Magnetic Resonance Imaging Radiomics-Driven Artificial Neural Network Model for Advanced Glioma Grading Assessment
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Image Acquisition
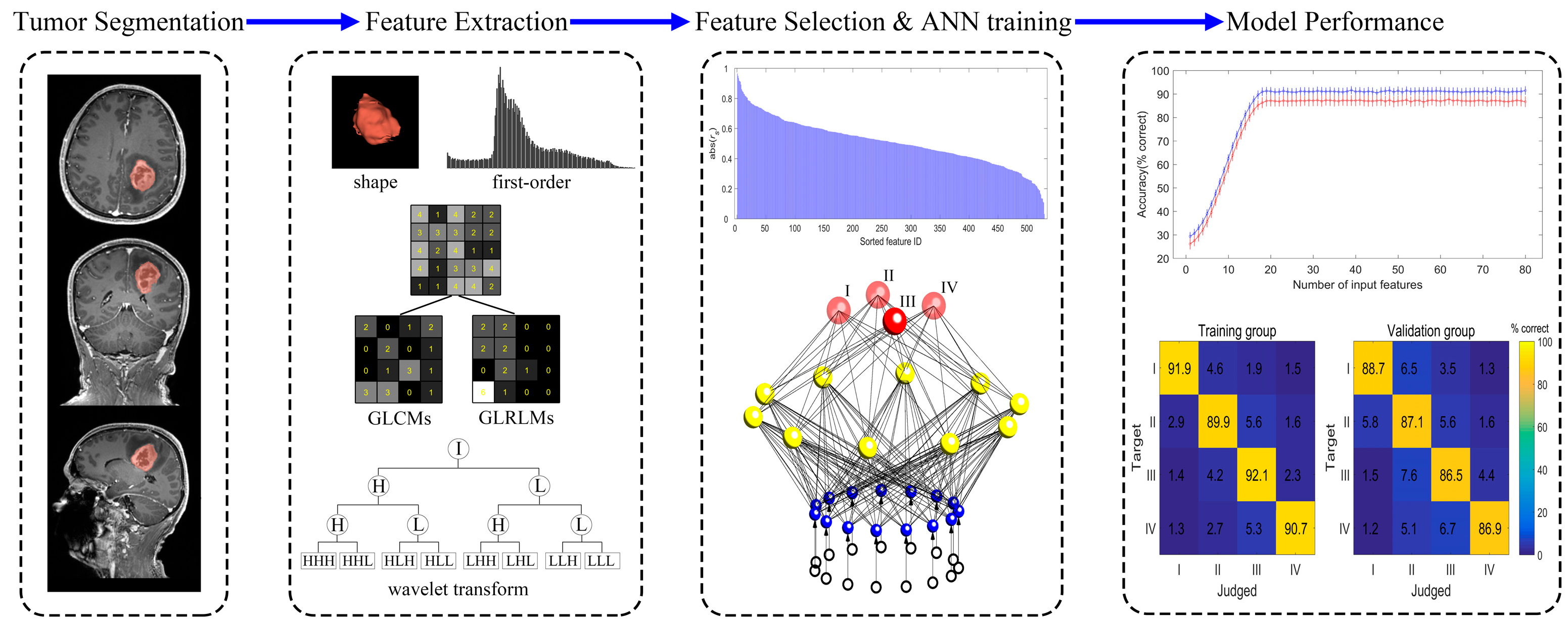
2.3. Image Segmentation
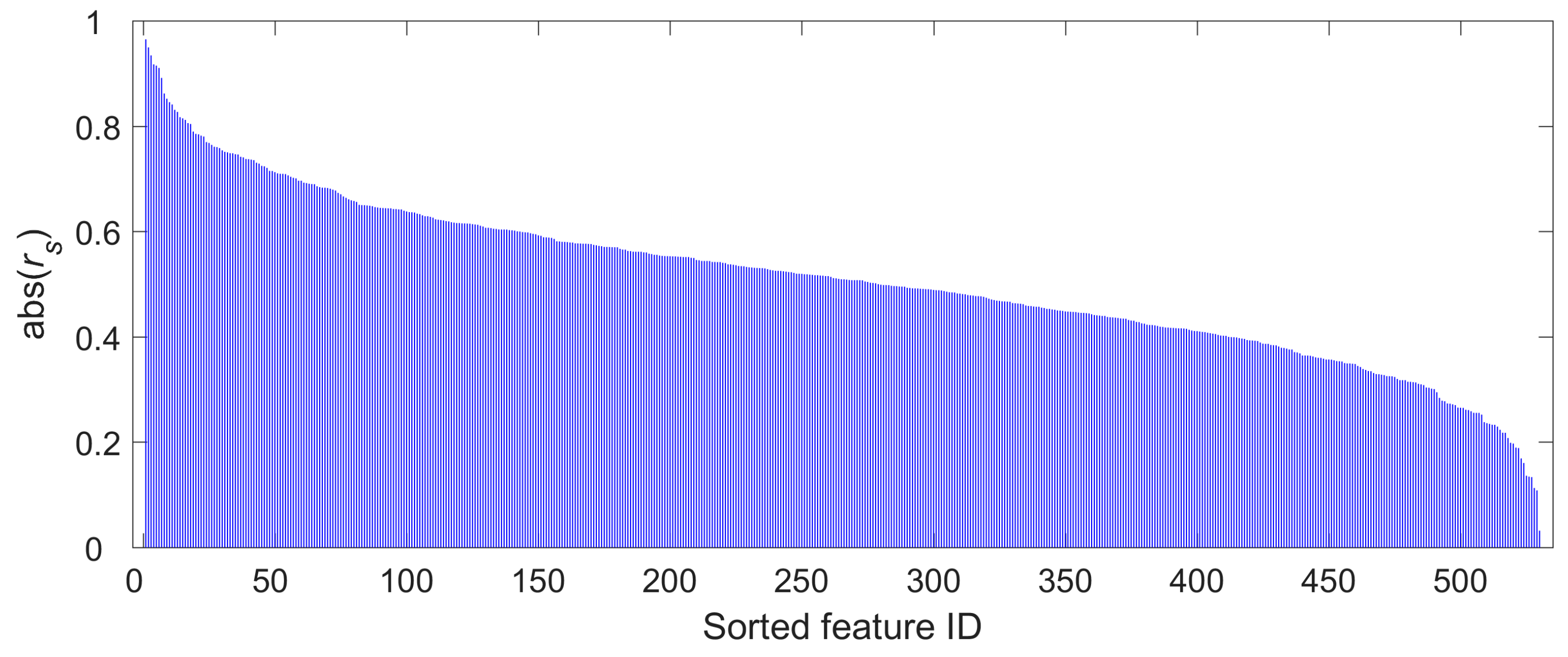
2.4. Radiomics Feature Selection
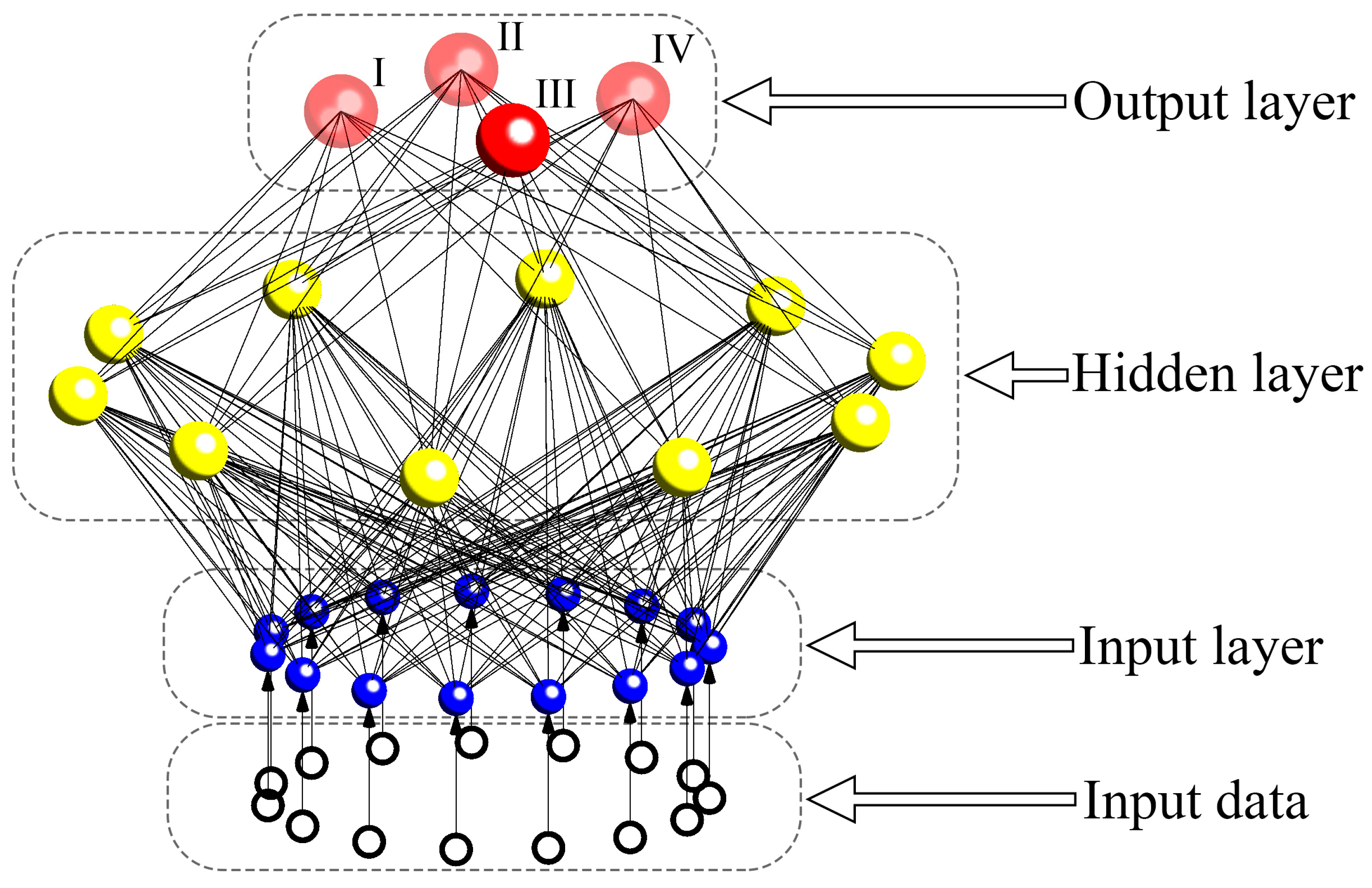
2.5. Development of ANN
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Radiomics Feature Selection
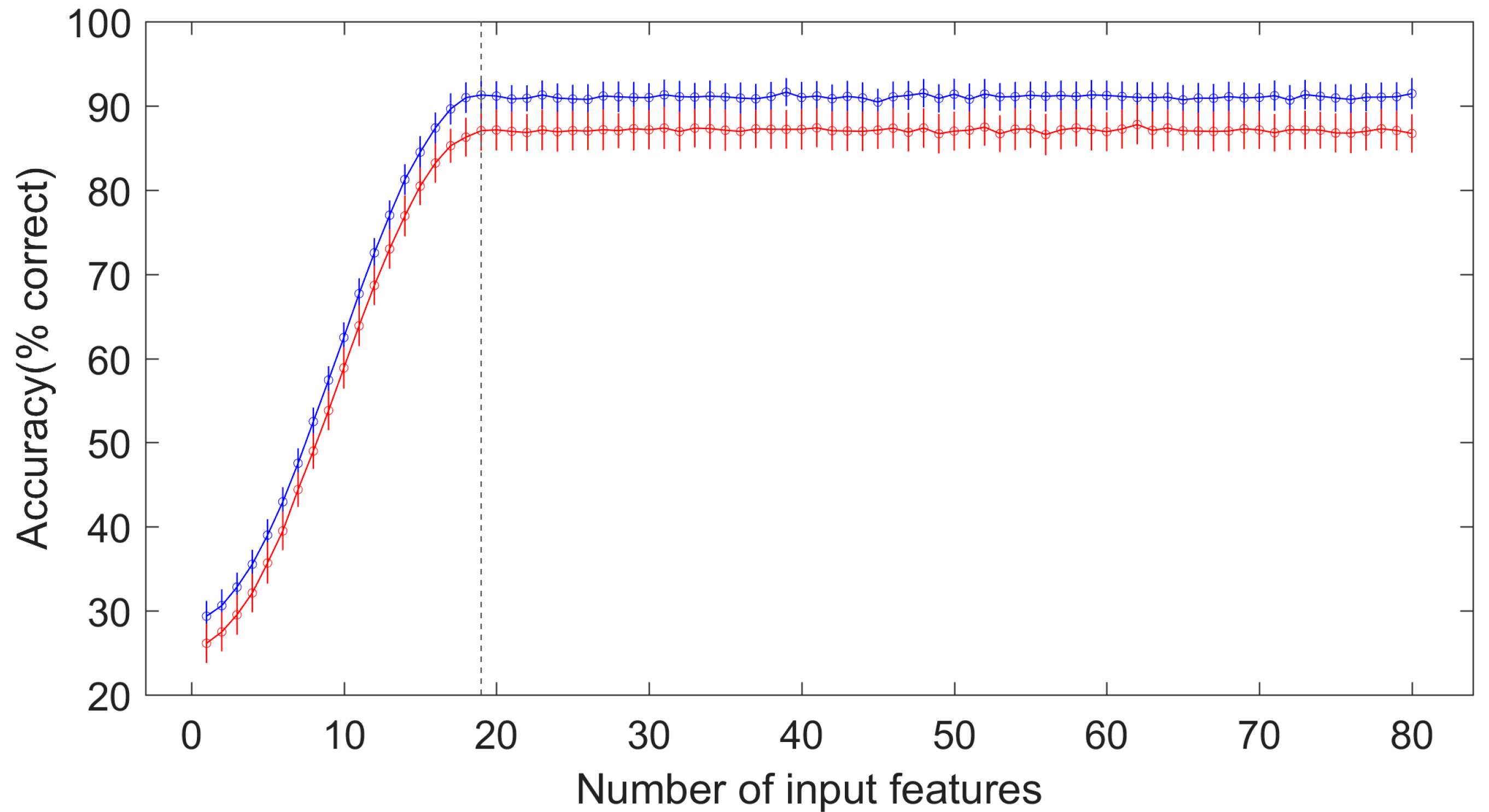
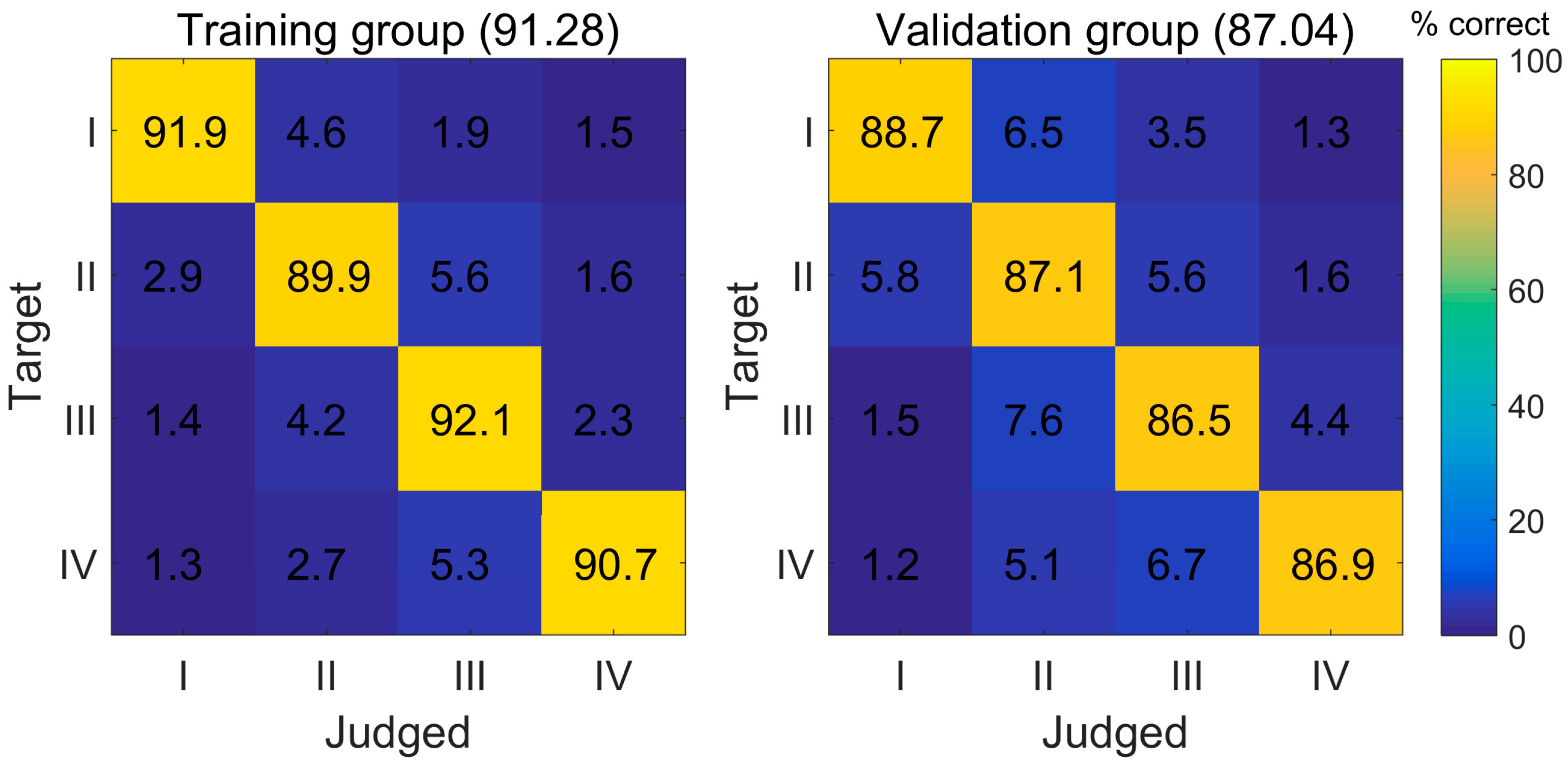
3.3. The Diagnostic Performance of the ANN Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MRI | Magnetic resonance imaging |
| ANN | Artificial neural network |
| CNS | Central nervous system |
| CE-T1WI | Contrast-enhanced T1-weighted images |
| T1WI | T1-weighted images |
| T2WI | T2-weighted images |
| FLAIR | Fluid-attenuated inversion recovery |
| ICC | Intra-observer correlation coefficient |
References
- Lapointe, S.; Perry, A.; Butowski, N.A. Primary brain tumours in adults. Lancet 2018, 392, 432–446. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Nam, D.H.; Ram, Z.; Poon, W.S.; Wang, J.; Boldbaatar, D.; Mao, Y.; Ma, W.; Mao, Q.; You, Y.; et al. Clinical practice guidelines for the management of adult diffuse gliomas. Cancer Lett. 2021, 499, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.; van den Bent, M.; Preusser, M.; Le Rhun, E.; Tonn, J.C.; Minniti, G.; Bendszus, M.; Balana, C.; Chinot, O.; Dirven, L.; et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat. Rev. Clin. Oncol. 2021, 18, 170–186. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Lombardi, G.; Barresi, V.; Castellano, A.; Tabouret, E.; Pasqualetti, F.; Salvalaggio, A.; Cerretti, G.; Caccese, M.; Padovan, M.; Zagonel, V.; et al. Clinical Management of Diffuse Low-Grade Gliomas. Cancers 2020, 12, 3008. [Google Scholar] [CrossRef]
- Nabors, L.B.; Portnow, J.; Ahluwalia, M.; Baehring, J.; Brem, H.; Brem, S.; Butowski, N.; Campian, J.L.; Clark, S.W.; Fabiano, A.J.; et al. Central Nervous System Cancers, Version 3.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc Netw. 2020, 18, 1537–1570. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.S.; Carter, B.S.; Chen, C.C. Role of Biopsies in the Management of Intracranial Gliomas. Prog. Neurol. Surg. 2018, 30, 232–243. [Google Scholar]
- D’Angelo, L.; Armocida, D.; Sampirisi, L.; Paglia, F.; Berra, L.V.; Santoro, A. Role of endoscopic surgical biopsy in diagnoses of intraventricular/periventricular tumors: Review of literature including a monocentric case series. Acta Neurol. Belg. 2020, 120, 517–530. [Google Scholar] [CrossRef]
- Lara-Almunia, M.; Hernandez-Vicente, J. Related factors with diagnostic yield and intracranial hemorrhagic complications in frame-based stereotactic biopsy. Review. Neurocirugia 2021, 32, 285–294. [Google Scholar] [CrossRef]
- Thust, S.C.; Heiland, S.; Falini, A.; Jager, H.R.; Waldman, A.D.; Sundgren, P.C.; Godi, C.; Katsaros, V.K.; Ramos, A.; Bargallo, N.; et al. Glioma imaging in Europe: A survey of 220 centres and recommendations for best clinical practice. Eur. Radiol. 2018, 28, 3306–3317. [Google Scholar] [CrossRef]
- Habib, A.; Jovanovich, N.; Hoppe, M.; Ak, M.; Mamindla, P.; RColen, R.; Zinn, P.O. MRI-Based Radiomics and Radiogenomics in the Management of Low-Grade Gliomas: Evaluating the Evidence for a Paradigm Shift. J. Clin. Med. 2021, 10, 1411. [Google Scholar] [CrossRef] [PubMed]
- Verburg, N.; de Witt Hamer, P.C. State-of-the-art imaging for glioma surgery. Neurosurg. Rev. 2021, 44, 1331–1343. [Google Scholar] [CrossRef] [PubMed]
- Tagliafico, A.S.; Piana, M.; Schenone, D.; Lai, R.; Massone, A.M.; Houssami, N. Overview of radiomics in breast cancer diagnosis and prognostication. Breast 2020, 49, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Lohmann, P.; Galldiks, N.; Kocher, M.; Heinzel, A.; Filss, C.P.; Stegmayr, C.; Mottaghy, F.M.; Fink, G.R.; Jon Shah, N.; Langen, K.J. Radiomics in neuro-oncology: Basics, workflow, and applications. Methods 2021, 188, 112–121. [Google Scholar] [CrossRef]
- Liu, L.; Yi, X.; Lu, C.; Qi, L.; Zhang, Y.; Li, M.; Xiao, Q.; Wang, C.; Zhang, L.; Pang, Y.; et al. Applications of radiomics in genitourinary tumors. Am. J. Cancer Res. 2020, 10, 2293–2308. [Google Scholar]
- Mao, Y.; Wang, J.; Zhu, Y.; Chen, J.; Mao, L.; Kong, W.; Qiu, Y.; Wu, X.; Guan, Y.; He, J. Gd-EOB-DTPA-enhanced MRI radiomic features for predicting histological grade of hepatocellular carcinoma. Hepatobiliary Surg. Nutr. 2022, 11, 13–24. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, X.; Farewik, E.M.G.; Wang, R. Ankle Joint Torque Prediction Using an NMS Solver Informed-ANN Model and Transfer Learning. IEEE J. Biomed. Health Inform. 2022, 26, 5895–5906. [Google Scholar] [CrossRef]
- Fan, H.; Su, P.; Huang, J.; Liu, P.; Lu, H. Multi-band MR fingerprinting (MRF) ASL imaging using artificial-neural-network trained with high-fidelity experimental data. Magn. Reson. Med. 2021, 85, 1974–1985. [Google Scholar] [CrossRef]
- Ding, J.; Zhao, R.; Qiu, Q.; Chen, J.; Duan, J.; Cao, X.; Yin, Y. Developing and validating a deep learning and radiomic model for glioma grading using multiplanar reconstructed magnetic resonance contrast-enhanced T1-weighted imaging: A robust, multi-institutional study. Quant. Imaging Med. Surg. 2022, 12, 1517–1528. [Google Scholar] [CrossRef]
- Lin, K.; Cidan, W.; Qi, Y.; Wang, X. Glioma grading prediction using multiparametric magnetic resonance imaging-based radiomics combined with proton magnetic resonance spectroscopy and diffusion tensor imaging. Med. Phys. 2022, 49, 4419–4429. [Google Scholar] [CrossRef]
- Tian, Q.; Yan, L.F.; Zhang, X.; Zhang, X.; Hu, Y.C.; Han, Y.; Liu, Z.C.; Nan, H.Y.; Sun, Q.; Sun, Y.Z.; et al. Radiomics strategy for glioma grading using texture features from multiparametric MRI. J. Magn. Reson. Imaging 2018, 48, 1518–1528. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lu, S.H.; Mao, Y.T.; Tan, L.; Li, G.; Gao, Y.; Tan, P.Q.; Huang, D.H.; Zhang, X.; Qiu, Y.Z.; et al. An MRI-based radiomics-clinical nomogram for the overall survival prediction in patients with hypopharyngeal squamous cell carcinoma: A multi-cohort study. Eur. Radiol. 2022, 32, 1548–1557. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Mao, Y.T.; Lu, S.H.; Tan, L.; Li, G.; Chen, J.; Huang, D.H.; Zhang, X.; Qiu, Y.Z.; Liu, Y. Magnetic resonance imaging-based radiogenomics analysis for predicting prognosis and gene expression profile in advanced nasopharyngeal carcinoma. Head Neck 2021, 43, 3730–3742. [Google Scholar] [CrossRef] [PubMed]
- Mou, Z.W.; Ye, W.J.; Chang, C.C.; Mao, Y.T. The Application Analysis of Neural Network Techniques on Lexical Tone Rehabilitation of Mandarin-Speaking Patients with Post-Stroke Dysarthria. IEEE Access 2020, 8, 90709–90717. [Google Scholar] [CrossRef]
- Lin, B.; Lin, G.T.; Liu, X.Y.; Ma, J.S.; Wang, X.C.; Lin, F.Y.; Hu, L.F. Application of back-propagation artificial neural network and curve estimation in pharmacokinetics of losartan in rabbit. Int. J. Clin. Exp. Med. 2015, 8, 22352–22358. [Google Scholar]
- Ma, J.S.; Cai, J.Z.; Lin, G.Y.; Chen, H.L.; Wang, X.Q.; Wang, X.C.; Hu, L.F. Development of LC-MS determination method and back-propagation ANN pharmacokinetic model of corynoxeine in rat. J. Chromatogr. B 2014, 959, 10–15. [Google Scholar] [CrossRef]
- Papanikolaou, N.; Matos, C.; Koh, D.M. How to develop a meaningful radiomic signature for clinical use in oncologic patients. Cancer Imaging 2020, 20, 33. [Google Scholar] [CrossRef]
- Lo Greco, M.C.; Marano, G.; La Rocca, M.; Acquaviva, G.; Milazzotto, R. Latest Advancements in the Management of H3K27M-Mutant Diffuse Intrinsic Pontine Glioma: A Narrative Review. Cancers 2025, 17, 420. [Google Scholar] [CrossRef]
- Asteris, P.G.; Gavriilaki, E.; Touloumenidou, T.; Koravou, E.E.; Koutra, M.; Papayanni, P.G.; Pouleres, A.; Karali, V.; Lemonis, M.E.; Mamou, A.; et al. Genetic prediction of ICU hospitalization and mortality in COVID-19 patients using artificial neural networks. J. Cell Mol. Med. 2022, 26, 1445–1455. [Google Scholar] [CrossRef]
- Bede, P.; Murad, A.; Hardiman, O. Pathological neural networks and artificial neural networks in ALS: Diagnostic classification based on pathognomonic neuroimaging features. J. Neurol. 2022, 269, 2440–2452. [Google Scholar] [CrossRef]
- Zhou, M.; Ji, J.; Xie, N.; Chen, D. Prediction of birth weight in pregnancy with gestational diabetes mellitus using an artificial neural network. J. Zhejiang Univ. Sci. B 2022, 23, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Mai, R.Y.; Lu, H.Z.; Bai, T.; Liang, R.; Lin, Y.; Ma, L.; Xiang, B.D.; Wu, G.B.; Li, L.Q.; Ye, J.Z. Artificial neural network model for preoperative prediction of severe liver failure after hemihepatectomy in patients with hepatocellular carcinoma. Surgery 2020, 168, 643–652. [Google Scholar] [CrossRef]
- Pathak, P.; Panday, S.B.; Ahn, J. Artificial neural network model effectively estimates muscle and fat mass using simple demographic and anthropometric measures. Clin. Nutr. 2022, 41, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Gutta, S.; Acharya, J.; Shiroishi, M.S.; Hwang, D.; Nayak, K.S. Improved Glioma Grading Using Deep Convolutional Neural Networks. AJNR Am. J. Neuroradiol. 2021, 42, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Tabatabaei, M.; Razaei, A.; Sarrami, A.H.; Saadatpour, Z.; Singhal, A.; Sotoudeh, H. Current Status and Quality of Machine Learning-Based Radiomics Studies for Glioma Grading: A Systematic Review. Oncology 2021, 99, 433–443. [Google Scholar] [CrossRef]
- Zhou, H.; Xu, R.; Mei, H.; Zhang, L.; Yu, Q.; Liu, R.; Fan, B. Application of Enhanced T1WI of MRI Radiomics in Glioma Grading. Int. J. Clin. Pract. 2022, 2022, 3252574. [Google Scholar] [CrossRef]
- Li, G.; Li, L.; Li, Y.; Qian, Z.; Wu, F.; He, Y.; Jiang, H.; Li, R.; Wang, D.; Zhai, Y.; et al. An MRI radiomics approach to predict survival and tumour-infiltrating macrophages in gliomas. Brain 2022, 145, 1151–1161. [Google Scholar] [CrossRef]
- Choi, Y.S.; Bae, S.; Chang, J.H.; Kang, S.G.; Kim, S.H.; Kim, J.; Rim, T.H.; Choi, S.H.; Jain, R.; Lee, S.K. Fully automated hybrid approach to predict the IDH mutation status of gliomas via deep learning and radiomics. Neuro-Oncology 2021, 23, 304–313. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, B.; Zhang, S.; Cheng, J.; Liu, X.; Wang, W.; Dong, Y.; Zhang, L.; Mo, X.; Chen, Q.; et al. Quantitative MRI-based radiomics for noninvasively predicting molecular subtypes and survival in glioma patients. NPJ Precis. Oncol. 2021, 5, 72. [Google Scholar] [CrossRef]
- Zhuo, Z.; Qu, L.; Zhang, P.; Duan, Y.; Cheng, D.; Xu, X.; Sun, T.; Ding, J.; Xie, C.; Liu, X.; et al. Prediction of H3K27M-mutant brainstem glioma by amide proton transfer-weighted imaging and its derived radiomics. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4426–4436. [Google Scholar] [CrossRef]
- Su, C.; Jiang, J.; Zhang, S.; Shi, J.; Xu, K.; Shen, N.; Zhang, J.; Li, L.; Zhao, L.; Zhang, J.; et al. Radiomics based on multicontrast MRI can precisely differentiate among glioma subtypes and predict tumour-proliferative behaviour. Eur. Radiol. 2019, 29, 1986–1996. [Google Scholar] [CrossRef] [PubMed]
- Vidyadharan, S.; Rao, B.P.; Yogeeswari, P.; Kesavadas, C.; Rajagopalan, V. Accurate low and high grade glioma classification using free water eliminated diffusion tensor metrics and ensemble machine learning. Sci. Rep. 2024, 14, 19844. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Wu, Y.; Bai, Y.; Han, H.; Chen, L.; Gao, H.; Wei, H.; Wang, M. A lightweight 3D UNet model for glioma grading. Phys. Med. Biol. 2022, 67, 155006. [Google Scholar] [CrossRef] [PubMed]
| WHO I | WHO II | WHO III | WHO IV | |
|---|---|---|---|---|
| Number | 23 | 139 | 81 | 119 |
| Age (years) | 40.8 (16.5–54.7) | 43.5 (19.4–60.5) | 49.7 (31.7–66.8) | 52.2 (29.8–71.5) |
| Gender | ||||
| Male | 15 | 82 | 46 | 66 |
| Female | 8 | 57 | 35 | 53 |
| Selected Features | |rs| |
|---|---|
| Wavelet–HLL–First order–Skewness | 0.9648 |
| Original–GLRLM–Run-length non-uniformity normalized | 0.9494 |
| Wavelet–LHH–GLCM–Joint energy | 0.9342 |
| Wavelet–LHL–First order–Maximum | 0.9171 |
| Wavelet–HHH–First order–Kurtosis | 0.9147 |
| Wavelet–HHH–First order–Median | 0.9104 |
| Wavelet–LLH–First order–Skewness | 0.8915 |
| Original–GLRLM–Gray-level non-uniformity | 0.8619 |
| Wavelet–LLL–GLCM–MCC | 0.852 |
| Wavelet–HLH–GLRLM–Run entropy | 0.8454 |
| Wavelet–HLH–First order–Kurtosis | 0.841 |
| Original–GLCM–Inverse variance | 0.8309 |
| Wavelet–LHH–GLCM–Idn | 0.827 |
| Original–GLRLM–LRLGLE | 0.817 |
| Wavelet–HHH–GLCM–Difference variance | 0.8146 |
| Wavelet–LLH–GLCM–Maximum probability | 0.8118 |
| Wavelet–LHL–GLRLM–LRLGLE | 0.8056 |
| Wavelet–HHL–GLCM–Inverse variance | 0.804 |
| Wavelet–LLL–First order–Kurtosis | 0.7896 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, Y.; You, W.; Wang, Y.; Zhang, Y.; Xu, Z.; Li, Q.; Zhao, Y.; Mou, Z.; Mao, Y. Magnetic Resonance Imaging Radiomics-Driven Artificial Neural Network Model for Advanced Glioma Grading Assessment. Medicina 2025, 61, 1034. https://doi.org/10.3390/medicina61061034
Qin Y, You W, Wang Y, Zhang Y, Xu Z, Li Q, Zhao Y, Mou Z, Mao Y. Magnetic Resonance Imaging Radiomics-Driven Artificial Neural Network Model for Advanced Glioma Grading Assessment. Medicina. 2025; 61(6):1034. https://doi.org/10.3390/medicina61061034
Chicago/Turabian StyleQin, Yan, Wei You, Yulong Wang, Yu Zhang, Zhijie Xu, Qingling Li, Yuelong Zhao, Zhiwei Mou, and Yitao Mao. 2025. "Magnetic Resonance Imaging Radiomics-Driven Artificial Neural Network Model for Advanced Glioma Grading Assessment" Medicina 61, no. 6: 1034. https://doi.org/10.3390/medicina61061034
APA StyleQin, Y., You, W., Wang, Y., Zhang, Y., Xu, Z., Li, Q., Zhao, Y., Mou, Z., & Mao, Y. (2025). Magnetic Resonance Imaging Radiomics-Driven Artificial Neural Network Model for Advanced Glioma Grading Assessment. Medicina, 61(6), 1034. https://doi.org/10.3390/medicina61061034









