Abstract
Background and Objectives: Osteoarthritis (OA) of the knee and hip is a major cause of pain and functional impairment. This study evaluated the effects of non-immersive virtual reality (NIVR) combined with conventional physical therapy (CPT) on pain intensity, mechanical hyperalgesia, and perceived recovery in older adults with OA. Materials and Methods: Sixty older adults with mild-to-moderate knee or hip OA were randomly assigned to a NIVR group (NIVR-G; n = 30) or a CPT group (CPT-G; n = 30). Both groups completed 30 sessions over 10 weeks (3 sessions/week). The NIVR-G performed 20 minutes of exergames integrated into CPT. Pain intensity was assessed using the Visual Analog Scale (VAS), and mechanical hyperalgesia was evaluated through pressure pain thresholds (PPTs). Secondary outcomes included the Global Rating of Change (GRoC) and the minimal clinically important difference (MCID) for the VAS. This study is a secondary analysis of a randomized controlled trial registered at ClinicalTrials.gov (ID: NCT05839262). Results: The NIVR-G demonstrated significant reductions in pain intensity after 30 sessions (p < 0.05, d = 1.50), with greater improvements compared to the CPT-G (p < 0.05, d = 1.17). The MCID for the VAS was established at 9.2 mm, with a higher proportion of responders in the NIVR-G (p < 0.05). The NIVR-G also reported superior recovery perception on the GRoC scale (p < 0.05). No significant changes in PPTs were observed in either group. However, the improvements in the NIVR-G diminished four weeks post-intervention. Conclusions: NIVR exergames combined with CPT significantly reduced pain intensity, improved perceived recovery, and resulted in a higher proportion of responders compared to CPT alone. These findings support the use of NIVR as an effective adjunct to CPT in older adults with OA; however, further research is needed to optimize its long-term benefits.
1. Introduction
Knee and hip osteoarthritis (OA) are prevalent degenerative joint diseases that significantly impact global health, affecting over 300 million people and ranking as a leading cause of disability worldwide [1,2]. Knee OA has a global prevalence of approximately 3.8%, while hip OA affects around 0.85% of the population [3]. The disease burden increases with age, affecting up to 13% of women and 10% of men over 60 [4]. Key risk factors for OA include age, female sex, obesity, joint injuries, and genetic predisposition [1,2]. Rising life expectancy and obesity rates are projected to further increase OA prevalence, emphasizing the need for public health interventions and healthcare preparedness [2,5].
Pain is the most common symptom in knee and hip OA, involving a range of mechanisms and interactions, and is associated with deterioration in physical function, future disability, and a significant impact on patients’ quality of life [1,6]. OA pain typically presents as a dull, aching discomfort that becomes more persistent over time, with intermittent episodes of intense and unpredictable pain, disrupting social and recreational activities [7]. Pain perception is influenced by both age and sex, with younger patients reporting higher pain intensity and using more affective descriptors despite having less severe disease, while women tend to report more pain and use more analgesics compared to men [8]. Radiographic severity does not always correlate with pain intensity, particularly in hip OA, where pain can be more severe despite less advanced radiographic changes [9,10]. Therefore, comprehensive pain management strategies should address the multifaceted nature of OA pain to improve patient outcomes.
In OA, pain is driven not only by joint pathology but also by altered nociceptive processing, such as mechanical hyperalgesia [11]. Patients with knee OA, for example, exhibit lower pressure pain thresholds (PPTs) not only at the knee but also at remote sites, suggesting widespread hyperalgesia [12]. This phenomenon has been described as pain sensitization and could imply the presence of central sensitization (CS), where the central nervous system amplifies pain signals, lowering pain thresholds throughout the body [13,14,15]. This is consistent with findings from other chronic musculoskeletal pain conditions, supporting the idea that OA pain extends beyond the affected joint [16]. CS has been proposed as one of the mechanisms underlying the lack of correlation with structural damage observed in imaging studies [17]. Furthermore, neuropathic pain may be present in OA patients, being more common in knee OA than in hip OA, with up to 40% of knee OA patients affected [13]. As such, pain management for OA must address both local and central mechanisms to improve pain control and enhance overall patient outcomes [12].
The management of knee and hip OA involves a combination of non-pharmacologic, pharmacologic, and surgical treatments tailored to the severity of the disease and individual patient needs, with non-pharmacologic treatments being foundational, and exercise and weight loss as key components [18]. Exercise, including both aerobic and resistance training, is highly recommended, as it improves pain and function without significant adverse effects [19]. In addition to exercise, common self-management strategies such as the use of knee braces or supports have shown efficacy in symptom relief and functional improvement [20]. Other non-pharmacologic therapies, including physical modalities, life-style modification, and patient education, also contribute to comprehensive OA management [21]. Pharmacologic treatments, such as nonsteroidal anti-inflammatory drugs, serve as first-line options for pain relief, intra-articular corticosteroid injections offer short-term pain management for both knee and hip OA, and for advanced OA that does not respond to conservative treatments, total joint arthroplasty is the most effective surgical intervention [19,22]. However, many patients continue to experience persistent pain and functional limitations after conventional treatments, which has led to the exploration of new therapeutic approaches.
Virtual reality (VR), defined as the creation of digital environments that allow interaction, can be classified based on its level of immersion into fully immersive, semi-immersive, and non-immersive [23]. Physical exercise performed through non-immersive virtual reality (NIVR) systems (also known as exergames) and delivered either as a standalone intervention or combined with conventional physical therapy (CPT) has emerged as a promising tool for managing pain and improving various outcomes in individuals with OA, including postural balance, proprioception, gait, range of motion, fall risk, depression, and quality of life [24,25,26,27]. Several studies have specifically examined the effects of NIVR interventions on pain reduction, yielding encouraging results as measured by instruments such as the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) and the Visual Analog Scale (VAS) in patients with knee OA [24,25,26,28]. Additionally, VR rehabilitation has demonstrated potential in reducing kinesiophobia and pain catastrophizing in patients undergoing total knee arthroplasty [29]. Despite these positive findings, the effectiveness of exergames relative to conventional rehabilitation remains unclear, and more high-quality studies are needed to establish definitive clinical guidelines [25,30]. In summary, NIVR shows promise as a complementary approach to OA pain rehabilitation, though further research is required to refine and standardize its clinical application.
VR systems seem to reduce pain through a combination of psychological and physiological mechanisms. One key mechanism is distraction and immersion, where the multisensory nature of VR diverts attention from pain, lowering its intensity and unpleasantness [31]. Research shows that immersive VR environments, especially those involving interactive elements like avatar embodiment, effectively reduce pain perception [32,33]. Additionally, physical activity during exergaming induces hypoalgesic effects [34]. Active VR games that require movement have been shown to increase pain thresholds and reduce sensitivity, particularly when they elicit cardiovascular responses [35,36]. Neural mechanisms also play a role, with VR altering neural oscillations, such as gamma and alpha waves, which are linked to reduced pain intensity [33]. Furthermore, tactile feedback enhances the sense of avatar ownership, which increases VR’s effectiveness in alleviating pain [37]. These mechanisms together make NIVR a promising non-pharmacological approach to pain management [25].
Despite promising findings, a comprehensive understanding of the mechanisms through which NIVR modulates pain and influences pain perception is still lacking, requiring further investigation [31]. Moreover, additional controlled trials comparing NIVR with conventional interventions are needed to definitively establish its efficacy in pain management [25,38], which could contribute to developing more personalized and effective treatment strategies in older adults with knee and hip OA [24,25,28,31]. Furthermore, to the authors’ knowledge, no studies have yet examined the effect of NIVR on clinically meaningful measures, such as the minimum clinically important difference (MCID) and the Global Rating of Change (GRoC) scale for pain in this population. Therefore, this study aimed to evaluate the effect of an NIVR intervention on self-reported pain intensity and the response of local and remote mechanical hyperalgesia in patients with knee and/or hip OA. We hypothesized that NIVR exercise combined with CPT would reduce self-reported pain intensity and mechanical hyperalgesia in patients with knee and/or hip OA. Secondarily, an aim was to establish the MCID threshold for the VAS to identify responders and non-responders to the NIVR treatment in terms of pain intensity.
2. Materials and Methods
2.1. Study Overview
This study was a secondary analysis of a parallel, two-arm randomized controlled trial with a 1:1 allocation [39]. The experimental group (NIVR-G) engaged in NIVR complementarily to CPT, while the control group (CPT-G) followed the CPT protocol exclusively. The protocol received ethical approval from the Scientific Ethics Committee of the Concepción Health Service (Approval No. 22-12-59) and was registered at clinicaltrials.gov (NCT05839262) on 15 March 2023.
2.2. Participant Recruitment
The participants were selected from Lorenzo Arenas Primary Health Care Center, Bíobío, Chile. A recruitment process was conducted by a professional who contacted eligible patients referred for physical therapy, either in person or by phone. Those expressing interest attended a briefing session where the study’s details were explained, and written informed consent was obtained in accordance with the Declaration of Helsinki.
Demographic and clinical data were collected during the initial session (Table 1), and the participants were introduced to the study procedures to ensure familiarity. The inclusion criteria encompassed adults aged 60–84 years with mild-to-moderate knee or hip OA diagnosed using the American College of Rheumatology (ACR) criteria and graded 2–3 on the Kellgren–Lawrence scale [40,41]. The exclusion criteria included uncontrolled physical or cognitive conditions, opioid use, scores below 13 on the abbreviated Mini-Mental State Examination (MMSE-EFAM) [42], OA from infectious or autoimmune causes, recent surgeries or fractures, and participation in other rehabilitation programs within the past three months. The participant flow throughout the study phases is shown in Figure 1.

Table 1.
Sociodemographic characterization of the sample.
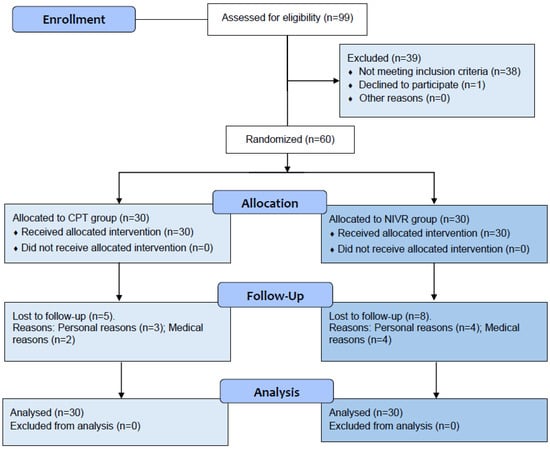
Figure 1.
Study flow chart.
Sample size calculations were conducted using G*Power (v3.1.9.7), targeting a moderate effect size (α = 0.05, power = 0.8). To account for potential attrition, 60 participants were recruited, exceeding the estimated 50 required.
2.3. Group Allocation
The participants were randomized by an independent researcher external to the research group, using a stratified randomization strategy in R software (v4.1.2), ensuring balance by age and sex. Group assignments were concealed in sequentially numbered, opaque envelopes, which were opened by a healthcare professional at the time of allocation.
2.4. Intervention Protocol
The 10-week intervention consisted of three supervised sessions per week for both groups, totaling 30 sessions. Each session included 50 min of effective exercise time. Adherence was defined as attendance at a minimum of 20 sessions. Exercise intensity was monitored using a 0–10 perceived exertion scale, with the participants instructed to maintain a light (ratings of 3–4) to moderate (ratings of 5–6) effort. The intervention was designed to meet a target weekly exercise volume of 150 min, in alignment with the recommendations of the American College of Sports Medicine (ACSM) [43].
- Control group: CPT sessions included physical agents (e.g., TENS, hot packs), warm-up, structured exercises (aerobics, strength, balance, and flexibility), and a cool-down. Individualized adaptations ensured the exercises matched participants’ capacities.
- Experimental group: CPT was complemented with 20 min of NIVR, replacing part of the CPT exercise block to maintain an equal session duration of 50 min. The NIVR included activities targeting strength, balance, flexibility, and aerobic endurance, delivered through Ring Fit Adventure (Nintendo Switch®, Nintendo Co., Ltd., Kyoto, Japan) on a 43-inch TV with real-time feedback (Figure 2). Specifically, the exercises performed using the NIVR device were as follows: Dorsal rotation, Rotation with inclination, Knee raises, Squats, Lunge with rotation, Lateral inclination, Squats with extension, The warrior, The chair, Crescent moon, Equilibrium, Moto adductors, Trunk swinging, Running path, Monster’s lair, and Jogging bridge.
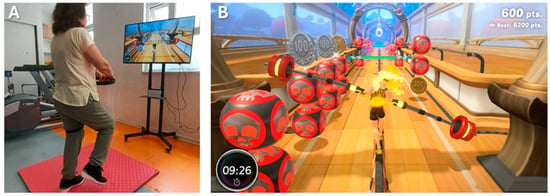 Figure 2. Experimental group subject performing NIVR intervention (A) and their avatar for feedback (B).
Figure 2. Experimental group subject performing NIVR intervention (A) and their avatar for feedback (B).
2.5. Outcome Assessments
A trained health professional, blinded to the group assignment, conducted assessments at five time points: baseline (one week prior to the start of the intervention), after 10 sessions, after 20 sessions, after 30 sessions, and at follow-up four weeks after the intervention ended.
Pain intensity was measured using the VAS, a widely recognized and reliable tool. The participants were asked to rate their actual pain intensity on a scale from 0 (no pain) to 10 (worst possible pain) [44].
The PPT, a reliable and reproducible measurement tool [45,46], was used to assess the activation of endogenous analgesic mechanisms and mechanical hyperalgesia, a marker of generalized mechanical pain sensitization. These measurements were taken at three anatomical sites: the upper trapezius muscle (PPT-T, representing remote or widespread hyperalgesia), the knee (PPT-K, midway between the medial epicondyle and the patellar tendon at the joint line), and the greater trochanter of the hip (PPT-H). The assessments followed validated protocols [47,48] and used a Wagner FPX 25 digital algometer (Wagner Instruments, Greenwich, CT, USA). Measurements were performed on the affected side or the dominant side in cases of bilateral involvement. The evaluator, consistent throughout the study, applied the algometer perpendicularly to the skin at a standardized pressure increase rate (0.5 kg/cm2 per second) until the participant reported pain onset. Each site was measured three times, with a three-minute rest between repetitions. The mean value from the three measurements at each site was used for the analysis.
Changes in perceived pain were assessed solely at the final evaluation (after 30 sessions) using the GRoC scale. This 15-point scale, commonly used as both an outcome measure and an external anchor, ranges from +7 (“a very great deal better”) to −7 (“a very great deal worse”), with 0 indicating no change. The change was categorized as small (1–3 points), moderate (4–5 points), or large (6–7 points) [49].
2.6. Statistical Anlysis
The data were analyzed using an intention-to-treat approach. This analysis was conducted by an investigator who was blinded to the group allocation. Missing values were managed with predictive mean matching multiple imputation [50]. Normality was assessed using the Shapiro–Wilk test, and homoscedasticity was evaluated with Levene’s test. A significance level of p < 0.05 was applied to all analyses. A two-way repeated measures analysis of variance (ANOVA), followed by Bonferroni post-hoc tests, was performed for the VAS and PPT scores. To address unequal variances between groups (as assessed by the Brown–Forsythe test, p < 0.05), the Welch test was applied to evaluate differences in the GRoC of pain. Effect sizes were calculated using Cohen’s d and categorized as negligible (<0.2), small (0.2–0.49), moderate (0.5–0.79), or large (≥0.8) [51].
For the calculation of the MCID, the anchor-based method using the GRoC was employed. The patients were categorized according to their score on this scale as follows: (i) No change (GRoC = −1, 0, and 1), (ii) Minimal change (GRoC = −2, −3, 2, and 3), (iii) Moderate change (GRoC = −4, −5, 4, and 5), (iv) Large change (GRoC = −6, −7, 6, and 7). Subsequently, the absolute mean changes in the VAS scores were calculated based on the change perceived by the patient using the GRoC scale. To identify responders and non-responders, the MCID threshold for the VAS was calculated using a standardized effect size multiplied by the standard deviation [52]. This method accounts for inter-individual variability and allows for the identification of responders whose improvements exceed the MCID, reflecting clinically meaningful benefits. The participants were classified based on the change (Δ) in scores (post-test 3 vs. pre-test) into two groups: responders (Rs) and non-responders (NRs) [53]. The classification criteria were defined as follows: Rs if Δ score ≥ MCID, and NRs if Δ score < MCID.
3. Results
At the beginning of the study, the groups exhibited comparable sociodemographic characteristics (Table 1) and baseline levels of the study outcomes. Of the 99 individuals initially screened, 60 participants were enrolled, with 13 dropouts for reasons unrelated to the study protocol (Figure 1). Recruitment and follow-up occurred between April 2023 and March 2024. No adverse events, such as falls, fainting, nausea, or severe pain, were reported. Adherence rates were 73.3% in the CPT-G and 76.7% in the NIVR-G.
The VAS, PPT-T, PPT-K, and PPT-H values are presented in Table 2. For VAS pain intensity (Figure 3A), the within-subjects ANOVA revealed significant changes over time (p < 0.001) and a significant interaction between time and group (p < 0.001). The between-group comparisons indicated a significant difference between the NIVR-G and CPT-G (p = 0.029). The post-hoc intragroup analysis showed no significant differences in pain intensity over time within the CPT-G. However, the NIVR-G demonstrated significant differences after 10 (p < 0.05; mean difference [MD] = 18.00; 95% CI [3.89–32.11]) and 30 sessions (p < 0.05; MD = 32.63; 95% CI [17.04–48.23]), with large effect sizes (d = 0.825, and 1.496, respectively). The post-hoc comparisons between the groups showed that only after 30 sessions did the NIVR-G demonstrate a significant reduction in pain intensity compared to the CPT-G (p < 0.001; MD = 25.43; 95% CI [12.83–38.03]), with a large effect size (d = 1.166).

Table 2.
Comparison of pain intensity (VAS, mm) and pressure pain thresholds (kg/cm2) values in the study groups.
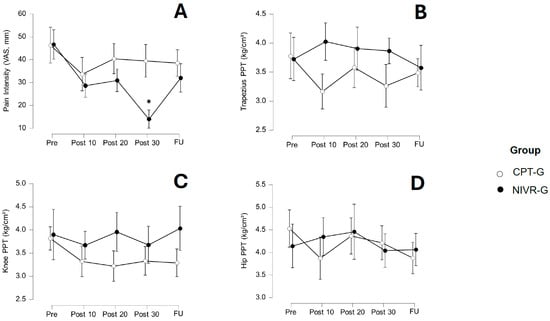
Figure 3.
Changes in pain intensity (A) Visual Analog Scale [VAS], trapezius pressure pain threshold [PPT] (B), knee PPT (C), and hip PPT (D) after 10, 20, and 30 sessions, as well as at follow-up (FU), in the conventional physical therapy group (CPT-G) and the non-immersive virtual reality group (NIVR-G). The means and 95% confidence intervals are shown for each group at each time point. An asterisk (*) indicates a statistically significant difference between groups, in favor of the NIVR-G.
The within-subjects ANOVA for the PPT-T revealed no significant main effect of time (p = 0.540), though the interaction between time and group was significant (p = 0.039). However, the post-hoc Bonferroni’s tests did not show significant differences between the comparisons. The between-subjects analysis also did not find a significant group difference (p = 0.156). Figure 3B illustrates the changes in these variables after both interventions.
For the PPT-K (Figure 3C), the within-subjects ANOVA indicated no significant main effect of time (p = 0.267), and the interaction between time and group was not significant (p = 0.311). The between-subjects analysis revealed no significant group difference for the PPT-K (p = 0.069), suggesting no overall differences between the groups in PPT-K scores.
For the PPT-H, no significant main effect of time was observed (p = 0.223). Similarly, the interaction between time and group was non-significant (p = 0.296), and the between-subjects analysis found no significant group differences (p = 0.901). These results suggest that the intervention had no significant impact on the PPT-H, either across time or between the groups (Figure 3D).
Finally, after 30 sessions, the GRoC scores for the CPT-G showed a mean of 3.27 ± 1.57, while the NIVR-G exhibited a mean score of 4.87 ± 1.01. A statistically significant difference was found between the groups (p < 0.001; MD = 1.60; 95% CI [0.91–2.29]), with a large effect size (Cohen’s d= −1.210), indicating a meaningful difference in favor of NIVR-G (Figure 4).
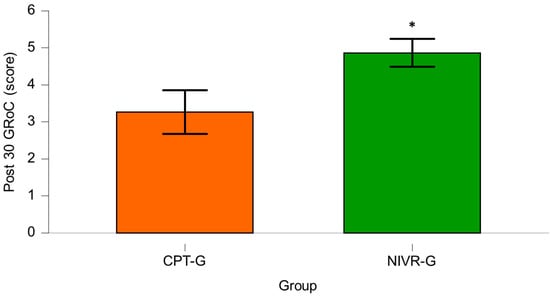
Figure 4.
Comparison of Global Rating of Change for Pain (GRoC) after 30 sessions in the conventional physical therapy group (CPT-G) and the non-immersive virtual reality group (NIVR-G). An asterisk (*) indicates a statistically significant difference in favor of the NIVR-G.
The Supplementary Material 1 (Table S1) presents the absolute mean changes (95% CI) in the VAS scores based on the patient’s perceived change. The anchor-based estimation of the MCID for VAS was 9.2 mm.
The Supplementary Material 2 (Figure S1) presents the ±MCID thresholds for the VAS scores, along with bars representing each participant’s Δ scores for their classification as Rs and NRs. In the NIVR-G, the most frequent category was Rs, whereas in the CPT-G, both categories were equivalent. Compared to the CPT-G, the NIVR-G had higher frequencies of Rs (76.67%), with a statistically significant difference in the VAS (chi-square test, p < 0.05; z-test, p < 0.05). For the NRs category, the NVIR-G had lower frequencies, with a statistically significant difference in the VAS (chi-square test, p < 0.05; z-test, p < 0.05).
4. Discussion
The results suggest that adding NIVR enhances the effect of CPT on pain intensity in older adults with hip or knee OA after 30 sessions. Furthermore, this aligned with the patients’ perceptions of improvement in their pain condition following the intervention. However, this improvement was not maintained at the 4-week follow-up. Additionally, the results show that local and remote PPTs did not significantly change after 10, 20, or 30 sessions, nor at the 4-week post-intervention follow-up. Therefore, CPT, whether complemented with NIVR or not, did not significantly alter local or remote mechanical hyperalgesia in the sample studied. Finally, the MCID for VAS after 30 sessions was estimated at 9.2 mm for this population.
4.1. Self-Reported Pain Intensity
The findings of this study regarding pain intensity reduction are aligned with previous research on the effects of rehabilitation using various VR modalities. These include studies on knee and hip OA, as well as other chronic painful conditions [24,54,55]. Additionally, the lack of long-term results observed in the present study is consistent with previous reports by Mallari et al. [56]. A recent systematic review showed that both NIVR and immersive VR training are effective for alleviating chronic musculoskeletal pain [57]. Specifically for knee OA, two recent reviews concluded that VR-based exercise therapy is effective in improving pain [25,28]. Wei et al. [28] analyzed nine studies, five of which used NIVR for 4 to 12 weeks, with a frequency of 2 or 3 times per week. Subgroup analysis based on the VR immersion level showed that immersive VR was more effective at relieving pain compared to NIVR, which could be attributed to a lower level of sensory substitution in NIVR, leading to a reduced capacity to attenuate pain perception. On the other hand, in their review on the effects of exergames in patients with OA, Guede-Rojas et al. [25] found that in three out of four studies reviewed, NIVR yielded better results than the control group in reducing pain intensity in knee OA, which is consistent with our findings after 30 sessions. Finally, although VR interventions have the potential to reduce attention to pain and improve pain perception by stimulating visual, auditory, sensorimotor, cognitive, and affective networks [31,58], further clinical studies are required to elucidate the mechanisms underlying our findings, specifically, the mechanisms involved in pain intensity reduction in individuals with OA after completing a NIVR protocol and the reasons why these improvements do not persist over time.
Regarding treatment duration, the systematic review and meta-analysis by Wei et al. concluded that treatments lasting less than 6 weeks are more effective than those lasting 6 weeks or longer [28]. Furthermore, the best results are observed with treatment frequencies of ≤3 times per week and session durations of 20 min each [28]. Despite these reported values, our study demonstrated significant results with a large effect size when complementing CPT with NIVR applied at a frequency of 3 times per week over a 10-week period, with a session duration of 20 min, immediately following the completion of the treatment. These differences could be attributed to the characteristics of the devices used, the type of exercise performed, the protocol parameters, or the level of immersion in the interventions.
Besides its therapeutic utility on its own, adding NIVR to CPT appears to provide greater short-term benefits for pain intensity compared to CPT alone in patients with hip or knee OA in the short term. The combination of locally applied interventions (e.g., exercises) and centrally-targeted interventions (e.g., NIVR) may produce synergistic effects, resulting in better outcomes [28,31,38,58]. Several studies have reported that adding a new therapeutic tool to CPT could improve the outcomes achieved. For instance, a recent review found low-to-moderate-certainty evidence supporting manual therapy as an adjunct to exercise therapy for pain in patients with knee or hip OA in the short term [59]. However, the effectiveness of other tools, such as bracing [60], orthotics [61] and dry needling [62], as part of a multidisciplinary approach, remains questionable. The results of this clinical trial support the idea of complementing CPT with tools like NIVR. However, further studies are needed to better characterize the interventions and patients who would benefit from this therapeutic synergy, especially regarding long-term outcomes.
The severity of reported pain intensity has been associated with the degree of pain sensitization, and this association is independent of the radiologically-reported severity of OA [14]. This gives significant clinical relevance to our NIVR results, particularly considering that acute OA flare-ups can reduce adherence to active exercise treatment [63,64]. In our study, the additional improvement in pain intensity offered by NIVR occurred after approximately 30 sessions, suggesting that NIVR would not be the first choice for the rapid management of acute OA flare-ups. This does not mean that NIVR cannot help improve patient adherence to treatment, as it may be the most motivating way for some individuals to engage in exercise [65]. OA is a heterogeneous disease, so subgroups of patients may respond differently to various treatment modalities. In this regard, clinicians must consider the patient’s perspective when setting therapeutic goals [66], as these may differ from the goals of a study group (e.g., the mean change in a variable). This supports the use of clinical measurements in such studies, and in this sense, the differences observed between the groups for the post-treatment GRoC variable—positive in favor of the NIVR-G—suggest recommending its use both in clinical practice and in outcome assessments in future studies.
Most studies on OA have primarily focused on short-term outcomes, leaving the long-term effects of VR-based therapies insufficiently explored [28]. In our study, although the combination of NIVR with CPT produced significant reductions in pain intensity after 30 sessions—with large between-group effect sizes—these benefits were not maintained at the 4-week follow-up. Similar patterns have been observed in previous research on exercise programs based on other digital technologies (such as mobile applications or telerehabilitation), where clinical improvements tend to diminish over the long-term following treatment cessation [67]. This suggests that ongoing engagement or booster sessions may be necessary to sustain therapeutic gains. Future studies should investigate the feasibility and efficacy of reinforcement strategies or home-based NIVR programs to improve the long-term management of knee OA.
4.2. Mechanical Hyperalgesia
Patients with different types of OA may exhibit pain sensitization, characterized—among other variables—by a decrease in baseline PPTs, both locally (peripheral sensitization) and remotely (central sensitization) [68]. Specifically, in patients with knee and hip OA, a robust body of literature has shown the presence of both local and remote mechanical hyperalgesia when compared to healthy controls [11,12,14,69,70,71,72], which is related to functionality levels [73]. Increased mechanical pain sensitization is a pre-morbid risk factor for worsening knee and hip OA symptoms [74,75,76], and a predictor of poor treatment response [11,76]. Interestingly, several studies have reported that alterations in PPTs can normalize once the nociceptive source is surgically treated [77,78,79], while Hattori et al. reported that therapeutic exercise may reduce pain intensity in people with knee or hip OA, depending on baseline symptom severity and mechanical hyperalgesia [11]. Fingleton et al. observed that PPTs’ responses to exercise in people with OA are related to conditioned pain modulation (CPM) test behavior, suggesting that exercise and CPM share equivalent mechanisms [80]. Specifically, they found that individuals with normal CPM showed improvements in their PPTs, whereas those with impaired CPM exhibited decreased PPTs. In contrast, our study found no significant changes in local and remote PPTs values following the intervention.
The baseline PPTs obtained in our sample were similar to those reported in previous studies for individuals with knee or hip OA; moreover, they were more than 20% lower than those measured in healthy individuals [73,81,82]. This clinically significant difference indicates the presence of both local and remote mechanical hyperalgesia [14,83,84]. Remote mechanical hyperalgesia has been described as an indicator of generalized pain sensitization [12] and alterations in nociceptive processing, which are associated with poor responses to standard treatment in OA patients [85]. This highlights the therapeutic potential of the descending modulatory system to influence pain thresholds. This system works by releasing neurotransmitters from the brain that can either inhibit or enhance nociceptive transmission in the spinal cord [86]. Thus, emotional states and sensory experiences can influence pain perception through limbic and thalamic regions of the brain.
Based on the reasoning outlined above, it is important to highlight that this study observed a paradoxical finding: adding NIVR to the standard treatment did not modify local or remote mechanical hyperalgesia, but it did significantly reduce the pain intensity perceived after 30 sessions of intervention. The cognitive-emotional stimuli associated with VR exposure, such as distraction, affective response, ritualization, positive experience, learning through exposure, and anxiety control, may have acted as the main modulators of the analgesic response perceived by the patients at the end of the intervention [25,31]. NIVR has been shown to induce improvements in various positive emotions, such as happiness, self-esteem, self-worth, self-efficacy, vitality, motivation, and relaxation [87], which could have facilitated the attenuation of the self-reported pain perception through mechanisms such as distraction and activation of motivational systems that counteract the perception of pain [88]. Additionally, dysfunction in exercise-induced analgesic mechanisms has been reported in individuals with chronic pain and pain sensitization [11,89,90], which, according to Previtali et al., may be present in up to one-fifth of patients with knee OA [71]. As mentioned, this dysfunction is reflected in increased pain intensity and duration, influenced by factors such as emotions, stress, and cognitive processes. However, although the presence of sensitization has been identified in individuals with knee or hip OA, the reasons why some patients experience pain only associated with activity, while others suffer from persistent pain, remain unknown.
It is tempting to speculate that new VR modalities may more effectively activate descending inhibitory pathways through distraction with cognitively demanding tasks. However, our study did not show a sustained improvement in pain intensity when assessed 4 weeks post-intervention. Future studies should examine whether VR can achieve this effect and what conditions are required to facilitate it. Additionally, to increase clinical relevance and measurement accuracy, it is suggested that future research include CPM and temporal summation. These are dynamic tests used to investigate central pain processing by assessing an individual’s response to experimental pain stimuli [91]. These quantitative sensory tests, along with PPTs, are the most commonly used measures to assess pain sensitization and may contribute to identifying individuals at risk of chronic pain, guiding a more personalized and effective treatment for OA [92]. Finally, although NIVR showed benefits in pain intensity perception, the protocol may not have been sufficient or optimal to induce significant changes in PPTs, considering that the appropriate parameters for prescribing exercise to improve pain sensitization in chronic pain populations, including OA, are still unknown [93,94].
4.3. Minimal Clinically Important Difference
Research on the MCID for pain intensity in OA has provided valuable insights into interpreting treatment outcomes. For knee and hip OA, a reduction of 1 point or 15% on the numerical rating scale is considered clinically important. Additionally, changes of 19.9 mm for knee OA and 15.3 mm for hip OA on the VAS are also recognized as clinically meaningful improvements [95,96]. In our study, we estimated the MCID for pain intensity (VAS) at 9.2 mm. This value serves as an important benchmark for assessing clinically relevant pain reductions in OA patients undergoing NIVR in conjunction with CPT. The discrepancy between our findings and previous research may be attributed to differences in sample characteristics, including factors such as baseline symptom severity, age, sex distribution, and the specific treatment applied. These factors can significantly influence the achievement of MCID, with greater improvements typically observed in younger patients, females, and those with more severe baseline symptoms [97].
4.4. Strengths and Limitations
This study has several limitations that should be considered when interpreting the results. First, the follow-up period post-treatment was limited to one month, which prevents an assessment of the long-term effects of NIVR on pain intensity and mechanical hyperalgesia. Additionally, the measurement of PPTs can be challenging in certain patients due to the difficulty in accurately identifying the transition point between pressure and perceived pain [98]. Another notable aspect is that the sample, although calculated using G*Power (α = 0.05, power = 0.8), was relatively small and included individuals with both unilateral and bilateral hip and knee OA, as well as exclusively mild-to-moderate disease severity. These clinical characteristics may have influenced individual responses to the intervention and limit the generalizability of the findings to patients with more advanced OA or more uniform clinical profiles. Moreover, the predominance of female participants and minor imbalances in baseline characteristics—such as educational level and OA location—could also have affected pain perception or treatment response. Therefore, the findings should be interpreted with caution and may not be generalizable to the broader OA population. Finally, although both groups received equivalent total exercise time and matched intensity levels, the substitution of 20 min of CPT with NIVR may limit the interpretation of the additive effects of VR. These limitations highlight the need for future studies with larger sample sizes, extended follow-up periods, and multi-arm designs that allow for isolating the specific contribution of NIVR in addition to full-dose conventional physiotherapy.
The strengths of this study include its randomized controlled design, recognized as the gold standard in clinical research, which enhances internal validity and minimizes the risk of bias. Additionally, strategies such as stratified randomization and concealed allocation were implemented, ensuring balance between the groups and reducing the possibility of bias in participant assignment. The pragmatic application of the protocol in a real-world clinical setting strengthens the external validity of the findings, facilitating their extrapolation to similar clinical contexts. Moreover, established, validated, and reliable measurement tools, such as the VAS and PPTs, were used. The inclusion of clinically significant outcome measures, such as the MCID and GRoC, emphasizes the patient-centered approach and reinforces the applicability of the findings. On the other hand, the comparison between groups was appropriate due to the equivalent weekly volume of physical exercise, which allowed for the evaluation of the effects of the interventions without introducing confounding by differences in total physical activity load. Finally, the study considered an intention-to-treat analysis, ensuring that the results reflect the effect of the intervention in the originally assigned population, thereby reducing the influence of potential biases due to data loss during the intervention.
5. Conclusions
The application of 30 sessions of NIVR combined with CPT in older adults with knee and hip OA resulted in significant short-term improvements in pain intensity and global perceived recovery (GRoC). However, no changes were observed in local or remote PPTs. Additionally, pain intensity improvements were not sustained, as they reverted one month after the intervention. These findings suggest that while NIVR can enhance the effects of CPT on subjective pain and recovery during the intervention, its impact may be transient. Moreover, the lack of effect on mechanical hyperalgesia highlights the need for further research to develop strategies that promote long-term pain relief and address the underlying mechanisms of pain sensitization in this population. Future studies should also explore the key characteristics of intervention protocols that drive these analgesic responses.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/medicina61071122/s1, Table S1: “Mean change in pain Visual Analog Scale scores according to patient-rated change”; Figure S1: “Minimum clinically important difference thresholds”.
Author Contributions
Conceptualization, F.G.-R., C.M., and C.C.-P.; methodology, F.G.-R., A.S.-M., and C.C.-P.; software, A.S.-M. and C.C.-P.; validation, F.G.-R., C.M., L.R.-L., A.S.-M., D.U.-D., C.J.-A., and C.C.-P.; formal analysis, F.G.-R., A.S.-M., L.R.-L., and C.C.-P.; investigation, F.G.-R., C.M., and C.C.-P.; resources, F.G.-R., C.M., D.U.-D., C.J.-A., and C.C.-P.; data curation, F.G.-R. and C.C.-P.; writing—original draft preparation, F.G.-R., A.S.-M., L.R.-L., and C.C.-P.; writing—review and editing, F.G.-R., C.M., L.R.-L., A.S.-M., D.U.-D., C.J.-A., and C.C.-P.; visualization, F.G.-R., C.M., L.R.-L., A.S.-M., D.U.-D., C.J.-A., and C.C.-P.; supervision, F.G.-R., and C.C.-P.; project administration, F.G.-R. and C.M.; funding acquisition, F.G.-R., C.M., and C.C.-P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Agencia Nacional de Investigación y Desarrollo, Ministerio de Ciencia, Tecnología, Conocimiento e Innovación of Chile (Fondo de Investigación y Desarrollo en Salud [FONIS] 2022, Subdirección de investigación aplicada, FONDEF), grant code SA22I0092.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, approved by the Scientific Ethics Committee of the Concepción Health Service (No. 22-12-59, date: 21 December 2022), and registered at clinicaltrials.gov (NCT05839262) on 15 March 2023.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All relevant data are presented in the manuscript. The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors would like to thank the rehabilitation professionals at CESFAM Lorenzo Arenas, under the Dirección de Administración de Salud of Concepción, Chile.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| OA | Osteoarthritis |
| PPTs | Pressure pain thresholds |
| CS | Central Sensitization |
| VR | Virtual Reality |
| NIVR | Non-immersive virtual reality |
| CPT | Conventional physical therapy |
| VAS | Visual Analog Scale |
| MCID | Minimum clinically important difference |
| GRoC | Global Rating of Change |
| ACR | American College of Rheumatology |
| ACSM | American College of Sports Medicine |
| ANOVA | Analysis of variance |
| CPM | Conditioned pain modulation |
References
- Katz, J.N.; Arant, K.R.; Loeser, R.F. Diagnosis and Treatment of Hip and Knee Osteoarthritis: A Review. JAMA 2021, 325, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Safiri, S.; Kolahi, A.-A.; Hoy, D.; Buchbinder, R.; Mansournia, M.A.; Bettampadi, D.; Ashrafi-Asgarabad, A.; Almasi-Hashiani, A.; Smith, E.; Sepidarkish, M.; et al. Global, Regional, and National Burden of Neck Pain in the General Population, 1990-2017: Systematic Analysis of the Global Burden of Disease Study 2017. BMJ 2020, 368, m791. [Google Scholar] [CrossRef] [PubMed]
- Cross, M.; Smith, E.; Hoy, D.; Nolte, S.; Ackerman, I.; Fransen, M.; Bridgett, L.; Williams, S.; Guillemin, F.; Hill, C.L.; et al. The Global Burden of Hip and Knee Osteoarthritis: Estimates from the Global Burden of Disease 2010 Study. Ann. Rheum. Dis. 2014, 73, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jordan, J.M. Epidemiology of Osteoarthritis. Clin. Geriatr. Med. 2010, 26, 355–369. [Google Scholar] [CrossRef]
- Yates, A.J.J.; McGrory, B.J.; Starz, T.W.; Vincent, K.R.; McCardel, B.; Golightly, Y.M. AAOS Appropriate Use Criteria: Optimizing the Non-Arthroplasty Management of Osteoarthritis of the Knee. JAAOS—J. Am. Acad. Orthop. Surg. 2014, 22, 261. [Google Scholar] [CrossRef]
- Wojcieszek, A.; Kurowska, A.; Majda, A.; Liszka, H.; Gądek, A. The Impact of Chronic Pain, Stiffness and Difficulties in Performing Daily Activities on the Quality of Life of Older Patients with Knee Osteoarthritis. Int. J. Environ. Res. Public Health 2022, 19, 16815. [Google Scholar] [CrossRef]
- Hawker, G.A.; Stewart, L.; French, M.R.; Cibere, J.; Jordan, J.M.; March, L.; Suarez-Almazor, M.; Gooberman-Hill, R. Understanding the Pain Experience in Hip and Knee Osteoarthritis—An OARSI/OMERACT Initiative. Osteoarthr. Cartil. 2008, 16, 415–422. [Google Scholar] [CrossRef]
- Skogö Nyvang, J.; Naili, J.E.; Iversen, M.D.; Broström, E.W.; Hedström, M. Younger Age Is Associated with Greater Pain Expression among Patients with Knee or Hip Osteoarthritis Scheduled for a Joint Arthroplasty. BMC Musculoskelet. Disord. 2019, 20, 365. [Google Scholar] [CrossRef]
- Pereira, D.; Severo, M.; Santos, R.A.; Barros, H.; Branco, J.; Lucas, R.; Costa, L.; Ramos, E. Knee and Hip Radiographic Osteoarthritis Features: Differences on Pain, Function and Quality of Life. Clin. Rheumatol. 2016, 35, 1555–1564. [Google Scholar] [CrossRef]
- Finan, P.H.; Buenaver, L.F.; Bounds, S.C.; Hussain, S.; Park, R.J.; Haque, U.J.; Campbell, C.M.; Haythornthwaite, J.A.; Edwards, R.R.; Smith, M.T. Discordance between Pain and Radiographic Severity in Knee Osteoarthritis: Findings from Quantitative Sensory Testing of Central Sensitization. Arthritis Rheum. 2013, 65, 363–372. [Google Scholar] [CrossRef]
- Hattori, T.; Shimo, K.; Niwa, Y.; Katsura, Y.; Tokiwa, Y.; Ohga, S.; Matsubara, T. Pain Sensitization and Neuropathic Pain-like Symptoms Associated with Effectiveness of Exercise Therapy in Patients with Hip and Knee Osteoarthritis. Pain Res. Manag. 2022, 2022, 4323045. [Google Scholar] [CrossRef] [PubMed]
- Moss, P.; Knight, E.; Wright, A. Subjects with Knee Osteoarthritis Exhibit Widespread Hyperalgesia to Pressure and Cold. PLoS ONE 2016, 11, e0147526. [Google Scholar] [CrossRef] [PubMed]
- Zolio, L.; Lim, K.Y.; McKenzie, J.E.; Yan, M.K.; Estee, M.; Hussain, S.M.; Cicuttini, F.; Wluka, A. Systematic Review and Meta-Analysis of the Prevalence of Neuropathic-like Pain and/or Pain Sensitization in People with Knee and Hip Osteoarthritis. Osteoarthr. Cartil. 2021, 29, 1096–1116. [Google Scholar] [CrossRef] [PubMed]
- Fingleton, C.; Smart, K.; Moloney, N.; Fullen, B.M.; Doody, C. Pain Sensitization in People with Knee Osteoarthritis: A Systematic Review and Meta-Analysis. Osteoarthr. Cartil. 2015, 23, 1043–1056. [Google Scholar] [CrossRef]
- Woolf, C.J. Central Sensitization: Implications for the Diagnosis and Treatment of Pain. Pain 2011, 152, S2–S15. [Google Scholar] [CrossRef]
- Imamura, M.; Imamura, S.T.; Kaziyama, H.H.S.; Targino, R.A.; Hsing, W.T.; De Souza, L.P.M.; Cutait, M.M.; Fregni, F.; Camanho, G.L. Impact of Nervous System Hyperalgesia on Pain, Disability, and Quality of Life in Patients with Knee Osteoarthritis: A Controlled Analysis. Arthritis Rheum. 2008, 59, 1424–1431. [Google Scholar] [CrossRef]
- Arendt-Nielsen, L.; Nie, H.; Laursen, M.B.; Laursen, B.S.; Madeleine, P.; Simonsen, O.H.; Graven-Nielsen, T. Sensitization in Patients with Painful Knee Osteoarthritis. Pain 2010, 149, 573–581. [Google Scholar] [CrossRef]
- Moseng, T.; Vliet Vlieland, T.P.M.; Battista, S.; Beckwée, D.; Boyadzhieva, V.; Conaghan, P.G.; Costa, D.; Doherty, M.; Finney, A.G.; Georgiev, T.; et al. EULAR Recommendations for the Non-Pharmacological Core Management of Hip and Knee Osteoarthritis: 2023 Update. Ann. Rheum. Dis. 2024, 83, 730–740. [Google Scholar] [CrossRef]
- Kolasinski, S.L.; Neogi, T.; Hochberg, M.C.; Oatis, C.; Guyatt, G.; Block, J.; Callahan, L.; Copenhaver, C.; Dodge, C.; Felson, D.; et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Rheumatol. 2020, 72, 220–233. [Google Scholar] [CrossRef]
- Cudejko, T.; van der Esch, M.; Schrijvers, J.; Richards, R.; van den Noort, J.C.; Wrigley, T.; van der Leeden, M.; Roorda, L.D.; Lems, W.; Harlaar, J.; et al. The Immediate Effect of a Soft Knee Brace on Dynamic Knee Instability in Persons with Knee Osteoarthritis. Rheumatol. Oxf. Engl. 2018, 57, 1735–1742. [Google Scholar] [CrossRef]
- Poenaru, D.; Sandulescu, M.I.; Potcovaru, C.G.; Cinteza, D. High-Intensity Laser Therapy in Pain Management of Knee Osteoarthritis. Biomedicines 2024, 12, 1679. [Google Scholar] [CrossRef] [PubMed]
- Madry, H. Surgical Therapy in Osteoarthritis. Osteoarthr. Cartil. 2022, 30, 1019–1034. [Google Scholar] [CrossRef] [PubMed]
- Wohlgenannt, I.; Simons, A.; Stieglitz, S. Virtual Reality. Bus. Inf. Syst. Eng. 2020, 62, 455–461. [Google Scholar] [CrossRef]
- Byra, J.; Czernicki, K. The Effectiveness of Virtual Reality Rehabilitation in Patients with Knee and Hip Osteoarthritis. J. Clin. Med. 2020, 9, 2639. [Google Scholar] [CrossRef]
- Guede-Rojas, F.; Andrades-Torres, B.; Aedo-Díaz, N.; González-Koppen, C.; Muñoz-Fuentes, M.; Enríquez-Enríquez, D.; Carvajal-Parodi, C.; Mendoza, C.; Alvarez, C.; Fuentes-Contreras, J. Effects of Exergames on Rehabilitation Outcomes in Patients with Osteoarthritis. A Systematic Review. Disabil. Rehabil. 2025, 47, 1100–1113. [Google Scholar] [CrossRef]
- Mete, E.; Sari, Z. The Efficacy of Exergaming in Patients with Knee Osteoarthritis: A Randomized Controlled Clinical Trial. Physiother. Res. Int. 2022, 27, e1952. [Google Scholar] [CrossRef]
- Manlapaz, D.G.; Sole, G.; Jayakaran, P.; Chapple, C.M. Risk Factors for Falls in Adults with Knee Osteoarthritis: A Systematic Review. PM R 2019, 11, 745–757. [Google Scholar] [CrossRef]
- Wei, W.; Tang, H.; Luo, Y.; Yan, S.; Ji, Q.; Liu, Z.; Li, H.; Wu, F.; Yang, S.; Yang, X. Efficacy of Virtual Reality Exercise in Knee Osteoarthritis Rehabilitation: A Systematic Review and Meta-Analysis. Front. Physiol. 2024, 15, 1424815. [Google Scholar] [CrossRef]
- Gür, O.; Başar, S. The Effect of Virtual Reality on Pain, Kinesiophobia and Function in Total Knee Arthroplasty Patients: A Randomized Controlled Trial. Knee 2023, 45, 187–197. [Google Scholar] [CrossRef]
- Su, S.; He, J.; Wang, R.; Chen, Z.; Zhou, F. The Effectiveness of Virtual Reality, Augmented Reality, and Mixed Reality Rehabilitation in Total Knee Arthroplasty: A Systematic Review and Meta-Analysis. J. Arthroplast. 2024, 39, 582–590.e4. [Google Scholar] [CrossRef]
- Pretat, T.; Koller, C.; Hügle, T. Virtual Reality as a Treatment for Chronic Musculoskeletal Pain Syndromes. Jt. Bone Spine 2025, 92, 105769. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, H.G. Interacting with Virtual Objects via Embodied Avatar Hands Reduces Pain Intensity and Diverts Attention. Sci. Rep. 2021, 11, 10672. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, H.; Xiao, Y.; Liu, X.; Ma, B.; Ma, K.; Hu, L.; Lu, X. The Analgesic Effects and Neural Oscillatory Mechanisms of Virtual Reality Scenes Based on Distraction and Mindfulness Strategies in Human Volunteers. Br. J. Anaesth. 2023, 131, 1082–1092. [Google Scholar] [CrossRef] [PubMed]
- Ditchburn, J.-L.; van Schaik, P.; Dixon, J.; MacSween, A.; Martin, D. The Effects of Exergaming on Pain, Postural Control, Technology Acceptance and Flow Experience in Older People with Chronic Musculoskeletal Pain: A Randomised Controlled Trial. BMC Sports Sci. Med. Rehabil. 2020, 12, 63. [Google Scholar] [CrossRef]
- Rodriguez, S.T.; Makarewicz, N.; Wang, E.Y.; Zuniga-Hernandez, M.; Titzler, J.; Jackson, C.; Suen, M.Y.; Rosales, O.; Caruso, T.J. Virtual Reality Facilitated Exercise Improves Pain Perception: A Crossover Study. J. Clin. Anesth. 2023, 91, 111257. [Google Scholar] [CrossRef]
- Naugle, K.E.; Cervantes, X.A.; Boone, C.L.; Wind, B.; Naugle, K.M. The Acute Hypoalgesic Effects of Active Head-Mounted Display Virtual Reality Games. PLoS ONE 2024, 19, e0308064. [Google Scholar] [CrossRef]
- Hoffman, H.G.; Fontenot, M.R.; Garcia-Palacios, A.; Greenleaf, W.J.; Alhalabi, W.; Curatolo, M.; Flor, H. Adding Tactile Feedback Increases Avatar Ownership and Makes Virtual Reality More Effective at Reducing Pain in a Randomized Crossover Study. Sci. Rep. 2023, 13, 7915. [Google Scholar] [CrossRef]
- Mo, N.; Feng, J.y.; Liu, H.x.; Chen, X.y.; Zhang, H.; Zeng, H. Effects of Exergaming on Musculoskeletal Pain in Older Adults: Systematic Review and Meta-Analysis. JMIR Serious Games 2023, 11, e42944. [Google Scholar] [CrossRef]
- Carvajal-Parodi, C.; Mendoza, C.; Alvarez, C.; Soto-Martínez, A.; Ulloa-Díaz, D.; Jorquera-Aguilera, C.; Guede-Rojas, F. Effectiveness of Exergames on Functional Physical Performance in Older Adults with Knee/Hip Osteoarthritis: A Randomized Controlled Trial. J. Clin. Med. 2025, 14, 2968. [Google Scholar] [CrossRef]
- Wang, Q.; Runhaar, J.; Kloppenburg, M.; Boers, M.; Bijlsma, J.W.J.; Bierma-Zeinstra, S.M.A.; CREDO Expert Group. Evaluation of the Diagnostic Performance of American College of Rheumatology, EULAR, and National Institute for Health and Clinical Excellence Criteria Against Clinically Relevant Knee Osteoarthritis: Data From the CHECK Cohort. Arthritis Care Res. 2024, 76, 511–516. [Google Scholar] [CrossRef]
- Bierma-Zeinstra, S.; Bohnen, A.; Ginai, A.; Prins, A.; Verhaar, J. Validity of American College of Rheumatology Criteria for Diagnosing Hip Osteoarthritis in Primary Care Research. J. Rheumatol. 1999, 26, 1129–1133. [Google Scholar] [PubMed]
- Jiménez, D.; Lavados, M.; Rojas, P.; Henríquez, C.; Silva, F.; Guillón, M.; Jiménez, D.; Lavados, M.; Rojas, P.; Henríquez, C.; et al. Performance of an Abbreviated Mini Mental Examination to Detect Dementia in Older People. Rev. Méd. Chile 2017, 145, 862–868. [Google Scholar] [CrossRef] [PubMed]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.-M.; Nieman, D.C.; Swain, D.P. Quantity and Quality of Exercise for Developing and Maintaining Cardiorespiratory, Musculoskeletal, and Neuromotor Fitness in Apparently Healthy Adults: Guidance for Prescribing Exercise. Med. Sci. Sports Exerc. 2011, 43, 1334. [Google Scholar] [CrossRef] [PubMed]
- Alghadir, A.; Anwer, S.; Iqbal, A.; Iqbal, Z. Test-Retest Reliability, Validity, and Minimum Detectable Change of Visual Analog, Numerical Rating, and Verbal Rating Scales for Measurement of Osteoarthritic Knee Pain. J. Pain Res. 2018, 11, 851–856. [Google Scholar] [CrossRef]
- O’Leary, H.; Smart, K.M.; Moloney, N.A.; Blake, C.; Doody, C.M. Pain Sensitization Associated with Nonresponse after Physiotherapy in People with Knee Osteoarthritis. PAIN 2018, 159, 1877. [Google Scholar] [CrossRef]
- Dua, A.B.; Neogi, T.; Mikolaitis, R.A.; Block, J.A.; Shakoor, N. Somatosensation in OA: Exploring the Relationships of Pain Sensitization, Vibratory Perception and Spontaneous Pain. BMC Musculoskelet. Disord. 2018, 19, 307. [Google Scholar] [CrossRef]
- Burrows, N.J.; Booth, J.; Sturnieks, D.L.; Barry, B.K. Acute Resistance Exercise and Pressure Pain Sensitivity in Knee Osteoarthritis: A Randomised Crossover Trial. Osteoarthr. Cartil. 2014, 22, 407–414. [Google Scholar] [CrossRef]
- Sayed-Noor, A.S.; Englund, E.; Wretenberg, P.; Sjödén, G.O. Pressure-Pain Threshold Algometric Measurement in Patients With Greater Trochanteric Pain After Total Hip Arthroplasty. Clin. J. Pain 2008, 24, 232–236. [Google Scholar] [CrossRef]
- Kamper, S.J.; Maher, C.G.; Mackay, G. Global Rating of Change Scales: A Review of Strengths and Weaknesses and Considerations for Design. J. Man. Manip. Ther. 2009, 17, 163. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; White, I.R.; Carlin, J.B.; Spratt, M.; Royston, P.; Kenward, M.G.; Wood, A.M.; Carpenter, J.R. Multiple Imputation for Missing Data in Epidemiological and Clinical Research: Potential and Pitfalls. BMJ 2009, 338, b2393. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge: New York, NY, USA, 1988; ISBN 978-0-203-77158-7. [Google Scholar]
- da Cunha Nascimento, D.; Rolnick, N.; da Silva Almeida, I.; Junior, G.C.; Durigan, J.L. Frequentist, Bayesian Analysis and Complementary Statistical Tools for Geriatric and Rehabilitation Fields: Are Traditional Null-Hypothesis Significance Testing Methods Sufficient? Clin. Interv. Aging 2024, 19, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, G.; Williamson, P.; Batterham, A.M. Issues in the Determination of ‘Responders’ and ‘Non-Responders’ in Physiological Research. Exp. Physiol. 2019, 104, 1215–1225. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Zhang, L.; Gui, C.; Chen, G.; Chen, Y.; Tan, H.; Su, W.; Zhang, R.; Gao, Q. Virtual Reality Intervention for Patients With Neck Pain: Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Med. Internet Res. 2023, 25, e38256. [Google Scholar] [CrossRef] [PubMed]
- Brea-Gómez, B.; Laguna-González, A.; Pérez-Gisbert, L.; Valenza, M.C.; Torres-Sánchez, I. Virtual Reality Based Rehabilitation in Adults with Chronic Neck Pain: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Virtual Real. 2024, 28, 86. [Google Scholar] [CrossRef]
- Mallari, B.; Spaeth, E.K.; Goh, H.; Boyd, B.S. Virtual Reality as an Analgesic for Acute and Chronic Pain in Adults: A Systematic Review and Meta-Analysis. J. Pain Res. 2019, 12, 2053–2085. [Google Scholar] [CrossRef]
- Lo, H.H.M.; Zhu, M.; Zou, Z.; Wong, C.L.; Lo, S.H.S.; Chung, V.C.-H.; Wong, S.Y.-S.; Sit, R.W.S. Immersive and Nonimmersive Virtual Reality–Assisted Active Training in Chronic Musculoskeletal Pain: Systematic Review and Meta-Analysis. J. Med. Internet Res. 2024, 26, e48787. [Google Scholar] [CrossRef]
- Ahmadpour, N.; Randall, H.; Choksi, H.; Gao, A.; Vaughan, C.; Poronnik, P. Virtual Reality Interventions for Acute and Chronic Pain Management. Int. J. Biochem. Cell Biol. 2019, 114, 105568. [Google Scholar] [CrossRef]
- Runge, N.; Aina, A.; May, S. The Benefits of Adding Manual Therapy to Exercise Therapy for Improving Pain and Function in Patients With Knee or Hip Osteoarthritis: A Systematic Review With Meta-Analysis. J. Orthop. Sports Phys. Ther. 2022, 52, 675-A13. [Google Scholar] [CrossRef]
- Yu, S.P.; Williams, M.; Eyles, J.P.; Chen, J.S.; Makovey, J.; Hunter, D.J. Effectiveness of Knee Bracing in Osteoarthritis: Pragmatic Trial in a Multidisciplinary Clinic. Int. J. Rheum. Dis. 2016, 19, 279–286. [Google Scholar] [CrossRef]
- Duivenvoorden, T.; van Raaij, T.M.; Horemans, H.L.D.; Brouwer, R.W.; Bos, P.K.; Bierma-Zeinstra, S.M.A.; Verhaar, J.A.N.; Reijman, M. Do Laterally Wedged Insoles or Valgus Braces Unload the Medial Compartment of the Knee in Patients with Osteoarthritis? Clin. Orthop. 2015, 473, 265–274. [Google Scholar] [CrossRef]
- Sánchez Romero, E.A.; Fernández-Carnero, J.; Calvo-Lobo, C.; Ochoa Sáez, V.; Burgos Caballero, V.; Pecos-Martín, D. Is a Combination of Exercise and Dry Needling Effective for Knee OA? Pain Med. 2020, 21, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Collado-Mateo, D.; Lavín-Pérez, A.M.; Peñacoba, C.; Del Coso, J.; Leyton-Román, M.; Luque-Casado, A.; Gasque, P.; Fernández-del-Olmo, M.Á.; Amado-Alonso, D. Key Factors Associated with Adherence to Physical Exercise in Patients with Chronic Diseases and Older Adults: An Umbrella Review. Int. J. Environ. Res. Public Health 2021, 18, 2023. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.J.; Rathod-Mistry, T.; Parry, E.L.; Pope, C.; Neogi, T.; Peat, G. Triggers for Acute Flare in Adults with, or at Risk of, Knee Osteoarthritis: A Web-Based Case-Crossover Study in Community-Dwelling Adults. Osteoarthr. Cartil. 2021, 29, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Asadzadeh, A.; Samad-Soltani, T.; Salahzadeh, Z.; Rezaei-Hachesu, P. Effectiveness of Virtual Reality-Based Exercise Therapy in Rehabilitation: A Scoping Review. Inform. Med. Unlocked 2021, 24, 100562. [Google Scholar] [CrossRef]
- Thom, J.M.; Dennis, S.; Gibson, K.A.; Livings, R.; Mills, K.; Schabrun, S.M.; Sun, H.; Naylor, J.M. Knee Osteoarthritis Patient Perspectives of Their Care in an Australian Private Physiotherapy Setting: A Qualitative Exploratory Interview Study. BMC Musculoskelet. Disord. 2023, 24, 564. [Google Scholar] [CrossRef]
- Long, J.; You, J.; Yang, Y. Effect of Digital Exercise Therapy on the Pain and Physical Function of Patients With Osteoarthritis: Systematic Review and Meta-Analysis. J. Med. Internet Res. 2025, 27, e66037. [Google Scholar] [CrossRef]
- Suokas, A.K.; Walsh, D.A.; McWilliams, D.F.; Condon, L.; Moreton, B.; Wylde, V.; Arendt-Nielsen, L.; Zhang, W. Quantitative Sensory Testing in Painful Osteoarthritis: A Systematic Review and Meta-Analysis. Osteoarthr. Cartil. 2012, 20, 1075–1085. [Google Scholar] [CrossRef]
- Hattori, T.; Shimo, K.; Niwa, Y.; Tokiwa, Y.; Matsubara, T. Association of Chronic Pain with Radiologic Severity and Central Sensitization in Hip Osteoarthritis Patients. J. Pain Res. 2021, 14, 1153–1160. [Google Scholar] [CrossRef]
- Lluch, E.; Torres, R.; Nijs, J.; Van Oosterwijck, J. Evidence for Central Sensitization in Patients with Osteoarthritis Pain: A Systematic Literature Review. Eur. J. Pain 2014, 18, 1367–1375. [Google Scholar] [CrossRef]
- Previtali, D.; Capone, G.; Marchettini, P.; Candrian, C.; Zaffagnini, S.; Filardo, G. High Prevalence of Pain Sensitization in Knee Osteoarthritis: A Meta-Analysis with Meta-Regression. Cartilage 2022, 13, 19476035221087698. [Google Scholar] [CrossRef]
- Cibulka, M.T.; Bloom, N.J.; Enseki, K.R.; Macdonald, C.W.; Woehrle, J.; McDonough, C.M. Hip Pain and Mobility Deficits-Hip Osteoarthritis: Revision 2017. J. Orthop. Sports Phys. Ther. 2017, 47, A1–A37. [Google Scholar] [CrossRef] [PubMed]
- Kuni, B.; Wang, H.; Rickert, M.; Ewerbeck, V.; Schiltenwolf, M. Pain Threshold Correlates with Functional Scores in Osteoarthritis Patients. Acta Orthop. 2015, 86, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Izumi, M.; Petersen, K.K.; Laursen, M.B.; Arendt-Nielsen, L.; Graven-Nielsen, T. Facilitated Temporal Summation of Pain Correlates with Clinical Pain Intensity after Hip Arthroplasty. Pain 2017, 158, 323–332. [Google Scholar] [CrossRef] [PubMed]
- King, C.D.; Sibille, K.T.; Goodin, B.R.; Cruz-Almeida, Y.; Glover, T.L.; Bartley, E.; Riley, J.L.; Herbert, M.S.; Sotolongo, A.; Schmidt, J.; et al. Experimental Pain Sensitivity Differs as a Function of Clinical Pain Severity in Symptomatic Knee Osteoarthritis. Osteoarthr. Cartil. 2013, 21, 1243–1252. [Google Scholar] [CrossRef]
- Carlesso, L.C.; Segal, N.A.; Frey-Law, L.; Zhang, Y.; Na, L.; Nevitt, M.; Lewis, C.E.; Neogi, T. Pain Susceptibility Phenotypes in Those Free of Knee Pain with or at Risk of Knee Osteoarthritis: The Multicenter Osteoarthritis Study. Arthritis Rheumatol. 2019, 71, 542–549. [Google Scholar] [CrossRef]
- Aranda-Villalobos, P.; Fernández-de-Las-Peñas, C.; Navarro-Espigares, J.L.; Hernández-Torres, E.; Villalobos, M.; Arendt-Nielsen, L.; Arroyo-Morales, M. Normalization of Widespread Pressure Pain Hypersensitivity after Total Hip Replacement in Patients with Hip Osteoarthritis Is Associated with Clinical and Functional Improvements. Arthritis Rheum. 2013, 65, 1262–1270. [Google Scholar] [CrossRef]
- Graven-Nielsen, T.; Wodehouse, T.; Langford, R.M.; Arendt-Nielsen, L.; Kidd, B.L. Normalization of Widespread Hyperesthesia and Facilitated Spatial Summation of Deep-Tissue Pain in Knee Osteoarthritis Patients after Knee Replacement. Arthritis Rheum. 2012, 64, 2907–2916. [Google Scholar] [CrossRef]
- Petersen, K.K.; Arendt-Nielsen, L.; Simonsen, O.; Wilder-Smith, O.; Laursen, M.B. Presurgical Assessment of Temporal Summation of Pain Predicts the Development of Chronic Postoperative Pain 12 Months after Total Knee Replacement. Pain 2015, 156, 55–61. [Google Scholar] [CrossRef]
- Fingleton, C.; Smart, K.M.; Doody, C.M. Exercise-Induced Hypoalgesia in People With Knee Osteoarthritis With Normal and Abnormal Conditioned Pain Modulation. Clin. J. Pain 2017, 33, 395–404. [Google Scholar] [CrossRef]
- Lee, Y.C.; Lu, B.; Bathon, J.M.; Haythornthwaite, J.A.; Smith, M.T.; Page, G.G.; Edwards, R.R. Pain Sensitivity and Pain Reactivity in Osteoarthritis. Arthritis Care Res. 2011, 63, 320–327. [Google Scholar] [CrossRef]
- Jakorinne, P.; Haanpää, M.; Arokoski, J. Reliability of Pressure Pain, Vibration Detection, and Tactile Detection Threshold Measurements in Lower Extremities in Subjects with Knee Osteoarthritis and Healthy Controls. Scand. J. Rheumatol. 2018, 47, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Geri, T.; Botticchio, A.; Rossettini, G.; Pournajaf, S.; Pellicciari, L.; Di Antonio, S.; Castaldo, M. Pressure Pain Threshold of the Upper Trapezius Trigger Point: A Systematic Review with Meta-Analysis of Baseline Values and Their Modification after Physical Therapy. J. Clin. Med. 2022, 11, 7243. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.A. Pressure Algometry over Normal Muscles. Standard Values, Validity and Reproducibility of Pressure Threshold. PAIN 1987, 30, 115. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.K.; Graven-Nielsen, T.; Simonsen, O.; Laursen, M.B.; Arendt-Nielsen, L. Preoperative Pain Mechanisms Assessed by Cuff Algometry Are Associated with Chronic Postoperative Pain Relief after Total Knee Replacement. PAIN 2016, 157, 1400. [Google Scholar] [CrossRef]
- Ossipov, M.H.; Morimura, K.; Porreca, F. Descending Pain Modulation and Chronification of Pain. Curr. Opin. Support. Palliat. Care 2014, 8, 143–151. [Google Scholar] [CrossRef]
- Marques, L.M.; Uchida, P.M.; Barbosa, S.P. The Impact of Exergames on Emotional Experience: A Systematic Review. Front. Public Health 2023, 11, 1209520. [Google Scholar] [CrossRef]
- Mikkelsen, M.B.; Neumann, H.; Buskbjerg, C.R.; Johannsen, M.; O’Toole, M.S.; Arendt-Nielsen, L.; Zachariae, R. The Effect of Experimental Emotion Induction on Experimental Pain: A Systematic Review and Meta-Analysis. Pain 2024, 165, e17–e38. [Google Scholar] [CrossRef]
- Rice, D.; Nijs, J.; Kosek, E.; Wideman, T.; Hasenbring, M.I.; Koltyn, K.; Graven-Nielsen, T.; Polli, A. Exercise-Induced Hypoalgesia in Pain-Free and Chronic Pain Populations: State of the Art and Future Directions. J. Pain 2019, 20, 1249–1266. [Google Scholar] [CrossRef]
- Nijs, J.; Kosek, E.; Van Oosterwijck, J.; Meeus, M. Dysfunctional Endogenous Analgesia during Exercise in Patients with Chronic Pain: To Exercise or Not to Exercise? Pain Physician 2012, 15, ES205–ES213. [Google Scholar] [CrossRef]
- Mackey, I.G.; Dixon, E.A.; Johnson, K.; Kong, J.-T. Dynamic Quantitative Sensory Testing to Characterize Central Pain Processing. J. Vis. Exp. JoVE 2017, 120, 54452. [Google Scholar] [CrossRef]
- Arant, K.R.; Katz, J.N.; Neogi, T. Quantitative Sensory Testing: Identifying Pain Characteristics in Patients with Osteoarthritis. Osteoarthr. Cartil. 2022, 30, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Belavy, D.L.; Van Oosterwijck, J.; Clarkson, M.; Dhondt, E.; Mundell, N.L.; Miller, C.T.; Owen, P.J. Pain Sensitivity Is Reduced by Exercise Training: Evidence from a Systematic Review and Meta-Analysis. Neurosci. Biobehav. Rev. 2021, 120, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Song, J.S.; Yamada, Y.; Kataoka, R.; Wong, V.; Spitz, R.W.; Bell, Z.W.; Loenneke, J.P. Training-Induced Hypoalgesia and Its Potential Underlying Mechanisms. Neurosci. Biobehav. Rev. 2022, 141, 104858. [Google Scholar] [CrossRef] [PubMed]
- Salaffi, F.; Stancati, A.; Silvestri, C.A.; Ciapetti, A.; Grassi, W. Minimal Clinically Important Changes in Chronic Musculoskeletal Pain Intensity Measured on a Numerical Rating Scale. Eur. J. Pain 2004, 8, 283–291. [Google Scholar] [CrossRef]
- Tubach, F. Evaluation of Clinically Relevant Changes in Patient Reported Outcomes in Knee and Hip Osteoarthritis: The Minimal Clinically Important Improvement. Ann. Rheum. Dis. 2005, 64, 29–33. [Google Scholar] [CrossRef]
- Han, S.; Li, T.; Cao, Y.; Li, Z.; Mai, Y.; Fan, T.; Zeng, M.; Wen, X.; Han, W.; Lin, L.; et al. Quantitative Analysis of Effectiveness and Associated Factors of Exercise on Symptoms in Osteoarthritis: A Pharmacodynamic Model-Based Meta-Analysis. Br. J. Sports Med. 2024, 58, 1539–1550. [Google Scholar] [CrossRef]
- Pelfort, X.; Torres-Claramunt, R.; Sánchez-Soler, J.F.; Hinarejos, P.; Leal-Blanquet, J.; Valverde, D.; Monllau, J.C. Pressure Algometry Is a Useful Tool to Quantify Pain in the Medial Part of the Knee: An Intra- and Inter-Reliability Study in Healthy Subjects. Orthop. Traumatol. Surg. Res. 2015, 101, 559–563. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).