef] [PubMed]
- Petri, K.C.C.; Ingwersen, S.H.; Flint, A.; Zacho, J.; Overgaard, R.V. Exposure-response analysis for evaluation of semaglutide dose levels in type 2 diabetes. Diabetes Obes. Metab. 2018, 20, 2238–2245. [Google Scholar] [CrossRef] [PubMed]
- Gudzune, K.; Lingvay, I.; Cao, D.; Mojdami, D.; Stefanski, A.; Mast, C.; Lee, M. #1696673 Weight reduction over time in tirzepatide-treated participants by early weight loss response—Post hoc analysis in RMOUNT-1. Endocr Pract. 2024, 30, S66. [Google Scholar]
- National Institute for Health and Care Excellence (NICE). NICE Guideline: Type 2 Diabetes in Adults: Management. Available online: https://www.nice.org.uk/guidance/ng28/resources/type-2-diabetes-in-adults-management-pdf-1837338615493 (accessed on 10 July 2025).
- Brown, E.; Wilding, J.P.H.; Barber, T.M.; Alam, U.; Cuthbertson, D.J. Weight loss variability with SGLT2 inhibitors and, GLP-1 receptor agonists in type 2 diabetes mellitus and obesity: Mechanistic possibilities. Obes. Rev. 2019, 20, 816–828. [Google Scholar] [CrossRef] [PubMed]
- Franch-Nadal, J.; Granado-Casas, M.; Mata-Cases, M.; Ortega, E.; Vlacho, B.; Mauricio, D. Determinants of response to the glucagon-like peptide-1 receptor agonists in a type 2 diabetes population in the real-world. Prim. Care Diabetes 2022, 16, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Fowler, M.J.; Wells, Q.S.; Stafford, J.M.; Gannon, M. Predicting responsiveness to GLP-1 pathway drugs using real-world data. BMC Endocr. Disord. 2024, 24, 269. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Yang, J.; Huang, J.; Xu, L.; Lv, Y.; Wang, Y.; Ren, J.; Feng, Y.; Zheng, Q.; Li, L. Comparative efficacy and safety of, GLP-1 receptor agonists for weight reduction: A model-based meta-analysis of placebo-controlled trials. Obes. Pillars 2025, 13, 100162. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.S.; Frandsen, C.S.; Fleischer, J.; Vistisen, D.; Holst, J.J.; Tarnow, L.; Knop, F.K.; Madsbad, S.; Andersen, H.U.; Dejgaard, T.F. Liraglutide-Induced Weight Loss May be Affected by Autonomic Regulation in Type 1 Diabetes. Front. Endocrinol. 2019, 10, 242. [Google Scholar] [CrossRef] [PubMed]
- Drucker, D.J. Prevention of cardiorenal complications in people with type 2 diabetes and obesity. Cell Metab. 2024, 36, 338–353. [Google Scholar] [CrossRef] [PubMed]
- Lingvay, I.; Sumithran, P.; Cohen, R.V.; le Roux, C.W. Obesity management as a primary treatment goal for type 2 diabetes: Time to reframe the conversation. Lancet 2022, 399, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Perkovic, V.; Tuttle, K.R.; Rossing, P.; Mahaffey, K.W.; Mann, J.F.E.; Bakris, G.; Baeres, F.M.M.; Idorn, T.; Bosch-Traberg, H.; Lausvig, N.L.; et al. Effects of Semaglutide on Chronic Kidney Disease in Patients with Type 2 Diabetes. N. Engl. J. Med. 2024, 391, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Tofé, S.; Argüelles, I.; Mena, E.; Serra, G.; Codina, M.; Urgeles, J.R.; García, H.; Pereg, V. Real-world, GLP-1 RA therapy in type 2 diabetes: A long-term effectiveness observational study. Endocrinol. Diabetes Metab. 2018, 2, e00051. [Google Scholar] [CrossRef] [PubMed]
- Romera, I.; Rubio-de Santos, M.; Artola, S.; Suárez Fernández, C.; Conget, I. GLP-1 RAs in Spain: A Short Narrative Review of Their Use in Real Clinical Practice. Adv. Ther. 2023, 40, 1418–1429. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Quast, D.R.; Wefers, J.; Meier, J.J. GLP-1 receptor agonists in the treatment of type 2 diabetes—State-of-the-art. Mol. Metab. 2021, 46, 101102. [Google Scholar] [CrossRef] [PubMed]
- Divino, V.; Boye, K.S.; Lebrec, J.; DeKoven, M.; Norrbacka, K. GLP-1 RA Treatment and Dosing Patterns Among Type 2 Diabetes Patients in Six Countries: A Retrospective Analysis of Pharmacy Claims Data. Diabetes Ther. 2019, 10, 1067–1088. [Google Scholar] [CrossRef] [PubMed]
- Piccini, S.; Favacchio, G.; Morenghi, E.; Mazziotti, G.; A Lania, A.G.; Mirani, M. Real-world sex differences in type 2 diabetes patients treated with GLP-1 receptor agonists. Diabetes Res. Clin. Pract. 2024, 212, 111689. [Google Scholar] [CrossRef] [PubMed]
- Alsaqaaby, M.S.; Cooney, S.; le Roux, C.W.; Pournaras, D.J. Sex, race, and BMI in clinical trials of medications for obesity over the past three decades: A systematic review. Lancet Diabetes Endocrinol. 2024, 12, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, J.; Zhang, S.; Zhu, D.; Deng, F.; Zuo, R.; Hu, Y.; Zhao, Y.; Duan, Y.; Lin, B.; et al. Real-world effectiveness of, GLP-1 receptor agonist-based treatment strategies on “time in range” in patients with type 2 diabetes. Front. Pharmacol. 2024, 15, 1370594. [Google Scholar] [CrossRef] [PubMed]
- Aroda, V.R.; Bauer, R.; Christiansen, E.; Haluzík, M.; Kallenbach, K.; Montanya, E.; Rosenstock, J.; Meier, J.J. Efficacy and safety of oral semaglutide by subgroups of patient characteristics in the PIONEER phase 3 programme. Diabetes Obes. Metab. 2022, 24, 1338–1350. [Google Scholar] [CrossRef] [PubMed]
- Jensterle, M.; Rizzo, M.; Janež, A. Semaglutide in Obesity: Unmet Needs in Men. Diabetes Ther. 2023, 14, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Overgaard, R.V.; Petri, K.C.; Jacobsen, L.V.; Jensen, C.B. Liraglutide 3.0 mg for Weight Management: A Population Pharmacokinetic Analysis. Clin. Pharmacokinet. 2016, 55, 1413–1422. [Google Scholar] [CrossRef] [PubMed]
- Abd El Aziz, M.S.; Kahle, M.; Meier, J.J.; Nauck, M.A. A meta-analysis comparing clinical effects of short- or long-acting, GLP-1 receptor agonists versus insulin treatment from head-to-head studies in type 2 diabetic patients. Diabetes Obes. Metab. 2017, 19, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Dawed, A.Y.; Mari, A.; Brown, A.; McDonald, T.J.; Li, L.; Wang, S.; Hong, M.G.; Sharma, S.; Robertson, N.R.; Mahajan, A.; et al. Pharmacogenomics of, GLP-1 receptor agonists: A genome-wide analysis of observational data and large randomised controlled trials. Lancet Diabetes Endocrinol. 2023, 11, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Durden, E.; Liang, M.; Fowler, R.; Panton, U.H.; Mocevic, E. The Effect of Early Response to, GLP-1 RA Therapy on Long-Term Adherence and Persistence Among Type 2 Diabetes Patients in the United States. J. Manag. Care Spec. Pharm. 2019, 25, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Schlögl, H.; Kabisch, S.; Horstmann, A.; Lohmann, G.; Müller, K.; Lepsien, J.; Busse-Voigt, F.; Kratzsch, J.; Pleger, B.; Villringer, A.; et al. Exenatide-induced reduction in energy intake is associated with increase in hypothalamic connectivity. Diabetes Care 2013, 36, 1933–1940. [Google Scholar] [CrossRef] [PubMed]
- White, G.E.; Shu, I.; Rometo, D.; Arnold, J.; Korytkowski, M.; Luo, J. Real-world weight-loss effectiveness of glucagon-like peptide-1 agonists among patients with type 2 diabetes: A retrospective cohort study. Obesity 2023, 31, 537–544. [Google Scholar] [CrossRef] [PubMed]



| Total Sample n = 114 | Women n = 47 | Men n = 67 | p-Value | |
|---|---|---|---|---|
| Area of residence (urban) n (%) | 93 (81.6%) | 38 (80.9%) | 55 (82.1%) | 0.867 |
| Age at GLP-1 RA initiation, years | 60.4 ± 10.1 | 60.8 ± 10.0 | 60.2 ± 10.2 | 0.760 |
| Body mass index at GLP-1 RA initiation, kg/m2 | 35.1 ± 6.0 | 36.4 ± 5.6 | 34.1 ± 6.1 | 0.060 |
| Diabetes duration, years | 8.9 ± 6.7 | 12.3 ± 6.2 | 10.0 ± 7.5 | 0.087 |
| HbA1c at GLP-1 RA initiation, % | 8.5 ± 1.7 | 8.4 ± 1.3 | 8.6 ± 1.9 | 0.595 |
| Retinopathy, n (%) | 13 (11.4%) | 6 (12.8%) | 7 (10.4%) | 0.346 |
| Diabetic neuropathy, n (%) | 49 (43.0%) | 22 (46.8%) | 27 (40.3%) | 0.760 |
| Chronic kidney disease, n (%) | 19 (16.7%) | 2 (4.3%) | 17 (25.4%) | 0.007 |
| Stroke, n (%) | 7 (6.1%) | 3 (6.4%) | 4 (6.0%) | 0.916 |
| Peripheral artery disease, n (%) | 36 (31.6%) | 9 (19.1%) | 27 (40.3%) | 0.053 |
| Coronary artery disease, n (%) | 49 (43.0%) | 19 (40.4%) | 30 (44.8%) | 0.898 |
| Type of GLP-1 RA at initiation | ||||
| Exenatide, n (%) | 5 (4.4%) | 2 (4.3%) | 3 (4.5) | 0.987 |
| Semaglutide, n (%) | 78 (68.4%) | 31 (66.0%) | 47 (70.1%) | |
| Lixisenatide, n (%) | 2 (1.8%) | 1 (2.1%) | 1 (1.5%) | |
| Dulaglutide, n (%) | 25 (21.9%) | 11 (23.4%) | 14 (20.9) | |
| Liraglutide, n (%) | 4 (3.5%) | 2 (4.3%) | 2 (3.0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inceu, G.V.; Crăciun, A.-E.; Ciobanu, D.M.; Berchisan, A.; Fodor, A.; Bala, C.; Roman, G.; Rusu, A. Real-World Sex Differences in Response to Treatment with Glucagon-like Peptide-1 Receptor Agonists: Analysis of Single-Center Outpatient Case Series. Medicina 2025, 61, 1343. https://doi.org/10.3390/medicina61081343
Inceu GV, Crăciun A-E, Ciobanu DM, Berchisan A, Fodor A, Bala C, Roman G, Rusu A. Real-World Sex Differences in Response to Treatment with Glucagon-like Peptide-1 Receptor Agonists: Analysis of Single-Center Outpatient Case Series. Medicina. 2025; 61(8):1343. https://doi.org/10.3390/medicina61081343
Chicago/Turabian StyleInceu, Georgeta Victoria, Anca-Elena Crăciun, Dana Mihaela Ciobanu, Antonia Berchisan, Adriana Fodor, Cornelia Bala, Gabriela Roman, and Adriana Rusu. 2025. "Real-World Sex Differences in Response to Treatment with Glucagon-like Peptide-1 Receptor Agonists: Analysis of Single-Center Outpatient Case Series" Medicina 61, no. 8: 1343. https://doi.org/10.3390/medicina61081343
APA StyleInceu, G. V., Crăciun, A.-E., Ciobanu, D. M., Berchisan, A., Fodor, A., Bala, C., Roman, G., & Rusu, A. (2025). Real-World Sex Differences in Response to Treatment with Glucagon-like Peptide-1 Receptor Agonists: Analysis of Single-Center Outpatient Case Series. Medicina, 61(8), 1343. https://doi.org/10.3390/medicina61081343






