Advances in Minimally Invasive Esophagectomy—An Overview of Recent Developments and a Novel Classification of Innovations in Treatment of Thoracic Esophageal Cancer
Abstract
1. Introduction
2. Objectives
3. Methods
Literature Search Strategy
4. Results
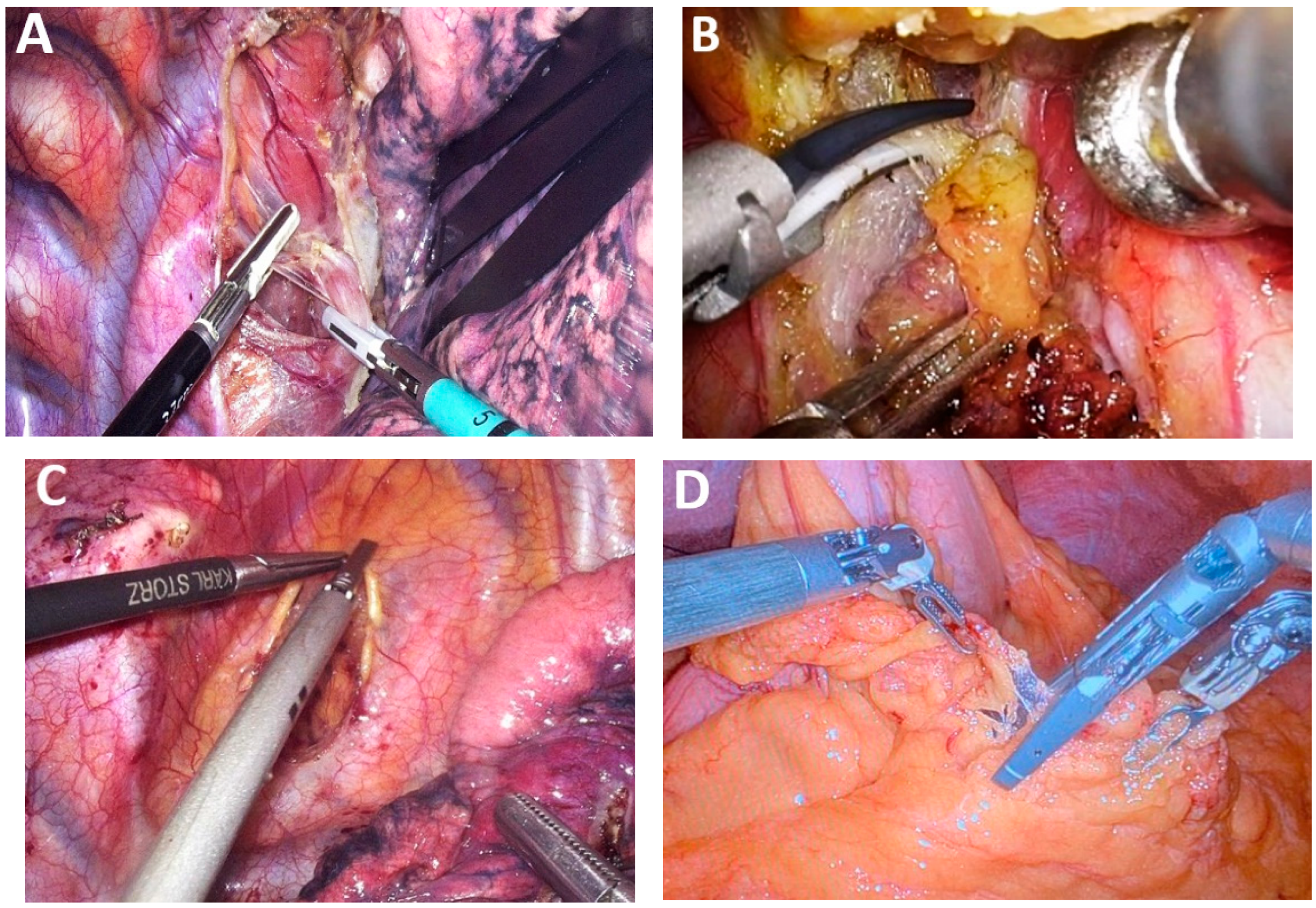
4.1. Class 1—Technological Innovations
- Improvement in visualization systems: high-definition cameras, 3D imaging, and fluorescence-guided or other dye techniques that optimize the surgical field and facilitate meticulous dissection.
- Development of advanced surgical instruments: ergonomic, articulating, energy-based tools and staplers that enable complex maneuvers in confined anatomical spaces.
- Auxiliary equipment and anesthetic innovations: enhanced insufflation systems, integrated operating platforms, and anesthetic protocols tailored to thoracoscopic and robotic procedures, all contributing to intraoperative stability and reduced complication rates.
4.2. Subclass 1A Advances in Camera Technology
4.2.1. Ultra-High Definition and Tridimensional Image in MIE
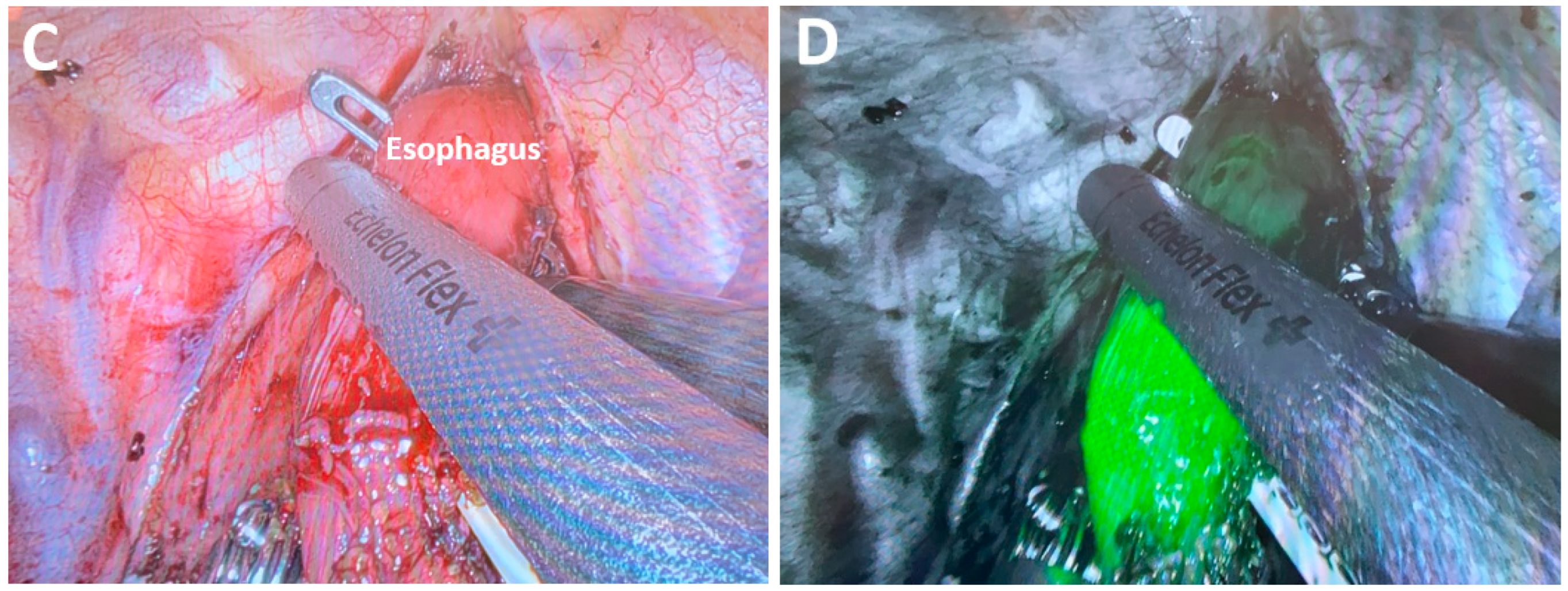
4.2.2. Fluorescence-Guided Surgery and MIE
4.2.3. Dye-Free Imaging
4.2.4. Artificial Intelligence
4.3. Subclass 1B Surgical Instruments
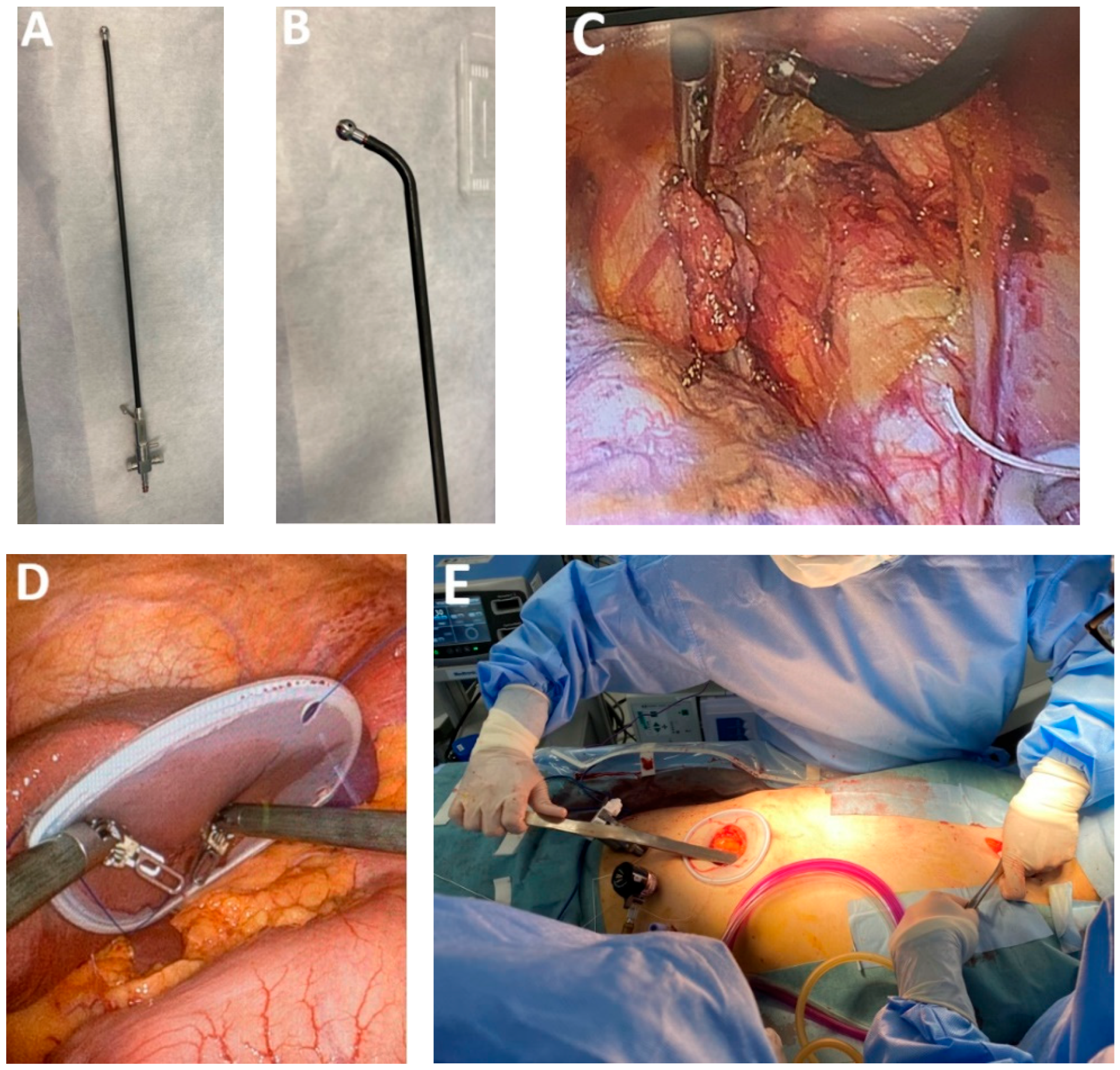
4.3.1. Ultra-Specialized Surgical Instruments for MIE
4.3.2. Advances in Endoscopic Stapling
4.4. Subclass 1C Auxiliary Equipment
4.5. Class 2—Advances in Surgical Approach
4.6. Subclass 2A Transthoracic MIE
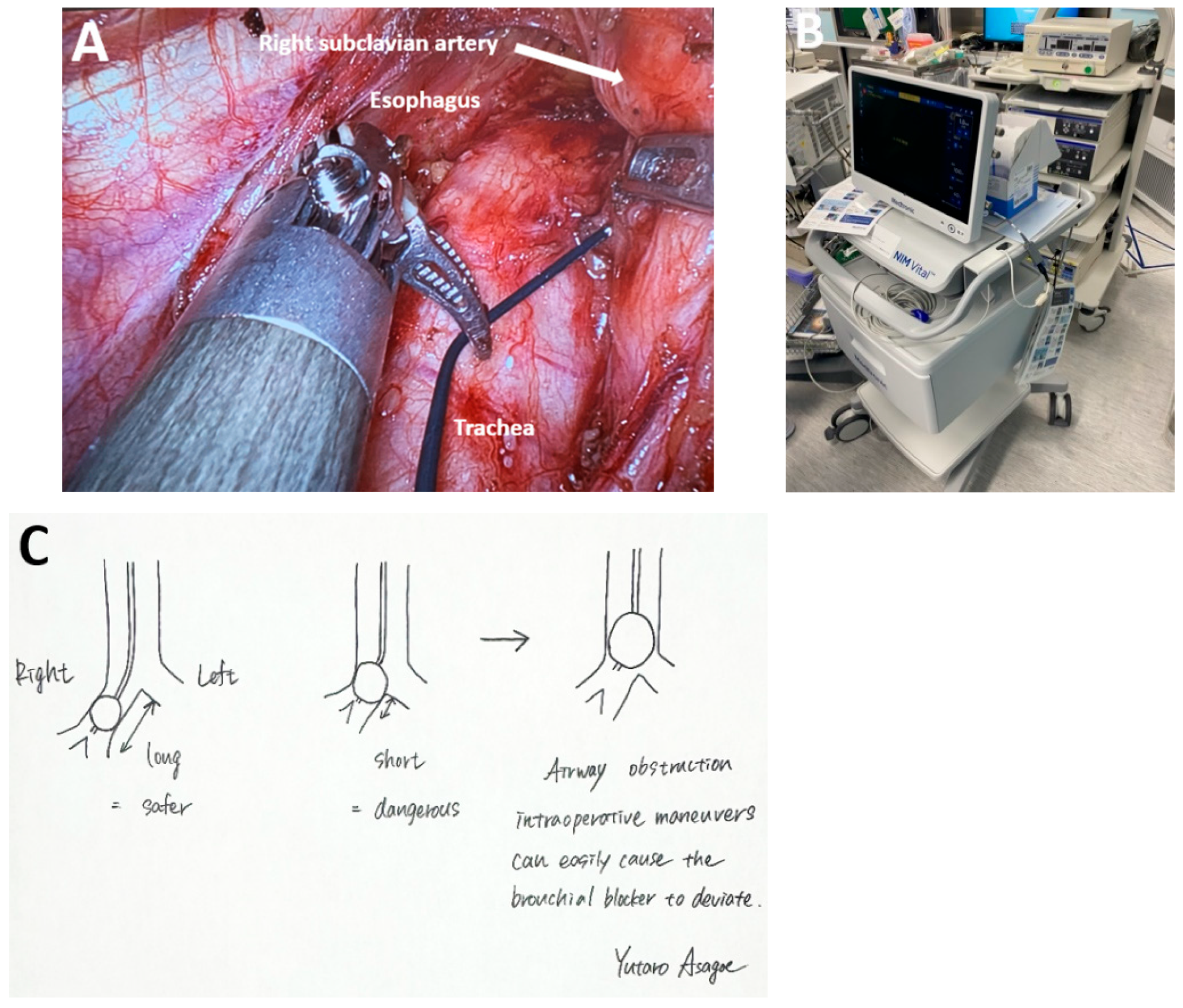
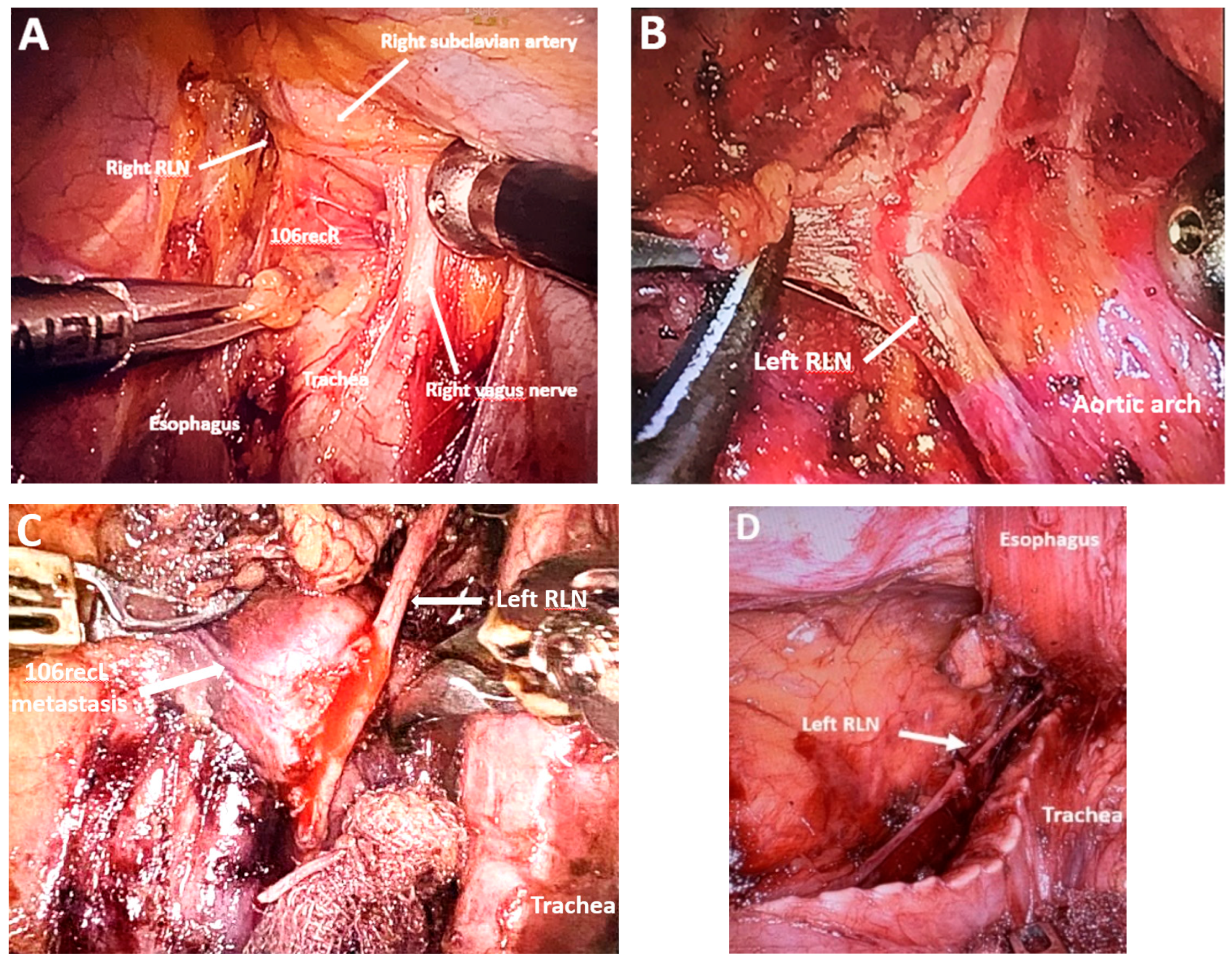
Reducing the Risk of RLN Damage
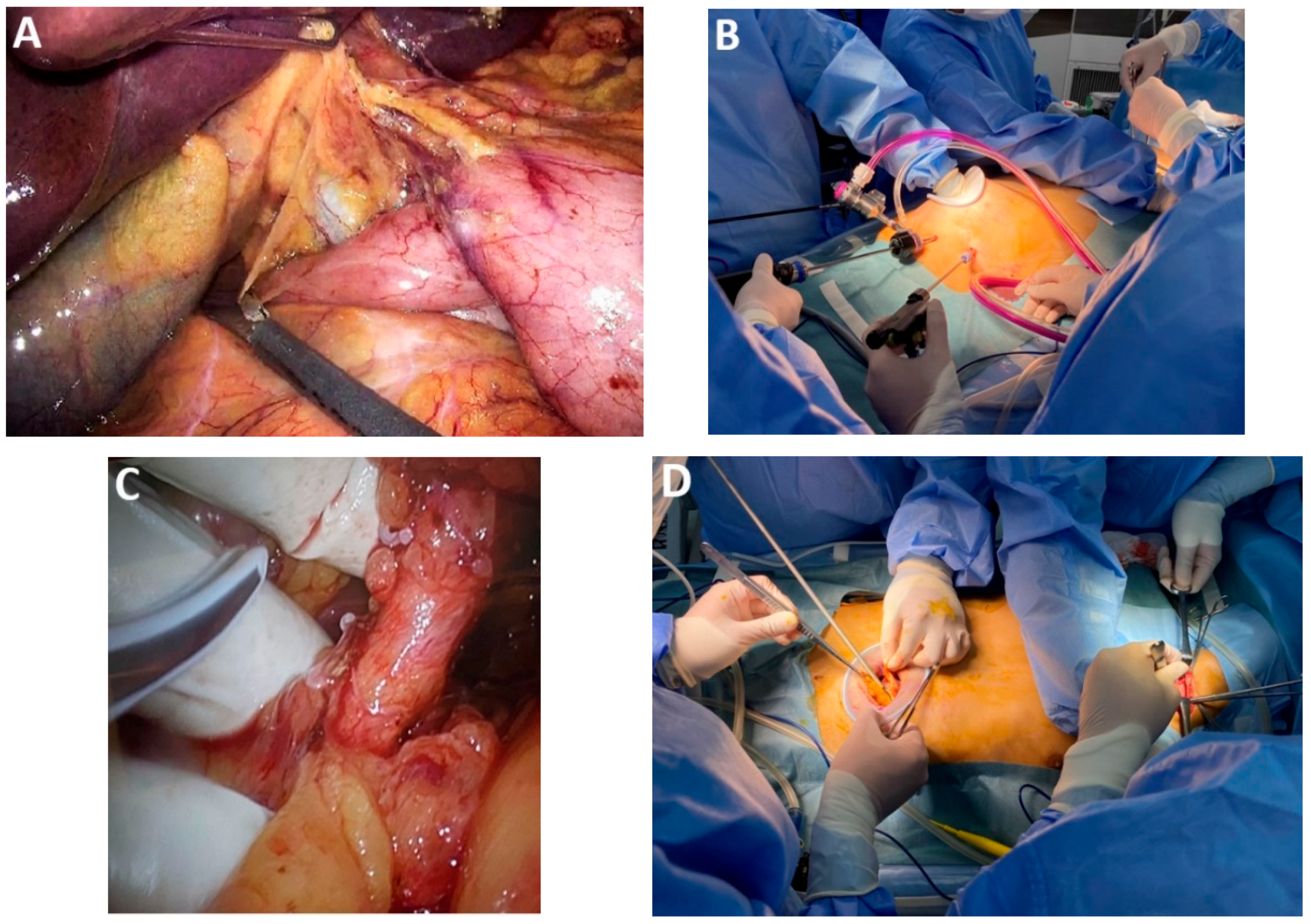
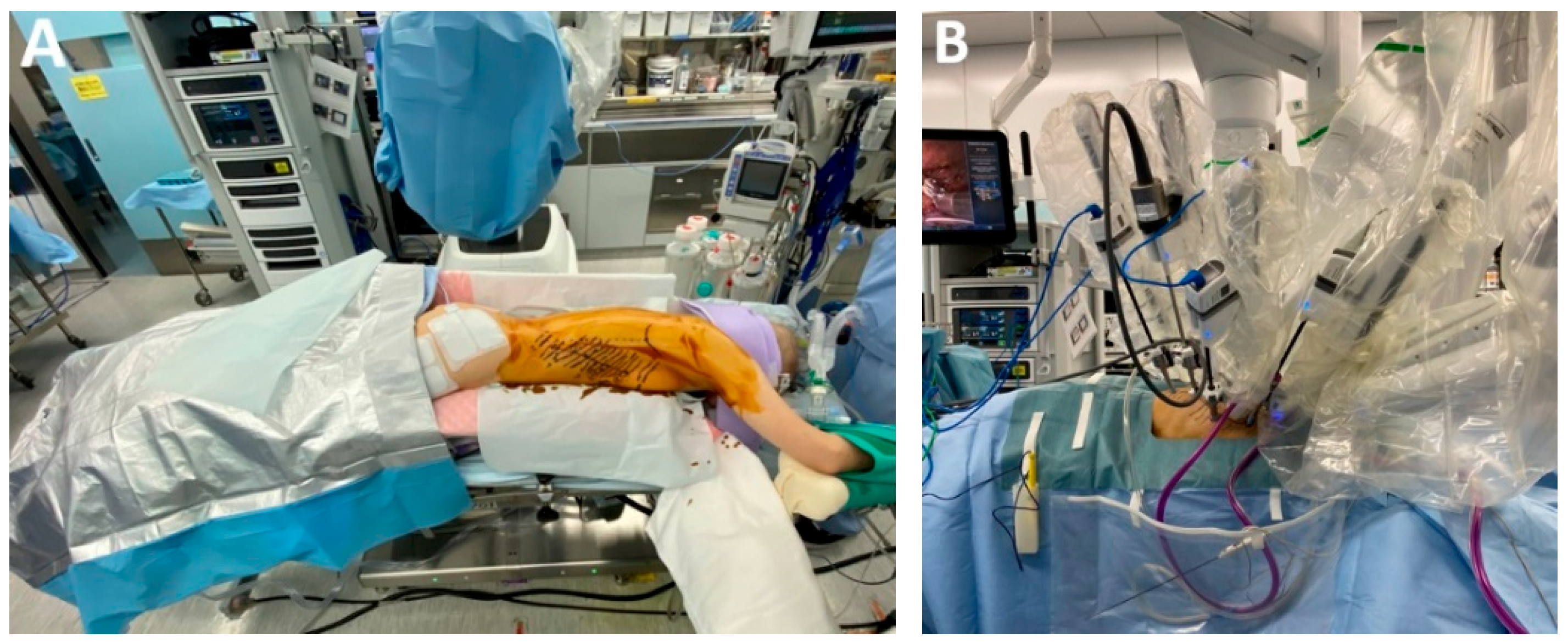
4.7. Subclass 2B: Transthoracic Robotic-Assisted Esophagectomy
4.8. Subclass 2C Transcervical Approach
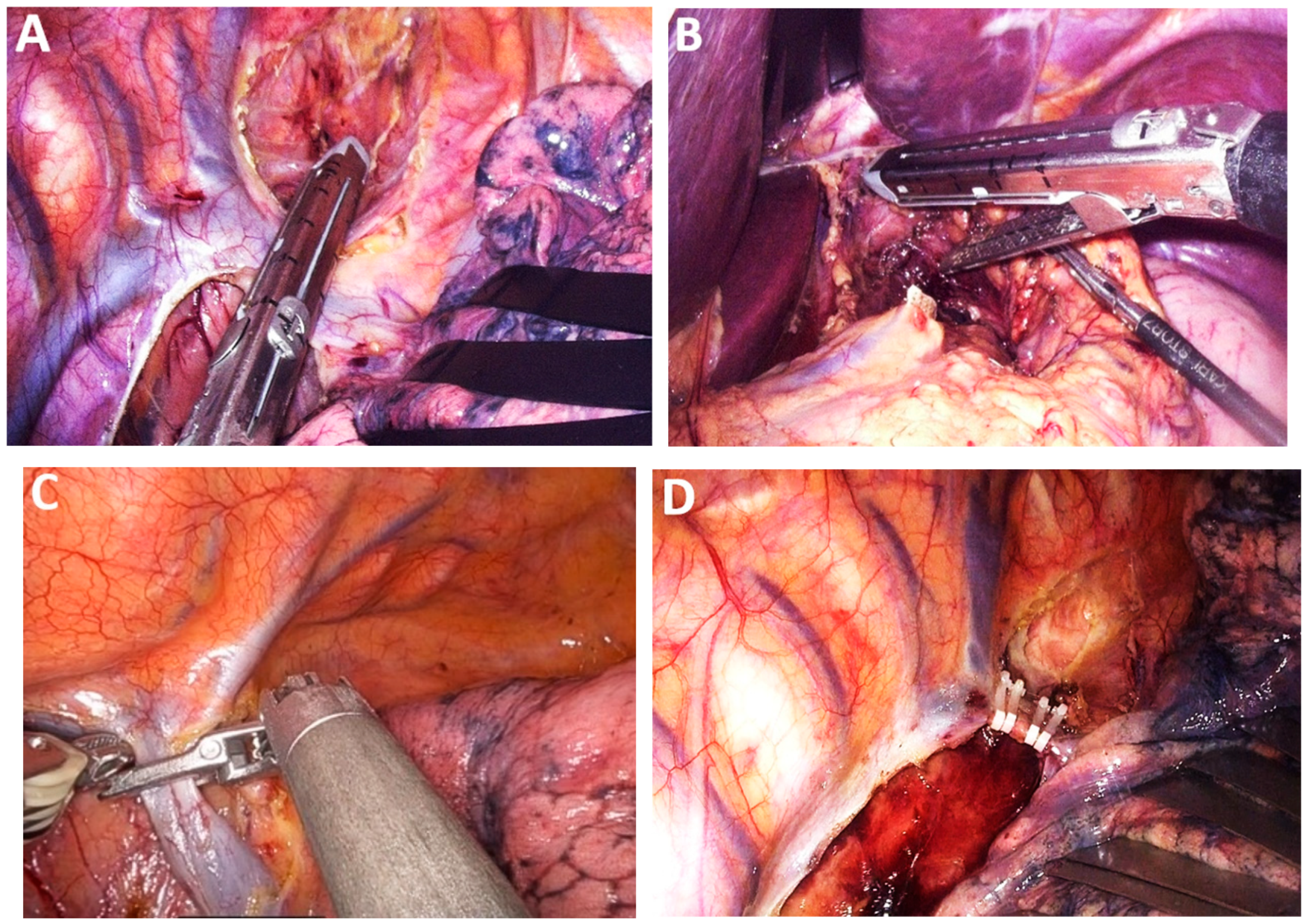
4.9. Class 3A—Lymph Node Dissection
4.10. Subclass 3A Mediastinal Lymphadenectomy
Advances in Mediastinal Lymphadenectomy
4.11. Subclass 4B Abdominal Lymph Node Resection
4.12. Subclass 4C Cervical Lymph Node Resection
4.13. Class 4—Advances in Esophageal Reconstruction
4.14. Subclass 4A Esophageal Reconstruction with Stomach
4.15. Subclass 4B Esophageal Reconstruction with Colon
4.16. Subclass 4C Ascension Route
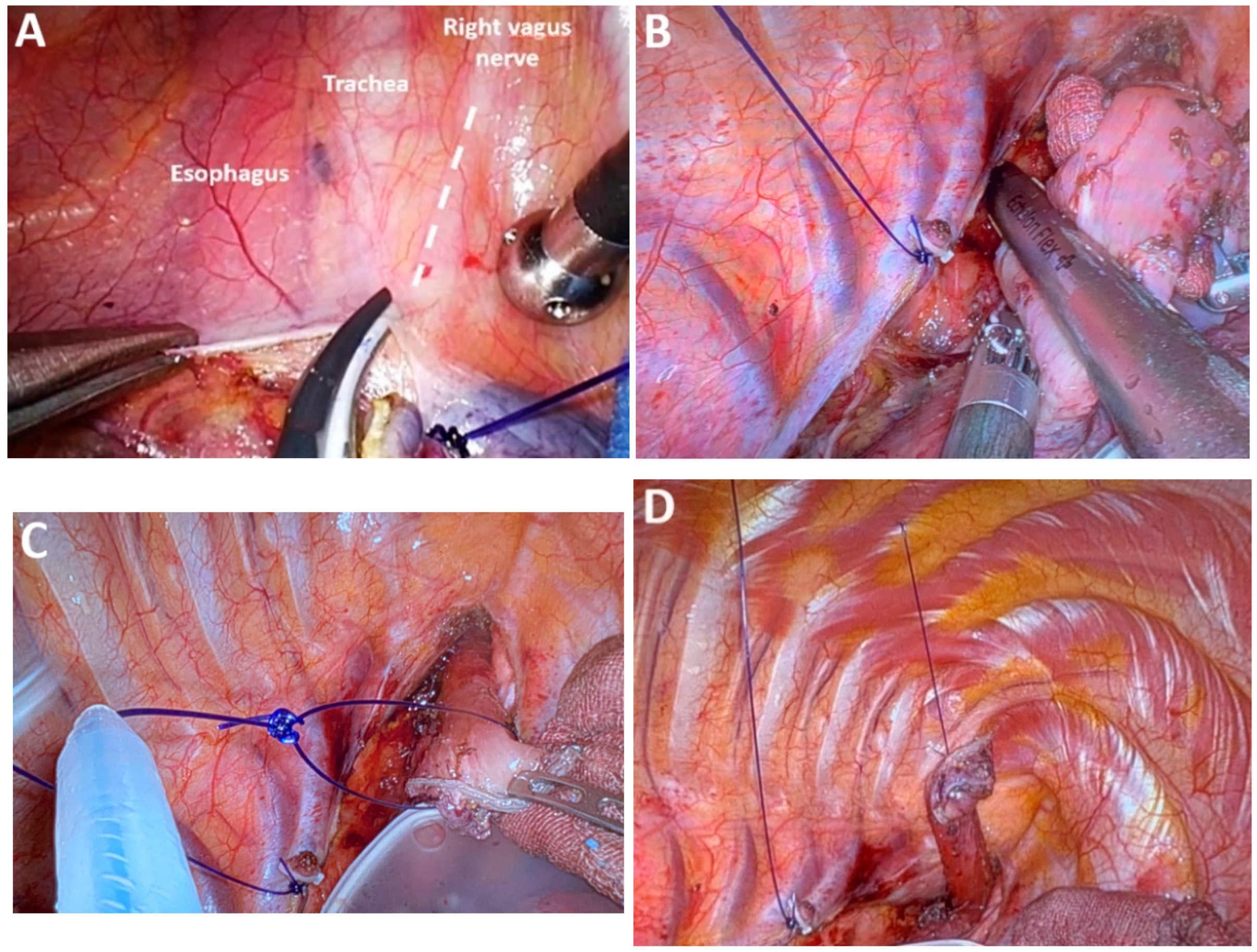
4.17. Class 5 Advances in Performing Esophagogastric Anastomosis
4.18. Subclass 5A Hand-Sewn Esophagogastric Anastomosis
4.19. Subclass 5B Mechanical Circular Anastomosis
4.20. Subclass 5C Mechanical Linear Anastomosis
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MIE | Minimally invasive esophagectomy |
| RAMIE | Robotic-assisted minimally invasive esophagectomy |
| MICE | Minimally invasive transcervical esophagectomy |
| EC | Esophageal cancer |
| RLN | Recurrent laryngeal nerves |
| ICG | Indocyanine green |
| ICG-TBF | Tracheobronchial fluorescence |
| AI | Artificial intelligence |
| TD | Thoracic duct |
| HSI | Hyperspectral imaging |
| OXEI | Oxygen saturation endoscopic imaging |
| RATME Robot- | Assisted Transcervical Esophagectomy |
| 2FL | Two-field lymphadenectomy |
| 3FL | Three-field Lymphadenectomy |
References
- International Agency for Research on Cancer—IARC; World Health Organization. GLOBOCAN 2022. Available online: http://gco.iarc.fr/today (accessed on 1 July 2025).
- Waters, J.K.; Reznik, S.I. Update on Management of Squamous Cell Esophageal Cancer. Curr. Oncol. Rep. 2022, 24, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Hauge, T.; Førland, D.T.; Johannessen, H.O.; Johnson, E. Short- and long-term outcomes in patients operated with total minimally invasive esophagectomy for esophageal cancer. Dis. Esophagus 2021, 7, doab061. [Google Scholar] [CrossRef] [PubMed]
- Müller-Stich, B.P.; Probst, P.; Nienhüser, H.; Fazeli, S.; Senft, J.; Kalkum, E.; Heger, P.; Warschkow, R.; Nickel, F.; Billeter, A.T.; et al. Meta-analysis of randomized controlled trials and individual patient data comparing minimally invasive with open oesophagectomy for cancer. Br. J. Surg. 2021, 108, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Helminen, O.; Kauppila, J.H.; Saviaro, H.; Yannopoulos, F.; Meriläinen, S.; Koivukangas, V.; Huhta, H.; Mrena, J.; Saarnio, J.; Sihvo, E. Minimally invasive esophagectomy learning curves with different types of background experience. J. Thorac. Dis. 2021, 13, 6261–6271. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Büdeyri, I.; El-Sourani, N.; Eichelmann, A.K.; Merten, J.; Juratli, M.A.; Pascher, A.; Hoelzen, J.P. Caseload per Year in Robotic-Assisted Minimally Invasive Esophagectomy: A Narrative Review. Cancers 2024, 16, 3538. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Murashima, Y.; Yamamoto, S.; Hirose, T.; Kadono, T.; Ikeda, G.; Ohara, A.; Itoyama, M.; Yokoyama, K.; Honma, Y.; Ishiyama, K.; et al. Efficacy and Safety of Salvage-line Nivolumab Monotherapy for Advanced Esophageal Squamous Cell Carcinoma: Comparison of 240 mg Versus 480 mg Doses. J. Gastrointest. Cancer 2024, 55, 1345–1351. [Google Scholar] [CrossRef] [PubMed]
- Sihag, S.; Ku, G.Y.; Tan, K.S.; Nussenzweig, S.; Wu, A.; Janjigian, Y.Y.; Jones, D.R.; Molena, D. Safety and feasibility of esophagectomy following combined immunotherapy and chemoradiotherapy for esophageal cancer. J. Thorac. Cardiovasc. Surg. 2021, 161, 836–843.e1. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, H.; Jin, D.; Wang, Q.; Cui, Z.; Zhang, L.; Wei, Y. Perioperative safety and short-term efficacy of functional minimally invasive esophagectomy. J. Int. Med. Res. 2021, 49, 3000605211010081. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Achim, F.; Gheorghe, M.; Constantin, A.; Hoara, P.; Popa, C.; Alkadour, A.; Vergu, I.; Birceanu, A.; Constantinoiu, S. Totally minimally invasive esophagectomy 3D HD for thoracic esophageal cancer after neoadjuvant chemoradiotherapy. J. Surg. Sci. 2018, 5, 133–145, 2360-30-38. [Google Scholar] [CrossRef]
- Bao, T.; Wang, Y.J.; Li, K.K.; Zhao, X.L.; Liu, B.; He, X.D.; Xie, X.F.; Zhang, L.; Li, K.L.; Guo, W. Safety and feasibility of three-dimensional McKeown minimally invasive esophagectomy. Surg. Endosc. 2023, 37, 6908–6914. [Google Scholar] [CrossRef] [PubMed]
- Kudo, T.; Oshikiri, T.; Takiguchi, G.; Urakawa, N.; Hasegawa, H.; Yamamoto, M.; Kanaji, S.; Matsuda, Y.; Yamashita, K.; Matsuda, T.; et al. Three-dimensional visualization system is one of the factors that improve short-term out-comes after minimally invasive esophagectomy. Langenbecks Arch. Surg. 2021, 406, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Singla, V.; Bhattacharjee, H.K.; Gupta, E.; Singh, D.; Mishra, A.K.; Kumar, D. Performance of three-dimensional and ultra-high-definition (4K) technology in laparoscopic surgery: A systematic review and meta-analysis. J. Minim. Access Surg. 2022, 18, 167–175. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shirakawa, Y.; Noma, K.; Maeda, N.; Tanabe, S.; Sakurama, K.; Fujiwara, T. Standardization of bilateral upper mediastinal lymph node dissection using microanatomical concepts in minimally invasive esophagectomy. Mini-Invasive Surg. 2020, 4, 33. [Google Scholar] [CrossRef]
- Kikuchi, H.; Hiramatsu, Y.; Matsumoto, T.; Soneda, W.; Kawata, S.; Hirotsu, A.; Kamiya, K.; Takeuchi, H. The hybrid position is superior to the prone position for thoracoscopic esophagectomy with upper mediastinal lymphadenectomy. Ann. Laparosc. Endosc. Surg. 2020, 5, 13. [Google Scholar] [CrossRef]
- Slooter, M.D.; de Bruin, D.M.; Eshuis, W.J.; Veelo, D.P.; van Dieren, S.; Gisbertz, S.S.; van Berge Henegouwen, M.I. Quantitative fluorescence-guided perfusion assessment of the gastric conduit to predict anastomotic complications after esophagectomy. Dis. Esophagus 2021, 34, doaa100. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Selvens, L.; Surendran, S.; Abraham, V.P.; Paul, N.; Arelly, S.P.D.; Myla, Y.; Samarasam, I. Evaluation of Gastric Conduit Perfusion Using Indocyanine Green Fluorescence During Radical Esophagectomy and Its Correlation With Anastomotic Leak: A Single-Center, Prospective Study. Cureus 2025, 17, e79989. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pollmann, L.; Weberskirch, S.; Petry, M.; Kubasch, S.; Pollmann, N.S.; Bormann, E.; Pascher, A.; Juratli, M.; Hölzen, J.P. Indocyanine green quantification in full robotic esophagectomy using an unsupervised learning approach. Surgery 2025, 184, 109405. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Koyanagi, K.; Ninomiya, Y.; Kazuno, A.; Yamamoto, M.; Shoji, Y.; Yatabe, K.; Kanamori, K.; Tajima, K.; Mori, M. Modification of the lesser curvature incision line enhanced gastric conduit perfusion as determined by indocyanine green fluorescence imaging and decreased the incidence of anastomotic leakage following esophagectomy. Esophagus 2025, 22, 68–76. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Banks, K.C.; Barnes, K.E.; Wile, R.K.; Hung, Y.Y.; Santos, J.; Hsu, D.S.; Choe, G.; Elmadhun, N.Y.; Ashiku, S.K.; Patel, A.R.; et al. Outcomes of Anastomotic Evaluation Using Indocyanine Green Fluorescence during Minimally Invasive Esophagectomy. Am. Surg. 2023, 89, 5124–5130. [Google Scholar] [CrossRef] [PubMed]
- Thammineedi, S.R.; Patnaik, S.C.; Nusrath, S.; Naik, V.; Rayani, B.; Ramalingam, P.R.; Vashist, Y.; Shukla, S. Evaluation of indocyanine green tracheobronchial fluorescence (ICG-TBF) via nebulization during minimally invasive esophagectomy. Dis. Esophagus 2024, 37, doad059. [Google Scholar] [CrossRef] [PubMed]
- Somashekhar, S.P.; Saldanha, E.; Kumar, R.; Monteiro, A.; Pillarisetti, S.R.; Ashwin, K.R. A comparative study of indocyanine green instillation in inguinal node versus foot web space using da Vinci indocyanine green FireFly™ technology in identifying thoracic duct during robotic-assisted transthoracic oesophagectomy. J. Minim. Access Surg. 2024, 20, 271–277. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Peristeri, D.V.; Baltatzis, M. Real-Time Fluorescence Imaging for Thoracic Duct Identification during Oesophagectomy: A Systematic Review of the Literature. J. Chest Surg. 2025, 58, 5–14. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Barnes, T.G.; MacGregor, T.; Sgromo, B.; Maynard, N.D.; Gillies, R.S. Near infra-red fluorescence identification of the thoracic duct to prevent chyle leaks during oesophagectomy. Surg. Endosc. 2022, 36, 5319–5325. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Müller, D.T.; Schiffmann, L.M.; Reisewitz, A.; Chon, S.H.; Eckhoff, J.A.; Babic, B.; Schmidt, T.; Schröder, W.; Bruns, C.J.; Fuchs, H.F. Mapping the Lymphatic Drainage Pattern of Esophageal Cancer with Near-Infrared Fluorescent Imaging during Robotic Assisted Minimally Invasive Ivor Lewis Esophagectomy (RAMIE)-First Results of the Prospective ESOMAP Feasibility Trial. Cancers 2023, 15, 2247. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ilgen, A.; Köhler, H.; Pfahl, A.; Stelzner, S.; Mehdorn, M.; Jansen-Winkeln, B.; Gockel, I.; Moulla, Y. Intraoperative Laparoscopic Hyperspectral Imaging during Esophagectomy—A Pilot Study Evaluating Esophagogastric Perfusion at the Anastomotic Sites. Bioengineering 2024, 11, 69. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gritsiuta, A.I.; Esper, C.J.; Parikh, K.; Parupudi, S.; Petrov, R.V. Anastomotic Leak After Esophagectomy: Modern Approaches to Prevention and Diagnosis. Cureus 2025, 17, e80091. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Furube, T.; Takeuchi, M.; Kawakubo, H.; Noma, K.; Maeda, N.; Daiko, H.; Ishiyama, K.; Otsuka, K.; Kishimoto, Y.; Koyanagi, K.; et al. Impact of Artificial Intelligence on the Timing of Recurrent Laryngeal Nerve Recognition during Robot-Assisted Minimally Invasive Esophagectomy. Ann. Surg. Oncol. 2025, 32, 6366–6373. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, J.M.; Jenke, A.C.; Stern, A.; Daum, M.T.J.; Schulze, A.; Younis, R.; Petrynowski, P.; Davitashvili, T.; Vanat, V.; Bhasker, N.; et al. Active learning for extracting surgomic features in robot-assisted minimally invasive esophagectomy: A prospective annotation study. Surg. Endosc. 2023, 37, 8577–8593. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ishige, F.; Nabeya, Y.; Hoshino, I.; Takayama, W.; Chiba, S.; Arimitsu, H.; Iwatate, Y.; Yanagibashi, H. Quantitative Assessment of the Blood Perfusion of the Gastric Conduit by Indocyanine Green Imaging. J. Surg. Res. 2019, 234, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Koyanagi, K.; Kato, F.; Nakanishi, K.; Ozawa, S. Lateral thermal spread and recurrent laryngeal nerve paralysis after minimally invasive esophagectomy in bipolar vessel sealing and ultrasonic energy devices: A comparative study. Esophagus 2018, 15, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Back, K.; Hur, N.; Kim, M.J.; Choe, J.H.; Kim, J.H.; Kim, J.S. A Prospective, Randomized, Controlled Comparative Study of Three Energy Devices in Open Thyroid Surgery: Thunderbeat, Harmonic, and Ligasure. J. Endocr. Surg. 2019, 19, 106–115. [Google Scholar] [CrossRef]
- Hayami, M.; Watanabe, M.; Mine, S.; Imamura, Y.; Okamura, A.; Yuda, M.; Yamashita, K.; Toihata, T.; Shoji, Y.; Ishizuka, N. Lateral thermal spread induced by energy devices: A porcine model to evaluate the influence on the recurrent laryngeal nerve. Surg. Endosc. 2019, 33, 4153–4163. [Google Scholar] [CrossRef] [PubMed]
- Applewhite, M.K.; White, M.G.; James, B.C.; Abdulrasool, L.; Kaplan, E.L.; Angelos, P.; Grogan, R.H. Ultrasonic, bipolar, and integrated energy devices: Comparing heat spread in collateral tissues. J. Surg. Res. 2017, 207, 249–254, 0022-4804. [Google Scholar] [CrossRef]
- Shivdekar, S.; Rojatkar, P.; Cummings, J.F.; Clymer, J.W.; Ricketts, C.D.; Petraiuolo, W.J. Comparison of Circular Staplers with Adjustable Staple Height vs. Fixed Graduated Staple Height. World J. Surg. Surg. Res. 2021, 4, 1344. [Google Scholar]
- Vanstraelen, S.; Coosemans, W.; Depypere, L.; Mandeville, Y.; Moons, J.; Van Veer, H.; Nafteux, P. Real-life introduction of powered circular stapler for esophagogastric anastomosis: Cohort and propensity matched score study. Dis. Esophagus 2023, 36, doac073. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Koji, O.; Masahiko, M.; Tomotake, A.; Masahiro, K.; Rei, K.; Takeshi, Y.; Satoru, G.; Kimiyasu, Y.; Akira, F.; Makoto, W.; et al. The Usefulness of Airseal® Intelligent Flow System in Video Assisted Thoracoscopic Esophagectomy. Nihon Rinsho Geka Gakkai Zasshi. (J. Jpn. Surg. Assoc.) 2015, 76, 1266–1271. [Google Scholar] [CrossRef][Green Version]
- Otsuka, K.; Goto, S.; Ariyoshi, T.; Yamashita, T.; Saito, A.; Kohmoto, M.; Kato, R.; Motegi, K.; Yajima, N.; Murakami, M. Long-Term Outcomes of Carbon Dioxide Insufflation in Thoracoscopic Esophagectomy After Neoadjuvant Chemotherapy for Esophageal Squamous Cell Carcinoma: A Retrospective Cohort Study. Cureus 2024, 16, e65053. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shigeno, T.; Okuno, K.; Ogo, T.; Fujiwara, H.; Tanioka, T.; Kawada, K.; Haruki, S.; Tokunaga, M.; Fushimi, K.; Kinugasa, Y. Intraoperative Recurrent Laryngeal Nerve Monitoring for Esophagectomy: A National Cohort Study. Ann. Thorac. Surg. 2025, 119, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Peyser Cardoso, R.; Agarwal, L.; Cardoso, S.A.; Agarwal, A.; Varshney, V.; Soni, S.; Selvakumar, B.; Varshney, P. Impact of intraoperative recurrent laryngeal nerve monitoring on minimally invasive esophagectomy outcomes for esophageal cancer: A meta-analysis of case-control studies. Dis. Esophagus 2025, 38, doae116. [Google Scholar] [CrossRef] [PubMed]
- Mine, S.; Tanaka, K.; Kawachi, H.; Shirakawa, Y.; Kitagawa, Y.; Toh, Y.; Yasuda, T.; Watanabe, M.; Kamei, T.; Oyama, T.; et al. Japanese Classification of Esophageal Cancer, 12th Edition: Part I. Esophagus 2024, 21, 179–215. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Doki, Y.; Tanaka, K.; Kawachi, H.; Shirakawa, Y.; Kitagawa, Y.; Toh, Y.; Yasuda, T.; Watanabe, M.; Kamei, T.; Oyama, T.; et al. Japanese Classification of Esophageal Cancer, 12th Edition: Part II. Esophagus 2024, 21, 216–269. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Otsuka, K.; Murakami, M.; Goto, S.; Ariyoshi, T.; Yamashita, T.; Saito, A.; Kohmoto, M.; Kato, R.; Lefor, A.K.; Aoki, T. Minimally invasive esophagectomy and radical lymph node dissection without recurrent laryngeal nerve paralysis. Surg. Endosc. 2020, 34, 2749–2757. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guo, D.; Jiang, Y.; Zhang, Q.; Xing, H.; Wang, Z. A novel technique for lymphadenectomy along the left recurrent laryngeal nerve during minimally invasive esophagectomy: A retrospective cohort study. BMC Surg. 2023, 23, 355. [Google Scholar] [CrossRef]
- Zhu, Z.; Luo, R.; Li, Z.; He, Z.; Xu, Y.; Xu, S.; Yan, P. A comparison of two methods of lymph node dissection along the left recurrent laryngeal nerve in McKeown minimally invasive esophagectomy. J. Gastrointest. Oncol. 2023, 14, 29–39. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nakauchi, M.; Shibasaki, S.; Suzuki, K.; Serizawa, A.; Akimoto, S.; Tanaka, T.; Inaba, K.; Uyama, I.; Suda, K. Robotic esophagectomy with outermost layer-oriented dissection for esophageal cancer: Technical aspects and a retrospective review of a single-institution database. Surg. Endosc. 2023, 37, 8879–8891. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; He, X.D.; He, Y.Q.; Bao, T.; Xie, X.F.; Li, K.K.; Guo, W. Comparison of two different methods for lymphadenectomy along the left recurrent laryngeal nerve by minimally invasive esophagectomy in patients with esophageal squamous cell carcinoma: A prospective randomized trial. Int. J. Surg. 2024, 110, 159–166. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lye, T.J.; Chiu, C.H.; Chao, Y.K. Association between recurrent laryngeal nerve diameter and postoperative palsy following robot-assisted minimally invasive esophagectomy. Dis Esophagus. 2025, 38, doaf048. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Otsuka, K.; Goto, S.; Ariyoshi, T.; Yamashita, T.; Aoki, T. Thoracoscopic and hand assisted laparoscopic esophagectomy with radical lymph node dissection for esophageal squamous cell carcinoma in the left lateral decubitus position: A single center retrospective analysis of 654 patients. BMC Cancer 2017, 17, 748. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zheng, F.; Yang, J.; Zhang, J.; Li, J.; Fang, W.; Chen, M. Efficacy and complications of single-port thoracoscopic minimally invasive esophagectomy in esophageal squamous cell carcinoma: A single-center experience. Sci. Rep. 2023, 13, 16325. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aslan, S.; Tiryaki, G.G.; Pashayev, J.; Cetinkaya, C.; Durusoy, A.F.; Ermerak, N.O.; Batirel, H.F. Uniportal video-assisted thoracoscopic surgery esophagectomy outcomes in 40 consecutive patients. Interdiscip. Cardiovasc. Thorac. Surg. 2023, 36, ivad034. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huang, J.; Yu, Y.; Wu, Z.; Wu, C.; Li, J.; Lou, Z.; Wu, M. Thoracoscopic three-port single versus multiple intercostal for radical resection of esophageal cancer: A retrospective analysis. BMC Cancer 2024, 24, 1140. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xiao, X.; Luan, S.Y.; Zhang, S.H.; Shang, Q.X.; Yang, Y.S.; Wen, Y.; Fang, P.H.; Zhou, J.F.; Li, X.K.; Hu, Y.; et al. The comparison of uniportal versus multiportal video-assisted thoracic surgery for esophageal cancer: A propensity-weighted analysis. Surg. Endosc. 2025, 39, 1730–1739. [Google Scholar] [CrossRef] [PubMed]
- Weng, G.; Su, W.; Fiorelli, A.; Lin, Y.; Chen, L.; Zhang, H.; Fang, W. Single-port compared to multi-port video-assisted thoracoscopic esophagectomy: A propensity-matched study. J. Thorac. Dis. 2025, 17, 1626–1635. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tang, H.; Song, Z.; Wei, R.; Yan, K.; Chen, Z.; Huang, K.; Xin, N.; Hirahara, N.; Sarkaria, I.S.; Li, X.; et al. A preliminary study of modified inflatable mediastinoscopic and single-incision plus one-port laparoscopic esophagectomy. J. Thorac. Dis. 2024, 16, 2472–2481. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Matsunaga, T.; Shishido, Y.; Saito, H.; Sakano, Y.; Makinoya, M.; Miyauchi, W.; Shimizu, S.; Miyatani, K.; Kono, Y.; Murakami, Y.; et al. Impact of Robot-Assisted Minimally Invasive Esophagectomy for Esophageal Cancer: A Propensity Score-Matched Short-Term Analysis. Yonago Acta Medica 2023, 66, 239–245. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kuriyama, K.; Okamura, A.; Kanamori, J.; Imamura, Y.; Tamura, M.; Takahashi, N.; Terayama, M.; Kanie, Y.; Maruyama, S.; Watanabe, M. Anatomical factor associated with thoracic procedural difficulty in robot-assisted minimally invasive esophagectomy. Langenbecks Arch. Surg. 2024, 409, 190. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Yue, J.; Shang, X.; Chen, C.; Ma, Z.; Chen, Z.; Zhang, C.; Jiang, H. Learning Curve of Robot-Assisted Lymph Node Dissection of the Left Recurrent Laryngeal Nerve: A Retrospective Study of 417 Patients. Ann. Surg. Oncol. 2023, 30, 3991–4000. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Tamura, T.; Miki, Y.; Nishi, S.; Miyamoto, H.; Ishidate, T.; Kasashima, H.; Fukuoka, T.; Yoshii, M.; Shibutani, M.; et al. Robot-assisted minimally invasive esophagectomy for esophageal cancer in the left lateral decubitus position. Surg. Endosc. 2024, 38, 7208–7216. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Koterazawa, Y.; Goto, H.; Aoki, T.; Sawada, R.; Ikeda, T.; Harada, H.; Otowa, Y.; Urakawa, N.; Hasegawa, H.; Kanaji, S.; et al. Performing robot-assisted minimally invasive esophagectomy for patients with a narrow mediastinum and left-shifted esophagus for esophageal squamous cell carcinoma presents further challenges. Surg. Endosc. 2025, 39, 4156–4165. [Google Scholar] [CrossRef] [PubMed]
- Têtu, M.; Guimarães Rocha Lima, P.; Castano, W.; Maqueda, L.B.; Ferraro, P.; Nasir, B.; Liberman, M. Outside the Cage (OTC) Non-Intercostal Robotic-Assisted Esophagectomy. Ann. Thorac. Surg. Short Rep. 2024, 3, 216–218. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sato, K.; Fujita, T.; Otomo, M.; Shigeno, T.; Kajiyama, D.; Fujiwara, N.; Daiko, H. Total RAMIE with three-field lymph node dissection by a simultaneous two-team approach using a new docking method for esophageal cancer. Surg. Endosc. 2024, 38, 4887–4893. [Google Scholar] [CrossRef] [PubMed]
- Picozzi, P.; Nocco, U.; Labate, C.; Gambini, I.; Puleo, G.; Silvi, F.; Pezzillo, A.; Mantione, R.; Cimolin, V. Advances in Robotic Surgery: A Review of New Surgical Platforms. Electronics 2024, 13, 4675. [Google Scholar] [CrossRef]
- Puntambekar, S.; Bharambe, S.; Pawar, S.; Chitale, M.; Panse, M. Feasibility of transthoracic esophagectomy with a next-generation surgical robot. Sci. Rep. 2022, 12, 17925. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, J.H.; Gu, B.M.; Song, H.H.; Jang, Y.J.; Kim, H.K. Single-Port Robot-Assisted Minimally Invasive Esophagectomy Using the Single-Port Robotic System via the Subcostal Approach: A Single-Center Retrospective Study. Cancers 2025, 17, 1052. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ebihara, Y.; Hirano, S.; Shichinohe, T.; Morohashi, H.; Oki, E.; Hakamada, K.; Ikeda, N.; Mori, M. Tele-robot-assisted minimally invasive esophagectomy using a double-surgeon cockpit on a cadaver. Surg. Today 2025, 55, 1335–1339. [Google Scholar] [CrossRef] [PubMed]
- Boer, R.B.D.; de Jongh, C.; van Boxel, G.I.; Rouanet, P.; Mourregot, A.; Ruurda, J.P.; van Hillegersberg, R. Feasibility of Telementoring during Robot-Assisted Minimally Invasive Esophagectomy. Dig. Surg. 2025, 42, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lozanovski, V.J.; Hadzijusufovic, E.; Wandhoefer, C.; Gisbertz, S.; Lang, H.; Grimminger, P.P. Hinotori™ robotic esophagectomy: A feasibility cadaver study. Dis. Esophagus 2024, 37, doae091. [Google Scholar] [CrossRef] [PubMed]
- Rivero-Moreno, Y.; Rodriguez, M.; Losada-Muñoz, P.; Redden, S.; Lopez-Lezama, S.; Vidal-Gallardo, A.; Machado-Paled, D.; Cordova Guilarte, J.; Teran-Quintero, S. Autonomous Robotic Surgery: Has the Future Arrived? Cureus 2024, 16, e52243. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khaitan, P.G.; Lazar, J.F.; Margolis, M.; Henderson, H.R.; Watson, T.J. Robotic esophagectomy: How I do it? Mini-Invasive Surg. 2020, 4, 51. [Google Scholar] [CrossRef]
- Kanchodu, S.; Nag, H.H. Laparoscopic-assisted transhiatal oesophagectomy: An experience from a tertiary care centre over 10 years. J. Minimal Access Surg. 2023, 19, 378–383. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, C.L.; Dong, B.; Wu, B.; Li, S.H.; Qi, Y. The application of rigid and flexible mediastinoscopy in esophagectomy: Our experience and a new technology. World J. Surg. Oncol. 2021, 19, 234. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fujiwara, H.; Shiozaki, A.; Konishi, H.; Otsuji, E. Mediastinoscope and laparoscope-assisted esophagectomy. J. Vis. Surg. 2016, 2, 125. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dabsha, A.; Elkharbotly, I.A.M.H.; Yaghmour, M.; Badr, A.; Badie, F.; Khairallah, S.; Esmail, Y.M.; Shmushkevich, S.; Hossny, M.; Rizk, A.; et al. Novel Mediastinoscope-Assisted Minimally Invasive Esophagectomy for Esophageal Cancer: A Systematic Review and Meta-analysis. Ann. Surg. Oncol. 2023, 30, 4030–4039. [Google Scholar] [CrossRef] [PubMed]
- Furuke, H.; Konishi, H.; Fujiwara, H.; Shiozaki, A.; Ohashi, T.; Shimizu, H.; Arita, T.; Yamamoto, Y.; Morimura, R.; Kuriu, Y.; et al. Predictors of the difficulty of transcervical subcarinal lymph node dissection for esophageal cancer. Esophagus 2023, 20, 420–426. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Klarenbeek, B.R.; Fujiwara, H.; Scholte, M.; Rovers, M.; Shiozaki, A.; Rosman, C. Introduction of Minimally Invasive transCervical oEsophagectomy (MICE) according to the IDEAL framework. Br. J. Surg. 2023, 110, 1096–1099. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huang, J.-S.; Lin, W.-W.; Zhong, Q.-H.; Guo, F.-L.; Wu, J.-Y.; Zhang, Z.-Y.; Lin, J.-B. Single-port inflatable mediastinoscopy combined with laparoscopic esophagectomy via right cervical auxiliary operating port and sternal lifting: A safe and reliable surgical method. J. Thorac. Dis. 2025, 17, 1481–1490. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vercoulen, R.J.M.T.; van Veenendaal, L.; Kramer, I.F.; Hutteman, M.; Shiozaki, A.; Fujiwara, H.; Rosman, C.; Klarenbeek, B.R. Minimally Invasive transCervical oEsophagectomy (MICE) for oesophageal cancer: Prospective cohort study (IDEAL stage 2A). Br. J. Surg. 2024, 111, znae160. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Daiko, H.; Ishiyama, K.; Kurita, D.; Kubo, K.; Kubo, Y.; Utsunomiya, D.; Igaue, S.; Nozaki, R.; Akimoto, E.; Kakuta, R.; et al. Bilateral transcervical mediastinoscopic-assisted transhiatal laparoscopic esophagectomy compared with thoracolaparoscopic esophagectomy for esophageal cancer: A propensity score-matched analysis. Surg. Endosc. 2024, 38, 5746–5755. [Google Scholar] [CrossRef] [PubMed]
- Nakauchi, M.; Uyama, I.; Suda, K.; Shibasaki, S.; Kikuchi, K.; Kadoya, S.; Ishida, Y.; Inaba, K. Robot-assisted mediastinoscopic esophagectomy for esophageal cancer: The first clinical series. Esophagus 2019, 16, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Hadzijusufovic, E.; Lozanovski, V.J.; Griemert, E.-V.; Bellaio, L.; Lang, H.; Grimminger, P.P. Single-Port da Vinci Robot–Assisted Cervical Esophagectomy: How to Do It. Thorac. Cardiovasc. Surg. 2024, 72, 654–658. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fujita, T.; Sato, K.; Fujiwara, N.; Kajiyama, D.; Shigeno, T.; Otomo, M.; Daiko, H. Robot-assisted transcervical esophagectomy with a bilateral cervical approach for thoracic esophagectomy. Surg. Endosc. 2024, 38, 1617–1625. [Google Scholar] [CrossRef] [PubMed]
- Ketel, M.H.M.; van der Aa, D.C.; Henckens, S.P.G.; Rosman, C.; van Berge Henegouwen, M.I.; Klarenbeek, B.R.; Gisbertz, S.S.; DES Collaboration Group. Extent and Boundaries of Lymph Node Stations During Minimally Invasive Esophagectomy: A Survey Among Dutch Esophageal Surgeons. Ann. Surg. Oncol. 2024, 31, 5683–5696. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Koterazawa, Y.; Goto, H.; Saiga, H.; Kato, T.; Sawada, R.; Harada, H.; Urakawa, N.; Hasegawa, H.; Kanaji, S.; Yamashita, K.; et al. The number of resected lymph nodes from the upper mediastinal area predicts long-term outcomes of esophageal squamous cell carcinoma after minimally invasive esophagectomy. Surg. Endosc. 2024, 38, 3625–3635. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Fujita, T.; Nakajima, Y.; Okamura, A.; Kawada, K.; Watanabe, M.; Doki, Y. Validation of the cutoff values for the number of metastatic lymph nodes for esophageal cancer staging: A multi-institutional analysis of 655 patients in Japan. Esophagus 2024, 21, 464–471. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, B.; Qi, C.; Li, B.; Liu, Z.; Li, Z.; Li, C. Prognosis of Robot-Assisted Esophagectomy with Thoracic Duct Resection in Esophageal Squamous Cell Carcinoma. Ann. Surg. Oncol. 2025, 32, 5877–5886. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, S.; Takeuchi, H.; Kato, K.; Machida, R.; Ito, Y.; Tsubosa, Y.; Daiko, H.; Koyanagi, K.; Ogata, T.; Fukuda, T.; et al. Prognostic Impact of Thoracic Duct Resection in Patients Who Underwent Transthoracic Esophagectomy Following Neoadjuvant Therapy for Esophageal Squamous Cell Carcinoma: Exploratory Analysis of JCOG1109. Ann. Surg. Oncol. 2025, 32, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Igaue, S.; Fujita, T.; Oguma, J.; Ishiyama, K.; Sato, K.; Kurita, D.; Kubo, Y.; Kubo, K.; Utsunomiya, D.; Akimoto, E.; et al. Oncological outcomes of thoracic duct preservation and resection for esophageal carcinoma based on an understanding of its surgical microanatomy in the era of minimally invasive esophagectomy and neoadjuvant chemotherapy. Eur. J. Surg. Oncol. 2025, 51, 110062. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, H.; Zhang, L.; Jin, D.; Cui, Z.; Cai, R.; Huang, J.; Wei, Y. Two-rope method for dissecting esophagus in McKeown MIE. Front. Surg. 2023, 9, 1031142. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Daiko, H.; Oguma, J.; Ishiyama, K.; Kurita, D.; Kubo, K.; Kubo, Y.; Utsunomiya, D.; Igaue, S.; Nozaki, R.; Leng, X.F.; et al. Technical feasibility and oncological outcomes of robotic esophagectomy compared with conventional thoracoscopic esophagectomy for clinical T3 or T4 locally advanced esophageal cancer: A propensity-matched analysis. Surg. Endosc. 2024, 38, 3590–3601. [Google Scholar] [CrossRef] [PubMed]
- Chao, Y.K.; Li, Z.; Jiang, H.; Wen, Y.W.; Chiu, C.H.; Li, B.; Shang, X.; Fang, T.J.; Yang, Y.; Yue, J.; et al. Multicentre randomized clinical trial on robot-assisted versus video-assisted thoracoscopic oesophagectomy (REVATE trial). Br. J. Surg. 2024, 111, znae143. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lei, J.; Bai, Y.; Qiao, Z.; Ma, J. Robot-assisted minimally invasive esophagectomy versus minimally invasive esophagectomy for thoracic lymph node dissection in patients with squamous cell carcinoma: A retrospective comparative cohort study. J. Thorac. Dis. 2024, 16, 2115–2124. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ekeke, C.N.; Kuiper, G.M.; Luketich, J.D.; Ruppert, K.M.; Copelli, S.J.; Baker, N.; Levy, R.M.; Awais, O.; Christie, N.A.; Dhupar, R.; et al. Comparison of robotic-assisted minimally invasive esophagectomy versus minimally invasive esophagectomy: A propensity-matched study from a single high-volume institution. J. Thorac. Cardiovasc. Surg. 2022, 166, 374–382.e1. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tachimori, Y. Total mesoesophageal esophagectomy. Chin. Med. J. 2014, 127, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.H.; Xu, S.J.; Chen, C.; You, C.X.; Chen, R.Q.; Zhang, Z.F.; Kang, M.Q.; Chen, S.C. Impact of minimally invasive total mesoesophageal excision and minimally invasive esophagectomy on failure patterns of locally advanced esophageal squamous cell carcinoma: A matched cohort study with long-term follow-up. Surg. Endosc. 2023, 37, 7698–7708. [Google Scholar] [CrossRef] [PubMed]
- Cuesta, M.A.; van Jaarsveld, R.C.; Mingol, F.; Bleys, R.L.A.W.; van Hillegersberg, R.; Padules, C.; Bruna, M.; Ruurda, J.P. A novel anatomical description of the esophagus: The supracarinal mesoesophagus. Surg. Endosc. 2023, 37, 6895–6900. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Akagawa, S.; Hosogi, H.; Yoshimura, F.; Kawada, H.; Kanaya, S. Mesenteric excision for esophageal cancer surgery: Based on the concept of mesotracheoesophagus. Int. Cancer Conf. J. 2018, 7, 117–120. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fujiwara, H.; Kanamori, J.; Nakajima, Y.; Kawano, T.; Miura, A.; Fujita, T.; Akita, K.; Daiko, H. An anatomical hypothesis: A “concentric-structured model” for the theoretical understanding of the surgical anatomy in the upper mediastinum required for esophagectomy with radical mediastinal lymph node dissection. Dis. Esophagus 2019, 32, doy119. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, H.; Kitagawa, Y. Sentinel node navigation surgery in esophageal cancer. Ann. Gastroenterol. Surg. 2018, 3, 7–13. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Qi, F.Q.; Sun, Y. Efficacy and prognostic analysis of carbon nanotracers combined with the da Vinci robot in the treatment of esophageal cancer. World J. Clin. Cases 2024, 12, 4924–4931. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kalayarasan, R.; Sai Krishna, P. Robotic esophagectomy with function-preserving radical mediastinal lymphadenectomy for esophageal cancer. Ann. Gastroenterol. Surg. 2024, 9, 12–23. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sun, Z.F.; Fan, B.S.; Liu, J.Q.; Di, S.Y.; Yue, C.Y.; Zhao, J.H.; Wang, J.S.; Song, W.A.; Lu, J.; Zhang, J.L.; et al. Short-term outcomes of three- and two-field lymphadenectomy with minimally invasive esophagectomy for esophageal cancer: A propensity score-matching analysis. Transl. Cancer Res. 2024, 13, 3437–3445. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nguyen, A.T.; Pham, V.H.; Tran, M.T.; Nguyen, P.N.D. Lymph node metastases status in esophageal squamous cell carcinoma following neoadjuvant chemoradiotherapy: A single-center cross-sectional study. Transl. Gastroenterol. Hepatol. 2025, 10, 8. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mao, Y.; Liu, S.; Han, Y.; Guo, S.; Chen, C.; Gao, S.; Hao, A.; Duan, H.; Fang, W.; Zhang, R.; et al. Three-field vs two-field lymphadenectomy in thoracic ESCC patients: A multicenter randomized study (NST 1503). J. Natl. Cancer Cent. 2025, 5, 203–211. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- van der Horst, S.; Weijs, T.J.; Braunius, W.W.; Mook, S.; Mohammed, N.H.; Brosens, L.; van Rossum, P.S.N.; Weusten, B.L.A.M.; Ruurda, J.P.; van Hillegersberg, R. Safety and Feasibility of Robot-Assisted Minimally Invasive Esophagectomy (RAMIE) with Three-Field Lymphadenectomy and Neoadjuvant Chemoradiotherapy in Patients with Resectable Esophageal Cancer and Cervical Lymph Node Metastasis. Ann. Surg. Oncol. 2023, 30, 2743–2752. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.; Sun, Z.; Lu, J.; Liu, J.; Zhao, J.; Zhou, S.; Di, S.; Song, W.; Gong, T. Three-Field Versus Two-Field Lymphadenectomy in Minimally Invasive Esophagectomy: 3-Year Survival Outcomes of a Randomized Trial. Ann. Surg. Oncol. 2023, 30, 6730–6736. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Otsuka, K. Basic Technique of Reconstruction after Esophagectomy by Gastric Tube. Jpn. J. Thorac. Surg. 2019, 72, 869–873. [Google Scholar] [PubMed]
- Pather, K.; Deladisma, A.M.; Guerrier, C.; Kriley, I.R.; Awad, Z.T. Indocyanine green perfusion assessment of the gastric conduit in minimally invasive Ivor Lewis esophagectomy. Surg. Endosc. 2022, 36, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Shan, L.; Peng, C.; Cong, B.; Zhao, X. Learning curve for minimally invasive oesophagectomy of oesophageal cancer and survival analysis. J. Cardiothorac. Surg. 2021, 16, 328. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mingol-Navarro, F.; Ballester-Pla, N.; Jimenez-Rosellon, R. Ischaemic conditioning of the stomach previous to esophageal surgery. J. Thorac. Dis. 2019, 11, S663–S674. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- de Groot, E.M.; Goense, L.; Kingma, B.F.; van den Berg, J.W.; Ruurda, J.P.; van Hillegersberg, R. Implementation of the robotic abdominal phase during robot-assisted minimally invasive esophagectomy (RAMIE): Results from a high-volume center. Surg. Endosc. 2023, 37, 1357–1365. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shin, K.; Kim, I.H.; Jeon, Y.H.; Gong, C.S.; Kim, C.W.; Kim, Y.H. Two Cases of Robot-Assisted Totally Minimally Invasive Esophagectomy with Colon Interposition for Gastroesophageal Junction Cancer: Surgical Considerations. J. Chest Surg. 2024, 57, 323–327. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Long, V.D.; Thong, D.Q.; Hai, N.V.; Dat, T.Q.; Le Minh Quoc, H.; Nguyen, D.T.; Anh, N.V.T.; Minh, T.A.; Vuong, N.L.; So, J.B.; et al. Surgical outcomes and quality of life assessment of esophagectomy for cancer with colon conduit via retrosternal route. Esophagus 2023, 20, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Horikawa, M.; Oshikiri, T.; Kato, T.; Sawada, R.; Harada, H.; Urakawa, N.; Goto, H.; Hasegawa, H.; Kanaji, S.; Yamashita, K.; et al. Efficacy and Postoperative Outcomes of Laparoscopic Retrosternal Route Creation for the Gastric Conduit: Propensity Score–Matched Comparison to Posterior Mediastinal Reconstruction. Ann. Surg. Oncol. 2023, 30, 4044–4053. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, Y.; Otsuka, K.; Yamashita, T.; Saito, A.; Kohmoto, M.; Motegi, K.; Ariyoshi, T.; Goto, S.; Murakami, M.; Aoki, T. The correlation between intrathoracic herniation of the gastric tube and postoperative complications and the efficacy of laparoscopic retrosternal route creation. Esophagus 2025, 22, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Horie, K.; Oshikiri, T.; Kitamura, Y.; Shimizu, M.; Yamazaki, Y.; Sakamoto, H.; Ishida, S.; Koterazawa, Y.; Ikeda, T.; Yamamoto, M.; et al. Thoracoscopic retrosternal gastric conduit resection in the supine position for gastric tube cancer. Asian J. Endosc. Surg. 2020, 13, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, H.; Endo, H.; Yamamoto, H.; Ozawa, S.; Miyata, H.; Kakeji, Y.; Matsubara, H.; Doki, Y.; Kitagawa, Y.; Takeuchi, H. Impact of Reconstruction Route on Postoperative Morbidity After Esophagectomy: Analysis of Esophagectomies in the Japanese National Clinical Database. Ann. Gastroenterol. Surg. 2021, 6, 46–53. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Booka, E.; Takeuchi, H.; Morita, Y.; Hiramatsu, Y.; Kikuchi, H. What is the best reconstruction procedure after esophagectomy? A meta-analysis comparing posterior mediastinal and retrosternal approaches. Ann. Gastroenterol. Surg. 2023, 7, 553–564. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yan, Z.; Xu, X.; Guo, B.; Wang, P.; Niu, L.; Gao, Z.; Yuan, Y.; Li, F.; He, M. A approach of gastric conduit via the anterior of pulmonary hilum route during minimally invasive McKeown esophagectomy. J. Cardiothorac. Surg. 2024, 19, 232. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thomas, P.A. Milestones in the History of Esophagectomy: From Torek to Minimally Invasive Approaches. Medicina 2023, 59, 1786. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- He, X.; Mao, T.; Peng, L.; Wang, S.; Deng, T.; He, W. Redefining Esophagectomy: The Manual Layered Insertion Method That May Reduce Anastomotic Leakage. J. Surg. Res. 2024, 296, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Carr, R.A.; Molena, D. Minimally invasive esophagectomy: Anastomotic techniques. Ann. Esophagus 2021, 4, 19. [Google Scholar] [CrossRef]
- Müller, D.T.; Babic, B.; Herbst, V.; Gebauer, F.; Schlößer, H.; Schiffmann, L.; Chon, S.-H.; Schröder, W.; Bruns, C.J.; Fuchs, H.F. Does Circular Stapler Size in Surgical Management of Esophageal Cancer Affect Anastomotic Leak Rate? 4-Year Experience of a European High-Volume Center. Cancers 2020, 12, 3474. [Google Scholar] [CrossRef]
- Fujimoto, D.; Taniguchi, K.; Takashima, J.; Miura, F.; Kobayashi, H. Hybrid esophagogastric tube anastomosis after minimally invasive McKeown esophagectomy to prevent stenosis in patients with esophageal cancer. Langenbecks Arch. Surg. 2023, 408, 7. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.Y.L.; Goense, L.; Van De Horst, S.; Van Den Berg, J.W.; Ruurda, J.P.; Van Hillegersberg, R. Robotic- assisted minimally invasive Ivor-Lewis handsewn anastomosis technique and outcomes from a large-volume European centre. Dis. Esophagus 2025, 38, doaf019. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wong, L.Y.; Kamtam, D.; Kim, J.; Wallen, B.; Elliott, I.A.; Guenthart, B.A.; Liou, D.Z.; Backhus, L.M.; Berry, M.F.; Shrager, J.B.; et al. Novel Robotic Esophagogastric Anastomosis Simulation Model for Skill Development and Training. Ann. Thorac. Surg. Short Rep. 2024, 3, 206–211. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Takeda, F.R.; Tutihashi, R.; Tustumi, F.; Sallum, R.A.A.; de Freitas Busnardo, F.; Ribeiro, U., Jr.; Cecconello, I. Supercharged cervical anastomosis for esophagectomy and gastric pull-up. J. Thorac. Cardiovasc. Surg. 2021, 162, 688–697.e3. [Google Scholar] [CrossRef] [PubMed]
- Davey, M.G.; Donlon, N.E.; Elliott, J.A.; Robb, W.B.; Bolger, J.C. Optimal oesophagogastric anastomosis techniques for oesophageal cancer surgery—A systematic review and network meta-analysis of randomised clinical trials. Eur. J. Surg. Oncol. 2025, 51, 109600. [Google Scholar] [CrossRef] [PubMed]
- ROMIO Study Group. Laparoscopic or open abdominal surgery with thoracotomy for patients with oesophageal cancer: ROMIO randomized clinical trial. Br. J. Surg. 2024, 111, znae023. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- de Groot, E.M.; Goense, L.; Kingma, B.F.; Haverkamp, L.; Ruurda, J.P.; van Hillegersberg, R. Trends in surgical techniques for the treatment of esophageal and gastroesophageal junction cancer: The 2022 update. Dis. Esophagus 2023, 36, doac099. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Seika, P.; Maurer, M.M.; Winter, A.; Ossami-Saidy, R.R.; Serwah, A.; Ritschl, P.V.; Raakow, J.; Dobrindt, E.; Kurreck, A.; Pratschke, J.; et al. Textbook outcome after robotic and laparoscopic Ivor Lewis esophagectomy is associated with improved survival: A propensity score–matched analysis. J. Thorac. Cardiovasc. Surg. 2025, 169, 1604–1615. [Google Scholar] [CrossRef] [PubMed]
- Bolger, J.C.; Castro, P.P.; Marwah, A.; Tavakoli, I.; Espin-Garcia, O.; Darling, G.E.; Yeung, J.C. Nodal Yield <15 Is Associated With Reduced Survival in Esophagectomy and Is a Quality Metric. Ann. Thorac. Surg. 2023, 116, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Faermark, N.; Fuks, D.; Nassar, A.; Ferraz, J.-M.; Lamer, C.; Lefevre, M.; Gayet, B.; Bonnet, S. Quality of oncological resection criteria in minimally invasive esophagectomy. Surg. Endosc. 2021, 36, 3940–3946. [Google Scholar] [CrossRef] [PubMed]
- Blears, E.; Fernando, H.C.; Shahoud, J.; Weksler, B. Factors associated with access and approach to esophagectomy for cancer: A National Cancer Database study. Surg. Endosc. 2022, 36, 7016–7024. [Google Scholar] [CrossRef] [PubMed]
- Solomon, D.; Sarfaty, E.; Menasherov, N.; Bard, V.; Bueno, R.; Kashtan, H. Implementing the first program of minimally invasive esophagectomy for cancer in Israel: Shifting the paradigm in a high-volume center—A cohort study. Int. J. Surg. 2023, 109, 3467–3475. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Goense, L.; van der Sluis, P.C.; van der Horst, S.; Tagkalos, E.; Grimminger, P.P.; van Dijk, W.; Ruurda, J.P.; van Hillegersberg, R. Cost analysis of robot-assisted versus open transthoracic esophagectomy for resectable esophageal cancer. Results of the ROBOT randomized clinical trial. Eur. J. Surg. Oncol. 2023, 49, 106968. [Google Scholar] [CrossRef] [PubMed]
- Di Bello, F.; Rodriguez Peñarand, N.; Marmiroli, A.; Longoni, M.; Falkenbach, F.; Le, Q.C.; Nicolazzini, M.; Catanzaro, C.; Tian, Z.; Goyal, J.A.; et al. Costs of robot-assisted vs. open approaches for 5 major cancers. J. Robot. Surg. 2025, 19, 205. [Google Scholar] [CrossRef]
- Shimamoto, Y.; Ishihara, R.; Kato, Y.; Shoji, A.; Inoue, T.; Matsueda, K.; Miyake, M.; Waki, K.; Kono, M.; Fukuda, H.; et al. Real-time assessment of video images for esophageal squamous cell carcinoma invasion depth using artificial intelligence. J. Gastroenterol. 2020, 55, 1037–1045. [Google Scholar] [CrossRef] [PubMed]
- Theocharopoulos, C.; Davakis, S.; Ziogas, D.C.; Theocharopoulos, A.; Foteinou, D.; Mylonakis, A.; Katsaros, I.; Gogas, H.; Charalabopoulos, A. Deep Learning for Image Analysis in the Diagnosis and Management of Esophageal Cancer. Cancers 2024, 16, 3285. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Furube, T.; Takeuchi, M.; Kawakubo, H.; Noma, K.; Maeda, N.; Daiko, H.; Ishiyama, K.; Otsuka, K.; Sato, Y.; Koyanagi, K.; et al. Usefulness of an Artificial Intelligence Model in Recognizing Recurrent Laryngeal Nerves During Robot-Assisted Minimally Invasive Esophagectomy. Ann. Surg. Oncol. 2024, 31, 9344–9351. [Google Scholar] [CrossRef] [PubMed]
- Furube, T.; Kawakubo, H.; Takeuchi, M.; Ikeda, J.; Matsuda, S.; Kitagawa, Y. Real-time AI-based detection of excessive traction on the recurrent laryngeal nerve during robotic-assisted minimally invasive esophagectomy: A proof-of-concept study. Int. J. Surg. 2025, 111, 7336–7340. [Google Scholar] [CrossRef] [PubMed]
- Nakanoko, T.; Kimura, Y.; Natsugoe, K.; Nonaka, K.; Nambara, S.; Hu, Q.; Nakanishi, R.; Ota, M.; Oki, E.; Yoshizumi, T. Left recurrent nerve lymph node dissection in robotic esophagectomy for esophageal cancer without esophageal traction. World J. Surg. Oncol. 2023, 21, 223. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hu, Z.H.; Li, R.X.; Wang, J.T.; Wang, G.J.; Deng, X.M.; Zhu, T.Y.; Gao, B.L.; Zhang, Y.F. Thoracolaparoscopic esophagectomy for esophageal cancer with a cervical incision to extract specimen. Asian J. Surg. 2022, 46, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.O.; de Groot, E.M.; Kingma, B.F.; Babic, B.; Ruurda, J.P.; Grimminger, P.P.; Hölzen, J.P.; Chao, Y.K.; Haveman, J.W.; van Det, M.J.; et al. Hybrid laparoscopic versus fully robot-assisted minimally invasive esophagectomy: An international propensity-score matched analysis of perioperative outcome. Surg. Endosc. 2023, 37, 4466–4477. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vincke, A.; Miftode, S.; Alfarawan, F.; Bockhorn, M.; El-Sourani, N. Hybrid Minimally Invasive Esophagectomy vs. Open Esophagectomy: A Retrospective Propensity Score Matched Comparison. Medicina 2023, 59, 434. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lin, C.H.; Chuang, C.Y.; Ko, J.L.; Hsu, C.P. Experiences in reverse sequence esophagectomy: A promising alternative for esophageal cancer surgery. Surg. Endosc. 2023, 37, 6749–6760. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sugita, Y.; Nakamura, T.; Sawada, R.; Takiguchi, G.; Urakawa, N.; Hasegawa, H.; Yamamoto, M.; Kanaji, S.; Matsuda, Y.; Yamashita, K.; et al. Safety and feasibility of minimally invasive esophagectomy for elderly esophageal cancer patients. Dis. Esophagus 2020, 34, doaa083. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Akutsu, Y.; Miyata, H.; Toh, Y.; Toyozumi, T.; Kakeji, Y.; Seto, Y.; Matsubara, H. Essential risk factors for operative mortality in elderly esophageal cancer patients registered in the National Clinical Database of Japan. Esophagus 2023, 20, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Riccio, F.; Klarenbeek, B.R.; Fujiwara, H.; Seto, Y.; Cuesta, M.A.; International CMIE Collaborative Group. Worldwide survey on the transcervical approach for minimally invasive treatment of esophageal cancer: Results of questionnaire of the international collaborative group on transCervical Minimally Invasive Esophagectomy. Dis. Esophagus 2025, 38, doaf035. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hu, W.; Yuan, P.; Yuan, Y.; Chen, L.; Hu, Y. Learning curve for inflatable mediastinoscopic and laparoscopic-assisted esophagectomy. Surg. Endosc. 2023, 37, 4486–4494. [Google Scholar] [CrossRef] [PubMed]
- de Groot, E.M.; Kuiper, G.M.; van der Veen, A.; Fourie, L.; Goense, L.; van der Horst, S.; van den Berg, J.; van Hillegersberg, R.; Ruurda, J.P. Indocyanine green fluorescence in robot-assisted minimally invasive esophagectomy with intrathoracic anastomosis: A prospective study. Updat. Surg. 2023, 75, 409–418. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nguyen, D.T.; Dat, T.Q.; Thong, D.Q.; Hai, N.V.; Bac, N.H.; Long, V.D. Role of indocyanine green fluorescence imaging for evaluating blood supply in the gastric conduit via the substernal route after McKeown minimally invasive esophagectomy. J. Gastrointest. Surg. 2024, 28, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Khaitan, P.G.; Vekstein, A.M.; Thibault, D.; Kosinski, A.; Hartwig, M.G.; Block, M.; Gaissert, H.; Wolf, A.S. Robotic Esophagectomy Trends and Early Surgical Outcomes: The US Experience. Ann. Thorac. Surg. 2023, 115, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Matthée, E.; Ubels, S.; Klarenbeek, B.; Verstegen, M.H.P.; Hannink, G.; van Workum, F.; Rosman, C.; Grutters, J.P.C.; ICAN Collaborative Research Group. Intrathoracic versus cervical anastomosis after totally or hybrid minimally invasive transthoracic oesophagectomy for oesophageal cancer: Cost-effectiveness analysis alongside the randomized ICAN trial. BJS Open 2025, 9, zraf061. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Perez, C.; Weiser, L.; Razavi, A.; Bolster, D.; Fuller, C.; Knabe, K.; Soukiasian, S.; Rocco, R.; Brownlee, A.R.; Soukiasian, H.J. Standardized Approach to Robotic-Assisted Esophagectomy. Ann. Thorac. Surg. Short Rep. 2025, 3, 365–367. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kitagami, H.; Poudel, S.; Kitayama, Y.; Koinuma, J.; Ebihara, Y.; Hirano, S. A standardized protocol for robot-assisted minimally invasive esophagectomy: Improving efficiency and reducing costs. J. Robot. Surg. 2025, 19, 107. [Google Scholar] [CrossRef] [PubMed]
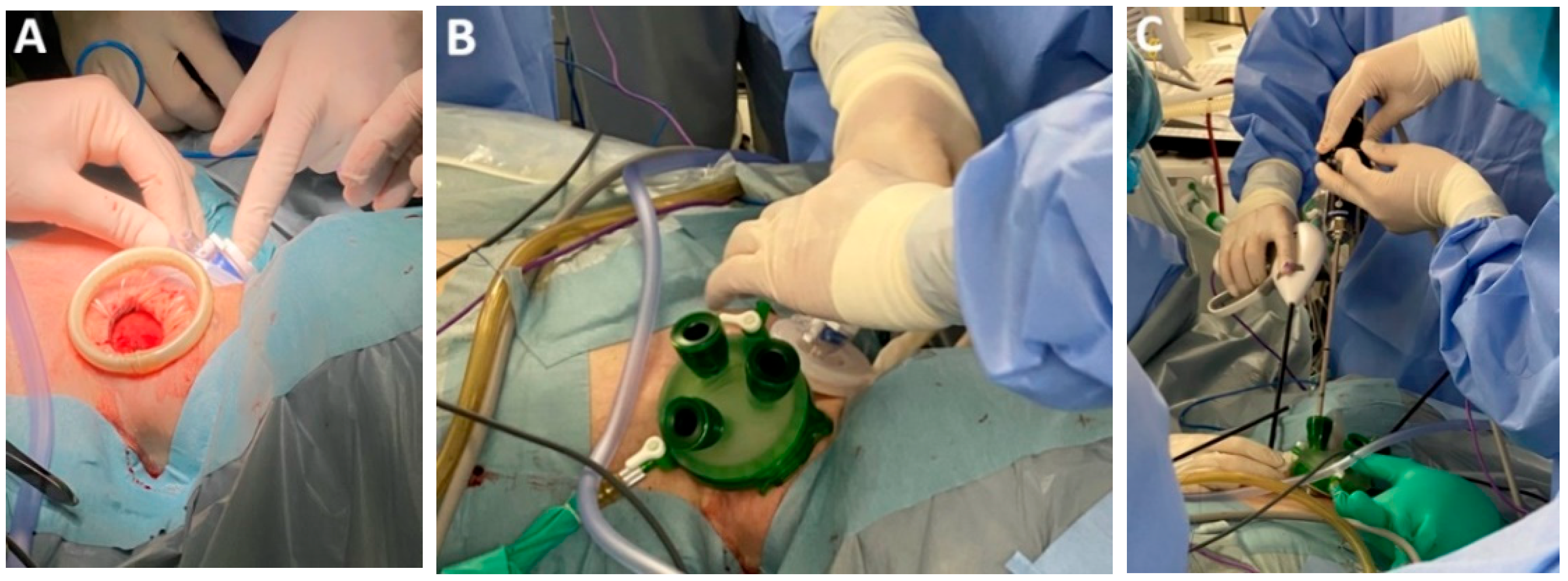
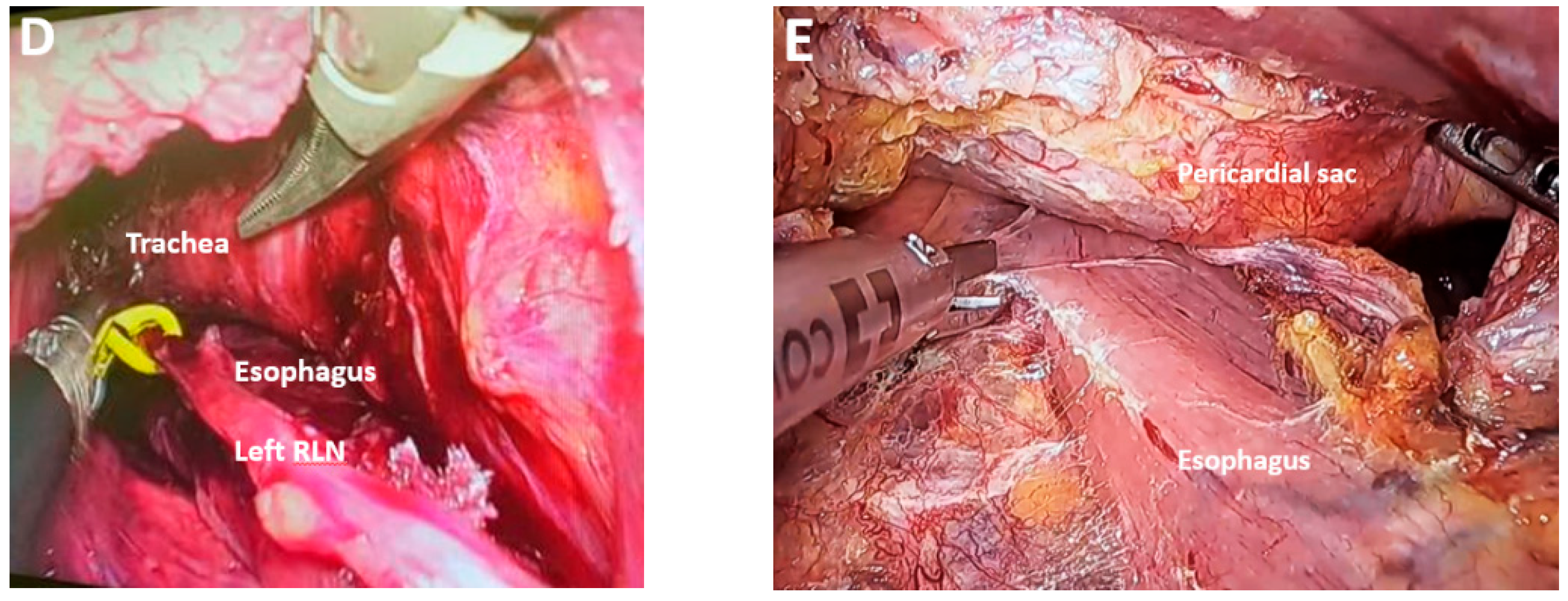
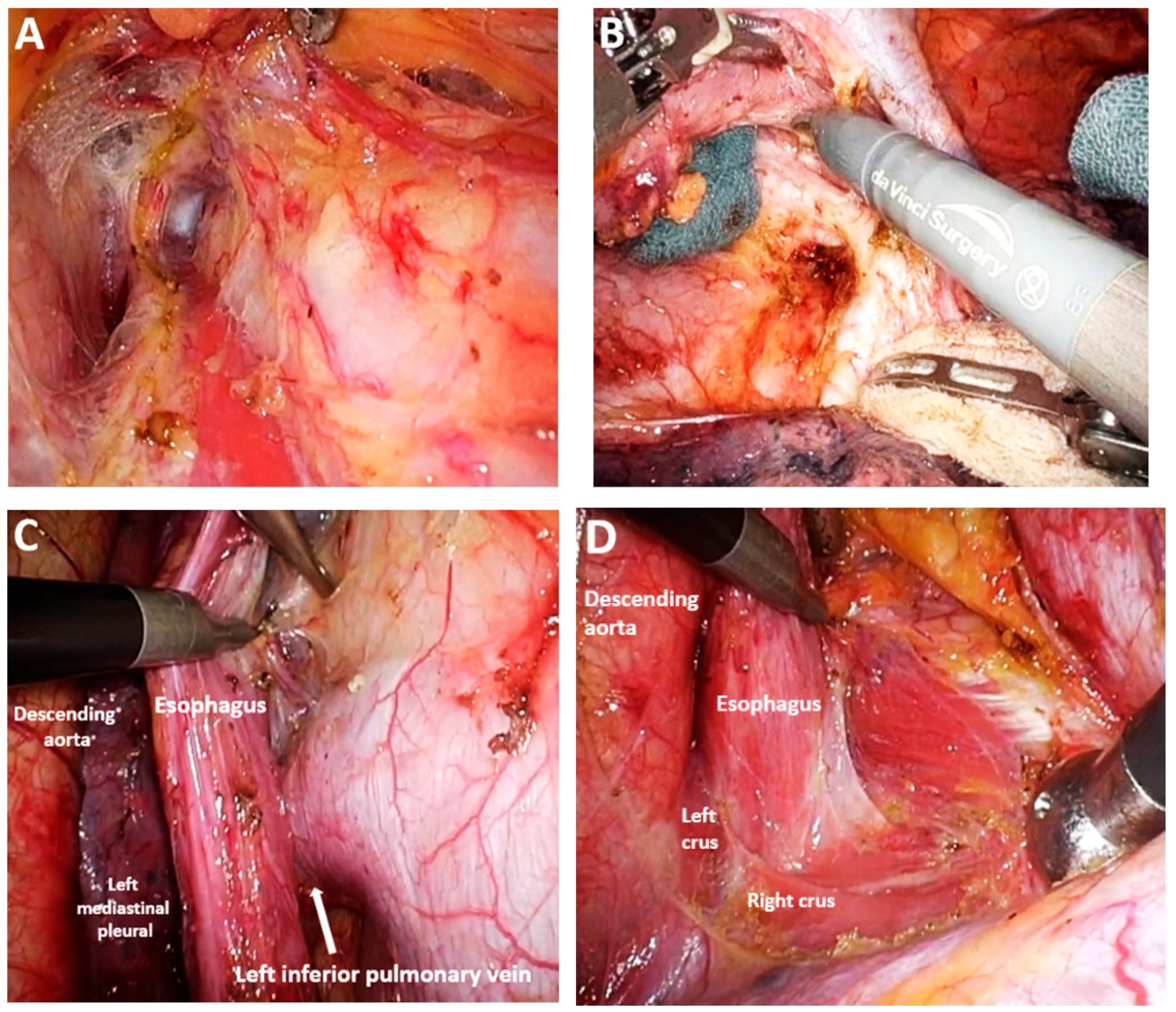
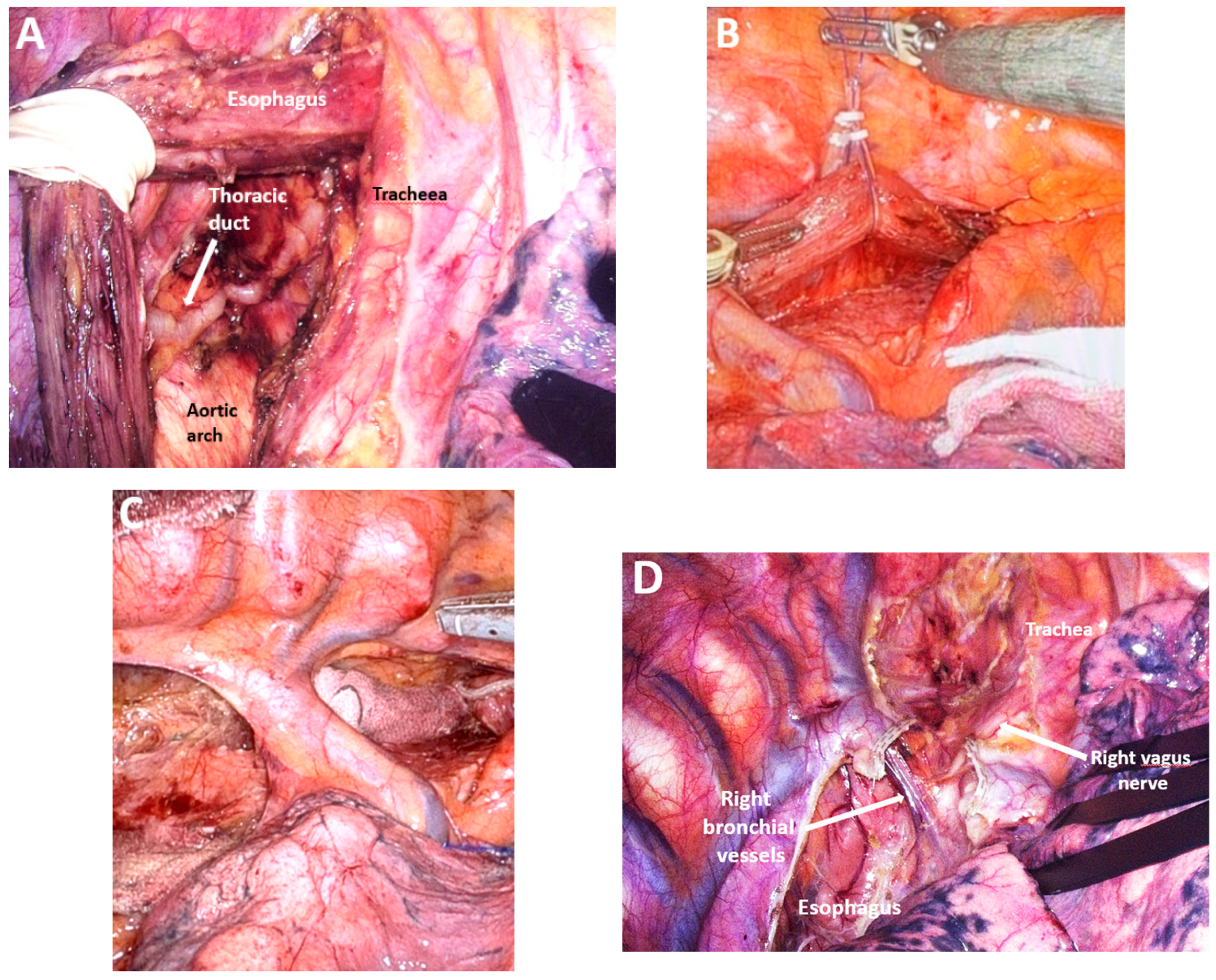
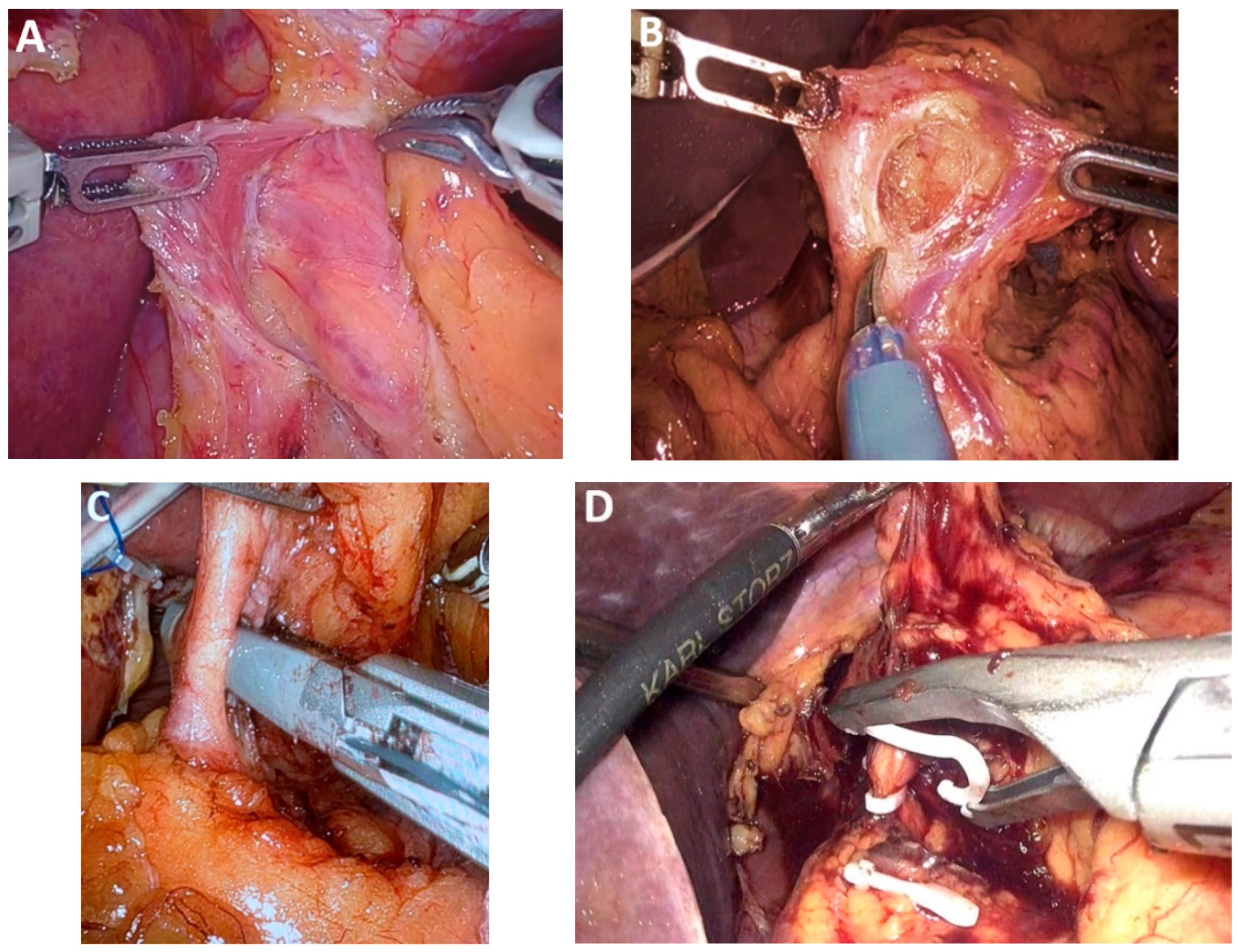
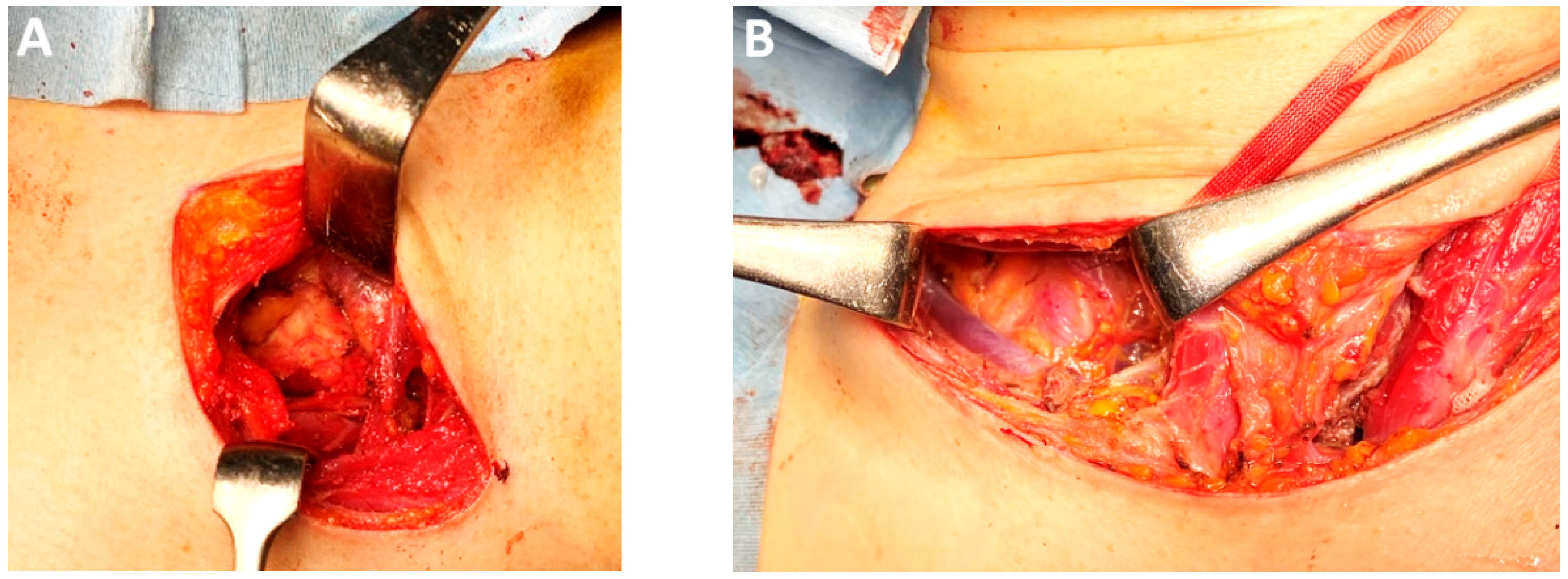
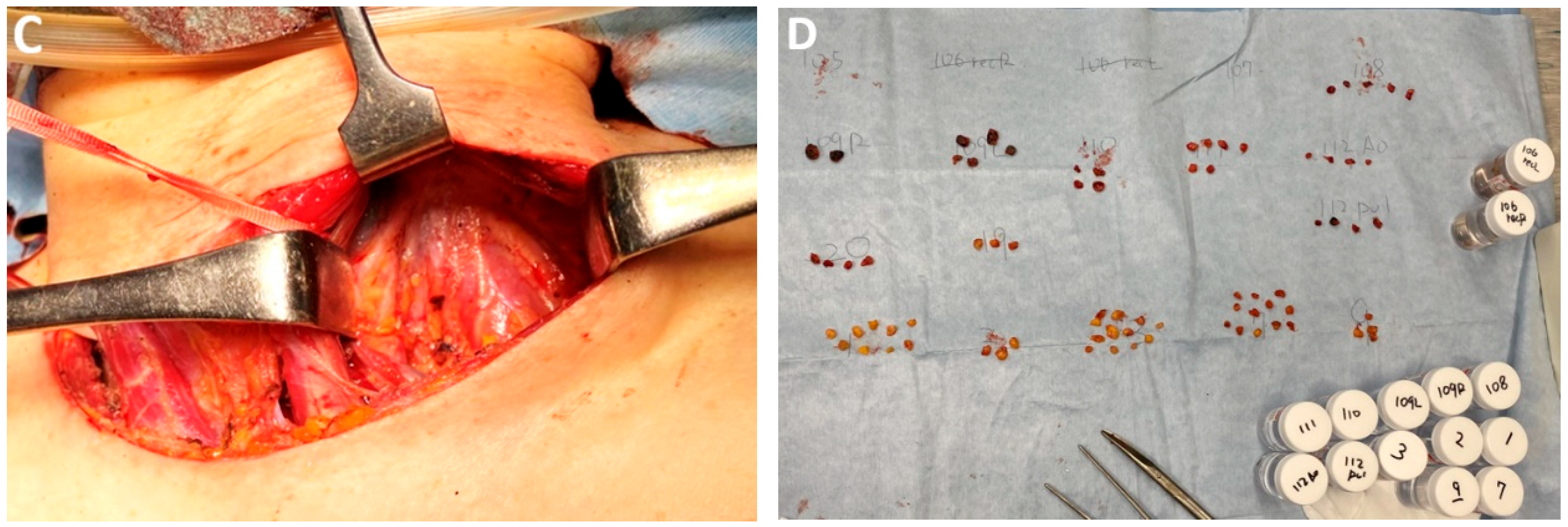
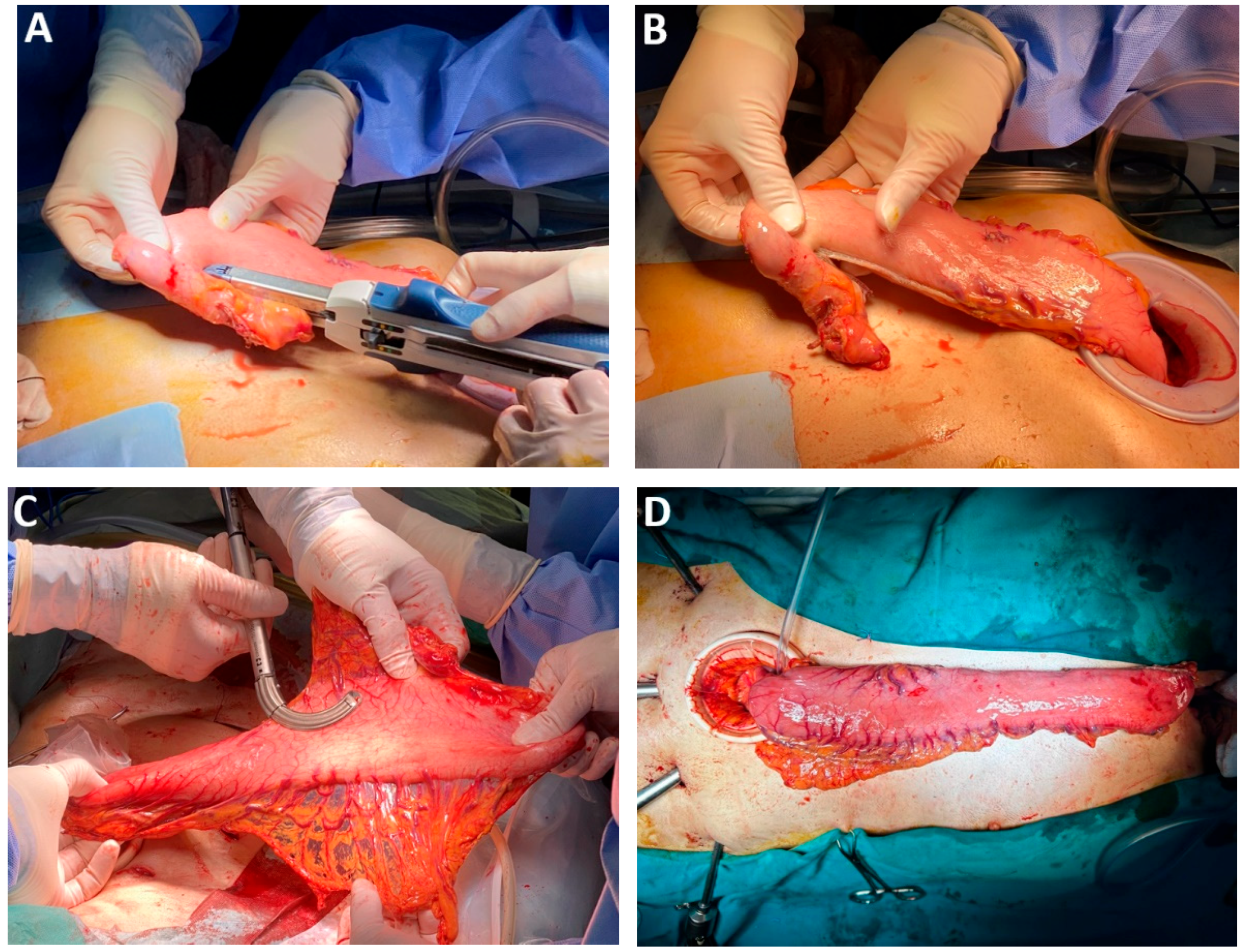
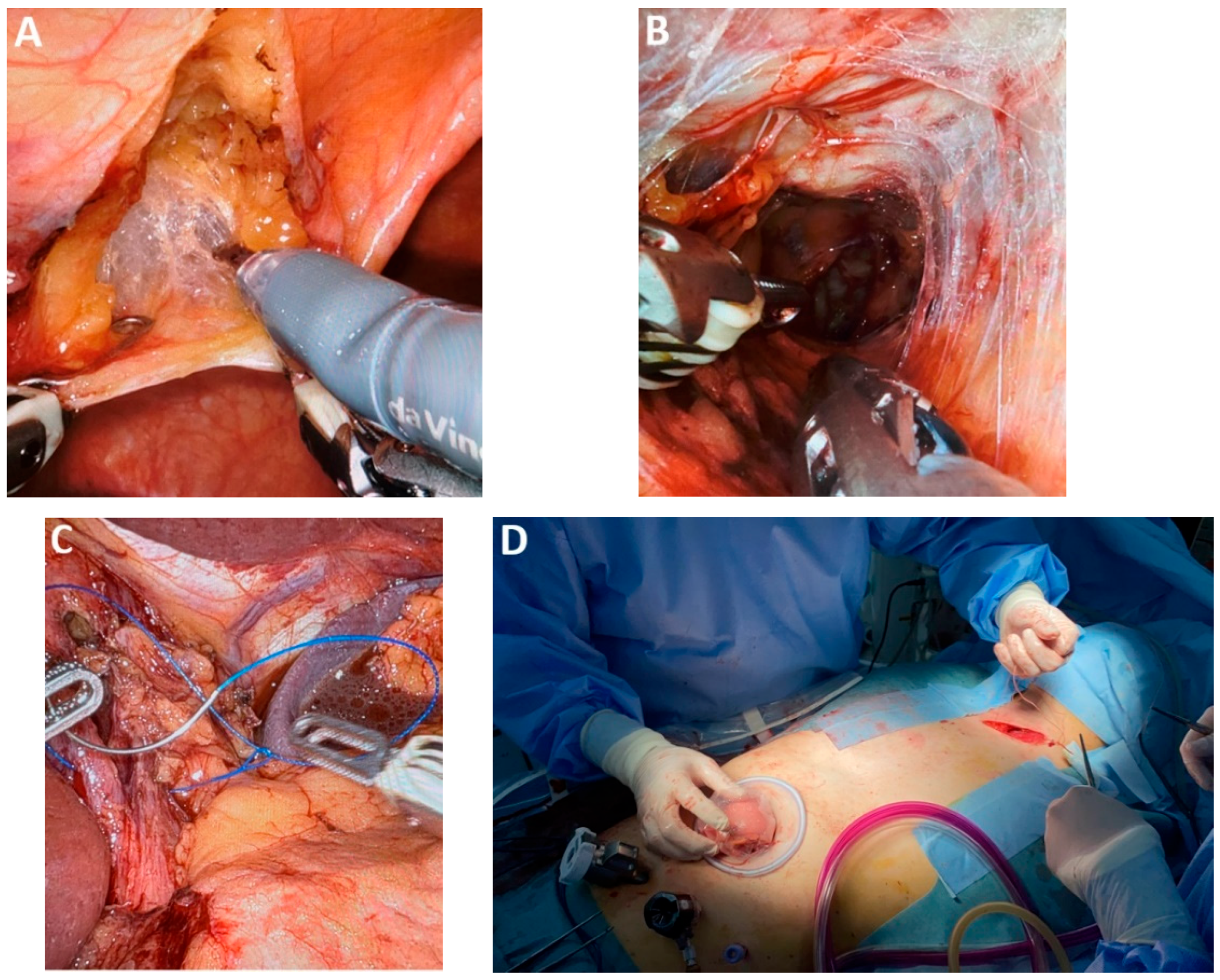
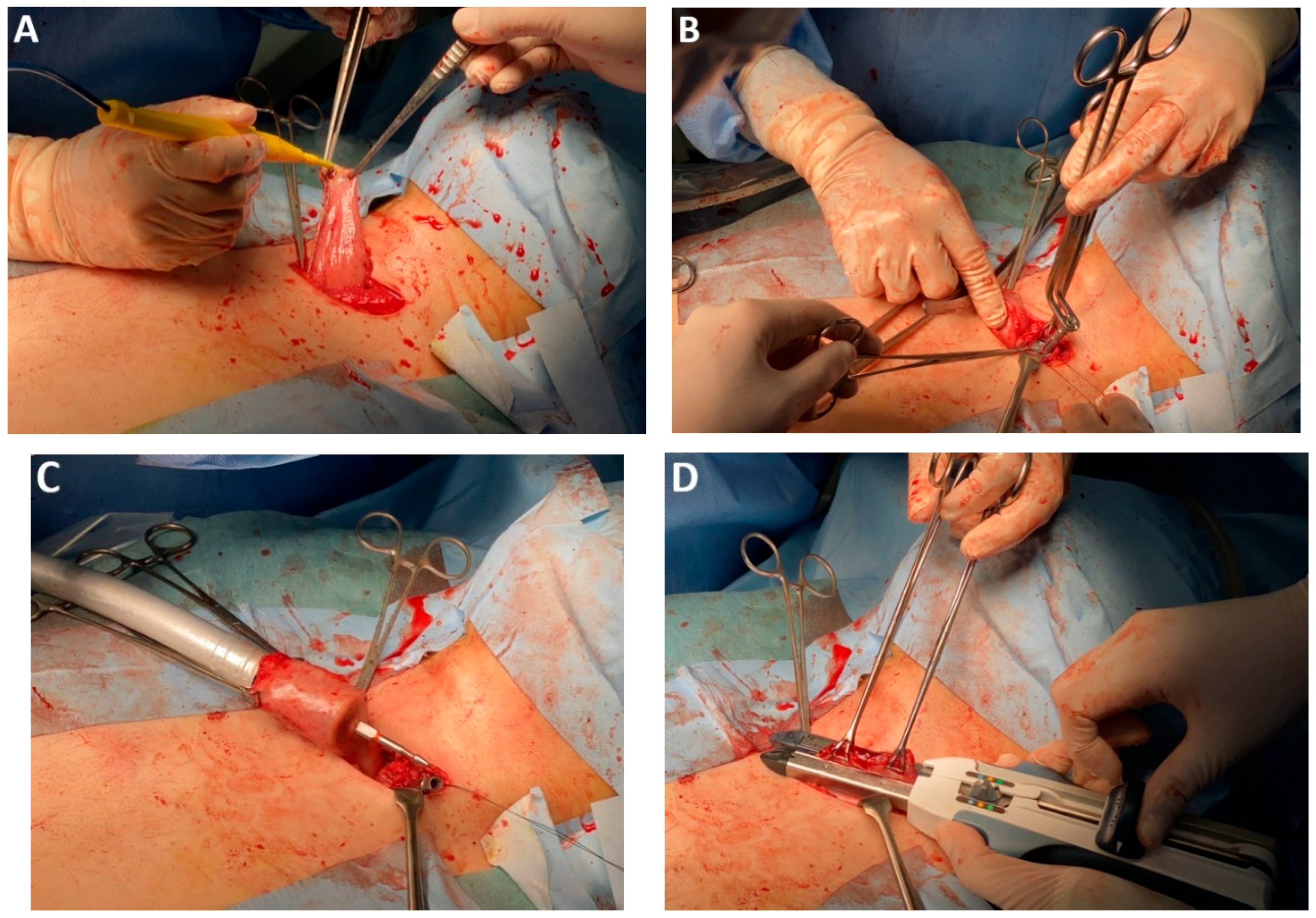
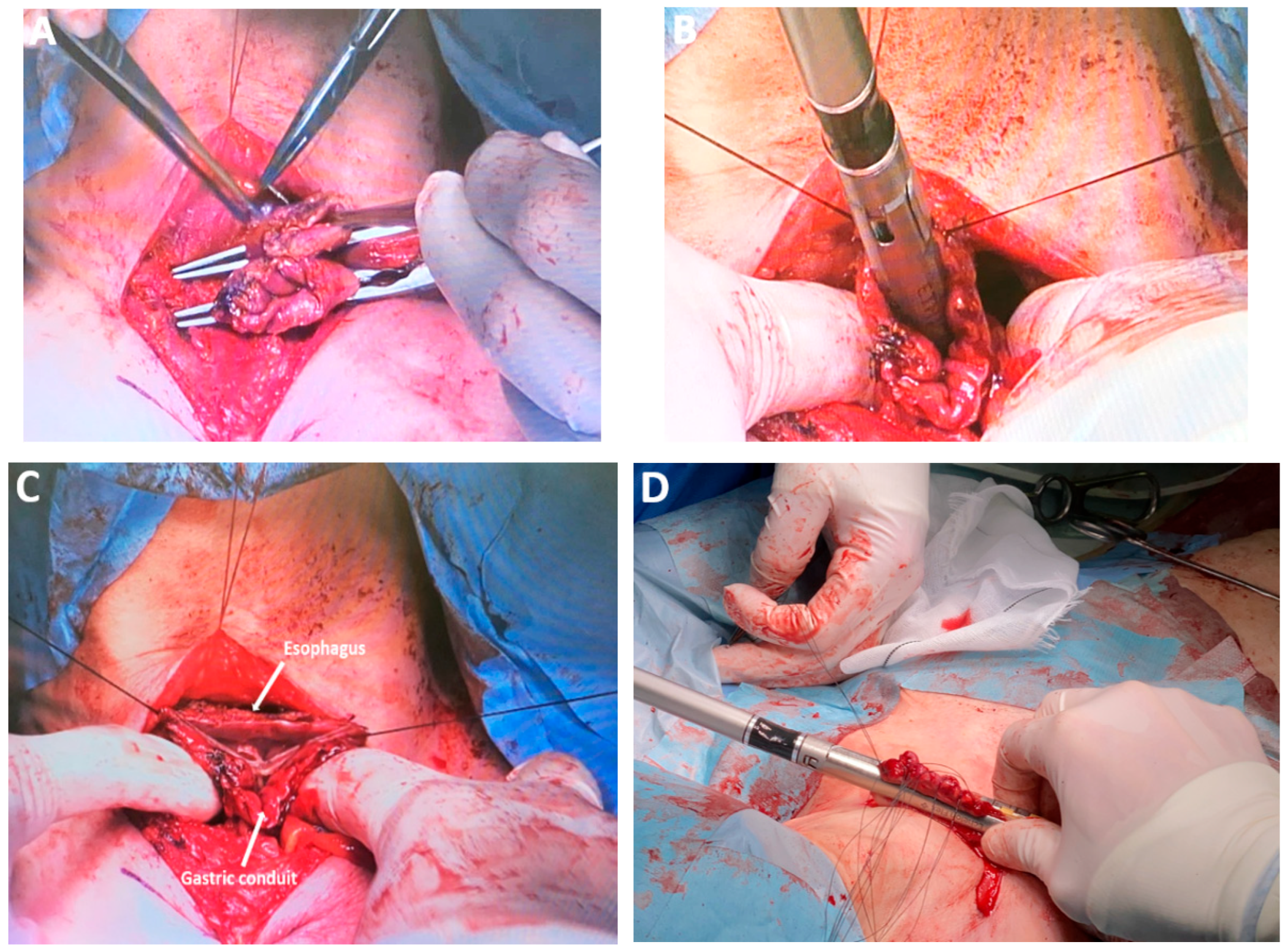
| Classification of Innovations | Subclasses | Definition | Objectives |
|---|---|---|---|
| 1—Technological innovations | 1a—camera technology 1b—surgical instruments 1c—auxiliary equipment | Improvements and innovations in camera, Development of new instruments and other auxiliary equipment, including anesthetic measures | Optimization of intraoperative visualization to enhance surgical precision and reduce operative time Integration of ergonomic and functionally advanced surgical instruments to facilitate dissection and improve surgeon efficiency Deployment of surgical and anesthetic equipment designed to enhance procedural safety and minimize intraoperative and postoperative complications |
| 2—Advances in surgical approach | 2a—transthoracic MIE 2b—transthoracic RAMIE 2c—transcervical MICE | Improvements in surgical approach Optimization of surgical technique Improvements of MIE, RAMIE and MICE | Reducing surgical trauma Improved access to difficult anatomical areas Reduced risk of intraoperative incidents and accidents Shorter learning curve |
| 3—Advances in lymph nodes dissection | 3a—thoracic 3b—abdominal 3c—cervical | New modalities of performing lymph node dissection | Effectiveness of operating stages facilitating radical lymphadenectomy Reducing complications associated with lymphadenectomy |
| 4—Advances in graft preparation | 4a—stomach 4b—colon 4c—ascension route | New modalities to prepare esophageal substitute New ascension routes | Minimizing the occurrence of graft necrosis |
| 5—Advances in performing esophagogastric anastomosis | 5a—hand-sewn 5b—mechanical circular 5c—mechanical linear | New techniques for performing cervical or intrathoracic anastomoses | Reducing the risk of anastomotic leakage |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Achim, F.; Otsuka, K.; Yamashita, T.; Asagoe, Y.; Kurita, D.; Constantin, A.; Constantinoiu, S.; Mohssen, A.; Rosianu, C.; Rotariu, A.; et al. Advances in Minimally Invasive Esophagectomy—An Overview of Recent Developments and a Novel Classification of Innovations in Treatment of Thoracic Esophageal Cancer. Medicina 2025, 61, 2176. https://doi.org/10.3390/medicina61122176
Achim F, Otsuka K, Yamashita T, Asagoe Y, Kurita D, Constantin A, Constantinoiu S, Mohssen A, Rosianu C, Rotariu A, et al. Advances in Minimally Invasive Esophagectomy—An Overview of Recent Developments and a Novel Classification of Innovations in Treatment of Thoracic Esophageal Cancer. Medicina. 2025; 61(12):2176. https://doi.org/10.3390/medicina61122176
Chicago/Turabian StyleAchim, Florin, Koji Otsuka, Takeshi Yamashita, Yutaro Asagoe, Daisuke Kurita, Adrian Constantin, Silviu Constantinoiu, Ahmed Mohssen, Cristian Rosianu, Alexandru Rotariu, and et al. 2025. "Advances in Minimally Invasive Esophagectomy—An Overview of Recent Developments and a Novel Classification of Innovations in Treatment of Thoracic Esophageal Cancer" Medicina 61, no. 12: 2176. https://doi.org/10.3390/medicina61122176
APA StyleAchim, F., Otsuka, K., Yamashita, T., Asagoe, Y., Kurita, D., Constantin, A., Constantinoiu, S., Mohssen, A., Rosianu, C., Rotariu, A., Moraru, A.-C., Rasuceanu, A., & Predescu, D. (2025). Advances in Minimally Invasive Esophagectomy—An Overview of Recent Developments and a Novel Classification of Innovations in Treatment of Thoracic Esophageal Cancer. Medicina, 61(12), 2176. https://doi.org/10.3390/medicina61122176





