Indoximod Attenuates Inflammatory Responses in Acetic Acid-Induced Acute Colitis by Modulating Toll-like Receptor 4 (TLR4) Signaling and Proinflammatory Cytokines in Rats
Abstract
1. Introduction
2. Materials and Methods
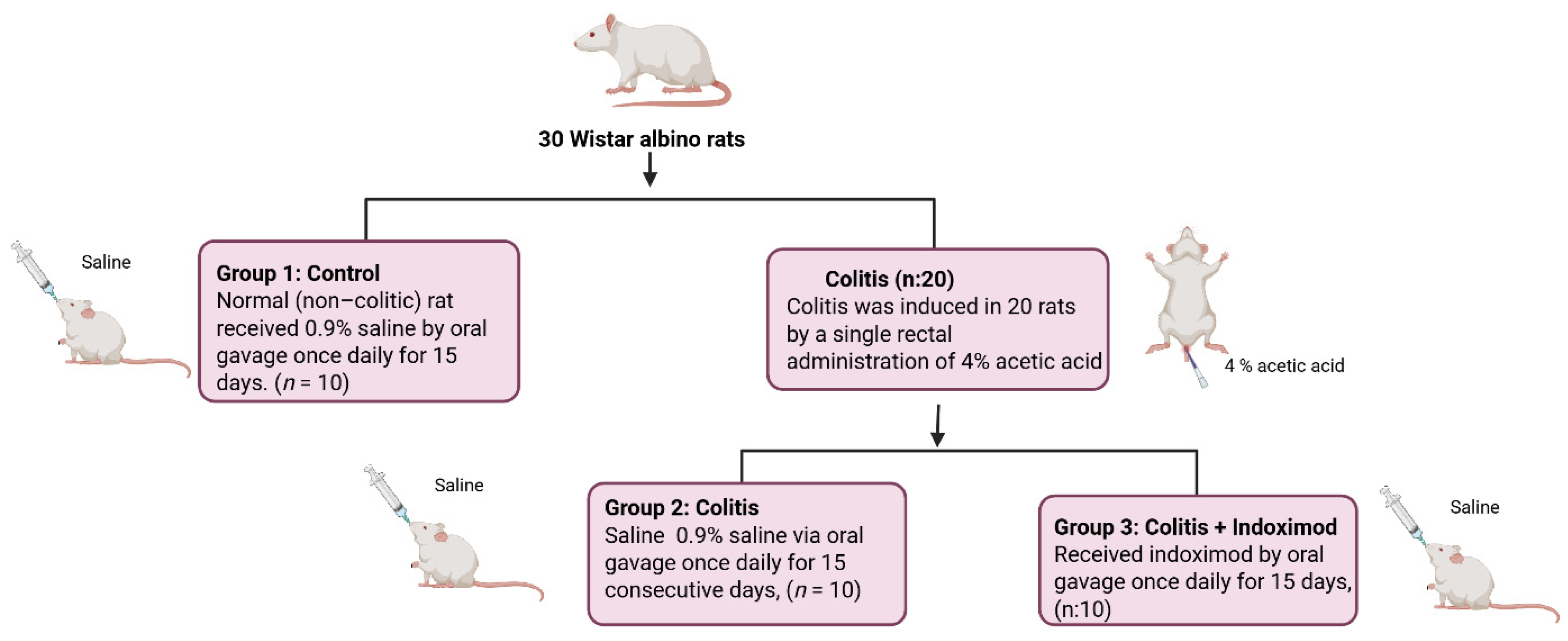
2.1. Animals
Safety and Tolerability
2.2. Drug
2.3. Induction of Experimental Colitis and Treatment Protocol
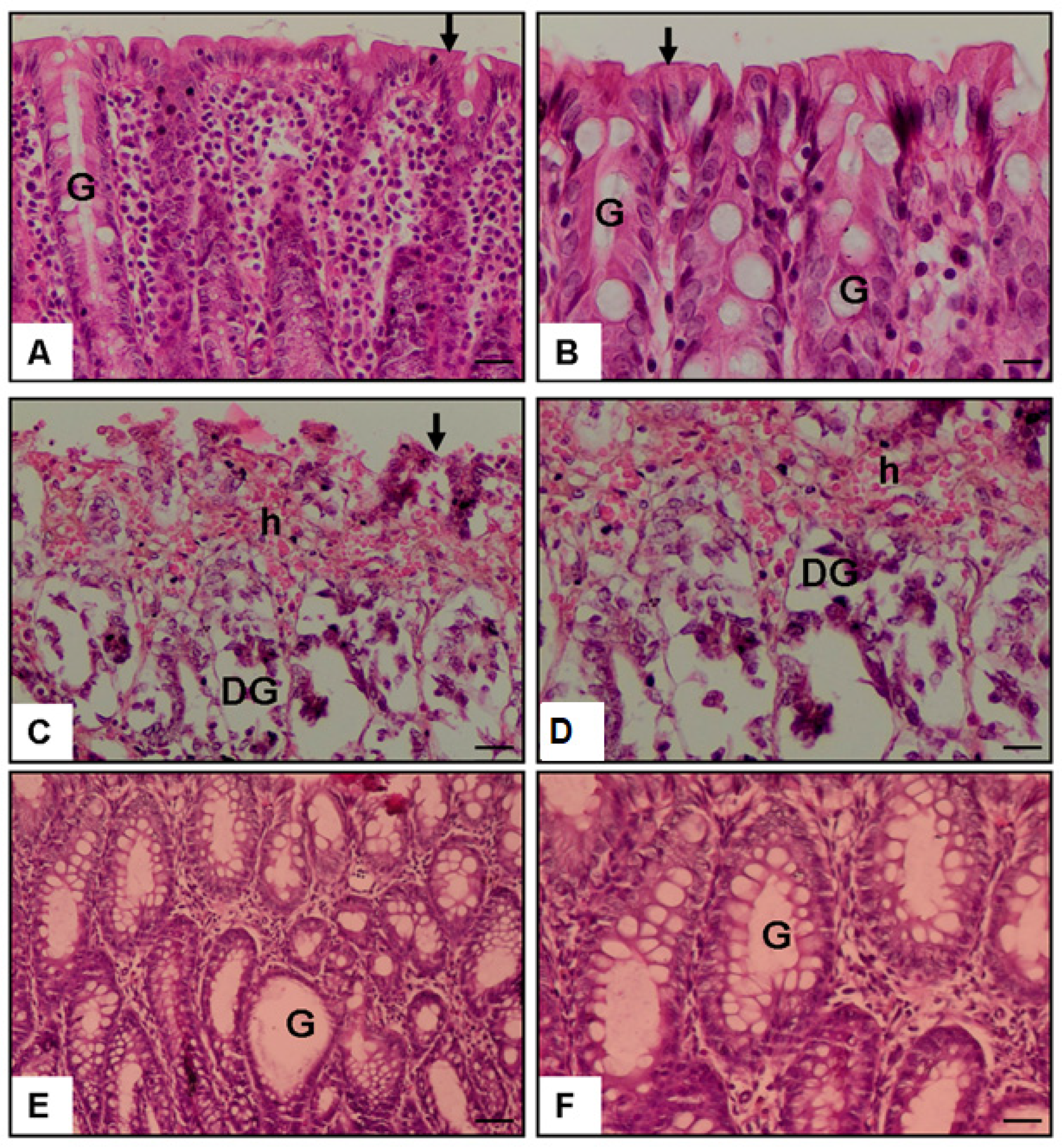
2.4. Histological Examination of Colonic Tissue
2.5. Quantification of TLR-4 in Colon Tissue
2.6. Measurement of Plasma TNF-α, PTX3, and PAF
2.7. Statistical Analysis
G*Power Analysis
3. Result
3.1. Histopathological Score
3.2. Colon TLR-4 Level (pg/mg Tissue)
3.3. Plasma TNF-α Level (pg/mL)
3.4. Plasma Pentraxin-3 Level (ng/mL)
3.5. Plasma PAF Level (pg/mL)
3.6. General Health Observations and Tolerability
4. Discussion
5. Conclusions
Limitation
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Gajendran, M.; Loganathan, P.; Jimenez, G.; Catinella, A.P.; Ng, N.; Umapathy, C.; Ziade, N.; Hashash, J.G. A comprehensive review and update on ulcerative colitis. Disease-a-Month 2019, 65, 100851. [Google Scholar] [CrossRef] [PubMed]
- Alatab, S.; Sepanlou, S.G.; Ikuta, K.; Vahedi, H.; Bisignano, C.; Safiri, S.; Sadeghi, A.; Nixon, M.R.; Abdoli, A.; Abolhassani, H.; et al. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Xavier, R.J.; Podolsky, D.K. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007, 448, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Yukitake, H.; Kimura, H.; Suzuki, H.; Tajima, Y.; Sato, Y.; Imaeda, T.; Kajino, M.; Takizawa, M. BTZO-15, an ARE-activator, ameliorates DSS- and TNBS-induced colitis in rats. PLoS ONE 2011, 6, e23256. [Google Scholar] [CrossRef]
- Mukherjee, T.; Kumar, N.; Chawla, M.; Philpott, D.J.; Basak, S. The NF-κB signaling system in the immunopathogenesis of inflammatory bowel disease. Sci. Signal. 2024, 17, eadh1641. [Google Scholar] [CrossRef]
- Fox, E.; Oliver, T.; Rowe, M.; Thomas, S.; Zakharia, Y.; Gilman, P.B.; Muller, A.J.; Prendergast, G.C. Indoximod: An immunometabolic adjuvant that empowers T cell activity in cancer. Front. Oncol. 2018, 8, 370. [Google Scholar] [CrossRef]
- Ciorba, M.A. Indoleamine 2, 3 dioxygenase in intestinal disease. Curr. Opin. Gastroenterol. 2013, 29, 146–152. [Google Scholar] [CrossRef]
- Gao, Z.; Shao, S.; Xu, Z.; Nie, J.; Li, C.; Du, C. IDO1 induced macrophage M1 polarization via ER stress-associated GRP78-XBP1 pathway to promote ulcerative colitis progression. Front. Med. 2025, 12, 1524952. [Google Scholar] [CrossRef]
- Ala, M.; Jafari, R.M.; Nematian, H.; Shadboorestan, A.; Dehpour, A.R. Sodium selenite modulates IDO1/Kynurenine, TLR4, NF-κB and Bcl2/Bax pathway and mitigates acetic acid-induced colitis in rat. Cell Physiol. Biochem. 2022, 56, 24–35. [Google Scholar]
- Shon, W.J.; Lee, Y.K.; Shin, J.H.; Choi, E.Y.; Shin, D.M. Severity of DSS-induced colitis is reduced in Ido1-deficient mice with down-regulation of TLR-MyD88-NF-kB transcriptional networks. Sci. Rep. 2015, 5, 17305. [Google Scholar] [CrossRef]
- Wu, P.; Yao, S.; Wang, X.; Yang, L.; Wang, S.; Dai, W.; Zhang, H.; He, B.; Wang, X.; Wang, S.; et al. Oral administration of nanoformulated indoximod ameliorates ulcerative colitis by promoting mitochondrial function and mucosal healing. Int. J. Pharm. 2023, 637, 122813. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Schweikart, K.; Tomaszewski, J.; Page, J.G.; Noker, P.E.; Buhrow, S.A.; Reid, J.M.; Ames, M.M.; Munn, D.H. Toxicology and pharmacokinetics of 1-methyl-d-tryptophan: Absence of toxicity due to saturating absorption. Food Chem. Toxicol. 2008, 46, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Ahlstedt, J.; Konradsson, E.; Ceberg, C.; Redebrandt, H.N. Increased effect of two-fraction radiotherapy in conjunction with IDO1 inhibition in experimental glioblastoma. PLoS ONE 2020, 15, e0233617. [Google Scholar] [CrossRef] [PubMed]
- Ercan, G.; Yigitturk, G.; Erbas, O. Therapeutic effect of adenosine on experimentally induced acute ulcerative colitis model in rats. Acta Cir. Bras. 2020, 34, e201901204. [Google Scholar] [CrossRef]
- MacPherson, B.; Pfeiffer, C.J. Experimental colitis. Digestion 1976, 14, 424–452. [Google Scholar] [CrossRef]
- Ercan, G.; Aygün, H.; Akbaş, A.; Çınaroğlu, O.S.; Erbas, O. Suramin Exerts an Ameliorative Effect on Acetic Acid-Induced Acute Colitis in Rats by Demonstrating Potent Antioxidant and Anti-Inflammatory Properties. Medicina 2025, 61, 829. [Google Scholar] [CrossRef]
- Cinpolat, H.Y.; Buğdaycı, G.; Şengül, N.; Astarcı, H.M. A chemically induced experimental colitis model with a simple combination of acetic acid and trinitrobenzene sulphonic acid. Turk. J. Gastroenterol. 2023, 34, 196. [Google Scholar] [CrossRef]
- Karaca, T.; Şimşek, N.; Uslu, S.; Kalkan, Y.; Can, I.; Kara, A.; Yörük, M. The effect of royal jelly on CD3+, CD5+, CD45+ T-cell and CD68+ cell distribution in the colon of rats with acetic acid-induced colitis. Allergol. Immunopathol. 2012, 40, 357–361. [Google Scholar] [CrossRef]
- Bertevello, P.L.; Logullo, Â.F.; Nonogaki, S.; Campos, F.M.; Chiferi, V.; Alves, C.C.; Torrinhas, R.S.; Gama-Rodrigues, J.J.; Waitzberg, D.L. Immunohistochemical assessment of mucosal cytokine profile in acetic acid experimental colitis. Clinics 2005, 60, 277–286. [Google Scholar] [CrossRef]
- Randhawa, P.K.; Singh, K.; Singh, N.; Jaggi, A.S. A review on chemical-induced inflammatory bowel disease models in rodents. Korean J. Physiol. Pharmacol. 2014, 18, 279. [Google Scholar] [CrossRef]
- Colombo, B.B.; Fattori, V.; Guazelli, C.F.; Zaninelli, T.H.; Carvalho, T.T.; Ferraz, C.R.; Bussmann, A.J.C.; Ruiz-Miyazawa, K.W.; Baracat, M.M.; Casagrande, R.; et al. Vinpocetine ameliorates acetic acid-induced colitis by inhibiting NF-κB activation in mice. Inflammation 2018, 41, 1276–1289. [Google Scholar] [CrossRef] [PubMed]
- Alsharif, I.A.; Fayed, H.M.; Abdel-Rahman, R.F.; Abd-Elsalam, R.M.; Ogaly, H.A. Miconazole mitigates acetic acid-induced experimental colitis in rats: Insight into inflammation, oxidative stress and Keap1/Nrf-2 signaling crosstalk. Biology 2022, 11, 303. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Kong, J.; Wu, Q.; Yang, Y.; Ji, P. Effect of TLR4/MyD88 signaling pathway on expression of IL-1β and TNF-α in synovial fibroblasts from temporomandibular joint exposed to lipopolysaccharide. Mediat. Inflamm. 2015, 2015, 329405. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Lai, J.; Li, Y.; Deng, J.; Zhao, C.; Huang, Q.; Yang, F.; Yang, S.; Wu, Y.; Tang, X.; et al. Methyl gallate alleviates acute ulcerative colitis by modulating gut microbiota and inhibiting TLR4/NF-κB pathway. Int. J. Mol. Sci. 2022, 23, 14024. [Google Scholar] [CrossRef]
- Oubaid, E.N.; Abu-Raghif, A.; Al-Sudani, I.M. Ibudilast ameliorates experimentally induced colitis in rats via down-reg-ulation of proinflammatory cytokines and myeloperoxidase enzyme activity. Pharmacia 2023, 70, 187–195. [Google Scholar] [CrossRef]
- Dejban, P.; Sahraei, M.; Chamanara, M.; Dehpour, A.; Rashidian, A. Anti-inflammatory effect of amitriptyline in a rat model of acetic acid-induced colitis: The involvement of the TLR4/NF-kB signaling pathway. Fundam. Clin. Pharmacol. 2021, 35, 843–851. [Google Scholar] [CrossRef]
- Abdel-Wahab, B.A.; Alkahtani, S.A.; Alqahtani, A.A.; Hassanein, E.H. Umbelliferone ameliorates ulcerative colitis induced by acetic acid via modulation of TLR4/NF-κB-p65/iNOS and SIRT1/PPARγ signaling pathways in rats. Environ. Sci. Pollut. Res. 2022, 29, 37644–37659. [Google Scholar] [CrossRef]
- Hassan, H.A.; Samy, W.; Mohammed, H.O.; Mahmoud, S.M.; Abbas, N.A. Ameliorative effects of androstenediol against acetic acid-induced colitis in male wistar rats via inhibiting TLR4-mediated PI3K/Akt and NF-κB pathways through estrogen receptor β activation. Int. Immunopharmacol. 2024, 127, 111414. [Google Scholar] [CrossRef]
- Brincks, E.L.; Adams, J.; Wang, L.; Turner, B.; Marcinowicz, A.; Ke, J.; Essmann, M.; Mautino, L.M.; Van Allen, C.; Kumar, S.; et al. Indoximod opposes the immunosuppressive effects mediated by IDO and TDO via modulation of AhR function and activation of mTORC1. Oncotarget 2020, 11, 2438. [Google Scholar] [CrossRef]
- Wu, W.; Zhong, W.; Lin, Z.; Yan, J. Blockade of Indoleamine 2, 3-Dioxygenase attenuates lipopolysaccharide-induced kidney injury by inhibiting TLR4/NF-κB signaling. Clin. Exp. Nephrol. 2023, 27, 495–505. [Google Scholar] [CrossRef]
- Inoue, K.; Kodama, T.; Daida, H. Pentraxin 3: A novel biomarker for inflammatory cardiovascular disease. Int. J. Vasc. Med. 2012, 2012, 657025. [Google Scholar] [CrossRef] [PubMed]
- Doni, A.; Stravalaci, M.; Inforzato, A.; Magrini, E.; Mantovani, A.; Garlanda, C.; Bottazzi, B. The long pentraxin PTX3 as a link between innate immunity, tissue remodeling, and cancer. Front. Immunol. 2019, 10, 712. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Zhao, F.; Li, Z.; Liang, F.; Yu, S. Silencing of PTX3 alleviates LPS-induced inflammatory pain by regulating TLR4/NF-κB signaling pathway in mice. Biosci. Rep. 2020, 40, BSR20194208. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, I.; Rizzo, A.; Sarra, M.; Sica, G.; Sileri, P.; Biancone, L.; Macdonald, T.T.; Pallone, F.; Monteleone, G. Aryl hydrocarbon receptor-induced signals up-regulate IL-22 production and inhibit inflammation in the gastrointestinal tract. Gastroenterology 2011, 141, 237–248. [Google Scholar] [CrossRef]
- Huynh, L.; Kusnadi, A.; Park, S.H.; Murata, K.; Park-Min, K.H.; Ivashkiv, L.B. Opposing regulation of the late phase TNF response by mTORC1-IL-10 signaling and hypoxia in human macrophages. Sci. Rep. 2016, 6, 31959. [Google Scholar] [CrossRef]
- Elson, C.O.; Cong, Y.; McCracken, V.J.; Dimmitt, R.A.; Lorenz, R.G.; Weaver, C.T. Experimental models of inflammatory bowel disease reveal innate, adaptive, and regulatory mechanisms of host dialogue with the microbiota. Immunol. Rev. 2005, 206, 260–276. [Google Scholar] [CrossRef]
- Kawada, M.; Arihiro, A.; Mizoguchi, E. Insights from advances in research of chemically induced experimental models of human inflammatory bowel disease. World J. Gastroenterol. 2007, 13, 5581. [Google Scholar] [CrossRef]
- Mascolo, N.; Izzo, A.A.; Autore, G.; Maiello, F.M.; Di Carlo, G.; Capasso, F. Acetic acid-induced colitis in normal and essential fatty acid deficient rats. J. Pharmacol. Exp. Ther. 1995, 272, 469–475. [Google Scholar] [CrossRef]
- Will, P.C.; Thomas, T.K.; Iverson, L.; Buckman, D.; Weis, W.; Wilson, C.; Srivastava, A. Platelet activating factor as a proinflammatory mediator in acetic-induced colitis in the rat. Agents Actions 1991, 34, 181–184. [Google Scholar] [CrossRef]
- Lacasse, C.; Turcotte, S.; Gingras, D.; Stankova, J.; Rola-Pleszczynski, M. Platelet-activating factor stimulates interleukin-6 production by human endothelial cells and synergizes with tumor necrosis factor for enhanced production of granulocyte-macrophage colony stimulating factor. Inflammation 1997, 21, 145–158. [Google Scholar] [CrossRef]
- Rehman, I.U.; Saleem, M.; Raza, S.A.; Bashir, S.; Muhammad, T.; Asghar, S.; Qamar, M.U.; Shah, T.A.; Bin Jardan, Y.A.; Mekonnen, A.B.; et al. Anti-ulcerative colitis effects of chemically characterized extracts from C alliandra haematocephala in acetic acid-induced ulcerative colitis. Front. Chem. 2024, 12, 1291230. [Google Scholar] [CrossRef] [PubMed]
- Vlachogianni, I.C.; Fragopoulou, E.; Stamatakis, G.M.; Kostakis, I.K.; Antonopoulou, S. Platelet Activating Factor (PAF) biosynthesis is inhibited by phenolic compounds in U-937 cells under inflammatory conditions. Prostaglandins Other Lipid Mediat. 2015, 121, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Bábíčková, J.; Tóthová, Ľ.; Lengyelová, E.; Bartoňová, A.; Hodosy, J.; Gardlík, R.; Celec, P. Sex differences in experimentally induced colitis in mice: A role for estrogens. Inflammation 2015, 38, 1996–2006. [Google Scholar] [CrossRef] [PubMed]
- Ige, S.; Aremu, W.; Olateju, B.; Oladipupo, V.A.; Adekola, A.T. Effects of Age and Sex on the Healing of Acetic-Acid Induced Ulcerative Colitis in Adult Wistar Rats. Asian J. Med. Health 2021, 19, 63–73. [Google Scholar] [CrossRef]


| Control Group | Colitis Group | Colitis + Indoximod Group | |
|---|---|---|---|
| Histopathological score | 0.30 ± 0.15 | 3.30 ± 0.26 *** | 1.90 ± 0.23 ***,## |
| Colon TLR-4 level (pg/mg tissue) | 1.31 ± 0.20 | 3.55 ± 0.25 *** | 2.20 ± 0.27 *,## |
| Plasma TNF-alfa level (pg/mL) | 19.55 ± 1.63 | 58.69 ± 3.96 *** | 32.14 ± 3.45 *,### |
| Plasma Pentraxin-3 level (ng/mL) | 1.25 ± 0.19 | 2.51 ± 0.30 ** | 1.68 ± 0.14 # |
| Plasma PAF level (pg/mL) | 129.37 ± 5.83 | 361.95 ± 10.85 *** | 343.63 ± 11.33 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ercan, G.; Aygun, H.; Akbaş, A.; Çınaroğlu, O.S.; Erbas, O. Indoximod Attenuates Inflammatory Responses in Acetic Acid-Induced Acute Colitis by Modulating Toll-like Receptor 4 (TLR4) Signaling and Proinflammatory Cytokines in Rats. Medicina 2025, 61, 1033. https://doi.org/10.3390/medicina61061033
Ercan G, Aygun H, Akbaş A, Çınaroğlu OS, Erbas O. Indoximod Attenuates Inflammatory Responses in Acetic Acid-Induced Acute Colitis by Modulating Toll-like Receptor 4 (TLR4) Signaling and Proinflammatory Cytokines in Rats. Medicina. 2025; 61(6):1033. https://doi.org/10.3390/medicina61061033
Chicago/Turabian StyleErcan, Gulcin, Hatice Aygun, Ahmet Akbaş, Osman Sezer Çınaroğlu, and Oytun Erbas. 2025. "Indoximod Attenuates Inflammatory Responses in Acetic Acid-Induced Acute Colitis by Modulating Toll-like Receptor 4 (TLR4) Signaling and Proinflammatory Cytokines in Rats" Medicina 61, no. 6: 1033. https://doi.org/10.3390/medicina61061033
APA StyleErcan, G., Aygun, H., Akbaş, A., Çınaroğlu, O. S., & Erbas, O. (2025). Indoximod Attenuates Inflammatory Responses in Acetic Acid-Induced Acute Colitis by Modulating Toll-like Receptor 4 (TLR4) Signaling and Proinflammatory Cytokines in Rats. Medicina, 61(6), 1033. https://doi.org/10.3390/medicina61061033






