Extraperitoneal Robot-Assisted Laparoscopic Radical Prostatectomy in Continuous Spinal Anesthesia: A New Approach to an Established Technique
Abstract
1. Introduction
2. Materials and Methods
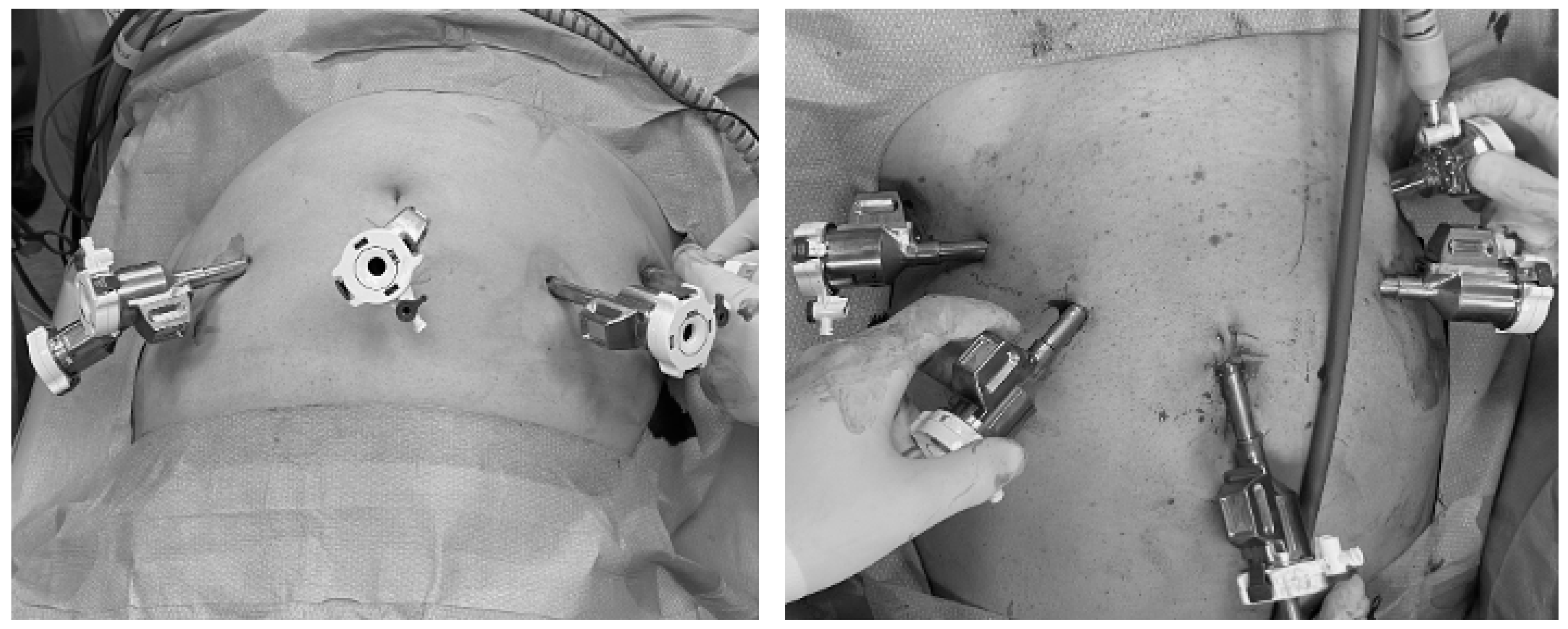
2.1. Surgical Technique
2.2. Aspects of Continuous Spinal Anesthesia
2.3. Study Population and Inclusion and Exclusion Criteria
2.4. Outcomes Definition and Follow-Up
2.5. Statistical Analysis
2.6. Ethics and Inclusion Criteria
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cornford, P.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Brunckhorst, O.; Darraugh, J.; Eberli, D.; De Meerleer, G.; De Santis, M.; Farolfi, A.; et al. EAU-EANM-ESTRO-ESUR-ISUP-SIOG Guidelines on Prostate Cancer-2024 Update. Part I: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2024, 86, 148–163. [Google Scholar] [CrossRef]
- Hatzinger, M.; Hubmann, R.; Moll, F.; Sohn, M. [The history of prostate cancer from the beginning to DaVinci]. Aktuelle Urol. 2012, 43, 228–230. [Google Scholar] [CrossRef]
- Semerjian, A.; Pavlovich, C.P. Extraperitoneal Robot-Assisted Radical Prostatectomy: Indications, Technique and Outcomes. Curr. Urol. Rep. 2017, 18, 42. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, S.; Umemoto, Y.; Mizuno, K.; Okada, A.; Nakane, A.; Nishio, H.; Hamamoto, S.; Ando, R.; Kawai, N.; Tozawa, K.; et al. New steps of robot-assisted radical prostatectomy using the extraperitoneal approach: A propensity-score matched comparison between extraperitoneal and transperitoneal approach in Japanese patients. BMC Urol. 2017, 17, 106. [Google Scholar] [CrossRef]
- Horovitz, D.; Feng, C.; Messing, E.M.; Joseph, J.V. Extraperitoneal vs. transperitoneal robot-assisted radical prostatectomy in patients with a history of prior inguinal hernia repair with mesh. J. Robot. Surg. 2017, 11, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Bačak Kocman, I.; Mihaljević, S.; Goluža, E.; Peršin Beraković, A.; Margaretić Piljek, N.; Kuliš, T.; Hudolin, T.; Knežević, N.; Kaštelan, Ž. Anesthesia for robot-assisted radical prostatectomy—A challenge for anaesthesiologist. Acta Clin. Croat. 2022, 61, 76–80. [Google Scholar] [CrossRef]
- Bilbro, N.A.; Hirst, A.; Paez, A.; Vasey, B.; Pufulete, M.; Sedrakyan, A.; McCulloch, P.; IDEAL Collaboration Reporting Guidelines Working Group. The IDEAL Reporting Guidelines: A Delphi Consensus Statement Stage Specific Recommendations for Reporting the Evaluation of Surgical Innovation. Ann. Surg. 2021, 273, 82–85. [Google Scholar] [CrossRef]
- Van Velthoven, R.F.; Ahlering, T.E.; Peltier, A.; Skarecky, D.W.; Clayman, R.V. Technique for laparoscopic running urethrovesical anastomosis:the single knot method. Urology 2003, 61, 699–702. [Google Scholar] [CrossRef] [PubMed]
- Rocco, F.; Carmignani, L.; Acquati, P.; Gadda, F.; Dell’Orto, P.; Rocco, B.; Bozzini, G.; Gazzano, G.; Morabito, A. Restoration of Posterior Aspect of Rhabdosphincter Shortens Continence Time After Radical Retropubic Prostatectomy. J. Urol. 2006, 175, 2201–2206. [Google Scholar] [CrossRef]
- Epstein, J.I.; Zelefsky, M.J.; Sjoberg, D.D.; Nelson, J.B.; Egevad, L.; Magi-Galluzzi, C.; Vickers, A.J.; Parwani, A.V.; Reuter, V.E.; Fine, S.W.; et al. A Contemporary Prostate Cancer Grading System: A Validated Alternative to the Gleason Score. Eur. Urol. 2016, 69, 428–435. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Tubaro, A.; Zattoni, F.; Prezioso, D.; Scarpa, R.M.; Pesce, F.; Rizzi, C.A.; Santini, A.M.; Simoni, L.; Artibani, W.; Flow Study Group. Italian validation of the International Consultation on Incontinence Questionnaires. BJU Int. 2006, 97, 101–108. [Google Scholar] [CrossRef] [PubMed]
- McCormack, H.M.; Horne, D.J.; Sheather, S. Clinical applications of visual analogue scales: A critical review. Psychol. Med. 1988, 18, 1007–1019. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [CrossRef]
- Jacobs, B.L.; Montgomery, J.S.; Dunn, R.L.; Weizer, A.Z.; Miller, D.C.; Wood, D.P.; Wolf, J.S.; Zhang, Y.; Wei, J.T.; Hollenbeck, B.K. A comparison of extraperitoneal and intraperitoneal approaches for robotic prostatectomy. Surg. Innov. 2012, 19, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Uy, M.; Cassim, R.; Kim, J.; Hoogenes, J.; Shayegan, B.; Matsumoto, E.D. Extraperitoneal versus transperitoneal approach for robot-assisted radical prostatectomy: A contemporary systematic review and meta-analysis. J. Robot. Surg. 2022, 16, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Anesthesiologic Effects of Transperitoneal Versus Extraperitoneal Approach During Robot-Assisted Radical Prostatectomy: Results of a Prospective Randomized Study—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/26200539/ (accessed on 14 October 2024).
- Ragavan, N.; Dholakia, K.; Ramesh, M.; Stolzenburg, J.U. Extraperitoneal vs. transperitoneal robot-assisted laparoscopic radical prostatectomy-analysis of perioperative outcomes, a single surgeon’s experience. J. Robot. Surg. 2019, 13, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M.; STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. PLoS Med. 2007, 4, e297. [Google Scholar] [CrossRef]
| Patient Number | Age | PSA (ng/mL) | Contraindication to RALP | ISUP at Biopsy | Nerve Sparing | Global Operative Time (Minutes) | Day of Discharge | Day of Bladder Catheter Removal | Complications |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 63 | 5.3 | Extensive previous abdominal surgery | 2 | Bilateral | 120 | 3 | 8 | No |
| 2 | 73 | 6.8 | Glaucoma and recently treated aortic aneurism | 4 | No | 150 | 4 | 12 | Small entity urine leakage |
| 3 | 69 | 5.4 | Extensive previous abdominal surgery | 3 | Monolateral | 150 | 4 | 8 | No |
| Patient Number | Post-Operative PSA (ng/mL) | ISUP at Pathology | TNM Staging | Surgical Margins (mm) | Number of Removed Nodes | ICIQ-SF at 3 Months | Number of Pad/Day | Further Surgical Operations |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.01 | 1 | pT2cNxR0 | 0 | NA | 0 | 0 | No |
| 2 | 0.01 | 2 | pT2aN0R0 | 0 | 19 | 1 | 0 | No |
| 3 | 0.01 | 2 | pT2cNxR0 | 0 | NA | 0 | 0 | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morselli, S.; Zavatti, L.; Ferrari, R.; Gatti, L.; Micali, S.; Rabito, S.; Cindolo, L.; Ferrari, G. Extraperitoneal Robot-Assisted Laparoscopic Radical Prostatectomy in Continuous Spinal Anesthesia: A New Approach to an Established Technique. Medicina 2024, 60, 1973. https://doi.org/10.3390/medicina60121973
Morselli S, Zavatti L, Ferrari R, Gatti L, Micali S, Rabito S, Cindolo L, Ferrari G. Extraperitoneal Robot-Assisted Laparoscopic Radical Prostatectomy in Continuous Spinal Anesthesia: A New Approach to an Established Technique. Medicina. 2024; 60(12):1973. https://doi.org/10.3390/medicina60121973
Chicago/Turabian StyleMorselli, Simone, Laura Zavatti, Riccardo Ferrari, Lorenzo Gatti, Salvatore Micali, Salvatore Rabito, Luca Cindolo, and Giovanni Ferrari. 2024. "Extraperitoneal Robot-Assisted Laparoscopic Radical Prostatectomy in Continuous Spinal Anesthesia: A New Approach to an Established Technique" Medicina 60, no. 12: 1973. https://doi.org/10.3390/medicina60121973
APA StyleMorselli, S., Zavatti, L., Ferrari, R., Gatti, L., Micali, S., Rabito, S., Cindolo, L., & Ferrari, G. (2024). Extraperitoneal Robot-Assisted Laparoscopic Radical Prostatectomy in Continuous Spinal Anesthesia: A New Approach to an Established Technique. Medicina, 60(12), 1973. https://doi.org/10.3390/medicina60121973







