Embolic Stroke of Undetermined Source (ESUS): Exploring the Neurocardiological Axis and Its Clinical Implications
Abstract
1. Introduction
Unique Contributions and Novelty of This Review
2. Materials and Methods
2.1. Databases and Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Study Selection and Data Extraction
3. Definition and Diagnostic Criteria of ESUS
- Absence of ≥50% stenosis in arteries supplying the affected territory
- No major cardioembolic source (e.g., atrial fibrillation, intracardiac thrombus)
- Non-lacunar infarct on imaging
- Completion of minimum diagnostic tests: brain and vascular imaging, ≥24 h ECG monitoring, transthoracic echocardiography, and basic laboratory evaluation. Transesophageal echocardiography and thrombophilia testing may be added based on clinical suspicion.
4. ESUS in the Context of Ischemic Stroke Classification (Relation to TOAST)
5. Prevalence and Incidence of ESUS
5.1. Prevalence
5.2. Incidence
5.3. Recurrent Stroke Risk
6. Demographic Differences in ESUS
6.1. Age
6.2. Sex
6.3. Geographic and Ethnic Differences
6.4. Stroke Severity
6.5. Underlying Covert Pathologies
7. Risk Factors and Patient Profile in ESUS
7.1. Traditional Vascular Risk Factors
- Hypertension: Present in a majority of ESUS patients (~60–70%), but often not as long-standing or severe as in lacunar stroke patients [10,17]. Hypertension can cause small vessel disease, but in ESUS patients, the hypertension may contribute indirectly (e.g., to atrial enlargement or aortic stiffness) rather than directly.
- Diabetes mellitus: Seen in roughly 20–30% of ESUS patients [10]. This is slightly lower than in large artery atherosclerotic stroke populations. Poorly controlled diabetes tends to cause microvascular disease, which would manifest as lacunar infarcts (excluded from ESUS). Thus, ESUS diabetics might be those with relatively moderate disease.
- Smoking: Many ESUS patients have a history of smoking, though exact proportions vary by region. Smoking is a risk factor for atherosclerosis; heavy smokers might develop carotid plaques. In ESUS cohorts, smoking rates have been reported anywhere from ~20% to 40% [4,10]. If significant atherosclerosis from smoking is absent, its role might be through promoting hypercoagulability or cardiac arrhythmia triggers.
- Dyslipidemia: Hyperlipidemia is common but typically managed—many ESUS patients are found to have elevated cholesterol or are on statins for primary prevention. Dyslipidemia contributes to atherosclerosis; hence severe dyslipidemia would lead to an identified plaque source (and not ESUS). However, moderate dyslipidemia can be present without overt plaque >50%. The risk factor profiles often show that ESUS patients resemble the general stroke population [17].
- Prior stroke or transient ischemic attack (TIA): A history of a prior stroke or transient ischemic attack is noted in some ESUS patients (~15–20%) [10]. A prior unexplained stroke suggests an underlying persistent but undetected cause [14]. Patients with recurrent cryptogenic strokes warrant especially intensive evaluation for occult causes.
7.2. Potential Covert Embolic Sources and Associated Factors
- Atrial cardiopathy: Even without diagnosed atrial fibrillation (AF), patients may show signs of atrial dysfunction—referred to as atrial cardiopathy. Markers include left atrial enlargement on echocardiography, elevated NT-proBNP, abnormal P-wave indices on ECG (e.g., increased P-wave terminal force in V1), or atrial fibrosis on cardiac MRI. Studies report that 30–40% of ESUS patients exhibit one or more of these features, suggesting an underlying atrial substrate prone to thromboembolism [18]. Elevated NT-proBNP is associated with both cryptogenic stroke and future AF detection, and patients with atrial cardiopathy are typically older and may develop AF with longer monitoring.
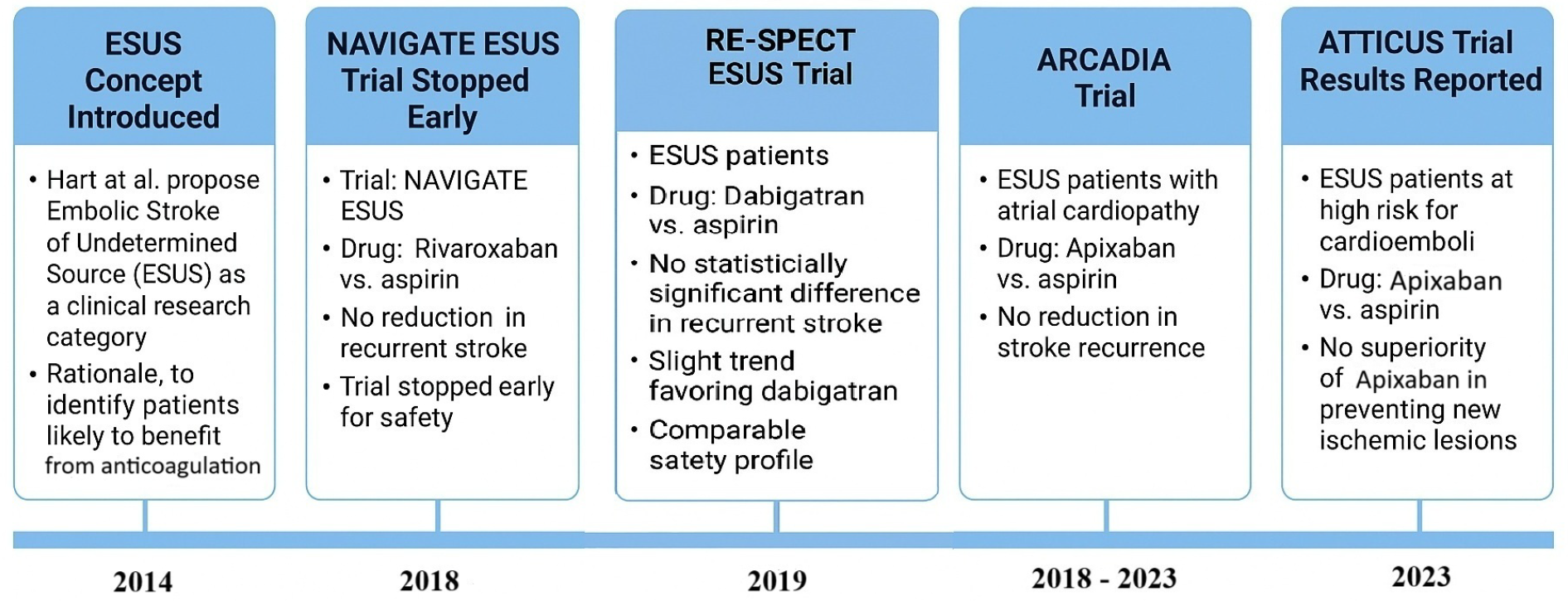
- Subclinical atrial fibrillation: Although AF is absent by definition in ESUS, prolonged cardiac monitoring often reveals paroxysmal AF. The CRYSTAL-AF study found AF in 12.4% of cryptogenic stroke patients at 12 months and about 30% at 3 years using an implantable loop recorder [5]. The EMBRACE trial similarly detected AF in 16% of patients with 30-day external monitors [19]. Risk factors for subclinical AF include older age, structural heart disease, and elevated NT-proBNP. In the NAVIGATE ESUS trial, only ~3% of patients had AF detected over a median 11 months of standard monitoring [20], though this likely underestimates the true prevalence due to limited follow-up duration.
- Patent foramen ovale (PFO): PFO, a potential route for paradoxical embolism, is present in about 25% of the general population and in 40–50% of younger patients (<55) with cryptogenic stroke [21]. In ESUS, PFOs are included unless proven causative (e.g., with associated DVT). High-risk features (e.g., large shunt, atrial septal aneurysm) increase the likelihood of causality, particularly in younger patients without vascular risk factors. Trials have shown that closure of high-risk PFOs reduces recurrence in cryptogenic stroke [21,22]. In NAVIGATE ESUS, patients with PFO did not show differential benefit from rivaroxaban vs. aspirin [14], suggesting that anticoagulation is not necessarily indicated unless PFO is clearly causal.
- Non-stenosing atherosclerotic plaques: Some ESUS patients have non-occlusive atherosclerotic plaques (<50% stenosis) in the aortic arch or carotid arteries that may still cause embolism. Aortic plaques ≥4 mm and complex carotid lesions (ulcerated or mobile) are associated with stroke risk despite falling below the threshold for large-artery categorization. These findings, more common in older individuals, smokers, and those with hyperlipidemia, may represent an arterio-embolic ESUS subgroup. Such patients may benefit more from intensive antiplatelet or statin therapy than anticoagulation [17].
- Left ventricular (LV) dysfunction: Subtle LV abnormalities—such as wall motion deficits or mildly reduced ejection fraction—may exist in ESUS patients without meeting criteria for cardioembolic stroke. These could reflect silent myocardial infarction or undetected ventricular thrombi. Studies such as that by Perkins et al. (2022) [17] found clusters of LV dysfunction and aortic plaque, hinting at a cardioaortic embolic mechanism. These patients may have coronary disease history, but no overt cause detected during acute stroke evaluation. Advanced imaging (e.g., cardiac MRI) may reveal missed sources [17].
- Hypercoagulability and cancer-associated stroke: While rare, prothrombotic conditions and occult cancer can present as ESUS. Malignancy may cause embolic-appearing strokes via nonbacterial thrombotic endocarditis or coagulation disturbances. If cancer is already known, the stroke is often classified as cancer-associated. However, undiagnosed malignancy may be present in ESUS patients, particularly older individuals. Up to 5–10% of cryptogenic strokes in elderly patients reveal cancer within a year [23]. Clues include high D-dimer levels, weight loss, or systemic symptoms. Treatment often involves anticoagulation and managing the underlying cancer.
7.3. Predictors of Recurrence
8. Current Controversies and Diagnostic Strategies
- Utility of the ESUS construct: A major controversy is whether labeling a stroke as ESUS meaningfully influences clinical decision-making. Proponents argue it emphasizes an embolic mechanism and promotes standardized workup and consideration of anticoagulation. Critics counter that the label does not change therapy—especially after trials failed to show benefit from empiric anticoagulation—and may discourage continued diagnostic efforts [6,8]. They highlight that ESUS is not fundamentally distinct from cryptogenic stroke, yet was widely adopted before evidence of clinical benefit. Many advocate instead for deeper diagnostics—e.g., prolonged monitoring, plaque imaging, or thrombophilia screening—to reclassify ESUS cases into specific stroke subtypes [12]. This sparked debate between empiric treatment vs. extended diagnostic strategies.
- Extent of diagnostic workup: Another area of debate is how far to go in evaluating a stroke before labeling it ESUS. While a minimum workup is defined, many experts recommend extended cardiac monitoring (≥30 days) in all patients with embolic-appearing cryptogenic strokes [3,5,19] due to the high yield of detecting paroxysmal AF. Transesophageal echocardiography (TEE), though not required by ESUS criteria, may uncover relevant findings such as PFO or complex aortic plaque. Likewise, cancer screening (e.g., CT, tumor markers) may be warranted in older patients with elevated D-dimer or other red flags. Genetic testing for thrombophilia is not routinely advised, but may be appropriate in younger patients with suggestive histories. Ultimately, the depth of evaluation remains a judgment call, with some clinicians stopping once ESUS criteria are met, and others pursuing exhaustive workup.
- Management of PFO in ESUS: The presence of a PFO in ESUS patients is a gray zone. Technically, patients with PFO but no DVT or alternate cause still qualify as ESUS. However, with the advent of PFO closure trials showing benefit in select patients, many clinicians now treat these as paradoxical embolisms, especially in younger patients with high-risk PFO features [21,22]. Patients <60 with isolated PFOs are often reclassified and considered for closure. In contrast, small or incidental PFOs in older adults remain controversial. Although ESUS definitions historically included PFO, evolving evidence is reshaping clinical management.
- Role of anticoagulation vs. antiplatelet: Initial ESUS trials (NAVIGATE ESUS, RE-SPECT ESUS) hypothesized that empiric anticoagulation would reduce recurrence. However, both trials found no benefit of DOACs over aspirin and highlighted bleeding risks [20,25]. This outcome spurred debate about whether the failure was due to ineffective treatment or the inclusion of a heterogeneous population. Subgroup analyses suggested possible benefit in specific populations—e.g., patients ≥75 years or those with LV dysfunction (EF < 50%) [26,27]. These findings support a move toward more individualized strategies, guided by markers such as atrial cardiopathy. While guidelines now generally favor antiplatelets for ESUS absent another indication [3]. The controversy reflects the tension between generalized treatment vs. personalized care.
- ESUS vs. further subdivision: Another debate is whether the umbrella of ESUS is too broad. Alternative approaches, such as the A-S-C-O-D system, grade potential etiologies rather than assign a binary label [28]. For example, a patient might have minor atrial cardiopathy and non-stenotic aortic plaque—both possible sources. This raises the question: should treatment be tailored based on these findings (e.g., anticoagulate atrial ESUS, intensify statins for arterial ESUS)? Since the original ESUS trials did not stratify by these features, benefits may have been diluted. Recent and ongoing trials now target narrower phenotypes. The debate continues as to whether ESUS should remain a clinical category or evolve into a transitional research term, with the ultimate goal being precise etiologic diagnosis [4,29].
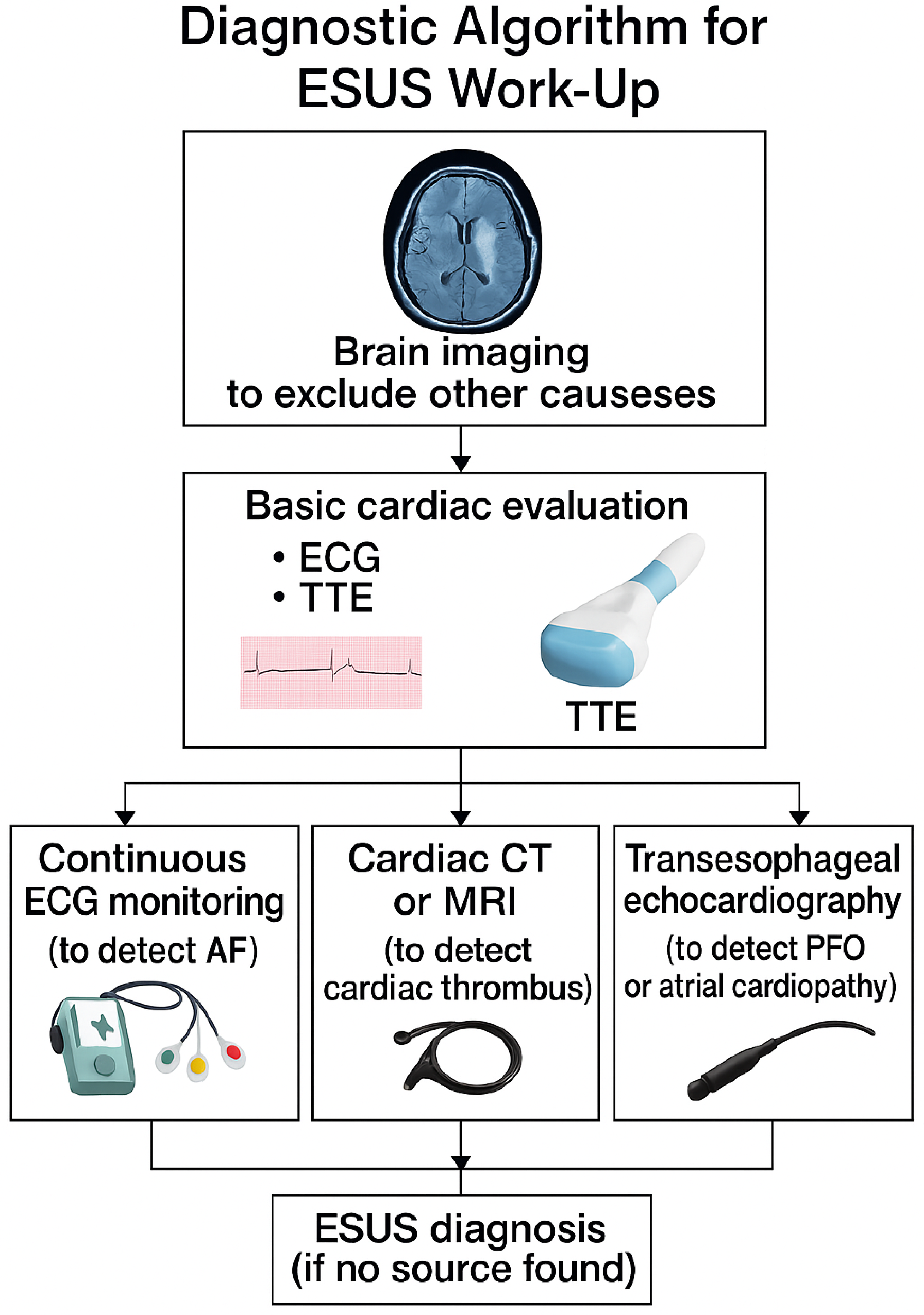
Diagnostic Strategies
- Prolonged cardiac monitoring: As mentioned, detecting occult atrial fibrillation can be game-changing (because it shifts management to anticoagulation unequivocally). Current best practice for ESUS (cryptogenic stroke) is to perform at least several weeks of monitoring if initial telemetry and Holter are unrevealing [3]. This can be achieved via a 30-day event monitor, multiple shorter Holters, or an implantable loop recorder depending on patient risk and resources. Many stroke centers now place implantable cardiac monitors in patients with cryptogenic stroke who are at moderate-to-high risk for AF (for instance, age >55 or evidence of atrial cardiopathy). As an example, a CRYSTAL-AF trial showed the benefit of an implantable monitor, and thus in a patient who is willing and where AF suspicion is high, this is a reasonable step [5]. Even the ARCADIA trial, which required no known AF at baseline, found that about 25% of participants were diagnosed with AF during follow-up [30], underscoring how common subclinical AF is.
- Imaging for structural cardiac sources: Transthoracic echocardiography (TTE) is routine, but transesophageal echocardiography (TEE) is often more sensitive for detecting things such as PFO (via bubble study), atrial septal aneurysm, valvular strands, or aortic arch atheroma [5]. In an ESUS workup, a TEE can be very revealing and is recommended especially in patients <60 or in older patients if no other clue is found [2]. Likewise, if TEE confirms a PFO with a large shunt, that patient might be referred for PFO closure [5]. Thus, TEE plays a diagnostic and potentially therapeutic role. Additionally, if cardiomyopathy is possible, cardiac MRI provides tissue characterization (for example, late gadolinium enhancement in the left atrium might indicate fibrosis predisposing to AF) [11].
- Vascular imaging and novel techniques: Standard vascular imaging (carotid ultrasound, CTA, or MRA) is conducted to rule out >50% stenosis. However, newer techniques, such as high-resolution MRI of vessel walls, can sometimes identify features in non-stenotic plaques (such as intra-plaque hemorrhage or ulceration) that suggest them as the culprit [12]. Some research imaging can show plaque inflammation (using PET scans) or detect microembolic signals on transcranial Doppler, indicating an active embolic source in arteries [12]. These are not yet routine, but they represent how technology might pin down an arterial source in ESUS.
- Laboratory workup: All ESUS patients should have basic labs for hypercoagulability and other clues: e.g., antiphospholipid antibody panel in younger patients or if there is any autoimmune clue, coagulation factors, platelet count (for rare disorders), inflammatory markers (for vasculitis), or cancer screening labs if indicated (such as CEA, PSA, etc., not routinely conducted without suspicion). A particularly useful test is D-dimer; an elevated D-dimer in cryptogenic stroke has been associated with cancer-related strokes or prothrombotic states. Extremely high D-dimer might prompt screening for malignancy or deep vein thrombosis (with the idea that maybe a venous clot crossed a PFO) [23]. ESUS patients usually do not show gross lab abnormalities; if they do (such as very high antiphospholipid titers), then one would reclassify the stroke cause (e.g., “antiphospholipid syndrome” rather than ESUS) [4].
- Neuroimaging patterns: Radiologically, clinicians examine the stroke pattern on MRI to glean insights. For instance, an infarct in multiple vascular territories (different sides or anterior and posterior circulation simultaneously) strongly suggests an embolic shower, often cardiac [2]. ESUS patients with such patterns might be prioritized for arrhythmia monitoring. Conversely, a single small cortical infarct might raise consideration of local plaque. Diffusion-weighted MRI “lesion mapping” is being studied to differentiate likely cardioembolic vs. artery-to-artery mechanisms. Although not definitive, this is part of the art of ESUS evaluation [4] (Table 4).
9. Importance of Accurate Identification of Stroke Etiology
10. Future Directions and Innovations
10.1. Personalized Medicine and Subgroup Trials
10.2. Collaborative Multidisciplinary Approach
11. Limitations
11.1. Limitations and Controversies of the ESUS Concept
11.2. Limitations of Major ESUS Trials
11.3. Limitations of This Review
12. Conclusions
Key Takeaways and Implications
- -
- ESUS remains a useful diagnostic framework that prompts thorough evaluation but should not be treated as a definitive endpoint. Clinicians must recognize its limitations and seek individualized explanations.
- -
- Routine anticoagulation is not recommended in unselected ESUS patients based on the neutral outcomes of NAVIGATE ESUS and RE-SPECT ESUS. Treatment should default to antiplatelet therapy unless a high-risk source is later identified.
- -
- Prolonged cardiac monitoring is essential, especially in older patients or those with signs of atrial cardiopathy, to detect covert atrial fibrillation and adjust secondary prevention accordingly.
- -
- Future research should prioritize the following:
- -
- Developing tools to stratify ESUS patients by a likely embolic source.
- -
- Identifying predictive biomarkers and imaging signatures.
- -
- Conducting trials focused on targeted subgroups (e.g., atrial cardiopathy, PFO).
- -
- The ultimate goal is precision medicine: shifting from treating ESUS as a single entity to tailoring prevention strategies based on individualized stroke mechanisms.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AF | Atrial fibrillation |
| ECG | Electrocardiogram |
| CEA | Carcinoembryonic antigen |
| CTA | CT-angiography |
| DOAC | Direct oral anticoagulants |
| ESUS | Embolic stroke of undetermined source |
| FDA | Food and Drug Administration |
| LV | Left Ventricular |
| MCA | Middle cerebral artery |
| MeSH | Medical subject headings |
| MI | Myocardial infarction |
| NBTE | Nonbacterial thrombotic endocarditis |
| NIHSS | National Institutes of Health Stroke Scale |
| NT-proBNP | Atrial natriuretic peptides |
| MRA | MR-angiography |
| PFO | Patent foramen ovale |
| PSA | Prostate-specific antigen |
| RCT | Randomized controlled trial |
| TEE | Transesophageal echocardiography |
| TIA | Transient ischemic attack |
| TOAST | The Trial of Org 10172 in Acute Stroke Treatment |
References
- Adams, H.P., Jr.; Bendixen, B.H.; Kappelle, L.J.; Biller, J.; Love, B.B.; Gordon, D.L.; Marsh, E.E., 3rd. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993, 24, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Hart, R.G.; Diener, H.C.; Coutts, S.B.; Easton, J.D.; Granger, C.B.; O’Donnell, M.J.; Sacco, R.L.; Connolly, S.J.; Cryptogenic Stroke/ESUS International Working Group. Embolic strokes of undetermined source: The case for a new clinical construct. Lancet Neurol. 2014, 13, 429–438. [Google Scholar] [CrossRef]
- Kleindorfer, D.O.; Towfighi, A.; Chaturvedi, S.; Cockroft, K.M.; Gutierrez, J.; Lombardi-Hill, D.; Kamel, H.; Kernan, W.N.; Kittner, S.J.; Leira, E.C.; et al. 2021 Guideline for the Prevention of Stroke in Patients With Stroke and Transient Ischemic Attack: A Guideline From the American Heart Association/American Stroke Association. Stroke 2021, 52, e364–e467. [Google Scholar] [CrossRef] [PubMed]
- Ntaios, G. Embolic Stroke of Undetermined Source: JACC Review Topic of the Week. J. Am. Coll Cardiol. 2020, 75, 333–340. [Google Scholar] [CrossRef]
- Sanna, T.; Diener, H.C.; Passman, R.S.; Di Lazzaro, V.; Bernstein, R.A.; Morillo, C.A.; Rymer, M.M.; Thijs, V.; Rogers, T.; Beckers, F.; et al. CRYSTAL AF Investigators. Cryptogenic stroke and underlying atrial fibrillation. N. Engl. J. Med. 2014, 370, 2478–2486. [Google Scholar] [CrossRef]
- Schulz, U.G. Cryptogenic stroke—How to make sense of a non-diagnostic entity. Maturitas 2019, 122, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Tang, X.; Liu, W.; Jia, K.; Zhao, X.; Yu, F. Clinical Features of Embolic Stroke of Undetermined Source. Front. Neurol. 2020, 11, 58. [Google Scholar] [CrossRef]
- Martí-Fàbregas, J.; Delgado-Mederos, R.; Crespo, J.; Peña, E.; Marín, R.; Jiménez-Xarrié, E.; Fernández-Arcos, A.; Pérez-Pérez, J.; Martínez-Domeño, A.; Camps-Renom, P.; et al. Circulating endothelial progenitor cells and the risk of vascular events after ischemic stroke. PLoS ONE 2015, 10, e0124895. [Google Scholar] [CrossRef]
- Hart, R.G.; Catanese, L.; Perera, K.S.; Ntaios, G.; Connolly, S.J. Embolic Stroke of Undetermined Source: A Systematic Review and Clinical Update. Stroke 2017, 48, 867–872. [Google Scholar] [CrossRef]
- Perera, K.S.; Vanassche, T.; Bosch, J.; Giruparajah, M.; Swaminathan, B.; Mattina, K.R.; Berkowitz, S.D.; Arauz, A.; O’Donnell, M.J.; Ameriso, S.F.; et al. ESUS Global Registry Investigators. Embolic strokes of undetermined source: Prevalence and patient features in the ESUS Global Registry. Int. J. Stroke 2016, 11, 526–533. [Google Scholar] [CrossRef]
- Jordan, K.; Yaghi, S.; Poppas, A.; Chang, A.D.; Mac Grory, B.; Cutting, S.; Burton, T.; Jayaraman, M.; Tsivgoulis, G.; Sabeh, M.K.; et al. Left Atrial Volume Index Is Associated With Cardioembolic Stroke and Atrial Fibrillation Detection After Embolic Stroke of Undetermined Source. Stroke 2019, 50, 1997–2001. [Google Scholar] [CrossRef] [PubMed]
- Bang, O.Y.; Ovbiagele, B.; Kim, J.S. Evaluation of cryptogenic stroke with advanced diagnostic techniques. Stroke 2014, 45, 1186–1194. [Google Scholar] [CrossRef]
- Kaul, S. Anticoagulation in subclinical atrial fibrillation: To treat or not to treat? J. Am. Coll. Cardiol. 2019, 74, 2805–2807. [Google Scholar]
- Kasner, S.E.; Swaminathan, B.; Lavados, P.; Sharma, M.; Muir, K.; Veltkamp, R.; Ameriso, S.F.; Endres, M.; Lutsep, H.; Messé, S.R.; et al. NAVIGATE ESUS Investigators. Rivaroxaban or aspirin for patent foramen ovale and embolic stroke of undetermined source: A prespecified subgroup analysis from the NAVIGATE ESUS trial. Lancet Neurol. 2018, 17, 1053–1060. [Google Scholar] [CrossRef]
- Veltkamp, R.; Pearce, L.A.; Korompoki, E.; Sharma, M.; Kasner, S.E.; Toni, D.; Ameriso, S.F.; Mundl, H.; Tatlisumak, T.; Hankey, G.J.; et al. Characteristics of Recurrent Ischemic Stroke After Embolic Stroke of Undetermined Source: Secondary Analysis of a Randomized Clinical Trial. JAMA Neurol. 2020, 77, 1233–1240. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Elmashad, A.; Staff, I.; Alberts, M.; Nouh, A. Potential Embolic Sources Differ in Patients With Embolic Stroke of Undetermined Source According to Age: A 15-Year Study. Front. Neurol. 2022, 13, 860827. [Google Scholar] [CrossRef]
- Perkins, J.D.; Akhtar, N.; Singh, R.; Kamran, A.; Ilyas, S. Partitioning risk factors for embolic stroke of undetermined source using exploratory factor analysis. Int. J. Stroke 2022, 17, 407–414. [Google Scholar] [CrossRef]
- Kamel, H.; Bartz, T.M.; Elkind, M.S.V.; Okin, P.M.; Thacker, E.L.; Patton, K.K.; Stein, P.K.; de Filippi, C.R.; Gottesman, R.F.; Heckbert, S.R.; et al. Atrial Cardiopathy and the Risk of Ischemic Stroke in the CHS (Cardiovascular Health Study). Stroke 2018, 49, 980–986. [Google Scholar] [CrossRef] [PubMed]
- Gladstone, D.J.; Spring, M.; Dorian, P.; Panzov, V.; Thorpe, K.E.; Hall, J.; Vaid, H.; O’Donnell, M.; Laupacis, A.; Côté, R.; et al. EMBRACE Investigators and Coordinators. Atrial fibrillation in patients with cryptogenic stroke. N. Engl. J. Med. 2014, 370, 2467–2477. [Google Scholar] [CrossRef]
- Hart, R.G.; Sharma, M.; Mundl, H.; Kasner, S.E.; Bangdiwala, S.I.; Berkowitz, S.D.; Swaminathan, B.; Lavados, P.; Wang, Y.; Wang, Y.; et al. NAVIGATE ESUS Investigators. Rivaroxaban for Stroke Prevention after Embolic Stroke of Undetermined Source. N. Engl. J. Med. 2018, 378, 2191–2201. [Google Scholar] [CrossRef]
- Mas, J.L.; Derumeaux, G.; Guillon, B.; Massardier, E.; Hosseini, H.; Mechtouff, L.; Arquizan, C.; Béjot, Y.; Vuillier, F.; Detante, O.; et al. CLOSE Investigators. Patent Foramen Ovale Closure or Anticoagulation vs. Antiplatelets after Stroke. N. Engl. J. Med. 2017, 377, 1011–1021. [Google Scholar] [CrossRef] [PubMed]
- Saver, J.L.; Carroll, J.D.; Thaler, D.E.; Smalling, R.W.; MacDonald, L.A.; Marks, D.S.; Tirschwell, D.L. RESPECT Investigators. Long-Term Outcomes of Patent Foramen Ovale Closure or Medical Therapy after Stroke. N. Engl. J. Med. 2017, 377, 1022–1032. [Google Scholar] [CrossRef] [PubMed]
- Navi, B.B.; Kasner, S.E.; Elkind, M.S.V.; Cushman, M.; Bang, O.Y.; DeAngelis, L.M. Cancer and Embolic Stroke of Undetermined Source. Stroke 2021, 52, 1121–1130. [Google Scholar] [CrossRef]
- Kasner, S.E.; Lavados, P.; Sharma, M.; Wang, Y.; Wang, Y.; Dávalos, A.; Shamalov, N.; Cunha, L.; Lindgren, A.; Mikulik, R.; et al. NAVIGATE ESUS Steering Committee and Investigators. Characterization of Patients with Embolic Strokes of Undetermined Source in the NAVIGATE ESUS Randomized Trial. J. Stroke Cerebrovasc. Dis. 2018, 27, 1673–1682. [Google Scholar] [CrossRef] [PubMed]
- Diener, H.C.; Sacco, R.L.; Easton, J.D.; Granger, C.B.; Bernstein, R.A.; Uchiyama, S.; Kreuzer, J.; Cronin, L.; Cotton, D.; Grauer, C.; et al. RE-SPECT ESUS Steering Committee and Investigators. Dabigatran for Prevention of Stroke after Embolic Stroke of Undetermined Source. N. Engl. J. Med. 2019, 380, 1906–1917. [Google Scholar] [CrossRef]
- Pirera, E.; D’Anna, L.; Di Raimondo, D.; Tuttolomondo, A. Direct Oral Anticoagulants Versus Aspirin for Stroke Prevention After Embolic Stroke of Undetermined Source: An Updated Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2024, 13, 6730. [Google Scholar] [CrossRef]
- Healey, J.S.; Connolly, S.J.; Gold, M.R.; Israel, C.W.; Van Gelder, I.C.; Capucci, A.; Lau, C.P.; Fain, E.; Yang, S.; Bailleul, C.; et al. ASSERT Investigators. Subclinical atrial fibrillation and the risk of stroke. N. Engl. J. Med. 2012, 366, 120–129, Erratum in N. Engl. J. Med. 2016, 374, 998. [Google Scholar] [CrossRef]
- Amarenco, P.; Cohen, A.; Tzourio, C.; Bertrand, B.; Hommel, M.; Besson, G.; Chauvel, C.; Touboul, P.J.; Bousser, M.G. Atherosclerotic disease of the aortic arch and the risk of ischemic stroke. N. Engl. J. Med. 1994, 331, 1474–1479. [Google Scholar] [CrossRef]
- Fuentes, B.; Gutiérrez-Zúñiga, R.; Díez-Tejedor, E. It’s Time to Say Goodbye to the ESUS Construct. Front. Neurol. 2020, 11, 653. [Google Scholar] [CrossRef]
- McIntyre, W.F.; Benz, A.P.; Becher, N.; Healey, J.S.; Granger, C.B.; Rivard, L.; Camm, A.J.; Goette, A.; Zapf, A.; Alings, M.; et al. Direct Oral Anticoagulants for Stroke Prevention in Patients with Device-Detected Atrial Fibrillation: A Study-Level Meta-Analysis of the NOAH-AFNET 6 and ARTESiA Trials. Circulation 2024, 149, 981–988. [Google Scholar] [CrossRef]
- Caso, V.; Turc, G.; Abdul-Rahim, A.H.; Castro, P.; Hussain, S.; Lal, A.; Mattle, H.; Korompoki, E.; Søndergaard, L.; Toni, D.; et al. European Stroke Organisation (ESO) Guidelines on the Diagnosis and Management of Patent Foramen Ovale (PFO) After Stroke. Eur. Stroke J. 2024, 9, 800–834. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.S.; Ho, E.S.; Yeo, L.L.; Kong, W.K.; Li, T.Y.; Tan, B.Y.; Chan, M.Y.; Sharma, V.K.; Poh, K.K.; Sia, C.H. Use of Wearable Technology in Cardiac Monitoring After Cryptogenic Stroke or Embolic Stroke of Undetermined Source: A Systematic Review. Singapore Med. J. 2024, 65, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Kamel, H.; Longstreth, W.T., Jr.; Tirschwell, D.L.; Kronmal, R.A.; Marshall, R.S.; Broderick, J.P.; Aragón García, R.; Plummer, P.; Sabagha, N.; Pauls, Q.; et al. ARCADIA Investigators. Apixaban to Prevent Recurrence After Cryptogenic Stroke in Patients With Atrial Cardiopathy: The ARCADIA Randomized Clinical Trial. JAMA 2024, 331, 573–581. [Google Scholar] [CrossRef]
- Bahit, M.C.; Sacco, R.L.; Easton, J.D.; Meyerhoff, J.; Cronin, L.; Kleine, E.; Grauer, C.; Brueckmann, M.; Diener, H.C.; Lopes, R.D.; et al. RE-SPECT ESUS Steering Committee and Investigators. Predictors of Atrial Fibrillation Development in Patients With Embolic Stroke of Undetermined Source: An Analysis of the RE-SPECT ESUS Trial. Circulation 2021, 144, 1738–1746. [Google Scholar] [CrossRef] [PubMed]
- Ntaios, G.; Baumgartner, H.; Doehner, W.; Donal, E.; Edvardsen, T.; Healey, J.S.; Iung, B.; Kamel, H.; Kasner, S.E.; Korompoki, E.; et al. Embolic strokes of undetermined source: A clinical consensus statement of the ESC Council on Stroke, the European Association of Cardiovascular Imaging and the European Heart Rhythm Association of the ESC. Eur. Heart J. 2024, 45, 1701–1715. [Google Scholar] [CrossRef]
- Paciaroni, M.; Kamel, H. Do the Results of RE-SPECT ESUS Call for a Revision of the Embolic Stroke of Undetermined Source Definition? Stroke 2019, 50, 1032–1033. [Google Scholar] [CrossRef]
- Geisler, T.; Keller, T.; Martus, P.; Poli, K.; Serna-Higuita, L.M.; Schreieck, J.; Gawaz, M.; Tünnerhoff, J.; Bombach, P.; Nägele, T.; et al. Apixaban versus Aspirin for Embolic Stroke of Undetermined Source. NEJM Evid. 2024, 3, EVIDoa2300235. [Google Scholar] [CrossRef]
| Classification | Definition | Required Diagnostic Workup | Reference |
|---|---|---|---|
| Cryptogenic stroke (TOAST) | Ischemic stroke of undetermined etiology: Either:
| Brain imaging (CT/MRI) Electrocardiogram (ECG) Echocardiogram Vascular imaging Relevant laboratory tests | Adams et al. (1993) [1] |
| Embolic stroke of undetermined source (ESUS) | Non-lacunar ischemic stroke without
| Brain CT/MRI Vascular imaging of head/neck 12-lead ECG and ≥24 h cardiac monitoring (telemetry/Holter) Transthoracic echo (±TEE) Laboratory testing | (Hart et al., 2014) [2]; (Ntaios et al., 2020) [4] |
| Study (Year) | Population | Prevalence of ESUS | Key Characteristics of ESUS Patients | Reference |
|---|---|---|---|---|
| Hart et al. (2017)—Systematic Review | 9 studies (multi-national) of ischemic stroke patients (2014–2016 data) | 9–25% (average ~17% of ischemic strokes) | Mean age ~65 years ~42% female mostly mild strokes (mean NIHSS ~5). Average annual recurrence ~4.5% on antiplatelet therapy. | (Hart et al., 2017) [9] |
| Perera et al. (2016)—ESUS Global Registry | 2144 stroke patients from 19 countries; hospital-based registries | 16% met ESUS criteria 14% cryptogenic with incomplete workup) | Mean age 62 for ESUS vs. 68 for non-ESUS strokes 64% hypertension 25% diabetes in ESUS median NIHSS 4 (mild). 90% discharged on antiplatelet, 7% on anticoagulant. | (Perera et al., 2016) [10] |
| Ntaios (2020)—JACC Review (summary of prior data) | Overview of multiple cohorts | ~17% of all ischemic strokes (consistent with prior findings) | ESUS patients typically younger than those with cardioembolic or atherothrombotic strokes fewer conventional risk factors lower stroke severity Annual recurrence ~4–5%. | (Ntaios, 2020) [4] |
| Yaghi et al. (2019)—Young ESUS Cohort (post-hoc analysis) | Young adult (≤50) stroke patients (multi-center) | Varied (ESUS was a common cryptogenic subtype in young) | Young ESUS patients had lower recurrence risk than older ESUS risk factor profile differed by age (younger had fewer vascular risk factors, more often PFO). | (Yaghi et al., 2019) * [11] |
| Various Regional Registries—e.g., China, India (2018–2022) | Country-specific hospital stroke registries | ~15–20% (similar to global data, with some variability) | Some ethnic differences noted: e.g., South Asian ESUS patients present at a younger age and may more often have covert cardiogenic sources (Bang et al., 2014 [12]; Kaul et al., 2019 [13]) *. Overall risk factors (hypertension, etc.) present but slightly less prevalent than in non-ESUS stroke. | (Bang et al., 2014) [12] [for approach]; (Schulz, 2019) * [6] |
| Category | AHA/ASA 2021 Guidelines | ESO 2022 Guidelines |
|---|---|---|
| Definition of ESUS | Defined as a non-lacunar infarct with no identifiable cause despite standard evaluation. | Adopts same ESUS definition but emphasizes the need for comprehensive diagnostic workup. |
| Initial treatment | Recommends antiplatelet therapy (aspirin) as standard secondary prevention. | Supports antiplatelet therapy; routine use of anticoagulation not recommended. |
| Anticoagulation in ESUS | DOACs not recommended unless specific indication (e.g., detected AF). | Recommends against routine DOAC use based on NAVIGATE/RE-SPECT outcomes. |
| AF monitoring | Reasonable to pursue extended monitoring (≥30 days) in cryptogenic stroke. | Advocates for extended monitoring (e.g., ILR) in selected patients, especially those ≥60 years. |
| Role of PFO | Considers PFO closure in patients <60 with high-risk PFO features and no other cause. | Similar stance: supports closure in young patients with high-risk PFO. |
| Workup recommendations | Suggests imaging, cardiac testing, and prolonged monitoring to identify stroke cause. | Emphasizes exhaustive diagnostics including TEE and MRI if suspicion for hidden cause. |
| Use of ESUS label | Used as an operational term, not as an endpoint. Continual diagnostic pursuit recommended. | Warns against overreliance on ESUS label; encourages refining cause where possible. |
| Diagnostic Domain | Tool/Technique | Purpose | Key Insights/Examples |
|---|---|---|---|
| 1. Cardiac monitoring |
| Detect occult atrial fibrillation (AF) |
|
| 2. Structural cardiac imaging |
| Identify PFO, atrial septal aneurysm, aortic atheroma, tumors, thrombi, cardiomyopathy |
|
| 3. Vascular imaging |
| Rule out large artery disease; detect culprit non-stenotic plaques |
|
| 4. Laboratory evaluation |
| Identify hypercoagulable states, malignancy, or autoimmune vasculopathy |
|
| 5. Neuroimaging patterns |
| Infer embolic mechanism based on lesion distribution |
|
| Trial | Intervention | Sample Size | Primary Outcome (Recurrent Stroke) | Major Bleeding Rate | Conclusion |
|---|---|---|---|---|---|
| NAVIGATE ESUS | Rivaroxaban vs. aspirin | 7213 | No significant difference 4.7%/yr vs. 4.7%/yr | Higher with rivaroxaban (1.8% vs. 0.7%) | No benefit of anticoagulation; higher bleeding risk |
| RE-SPECT ESUS | Dabigatran vs. aspirin | 5390 | No significant difference 4.1%/yr vs. 4.8%/yr HR 0.85, p = 0.10 | Similar (dabigatran had more minor bleeds) | Trend toward benefit with dabigatran; not significant |
| ARCADIA | Apixaban vs. aspirin | ~1100 | No significant difference ~4.4%/yr in both groups | No significant difference | No benefit in atrial cardiopathy subgroup |
| ATTICUS | Apixaban vs. aspirin | 373 | No significant difference ~13.6% vs. ~16.0% | 2.8% in apixaban group vs. 4.0% in aspirin group | No benefit of apixaban over aspirin; similar bleeding risk |
| Controversy | Clinical Approach | Pros | Cons |
|---|---|---|---|
| Empiric anticoagulation | Direct oral anticoagulants (DOACs) vs. antiplatelets |
|
|
| PFO closure | Percutaneous device closure in select ESUS cases |
|
|
| Atrial cardiopathy | Use of biomarkers (e.g., LA size, NT-proBNP) to guide therapy |
|
|
| Duration of cardiac monitoring | 24 h Holter vs. prolonged or implantable loop recorders |
|
|
| ESUS as a clinical entity | Whether ESUS should be retained as a diagnostic label |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sargu, G.D.; Covali, R.; Filip, C.; Butureanu, T.; Akad, M.; Păvăleanu, I.; Cucu, A.I.; Bobu, A.M.; Riscanu, L.; Lacatusu, D.; et al. Embolic Stroke of Undetermined Source (ESUS): Exploring the Neurocardiological Axis and Its Clinical Implications. Medicina 2025, 61, 1252. https://doi.org/10.3390/medicina61071252
Sargu GD, Covali R, Filip C, Butureanu T, Akad M, Păvăleanu I, Cucu AI, Bobu AM, Riscanu L, Lacatusu D, et al. Embolic Stroke of Undetermined Source (ESUS): Exploring the Neurocardiological Axis and Its Clinical Implications. Medicina. 2025; 61(7):1252. https://doi.org/10.3390/medicina61071252
Chicago/Turabian StyleSargu, Gabriela Dumachita, Roxana Covali, Cristiana Filip, Tudor Butureanu, Mona Akad, Ioana Păvăleanu, Andrei Ionuț Cucu, Amelian Mădălin Bobu, Laura Riscanu, Diana Lacatusu, and et al. 2025. "Embolic Stroke of Undetermined Source (ESUS): Exploring the Neurocardiological Axis and Its Clinical Implications" Medicina 61, no. 7: 1252. https://doi.org/10.3390/medicina61071252
APA StyleSargu, G. D., Covali, R., Filip, C., Butureanu, T., Akad, M., Păvăleanu, I., Cucu, A. I., Bobu, A. M., Riscanu, L., Lacatusu, D., Irina Smihor, M., & Popa, R. (2025). Embolic Stroke of Undetermined Source (ESUS): Exploring the Neurocardiological Axis and Its Clinical Implications. Medicina, 61(7), 1252. https://doi.org/10.3390/medicina61071252





