Innovations in Chronic Pain Treatment: A Narrative Review on the Role of Cryoneurolysis
Abstract
1. Introduction
- The distance between the probe and the target nerve;
- The cryoprobe diameter;
- The size of the resulting ice ball;
- The temperature of the immediately surrounding tissue (such as blood, which acts as a heat sink);
- The rate and duration of cold application. The latter two factors are highly dependent upon the gas flow rate and the number of ‘freeze cycles’ applied, usually with 2–3 min of freezing followed by 0.5–2 min of thawing [11].
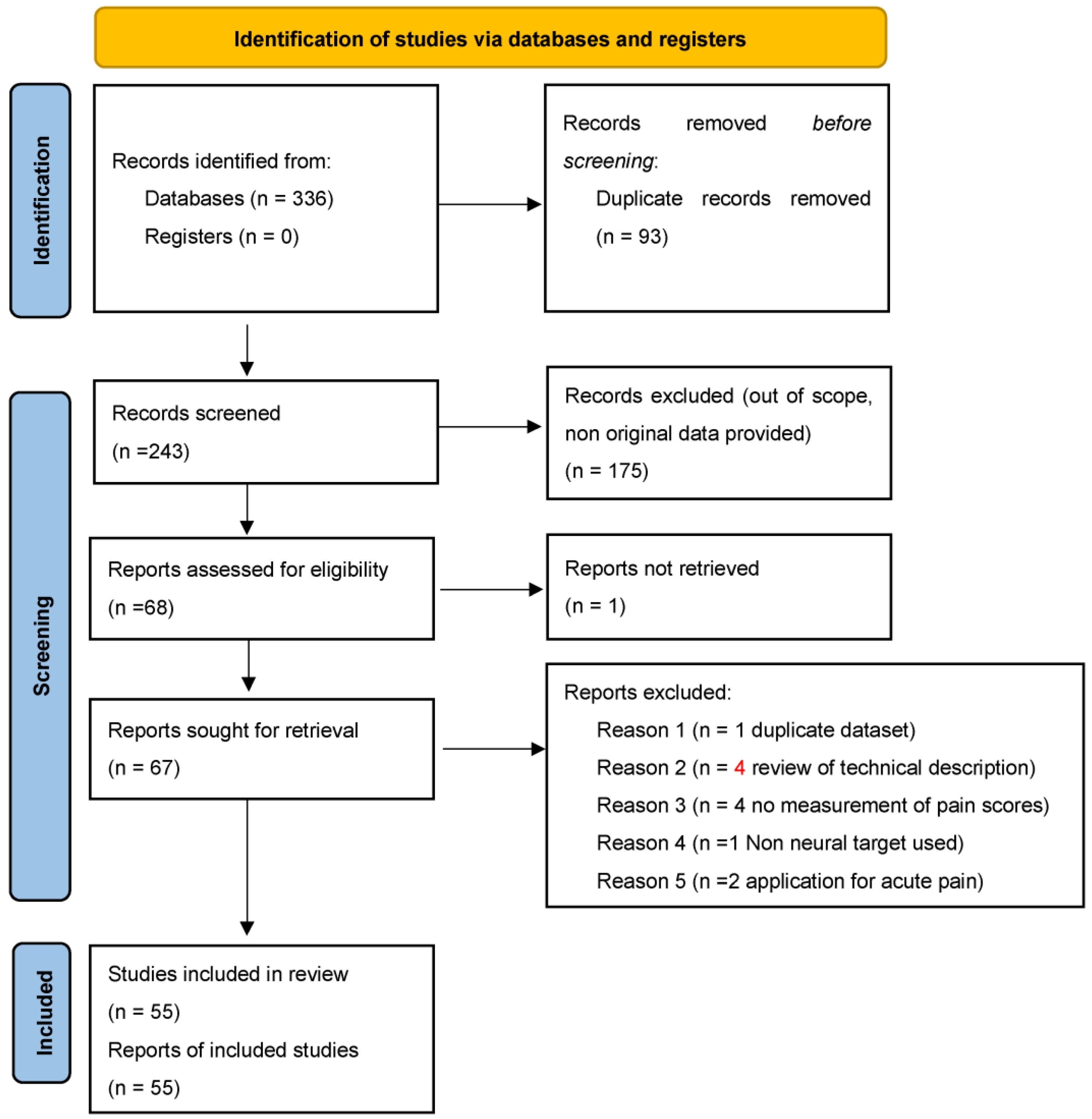
2. Materials and Methods
- -
- Papers describing the application of cryoneurolysis during a surgical procedure (i.e., intraoperative application on the intercostal nerves for post-thoracotomy pain).
- -
- Papers which did not report a statistical analysis (apart from case reports) or simply described a study protocol.
- -
- Reviews and all studies not including novel data.
- -
- Papers written in a non-English language.
3. Results
4. Discussion
5. Conclusions
- -
- Precise protocol description for cryoneurolysis application (temperature, duration of application, number of freezing cycles used);
- -
- Specific neural targets;
- -
- Specific patient population (i.e., chronic post-surgical pain after total joint replacement).
Author Contributions
Funding
Conflicts of Interest
References
- Werner, M.U.; Kongsgaard, U.E. I. Defining Persistent Post-Surgical Pain: Is an Update Required? Br. J. Anaesth. 2014, 113, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Cui, A.; Li, H.; Wang, D.; Zhong, J.; Chen, Y.; Lu, H. Global, Regional Prevalence, Incidence and Risk Factors of Knee Osteoarthritis in Population-Based Studies. EClinicalMedicine 2020, 29–30, 100587. [Google Scholar] [CrossRef]
- Baker, P.N.; Van Der Meulen, J.H.; Lewsey, J.; Gregg, P.J. The Role of Pain and Function in Determining Patient Satisfaction after Total Knee Replacement: Data from the National Joint Registry for England and Wales. J. Bone Joint Surg. Br. 2007, 89, 893–900. [Google Scholar] [CrossRef]
- Martel, M.O.; Finan, P.H.; Dolman, A.J.; Subramanian, S.; Edwards, R.R.; Wasan, A.D.; Jamison, R.N. Self-Reports of Medication Side Effects and Pain-Related Activity Interference in Patients with Chronic Pain: A Longitudinal Cohort Study. Pain 2015, 156, 1092–1100. [Google Scholar] [CrossRef]
- Poenaru, D.; Sandulescu, M.I.; Cinteza, D. Pain Modulation in Chronic Musculoskeletal Disorders: Botulinum Toxin, a Descriptive Analysis. Biomedicines 2023, 11, 1888. [Google Scholar] [CrossRef]
- Wylde, V.; Beswick, A.; Bruce, J.; Blom, A.; Howells, N.; Gooberman-Hill, R. Chronic Pain after Total Knee Arthroplasty. EFORT Open Rev. 2018, 3, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Panagopoulos, A.; Tsiplakos, P.; Katsanos, K.; Antzoulas, P.; Lakoumentas, J. Cooled Radiofrequency Ablation versus Cryoneurolysis of the Genicular Nerves for the Symptomatic Pain Management in Knee Osteoarthritis: A Study Protocol of a Prospective, Randomized, Single-Blinded Clinical Trial. J. Orthop. Surg. 2023, 18, 295. [Google Scholar] [CrossRef]
- Slavin, B.R.; Markowitz, M.I.; Klifto, K.M.; Prologo, F.J.; Taghioff, S.M.; Dellon, A.L. Cryoanalgesia: Review with Respect to Peripheral Nerve. J. Reconstr. Microsurg. 2024, 40, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.; Bremer, N.; Weyker, P.D.; Webb, C.A.J. Ultrasound-Guided Genicular Nerve Thermal Radiofrequency Ablation for Chronic Knee Pain. Case Rep. Anesthesiol. 2016, 2016, 8292450. [Google Scholar] [CrossRef]
- Ilfeld, B.M.; Finneran, J.J. Cryoneurolysis and Percutaneous Peripheral Nerve Stimulation to Treat Acute Pain: A Narrative Review. Anesthesiology 2020, 133, 1127–1149. [Google Scholar] [CrossRef]
- Ilfeld, B.M.; Gabriel, R.A.; Trescot, A.M. Ultrasound-Guided Percutaneous Cryoneurolysis for Treatment of Acute Pain: Could Cryoanalgesia Replace Continuous Peripheral Nerve Blocks? Br. J. Anaesth. 2017, 119, 709–712. [Google Scholar] [CrossRef] [PubMed]
- Radnovich, R.; Scott, D.; Patel, A.T.; Olson, R.; Dasa, V.; Segal, N.; Lane, N.E.; Shrock, K.; Naranjo, J.; Darr, K.; et al. Cryoneurolysis to Treat the Pain and Symptoms of Knee Osteoarthritis: A Multicenter, Randomized, Double-Blind, Sham-Controlled Trial. Osteoarthritis Cartilage 2017, 25, 1247–1256. [Google Scholar] [CrossRef]
- Truong, K.; Meier, K.; Ahrens, L.C.; Wichmann, T.O.; Zaer, H.; Tiroke, L.H.; Arvin, S.; Bazys, M.; Duel, P.; Gudmundsdottir, G.; et al. Cryoneurolysis versus Radiofrequency Ablation Outcome on Pain Experience in Chronic Low Back Pain (COPE): A Single-Blinded Randomised Controlled Trial. RMD Open 2024, 10, e004196. [Google Scholar] [CrossRef] [PubMed]
- Ilfeld, B.M.; Smith, C.R.; Turan, A.; Mariano, E.R.; Miller, M.E.; Fisher, R.L.; Trescot, A.M.; Cohen, S.P.; Eisenach, J.C.; Sessler, D.I.; et al. Ultrasound-Guided Percutaneous Cryoneurolysis to Treat Chronic Postamputation Phantom Limb Pain: A Multicenter Randomized Controlled Trial. Anesthesiology 2023, 138, 82–97. [Google Scholar] [CrossRef] [PubMed]
- Kvarstein, G.; Högström, H.; Allen, S.M.; Rosland, J.H. Cryoneurolysis for Cervicogenic Headache—A Double Blinded Randomized Controlled Study. Scand. J. Pain 2019, 20, 39–50. [Google Scholar] [CrossRef]
- Grigsby, E.; Radnovich, R.; Nalamachu, S. Efficacy and Safety of Cryoneurolysis for Treatment of Chronic Head Pain Secondary to Occipital Neuralgia: A Pilot Study. Local Reg. Anesth. 2021, 14, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Perry, T.A.; Segal, N.A. An Open-Label, Single-Arm Trial of Cryoneurolysis for Improvements in Pain, Activities of Daily Living and Quality of Life in Patients with Symptomatic Ankle Osteoarthritis. Osteoarthr. Cartil. Open 2022, 4, 100272. [Google Scholar] [CrossRef]
- Yoon, J.H.E.; Grechushkin, V.; Chaudhry, A.; Bhattacharji, P.; Durkin, B.; Moore, W. Cryoneurolysis in Patients with Refractory Chronic Peripheral Neuropathic Pain. J. Vasc. Interv. Radiol. 2016, 27, 239–243. [Google Scholar] [CrossRef]
- Zhan, C.; Yoon, J.; Baghai Kermani, A.; Gupta, A.; Moore, W. Abstract No. 587 Safety and Efficacy of Computed Tomography–Guided Percutaneous Cryoneurolysis for Chronic Intercostal Pain Syndrome. J. Vasc. Interv. Radiol. 2020, 31, S256. [Google Scholar] [CrossRef]
- Das, G.; Das, S.; Sahoo, R.; Shreyas, S.; Kanthi, B.; Sharma, V.S. Efficacy of Cryoneurolysis versus Intra-Articular Steroid in Sacroiliac Joint Pain: A Retrospective, Case-Control Study. Indian J. Anaesth. 2023, 67, 1004–1008. [Google Scholar] [CrossRef]
- Sidebottom, A.J.; Carey, E.C.; Madahar, A.K. Cryoanalgesia in the Management of Intractable Pain in the Temporomandibular Joint: A Five-Year Retrospective Review. Br. J. Oral Maxillofac. Surg. 2011, 49, 653–656. [Google Scholar] [CrossRef] [PubMed]
- Prologo, J.D.; Gilliland, C.A.; Miller, M.; Harkey, P.; Knight, J.; Kies, D.; Hawkins, C.M.; Corn, D.; Monson, D.K.; Edalat, F.; et al. Percutaneous Image-Guided Cryoablation for the Treatment of Phantom Limb Pain in Amputees: A Pilot Study. J. Vasc. Interv. Radiol. 2017, 28, 24–34.e4. [Google Scholar] [CrossRef] [PubMed]
- Yasin, J.; Thimmappa, N.; Kaifi, J.T.; Avella, D.M.; Davis, R.; Tewari, S.O.; Saboo, S.S.; Bhat, A. CT-Guided Cryoablation for Post-Thoracotomy Pain Syndrome: A Retrospective Analysis. Diagn. Interv. Radiol. 2020, 26, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Moore, W.; Kolnick, D.; Tan, J.; Yu, H.S. CT Guided Percutaneous Cryoneurolysis for Post Thoracotomy Pain Syndrome. Acad. Radiol. 2010, 17, 603–606. [Google Scholar] [CrossRef] [PubMed]
- Wolter, T.; Deininger, M.; Hubbe, U.; Mohadjer, M.; Knoeller, S. Cryoneurolysis for Zygapophyseal Joint Pain: A Retrospective Analysis of 117 Interventions. Acta Neurochir. 2011, 153, 1011–1019. [Google Scholar] [CrossRef]
- Kim, C.H.; Hu, W.; Gao, J.; Dragan, K.; Whealton, T.; Julian, C. Cryoablation for the Treatment of Occipital Neuralgia. Pain Physician 2015, 18, E363–E368. [Google Scholar] [CrossRef]
- Nemecek, Z.; Sturm, C.; Rauen, A.C.; Reisig, F.; Streitberger, K.; Harnik, M.A. Ultrasound-Controlled Cryoneurolysis for Peripheral Mononeuropathies: A Retrospective Cohort Study. Pain Manag. 2023, 13, 363–372. [Google Scholar] [CrossRef]
- Parekattil, S.; Gudeloglu, A.; Ergun, O.; Galante, A.; Etafy, M.; Mendelson, R. MP31-10 A Cost Effective Office Based Technique for Ultrasound Guided Peri-Spermatic Cord Cryoablation for Chronic Scrotal Content Pain. J. Urol. 2021, 206, e558–e559. [Google Scholar] [CrossRef]
- Tinnirello, A. Genicular Nerves Ablation for Chronic Knee Pain: A Single-Center Retrospective Evaluation Comparing Four Ablation Modalities. Pain Pract. 2020, 20, 7–95. [Google Scholar] [CrossRef]
- Nezami, N.; Behi, A.; Manyapu, S.; Meisel, J.L.; Resnick, N.; Corn, D.; Prologo, J.D. Percutaneous CT-Guided Cryoneurolysis of the Intercostobrachial Nerve for Management of Postmastectomy Pain Syndrome. J. Vasc. Interv. Radiol. 2023, 34, 807–813. [Google Scholar] [CrossRef]
- McLean, B.C.; Nguyen, C.D.; Newman, D.P. Cryoablation of the Infrapatellar Branch of the Saphenous Nerve Identified by Non-Invasive Peripheral Nerve Stimulator for the Treatment of Non-Surgical Anterior Knee Pain: A Case Series and Review of the Literature. Cureus 2020, 12, e8747. [Google Scholar] [CrossRef] [PubMed]
- Calixte, N.; Kartal, I.G.; Tojuola, B.; Gudeloglu, A.; Etafy, M.; Brahmbhatt, J.V.; Mendelson, R.A.; Chetta, M.; Parekattil, S.J. Salvage Ultrasound-Guided Targeted Cryoablation of The Perispermatic Cord For Persistent Chronic Scrotal Content Pain After Microsurgical Denervation Of The Spermatic Cord. Urology 2019, 130, 181–185. [Google Scholar] [CrossRef]
- Bellini, M.; Barbieri, M. Percutaneous Cryoanalgesia in Pain Management: A Case-Series. Anestezjol. Intensywna Ter. 2015, 47, 131–133. [Google Scholar] [CrossRef]
- Prologo, J.; Mittal, A.; Knight, J.; Hsu, D.; Dolan, R.; Com, D. Percutaneous CT-Guided Cryoablation for the Management of Pudendal Neuralgia: Long-Term Outcomes. J. Vasc. Interv. Radiol. 2018, 29, S241–S242. [Google Scholar]
- Lo Bianco, G.; D’angelo, F.P.; Dos Santos, G.F.; Stogicza, A.; Leoni, M.L.G.; Trescot, A.M.; Yong, R.J.; Robinson, C.L. Genicular Nerve Ultrasound-Guided Cryoanalgesia for the Treatment of Chronic Knee Joint Pain: An Observational Retrospective Study. Pain Ther. 2025, 14, 985–998. [Google Scholar] [CrossRef]
- Filipovski, I.; Gabriel, R.A.; Kestenholz, R. Ultrasound-Guided Cryoneurolysis for the Treatment of Painful Diabetic Neuropathy of the Foot: A Case Series. Cureus 2024, 16, e56267. [Google Scholar] [CrossRef]
- Stogicza, A.R.; Peng, P. Cryoanalgesia for Shoulder Pain: A Motor-Sparing Approach to Rotator Cuff Disease. Reg. Anesth. Pain Med. 2022, 47, 576–580. [Google Scholar] [CrossRef] [PubMed]
- Dalili, D.; Ahlawat, S.; Rashidi, A.; Belzberg, A.J.; Fritz, J. Cryoanalgesia of the Anterior Femoral Cutaneous Nerve (AFCN) for the Treatment of Neuropathy-Mediated Anterior Thigh Pain: Anatomy and Technical Description. Skeletal Radiol. 2021, 50, 1227–1236. [Google Scholar] [CrossRef]
- Moesker, A.A.; Karl, H.W.; Trescot, A.M. Treatment of Phantom Limb Pain by Cryoneurolysis of the Amputated Nerve. Pain Pract. 2014, 14, 52–56. [Google Scholar] [CrossRef]
- Sahoo, R.K.; Das, G.; Pathak, L.; Dutta, D.; Roy, C.; Bhatia, A. Cryoneurolysis of Innervation to Sacroiliac Joints: Technical Description and Initial Results—A Case Series. AA Pract. 2021, 15, e01427. [Google Scholar] [CrossRef]
- Mendes-Andrade, I.; Pagan-Rosado, R.; Ferreira-Silva, N.; Hurdle, M.F. A Novel Approach to Refractory Coccydynia: Ultrasound- Fluoroscopy-Guided Cryoablation of Sacrococcygeal Nerve. Pain Manag. 2024, 14, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, J.P.; Williams, V.B.; Shin, S.S. Cryoneurolysis for Digital Neuralgia in Professional Baseball Players: A Case Series. Orthop. J. Sports Med. 2022, 10, 23259671221096095. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, R.A.; Kestenholz, R.; Filipovski, I. Repeated Ultrasound-Guided Percutaneous Intercostal Cryoneurolysis for the Management of Chronic Postmastectomy Pain Syndrome: A Case Report. AA Pract. 2024, 18, e01764. [Google Scholar] [CrossRef] [PubMed]
- Cachemaille, M.; Geering, S.; Broome, M. Cryoneurolysis of Alveolar Nerves for Chronic Dental Pain: A New Technique and a Case Series. Pain Pract. 2023, 23, 851–854. [Google Scholar] [CrossRef]
- Kočan, L.; Rapčan, R.; Sudzina, R.; Rapčanová, S.; Rybár, D.; Mláka, J.; Kočanová, H.; Buriánek, M.; Vašková, J. Radiofrequency Denervation and Cryoablation of the Lumbar Zygapophysial Joints in the Treatment of Positive Lumbar Facet Joint Syndrome—A Report of Three Cases. Radiol. Case Rep. 2022, 17, 4515–4520. [Google Scholar] [CrossRef]
- Connelly, N.R.; Malik, A.; Madabushi, L.; Gibson, C. Use of Ultrasound-Guided Cryotherapy for the Management of Chronic Pain States. J. Clin. Anesth. 2013, 25, 634–636. [Google Scholar] [CrossRef]
- Matelich, B.; Berg, A.; Habeck, J.; Hutchins, J. B32 Ultrasound-Guided Cryoneurolysis of the Supra Scapular Nerve for Chronic Shoulder Pain: A Case Series. Reg. Anesth. Pain Med. 2022, 47, A99. [Google Scholar]
- Fox, S. Pudendal Nerve Cryoablation for Chronic Pelvic Pain. Dis. Colon Rectum 2019, 62, e39–e401. [Google Scholar] [CrossRef]
- Sarridou, D.; Papadopoulou, D.; Paraskevopoulos, T.; Stavropoulou, E. Successful Treatment of Complex Regional Pain Syndrome Type 1 of Upper Limb with Cryoneurolysis of the Stellate Ganglion: A Rare Case Report. Pain Pract. 2022, 22, 285–287. [Google Scholar] [CrossRef]
- Ramsook, R.; Spinner, D. (468) Cryoablation of a Hip Disarticulation Residual Limb Neuroma Allowing for Ambulation and Pain Relief: A Case Report. J. Pain 2016, 17, S91. [Google Scholar] [CrossRef]
- Joshi, D.H.; Thawait, G.K.; Del Grande, F.; Fritz, J. MRI-Guided Cryoablation of the Posterior Femoral Cutaneous Nerve for the Treatment of Neuropathy-Mediated Sitting Pain. Skeletal Radiol. 2017, 46, 983–987. [Google Scholar] [CrossRef] [PubMed]
- Kalava, A.; Pham, K.; Okon, S. Cryoneurolysis of the Subcostal Nerve: A Technical Description and Case Report. Cureus 2024, 16, e57521. [Google Scholar] [CrossRef]
- Sen, S.; De Haan, J.B.; Mehrafza, M.; Hernandez, N. Ultrasound-Guided Percutaneous Intercostal Cryoneurolysis for Acute-on-Chronic Pain in CLOVES Syndrome. Cureus 2023, 15, e34066. [Google Scholar] [CrossRef]
- Jung, E.Y.; Lee, S.S. Treatment of Chronic Mini-Thoracotomy Wound Pain and Lung Herniation with Intercostal Cryoablation and Surgical Mesh Repair: A Case Report. J. Cardiothorac. Surg. 2024, 19, 348. [Google Scholar] [CrossRef]
- MacRae, F.; Speirs, A.; Bursuc, A.; Hashemi, M.; Winston, P. A Case Report of Cryoneurolysis for Dorsal Foot Pain and Toe Clawing in a Patient With Multiple Sclerosis. Arch. Rehabil. Res. Clin. Transl. 2023, 5, 100286. [Google Scholar] [CrossRef] [PubMed]
- Perese, J.; Oswald, J.; Gabriel, R.A. Ultrasound-Guided Percutaneous Cryoneurolysis for Post-Thoracotomy Pain Syndrome: A Case Report. Cureus 2022, 14, e328. [Google Scholar] [CrossRef]
- Koethe, Y.; Mannes, A.J.; Wood, B.J. Image-Guided Nerve Cryoablation for Post-Thoracotomy Pain Syndrome. Cardiovasc. Intervent. Radiol. 2014, 37, 843–846. [Google Scholar] [CrossRef]
- Rhame, E.E.; DeBonet, A.F.; Simopoulos, T.T. Ultrasonographic Guidance and Characterization of Cryoanalgesic Lesions in Treating a Case of Refractory Sural Neuroma. Case Rep. Anesthesiol. 2011, 2011, 691478. [Google Scholar] [CrossRef] [PubMed]
- MacRae, F.; Boissonnault, E.; Hashemi, M.; Winston, P. Bilateral Suprascapular Nerve Cryoneurolysis for Pain Associated With Glenohumeral Osteoarthritis: A Case Report. Arch. Rehabil. Res. Clin. Transl. 2023, 5, 100256. [Google Scholar] [CrossRef]
- Weber, G.; Saad, K.; Awad, M.; Wong, T.H. Case Report Of Cryoneurolysis For The Treatment of Refractory Intercostobrachial Neuralgia With Postherpetic Neuralgia. Local Reg. Anesth. 2019, 12, 103–107. [Google Scholar] [CrossRef]
- Yarmohammadi, H.; Nakamoto, D.; Azar, N.; Hayek, S.; Haaga, J. Percutaneous Computed Tomography Guided Cryoablation of the Celiac Plexus as an Alternative Treatment for Intractable Pain Caused by Pancreatic Cancer. J. Cancer Res. Ther. 2011, 7, 481. [Google Scholar] [CrossRef] [PubMed]
- Rupp, A.; Panchang, P.; Smith, M. A Case Report of Cryoablation for Chronic Shoulder Pain Due to Osteoarthritis. Interv. Pain Med. 2022, 1, 100146. [Google Scholar] [CrossRef]
- Kalava, A.; Kassie, R.; Borick, E. Cryoneurolysis of Intercostal Nerves for Postherpetic Neuralgia: A Case Report. Cureus 2024, 16, e70557. [Google Scholar] [CrossRef] [PubMed]
- Hampton, H.; Kalava, A. Ischiorectal Approach to Cryoablation of the Pudendal Nerve Using a Handheld Device: A Report of Two Cases. Cureus 2023, 15, e44377. [Google Scholar] [CrossRef]
- Fiala, M.; Azariah, A.; Woo, J.; Aal, A.K.A.; Levey, A. Treating Phantom Limb Pain: Cryoablation of the Posterior Tibial Nerve. Radiol. Case Rep. 2022, 17, 3168–3171. [Google Scholar] [CrossRef]
- Davis, T.; Loudermilk, E.; DePalma, M.; Hunter, C.; Lindley, D.A.; Patel, N.; Choi, D.; Soloman, M.; Gupta, A.; Desai, M.; et al. Twelve-Month Analgesia and Rescue, by Cooled Radiofrequency Ablation Treatment of Osteoarthritic Knee Pain: Results from a Prospective, Multicenter, Randomized, Cross-over Trial. Reg. Anesth. Pain Med. 2019, 44, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Santi, C.; Haag, T.; Cooke, C.; Schatman, M.; Tinnirello, A. Two-Centre Retrospective Analysis on Selective Sensory Denervation of Shoulder Joint by Means of Cooled Radiofrequency in Chronic Shoulder Pain. J. Pain Res. 2024, 17, 3139–3150. [Google Scholar] [CrossRef]
- Klessinger, S.; Casser, H.-R.; Gillner, S.; Koepp, H.; Kopf, A.; Legat, M.; Meiler, K.; Norda, H.; Schneider, M.; Scholz, M.; et al. Radiofrequency Denervation of the Spine and the Sacroiliac Joint: A Systematic Review Based on the Grades of Recommendations, Assesment, Development, and Evaluation Approach Resulting in a German National Guideline. Glob. Spine J. 2024, 14, 2124–2154. [Google Scholar] [CrossRef]
- Cahani, D.; Chacko, J.; Hahn, B. Myonecrosis: A Rare Complication of Cryoneurolysis. J. Emerg. Med. 2019, 57, e73–e76. [Google Scholar] [CrossRef]
- Fleischmann, E.; Lenhardt, R.; Kurz, A.; Herbst, F.; Fülesdi, B.; Greif, R.; Sessler, D.I.; Akça, O. Nitrous Oxide and Risk of Surgical Wound Infection: A Randomised Trial. Lancet 2005, 366, 1101–1107. [Google Scholar] [CrossRef]
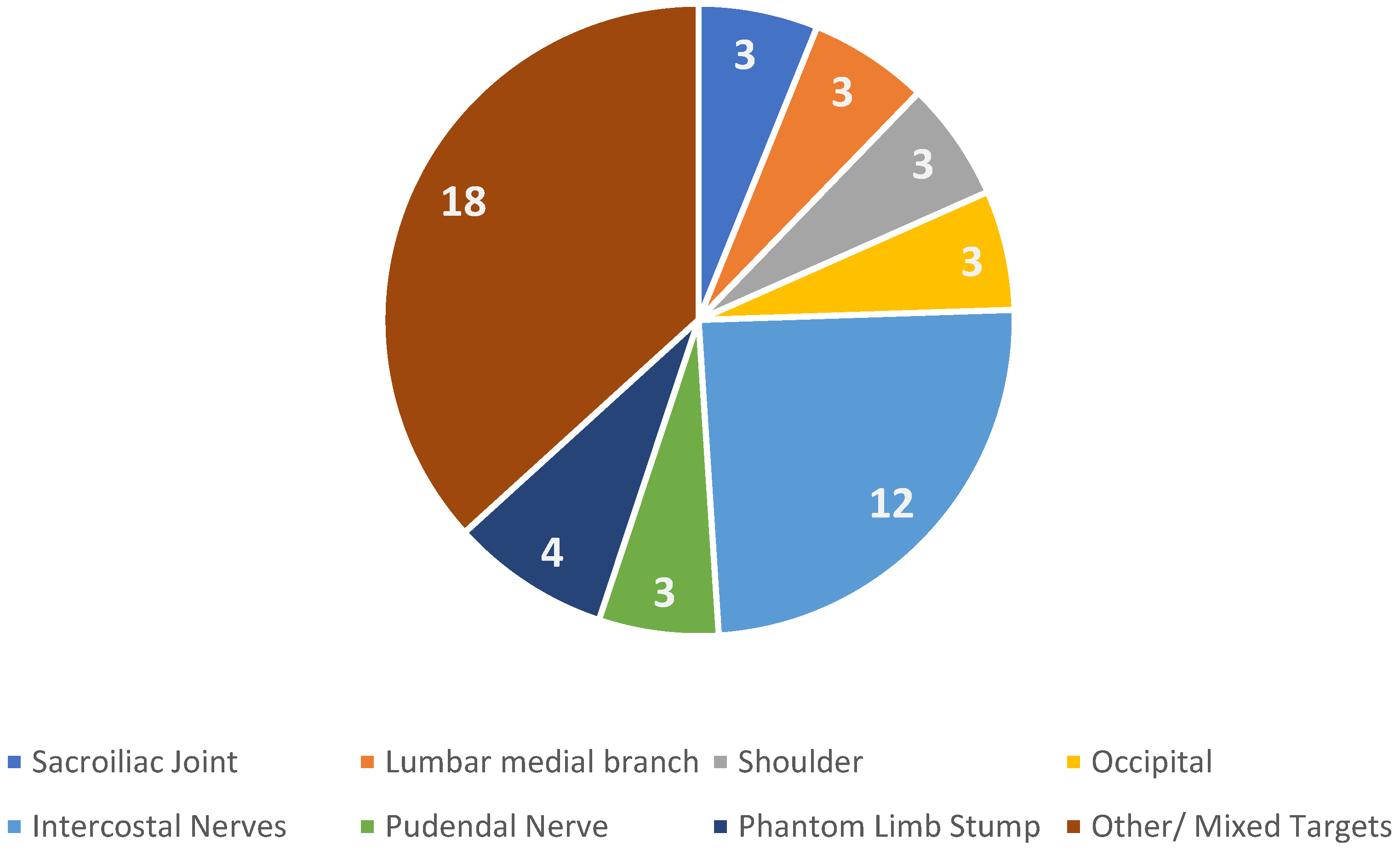
| Author, Year | Design | Patients | Targets | Guidance | Follow Up | Outcomes | Notes |
|---|---|---|---|---|---|---|---|
| Radnovich, 2017 [12] | RCT vs. Sham | 180 | Saphenous Nerve | Landmark | 120 days | 87.5% responders at 120 days (VAS reduction > 50%) | 61.2% of responders in the sham group at 120 days. No adverse events |
| Truong, 2024 [13] | RCT vs. Sham and RF | 120 | Lumbar medial branch | Fluoroscopic | 6 months | No improvement compared to RF or Placebo | |
| Ilfeld, 2023 [14] | RCT vs. Sham | 144 | Sciatic (post-amputation pain) | US | 4 months | No improvement compared to Placebo | |
| Kvarstein, 2019 [15] | RCT vs. Steroid Injection | 52 | Occipital Nerve | US | 18 weeks | Pain relief > 50% in 35% of patients (no difference) | |
| Grigsby, 2021 [16] | OP | 26 | Occipital Nerve | Blind | 56 days | NRS reduction > 2 points in 35% of patients | |
| Perry, 2022 [17] | OP | 40 | Sural, Saphenous, Superficial, and/or Deep fibular nerves | US | 6 months | Mean NRS reduction 2.8 (<than 50% from baseline) | 22 patients completed follow-up |
| Yoon, 2016 [18] | OP | 22 | Peripheral neuropathy (mixed 3 plantar neuromas, 3 ileoinguinal, 4 posterior tibial, 7 saphenous, 1 gluteal, 1 sural, 1 genicular, 2 digital nerves) | US | 12 months | 3.2 mean VAS reduction at 12 months. Mean pain relief > 50% at 3 months | |
| Zhan, 2020 [19] | OP | 18 | Intercostal | CT | 12 months | 2.3 and 1.3 mean VAS reduction at 6 and 9 months, respectively | No significant reduction at 12 months |
| Das, 2023 [20] | OR | 83 | Sacroiliac Joint | US | 6 months | 69% of patients with pain relief of > 50% after 6 months | |
| Sidebottom, 2011 [21] | OR | 17 | TMJ (auricular nerve and TMJ capsule) | Surgical | NS | Mean duration of pain relief 7 months; 3/17 patients were pain-free at 12 months | 2 temporary numbness |
| Prologo, 2017 [22] | OR | 21 | Phantom Limb Neuroma | US | 194 ± 99 Days | Mean NRS reduction 4.2 | |
| Yasin, 2019 [23] | OR | 13 | Intercostal Nerves | CT | Various, 2–18 months | 61.5% of patients with pain relief > 50% | 1 pneumothorax and 3 pseudohernia |
| Moore, 2010 [24] | OR | 18 | Intercostal Nerves | CT | Variable (mean 51 days) | Mean pain relief < 50% | |
| Wolter, 2011 [25] | OR | 91 | Lumbar Medial Branch | CT | 3 months | Mean NRS decrease 3.50 points | |
| Kim, 2015 [26] | OR | 38 | Occipital Nerve | Landmark | 6 months | Mean NRS Improvement 3.8 | 2 post op neuritis and 1 hematoma |
| Nemecek, 2023 [27] | OR | 24 | Various (intercostal, saphenous, peroneal, | US | 6 months | Pain reduction > 30% in 2/24 Patients | |
| Parekattil, 2021 [28] | OR | 35 | Genitofemoral, ilioinguinal and inferior hypogastric nerve | Blind (surgical) | 1 month | Pain reduction > 50% in 68% of patients | |
| Tinnirello, 2020 [29] | OR | 10 | Genicular nerves | US | 6 months | Pain relief > 50% in 50% of patients at 6 months | Comparison between Cryo, Pulsed, Cooled, and Conventional RF |
| Nezami, 2022 [30] | OR | 14 | Intercostobrachial nerve | CT | 6 months | Mean NRS decrease 2.9 | |
| McLean, 2020 [31] | OR | 23 | Saphenous nerve, infrapatellar branch | Blind | Variable | Pain relief > 50% in all patients | Follow up at 6 months for 4 patients, NS for others. |
| Calixte, 2019 [32] | OR | 279 | Genitofemoral, ilioinguinal and inferior hypogastric nerve | Blind (Surgical) | 5 years | Pain relief > 50% in 64% of patients | |
| Bellini, 2015 [33] | OR | 18 | Facet, knee, sacroiliac joint | 4 months | Mean NRS decrease 4/10 | ||
| Prologo, 2018 [34] | OR | 14 | Pudendal nerve | CT | 18 months | Responder rate 63% | Responder definition not stated |
| Lo Bianco, 2025 [35] | OR | 90 | Genicular nerves | US | 9 months | Mean NRS 5 ± 1 from 7 ± 2 at Baseline | Pain relief > 50% in 56% of Patients at 3 months (NS at 9 Months) |
| Filipovski, 2024 [36] | CS | 3 | Superficial peroneal nerve | US | 5 years | 2 patients pain-free after 5 years, 1 without results | |
| Stogicza, 2024 [37] | CS | 4 | Suprascapular, axillary, lateropectoral nerves | US | 6 months | 3 patients with pain relief > 60% | |
| Dalili, 2021 [38] | CS | 3 | Anterior femoral cutaneous nerve | MRI | 12 months | 50% VAS reduction at 12 months in all patients | No adverse events |
| Moesker, 2014 [39] | CS | 5 | Phantom limb | US | 5–30 months | 60% of patients with > 50% pain relief | No adverse events |
| Sahoo, 2021 [40] | CS | 5 | Lateral branches of sacral dorsal nerve roots | US and Fluoro | 6 months | 100% of patients with >50% pain relief | |
| Mendes-Andrade, 2024 [41] | CS | 2 | Sacrococcygeal nerve | Fluoro | >50% pain relief in 100% of patients | ||
| Shaffer, 2022 [42] | CS | 3 | Digital nerves | US | 1 year | Pain relief 100% | |
| Gabriel, 2024 [43] | CR | 1 | Intercostal nerves | US | |||
| Cachemaille, 2023 [44] | CS | 4 | Alveolar nerves | Blind | 3 months | Pain relief > 50% in 2 patients | |
| Kocân, 2022 [45] | CS | 2 | Lumbar medial branches | Fluoroscopic | 6 months | Pain relief 50% at 3 months (<50% at 6 months, better result with RF) | 1 patient treated with RF, 2 with cryo |
| Connelly, 2013 [46] | CS | 3 | Intercostal nerves | US | Variable | Pain relief > 50% in 2 patients for 9 months, in 1 patient for 3 months | |
| Matelich, 2022 [47] | CS | 3 | Suprascapular nerve | US | 3–6 months | Duration of pain relief: 3–6 months | Pain scores not recorded |
| Fox, 2019 [48] | CS | 3 | Pudendal nerve | CT | NS | 2 patients with > 50% pain relief | |
| Sarridou, 2022 [49] | CR | 1 | Stellate ganglion | US | 6 months | Pain relief > 50% for 6 months | |
| Ramsook, 2016 [50] | CR | 1 | Hip stump neuroma | US | NS | Pain relief | |
| Joshi, 2017 [51] | CR | 1 | Posterior femoral cutaneous nerve | MRI | 5 months | Pain relief 100% at 6 months | |
| Kalava, 2024 [52] | CR | 1 | Intercostal nerves | CT | 3 months | 100% pain relief | |
| Sen, 2023 [53] | CR | 1 | Intercostal nerves | US | 5 days | Pain relief 100% | |
| Jung, 2024 [54] | CR | 1 | Intercostal nerves | Surgical | 8 weeks | Pain relief > 50% | |
| MacRae, 2023 [55] | CR | 1 | Superficial fibular nerve | US | 5 months | Pain relief (no NRS reported) | |
| Perese, 2022 [56] | CR | 1 | Intercostal nerves | US | 2 months | Pain relief > 50% | |
| Koethe, 2014 [57] | CR | 1 | Intercostal nerves | CT | 8 weeks | Paine relief > 50% | |
| Rhame, 2011 [58] | CR | 1 | Sural nerve | NS | 3 months | “Excellent pain relief” | |
| MacRae, 2023 [59] | CR | 1 | Suprascapular nerve | US | 7 months | NRS < 2 | |
| Weber, 2019 [60] | CR | 1 | Intercostobrachial nerve | Blind | 1 month | Pain relief > 50% | |
| Yarmohammadi, 2011 [61] | CR | 1 | Celiac plexus | CT | 6 months | Pain relief 70% | |
| Rupp, 2022 [62] | CR | 1 | Suprascapular nerve | US | 3 months | Pain relief > 50% | |
| Kalava, 2024 [63] | CR | 1 | Intercostal nerves | US | 2 months | Pain relief > 50% | |
| Hampton, 2023 [64] | CR | 2 | Pudendal nerve | Blind | NS | Pain relief for 3–4 Months | NRS not measured |
| Fiala, 2022 [65] | CR | 1 | Phantom limb stump | US | NS | Pain relief < 6 weeks | |
| Gabriel, 2024 [43] | CR | 1 | Intercostal nerves | US | 6 months | Pain relief > 50% |
| Author, Year. | Patients | Targets | Guidance | Positive Outcome at 6 Months |
|---|---|---|---|---|
| Truong, 2024 [13] | 120 | Lumbar medial branch | Fluoroscopic | NO |
| Perry, 2022 [17] | 40 | sural, saphenous, superficial, and/or deep fibular nerves | US | NO |
| Yoon, 2016 [18] | 22 | Peripheral neuropathy (mixed 3 plantar neuromas, 3 ileoinguinal, 4 posterior tibial, 7 saphenous, 1 gluteal, 1 sural, 1 genicular, 2 digital nerves) | US | NO |
| Das, 2023 [20] | 83 | Sacroiliac joint | US | YES |
| Tinnirello, 2020 [29] | 10 | Genicular nerves | US | YES |
| McLean, 2020 [31] | 23 | Saphenous nerve, Infrapatellar branch | Blind | YES |
| Calixte, 2019 [32] | 279 | Genitofemoral, ilioinguinal and inferior hypogastric | Surgical | YES |
| Stogicza, 2024 [37] | 4 | Suprascapular, axillary, lateropectoral | US | YES |
| Dalili, 2021 [38] | 3 | Anterior femoral cutaneous nerve | MRI | YES |
| Moesker, 2014 [39] | 5 | Phantom limb | US | YES |
| Sahoo, 2021 [40] | 5 | Lateral branches of sacral dorsal nerve roots | US and Fluoroscopic | YES |
| Shaffer, 2022 [42] | 3 | Digital nerves | US | YES |
| Kocân, 2022 [45] | 2 | Lumbar medial branches | Fluoroscopic | NO |
| Connelly, 2013 [46] | 3 | Intercostal | US | YES |
| Sarridou, 2022 [49] | 1 | Stellate ganglion | US | YES |
| Yarmohammadi, 2011 [61] | 1 | Celiac plexus | CT | YES |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tinnirello, A.; Marchesini, M.; Mazzoleni, S.; Santi, C.; Lo Bianco, G. Innovations in Chronic Pain Treatment: A Narrative Review on the Role of Cryoneurolysis. Medicina 2025, 61, 1090. https://doi.org/10.3390/medicina61061090
Tinnirello A, Marchesini M, Mazzoleni S, Santi C, Lo Bianco G. Innovations in Chronic Pain Treatment: A Narrative Review on the Role of Cryoneurolysis. Medicina. 2025; 61(6):1090. https://doi.org/10.3390/medicina61061090
Chicago/Turabian StyleTinnirello, Andrea, Maurizio Marchesini, Silvia Mazzoleni, Carola Santi, and Giuliano Lo Bianco. 2025. "Innovations in Chronic Pain Treatment: A Narrative Review on the Role of Cryoneurolysis" Medicina 61, no. 6: 1090. https://doi.org/10.3390/medicina61061090
APA StyleTinnirello, A., Marchesini, M., Mazzoleni, S., Santi, C., & Lo Bianco, G. (2025). Innovations in Chronic Pain Treatment: A Narrative Review on the Role of Cryoneurolysis. Medicina, 61(6), 1090. https://doi.org/10.3390/medicina61061090






