Exposure to Fine Particulate Matter (PM2.5) and Heavy Metals During the Second Trimester of Pregnancy Increases the Risk of Preeclampsia and Eclampsia: An Analysis of National Health Insurance Claims Data from South Korea
Abstract
1. Introduction
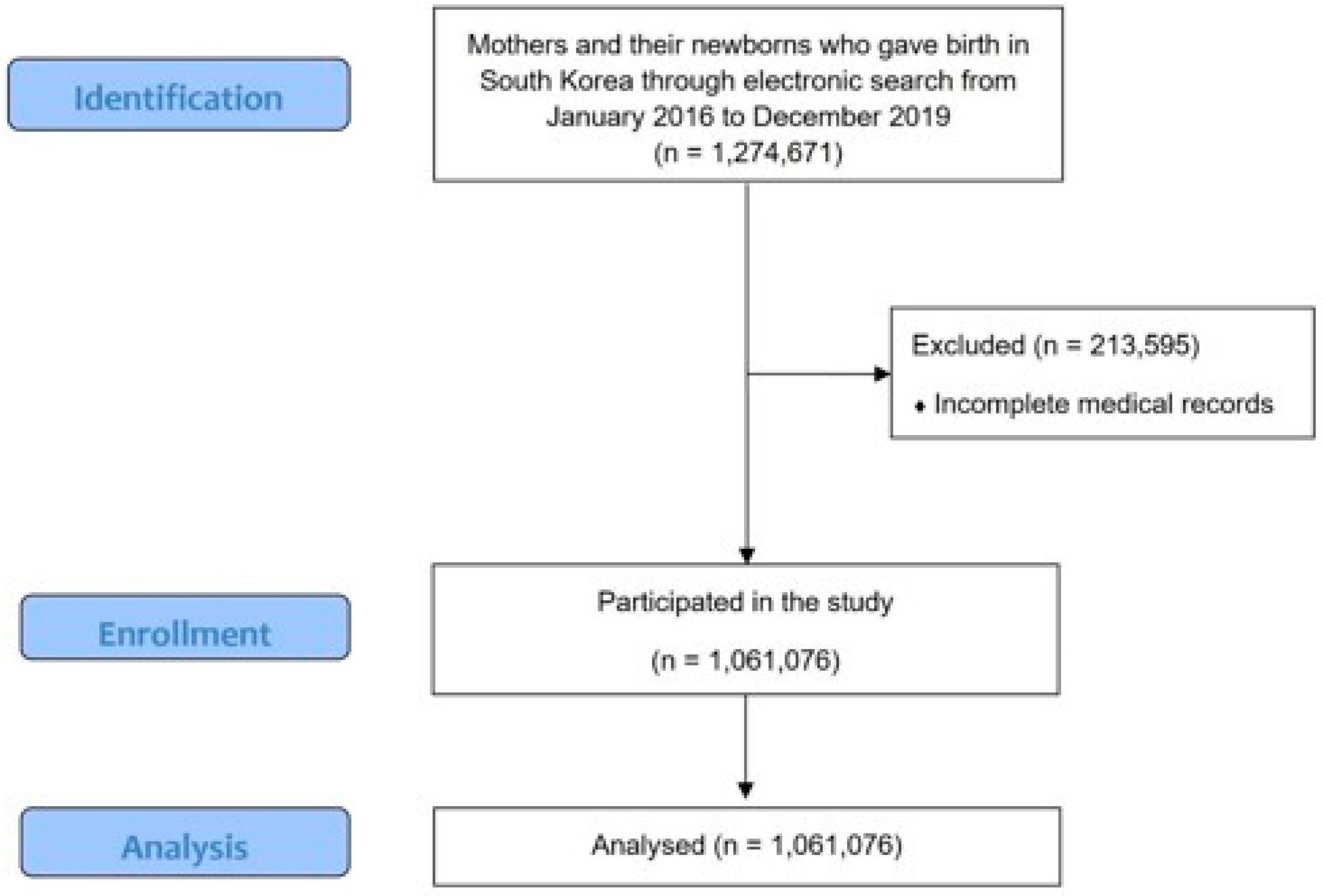
2. Materials and Methods
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PM2.5 | Particulate matter |
| NHI | National Health Insurance |
| ORs | Odds ratios |
| CIs | Confidence intervals |
References
- Garovic, V.D.; White, W.M.; Vaughan, L.; Saiki, M.; Parashuram, S.; Garcia-Valencia, O.; Weissgerber, T.L.; Milic, N.; Weaver, A.; Mielke, M.M. Incidence and Long-Term Outcomes of Hypertensive Disorders of Pregnancy. J. Am. Coll. Cardiol. 2020, 75, 2323–2334. [Google Scholar] [CrossRef] [PubMed]
- Torres-Torres, J.; Villafan-Bernal, J.R.; Martinez-Portilla, R.J.; Hidalgo-Carrera, J.A.; Estrada-Gutierrez, G.; Adalid-Martinez-Cisneros, R.; Rojas-Zepeda, L.; Acevedo-Gallegos, S.; Camarena-Cabrera, D.M.; Cruz-Martinez, M.Y.; et al. Performance of machine-learning approach for prediction of pre-eclampsia in a middle-income country. Ultrasound Obs. Gynecol. 2024, 63, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Dimitriadis, E.; Rolnik, D.L.; Zhou, W.; Estrada-Gutierrez, G.; Koga, K.; Francisco, R.P.V.; Whitehead, C.; Hyett, J.; da Silva Costa, F.; Nicolaides, K.; et al. Pre-eclampsia. Nat. Rev. Dis. Primers 2023, 9, 8. [Google Scholar] [CrossRef]
- Shah, D.M. The role of RAS in the pathogenesis of preeclampsia. Curr. Hypertens. Rep. 2006, 8, 144–152. [Google Scholar] [CrossRef]
- Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin, Number 222. Obs. Gynecol. 2020, 135, e237–e260. [CrossRef] [PubMed]
- Brown, M.A.; Magee, L.A.; Kenny, L.C.; Karumanchi, S.A.; McCarthy, F.P.; Saito, S.; Hall, D.R.; Warren, C.E.; Adoyi, G.; Ishaku, S. Hypertensive disorders of pregnancy: ISSHP classification, diagnosis, and management recommendations for international practice. Hypertension 2018, 72, 24–43. [Google Scholar] [CrossRef]
- Vuong, A.D.B.; Pham, X.T.T.; Nguyen, P.N. Posterior reversible encephalopathy syndrome (PRES) on the second postpartum day: Learning experience from a case report and literature review. Int. J. Emerg. Med. 2024, 17, 118. [Google Scholar] [CrossRef]
- North, R.A.; McCowan, L.M.; Dekker, G.A.; Poston, L.; Chan, E.H.; Stewart, A.W.; Black, M.A.; Taylor, R.S.; Walker, J.J.; Baker, P.N.; et al. Clinical risk prediction for pre-eclampsia in nulliparous women: Development of model in international prospective cohort. BMJ 2011, 342, d1875. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, G.; Breborowicz, A.; Brazert, M.; Maczkiewicz, M.; Kobelski, M.; Dubiel, M.; Gudmundsson, S. Evaluation of third trimester uterine artery flow velocity indices in relationship to perinatal complications. J. Matern. Fetal Neonatal Med. 2006, 19, 551–555. [Google Scholar] [CrossRef]
- La Verde, M.; Torella, M.; Ronsini, C.; Riemma, G.; Cobellis, L.; Marrapodi, M.M.; Capristo, C.; Rapisarda, A.M.C.; Morlando, M.; De Franciscis, P. The association between fetal Doppler and uterine artery blood volume flow in term pregnancies: A pilot study. Ultraschall Med. 2024, 45, 184–189. [Google Scholar] [CrossRef]
- Ju, M.J.; Oh, J.; Choi, Y.-H. Changes in air pollution levels after COVID-19 outbreak in Korea. Sci. Total Environ. 2021, 750, 141521. [Google Scholar] [CrossRef]
- Ngarambe, J.; Joen, S.J.; Han, C.-H.; Yun, G.Y. Exploring the relationship between particulate matter, CO, SO2, NO2, O3 and urban heat island in Seoul, Korea. J. Hazard. Mater. 2021, 403, 123615. [Google Scholar] [CrossRef]
- Choi, E.; Yi, S.-M.; Lee, Y.S.; Jo, H.; Baek, S.-O.; Heo, J.-B. Sources of airborne particulate matter-bound metals and spatial-seasonal variability of health risk potentials in four large cities, South Korea. Environ. Sci. Pollut. Res. 2022, 29, 28359–28374. [Google Scholar] [CrossRef] [PubMed]
- Savitz, D.A.; Elston, B.; Bobb, J.F.; Clougherty, J.E.; Dominici, F.; Ito, K.; Johnson, S.; McAlexander, T.; Ross, Z.; Shmool, J.L.; et al. Ambient Fine Particulate Matter, Nitrogen Dioxide, and Hypertensive Disorders of Pregnancy in New York City. Epidemiology 2015, 26, 748–757. [Google Scholar] [CrossRef]
- Veras, M.M.; Damaceno-Rodrigues, N.R.; Caldini, E.G.; Maciel Ribeiro, A.A.; Mayhew, T.M.; Saldiva, P.H.; Dolhnikoff, M. Particulate urban air pollution affects the functional morphology of mouse placenta. Biol. Reprod. 2008, 79, 578–584. [Google Scholar] [CrossRef]
- Yoder, S.R.; Thornburg, L.L.; Bisognano, J.D. Hypertension in pregnancy and women of childbearing age. Am. J. Med. 2009, 122, 890–895. [Google Scholar] [CrossRef]
- Redman, C.W. Current topic: Pre-eclampsia and the placenta. Placenta 1991, 12, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.M.; Redman, C.W. Pre-eclampsia: More than pregnancy-induced hypertension. Lancet 1993, 341, 1447–1451. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Wang, L.; Wu, L.; Wang, T.; Cui, X.; Yu, L.; Diao, R.; Mao, H. Particulate matter and hypertensive disorders in pregnancy: Systematic review and meta-analysis. Public Health 2021, 200, 22–32. [Google Scholar] [CrossRef]
- Melody, S.M.; Wills, K.; Knibbs, L.D.; Ford, J.; Venn, A.; Johnston, F. Maternal Exposure to Ambient Air Pollution and Pregnancy Complications in Victoria, Australia. Int J Environ. Res Public Health 2020, 17, 2572. [Google Scholar] [CrossRef]
- Bearblock, E.; Aiken, C.E.; Burton, G.J. Air pollution and pre-eclampsia; associations and potential mechanisms. Placenta 2021, 104, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Kim, T.; Kim, H. Effect of indoor and outdoor sources on indoor particle concentrations in South Korean residential buildings. J. Hazard. Mater. 2021, 416, 125852. [Google Scholar] [CrossRef]
- Chen, H.; Fang, X.; Wong, T.H.; Chan, S.N.; Akinwunmi, B.; Ming, W.K.; Zhang, C.J.P.; Wang, Z. Physical Activity during Pregnancy: Comparisons between Objective Measures and Self-Reports in Relation to Blood Glucose Levels. Int. J. Environ. Res. Public Health 2022, 19, 8064. [Google Scholar] [CrossRef]
- Chon, H.T.; Kim, K.W.; Kim, J.Y. Metal contamination of soils and dusts in Seoul metropolitan city, Korea. Environ. Geochem. Health 1995, 17, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.-M.; Lee, J.-H.; Moon, J.-H.; Chung, Y.-S.; Kim, K.-H. Airborne PM10 and metals from multifarious sources in an industrial complex area. Atmos. Res. 2010, 96, 53–64. [Google Scholar] [CrossRef]
- Hieu, N.T.; Lee, B.-K. Characteristics of particulate matter and metals in the ambient air from a residential area in the largest industrial city in Korea. Atmos. Res. 2010, 98, 526–537. [Google Scholar] [CrossRef]
- Mol, B.W.J.; Roberts, C.T.; Thangaratinam, S.; Magee, L.A.; de Groot, C.J.M.; Hofmeyr, G.J. Pre-eclampsia. Lancet 2016, 387, 999–1011. [Google Scholar] [CrossRef]
- Fasanya, H.O.; Hsiao, C.J.; Armstrong-Sylvester, K.R.; Beal, S.G. A Critical Review on the Use of Race in Understanding Racial Disparities in Preeclampsia. J. Appl. Lab. Med. 2021, 6, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.J.; Seow, K.M.; Chen, K.H. Preeclampsia: Recent Advances in Predicting, Preventing, and Managing the Maternal and Fetal Life-Threatening Condition. Int. J. Environ. Res. Public Health 2023, 20, 2994. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Fujiwara, H.; Konishi, I. Mechanism of maternal vascular remodeling during human pregnancy. Reprod. Med. Biol. 2012, 11, 27–36. [Google Scholar] [CrossRef]
- Sun, Y.; Bhuyan, R.; Jiao, A.; Avila, C.C.; Chiu, V.Y.; Slezak, J.M.; Sacks, D.A.; Molitor, J.; Benmarhnia, T.; Chen, J.-C. Association between particulate air pollution and hypertensive disorders in pregnancy: A retrospective cohort study. PLoS Med. 2024, 21, e1004395. [Google Scholar] [CrossRef] [PubMed]
- Nobles, C.J.; Williams, A.; Ouidir, M.; Sherman, S.; Mendola, P. Differential Effect of Ambient Air Pollution Exposure on Risk of Gestational Hypertension and Preeclampsia. Hypertension 2019, 74, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Liu, Q.; Hou, H.; Guo, G.; Zhang, T.; Fan, S.; Wang, L. Association of Ambient air Pollution with risk of preeclampsia during pregnancy: A retrospective cohort study. BMC Public Health 2020, 20, 1663. [Google Scholar] [CrossRef]
- Liu, N.; Miyashita, L.; Mcphail, G.; Thangaratinam, S.; Grigg, J. Late Breaking Abstract-Do inhaled carbonaceous particles translocate from the lung to the placenta? In Proceedings of the ERS International Congress, Paris, France, 15–19 September 2018. [Google Scholar]
- Terzano, C.; Di Stefano, F.; Conti, V.; Graziani, E.; Petroianni, A. Air pollution ultrafine particles: Toxicity beyond the lung. Eur. Rev. Med. Pharmacol. Sci. 2010, 14, 809–821. [Google Scholar] [PubMed]
- Naav, A.; Erlandsson, L.; Isaxon, C.; Asander Frostner, E.; Ehinger, J.; Sporre, M.K.; Krais, A.M.; Strandberg, B.; Lundh, T.; Elmer, E.; et al. Urban PM2.5 Induces Cellular Toxicity, Hormone Dysregulation, Oxidative Damage, Inflammation, and Mitochondrial Interference in the HRT8 Trophoblast Cell Line. Front. Endocrinol. 2020, 11, 75. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Yin, Y.; Zhang, J.; Zhou, R. The impact of particulate matter 2.5 on the risk of preeclampsia: An updated systematic review and meta-analysis. Environ. Sci. Pollut. Res. 2020, 27, 37527–37539. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, J.; Wang, K.; Yan, H.; Liu, Q.; Lan, Y.; Ren, L.; Wu, S. Exposure to ambient black carbon and particulate matter during pregnancy in associations with risk of pre-eclampsia: A meta-analysis based on population-based studies. Environ. Pollut. 2024, 343, 123230. [Google Scholar] [CrossRef]
- Gogna, P.; Villeneuve, P.J.; Borghese, M.M.; King, W.D. An exposure-response meta-analysis of ambient PM(2.5) during pregnancy and preeclampsia. Environ. Res. 2022, 210, 112934. [Google Scholar] [CrossRef]
- Rasmussen, L.G.; Lykke, J.A.; Staff, A.C. Angiogenic biomarkers in pregnancy: Defining maternal and fetal health. Acta Obs. Gynecol. Scand. 2015, 94, 820–832. [Google Scholar] [CrossRef][Green Version]
- Zheng, Y.; McElrath, T.; Cantonwine, D.; Hu, H. Longitudinal associations between ambient air pollution and angiogenic biomarkers among pregnant women in the LIFECODES Study, 2006–2008. Environ. Health Perspect. 2023, 131, 087005. [Google Scholar] [CrossRef]
- Hunter, R.; Baird, B.; Garcia, M.; Begay, J.; Goitom, S.; Lucas, S.; Herbert, G.; Scieszka, D.; Padilla, J.; Brayer, K.; et al. Gestational ozone inhalation elicits maternal cardiac dysfunction and transcriptional changes to placental pericytes and endothelial cells. Toxicol. Sci. 2023, 196, 238–249. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, P.; Zhang, L.; Shi, H.; Li, J.; Meng, X.; Xiao, X.; Dai, H.; Zhang, Y. Ozone Exposure During Pregnancy and Risk of Gestational Hypertension or Preeclampsia in China. JAMA Netw. Open 2023, 6, e236347. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Liu, N.; Fan, Y.; Ma, L.; Guan, T. Associations of pregnancy complications with ambient air pollution in China. Ecotoxicol. Environ. Saf. 2022, 241, 113727. [Google Scholar] [CrossRef]
- Hu, H.; Ha, S.; Roth, J.; Kearney, G.; Talbott, E.O.; Xu, X. Ambient Air Pollution and Hypertensive Disorders of Pregnancy: A Systematic Review and Meta-analysis. Atmos. Environ. 2014, 97, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Yu, G.; Wang, C.; Yin, S.; Huang, Y.; Chen, Q.; Sun, K.; Zhang, J. Exposure to multiple air pollutant mixtures and the subtypes of hypertensive disorders in pregnancy: A multicenter study. Int. J. Hyg. Environ. Health 2023, 253, 114238. [Google Scholar] [CrossRef]
- Freire, B.M.; Gonzaga, R.G.; Pedron, T.; Monteiro, L.R.; Lange, C.N.; Pedreira Filho, W.D.R.; Batista, B.L. Occupational exposure to potentially toxic elements in the foundry industry: An integrated environmental and biological monitoring. Environ. Sci. Pollut. Res. Int. 2021, 28, 34630–34641. [Google Scholar] [CrossRef] [PubMed]
- Galarneau, J.M.; Beach, J.; Cherry, N. Urinary Metals as a Marker of Exposure in Men and Women in the Welding and Electrical Trades: A Canadian Cohort Study. Ann. Work Expo. Health 2022, 66, 1111–1121. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.-C.; Ko, J.-W.; Park, S.-H.; Lim, J.-O.; Shin, I.-S.; Moon, C.; Kim, S.-H.; Heo, J.-D.; Kim, J.-C. Comparative toxicity and biodistribution of copper nanoparticles and cupric ions in rats. Int. J. Nanomed. 2016, 11, 2883–2900. [Google Scholar]
- Alarifi, S.; Ali, D.; Verma, A.; Alakhtani, S.; Ali, B.A. Cytotoxicity and genotoxicity of copper oxide nanoparticles in human skin keratinocytes cells. Int. J. Toxicol. 2013, 32, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, H.L.; Cronholm, P.; Gustafsson, J.; Möller, L. Copper oxide nanoparticles are highly toxic: A comparison between metal oxide nanoparticles and carbon nanotubes. Chem. Res. Toxicol. 2008, 21, 1726–1732. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Liu, C.; Yang, D.; Zhang, H.; Xi, Z. Comparative study of cytotoxicity, oxidative stress and genotoxicity induced by four typical nanomaterials: The role of particle size, shape and composition. J. Appl. Toxicol. 2009, 29, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Ameh, T.; Sayes, C.M. The potential exposure and hazards of copper nanoparticles: A review. Environ. Toxicol. Pharmacol. 2019, 71, 103220. [Google Scholar] [CrossRef] [PubMed]
- Tadesse, S.; Kidane, D.; Guller, S.; Luo, T.; Norwitz, N.G.; Arcuri, F.; Toti, P.; Norwitz, E.R. In Vivo and In Vitro Evidence for Placental DNA Damage in Preeclampsia. PLoS ONE 2014, 9, e86791. [Google Scholar] [CrossRef] [PubMed]
- Sak, S.; Barut, M.; Celik, H.; Incebiyik, A.; Agacayak, E.; Uyanikoglu, H.; Kirmit, A.; Sak, M. Copper and ceruloplasmin levels are closely related to the severity of preeclampsia. J. Matern. Fetal Neonatal Med. 2020, 33, 96–102. [Google Scholar] [CrossRef]
- Gul, A.Z.; Atakul, N.; Selek, S.; Atamer, Y.; Sarikaya, U.; Yildiz, T.; Demirel, M. Maternal Serum Levels of Zinc, Copper, and Thiols in Preeclampsia Patients: A Case-Control Study. Biol. Trace Elem. Res. 2022, 200, 464–472. [Google Scholar] [CrossRef]
- Salima, S.; Daundy, K.H.; Mose, J.C.; Pramatirta, A.Y.; Suardi, D.; Pusianawati, D. Comparison Levels of Copper, Zinc, and Cu/Zn Ratio of in Pre-eclampsia and Normal Pregnancy. Open Access Maced. J. Med. Sci. 2022, 10, 2392–2398. [Google Scholar] [CrossRef]
- Farzin, L.; Sajadi, F. Comparison of serum trace element levels in patients with or without pre-eclampsia. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2012, 17, 938. [Google Scholar]
- Chen, Y.; Ou, Q.X.; Chen, Y.; Zhu, Q.L.; Tan, M.H.; Zhang, M.M.; Wu, S.Z.; Xu, H.Y. Association between trace elements and preeclampsia: A retrospective cohort study. J. Trace Elem. Med. Biol. 2022, 72, 126971. [Google Scholar] [CrossRef]
- El Desouky, E.; Raslan, O.K.; El Magd, I.A.; Al Sheikh, W.; Eltonsy, M. Comparative study for serum zinc and copper levels in cases with normal pregnancy versus preeclampsia. Nat. Sci. 2020, 18, 180–184. [Google Scholar]
- Zhong, Z.; Yang, Q.; Sun, T.; Wang, Q. A global perspective of correlation between maternal copper levels and preeclampsia in the 21st century: A systematic review and Meta-analysis. Front. Public Health 2022, 10, 924103. [Google Scholar] [CrossRef] [PubMed]
- Choi, R.; Sun, J.; Yoo, H.; Kim, S.; Cho, Y.Y.; Kim, H.J.; Kim, S.W.; Chung, J.H.; Oh, S.Y.; Lee, S.Y. A Prospective Study of Serum Trace Elements in Healthy Korean Pregnant Women. Nutrients 2016, 8, 749. [Google Scholar] [CrossRef] [PubMed]
- Hee-Yun, K.; Jae-In, K.; Jin-Chul, K.; Ji-Eun, P.; Kyung-Jin, L.; Sung-Il, K.; Jae-Ho, O.; Young-Mi, J. Survey of Heavy Metal Contents of Circulating Agricultural Products in Korea. Korean J. Food Sci. Technol. 2009, 41, 238–244. [Google Scholar]
- Kim, M.; Kim, J.S.; Sho, Y.S.; Chung, S.-Y.; Lee, J.O. The Study on Heavy Metal Contents in Various Foods. Korean J. Food Sci. Technol. 2003, 35, 561–567. [Google Scholar]
- Jeon, J.-I.; Jung, J.-Y.; Park, S.-Y.; Lee, H.-W.; Lee, J.-I.; Lee, C.-M. A Comparison of Health Risks from PM2.5 and Heavy Metal Exposure in Industrial Complexes in Dangjin and Yeosu·Gwangyang. Toxics 2024, 12, 158. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhou, Q.; Wang, Y.; Wu, Y.; Li, M.; Wang, H.; Zheng, G.; Hao, Y.; Cao, X.; Yang, W.; et al. Association of PM2.5-bound multiple metals co-exposure with early cardiovascular damage: A panel study in young adults combining metabolomics. Environ. Pollut. 2025, 371, 125964. [Google Scholar] [CrossRef]
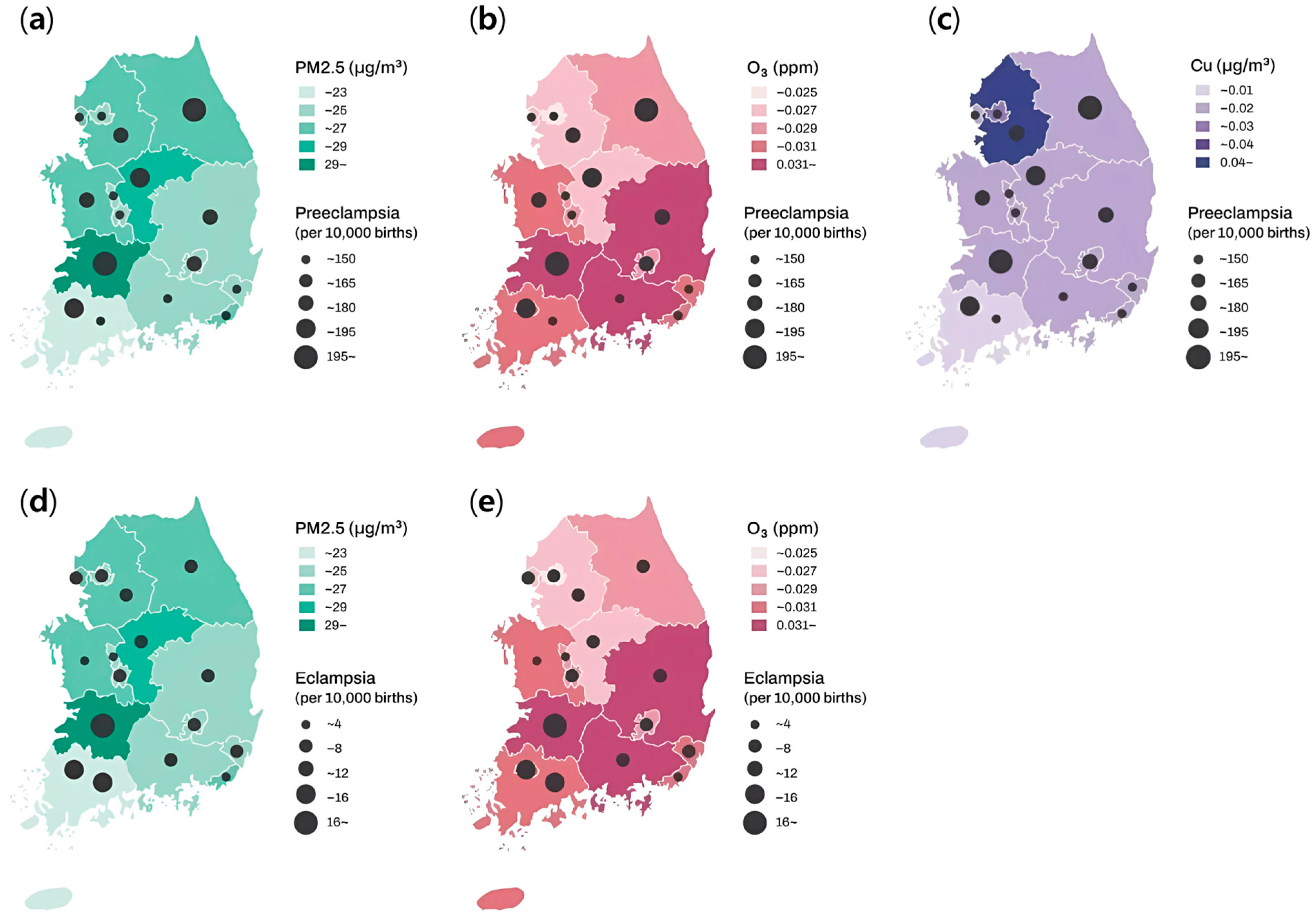
| Total | With Preeclampsia | With Eclampsia | |
|---|---|---|---|
| Maternal age (years) | |||
| Total | 1,061,076 | 16,920 | 592 |
| <20 *† | 203 (0.0) | 9 (0.1) | 1 (0.2) |
| 20–30 | 107,623 (10.1) | 1578 (9.3) | 75 (12.7) |
| 30–40 | 783,542 (73.8) | 11,780 (69.6) | 401 (67.7) |
| 40< | 169,708 (16.0) | 3553 (21.0) | 115 (19.4) |
| Occupational status *† | |||
| Worked outside home | 429,667 (40.5) | 7191 (42.5) | 272 (45.9) |
| Household income | |||
| Low (40%) * | 388,197 (36.6) | 6811 (40.3) | 234 (39.5) |
| Middle (40–95%) | 649,430 (61.2) | 9783 (57.8) | 351 (59.3) |
| High (95%<) | 23,449 (2.2) | 326 (1.9) | 7 (1.2) |
| Infant sex | |||
| Female *† | 516,809 (48.7) | 8486 (50.2) | 316 (53.4) |
| Season | |||
| Winter *† | 267,164 (25.2) | 4353 (25.7) | 152 (25.7) |
| Spring | 279,390 (26.3) | 4273 (25.3) | 148 (25.0) |
| Summer | 261,856 (24.7) | 4048 (23.9) | 137 (23.1) |
| Fall | 252,666 (23.8) | 4246 (25.1) | 155 (26.2) |
| Body weight | |||
| <1 kg *† | 2060 (0.2) | 442 (2.6) | 11 (1.9) |
| 1–2.5 kg | 35,697 (3.4) | 4038 (23.9) | 109 (18.4) |
| 2.5 kg< | 1,023,319 (96.4) | 12,440 (73.5) | 472 (79.7) |
| Premature | |||
| <28 weeks *† | 1661 (0.2) | 192 (1.1) | 5 (0.8) |
| 28–36 weeks | 41,341 (3.9) | 4614 (27.3) | 120 (20.3) |
| 36 weeks< | 1,018,074 (95.9) | 12,114 (71.6) | 467 (78.9) |
| Multiple birth | |||
| Singleton pregnancy *† | 1,039,880 (98.0) | 15,801 (93.4) | 557 (94.1) |
| Twin pregnancy | 20,433 (1.9) | 1072 (6.3) | 32 (5.4) |
| Multiple pregnancy | 763 (0.1) | 47 (0.3) | 3 (0.5) |
| Pre-existing hypertension *† | 37,236 (3.5) | 5759 (34.0) | 229 (38.7) |
| Gestational hypertension *† | 5508 (0.5) | 1003 (5.9) | 56 (9.5) |
| Superimposed preeclampsia *† | 1485 (0.1) | 600 (3.5) | 18 (3.0) |
| Pre-existing diabetes *† | 2877 (0.3) | 242 (1.4) | 10 (1.7) |
| Gestational diabetes *† | 229,918 (21.7) | 5026 (29.7) | 157 (26.5) |
| Kidney disease *† | 6438 (0.6) | 373 (2.2) | 15 (2.5) |
| Autoimmune disease * | 594 (0.1) | 16 (0.1) | 0 (0.0) |
| Thyroid disease *† | 374,703 (35.3) | 7683 (45.4) | 267 (45.1) |
| Dyslipidemia *† | 221,737 (20.9) | 6226 (36.8) | 234 (39.5) |
| Infertility *† | 157,150 (14.8) | 4210 (24.9) | 134 (22.6) |
| Air Pollutants and Heavy Metals | Mean | SD |
|---|---|---|
| PM10 (μg/m3) | 46.293 | 11.113 |
| PM2.5 (μg/m3) | 25.587 | 5.564 |
| SO2 (ppm) | 0.005 | 0.001 |
| NO2 (ppm) | 0.023 | 0.007 |
| O3 (ppm) | 0.027 | 0.008 |
| CO (ppm) | 0.503 | 0.110 |
| Pb (μg/m3) | 0.026 | 0.013 |
| Cd (μg/m3) | 0.001 | 0.001 |
| Cr (μg/m3) | 0.004 | 0.003 |
| Cu (μg/m3) | 0.025 | 0.014 |
| Mn (μg/m3) | 0.032 | 0.015 |
| Fe (μg/m3) | 0.664 | 0.267 |
| Ni (μg/m3) | 0.005 | 0.002 |
| As (μg/m3) | 0.004 | 0.002 |
| With Preeclampsia | With Eclampsia | |
|---|---|---|
| OR (95% CI) | OR (95% CI) | |
| PM10 | 0.995 (0.993–0.997) | 1.003 (0.993–1.013) |
| PM2.5 | 1.004 (1–1.008) | 1.017 (0.997–1.037) |
| SO2 | 0.232 (0.201–0.268) | 0.337 (0.158–0.718) |
| NO2 | 0.916 (0.893–0.94) | 0.826 (0.72–0.947) |
| O3 | 1.05 (1.021–1.081) | 1.312 (1.132–1.521) |
| CO | 0.995 (0.993–0.997) | 0.998 (0.988–1.008) |
| Pb | 0.949 (0.936–0.962) | 0.894 (0.831–0.962) |
| Cd | 0.845 (0.693–1.031) | 0.17 (0.048–0.607) |
| Cr | 1.036 (0.98–1.095) | 0.541 (0.387–0.755) |
| Cu | 1.013 (1.002–1.024) | 0.968 (0.913–1.027) |
| Mn | 0.979 (0.968–0.989) | 0.891 (0.837–0.948) |
| Fe | 1 (0.999–1) | 0.996 (0.992–0.999) |
| Ni | 0.977 (0.917–1.042) | 0.49 (0.341–0.705) |
| As | 0.958 (0.897–1.024) | 0.693 (0.476–1.009) |
| Univariate | Multivariate | |
|---|---|---|
| OR (95% CI) | OR (95% CI) | |
| Pre-existing hypertension | 15.187 (14.663–15.731) | 15.158 (14.643–15.692) |
| Twin and multiple pregnancy | 2.541 (2.372–2.723) | 2.537 (2.368–2.718) |
| Pre-existing diabetes and gestational diabetes | 1.305 (1.26–1.351) | 1.312 (1.267–1.358) |
| Age | 1.001 (0.997–1.004) | |
| Kidney disease | 1.482 (1.32–1.665) | 1.483 (1.321–1.664) |
| Autoimmune disease | 1.221 (0.727–2.051) | |
| Infertility | 1.499 (1.442–1.558) | 1.506 (1.45–1.564) |
| PM2.5 | 1.003 (1.001–1.006) | |
| O3 | 1.005 (0.985–1.025) | 1.003 (0.984–1.023) |
| Cu | 1.006 (0.995–1.018) | 1.011 (1.001–1.023) |
| Univariate | Multivariate | |
|---|---|---|
| OR (95% CI) | OR (95% CI) | |
| Pre-existing hypertension | 16.549 (13.934–19.654) | 16.538 (13.984–19.558) |
| Twin and multiple pregnancy | 2.065 (1.447–2.947) | 2.056 (1.441–2.933) |
| Pre-existing diabetes and gestational diabetes | 1.102 (0.918–1.324) | |
| Age | 0.984 (0.966–1.002) | |
| Kidney disease | 1.433 (0.837–2.453) | |
| Infertility | 1.318 (1.076–1.613) | 1.303 (1.067–1.593) |
| PM2.5 | 1.014 (0.998–1.029) | 1.013 (0.997–1.028) |
| O3 | 1.111 (1.005–1.228) | 1.113 (1.007–1.23) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.S.; Min, W.K.; Choi, Y.J.; Cho, J.; Kim, S.H.; Shin, H.W. Exposure to Fine Particulate Matter (PM2.5) and Heavy Metals During the Second Trimester of Pregnancy Increases the Risk of Preeclampsia and Eclampsia: An Analysis of National Health Insurance Claims Data from South Korea. Medicina 2025, 61, 1146. https://doi.org/10.3390/medicina61071146
Lee KS, Min WK, Choi YJ, Cho J, Kim SH, Shin HW. Exposure to Fine Particulate Matter (PM2.5) and Heavy Metals During the Second Trimester of Pregnancy Increases the Risk of Preeclampsia and Eclampsia: An Analysis of National Health Insurance Claims Data from South Korea. Medicina. 2025; 61(7):1146. https://doi.org/10.3390/medicina61071146
Chicago/Turabian StyleLee, Kuen Su, Won Kee Min, Yoon Ji Choi, Jeongun Cho, Sang Hun Kim, and Hye Won Shin. 2025. "Exposure to Fine Particulate Matter (PM2.5) and Heavy Metals During the Second Trimester of Pregnancy Increases the Risk of Preeclampsia and Eclampsia: An Analysis of National Health Insurance Claims Data from South Korea" Medicina 61, no. 7: 1146. https://doi.org/10.3390/medicina61071146
APA StyleLee, K. S., Min, W. K., Choi, Y. J., Cho, J., Kim, S. H., & Shin, H. W. (2025). Exposure to Fine Particulate Matter (PM2.5) and Heavy Metals During the Second Trimester of Pregnancy Increases the Risk of Preeclampsia and Eclampsia: An Analysis of National Health Insurance Claims Data from South Korea. Medicina, 61(7), 1146. https://doi.org/10.3390/medicina61071146






