Sex-Related Differences in Chronic Pain: A Narrative Review by a Multidisciplinary Task Force
Abstract
1. Introduction
- Summarize current evidence on sex-related differences in chronic pain epidemiology, mechanisms, and treatment response;
- Identify existing research gaps and barriers in clinical practice;
- Provide expert-driven suggestions for implementing more personalized and equitable approaches in chronic pain management.
2. Materials and Methods
3. Results and Discussion
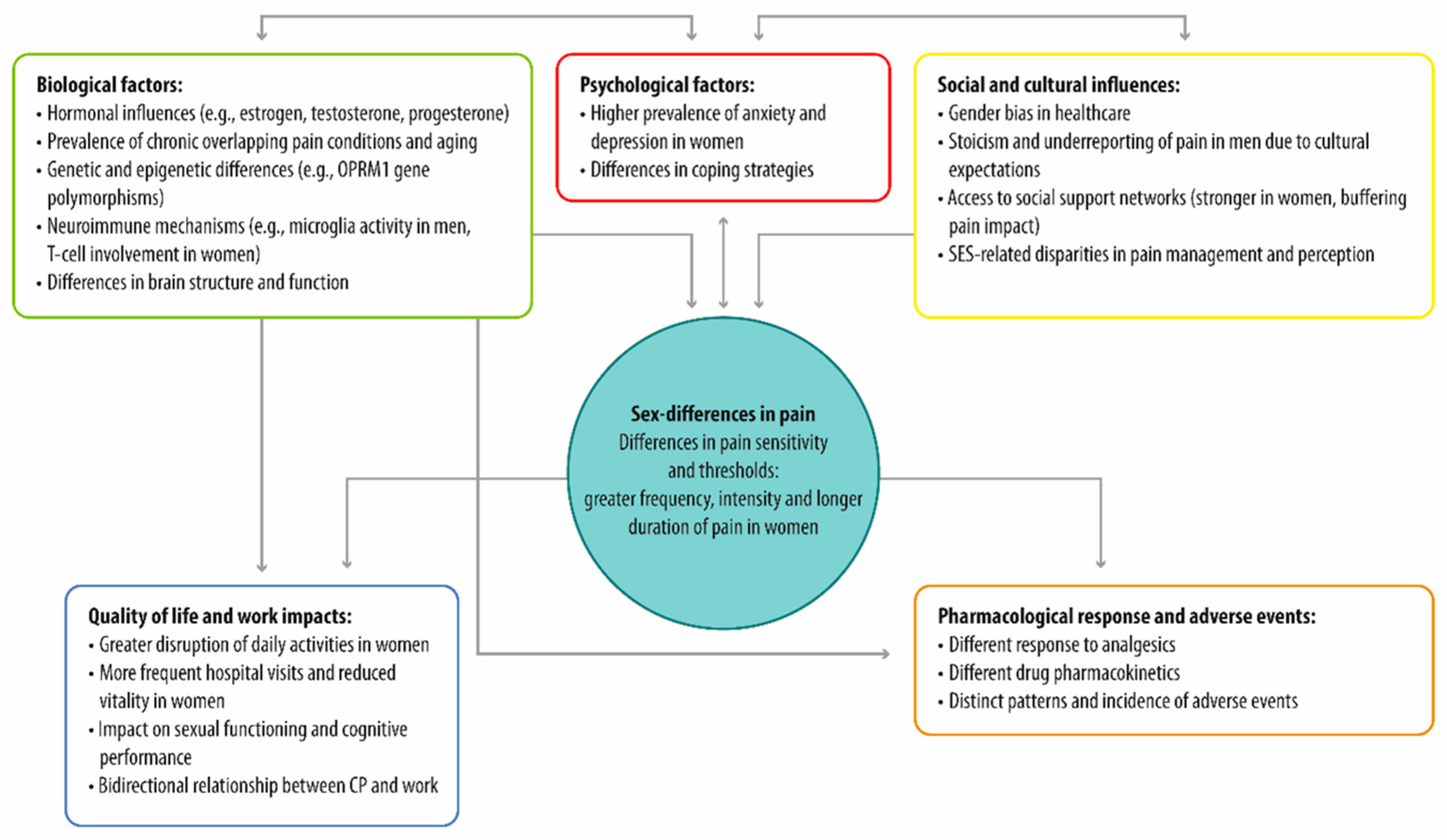
3.1. Sex Differences in Chronic Pain: Prevalence, Perception, and Underlying Factors
3.2. Biological and Psycho-Social Factors Could Drive Sex Differences in Chronic Pain
3.2.1. Biological Factors
3.2.2. Psycho-Social Factors
3.3. Pharmacological Approaches to Chronic Pain Management
3.3.1. Sex Differences in Chronic Pain Treatment Response
3.3.2. Sex Differences in Adverse Drug Reactions and Pharmacokinetics in Chronic Pain Management
3.3.3. Psychological Interventions in Chronic Pain: Enhancing Quality of Life Through Tailored Therapies and a Multidisciplinary Approach
- Operant-behavioral therapy: Focuses on modifying behaviors that reinforce the pain experience [153].
- Cognitive behavioral therapy (CBT): Helps patients reframe negative thoughts about pain and develop coping strategies [154].
- Mindfulness-based stress reduction (MBSR): Uses mindfulness techniques to reduce stress and promote emotional well-being [155].
- Acceptance and commitment therapy (ACT): Encourages patients to accept pain and commit to living a meaningful life despite it [155].
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACT | Acceptance and commitment therapy |
| AE | Adverse effect |
| CBT | Cognitive behavioral therapy |
| COMT | Catechol-O-methyltransferase |
| CP | Chronic pain |
| HPA | Hypothalamic–pituitary–adrenal |
| IASP | International Association for the Study of Pain |
| MBSR | Mindfulness-based stress reduction |
| MOR | µ-opioid receptors |
| NIH | National Institutes of Health |
| NSAIDs | Non-steroidal anti-inflammatory drugs |
| OPRM1 | Opioid receptor M 1 |
| OXN | Oxycodone/naloxone |
| PCA | Patient-controlled analgesia |
| QoL | Quality of life |
| SES | Socio-economic status |
References
- IASP Revises Its Definition of Pain for the First Time Since 1979. Available online: https://www.iasp-pain.org/wp-content/uploads/2022/04/revised-definition-flysheet_R2-1-1-1.pdf (accessed on 23 May 2024).
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The Revised IASP definition of pain: Concepts, challenges, and compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef]
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef]
- Zimmer, Z.; Fraser, K.; Grol-Prokopczyk, H.; Zajacova, A. A global study of pain prevalence across 52 countries: Examining the role of country-level contextual factors. Pain 2022, 163, 1740–1750. [Google Scholar] [CrossRef]
- Rikard, S.M.; Strahan, A.E.; Schmit, K.M.; Gery, P.; Guy, J. Chronic Pain Among Adults—United States, 2019–2021. Morb. Mortal. Wkly. Rep. 2023, 72, 379. [Google Scholar] [CrossRef]
- Breivik, H.; Collett, B.; Ventafridda, V.; Cohen, R.; Gallacher, D. Survey of chronic pain in Europe: Prevalence, impact on daily life, and treatment. Eur. J. Pain. 2006, 10, 287–333. [Google Scholar] [CrossRef]
- Osborne, N.R.; Davis, K.D. Sex and gender differences in pain. Int. Rev. Neurobiol. 2022, 164, 277–307. [Google Scholar] [CrossRef]
- Mundal, I.; Gråwe, R.W.; Bjørngaard, J.H.; Linaker, O.M.; Fors, E.A. Prevalence and long-term predictors of persistent chronic widespread pain in the general population in an 11-year prospective study: The HUNT study. BMC Musculoskelet. Disord. 2014, 15, 213. [Google Scholar] [CrossRef]
- Andrews, P.; Steultjens, M.; Riskowski, J. Chronic widespread pain prevalence in the general population: A systematic review. Eur. J. Pain 2018, 22, 5–18. [Google Scholar] [CrossRef]
- Øverås, C.; Johansson, M.; de Campos, T.; Ferreira, M.L.; Natvig, B.; Mork, P.J.; Hartvigsen, J. Distribution and prevalence of musculoskeletal pain co-occurring with persistent low back pain: A systematic review. BMC Musculoskelet. Disord. 2021, 22, 91. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, K.E.; Sim, J.; Jordan, J.L.; Jordan, K.P. A systematic review and meta-analysis of the prevalence of chronic widespread pain in the general population. Pain 2016, 157, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Picavet, H.S.J.; Monique Verschuren, W.M.; Groot, L.; Schaap, L.; van Oostrom, S.H. Pain over the adult life course: 15-year pain trajectories—The Doetinchem Cohort Study. Eur. J. Pain 2019, 23, 1723–1732. [Google Scholar] [CrossRef]
- Pandelani, F.F.; Nyalunga, S.L.N.; Mogotsi, M.M.; Mkhatshwa, V.B. Chronic pain: Its impact on the quality of life and gender. Front. Pain Res. 2023, 4, 1253460. [Google Scholar] [CrossRef] [PubMed]
- Umeda, M.; Kim, Y. Gender Differences in the Prevalence of Chronic Pain and Leisure Time Physical Activity Among US Adults: A NHANES Study. Int. J. Environ. Res. Public Health 2019, 16, 988. [Google Scholar] [CrossRef] [PubMed]
- Casale, R.; Atzeni, F.; Bazzichi, L.; Beretta, G.; Costantini, E.; Sacerdote, P.; Tassorelli, C. Pain in Women: A Perspective Review on a Relevant Clinical Issue that Deserves Prioritization. Pain Ther. 2021, 10, 287–314. [Google Scholar] [CrossRef] [PubMed]
- 1° Rapporto Censis-Grünenthal. Vivere Senza dolore. Il Significato Sociale del Dolore e le Aspettative di Soluzioni Efficaci. 2023. Available online: https://www.censis.it/sites/default/files/downloads/Vivere%20senza%20dolore_rapporto%20integrale.pdf (accessed on 15 October 2024).
- Rapporti ISTISAN 23/28. Dolore Cronico in Italia e suoi Correlati Psicosociali dalla “Indagine Europea Sulla Salute” (European Health Interview Survey 2019). Toccaceli, V., Francia, N., Cascavilla, I., Tenti, M., per il Gruppo di Lavoro Interistituzionale ISS-ISTAT-ISAL per lo Studio e la Ricerca sul Dolore Cronico. Available online: https://www.iss.it/documents/20126/9340234/23-28+web.pdf/cbc3f0b5-80e2-eddd-a6fe-94a923e2e731?t=1711719747588 (accessed on 15 October 2024).
- Conaghan, P.G.; Peloso, P.M.; Everett, S.V.; Rajagopalan, S.; Black, C.M.; Mavros, P.; Arden, N.K.; Phillips, C.J.; Rannou, F.; van de Laar, M.A.; et al. Inadequate pain relief and large functional loss among patients with knee osteoarthritis: Evidence from a prospective multinational longitudinal study of osteoarthritis real-world therapies. Rheumatology 2015, 54, 270–277. [Google Scholar] [CrossRef]
- Vitaloni, M.; Botto-van Bemden, A.; Sciortino, R.; Carné, X.; Quintero, M.; Santos-Moreno, P.; Espinosa, R.; Rillo, O.; Monfort, J.; de Abajo, F.; et al. A patients’ view of OA: The Global Osteoarthritis Patient Perception Survey (GOAPPS), a pilot study. BMC Musculoskelet. Disord. 2020, 21, 727. [Google Scholar] [CrossRef]
- Majedi, H.; Dehghani, S.S.; Soleyman-Jahi, S.; Tafakhori, A.; Emami, S.A.; Mireskandari, M.; Hosseini, S.M. Assessment of Factors Predicting Inadequate Pain Management in Chronic Pain Patients. Anesth. Pain Med. 2019, 9, e9. [Google Scholar] [CrossRef]
- Costa, D.; Cruz, E.; da Nunes da Silva, C.; Lopes, D.; Henriques, A.; Luis, D.; Canhão, H.; Branco, J.; Nunes, C.; Rodrigues, A. Prevalence and factors associated with inadequate pain relief in people with hip or knee osteoarthritis: A cross-sectional population based study. Osteoarthr. Cartil. 2022, 30, S13–S14. [Google Scholar] [CrossRef]
- Mogil, J.S. Qualitative sex differences in pain processing: Emerging evidence of a biased literature. Nat. Rev. Neurosci. 2020, 21, 353–365. [Google Scholar] [CrossRef]
- Samulowitz, A.; Gremyr, I.; Eriksson, E.; Hensing, G. “Brave Men” and “Emotional Women”: A Theory-Guided Literature Review on Gender Bias in Health Care and Gendered Norms towards Patients with Chronic Pain. Pain Res. Manag. 2018, 2018, 6358624. [Google Scholar] [CrossRef]
- Bartley, E.J.; Fillingim, R.B. Sex differences in pain: A brief review of clinical and experimental findings. Br. J. Anaesth. 2013, 111, 52–58. [Google Scholar] [CrossRef]
- Melchior, M.; Poisbeau, P.; Gaumond, I.; Marchand, S. Insights into the mechanisms and the emergence of sex-differences in pain. Neuroscience 2016, 338, 63–80. [Google Scholar] [CrossRef]
- Breivik, H.; Eisenberg, E.; O’Brien, T. The individual and societal burden of chronic pain in Europe: The case for strategic prioritisation and action to improve knowledge and availability of appropriate care. BMC Public Health 2013, 13, 1229. [Google Scholar] [CrossRef] [PubMed]
- Allegri, M.; Lucioni, C.; Mazzi, S.; Serra, G. Social cost of chronic pain in Italy. Glob. Reg. Health Technol. Assess. 2015, 2, GRHTA.5000187. [Google Scholar] [CrossRef]
- Athnaiel, O.; Cantillo, S.; Paredes, S.; Knezevic, N.N. The Role of Sex Hormones in Pain-Related Conditions. Int. J. Mol. Sci. 2023, 24, 1866. [Google Scholar] [CrossRef]
- Barnett, K.; Mercer, S.W.; Norbury, M.; Watt, G.; Wyke, S.; Guthrie, B. Epidemiology of multimorbidity and implications for health care, research, and medical education: A cross-sectional study. Lancet 2012, 380, 37–43. [Google Scholar] [CrossRef]
- Dagnino, A.P.A.; Campos, M.M. Chronic Pain in the Elderly: Mechanisms and Perspectives. Front. Hum. Neurosci. 2022, 16, 736688. [Google Scholar] [CrossRef]
- Sorge, R.E.; Mapplebeck, J.C.S.; Rosen, S.; Beggs, S.; Taves, S.; Alexander, J.K.; Martin, L.J.; Austin, J.S.; Sotocinal, S.G.; Chen, D.; et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat. Neurosci. 2015, 18, 1081–1083. [Google Scholar] [CrossRef]
- Mogil, J.S.; Parisien, M.; Esfahani, S.J.; Diatchenko, L. Sex differences in mechanisms of pain hypersensitivity. Neurosci. Biobehav. Rev. 2024, 163, 105749. [Google Scholar] [CrossRef]
- Barcelon, E.; Chung, S.; Lee, J.; Lee, S.J. Sexual Dimorphism in the Mechanism of Pain Central Sensitization. Cells 2023, 12, 2028. [Google Scholar] [CrossRef]
- Mauvais-Jarvis, F.; Berthold, H.K.; Campesi, I.; Carrero, J.J.; Dakal, S.; Franconi, F.; Gouni-Berthold, I.; Heiman, M.L.; Kautzky-Willer, A.; Klein, S.L.; et al. Sex- and Gender-Based Pharmacological Response to Drugs. Pharmacol. Rev. 2021, 73, 730. [Google Scholar] [CrossRef]
- Fullerton, E.F.; Doyle, H.H.; Murphy, A.Z. Impact of sex on pain and opioid analgesia: A review. Curr. Opin. Behav. Sci. 2018, 23, 183–190. [Google Scholar] [CrossRef]
- Fillingim, R.B.; Gear, R.W. Sex differences in opioid analgesia: Clinical and experimental findings. Eur. J. Pain 2004, 8, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Aubrun, F.; Salvi, N.; Coriat, P.; Riou, B. Sex- and age-related differences in morphine requirements for post-operative pain relief. Anesthesiology 2005, 103, 156–160. [Google Scholar] [CrossRef]
- Cepeda, M.S.; Carr, D.B. Women experience more pain and require more morphine than men to achieve a similar degree of analgesia. Anesth. Analg. 2003, 97, 1464–1468. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Losin, E.A.R.; Ashar, Y.K.; Koban, L.; Wager, T.D. Gender Biases in Estimation of Others’ Pain. J. Pain 2021, 22, 1048–1059. [Google Scholar] [CrossRef]
- Wesolowicz, D.M.; Clark, J.F.; Boissoneault, J.; Robinson, M.E. The roles of gender and profession on gender role expectations of pain in health care professionals. J. Pain Res. 2018, 11, 1121–1128. [Google Scholar] [CrossRef]
- Alabas, O.A.; Tashani, O.A.; Tabasam, G.; Johnson, M.I. Gender role affects experimental pain responses: A systematic review with meta-analysis. Eur. J. Pain 2012, 16, 1211–1223. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.E.; Gagnon, C.M.; Riley, J.L.; Price, D.D. Altering gender role expectations: Effects on pain tolerance, pain threshold, and pain ratings. J. Pain 2003, 4, 284–288. [Google Scholar] [CrossRef]
- Janevic, M.R.; McLaughlin, S.J.; Heapy, A.A.; Thacker, C.; Piette, J.D. Racial and Socio-economic Disparities in Disabling Chronic Pain: Findings From the Health and Retirement Study. J. Pain 2017, 18, 1459–1467. [Google Scholar] [CrossRef]
- Khalatbari-Soltani, S.; Blyth, F.M. Socio-economic position and pain: A topical review. Pain 2022, 163, 1855–1861. [Google Scholar] [CrossRef]
- Diniz, E.; Castro, P.; Bousfield, A.; Figueira Bernardes, S. Classism and dehumanization in chronic pain: A qualitative study of nurses’ inferences about women of different socio-economic status. Br. J. Health Psychol. 2020, 25, 152–170. [Google Scholar] [CrossRef] [PubMed]
- Bernardes, S.F.; Tomé-Pires, C.; Brandão, T.; Campos, L.; Teixeira, F.; Goubert, L. Classism in pain assessment and management: The mediating role of female patient dehumanization and perceived life hardship. Pain 2021, 162, 2854–2864. [Google Scholar] [CrossRef] [PubMed]
- Dorner, T.E.; Stein, K.V.; Hahne, J.; Wepner, F.; Friedrich, M.; Mittendorfer-Rutz, E. How are socio-demographic and psycho-social factors associated with the prevalence and chronicity of severe pain in 14 different body sites? A cross-sectional population-based survey. Wien. Klin. Wochenschr. 2018, 130, 14–22. [Google Scholar] [CrossRef]
- Beery, A.K.; Zucker, I. Sex bias in neuroscience and biomedical research. Neurosci. Biobehav. Rev. 2011, 35, 565–572. [Google Scholar] [CrossRef]
- Becker, J.B.; Prendergast, B.J.; Liang, J.W. Female rats are not more variable than male rats: A meta-analysis of neuroscience studies. Biol. Sex. Differ. 2016, 7, 34. [Google Scholar] [CrossRef] [PubMed]
- Prendergast, B.J.; Onishi, K.G.; Zucker, I. Female mice liberated for inclusion in neuroscience and biomedical research. Neurosci. Biobehav. Rev. 2014, 40, 1–5. [Google Scholar] [CrossRef]
- Greenspan, J.D.; Craft, R.M.; LeResche, L.; Arendt-Nielsen, L.; Berkley, K.J.; Fillingim, R.B.; Gold, M.S.; Holdcroft, A.; Lautenbacher, S.; Mayer, E.A.; et al. Studying sex and gender differences in pain and analgesia: A consensus report. Pain 2007, 132 (Suppl. S1), S26–S45. [Google Scholar] [CrossRef]
- Arnegard, M.E.; Whitten, L.A.; Hunter, C.; Clayton, J.A. Sex as a Biological Variable: A 5-Year Progress Report and Call to Action. J. Women’s Health (Larchmt) 2020, 29, 858–864. [Google Scholar] [CrossRef]
- Todd, A.; McNamara, C.L.; Balaj, M.; Huijts, T.; Akhter, N.; Thomson, K.; Kasim, A.; Eikemo, T.A.; Bambra, C. The European epidemic: Pain prevalence and socio-economic inequalities in pain across 19 European countries. Eur. J. Pain 2019, 23, 1425–1436. [Google Scholar] [CrossRef]
- Tsang, A.; Von Korff, M.; Lee, S.; Alonso, J.; Karam, E.; Angermeyer, M.C.; Borges, G.L.; Bromet, E.J.; Demytteneare, K.; de Girolamo, G.; et al. Common chronic pain conditions in developed and developing countries: Gender and age differences and comorbidity with depression-anxiety disorders. J. Pain 2008, 9, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Dueñas, M.; Moral-Munoz, J.A.; Palomo-Osuna, J.; Salazar, A.; De Sola, H.; Failde, I. Differences in physical and psychological health in patients with chronic low back pain: A national survey in general Spanish population. Qual. Life Res. 2020, 29, 2935–2947. [Google Scholar] [CrossRef] [PubMed]
- Collado, A.; Gomez, E.; Coscolla, R.; Sunyol, R.; Solé, E.; Rivera, J.; Altarriba, E.; Carbonell, J.; Castells, X. Work, family and social environment in patients with Fibromyalgia in Spain: An epidemiological study: EPIFFAC study. BMC Health Serv. Res. 2014, 14, 513. [Google Scholar] [CrossRef] [PubMed]
- Kaergaard, A.; Hansen, A.M.; Rasmussen, K.; Andersen, J.H. Association between plasma testosterone and work-related neck and shoulder disorders among female workers. Scand. J. Work. Environ. Health 2000, 26, 292–298. [Google Scholar] [CrossRef]
- Maranini, B.; Bortoluzzi, A.; Silvagni, E.; Govoni, M. Focus on Sex and Gender: What We Need to Know in the Management of Rheumatoid Arthritis. J. Pers. Med. 2022, 12, 499. [Google Scholar] [CrossRef]
- Segal, N.A.; Nilges, J.M.; Oo, W.M. Sex differences in osteoarthritis prevalence, pain perception, physical function and therapeutics. Osteoarthr. Cartil. 2024, 32, 1045–1053. [Google Scholar] [CrossRef]
- Al Sharie, S.; Varga, S.J.; Al-Husinat, L.; Sarzi-Puttini, P.; Araydah, M.; Bal’awi, B.R.; Varrassi, G. Unraveling the Complex Web of Fibromyalgia: A Narrative Review. Medicina 2024, 60, 272. [Google Scholar] [CrossRef]
- Al-Hassany, L.; Haas, J.; Piccininni, M.; Kurth, T.; Maassen Van Den Brink, A.; Rohmann, J.L. Giving Researchers a Headache—Sex and Gender Differences in Migraine. Front. Neurol. 2020, 11, 549038. [Google Scholar] [CrossRef]
- Stovner, L.J.; Hagen, K.; Linde, M.; Steiner, T.J. The global prevalence of headache: An update, with analysis of the influences of methodological factors on prevalence estimates. J. Headache Pain 2022, 23, 34. [Google Scholar] [CrossRef]
- Bizzoca, D.; Solarino, G.; Pulcrano, A.; Brunetti, G.; Moretti, A.M.; Moretti, L.; Piazzolla, A.; Moretti, B. Gender-Related Issues in the Management of Low-Back Pain: A Current Concepts Review. Clin. Pract. 2023, 13, 1360–1368. [Google Scholar] [CrossRef]
- Zieliński, G.; Pająk-Zielińska, B.; Ginszt, M. A Meta-Analysis of the Global Prevalence of Temporomandibular Disorders. J. Clin. Med. 2024, 13, 1365. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.-S.; Noor, N.; Urits, I.; Paladini, A.; Sadhu, M.S.; Gibb, C.; Carlson, T.; Myrcik, D.; Varrassi, G.; Viswanath, O. Complex Regional Pain Syndrome: A Comprehensive Review. Pain Ther. 2021, 10, 875–892. [Google Scholar] [CrossRef] [PubMed]
- Kohorst, J.J.; Bruce, A.J.; Torgerson, R.R.; Schenck, L.A.; Davis, M.D.P. The prevalence of burning mouth syndrome: A population-based study. Br. J. Dermatol. 2015, 172, 1654–1656. [Google Scholar] [CrossRef]
- Salis, F.; Sardo, S.; Finco, G.; Gessa, G.L.; Franconi, F.; Agabio, R. Sex-Gender Differences Are Completely Neglected in Treatments for Neuropathic Pain. Pharmaceuticals 2024, 17, 838. [Google Scholar] [CrossRef]
- Ayorinde, A.A.; Bhattacharya, S.; Druce, K.L.; Jones, G.T.; Macfarlane, G.J. Chronic pelvic pain in women of reproductive and post-reproductive age: A population-based study. Eur. J. Pain 2017, 21, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Viganò, P.; Parazzini, F.; Somigliana, E.; Vercellini, P. Endometriosis: Epidemiology and aetiological factors. Best. Pract. Res. Clin. Obstet. Gynaecol. 2004, 18, 177–200. [Google Scholar] [CrossRef]
- Windgassen, S.S.; Sutherland, S.; Finn, M.T.M.; Bonnet, K.R.; Schlundt, D.G.; Reynolds, W.S.; Dmochowski, R.R.; McKernan, L.C. Gender differences in the experience of interstitial cystitis/bladder pain syndrome. Front. Pain Res. 2022, 3, 954967. [Google Scholar] [CrossRef]
- Kim, S.A.; Choi, S.Y.; Youn, M.S.; Pozo-Rosich, P.; Lee, M.J. Epidemiology, burden and clinical spectrum of cluster headache: A global update. Cephalalgia 2023, 43, 3331024231201577. [Google Scholar] [CrossRef]
- Schofield, P. The Assessment of Pain in Older People: UK National Guidelines. Age Ageing 2018, 47, i1–i22. [Google Scholar] [CrossRef]
- Larsson, C.; Hansson, E.E.; Sundquist, K.; Jakobsson, U. Chronic pain in older adults: Prevalence, incidence, and risk factors. Scand. J. Rheumatol. 2017, 46, 317–325. [Google Scholar] [CrossRef]
- Wettstein, M.; Ghisletta, P.; Gerstorf, D. Feeling older, feeling pain? Reciprocal between-person and within-person associations of pain and subjective age in the second half of life. Psychol. Aging 2024, 39, 510. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Gu, N.; Zhou, L.; Eyo, U.B.; Murugan, M.; Gan, W.B.; Wu, L.J. Microglia and monocytes synergistically promote the transition from acute to chronic pain after nerve injury. Nat. Commun. 2016, 7, 12029. [Google Scholar] [CrossRef]
- Yi, M.-H.; Liu, Y.U.; Umpierre, A.D.; Chen, T.; Ying, Y.; Zheng, J.; Dheer, A.; Bosco, D.B.; Dong, H.; Wu, L.J. Optogenetic activation of spinal microglia triggers chronic pain in mice. PLoS Biol. 2021, 19, e3001154. [Google Scholar] [CrossRef]
- Batti, L.; Sundukova, M.; Murana, E.; Pimpinella, S.; De Castro Reis, F.; Pagani, F.; Wang, H.; Pellegrino, E.; Perlas, E.; Di Angelantonio, S.; et al. TMEM16F Regulates Spinal Microglial Function in Neuropathic Pain States. Cell Rep. 2016, 15, 2608. [Google Scholar] [CrossRef]
- What Is Gender? What Is Sex? Available online: https://cihr-irsc.gc.ca/e/48642.html (accessed on 6 July 2024).
- Sandberg, D.E.; Gardner, M. Differences/Disorders of Sex Development: Medical Conditions at the Intersection of Sex and Gender. Annu. Rev. Clin. Psychol. 2022, 18, 201–231. [Google Scholar] [CrossRef] [PubMed]
- Guzikevits, M.; Gordon-Hecker, T.; Rekhtman, D.; Salameh, S.; Israel, S.; Shayo, M.; Gozal, D.; Perry, A.; Gileles-Hillel, A.; Choshen-Hillel, S. Sex bias in pain management decisions. Proc. Natl. Acad. Sci. USA 2024, 121, e2401331121. [Google Scholar] [CrossRef]
- Al Hamid, A.; Beckett, R.; Wilson, M.; Jalal, Z.; Cheema, E.; Al-Jumeily Obe, D.; Coombs, T.; Ralebitso-Senior, K.; Assi, S. Gender Bias in Diagnosis, Prevention, and Treatment of Cardiovascular Diseases: A Systematic Review. Cureus 2024, 16, e54264. [Google Scholar] [CrossRef]
- Jovani, V.; Blasco-Blasco, M.; Pascual, E.; Ruiz-Cantero, M.T. Challenges to conquer from the gender perspective in medicine: The case of spondyloarthritis. PLoS ONE 2018, 13, e0205751. [Google Scholar] [CrossRef] [PubMed]
- Collado-Lledó, E.; de la Cuerda, F.; Ariza-Solé, A. Sex Differences in Acute Heart Failure Management: Is There a Gap in Treatment Quality? Curr. Heart Fail. Rep. 2023, 20, 121–128. [Google Scholar] [CrossRef]
- Ruiz-Cantero, M.T.; Blasco-Blasco, M.; Chilet-Rosell, E.; Peiró, A.M. Gender bias in therapeutic effort: From research to health care. Farm. Hosp. 2020, 44, 109–113. [Google Scholar] [CrossRef]
- Myers, C.D.; Riley, J.L.; Robinson, M.E. Psycho-social contributions to sex-correlated differences in pain. Clin. J. Pain 2003, 19, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Blyth, F.M. Chronic pain--is it a public health problem? Pain 2008, 137, 465–466. [Google Scholar] [CrossRef] [PubMed]
- Poleshuck, E.L.; Green, C.R. Socio-economic disadvantage and pain. Pain 2008, 136, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Maly, A.; Vallerand, A.H. Neighborhood, Socio-economic, and Racial Influence on Chronic Pain. Pain Manag. Nurs. 2018, 19, 14–22. [Google Scholar] [CrossRef]
- Langley, P.C. The societal burden of pain in Germany: Health-related quality-of-life, health status and direct medical costs. J. Med. Econ. 2012, 15, 1201–1215. [Google Scholar] [CrossRef]
- Macfarlane, G.J.; Beasley, M.; Smith, B.H.; Jones, G.T.; Macfarlane, T.V. Can large surveys conducted on highly selected populations provide valid information on the epidemiology of common health conditions? An analysis of UK Biobank data on musculoskeletal pain. Br. J. Pain 2015, 9, 203–212. [Google Scholar] [CrossRef]
- Huang, G.; Le, A.-L.; Goddard, Y.; James, D.; Thavorn, K.; Payne, M.; Chen, I. A Systematic Review of the Cost of Chronic Pelvic Pain in Women. J. Obstet. Gynaecol. Can. 2022, 44, 286–293.e3. [Google Scholar] [CrossRef]
- Missmer, S.A.; Tu, F.F.; Agarwal, S.K.; Chapron, C.; Soliman, A.M.; Chiuve, S.; Eichner, S.; Flores-Caldera, I.; Horne, A.W.; Kimball, A.B.; et al. Impact of Endometriosis on Life-Course Potential: A Narrative Review. Int. J. Gen. Med. 2021, 14, 9–25. [Google Scholar] [CrossRef]
- de Sola, H.; Salazar, A.; Dueñas, M.; Ojeda, B.; Failde, I. Nationwide cross-sectional study of the impact of chronic pain on an individual’s employment: Relationship with the family and the social support. BMJ Open 2016, 6, e012246. [Google Scholar] [CrossRef]
- Mesas, A.E.; González, A.D.; Mesas, C.E.; de Andrade, S.M.; Magro, I.S.; del Llano, J. The association of chronic neck pain, low back pain, and migraine with absenteeism due to health problems in Spanish workers. Spine (Phila Pa 1976) 2014, 39, 1243–1253. [Google Scholar] [CrossRef][Green Version]
- Keefe, F.J.; Lefebvre, J.C.; Egert, J.R.; Affleck, G.; Sullivan, M.J.; Caldwell, D.S. The relationship of gender to pain, pain behavior, and disability in osteoarthritis patients: The role of catastrophizing. Pain 2000, 87, 325–334. [Google Scholar] [CrossRef]
- El-Shormilisy, N.; Strong, J.; Meredith, P.J. Associations between gender, coping patterns and functioning for individuals with chronic pain: A systematic review. Pain Res. Manag. 2015, 20, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Ferreira Kdos, S.; Speciali, J.G. Epidemiology of chronic pain in the office of a pain specialist neurologist. Arq. Neuropsiquiatr. 2015, 73, 582–585. [Google Scholar] [CrossRef]
- Werneck, A.O.; Stubbs, B. Bidirectional relationship between chronic pain and depressive symptoms in middle-aged and older adults. Gen. Hosp. Psychiatry 2024, 89, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Reckziegel, D.; Abdullah, T.; Wu, B.; Wu, B.; Huang, L.; Schnitzer, T.J.; Apkarian, A.V. Hippocampus shape deformation: A potential diagnostic biomarker for chronic back pain in women. Pain 2021, 162, 1457–1467. [Google Scholar] [CrossRef] [PubMed]
- Fasick, V.; Spengler, R.N.; Samankan, S.; Nader, N.D.; Ignatowski, T.A. The hippocampus and TNF: Common links between chronic pain and depression. Neurosci. Biobehav. Rev. 2015, 53, 139–159. [Google Scholar] [CrossRef]
- Mokhtari, T.; Tu, Y.; Hu, L. Involvement of the hippocampus in chronic pain and depression. Brain Sci. Adv. 2019, 5, 288–298. [Google Scholar] [CrossRef]
- Munce, S.E.P.; Stewart, D.E. Gender Differences in Depression and Chronic Pain Conditions in a National Epidemiologic Survey. Psychosomatics 2007, 48, 394–399. [Google Scholar] [CrossRef]
- Annagür, B.B.; Uguz, F.; Apiliogullari, S.; Kara, I.; Gunduz, S. Psychiatric disorders and association with quality of sleep and quality of life in patients with chronic pain: A SCID-based study. Pain Med. 2014, 15, 772–781. [Google Scholar] [CrossRef]
- Miller, L.R.; Cano, A. Comorbid chronic pain and depression: Who is at risk? J. Pain 2009, 10, 619–627. [Google Scholar] [CrossRef]
- de Heer, E.W.; Ten Have, M.; van Marwijk, H.W.J.; Dekker, J.; de Graaf, R.; Beekman, A.T.F.; van der Feltz-Cornelis, C.M. Pain as a risk factor for common mental disorders. Results from the Netherlands Mental Health Survey and Incidence Study-2: A longitudinal, population-based study. Pain 2018, 159, 712–718. [Google Scholar] [CrossRef]
- Dueñas, M.; Ojeda, B.; Salazar, A.; Mico, J.A.; Failde, I. A review of chronic pain impact on patients, their social environment and the health care system. J. Pain Res. 2016, 9, 457–467. [Google Scholar] [CrossRef]
- Azevedo, L.F.; Costa-Pereira, A.; Mendonça, L.; Dias, C.C.; Castro-Lopes, J.M. Epidemiology of chronic pain: A population-based nationwide study on its prevalence, characteristics and associated disability in Portugal. J. Pain 2012, 13, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Dahlhamer, J. Prevalence of Chronic Pain and High-Impact Chronic Pain Among Adults—United States, 2016. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 1001–1006. [Google Scholar] [CrossRef]
- Flegge, L.G.; Barr, A.; Craner, J.R. Sexual Functioning Among Adults with Chronic Pain: Prevalence and Association with Pain-Related Outcomes. Pain Med. 2023, 24, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Palomo-Osuna, J.; De Sola, H.; Dueñas, M.; Moral-Munoz, J.A.; Failde, I. Cognitive function in diabetic persons with peripheral neuropathy: A systematic review and meta-analysis. Expert. Rev. Neurother. 2022, 22, 269–281. [Google Scholar] [CrossRef]
- Löfgren, M.; Ekholm, J.; Ohman, A. “A constant struggle”: Successful strategies of women in work despite fibromyalgia. Disabil. Rehabil. 2006, 28, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Samulowitz, A.; Hensing, G.; Haukenes, I.; Bergman, S.; Grimby-Ekman, A. General self-efficacy and social support in men and women with pain—Irregular sex patterns of cross-sectional and longitudinal associations in a general population sample. BMC Musculoskelet. Disord. 2022, 23, 1026. [Google Scholar] [CrossRef]
- Rovner, G.S.; Sunnerhagen, K.S.; Björkdahl, A.; Gerdle, B.; Börsbo, B.; Johansson, F.; Gillanders, D. Chronic pain and sex-differences; women accept and move, while men feel blue. PLoS ONE 2017, 12, e0175737. [Google Scholar] [CrossRef]
- Sycha, T.; Gustorff, B.; Lehr, S.; Tanew, A.; Eichler, H.G.; Schmetterer, L. A simple pain model for the evaluation of analgesic effects of NSAIDs in healthy subjects. Br. J. Clin. Pharmacol. 2003, 56, 165–172. [Google Scholar] [CrossRef][Green Version]
- Walker, J.S.; Carmody, J.J. Experimental pain in healthy human subjects: Gender differences in nociception and in response to ibuprofen. Anesth. Analg. 1998, 86, 1257–1262. [Google Scholar] [CrossRef] [PubMed]
- Butcher, B.E.; Carmody, J.J. Sex differences in analgesic response to ibuprofen are influenced by expectancy: A randomized, crossover, balanced placebo-designed study. Eur. J. Pain 2012, 16, 1005–1013. [Google Scholar] [CrossRef]
- Averbuch, M.; Katzper, M. A Search for Sex Differences in Response to Analgesia. Arch. Intern. Med. 2000, 160, 3424–3428. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.; Jureidini, B.; Hodges, J.; Baisden, M.; Swift, J.Q.; Bowles, W.R. Gender differences in analgesia for endodontic pain. J. Endod. 2008, 34, 552–556. [Google Scholar] [CrossRef]
- Fillingim, R.B.; Ness, T.J.; Glover, T.L.; Campbell, C.M.; Price, D.D.; Staud, R. Experimental pain models reveal no sex differences in pentazocine analgesia in humans. Anesthesiology 2004, 100, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Zubieta, J.-K.; Smith, Y.R.; Bueller, J.A.; Xu, Y.; Kilbourn, M.R.; Jewett, D.M.; Meyer, C.R.; Koeppe, R.A.; Stohler, C.S. mu-opioid receptor-mediated anti-nociceptive responses differ in men and women. J. Neurosci. 2002, 22, 5100–5107. [Google Scholar] [CrossRef]
- Loyd, D.R.; Murphy, A.Z. The neuroanatomy of sexual dimorphism in opioid analgesia. Exp. Neurol. 2014, 259, 57–63. [Google Scholar] [CrossRef]
- Kelly, M.J.; Qiu, J.; Rønnekleiv, O.K. Estrogen modulation of G-protein-coupled receptor activation of potassium channels in the central nervous system. Ann. N. Y. Acad. Sci. 2003, 1007, 6–16. [Google Scholar] [CrossRef]
- Micevych, P.E.; Rissman, E.F.; Gustafsson, J.-A.; Sinchak, K. Estrogen receptor-alpha is required for estrogen-induced mu-opioid receptor internalization. J. Neurosci. Res. 2003, 71, 802–810. [Google Scholar] [CrossRef]
- Romanescu, M.; Buda, V.; Lombrea, A.; Andor, M.; Ledeti, I.; Suciu, M.; Danciu, C.; Dehelean, C.A.; Dehelean, L. Sex-Related Differences in Pharmacological Response to CNS Drugs: A Narrative Review. J. Pers. Med. 2022, 12, 907. [Google Scholar] [CrossRef]
- Niesters, M.; Dahan, A.; Kest, B.; Zacny, J.; Stijnen, T.; Aarts, L.; Sarton, E. Do sex differences exist in opioid analgesia? A systematic review and meta-analysis of human experimental and clinical studies. Pain 2010, 151, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Agulló, L.; Muriel, J.; Margarit, C.; Escorial, M.; Garcia, D.; Herrero, M.J.; Hervás, D.; Sandoval, J.; Peiró, A.M. Sex Differences in Opioid Response Linked to OPRM1 and COMT genes DNA Methylation/Genotypes Changes in Patients with Chronic Pain. J. Clin. Med. 2023, 12, 3449. [Google Scholar] [CrossRef] [PubMed]
- Muriel, J.; Escorial, M.; Margarit, C.; Barrachina, J.; Carvajal, C.; Morales, D.; Peiró, A.M. Long-term deprescription in chronic pain and opioid use disorder patients: Pharmacogenetic and sex differences. Acta Pharm. 2023, 73, 227–241. [Google Scholar] [CrossRef]
- Martin, R.M.; Biswas, P.N.; Freemantle, S.N.; Pearce, G.L.; Mann, R.D. Age and sex distribution of suspected adverse drug reactions to newly marketed drugs in general practice in England: Analysis of 48 cohort studies. Br. J. Clin. Pharmacol. 1998, 46, 505–511. [Google Scholar] [CrossRef]
- Planelles, B.; Margarit, C.; Inda, M.-D.-M.; Ballester, P.; Muriel, J.; Barrachina, J.; Ajo, R.; Esteban, M.D.; Peiró, A.M. Gender based differences, pharmacogenetics and adverse events in chronic pain management. Pharmacogenom. J. 2020, 20, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Farkouh, A.; Baumgärtel, C.; Gottardi, R.; Hemetsberger, M.; Czejka, M.; Kautzky-Willer, A. Sex-Related Differences in Drugs with Anti-Inflammatory Properties. J. Clin. Med. 2021, 10, 1441. [Google Scholar] [CrossRef]
- Zucker, I.; Prendergast, B.J. Sex differences in pharmacokinetics predict adverse drug reactions in women. Biol. Sex Differ. 2020, 11, 32. [Google Scholar] [CrossRef]
- Soldin, O.P.; Mattison, D.R. Sex differences in pharmacokinetics and pharmacodynamics. Clin. Pharmacokinet. 2009, 48, 143–157. [Google Scholar] [CrossRef]
- Mori, H.; Suzuki, H.; Matsuzaki, J.; Taniguchi, K.; Shimizu, T.; Yamane, T.; Masaoka, T.; Kanai, T. Gender Difference of Gastric Emptying in Healthy Volunteers and Patients with Functional Dyspepsia. Digestion 2017, 95, 72–78. [Google Scholar] [CrossRef]
- Sadik, R.; Abrahamsson, H.; Stotzer, P.O. Gender differences in gut transit shown with a newly developed radiological procedure. Scand. J. Gastroenterol. 2003, 38, 36–42. [Google Scholar] [CrossRef]
- Farkouh, A.; Riedl, T.; Gottardi, R.; Czejka, M.; Kautzky-Willer, A. Sex-Related Differences in Pharmacokinetics and Pharmacodynamics of Frequently Prescribed Drugs: A Review of the Literature. Adv. Ther. 2020, 37, 644–655. [Google Scholar] [CrossRef] [PubMed]
- Ofotokun, I. Sex differences in the pharmacologic effects of antiretroviral drugs: Potential roles of drug transporters and phase 1 and 2 metabolizing enzymes. Top. HIV Med. 2005, 13, 79–83. [Google Scholar]
- Saiz-Rodríguez, M.; Ochoa, D.; Herrador, C.; Belmonte, C.; Román, M.; Alday, E.; Koller, D.; Zubiaur, P.; Mejía, G.; Hernández-Martínez, M.; et al. Polymorphisms associated with fentanyl pharmacokinetics, pharmacodynamics and adverse effects. Basic. Clin. Pharmacol. Toxicol. 2019, 124, 321–329. [Google Scholar] [CrossRef]
- Barrachina, J.; Margarit, C.; Muriel, J.; López-Gil, V.; López-Gil, S.; Ballester, P.; Mira-Lorente, L.; Agulló, L.; Peiró, A.M. Sex Differences in Oxycodone/Naloxone vs. Tapentadol in Chronic Non-Cancer Pain: An Observational Real-World Study. Biomedicines 2022, 10, 2468. [Google Scholar] [CrossRef]
- Baron, R.; Jansen, J.-P.; Binder, A.; Pombo-Suarez, M.; Kennes, L.; Müller, M.; Falke, D.; Steigerwald, I. Tolerability, Safety, and Quality of Life with Tapentadol Prolonged Release (PR) Compared with Oxycodone/Naloxone PR in Patients with Severe Chronic Low Back Pain with a Neuropathic Component: A Randomized, Controlled, Open-label, Phase 3b/4 Trial. Pain Pract. 2016, 16, 600–619. [Google Scholar] [CrossRef] [PubMed]
- Lopes, G.S.; Bielinski, S.J.; Moyer, A.M.; Black Iii, J.L.; Jacobson, D.J.; Jiang, R.; Larson, N.B.; St Sauver, J.L. Sex Differences in Associations Between CYP2D6 Phenotypes and Response to Opioid Analgesics. Pharmacogenom. Pers. Med. 2020, 13, 71. [Google Scholar] [CrossRef]
- Melsom, T.; Norvik, J.V.; Enoksen, I.T.; Stefansson, V.; Mathisen, U.D.; Fuskevåg, O.M.; Jenssen, T.G.; Solbu, M.D.; Eriksen, B.O. Sex Differences in Age-Related Loss of Kidney Function. J. Am. Soc. Nephrol. 2022, 33, 1891. [Google Scholar] [CrossRef]
- Holdcroft, A. Pharmacological differences between men and women. Acta Anaesthesiol. Belg. 2002, 53, 299–303. [Google Scholar] [PubMed]
- Johannessen Landmark, C.; Beiske, G.; Baftiu, A.; Burns, M.L.; Johannessen, S.I. Experience from therapeutic drug monitoring and gender aspects of gabapentin and pregabalin in clinical practice. Seizure 2015, 28, 88–91. [Google Scholar] [CrossRef]
- Back, S.E.; Payne, R.A.; Waldrop, A.E.; Smith, A.; Reeves, S.; Brady, K.T. Prescription opioid aberrant behaviors: A pilot study of sex differences. Clin. J. Pain 2009, 25, 477–484. [Google Scholar] [CrossRef]
- Gosch, M.; Böhmdorfer, B.; Benvenuti-Falger, U.; Dovjak, P.; Iglseder, B.; Lechleitner, M.; Otto, R.; Roller, R.E.; Sommeregger, U. Polypharmacy and pain treatment. Wien. Med. Wochenschr. 2010, 160, 286–292. [Google Scholar] [CrossRef]
- Vuong, C.; Van Uum, S.H.M.; O’Dell, L.E.; Lutfy, K.; Friedman, T.C. The effects of opioids and opioid analogs on animal and human endocrine systems. Endocr. Rev. 2010, 31, 98–132. [Google Scholar] [CrossRef]
- Corsi, D.J.; Murphy, M.S.Q. The Effects of opioids on female fertility, pregnancy and the breastfeeding mother-infant dyad: A Review. Basic Clin. Pharmacol. Toxicol. 2021, 128, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Turner, S.; Allen, V.M.; Graves, L.; Tanguay, R.; Green, C.R.; Cook, J.L. Guideline No. 443a: Opioid Use Throughout Women’s Lifespan: Fertility, Contraception, Chronic Pain, and Menopause. J. Obstet. Gynaecol. Can. 2023, 45, 102143. [Google Scholar] [CrossRef]
- Maeda, A.; Bateman, B.T.; Clancy, C.R.; Creanga, A.A.; Leffert, L.R. Opioid abuse and dependence during pregnancy: Temporal trends and obstetrical outcomes. Anesthesiology 2014, 121, 1158–1165. [Google Scholar] [CrossRef] [PubMed]
- Yen, E.; Davis, J.M. The immediate and long-term effects of prenatal opioid exposure. Front. Pediatr. 2022, 10, 1039055. [Google Scholar] [CrossRef] [PubMed]
- Bhaumik, S.; Lockett, J.; Cuffe, J.; Clifton, V.L. Glucocorticoids and Their Receptor Isoforms: Roles in Female Reproduction, Pregnancy, and Foetal Development. Biology 2023, 12, 1104. [Google Scholar] [CrossRef]
- Sturgeon, J.A. Psychological therapies for the management of chronic pain. Psychol. Res. Behav. Manag. 2014, 7, 115–124. [Google Scholar] [CrossRef]
- Henschke, N.; Ostelo, R.W.; van Tulder, M.W.; Vlaeyen, J.W.; Morley, S.; Assendelft, W.J.; Main, C.J. Behavioural treatment for chronic low-back pain. Cochrane Database Syst. Rev. 2010, 2010, CD002014. [Google Scholar] [CrossRef]
- Williams, A.C.d.C.; Eccleston, C.; Morley, S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst. Rev. 2012, 11, CD007407. [Google Scholar] [CrossRef]
- Veehof, M.M.; Oskam, M.-J.; Schreurs, K.M.G.; Bohlmeijer, E.T. Acceptance-based interventions for the treatment of chronic pain: A systematic review and meta-analysis. Pain 2011, 152, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, E.S.; Miller, W.A.; Koffel, E.; Ullman, K.; Landsteiner, A.; Stroebel, B.; Hill, J.; Ackland, P.E.; Wilt, T.J.; Duan-Porter, W. Barriers and Facilitators of Evidence-Based Psychotherapies for Chronic Pain in Adults: A Systematic Review. J. Pain 2023, 24, 742–769. [Google Scholar] [CrossRef] [PubMed]
- Sex in Science: The NIH Gets it Wrong? Available online: https://www.brainfacts.org/in-the-lab/animals-in-research/2014/sex-in-science--the-nih-gets-it-wrong (accessed on 17 October 2024).
- NIH (National Institutes of Health). Consideration of Sex as a Biological Variable in NIH-Funded Research; NIH Notice NOT-OD-15-102; NIH: Bethesda, MA USA, 2015. Available online: https://grants.nih.gov/grants/guide/notice-files/not-od-15-102.html (accessed on 6 June 2024).
- Pieretti, S.; Di Giannuario, A.; Di Giovannandrea, R.; Marzoli, F.; Piccaro, G.; Minosi, P.; Aloisi, A.M. Gender differences in pain and its relief. Ann. Ist. Super. Sanita 2016, 52, 184–189. [Google Scholar] [CrossRef] [PubMed]
| Domain | Section | Search Terms Used | Cited References * |
|---|---|---|---|
| Epidemiological | 3.1 | “chronic pain” AND (“sex differences” OR “gender differences”) AND (“hormones” OR “prevalence” OR “epidemiology” OR “treatment satisfaction” OR “coping” OR “comorbidity”) | [4,6,7,8,9,10,11,12,13,14,15,16,17,22,23,24,28,29,48,49,50,51,52,53,54,55,56] |
| Biological mechanisms | 3.2.1 | “chronic pain” AND (“sex differences” OR “gender differences”) AND (“hormones” OR “hormonal fluctuations” OR “testosterone” OR “estrogen” OR “neuroimmune” OR “microglia” OR “T cells” OR “aging” OR “biomarkers”) | [15,16,17,28,29,30,31,32,33,49,50,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77] |
| Psycho-social | 3.2.2 | “chronic pain” AND (“sex differences” OR “gender differences”) AND (“gender bias” OR “healthcare disparities” OR “social norms” OR “andronormativity” OR “cultural roles” OR “socio-economic status” OR “pain perception” OR “pain tolerance”OR “mental health” OR “depression” OR “anxiety” OR “coping strategies” OR “quality of life” OR “social support” OR “functioning” OR “pain acceptance”)) | [6,16,17,23,24,39,40,41,42,43,44,45,46,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113] |
| Pharmacological strategies | 3.3.1 | “chronic pain” AND (“sex differences” OR “gender differences”) AND (“analgesics” OR “NSAIDs” OR “ibuprofen” OR “opioid efficacy” OR “treatment response” OR “pharmacokinetics” OR “pharmacodynamics” OR “adverse drug reactions” OR “pharmacokinetics” OR “pharmacodynamics” OR “opioid metabolism” OR “drug clearance” OR “reproductive health” OR “endocrine effects”) | [34,37,38,114,115,116,117,118,119,120,121,122,123,124,125,126,127] |
| 3.3.2 | “chronic pain” AND (“sex differences” OR “gender differences”) AND (“adverse drug reactions” OR “pharmacokinetics” OR “pharmacodynamics” OR “opioid metabolism” OR “drug clearance” OR “reproductive health” OR “endocrine effects”) | [39,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151] | |
| 3.3.3 | “chronic pain” AND (“psychological interventions” OR “psychotherapy” OR “cognitive behavioral therapy” OR “mindfulness-based stress reduction” OR “acceptance and commitment therapy” OR “quality of life” OR “coping strategies”) | [152,153,154,155,156] |
| Key Domains in CP Management | Sex-Specific Considerations and Strategies |
|---|---|
| Biological and psycho-social interplay | Sex-related differences in CP stem from a complex interaction of biological and psycho-social factors. Effective management requires tailored, interdisciplinary strategies that address both dimensions. |
| Pharmacological considerations | CP treatments, particularly opioids and NSAIDs, must account for sex-specific variations in drug response and adverse effects to optimize safety and efficacy. |
| Psychological interventions | The integration of psychological strategies enhances CP management outcomes. These interventions should be personalized according to sex and individual coping mechanisms. |
| Advancing precision medicine | Future approaches to pain management should prioritize gene therapy and the identification of sex-specific molecular targets to enhance precision and personalized treatment. |
| Equity and policy implications | Achieving equitable pain care necessitates awareness of sex differences in CP, addressing translational gaps, incorporating psycho-social factors, and considering sex-specific pharmacokinetics and therapeutic targets. Clinicians and policymakers are urged to advocate for sex-aware guidelines and individualized care pathways. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchesini, M.; Fornasari, D.; Natoli, S.; Vegni, E.; Cuomo, A. Sex-Related Differences in Chronic Pain: A Narrative Review by a Multidisciplinary Task Force. Medicina 2025, 61, 1172. https://doi.org/10.3390/medicina61071172
Marchesini M, Fornasari D, Natoli S, Vegni E, Cuomo A. Sex-Related Differences in Chronic Pain: A Narrative Review by a Multidisciplinary Task Force. Medicina. 2025; 61(7):1172. https://doi.org/10.3390/medicina61071172
Chicago/Turabian StyleMarchesini, Maurizio, Diego Fornasari, Silvia Natoli, Elena Vegni, and Arturo Cuomo. 2025. "Sex-Related Differences in Chronic Pain: A Narrative Review by a Multidisciplinary Task Force" Medicina 61, no. 7: 1172. https://doi.org/10.3390/medicina61071172
APA StyleMarchesini, M., Fornasari, D., Natoli, S., Vegni, E., & Cuomo, A. (2025). Sex-Related Differences in Chronic Pain: A Narrative Review by a Multidisciplinary Task Force. Medicina, 61(7), 1172. https://doi.org/10.3390/medicina61071172






