Impact of Low- Versus Standard-Pressure Pneumoperitoneum on Postoperative Recovery in Patients with Obesity Undergoing Robot-Assisted Radical Prostatectomy: A Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMI | Body mass index |
| ERAS | Enhanced recovery after surgery |
| MCID | Minimal clinically important difference |
| ISUP | International Society of Urological Pathology. |
| QOR | Quality of recovery |
| POD | Postoperative day |
| PP | Pneumoperitoneum |
| PSA | Prostate specific antigen |
| RARP | Robot-assisted radical prostatectomy |
| SWS | Surgical workspace |
References
- Mottet, N.; van den Bergh, R.C.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer—2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2021, 79, 243–262. [Google Scholar] [CrossRef]
- Neudecker, J.; Sauerland, S.; Neugebauer, E.; Bergamaschi, R.; Bonjer, H.J.; Cuschieri, A.; Fuchs, K.-H.; Jacobi, C.; Jansen, F.W.; Koivusalo, A.-M.; et al. The European Association for Endoscopic Surgery clinical practice guideline on the pneumoperitoneum for laparoscopic surgery. Surg. Endosc. 2002, 16, 1121–1143. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Lagergren, J. Clinical management of obese patients with cancer. Nat. Rev. Clin. Oncol. 2013, 10, 519–533. [Google Scholar] [CrossRef] [PubMed]
- Jaber, A.R.; Saikali, S.; Moschovas, M.C.; Gamal, A.; Patel, E.; Sandri, M.; Rogers, T.; Patel, V. Impact of Class III Obesity (Morbid Obesity) on the Perioperative, Functional, and Oncological Outcomes of Robotic-Assisted Radical Prostatectomy. Cancers 2025, 17, 709. [Google Scholar] [CrossRef] [PubMed]
- Barone, B.; Amicuzi, U.; Massanova, M.; Napolitano, L.; Reccia, P.; Mirto, B.F.; Balsamo, R.; Del Giudice, F.; Ferro, M.; Busetto, G.M.; et al. The Correlation Between Body Mass Index and Prostate Volume: A Retrospective Analysis of Pre and Postoperative Measurements in Prostate Cancer Patients. Prostate 2025, 85, 433–442. [Google Scholar] [CrossRef]
- Alhusseinawi, H.; Sander, L.; Rosenvinge, P.M.; Jensen, S.L.; Bruun, N.H.; Kingo, P.S.; Jensen, J.B.; Rasmussen, S. Low- versus standard-pneumoperitoneum in patients undergoing robot-assisted radical prostatectomy: A randomised, triple-blinded study. BJU Int. 2023, 132, 560–567. [Google Scholar] [CrossRef]
- Stark, P.A.; Myles, P.S.; Burke, J.A. Development and psychometric evaluation of a postoperative quality of recovery score: The QoR-15. Anesthesiology 2013, 118, 1332–1340. [Google Scholar] [CrossRef]
- Kehlet, H.; Wilmore, D.W. Evidence-based surgical care and the evolution of fast-track surgery. Ann. Surg. 2008, 248, 189–198. [Google Scholar] [CrossRef]
- Brokelman, W.J.A.; Lensvelt, M.; Rinkes, I.H.M.B.; Klinkenbijl, J.H.G.; Reijnen, M.M.P.I. Peritoneal changes due to laparoscopic surgery. Surg. Endosc. 2011, 25, 1–9. [Google Scholar] [CrossRef]
- Ng, C.S.; Whelan, R.L.; Lacy, A.M.; Yim, A.P. Is minimal access surgery for cancer associated with immunologic benefits? World J. Surg. 2005, 29, 975–981. [Google Scholar] [CrossRef]
- Albers, K.I.; Polat, F.; Helder, L.; Panhuizen, I.F.; Snoeck, M.M.; Polle, S.B.W.; de Vries, H.; Dias, E.M.; Slooter, G.D.; de Boer, H.D.; et al. Quality of recovery and innate immune homeostasis in patients undergoing low-pressure versus standard-pressure pneumoperitoneum during laparoscopic colorectal surgery (RECOVER): A randomized controlled trial. Ann. Surg. 2022, 276, 664–673. [Google Scholar] [CrossRef]
- Kleif, J.; Edwards, H.M.; Sort, R.; Vilandt, J.; Gögenur, I. Translation and validation of the Danish version of the postoperative quality of recovery score QoR-15. Acta Anaesthesiol. Scand. 2015, 59, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Ferroni, M.C.; Abaza, R. Feasibility of robot-assisted prostatectomy performed at ultra-low pneumoperitoneum pressure of 6 mmHg and comparison of clinical outcomes vs standard pressure of 15 mmHg. BJU Int. 2019, 124, 308–313. [Google Scholar] [CrossRef]
- Baltayian, S. A brief review: Anesthesia for robotic prostatectomy. J. Robot. Surg. 2008, 2, 59–66. [Google Scholar] [CrossRef]
- El-Taji, O.; Howell-Etienne, J.; Taktak, S.; Hanchanale, V. Lower vs standard pressure pneumoperitoneum in robotic-assisted radical prostatectomy: A systematic review and meta-analysis. J. Robot. Surg. 2023, 17, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Dexter, S.P.; Vucevic, M.; Gibson, J.; McMahon, M.J. Hemodynamic consequences of high- and low-pressure capnoperitoneum during laparoscopic cholecystectomy. Surg. Endosc. 1999, 13, 376–381. [Google Scholar] [CrossRef]
- Rohloff, M.; Peifer, G.; Shakuri-Rad, J.; Maatman, T.J. The impact of low pressure pneumoperitoneum in robotic assisted radical prostatectomy: A prospective, randomized, double blinded trial. World J. Urol. 2021, 39, 2469–2474. [Google Scholar] [CrossRef]
- Ficarra, V.; Novara, G.; Rosen, R.C.; Artibani, W.; Carroll, P.R.; Costello, A.; Menon, M.; Montorsi, F.; Patel, V.R.; Stolzenburg, J.-U.; et al. Systematic review and meta-analysis of studies reporting urinary continence recovery after robot-assisted radical prostatectomy. Eur. Urol. 2012, 62, 405–417. [Google Scholar] [CrossRef]
- Abaza, R.; Ferroni, M.C. Randomized trial of ultralow vs standard pneumoperitoneum during robotic prostatectomy. J. Urol. 2022, 208, 626–632. [Google Scholar] [CrossRef]
- Huynh, L.M.; Ahlering, T.E. Robot-assisted radical prostatectomy: A step-by-step guide. J. Endourol. 2018, 32, S28–S32. [Google Scholar] [CrossRef] [PubMed]
- Vickers, A.J.; Savage, C.J.; Hruza, M.; Tuerk, I.; Koenig, P.; Martínez-Piñeiro, L.; Janetschek, G.; Guillonneau, B. The surgical learning curve for laparoscopic radical prostatectomy: A retrospective cohort study. Lancet Oncol. 2009, 10, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Dell’oGlio, P.; van Willigen, D.M.; van Oosterom, M.N.; Bauwens, K.; Hensbergen, F.; Welling, M.M.; van der Stadt, H.; Bekers, E.; Pool, M.; van Leeuwen, P.; et al. Feasibility of fluorescence imaging at microdosing using a hybrid PSMA tracer during robot-assisted radical prostatectomy in a large animal model. EJNMMI Res. 2022, 12, 14. [Google Scholar] [CrossRef]
- Claps, F.; De Pablos-Rodríguez, P.; Gómez-Ferrer, Á.; Mascarós, J.M.; Marenco, J.; Collado Serra, A.; Ramón-Borja, J.C.; Fons, A.C.; Trombetta, C.; Rubio-Briones, J.; et al. Free-indocyanine green-guided pelvic lymph node dissection during radical prostatectomy. Urol. Oncol. Semin. Orig. Investig. 2022, 40, 489.e19–489.e26. [Google Scholar] [CrossRef]
- Tewari, A.; Sooriakumaran, P.; Bloch, D.A.; Seshadri-Kreaden, U.; Hebert, A.E.; Wiklund, P. Positive surgical margin and perioperative complication rates of primary surgical treatments for prostate cancer: A systematic review and meta-analysis comparing retropubic, laparoscopic and robotic prostatectomy. Eur. Urol. 2012, 62, 1–15. [Google Scholar] [CrossRef]
- Novara, G.; Ficarra, V.; Mocellin, S.; Ahlering, T.E.; Carroll, P.R.; Graefen, M.; Guazzoni, G.; Menon, M.; Patel, V.R.; Shariat, S.F.; et al. Systematic review and meta-analysis of studies reporting oncologic outcome after robot-assisted radical prostatectomy. Eur. Urol. 2012, 62, 382–404. [Google Scholar] [CrossRef]
- McSorley, S.T.; Watt, D.G.; Horgan, P.G.; McMillan, D.C. Postoperative systemic inflammatory response, complication severity, and survival following surgery for colorectal cancer. Ann. Surg. Oncol. 2016, 23, 2832–2840. [Google Scholar] [CrossRef] [PubMed]
- Rapisarda, S.; Bada, M.; Crocetto, F.; Barone, B.; Arcaniolo, D.; Polara, A.; Imbimbo, C.; Grosso, G. The role of multiparametric resonance and biopsy in prostate cancer detection: Comparison with definitive histological report after laparoscopic/robotic radical prostatectomy. Abdom. Radiol. 2020, 45, 4178–4184. [Google Scholar] [CrossRef]
- Veldkamp, R.; Kuhry, E.; Hop, W.C.J.; Jeekel, J.; Kazemier, G.; Bonjer, H.J.; Haglind, E.; Påhlman, L.; Cuesta, M.A.; Msika, S.; et al. Laparoscopic surgery versus open surgery for colon cancer: Short-term outcomes of a randomised trial. Lancet Oncol. 2005, 6, 477–484. [Google Scholar] [CrossRef]
- Yaxley, J.W.; Coughlin, G.D.; Chambers, S.K.; Occhipinti, S.; Samaratunga, H.; Zajdlewicz, L.; Dunglison, N.; Carter, R.; Williams, S.; Payton, D.J.; et al. Robot-assisted radical prostatectomy versus open radical retropubic prostatectomy: Early outcomes from randomized controlled phase 3 study. Lancet Oncol. 2016, 388, 1057–1066. [Google Scholar] [CrossRef]
- Martinez-Pérez, A.; Carra, M.C.; Brunetti, F.; de’Angelis, N. Short-term clinical outcomes of laparoscopic vs open rectal excision for rectal cancer: A systematic review and meta-analysis. World J. Gastroenterol. 2017, 23, 7906–7916. [Google Scholar] [CrossRef] [PubMed]
- Myles, P.S.; Myles, D.B. An updated minimal clinically important difference for the QoR-15 scale. Anesthesiology 2021, 135, 934–935. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Ficarra, V.; Novara, G.; Ahlering, T.E.; Costello, A.; Eastham, J.A.; Graefen, M.; Guazzoni, G.; Menon, M.; Mottrie, A.; Patel, V.R.; et al. Systematic review and meta-analysis of studies reporting potency rates after robot-assisted radical prostatectomy. Eur. Urol. 2012, 62, 418–430. [Google Scholar] [CrossRef]
- Ahlering, T.E.; Skarecky, D.; Lee, D.; Clayman, R.V. Successful Transfer of Open Surgical Skills to a Laparoscopic Environment Using a Robotic Interface: Initial Experience with Laparoscopic Radical Prostatectomy. J. Urol. 2003, 170, 1738–1741. [Google Scholar] [CrossRef]
| Parameter | Standard-Pressure PP (n = 70) | Low-Pressure PP (n = 60) | p * |
|---|---|---|---|
| Age, years, mean ± SD | 63.39 ± 6.4 | 62.65 ± 5.97 | 0.502 |
| BMI, kg/m2, mean ± SD | 32.16 ± 1.33 | 33.32 ± 2.42 | 0.001 |
| PSA, ng/mL, mean ± SD | 7.98 ± 4.33 | 8.73 ± 4.98 | 0.359 |
| Prostate volume, mL, mean ± SD | 57.21 ± 33 | 58.5 ± 29.47 | 0.816 |
| Hypertension, n (%) | 30 (42.9) | 34 (56.7) | 0.116 |
| Diabetes mellitus, n (%) | 36 (51.4) | 29 (48.3) | 0.725 |
| Prior abdominal surgery, n (%) | 8 (11.4) | 10 (16.7) | 0.389 |
| Clinical T stage, n (%) | 0.273 | ||
| T2a | 28 (40.0) | 27 (45.0) | |
| T2b | 11 (15.7) | 4 (6.7) | |
| T2c | 31 (44.3) | 29 (48.3) | |
| Clinical ISUP grade, n (%) | 0.181 | ||
| 1 | 38 (54.3) | 42 (70.0) | |
| 2 | 8 (11.4) | 3 (5.0) | |
| 3 | 17 (24.3) | 13 (21.7) | |
| 4 | 7 (10.0) | 2 (3.3) |
| Category | Time Point | Standard-Pressure PP (Mean ± SD) | Low-Pressure PP (Mean ± SD) | Difference (95% CI) | p * |
|---|---|---|---|---|---|
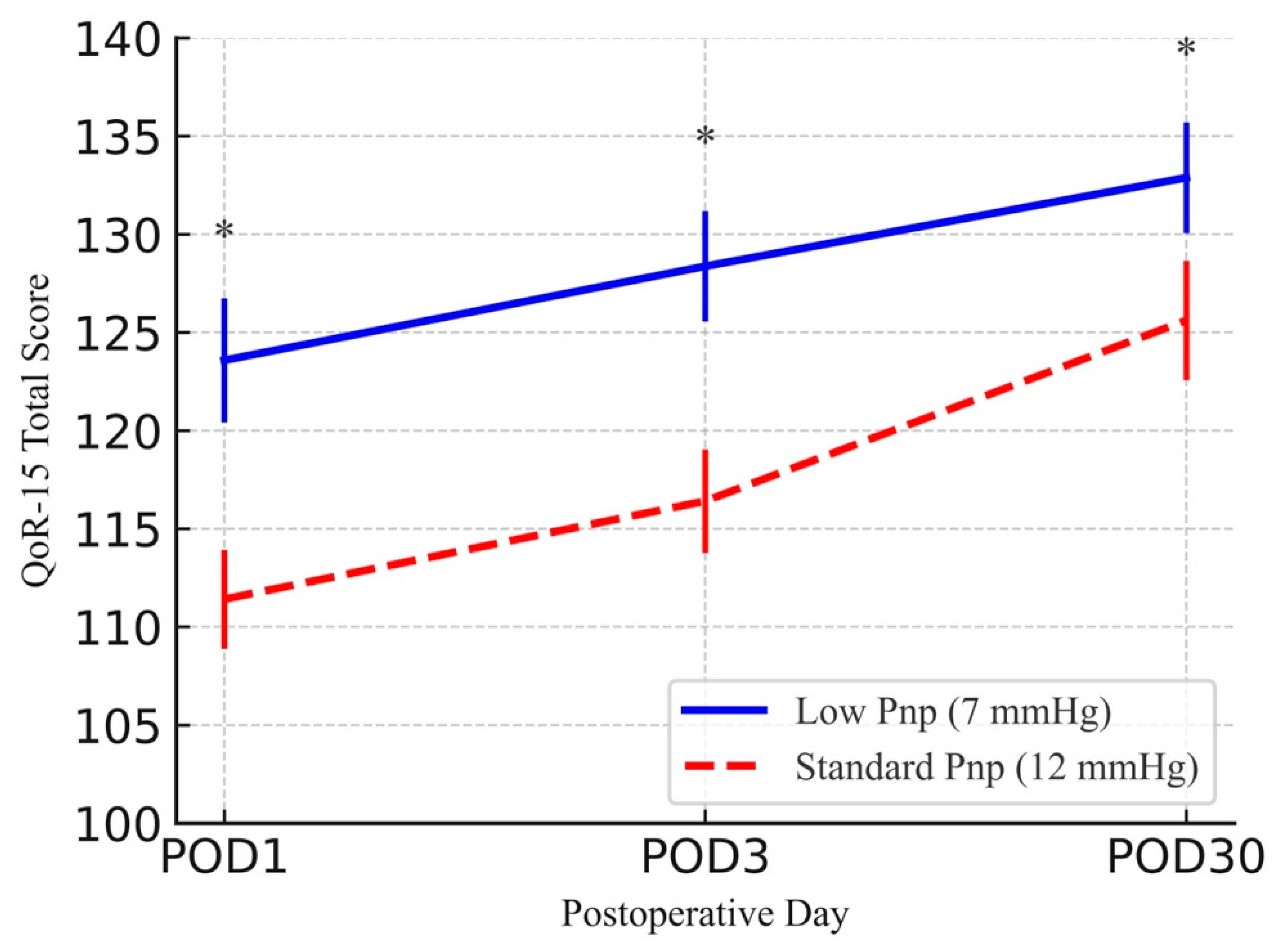
| QoR-15 total | POD1 | 111.41 ± 2.52 | 123.58 ± 3.16 | 12.17 (11.16, 13.17) | <0.001 |
| QoR-15 total | POD3 | 116.41 ± 2.64 | 128.37 ± 2.81 | 11.95 (11.01, 12.90) | <0.001 |
| QoR-15 total | POD30 | 125.61 ± 3.04 | 132.88 ± 2.82 | 7.27 (6.25, 8.29) | <0.001 |
| Pain | POD1 | 13.33 ± 0.85 | 15.60 ± 0.81 | 2.27 (1.98, 2.56) | <0.001 |
| Pain | POD3 | 14.46 ± 1.03 | 17.38 ± 0.76 | 2.93 (2.61, 3.24) | <0.001 |
| Pain | POD30 | 15.89 ± 0.84 | 18.22 ± 0.61 | 2.33 (2.07, 2.59) | <0.001 |
| Physical comfort | POD1 | 38.10 ± 1.48 | 42.85 ± 1.56 | 4.75 (4.22, 5.28) | <0.001 |
| Physical comfort | POD3 | 39.97 ± 1.47 | 44.63 ± 1.29 | 4.66 (4.18, 5.15) | <0.001 |
| Physical comfort | POD30 | 43.40 ± 1.95 | 45.93 ± 1.33 | 2.53 (1.96, 3.11) | <0.001 |
| Physical independence | POD1 | 12.27 ± 1.01 | 12.38 ± 1.29 | 0.11 (−0.29, 0.51) | 0.296 |
| Physical independence | POD3 | 13.70 ± 0.98 | 14.12 ± 1.01 | 0.42 (0.07, 0.76) | 0.005 |
| Physical independence | POD30 | 15.83 ± 1.08 | 15.80 ± 1.02 | −0.03 (−0.39, 0.34) | 0.622 |
| Psychological support | POD1 | 18.63 ± 0.68 | 19.27 ± 0.71 | 0.64 (0.40, 0.88) | <0.001 |
| Psychological support | POD3 | 18.44 ± 0.71 | 19.18 ± 0.70 | 0.74 (0.49, 0.99) | <0.001 |
| Psychological support | POD30 | 18.60 ± 0.71 | 19.12 ± 0.72 | 0.52 (0.27, 0.76) | <0.001 |
| Emotional state | POD1 | 29.09 ± 1.35 | 33.48 ± 1.85 | 4.40 (3.83, 4.97) | <0.001 |
| Emotional state | POD3 | 29.84 ± 1.50 | 33.05 ± 2.14 | 3.21 (2.55, 3.86) | <0.001 |
| Emotional state | POD30 | 31.90 ± 1.47 | 33.82 ± 1.73 | 1.92 (1.35, 2.48) | <0.001 |
| Outcome | Standard-Pressure PP (n = 70) | Low-Pressure PP (n = 60) | p * |
|---|---|---|---|
| Console time, min, mean ± SD | 71.57 ± 12.02 | 98.5 ± 16.5 | <0.001 |
| Hospital stay, days, mean ± SD | 2.09 ± 0.33 | 2.3 ± 0.83 | 0.064 |
| Intraoperative blood loss, mL, mean ± SD | 97.07 ± 48.4 | 129 ± 108.73 | 0.039 |
| Pathological T stage, n (%) | 0.017 | ||
| pT2a | 6 (8.6) | 14 (23.3) | |
| pT2b | 0 (0) | 3 (5.0) | |
| pT2c | 46 (65.7) | 27 (45.0) | |
| pT3a | 17 (24.3) | 15 (25.0) | |
| pT3b | 1 (1.4) | 1 (1.7) | |
| ISUP grade, n (%) | 0.037 | ||
| 1 | 33 (47.1) | 39 (65.0) | |
| 2 | 13 (18.6) | 4 (6.7) | |
| 3 | 12 (17.1) | 12 (20.0) | |
| 4 | 12 (17.1) | 4 (6.7) | |
| 5 | 0 (0) | 1 (1.7) | |
| Lymph node dissection, n (%) | 31 (44.3) | 30 (50.0) | 0.515 |
| Positive surgical margin, n (%) | 6 (8.6) | 8 (13.3) | 0.383 |
| Clavien–Dindo complications, n (%) | 0.185 | ||
| 0 | 57 (81.4) | 42 (70) | |
| 1 | 8 (11.4) | 7 (11.7) | |
| 2 | 5 (7.1) | 9 (15.0) | |
| 3a | 0 (0) | 2 (3.3) | |
| Postoperative transfusion, n (%) | 1 (1.4) | 3 (5.0) | 0.335 |
| SWS, n (%) | <0.001 | ||
| 3 | 0 (0) | 32 (53.3) | |
| 4 | 27 (38.6) | 28 (46.7) | |
| 5 | 43 (61.4) | 0 (0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobay, R.; Güngör, H.S.; İnkaya, A.; Beyatlı, M.; Tahra, A.; Küçük, E.V. Impact of Low- Versus Standard-Pressure Pneumoperitoneum on Postoperative Recovery in Patients with Obesity Undergoing Robot-Assisted Radical Prostatectomy: A Retrospective Cohort Study. Medicina 2025, 61, 1253. https://doi.org/10.3390/medicina61071253
Sobay R, Güngör HS, İnkaya A, Beyatlı M, Tahra A, Küçük EV. Impact of Low- Versus Standard-Pressure Pneumoperitoneum on Postoperative Recovery in Patients with Obesity Undergoing Robot-Assisted Radical Prostatectomy: A Retrospective Cohort Study. Medicina. 2025; 61(7):1253. https://doi.org/10.3390/medicina61071253
Chicago/Turabian StyleSobay, Resul, Hasan Samet Güngör, Abdurrahman İnkaya, Murat Beyatlı, Ahmet Tahra, and Eyüp Veli Küçük. 2025. "Impact of Low- Versus Standard-Pressure Pneumoperitoneum on Postoperative Recovery in Patients with Obesity Undergoing Robot-Assisted Radical Prostatectomy: A Retrospective Cohort Study" Medicina 61, no. 7: 1253. https://doi.org/10.3390/medicina61071253
APA StyleSobay, R., Güngör, H. S., İnkaya, A., Beyatlı, M., Tahra, A., & Küçük, E. V. (2025). Impact of Low- Versus Standard-Pressure Pneumoperitoneum on Postoperative Recovery in Patients with Obesity Undergoing Robot-Assisted Radical Prostatectomy: A Retrospective Cohort Study. Medicina, 61(7), 1253. https://doi.org/10.3390/medicina61071253






