Impact of Virtual Reality Alone and in Combination with Conventional Therapy on Balance in Parkinson’s Disease: A Systematic Review with a Meta-Analysis of Randomized Controlled Trials
Abstract
1. Introduction
- To assess the additional benefits of integrating VR with conventional therapy on balance in PD;
- To compare the efficacy of VR-based interventions versus conventional therapies on balance-related outcomes.
2. Methods
2.1. Design
2.2. Consulted Documentary Sources
2.3. Research Strategy
2.4. Eligibility Criteria
2.5. Study Selection Process
2.6. Data Extraction
- Population (3): adult, aged 65+, Parkinson’s disease;
- Intervention (1): virtual reality;
- Comparison (1): conventional physiotherapy;
- Outcomes (5): traditional exercise, Berg balance scale, sensory organization test score, six-minute walking test, Tinetti Performance-Oriented Mobility Assessment.
2.7. Risk-of-Bias Assessment Tool
2.8. Quality of Evidence
2.9. Treatment Effect Analysis
2.10. Data Synthesis
3. Results
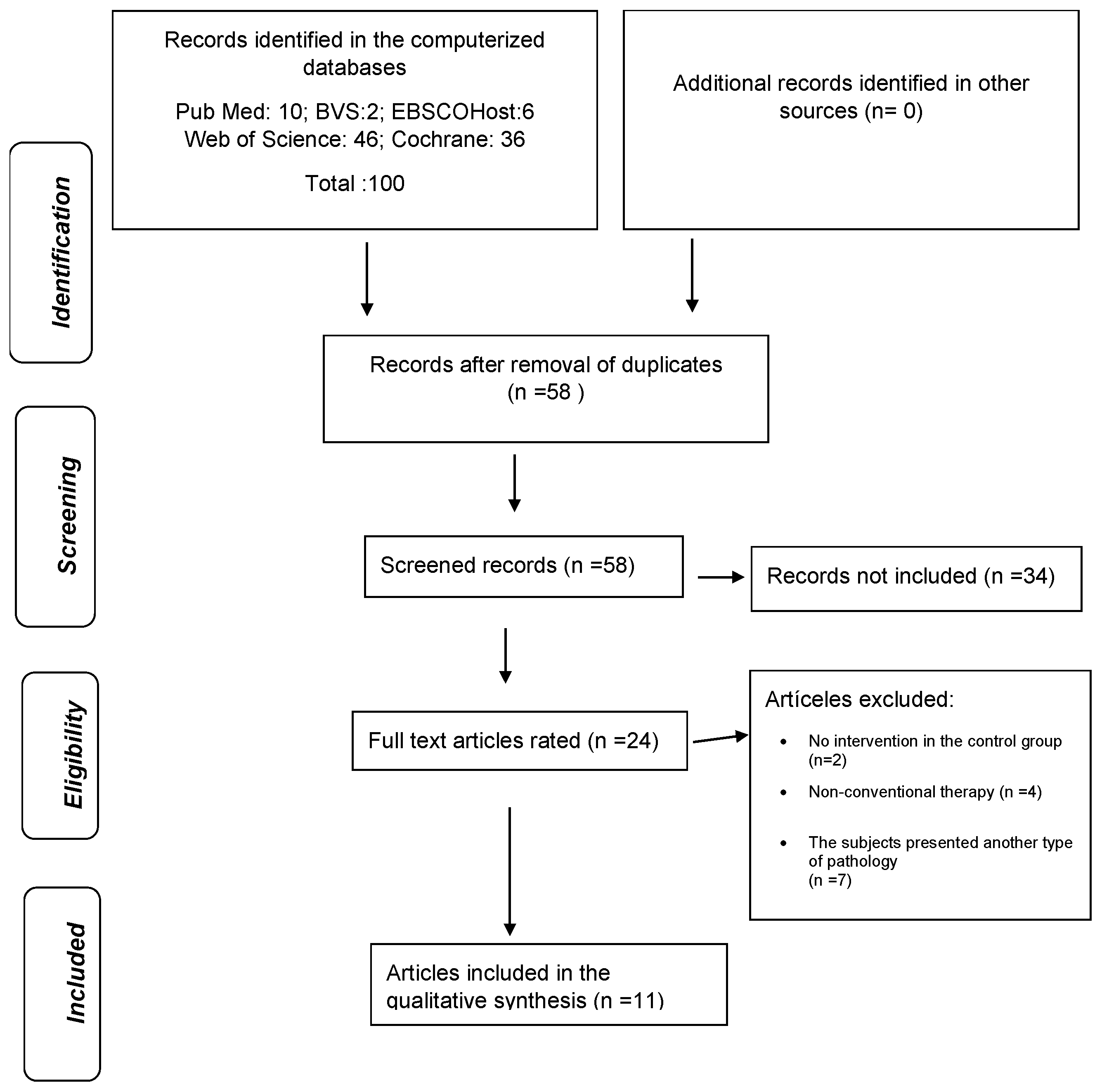
3.1. Study Selection and Identification Process
3.2. General Characteristics of the Included Studies
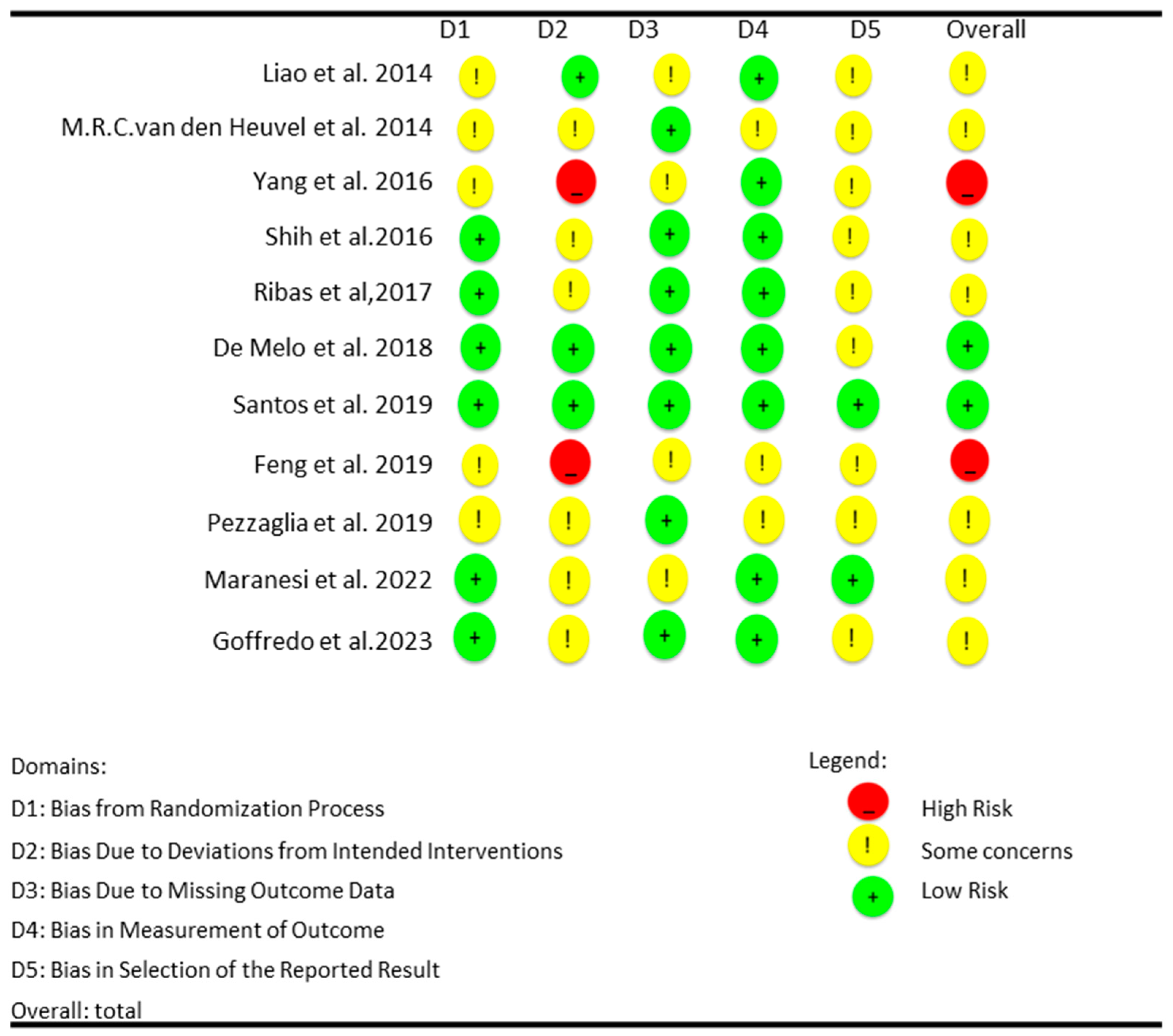
3.3. Risk of Bias in the Included Articles
3.4. Sample Characteristics
3.5. Quality of the Evidence
4. Discussion
5. Conclusions
- VR-based therapies were found to be as effective, if not superior, to conventional therapies at improving balance-related outcomes, with significant gains observed in dynamic balance, postural stability, and fall risk reduction;
- The combination of VR with conventional therapy demonstrated added benefits compared to standalone interventions, suggesting that an integrated approach may provide enhanced outcomes through the complementary strengths of both methods.
Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Olson, M.; Lockhart, T.E.; Lieberman, A. Motor Learning Deficits in Parkinson’s Disease (PD) and Their Effect on Training Response in Gait and Balance: A Narrative Review. Front. Neurol. 2019, 10, 62. [Google Scholar] [CrossRef]
- Canning, C.G.; Allen, N.E.; Nackaerts, E.; Paul, S.S.; Nieuwboer, A.; Gilat, M. Virtual Reality in Research and Rehabilitation of Gait and Balance in Parkinson Disease. Nat. Rev. Neurol. 2020, 16, 409–425. [Google Scholar] [CrossRef] [PubMed]
- Kraśnik, W.; Jankowska, O.; Kurzeja, J.; Piotrowicz, K.; Piotrowicz, H.; Bajkacz, A.; Osmólska, J. The Benefits and Challenges of Virtual Reality Application in Rehabilitation for Chronic Conditions. J. Educ. Health Sport 2024, 75, 56379. [Google Scholar] [CrossRef]
- Kwon, S.H.; Park, J.K.; Koh, Y.H. A Systematic Review and Meta-Analysis on the Effect of Virtual Reality-Based Rehabilitation for People with Parkinson’s Disease. J. NeuroEng. Rehabil. 2023, 20, 94. [Google Scholar] [CrossRef] [PubMed]
- Sarasso, E.; Gardoni, A.; Tettamanti, A.; Bonavia, M.; De Icco, R.; Casali, M.; Dalla Volta, G. Virtual Reality Balance Training to Improve Balance and Mobility in Parkinson’s Disease: A Systematic Review and Meta-Analysis. J. Neurol. 2022, 269, 1873–1888. [Google Scholar] [CrossRef] [PubMed]
- Kashif, M.; Ahmad, A.; Bandpei, M.A.M.; Farooq, M.; Iram, H.; Fatima, R. Systematic Review of the Application of Virtual Reality to Improve Balance, Gait, and Motor Function in Patients with Parkinson’s Disease. Medicine 2022, 101, e29212. [Google Scholar] [CrossRef]
- Dockx, K.; Bekkers, E.M.J.; Van den Bergh, V.; Ginis, P.; Rochester, L.; Hausdorff, J.M.; Mirelman, A.; Nieuwboer, A. Virtual Reality for Rehabilitation in Parkinson’s Disease. Cochrane Database Syst. Rev. 2016, 12, CD010760. [Google Scholar] [CrossRef]
- Wang, W.; Wong, S.S.-L.; Lai, F.H.-Y. The Effect of Virtual Reality Rehabilitation on Balance in Patients with Parkinson’s Disease: A Systematic Review and Meta-Analysis. Electronics 2021, 10, 1003. [Google Scholar] [CrossRef]
- Aderinto, N.; Olatunji, G.; Abdulbasit, M.O.; Edun, M.; Aboderin, G.; Egbunu, E. Exploring the Efficacy of Virtual Reality-Based Rehabilitation in Stroke: A Narrative Review of Current Evidence. Ann. Med. 2023, 55, 2285907. [Google Scholar] [CrossRef]
- Maggio, M.G.; Bonanno, M.; Manuli, A.; Maresca, G.; Calabrò, R.S. Advances in the Neuro-Rehabilitation of Parkinson’s Disease: Insights from a Personalized Multidisciplinary Innovative Pathway. Biomedicines 2024, 12, 2426. [Google Scholar] [CrossRef]
- Bekkers, E.M.J.; Mirelman, A.; Alcock, L.; Rochester, L.; Nieuwhof, F.; Bloem, B.R.; Pelosin, E.; Avanzino, L.; Cereatti, A.; Della Croce, U.; et al. Do Patients with Parkinson’s Disease with Freezing of Gait Respond Differently than Those without to Treadmill Training Augmented by Virtual Reality? Neurorehabil. Neural Repair 2020, 34, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, J.; Mehrabi, S.; Li, Y.; Basharat, A.; Middleton, L.E.; Cao, S.; Barnett-Cowan, M.; Boger, J. Immersive Virtual Reality Exergames for Persons Living with Dementia: User-Centered Design Study as a Multistakeholder Team during the COVID-19 Pandemic. JMIR Serious Games 2022, 10, e29987. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- da Costa Santos, C.M.; de Mattos Pimenta, C.A.; Nobre, M.R.C. The PICO Strategy for the Research Question Construction and Evidence Search. Rev. Lat. Am. Enferm. 2007, 15, 508–511. [Google Scholar] [CrossRef] [PubMed]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A Web and Mobile App for Systematic Reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V. Cochrane Handbook for Systematic Reviews of Interventions, 1st ed.; Wiley: Chichester, UK, 2019. [Google Scholar] [CrossRef]
- Aguayo-Albasini, J.L.; Flores-Pastor, B.; Soria-Aledo, V. Sistema GRADE: Clasificación de la Calidad de la Evidencia y Graduación de la Fuerza de la Recomendación. Cir. Esp. 2014, 92, 82–88. [Google Scholar] [CrossRef]
- Liao, Y.Y.; Yang, Y.R.; Cheng, S.J.; Wu, Y.R.; Fuh, J.L.; Wang, R.Y. Virtual Reality–Based Training to Improve Obstacle-Crossing Performance and Dynamic Balance in Patients with Parkinson’s Disease. Neurorehabil. Neural Repair 2015, 29, 658–667. [Google Scholar] [CrossRef]
- van den Heuvel, M.R.; Kwakkel, G.; Beek, P.J.; Berendse, H.W.; Daffertshofer, A.; van Wegen, E.E. Effects of augmented visual feedback during balance training in Parkinson’s disease: A pilot randomized clinical trial. Park. Relat. Disord. 2014, 20, 1352–1358. [Google Scholar] [CrossRef]
- Yang, W.C.; Wang, H.K.; Wu, R.M.; Lo, C.S.; Lin, K.H. Home-Based Virtual Reality Balance Training and Conventional Balance Training in Parkinson’s Disease: A Randomized Controlled Trial. J. Formos. Med. Assoc. 2016, 115, 734–743. [Google Scholar] [CrossRef]
- Shih, M.C.; Wang, R.Y.; Cheng, S.J.; Yang, Y.R. Effects of a Balance-Based Exergaming Intervention Using the Kinect Sensor on Posture Stability in Individuals with Parkinson’s Disease: A Single-Blinded Randomized Controlled Trial. J. Neuroeng. Rehabil. 2016, 13, 78. [Google Scholar] [CrossRef]
- Ribas, C.G.; Alves da Silva, L.; Corrêa, M.R.; Teive, H.G.; Valderramas, S. Effectiveness of Exergaming in Improving Functional Balance, Fatigue, and Quality of Life in Parkinson’s Disease: A Pilot Randomized Controlled Trial. Park. Relat. Disord. 2017, 38, 13–18. [Google Scholar] [CrossRef]
- de Melo, G.E.L.; Kleiner, A.F.R.; Lopes, J.B.P.; Dumont, A.J.L.; Lazzari, R.D.; Galli, M.; Oliveira, C.S. Effect of Virtual Reality Training on Walking Distance and Physical Fitness in Individuals with Parkinson’s Disease. NeuroRehabilitation 2018, 42, 473–480. [Google Scholar] [CrossRef]
- Santos, P.; Machado, T.; Santos, L.; Ribeiro, N.; Melo, A. Efficacy of the Nintendo Wii Combination with Conventional Exercises in the Rehabilitation of Individuals with Parkinson’s Disease: A Randomized Clinical Trial. NeuroRehabilitation 2019, 45, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Li, C.; Liu, J.; Wang, L.; Ma, J.; Li, G.; Gan, L.; Shang, X.; Wu, Z. Virtual Reality Rehabilitation versus Conventional Physical Therapy for Improving Balance and Gait in Parkinson’s Disease Patients: A Randomized Controlled Trial. Med. Sci. Monit. 2019, 25, 4186–4192. [Google Scholar] [CrossRef] [PubMed]
- Pazzaglia, C.; Imbimbo, I.; Tranchita, E.; Minganti, C.; Ricciardi, D.; Monaco, R.L.; Parisi, A.; Padua, L. Comparison of Virtual Reality Rehabilitation and Conventional Rehabilitation in Parkinson’s Disease: A Randomised Controlled Trial. Physiotherapy 2020, 106, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Maranesi, E.; Casoni, E.; Baldoni, R.; Barboni, I.; Rinaldi, N.; Tramontana, B.; Amabili, G.; Benadduci, M.; Barbarossa, F.; Luzi, R.; et al. The Effect of Non-Immersive Virtual Reality Exergames versus Traditional Physiotherapy in Parkinson’s Disease Older Patients: Preliminary Results from a Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2022, 19, 14818. [Google Scholar] [CrossRef]
- Goffredo, M.; Baglio, F.; De Icco, R.; Proietti, S.; Maggioni, G.; Turolla, A.; Pournajaf, S.; Jonsdottir, J.; Zeni, F.; Federico, S.; et al. RIN_TR_Group. Efficacy of Non-Immersive Virtual Reality-Based Telerehabilitation on Postural Stability in Parkinson’s Disease: A Multicenter Randomized Controlled Trial. Eur. J. Phys. Rehabil. Med. 2023, 59, 689–696. [Google Scholar] [CrossRef]
- Berg, K.O.; Maki, B.E.; Williams, J.I.; Holliday, P.J.; Wood-Dauphinee, S.L. Clinical and Laboratory Measures of Postural Balance in an Elderly Population. Arch. Phys. Med. Rehabil. 1992, 73, 1073–1080. [Google Scholar]
- Bailo, G.; Saibene, F.L.; Bandini, V.; Arcuri, P.; Salvatore, A.; Meloni, M.; Castagna, A.; Navarro, J.; Lencioni, T.; Ferrarin, M.; et al. Characterization of Walking in Mild Parkinson’s Disease: Reliability, Validity and Discriminant Ability of the Six-Minute Walk Test Instrumented with a Single Inertial Sensor. Sensors 2024, 24, 662. [Google Scholar] [CrossRef]
- Sadeghi, M.; Bristow, T.; Fakorede, S.; Liao, K.; Palmer, J.A.; Lyons, K.E.; Pahwa, R.; Huang, C.K.; Akinwuntan, A.; Devos, H. The Effect of Sensory Reweighting on Postural Control and Cortical Activity in Parkinson’s Disease: A Pilot Study. Arch. Rehabil. Res. Clin. Transl. 2024, 6, 100368. [Google Scholar] [CrossRef]
- The Unified Parkinson’s Disease Rating Scale (UPDRS). Status and Recommendations. Mov. Disord. 2003, 18, 738–750. [Google Scholar] [CrossRef] [PubMed]
- Hernández, R.M.C. Estudio Metaanalítico de Generalización de la Fiabilidad de la Escala de Equilibrio de Berg; Universidad de Murcia: Murcia, Spain, 2015; Available online: https://dialnet.unirioja.es/servlet/tesis?codigo=99098 (accessed on 9 March 2025).
- Faber, M.J.; Bosscher, R.J.; van Wieringen, P.C. Clinimetric properties of the performance-oriented mobility assessment. Phys. Ther. 2006, 86, 944–954. [Google Scholar] [CrossRef] [PubMed]
- Steffen, T.; Seney, M. Test-retest reliability and minimal detectable change on balance and ambulation tests, the 36-item short-form health survey, and the unified Parkinson disease rating scale in people with parkinsonism. Phys. Ther. 2008, 88, 733–746. [Google Scholar] [CrossRef] [PubMed]
- Bacha, J.M.; da Cunha, M.C.; de Freitas, T.B.; Nuvolini, R.A.; Doná, F.; da Silva, K.G.; Torriani-Pasin, C.; de Freitas Ganança, F.; Pompeu, J.E. Efeitos da Reabilitação Virtual no Controle Postural de Indivíduos com Doença de Parkinson. Motricidade 2021, 17, 3. [Google Scholar] [CrossRef]
- Lichter, D.G.; Benedict, R.H.B.; Hershey, L.A. Importance of balance-gait disorder as a risk factor for cognitive impairment, dementia and related non-motor symptoms in Parkinson’s disease. J. Park. Dis. 2018, 8, 539–552. [Google Scholar] [CrossRef]
- Savica, R.; Rocca, W.A.; Ahlskog, J.E. When Does Parkinson Disease Start? Arch. Neurol. 2010, 67, 798–801. [Google Scholar] [CrossRef]
- Cikajlo, I.; Peterlin Potisk, K. Advantages of Using 3D Virtual Reality-Based Training in Persons with Parkinson’s Disease: A Parallel Study. J. NeuroEng. Rehabil. 2019, 16, 119. [Google Scholar] [CrossRef]
- Kim, A.; Darakjian, N.; Finley, J.M. Walking in Fully Immersive Virtual Environments: An Evaluation of Potential Adverse Effects in Older Adults and Individuals with Parkinson’s Disease. J. NeuroEng. Rehabil. 2017, 14, 16. [Google Scholar] [CrossRef]
- Lei, C.; Sunzi, K.; Dai, F.; Liu, X.; Wang, Y.; Zhang, B.; Ju, M. Effects of virtual reality rehabilitation training on gait and balance in patients with Parkinson’s disease: A systematic review. PLoS ONE 2019, 14, e0224819. [Google Scholar] [CrossRef]
- Triegaardt, J.; Han, T.S.; Sada, C.; Sharma, S.; Sharma, P. The role of virtual reality on outcomes in rehabilitation of Parkinson’s disease: Meta-analysis and systematic review in 1031 participants. Neurol. Sci. 2020, 41, 529–536. [Google Scholar] [CrossRef]
| Author | Year | Design | Journal | Country |
|---|---|---|---|---|
| Liao et al. [18] | 2014 | RCT | Neurorehabilitation and Neural Repair | USA |
| M.R.C. van den Heuvel et al. [19] | 2014 | RCT | Elsevier | NETHERLANDS |
| Yang et al. [20] | 2016 | RCT | Journal of the Formosan Medical Association | TAIWAN |
| Shin et al. [21] | 2016 | RCT | Journal of NeuroEngineering and Rehabilitation | UK |
| Ribas et al. [22] | 2017 | RCT | Elsevier | NETHERLANDS |
| De Melo et al. [23] | 2018 | RCT | NeuroRehabilitation | NETHERLANDS |
| Santos et al. [24] | 2019 | RCT | NeuroRehabilitation | NETHERLANDS |
| Feng et al. [25] | 2019 | RCT | Medical Science Monitor | USA |
| Pazzaglia et al. [26] | 2019 | RCT | Elsevier | USA |
| Maranesi et al. [27] | 2022 | RCT | International Journal of Environmental Research and Public Health | SUISA |
| Goffredo et al. [28] | 2023 | RCT | European Journal of Physical and Rehabilitation Medicine | ITALY |
| Author | Year | Design | Size | Years | Gender | Severity |
|---|---|---|---|---|---|---|
| Liao et al. [18] | 2014 | RCT | EG pre: 12/post: 12 | 67 (7.1) | 6 M 6 F | H&Y = 2 (0.7) |
| CG: pre: 12/post: 11 | 64 (8.6) | 5 M 7 F | H&Y = 1.9 (0.8) | |||
| TE: pre: 12/post: 12 | 65 (6.7) | 6 M 6 F | H&Y = 2 (0.8) | |||
| M.R.C.van den Heuvel et al. [19] | 2014 | RCT | EG: pre: 17/post: 17 | 66.3 (6.39) | 12 M 5 F | H&Y = 2.5 |
| CG: pre: 16/post: 14 | 68.8 (9.68) | 8 M 8 F | H&T = 2.5 | |||
| Yang et al. [20] | 2016 | RCT | EG: pre: 12/post: 10 | 72.5 (8.4) | 7 M 4 F | H&Y = 3 (3.3) |
| CG: pre: 11/post: 10 | 75.4 (6.3) | 7 M 5 F | H&Y = 3 (3.3) | |||
| Shih et al. [21] | 2016 | RCT | EG: pre: 11/post: 10 | 67.5(9.9) | 9 M 1 F | H&Y = 1.6 (0.8) |
| CG: pre: 11/post: 10 | 68.8 (9.6) | 7 M 3 F | H&Y = 1.4 (0.52) | |||
| Ribas et al. [22] | 2017 | RCT | EG: pre: 10/post: 10 | 61.70 (6.8) | 4 M 6 F | H&Y = 1.25 |
| CG: pre: 10/post: 10 | 60.20 (11.2) | 4 M 6 F | H&Y = 1.5 | |||
| De Melo et al. [23] | 2018 | RCT | EG2: pre: 13/post: 12 | 60 (9.28) | 11 M 1 F | H&Y = 1.45 (0.51) |
| CG: pre: 14/post: 12 | 65 (13.04) | 5 M 7 F | H&Y = 2.08 (0.9) | |||
| EG1: pre: 15/post: 13 | 61 (10.72) | 12 M 1 F | H&Y = 1.53 (0.66) | |||
| Santos et al. [24] | 2019 | RCT | EG: pre: 15/post: 14 | 66.6 (8.2) | 9 M 5 F | H&Y = 1.5 (0.4) |
| EG1: pre: 15/post: 13 | 61.7 (7.3) | 11 M 2 F | H&Y = 1.4 (0.6) | |||
| GC2: pre: 15/post: 14 | 64.5 (9.8) | 11 M 3 F | H&Y = 1.3 (0.3) | |||
| Feng et al. [25] | 2019 | RCT | EG: pre: 14/post: 14 | 67 (4.7) | 8 M 7 F | H&Y = 3.03 (0.55) |
| GC: pre: 14/post: 14 | 66 (4.6) | 9 M 6 F | H&Y = 2.97 (0.58) | |||
| Pazzaglia et al. [26] | 2019 | RCT | EG: pre: 25/post: 25 | 72 (7) | 18 M 7 F | UPDRS III = 23 (9) |
| GC: pre: 26/post: 26 | 70 (10) | 17 M 9 F | UPDRS III = 25 (10) | |||
| Maranesi et al. [27] | 2022 | RCT | EG: pre: 16/post: 16 | 72 (6.3) | 6 M 10 F | H&Y = 2 |
| GC: pre: 16/post: 14 | 75 (5.4) | 9 M 5 F | H&Y = 2 | |||
| Goffredo at al. [28] | 2023 | RCT | EG: pre: 54/post: 49 | 67.8 (6.6) | 27 M 22 F | H&Y = 2 |
| GC: pre: 51/post: 48 | 68.2 (5.8) | 24 M 24 F | H&Y = 2 |
| Author | Year | Design | Intervention Characteristics | Intensity | Reps/Series | Frequency | Session Duration | Intervention Duration |
|---|---|---|---|---|---|---|---|---|
| Liao et al. [18] | 2014 | RCT | Fall prevention education | n/m | 10–15/3 | 2 sessions/week | 60 min | 6 weeks |
| Traditional exercise + treadmill | n/m | 10–15/3 | 2 sessions/week | 60 min | 6 weeks | |||
| Wii Fit VR + traditional exercise + treadmill | n/m | 10–15/3 | 2 sessions/week | 60 min | 6 weeks | |||
| M.R.C. van den Heuvel et al. [19] | 2014 | RCT | VR balance training Conventional balance training | n/m n/m | n/m n/m | 2 sessions/week 2 sessions/week | 60 min 60 min | 5 weeks 5 weeks |
| Shin et al. [20] | 2016 | Kinect sensor, Microsoft Conventional balance training | n/m n/m | n/m n/m | 2 sessions/week 2 sessions/week | 50 min 50 min | 8 weeks 8 weeks | |
| Yang et al. [21] | 2016 | RCT | VR balance training | n/m | 3 × 10 min | 2 sessions/week | 50 min | 6 weeks |
| Conventional balance training | n/m | 3 × 10 min | 2 sessions/week | 50 min | 6 weeks | |||
| Ribas et al. [22] | 2017 | RCT | Wii Fit games, Nintendo | n/m | n/m | 2 sessions/week | 30 min | 12 weeks |
| Warming + stretching and active exercises + resistance exercise and diagonal exercise for the trunk, neck, and limbs | n/m | n/m | 2 sessions/week | 30 min | 12 weeks | |||
| De Melo et al. [23] | 2018 | RCT | Kinect Xbox 360TM | 60–70% HB | n/m | 3 sessions/week | 20 min | 4 weeks |
| Traditional exercise | 60–70% HB | n/m | 3 sessions/week | 20 min | 4 weeks | |||
| Treadmill | 60–70% HB | n/m | 3 sessions/week | 20 min | 4 weeks | |||
| Santos et al. [24] | 2019 | RCT | Nintendo + Wii Balance Board platform + FNP | n/m | n/m | 2 sessions/week | 50 min | 8 weeks |
| Nintendo + Wii Balance Board platform + FNP | n/m | n/m | 2 sessions/week | 50 min | 8 weeks | |||
| FNP diagonals | n/m | n/m | 2 sessions/week | 50 min | 8 weeks | |||
| Feng et al. [25] | 2019 | RCT | RV | n/m | n/m | 5 sessions/week | 45 min | 12 weeks |
| Traditional exercise | n/m | n/m | 5 sessions/week | 45 min | 12 weeks | |||
| Pazzaglia et al. [26] | 2019 | RCT | VR session with multiple exercises | n/m | n/m | 3 sessions/week | 40 min | 6 weeks |
| Traditional exercise | n/m | n/m | 3 sessions/week | 40 min | 6 weeks | |||
| Maranesi et al. [27] | 2022 | RCT | Traditional exercise + Tymo system | n/m | n/m | 2 sessions/week | 50 min | 5 weeks |
| Traditional exercise | n/m | n/m | 2 sessions/week | 50 min | 5 weeks | |||
| Goffredo et al. [28] | 2023 | RCT | VRRS Tablet | n/m | n/m | 3–5 sessions/week | 45 min | 6–10 weeks |
| Stretching + active exercise | n/m | 10/1 | 3–5 sessions/week | 45 min | 6–10 weeks |
| Author | Year | Design | Equilibrium Outcomes |
|---|---|---|---|
| Liao et al. [18] | 2014 | RCT | EG pre–post: 16% improvement in SOT score |
| CG: no significant changes | |||
| TE pre–post: 9% improvement in SOT score | |||
| EG vs. CG: 15% more improvement than CG | |||
| EG vs. TE: 2% more improvement than TE | |||
| M.R.C.van den Heuvel et al. [19] | 2014 | EG pre–post: 96.43% reduction on UPDRS III MOTOR | |
| CG pre–post: 85.4% reduction on UPDRS III MOTOR EG vs. CG: −11.03% | |||
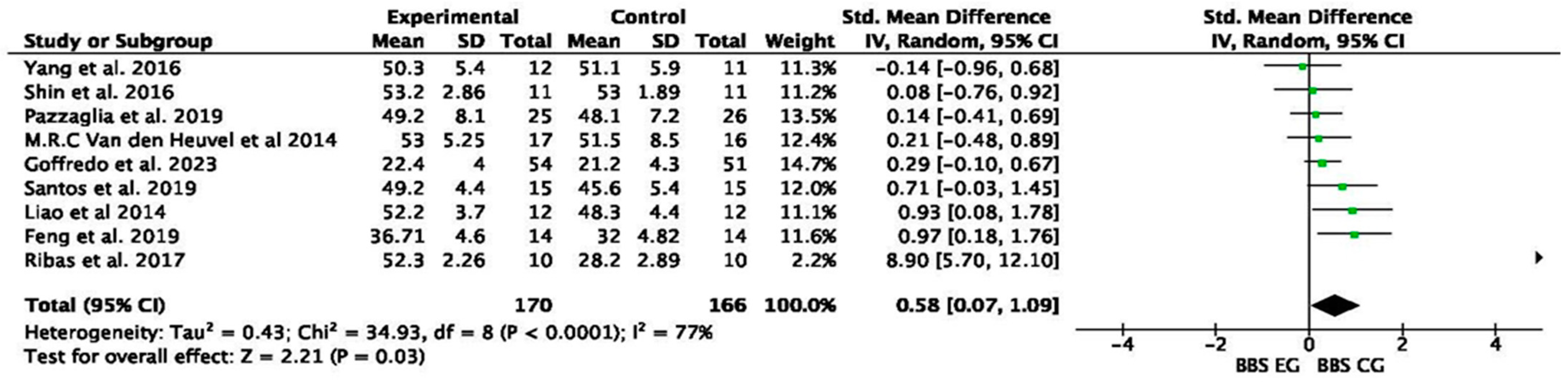
| Yang et al. [20] | 2016 | RCT | EG pre–post: 3 pt (7%) improvement on BBS |
| CG pre–post: 3 pt (6%) improvement on BBS | |||
| EG vs. CG: no differences on BBS | |||
| Shin et al. [21] | 2016 | EG pre–post: 2.3 pt (4.5%) improvement on BBS CG pre–post: 2.6 pt (5.1%) improvement on BSS EG vs. CG: 0.37% more improvement than GC | |
| Ribas et al. [22] | 2017 | EG pre–post: 1.9 pt (3.7%) improvement on BBS CG pre–post: 0.20 pt (0.41%) improvement on BBS EG vs. CG: 8.5% more improvement than CG | |
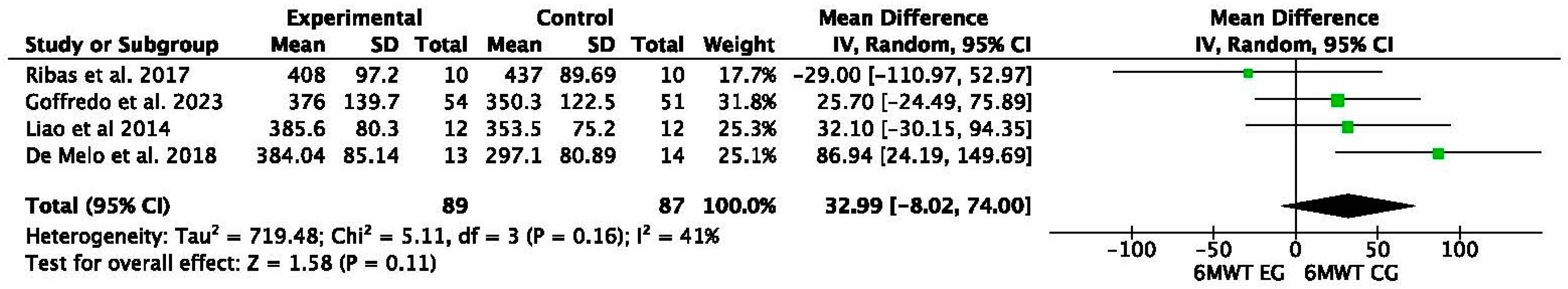
| De Melo et al. [23] | 2018 | RCT | EG pre–post: 6% improvement in 6 MWT HR |
| CG pre–post: no significant changes | |||
| CG 2 pre–post: 6% improvement in 6 MWT HR | |||
| EG vs. CG2: 3% more improvement than CG2 | |||
| Santos et al. [24] | 2019 | RCT | EG pre–post: 5.5 pt (13%) improvement on BBS |
| CG 1 pre–post: 5 pt (13%) improvement on BBS | |||
| CG 2 pre–post: 5 pt (12%) improvement on BBS | |||
| EG vs. CG 1: no differences on BBS | |||
| EG vs. CG 2: no differences on BBS | |||
| Feng et al. [25] | 2019 | RCT | EG pre–post: 6 pt (19%) improvement on BBS |
| CG pre–post: 2 pt (6%) improvement on BBS | |||
| EG vs. CG: 14% more improvement than GC | |||
| Pazzaglia et al. [26] | 2019 | RCT | EG pre–post: 5 pt (20%) improvement on BBS |
| CG pre–post: 4 pt (17%) improvement on BBS | |||
| EG vs. CG: no differences on BBS | |||
| Maranesi et al. [27] | 2022 | RCT | EG pre–post: 6% improvement in POMA balance |
| GC pre–post: 8% improvement in POMA balance | |||
| EG vs. CG: 8% more improvement than CG | |||
| Goffredo et al. [28] | 2023 | EG pre–post: 2.6 pt (7%) improvement on UPDRS III MOTOR CG pre–post: 0.3 pt (0.74%) improvement on UPDRS III MOTOR EG vs. CG: 18% more improvement than CG |
| Certainty Assessment | Impact | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of Studies | Study Design | Risk of Bias | Inconsistency | Indirect Evidence | Imprecision | Other Considerations | Certainty | Importance | |
| Balance Follow-Up: Range: 4 Weeks; Assessed with Various Scales (BBS, SOT Score, Tinetti POMA, 6 MWT HR) | |||||||||
| 11 | Randomized trial | Very serious | Not serious | Not serious | Extremely serious | Dose-response gradient | Two studies showed improvements in balance of 14% and 15% in favor of the virtual reality therapy group. Three studies did not show significant improvements, while two studies showed improvements of 8% and 3% in favor of the experimental group, the virtual reality group. | ⨁◯◯◯ Very low | Critical |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Natale, G.; Qorri, E.; Todri, J.; Lena, O. Impact of Virtual Reality Alone and in Combination with Conventional Therapy on Balance in Parkinson’s Disease: A Systematic Review with a Meta-Analysis of Randomized Controlled Trials. Medicina 2025, 61, 524. https://doi.org/10.3390/medicina61030524
De Natale G, Qorri E, Todri J, Lena O. Impact of Virtual Reality Alone and in Combination with Conventional Therapy on Balance in Parkinson’s Disease: A Systematic Review with a Meta-Analysis of Randomized Controlled Trials. Medicina. 2025; 61(3):524. https://doi.org/10.3390/medicina61030524
Chicago/Turabian StyleDe Natale, Giorgio, Erda Qorri, Jasemin Todri, and Orges Lena. 2025. "Impact of Virtual Reality Alone and in Combination with Conventional Therapy on Balance in Parkinson’s Disease: A Systematic Review with a Meta-Analysis of Randomized Controlled Trials" Medicina 61, no. 3: 524. https://doi.org/10.3390/medicina61030524
APA StyleDe Natale, G., Qorri, E., Todri, J., & Lena, O. (2025). Impact of Virtual Reality Alone and in Combination with Conventional Therapy on Balance in Parkinson’s Disease: A Systematic Review with a Meta-Analysis of Randomized Controlled Trials. Medicina, 61(3), 524. https://doi.org/10.3390/medicina61030524