The Burden of Esophageal Cancer and Its Correlation with Dietary, Metabolic, and Behavioral Risk Factors in 204 Countries and Territories: Results from the Global Burden of Disease Study 2021
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Statistical Analysis
3. Results
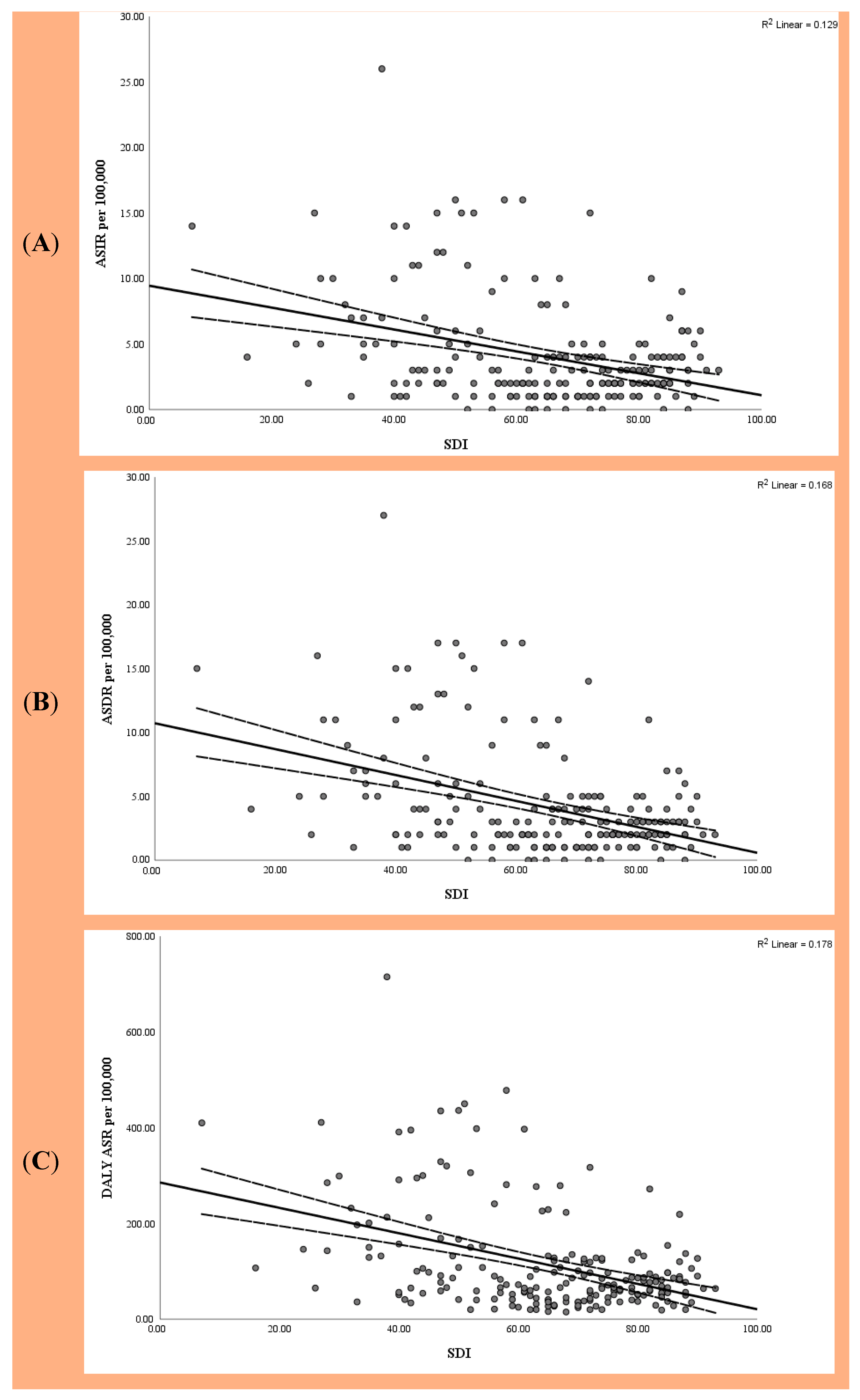
3.1. Global Burden of EC Based on SDI
3.2. National Correlation with SDI
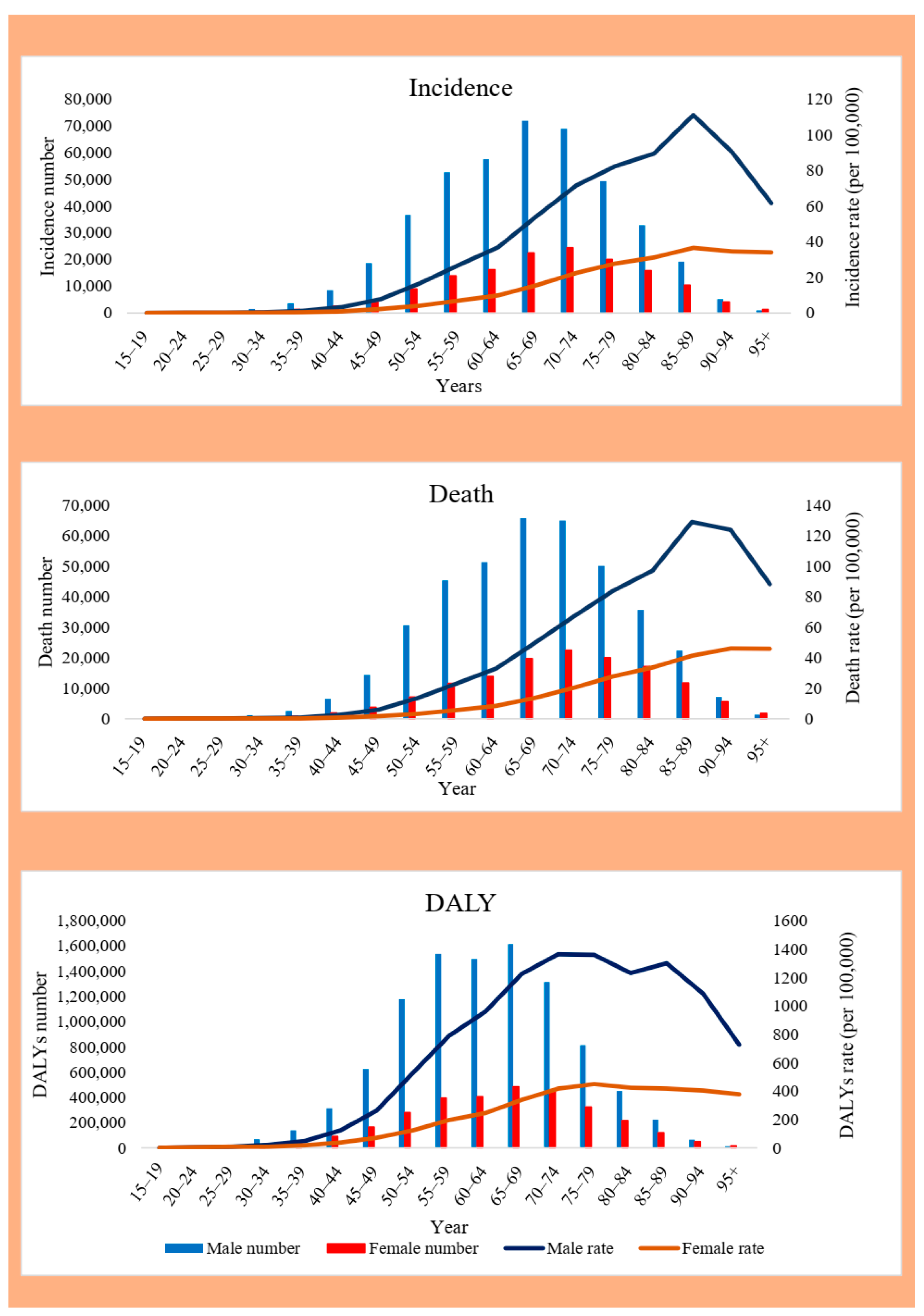
3.3. Sex and Age Distribution of EC
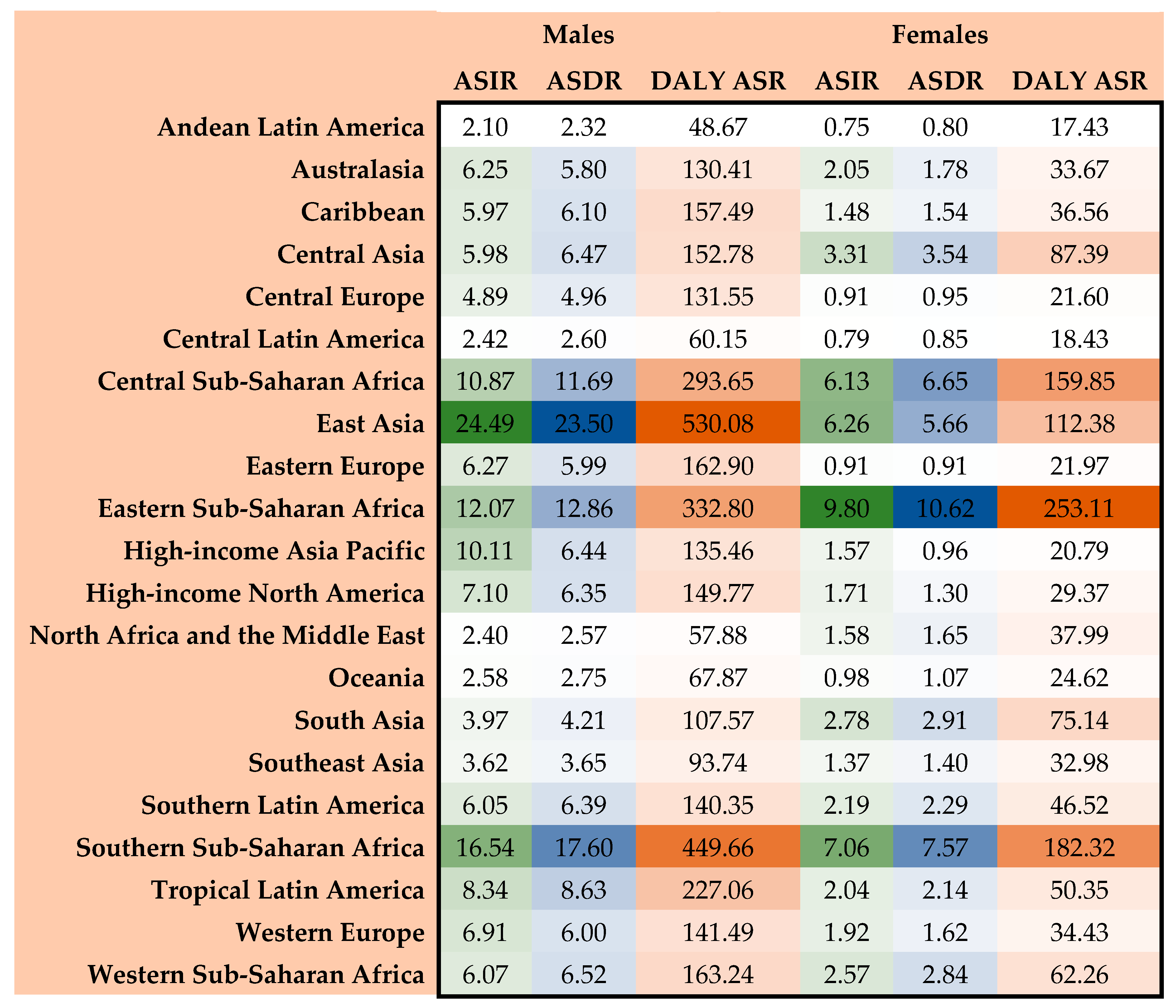
3.4. Regional Burden of EC
3.5. National Burden of EC
3.6. Risk Factors of EC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Teng, Y.; Xia, C.; Cao, M.; Yang, F.; Yan, X.; He, S.; Cao, M.; Zhang, S.; Li, Q.; Tan, N.; et al. Esophageal cancer global burden profiles, trends, and contributors. Cancer Biol. Med. 2024, 21, 656–666. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Karamanou, M.; Markatos, K.; Papaioannou, T.G.; Zografos, G.; Androutsos, G. Hallmarks in history of esophageal carcinoma. J. BUON 2017, 22, 1088–1091. [Google Scholar]
- Kamangar, F.; Nasrollahzadeh, D.; Safiri, S.; Sepanlou, S.G.; Fitzmaurice, C.; Ikuta, K.S.; Bisignano, C.; Islami, F.; Roshandel, G.; Lim, S.S. The global, regional, and national burden of oesophageal cancer and its attributable risk factors in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 582–597. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Q.; Ma, Y.L.; Qin, Q.; Wang, P.H.; Luo, Y.; Xu, P.F.; Cui, Y. Epidemiology of esophageal cancer in 2020 and projections to 2030 and 2040. Thorac. Cancer 2023, 14, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Liu, Z.; Mao, X.; Tong, X.; Zhang, T.; Suo, C.; Chen, X. Global trends in the incidence and mortality of esophageal cancer from 1990 to 2017. Cancer Med. 2020, 9, 6875–6887. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.C.; Hamilton, W.; Whiteman, D.C.; Jiang, J.Y.; Qiao, Y.; Fung, F.D.; Wang, H.H.; Chiu, P.W.; Ng, E.K.; Wu, J.C. Global Incidence and mortality of oesophageal cancer and their correlation with socioeconomic indicators temporal patterns and trends in 41 countries. Sci. Rep. 2018, 8, 4522. [Google Scholar] [CrossRef]
- Fitzmaurice, C.; Dicker, D.; Pain, A.; Hamavid, H.; Moradi-Lakeh, M.; MacIntyre, M.F.; Allen, C.; Hansen, G.; Woodbrook, R.; Wolfe, C. The global burden of cancer 2013. JAMA Oncol. 2015, 1, 505–527. [Google Scholar] [CrossRef]
- Morgan, E.; Soerjomataram, I.; Rumgay, H.; Coleman, H.G.; Thrift, A.P.; Vignat, J.; Laversanne, M.; Ferlay, J.; Arnold, M. The global landscape of esophageal squamous cell carcinoma and esophageal adenocarcinoma incidence and mortality in 2020 and projections to 2040: New estimates from GLOBOCAN 2020. Gastroenterology 2022, 163, 649–658.e2. [Google Scholar] [CrossRef]
- Huang, J.; Koulaouzidis, A.; Marlicz, W.; Lok, V.; Chu, C.; Ngai, C.H.; Zhang, L.; Chen, P.; Wang, S.; Yuan, J. Global burden, risk factors, and trends of esophageal cancer: An analysis of cancer registries from 48 countries. Cancers 2021, 13, 141. [Google Scholar] [CrossRef]
- Coleman, H.G.; Xie, S.-H.; Lagergren, J. The epidemiology of esophageal adenocarcinoma. Gastroenterology 2018, 154, 390–405. [Google Scholar] [CrossRef]
- Xie, S.-H.; Lagergren, J. Risk factors for oesophageal cancer. Best Pract. Res. Clin. Gastroenterol. 2018, 36, 3–8. [Google Scholar] [CrossRef]
- Beydoun, A.S.; Stabenau, K.A.; Altman, K.W.; Johnston, N. Cancer Risk in Barrett’s Esophagus: A Clinical Review. Int. J. Mol. Sci. 2023, 24, 6018. [Google Scholar] [CrossRef]
- Long, E.; Beales, I.L. The role of obesity in oesophageal cancer development. Ther. Adv. Gastroenterol. 2014, 7, 247–268. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.D. Impact of bariatric surgery on gastroesophageal reflux disease and esophageal motility. Curr. Opin. Gastroenterol. 2021, 37, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Fitzmaurice, C.; Allen, C.; Barber, R.M.; Barregard, L.; Bhutta, Z.A.; Brenner, H.; Dicker, D.J.; Chimed-Orchir, O.; Dandona, R.; Dandona, L. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: A systematic analysis for the global burden of disease study. JAMA Oncol. 2017, 3, 524–548. [Google Scholar]
- Global Burden of Disease Cancer Collaboration. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: A systematic analysis for the global burden of disease study. JAMA Oncol. 2019, 5, 1749–1768. [Google Scholar] [CrossRef]
- Uhlenhopp, D.J.; Then, E.O.; Sunkara, T.; Gaduputi, V. Epidemiology of esophageal cancer: Update in global trends, etiology and risk factors. Clin. J. Gastroenterol. 2020, 13, 1010–1021. [Google Scholar] [CrossRef]
- Gupta, B.; Kumar, N. Worldwide incidence, mortality and time trends for cancer of the oesophagus. Eur. J. Cancer Prev. 2017, 26, 107–118. [Google Scholar] [CrossRef]
- Arnold, M.; Soerjomataram, I.; Ferlay, J.; Forman, D. Global incidence of oesophageal cancer by histological subtype in 2012. Gut 2015, 64, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Baum, A.; Wisnivesky, J.; Basu, S.; Siu, A.L.; Schwartz, M.D. Association of Geographic Differences in Prevalence of Uncontrolled Chronic Conditions With Changes in Individuals’ Likelihood of Uncontrolled Chronic Conditions. JAMA 2020, 324, 1429–1438. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA: A Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Sharma, R.; Abbasi-Kangevari, M.; Abd-Rabu, R.; Abidi, H.; Abu-Gharbieh, E.; Acuna, J.M.; Adhikari, S.; Advani, S.M.; Afzal, M.S.; Aghaie Meybodi, M.; et al. Global, regional, and national burden of colorectal cancer and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol. Hepatol. 2022, 7, 627–647. [Google Scholar] [CrossRef]
- Mazidimoradi, A.; Momenimovahed, Z.; Allahqoli, L.; Tiznobaik, A.; Hajinasab, N.; Salehiniya, H.; Alkatout, I. The global, regional and national epidemiology, incidence, mortality, and burden of ovarian cancer. Health Sci. Rep. 2022, 5, e936. [Google Scholar] [CrossRef]
- Go, D.S.; Kim, Y.E.; Yoon, S.J. Subnational Burden of Disease According to the Sociodemographic Index in South Korea. Int. J. Environ. Res. Public Health 2020, 17, 5788. [Google Scholar] [CrossRef]
- Rezaei, F.; Mazidimoradi, A.; Rayatinejad, A.; Allahqoli, L.; Salehiniya, H. Temporal trends of tracheal, bronchus, and lung cancer between 2010 and 2019, in Asian countries by geographical region and sociodemographic index, comparison with global data. Thorac. Cancer 2023, 14, 1668–1706. [Google Scholar] [CrossRef]
- World Bank. The World by Income and Region. Available online: https://datatopics.worldbank.org/world-development-indicators/the-world-by-income-and-region.html (accessed on 7 July 2025).
- Allahqoli, L.; Mazidimoradi, A.; Momenimovahed, Z.; Rahmani, A.; Hakimi, S.; Tiznobaik, A.; Gharacheh, M.; Salehiniya, H.; Babaey, F.; Alkatout, I. The Global Incidence, Mortality, and Burden of Breast Cancer in 2019: Correlation with Smoking, Drinking, and Drug Use. Front. Oncol. 2022, 12, 921015. [Google Scholar] [CrossRef]
- Momenimovahed, Z.; Mazidimoradi, A.; Banakar, N.; Allahqoli, L.; Salehiniya, H. Temporal Trends of Ovarian Cancer Between 1990 and 2019, in Asian Countries by Geographical Region and SDI, Comparison with Global Data. Indian J. Gynecol. Oncol. 2023, 21, 38. [Google Scholar] [CrossRef]
- Mazidimoradi, A.; Ghavidel, F.; Momenimovahed, Z.; Allahqoli, L.; Salehiniya, H. Global incidence, mortality, and burden of esophageal cancer, and its correlation with SDI, metabolic risks, fasting plasma glucose, LDL cholesterol, and body mass index: An ecological study. Health Sci. Rep. 2023, 6, e1342. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, X.; Lin, S.; Yuan, J.; Yu, I.T. Dietary patterns and oesophageal squamous cell carcinoma: A systematic review and meta-analysis. Br. J. Cancer 2014, 110, 2785–2795. [Google Scholar] [CrossRef]
- Mao, W.-M.; Zheng, W.-H.; Ling, Z.-Q. Epidemiologic risk factors for esophageal cancer development. Asian Pac. J. Cancer Prev. 2011, 12, 2461–2466. [Google Scholar]
- Rezaianzadeh, A.; Azgomi, S.H.; Mokhtari, A.M.; Maghsoudi, A.; Nazarzadeh, M.; Dehghan, S.L.; Kazerooni, S.R. The incidence of breast cancer in Iran: A systematic review and meta-analysis. J. Anal. Oncol. 2016, 5, 139–145. [Google Scholar] [CrossRef]
- Rezaianzadeh, A.; Jalali, M.; Maghsoudi, A.; Mokhtari, A.M.; Azgomi, S.H.; Dehghani, S.L. The overall 5-year survival rate of breast cancer among Iranian women: A systematic review and meta-analysis of published studies. Breast Dis. 2017, 37, 63–68. [Google Scholar] [CrossRef]
- Cao, M.; Li, H.; Sun, D.; He, S.; Yan, X.; Yang, F.; Zhang, S.; Xia, C.; Lei, L.; Peng, J. Current cancer burden in China: Epidemiology, etiology, and prevention. Cancer Biol. Med. 2022, 19, 1121. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Weng, W.-C.; Huang, C.-W.; Su, C.-C.; Mukundan, A.; Karmakar, R.; Chen, T.-H.; Avhad, A.R.; Chou, C.-K.; Wang, H.-C. Optimizing Esophageal Cancer Diagnosis with Computer-Aided Detection by YOLO Models Combined with Hyperspectral Imaging. Diagnostics 2025, 15, 1686. [Google Scholar] [CrossRef] [PubMed]
- Vashist, Y.; Goyal, A.; Shetty, P.; Girnyi, S.; Cwalinski, T.; Skokowski, J.; Malerba, S.; Prete, F.P.; Mocarski, P.; Kania, M.K.; et al. Evaluating Postoperative Morbidity and Outcomes of Robotic-Assisted Esophagectomy in Esophageal Cancer Treatment—A Comprehensive Review on Behalf of TROGSS (The Robotic Global Surgical Society) and EFISDS (European Federation International Society for Digestive Surgery) Joint Working Group. Curr. Oncol. 2025, 32, 72. [Google Scholar] [PubMed]
- Li, S.; Chen, H.; Man, J.; Zhang, T.; Yin, X.; He, Q.; Yang, X.; Lu, M. Changing trends in the disease burden of esophageal cancer in China from 1990 to 2017 and its predicted level in 25 years. Cancer Med. 2021, 10, 1889–1899. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.-H.; Lagergren, J. The male predominance in esophageal adenocarcinoma. Clin. Gastroenterol. Hepatol. 2016, 14, 338–347.e1. [Google Scholar] [CrossRef]
- Cronin-Fenton, D.P.; Murray, L.J.; Whiteman, D.C.; Cardwell, C.; Webb, P.M.; Jordan, S.J.; Corley, D.A.; Sharp, L.; Lagergren, J. Reproductive and sex hormonal factors and oesophageal and gastric junction adenocarcinoma: A pooled analysis. Eur. J. Cancer 2010, 46, 2067–2076. [Google Scholar] [CrossRef] [PubMed]
- Gan, X.; Dai, G.; Li, Y.; Xu, L.; Liu, G. Intricate roles of estrogen and estrogen receptors in digestive system cancers: A systematic review. Cancer Biol. Med. 2024, 21, 898–915. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, P.; Liu, J.-C.; Zhao, Z.-A.; Guo, R.; Li, Y.; Liu, Y.-S.; Li, S.-G.; Zhao, Z.-G. Interaction of Estradiol and Endoplasmic Reticulum Stress in the Development of Esophageal Carcinoma. Front. Endocrinol. 2020, 11, 410. [Google Scholar] [CrossRef]
- Xie, S.-H.; Fang, R.; Huang, M.; Dai, J.; Thrift, A.P.; Anderson, L.A.; Chow, W.-H.; Bernstein, L.; Gammon, M.D.; Risch, H.A.; et al. Association Between Levels of Sex Hormones and Risk of Esophageal Adenocarcinoma and Barrett’s Esophagus. Clin. Gastroenterol. Hepatol. 2020, 18, 2701–2709.e3. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, R.; Arnold, M.; Abnet, C.; Zeng, H.; Zhang, S.; Chen, R.; Sun, K.; Li, L.; An, L.; et al. Global and national trends in the age-specific sex ratio of esophageal cancer and gastric cancer by subtype. Int. J. Cancer 2022, 151, 1447–1461. [Google Scholar] [CrossRef]
- Middleton, D.R.; Bouaoun, L.; Hanisch, R.; Bray, F.; Dzamalala, C.; Chasimpha, S.; Menya, D.; Mbalawa, C.G.; N’Da, G.; Woldegeorgis, M.A. Esophageal cancer male to female incidence ratios in Africa: A systematic review and meta-analysis of geographic, time and age trends. Cancer Epidemiol. 2018, 53, 119–128. [Google Scholar] [CrossRef]
- Mengistu, S.T.; Kesete, Y.; Achila, O.O.; Fikadu, G.T.; Abrhaley, F.; Fikadu, E.T.; Said, S.M.; Gheberehiwet, M.A.; Hamida, M.E.; Ghidei, Y.T. High Incidence of Esophageal Cancer in Women in Eritrea and Its Potential Link to Low Age at Menopause: Evidence from a 10-Year Retrospective Data Analysis. J. Cancer Epidemiol. 2024, 2024, 5566016. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.H.; Park, H.; Kim, D.H.; Na, H.K.; Ahn, J.Y.; Lee, J.H.; Jung, K.W.; Choi, K.D.; Song, H.J.; Lee, G.H.; et al. Sex Differences in Clinical Features and Survival Outcomes of Esophageal Cancer: A Comparative Study in the Korean Population. World J. Mens Health 2025, 43, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, X.; Zhang, A.; Liang, G.; Sun, Y.; Zhang, J. Age-period-cohort analysis of incidence, mortality and disability-adjusted life years of esophageal cancer in global, regional and national regions from 1990 to 2019. BMC Public Health 2024, 24, 212. [Google Scholar] [CrossRef]
- Li, B.; Liu, Y.; Peng, J.; Sun, C.; Rang, W. Trends of esophageal cancer incidence and mortality and its influencing factors in China. Risk Manag. Healthc. Policy 2021, 14, 4809–4821. [Google Scholar] [CrossRef]
- Xie, S.-H.; Ness-Jensen, E.; Rabbani, S.; Langseth, H.; Gislefoss, R.E.; Mattsson, F.; Lagergren, J. Circulating sex hormone levels and risk of esophageal adenocarcinoma in a prospective study in men. Off. J. Am. Coll. Gastroenterol.|ACG 2020, 115, 216–223. [Google Scholar] [CrossRef]
- Mantziari, S.; Teixeira Farinha, H.; Bouygues, V.; Vignal, J.C.; Deswysen, Y.; Demartines, N.; Schäfer, M.; Piessen, G. Esophageal Cancer in Elderly Patients, Current Treatment Options and Outcomes; A Systematic Review and Pooled Analysis. Cancers 2021, 13, 2104. [Google Scholar] [CrossRef]
- sadat Yousefi, M.; Sharifi-Esfahani, M.; Pourgholam-Amiji, N.; Afshar, M.; Sadeghi-Gandomani, H.; Otroshi, O.; Salehiniya, H. Esophageal cancer in the world: Incidence, mortality and risk factors. Biomed. Res. Ther. 2018, 5, 2504–2517. [Google Scholar] [CrossRef]
- Pakzad, R.; Mohammadian-Hafshejani, A.; Khosravi, B.; Soltani, S.; Pakzad, I.; Mohammadian, M.; Salehiniya, H.; Momenimovahed, Z. The incidence and mortality of esophageal cancer and their relationship to development in Asia. Ann. Transl. Med. 2016, 4, 29. [Google Scholar]
- Jiang, Y.; Lin, Y.; Wen, Y.; Fu, W.; Wang, R.; He, J.; Zhang, J.; Wang, Z.; Ge, F.; Huo, Z.; et al. Global trends in the burden of esophageal cancer, 1990-2019: Results from the Global Burden of Disease Study 2019. J. Thorac. Dis. 2023, 15, 348–364. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Lin, J.; Wei, W.; Chen, P.; Yao, K. Burden of esophageal cancer and its attributable risk factors in 204 countries and territories from 1990 to 2019. Front. Public Health 2022, 10, 952087. [Google Scholar] [CrossRef]
- Lim, R.Z.M.; Mahendran, H.A.; Ng, C.B.; Low, K.Y.; Thannimalai, S.; Ngo, C.W.; Mahmood, N.R.K.N.; Rajan, R.; Shuhaili, M.A.; Salleh, A.S.B.M. Esophageal squamous cell carcinoma and adenocarcinoma in Malaysia–Pooled data from upper gastrointestinal centers in a multiethnic Asian population. Cancer Epidemiol. 2022, 80, 102211. [Google Scholar] [CrossRef] [PubMed]
- Ran, X.; Zeng, H.; Zheng, R.; Sun, K.; Han, B.; Wang, S.; Chen, R.; Li, L.; Wei, W.; He, J. Geographic, sex and socioeconomic disparities in esophageal cancer incidence in China: A population-based study. Int. J. Cancer 2024, 154, 477–487. [Google Scholar] [CrossRef]
- Grille, V.J.; Campbell, S.; Gibbs, J.F.; Bauer, T.L. Esophageal cancer: The rise of adenocarcinoma over squamous cell carcinoma in the Asian belt. J. Gastrointest. Oncol. 2021, 12, S339. [Google Scholar] [CrossRef]
- Ghosh, N.R. Dietary Risk Factors for Esophageal Cancer Based on Who Regions. Master’s Thesis, Saint Louis University, St. Louis, MO, USA, 2020. [Google Scholar]
- Cook, M.B.; Kamangar, F.; Whiteman, D.C.; Freedman, N.D.; Gammon, M.D.; Bernstein, L.; Brown, L.M.; Risch, H.A.; Ye, W.; Sharp, L.; et al. Cigarette smoking and adenocarcinomas of the esophagus and esophagogastric junction: A pooled analysis from the international BEACON consortium. J. Natl. Cancer Inst. 2010, 102, 1344–1353. [Google Scholar] [CrossRef]
- McCain, R.S.; McManus, D.T.; McQuaid, S.; James, J.A.; Salto-Tellez, M.; Reid, N.B.; Craig, S.; Chisambo, C.; Bingham, V.; McCarron, E. Alcohol intake, tobacco smoking, and esophageal adenocarcinoma survival: A molecular pathology epidemiology cohort study. Cancer Causes Control 2020, 31, 1–11. [Google Scholar] [CrossRef]
- Schlottmann, F.; Dreifuss, N.H.; Patti, M.G. Obesity and esophageal cancer: GERD, Barrett s esophagus, and molecular carcinogenic pathways. Expert Rev. Gastroenterol. Hepatol. 2020, 14, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Elliott, J.A.; Reynolds, J.V. Visceral obesity, metabolic syndrome, and esophageal adenocarcinoma. Front. Oncol. 2021, 11, 627270. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gao, P.; Zhang, M.; Huang, Z.; Zhang, D.; Deng, Q.; Li, Y.; Zhao, Z.; Qin, X.; Jin, D. Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. Jama 2017, 317, 2515–2523. [Google Scholar] [CrossRef]
- Lindkvist, B.; Johansen, D.; Stocks, T.; Concin, H.; Bjørge, T.; Almquist, M.; Häggström, C.; Engeland, A.; Hallmans, G.; Nagel, G. Metabolic risk factors for esophageal squamous cell carcinoma and adenocarcinoma: A prospective study of 580 000 subjects within the Me-Can project. BMC Cancer 2014, 14, 103. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Incidence Cases (95% UI) | ASIR per 105 (95% UI) | Death Cases (95% UI) | ASDR per 105 (95% UI) | DALY Number (95% UI) | DALY ASR per 105 (95% UI) |
|---|---|---|---|---|---|---|
| Global | 576,529 | 6.65 | 538,602 | 6.25 | 12,999,265 | 148.56 |
| (509,492–645,648) | (5.88–7.45) | (475,944–603,406) | (5.53–7.00) | (11,522,861–14,605,268) | (131.71–166.82) | |
| Sex | ||||||
| Male | 428,387 | 10.63 | 399,796 | 10.08 | 9,889,701 | 237.75 |
| (367,888–495,196) | (9.15–12.23) | (343,473–459,871) | (8.69–11.56) | (8,502,607–11,434,422) | (204.75–274.29) | |
| Female | 148,142 | 3.20 | 138,806 | 2.99 | 3,109,564 | 67.79 |
| (113,641–172,538) | (2.46–3.72) | (107,414–161,288) | (2.32–3.48) | (2,478,471–3,575,202) | (54.15–77.99) | |
| SDI | ||||||
| High SDI | 102,510 | 4.94 | 85,652 | 4.02 | 1,825,459 | 93.95 |
| (95,224–107,348) | (4.63–5.16) | (79,160–89,950) | (3.75–4.2) | (1,719,026–1,902,714) | (89.28–97.92) | |
| High–middle SDI | 176,768 | 8.84 | 162,430 | 8.13 | 3,834,291 | 192.56 |
| (145,141–214,116) | (7.26–10.7) | (134,262–195,467) | (6.72–9.77) | (3,157,464–4,667,629) | (158.7–234.03) | |
| Low SDI | 27,960 | 5.49 | 28,924 | 5.89 | 830,121 | 148.67 |
| (23,834–32,184) | (4.7–6.32) | (24,611–33,445) | (5.02–6.8) | (701,259–964,024) | (126.11–172.2) | |
| Low–middle SDI | 52,104 | 3.59 | 53,724 | 3.79 | 1,491,634 | 97.10 |
| (47,166–59,926) | (3.24–4.15) | (48,513–61,806) | (3.42–4.39) | (1,348,142–1,724,084) | (87.74–111.84) | |
| Middle SDI | 216,951 | 8.10 | 207,634 | 7.91 | 5,011,783 | 180.65 |
| (182,212–258,446) | (6.78–9.62) | (174,863–246,498) | (6.65–9.34) | (4,233,898–5,964,294) | (153.17–214.63) | |
| GBD Regions | ||||||
| Andean Latin America | 802 | 1.38 | 866 | 1.51 | 19,168 | 32.27 |
| (656–987) | (1.14–1.7) | (712–1063) | (1.24–1.85) | (15,515–23,634) | (26.17–39.76) | |
| Australasia | 2202 | 4.05 | 2050 | 3.68 | 41,017 | 80.20 |
| (1983–2361) | (3.68–4.33) | (1849–2204) | (3.33–3.94) | (37,626–43,815) | (74.13–85.45) | |
| Caribbean | 1952 | 3.60 | 1997 | 3.68 | 51,045 | 94.29 |
| (1713–2207) | (3.17–4.08) | (1755–2254) | (3.24–4.16) | (44,533–58,194) | (82.3–107.49) | |
| Central Asia | 3573 | 4.42 | 3735 | 4.74 | 99,947 | 115.48 |
| (3191–3974) | (3.98–4.9) | (3342–4158) | (4.28–5.25) | (88,486–112,299) | (102.92–128.96) | |
| Central Europe | 5759 | 2.73 | 5926 | 2.76 | 146,485 | 73.05 |
| (5284–6214) | (2.5–2.95) | (5441–6394) | (2.54–2.98) | (134,849–158,439) | (67.15–79.02) | |
| Central Latin America | 3807 | 1.54 | 4029 | 1.65 | 95,847 | 37.71 |
| (3398–4285) | (1.37–1.73) | (3594–4524) | (1.47–1.85) | (85,462–107,643) | (33.67–42.31) | |
| Central Sub-Saharan Africa | 4536 | 8.26 | 4653 | 8.89 | 137,615 | 221.54 |
| (3325–5881) | (6.03–10.61) | (3398–6069) | (6.44–11.5) | (100,038–180,215) | (161.83–289.26) | |
| East Asia | 327,706 | 14.83 | 302,582 | 13.91 | 7,069,761 | 313.94 |
| (263,648–401,882) | (11.94–18.09) | (243,363–368,743) | (11.23–16.84) | (5,660,281–8,736,103) | (252.18–387.12) | |
| Eastern Europe | 10,710 | 3.09 | 10,305 | 2.94 | 274,133 | 81.25 |
| (9692–11,618) | (2.79–3.35) | (9378–11,159) | (2.68–3.19) | (247,183–298,314) | (73.21–88.53) | |
| Eastern Sub-Saharan Africa | 18,379 | 10.93 | 19,000 | 11.74 | 545,442 | 292.22 |
| (15,328–22,110) | (9.14–13.09) | (15,876–22,909) | (9.82–14.12) | (452,300–659,237) | (243.43–352.18) | |
| High-income Asia Pacific | 25,547 | 5.49 | 16,914 | 3.42 | 318,613 | 74.52 |
| (22,820–27,076) | (5–5.8) | (15,031–17,974) | (3.1–3.62) | (290,566–336,610) | (69.25–78.49) | |
| High-income North America | 27,331 | 4.20 | 23,960 | 3.62 | 535,321 | 86.13 |
| (25,620–28,409) | (3.96–4.36) | (22,394–24,952) | (3.4–3.76) | (510,487–552,767) | (82.45–88.83) | |
| North Africa and the Middle East | 8,684 | 1.99 | 8,846 | 2.11 | 230,171 | 48.01 |
| (7367–9766) | (1.71–2.22) | (7500–9959) | (1.81–2.36) | (191,966–262,991) | (40.39–54.41) | |
| Oceania | 131 | 1.81 | 134 | 1.95 | 3,906 | 47.10 |
| (103–166) | (1.43–2.28) | (106–170) | (1.54–2.45) | (3056–5016) | (37.29–59.95) | |
| South Asia | 50,081 | 3.36 | 51,542 | 3.54 | 1,434,797 | 91.08 |
| (44,230–59,870) | (2.95–4.03) | (45,651–61,688) | (3.12–4.26) | (1,268,757–1,704,574) | (80.54–108.48) | |
| Southeast Asia | 16,164 | 2.42 | 15,830 | 2.44 | 437,488 | 61.71 |
| (13,984–18,580) | (2.11–2.76) | (13,725–18,154) | (2.13–2.78) | (374,888–504,001) | (53.19–70.79) | |
| Southern Latin America | 3,432 | 3.89 | 3,627 | 4.07 | 76,790 | 88.92 |
| (3190–3667) | (3.62–4.15) | (3346–3879) | (3.77–4.35) | (72,068–82,063) | (83.56–94.99) | |
| Southern Sub-Saharan Africa | 6,410 | 11.01 | 6,602 | 11.69 | 185,849 | 297.67 |
| (5853–7006) | (10.06–11.99) | (6026–7225) | (10.68–12.72) | (169,360–204,414) | (271.83–326.47) | |
| Tropical Latin America | 12,767 | 4.91 | 13,113 | 5.07 | 348,544 | 132.10 |
| (12,077–13,280) | (4.64–5.11) | (12,383–13,661) | (4.78–5.29) | (331,533–362,398) | (125.59–137.34) | |
| Western Europe | 38,417 | 4.26 | 34,397 | 3.65 | 712,093 | 85.44 |
| (35,454–40,217) | (4–4.44) | (31,525–36,115) | (3.41–3.81) | (672,401–741,301) | (81.46–88.58) | |
| Western Sub-Saharan Africa | 8,137 | 4.22 | 8,494 | 4.58 | 235,235 | 110.00 |
| (6102–9760) | (3.15–5.02) | (6355–10,192) | (3.43–5.43) | (175,348–283,946) | (82.38–132.2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almasi, Z.; Mazidimoradi, A.; Shahabinia, Z.; Allahqoli, L.; Salehiniya, H.; Lee, D.-Y. The Burden of Esophageal Cancer and Its Correlation with Dietary, Metabolic, and Behavioral Risk Factors in 204 Countries and Territories: Results from the Global Burden of Disease Study 2021. Medicina 2025, 61, 1891. https://doi.org/10.3390/medicina61111891
Almasi Z, Mazidimoradi A, Shahabinia Z, Allahqoli L, Salehiniya H, Lee D-Y. The Burden of Esophageal Cancer and Its Correlation with Dietary, Metabolic, and Behavioral Risk Factors in 204 Countries and Territories: Results from the Global Burden of Disease Study 2021. Medicina. 2025; 61(11):1891. https://doi.org/10.3390/medicina61111891
Chicago/Turabian StyleAlmasi, Zeinab, Afrooz Mazidimoradi, Zahra Shahabinia, Leila Allahqoli, Hamid Salehiniya, and Do-Youn Lee. 2025. "The Burden of Esophageal Cancer and Its Correlation with Dietary, Metabolic, and Behavioral Risk Factors in 204 Countries and Territories: Results from the Global Burden of Disease Study 2021" Medicina 61, no. 11: 1891. https://doi.org/10.3390/medicina61111891
APA StyleAlmasi, Z., Mazidimoradi, A., Shahabinia, Z., Allahqoli, L., Salehiniya, H., & Lee, D.-Y. (2025). The Burden of Esophageal Cancer and Its Correlation with Dietary, Metabolic, and Behavioral Risk Factors in 204 Countries and Territories: Results from the Global Burden of Disease Study 2021. Medicina, 61(11), 1891. https://doi.org/10.3390/medicina61111891






