Choline in Adolescent Pregnancy: The Impact on Fetal Brain Development and Long-Term Cognitive Outcomes of Offspring
Abstract
1. Introduction
Evolutionary and Developmental Context of Adolescent Pregnancy
- maternal brain development continues until approximately 25 years of age, with the prefrontal cortex among the last regions to mature [20]. This ongoing neural maturation creates direct competition between maternal and fetal brains for essential nutrients, particularly those required for membrane synthesis, neurotransmitter production, and myelination.
- modern adolescents in developed countries typically experience menarche at ages when peak linear growth has not yet been achieved, creating simultaneous demands for nutrients to support both continued maternal skeletal growth and fetal development. This differs from the evolutionary pattern, where menarche typically occurred near the completion of growth.
- contemporary adolescents often have lower pre-pregnancy nutritional reserves compared to adult women, particularly for nutrients like choline that require hepatic storage. Studies demonstrate that adolescents have approximately 40–50% lower liver choline concentrations compared to adults, limiting the ability to mobilize reserves during pregnancy.
2. Choline Metabolism and Biochemical Pathways
2.1. The Kennedy Pathway: Phospholipid Synthesis
2.2. Acetylcholine Synthesis Pathway
2.3. Betaine Pathway: Methylation Reactions
2.4. Adolescent-Specific Metabolic Differences
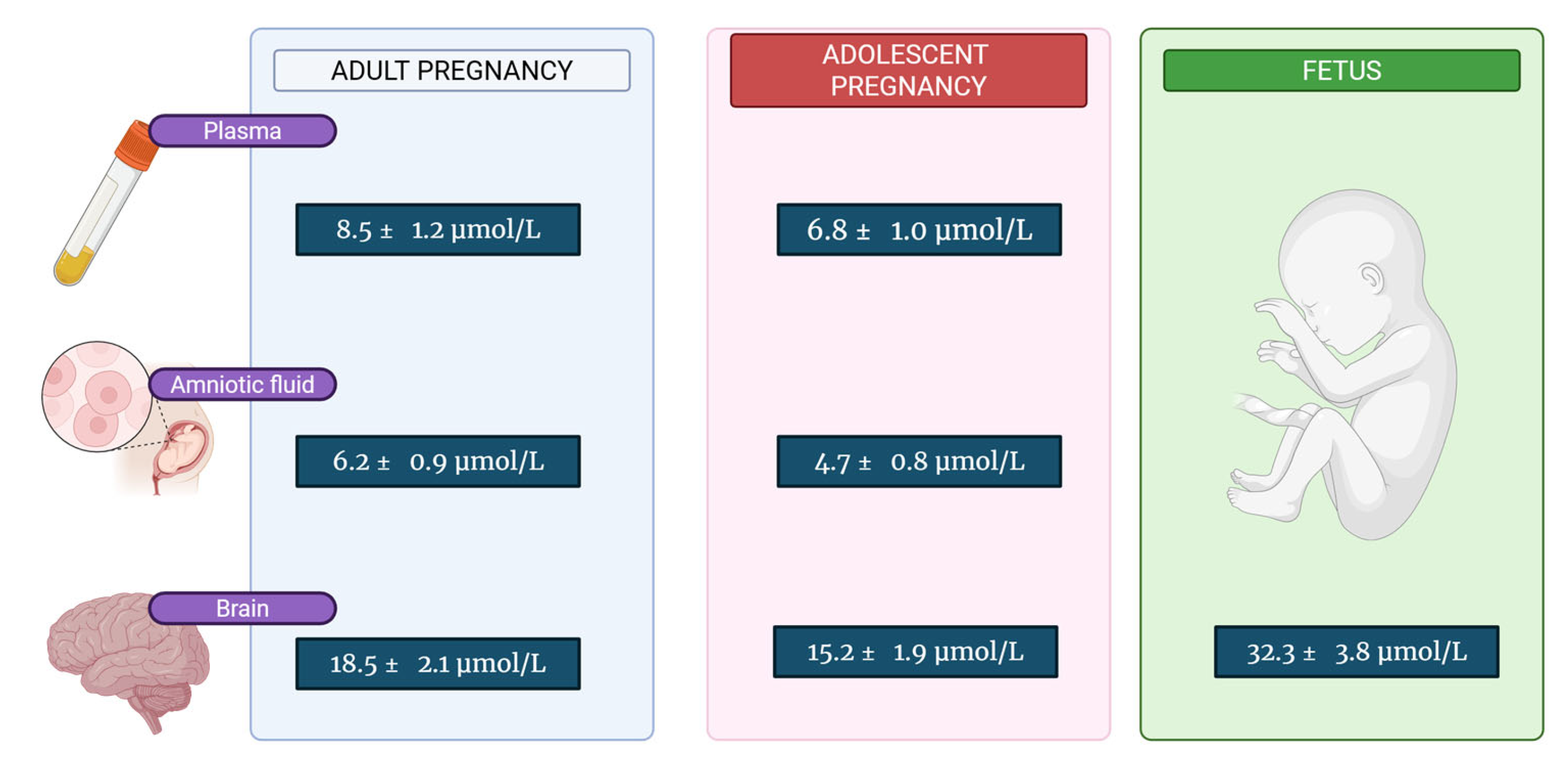
| Tissue/Fluid | Adult Pregnancy | Adolescent Pregnancy | Fetus (Third Trimester) | Ref. |
|---|---|---|---|---|
| Maternal Plasma | 8.5 ± 1.2 | 6.8 ± 1.0 | - | [44,45] |
| Fetal Plasma | 22.1 ± 3.5 | 18.3 ± 2.8 | 20.2 ± 3.1 | [52,53] |
| Amniotic Fluid | 6.2 ± 0.9 | 4.7 ± 0.8 | - | [54] |
| Maternal Brain | 18.5 ± 2.1 | 15.2 ± 1.9 | - | [55] |
| Fetal Brain | 35.8 ± 4.2 | 28.9 ± 3.6 | 32.3 ± 3.8 | [56,57] |
3. Placental Choline Transport Mechanisms
3.1. Choline Transporter-like Protein 1 (CTL1/SLC44A1)
3.2. Choline Transporter-like Protein 2 (CTL2/SLC44A2)
3.3. Organic Cation Transporters (OCT1, OCT3)
3.4. Transport Limitations in Adolescent Pregnancy
3.5. Mechanistic Basis of Transport Limitations in Adolescent Pregnancy
3.5.1. Placental Developmental Immaturity
3.5.2. Altered Endocrine Regulation
- placental growth hormone variant (GH-V) secretion follows a distinct pattern in adolescent versus adult pregnancy. GH-V normally increases exponentially from weeks 15–37 of gestation and plays a crucial role in upregulating placental nutrient transporter expression through STAT5 signaling pathways. Adolescent pregnancies may demonstrate altered GH-V patterns, potentially limiting transporter upregulation during critical developmental windows.
- the estrogen-to-progesterone ratio shows age-dependent variations that influence CTL1 expression. CTL1 gene promoter analysis reveals functional estrogen response elements (EREs) that mediate transcriptional activation. Adolescent pregnancies exhibit altered estrogen: progesterone ratios, particularly in early pregnancy, which may affect the hormonal drive for CTL1 upregulation.
- leptin and adiponectin—adipokines that regulate placental nutrient transport—show distinct patterns in adolescent pregnancies [5]. Leptin normally increases throughout pregnancy and enhances amino acid transporter expression through mTOR pathway activation. Adolescent pregnant women may demonstrate different leptin concentrations adjusted for body fat percentage, potentially reflecting ongoing competition between maternal growth needs and pregnancy adaptations.
3.5.3. Nutrient Prioritization and Maternal-Placental Competition
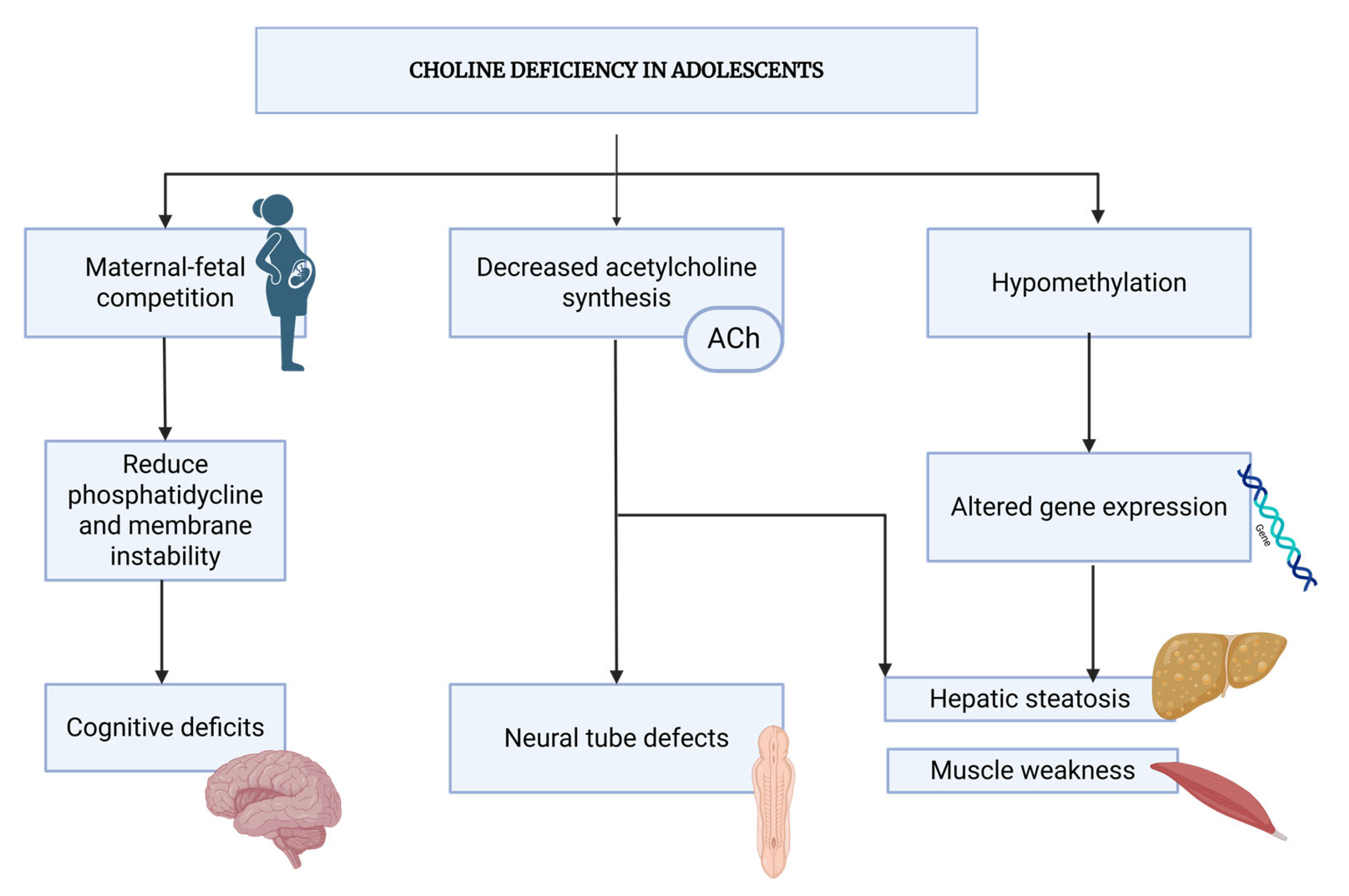
4. Pathophysiology of Choline Deficiency in Adolescent Pregnancy
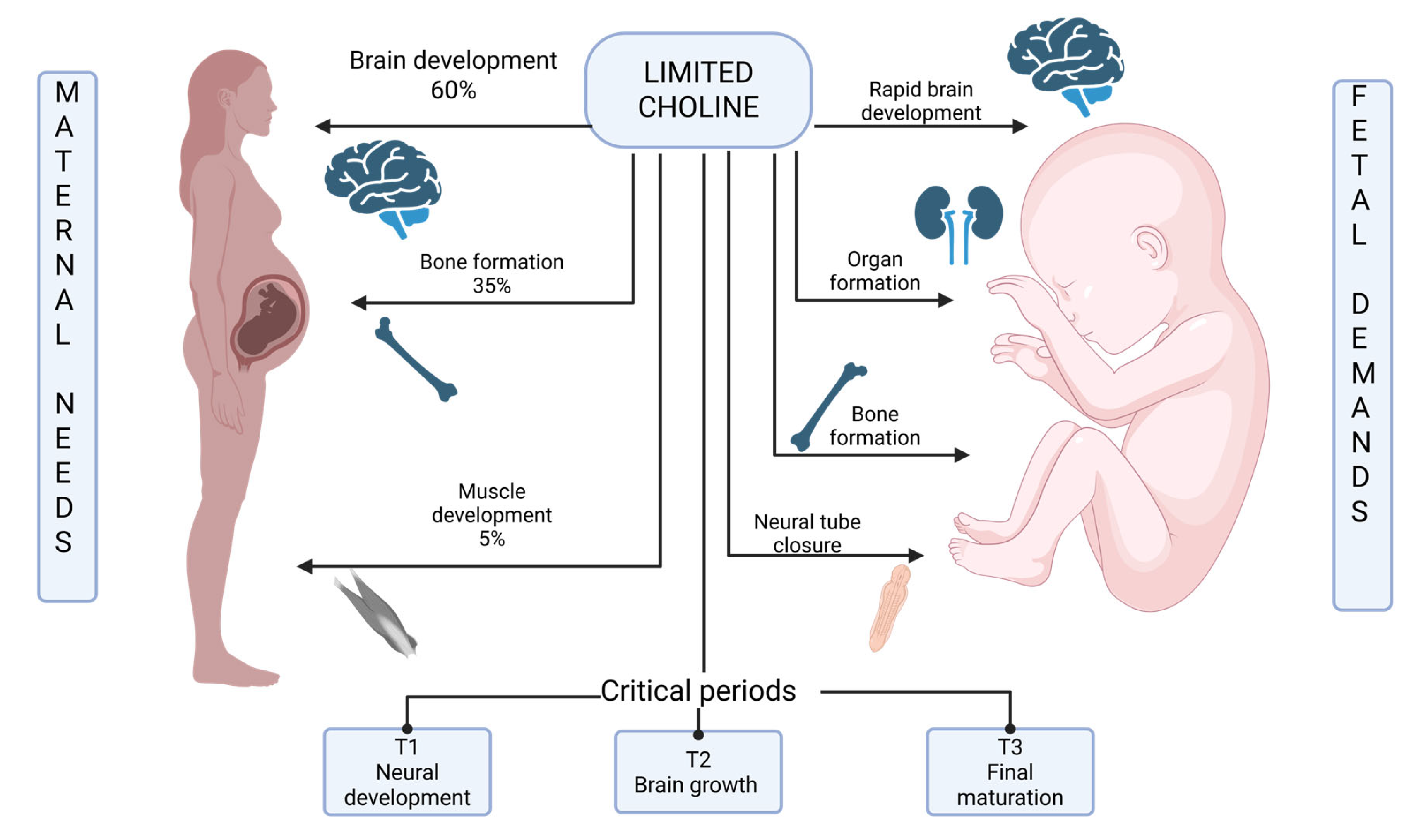
4.1. Maternal–Fetal Competition for Choline Resources
- Maternal basal metabolism: ~300 mg/day for ongoing cellular functions, including membrane phospholipid turnover, neurotransmitter synthesis (acetylcholine production), and hepatic lipid metabolism (VLDL secretion)
- Total estimated requirement for pregnant adolescents: 600–700 mg/day
4.2. Myelination in Adolescents
- -
- The placenta maintains a concentration gradient through energy-dependent transport mechanisms. CTL1 transporters (SLC44A1) function bidirectionally but show preferential transport from maternal to fetal circulation when coupled with Na+-K+-ATPase activity and membrane potential gradients [2,6]. This active transport system can concentrate choline against a concentration gradient, like active transport of amino acids and other essential nutrients. The transport capacity is estimated at 0.5–0.8 μmol/min/kg placental tissue, sufficient to maintain elevated fetal levels under normal conditions [2]. The fetal liver has substantially lower choline oxidase and choline dehydrogenase activity (approximately 30–40% of adult levels) compared to maternal liver, resulting in reduced catabolism and higher circulating levels.
- -
- While the fetal brain actively incorporates choline for rapid neurogenesis and membrane synthesis, the overall fetal metabolic rate for choline degradation is 40–50% lower than maternal rates [106]. This metabolic immaturity serves to conserve choline for biosynthetic purposes rather than oxidative metabolism. The fetus actively sequesters choline in developing neural tissues through high-affinity choline transporters (CHT1 and CTL1) expressed at the blood–brain barrier [2,12]. Fetal brain tissue concentrations reach 32–36 μmol/L during the third trimester, representing a 1.5–2-fold concentration gradient over fetal plasma [12]. This preferential accumulation reflects the critical importance of choline for neurodevelopment, with the fetal brain prioritizing choline uptake even when plasma levels are marginal. The placenta itself possesses enzymatic capacity for choline synthesis and metabolism. Placental tissue expresses PEMT (phosphatidylethanolamine N-methyltransferase), which can synthesize phosphatidylcholine de novo, and phospholipases that release free choline from phospholipids [2,109,110]. Placental choline oxidation is minimal (representing less than 5% of choline uptake), preserving choline for transfer to fetal circulation [2]. Additionally, the placenta releases choline metabolites including phosphocholine and glycerophosphocholine into fetal circulation, contributing to elevated total choline-containing compounds.
- -
- Maternal physiology appears to prioritize fetal choline delivery through several adaptive mechanisms, even at the expense of maternal stores [30,31,106]. During pregnancy, upregulation of placental choline transporters increases transfer capacity [37,38]. Maternal choline clearance decreases during pregnancy (from 1.5 mL/min/kg in non-pregnant state to 0.8–1.2 mL/min/kg during pregnancy), conserving choline in circulation for placental uptake [30,31]. Furthermore, maternal liver and skeletal muscle choline concentrations decline progressively during pregnancy while fetal concentrations remain stable or increase, demonstrating net maternal-to-fetal choline flux [30,106,107]. Fetal kidneys exhibit lower choline excretion rates compared to maternal kidneys, with fractional reabsorption exceeding 95% [106]. This efficient renal conservation mechanism contributes to maintaining elevated fetal plasma choline concentrations.
4.3. Biochemical Consequences of Deficiency
4.4. Developmental Impact on Fetal Brain
4.5. Systemic Consequences Beyond the Brain
| Author | Study Design | Population | Sample Size (n) | Choline Assessment Method | Main Outcome Measures | Key Findings |
|---|---|---|---|---|---|---|
| Bahnfleth [76] | RCT, double-blind, 7-year follow-up | Pregnant women (18–35 y), subset ages 18–21 | n = 140 total (24 young mothers) | Maternal plasma choline measured by LC-MS/MS | Child sustained attention at age 7 years | Supplementation (930 mg/day) improved attention: 15% faster reaction time, 23% fewer lapses (p < 0.01) |
| Jacobson [55] | RCT, double-blind, placebo-controlled | Pregnant women with alcohol exposure (18–35 y) | n = 62 (31 choline, 31 placebo) | Maternal plasma choline at baseline and throughout pregnancy | Infant growth and cognition at 12 months | Choline (2000 mg/day) improved recognition memory (p = 0.01) and reduced cognitive errors by 22% (p = 0.04) |
| Wu [14,129] | Prospective cohort | Pregnant women (19–42 y) | n = 154 | Maternal plasma free choline and betaine (LC-MS/MS) at 16 weeks | Infant cognitive development at 18 months (Bayley Scales) | Each 1 μmol/L increase in maternal choline associated with 0.11-point higher MDI (p = 0.02); 4.8-point difference between tertiles |
| Boeke [14] | Prospective cohort (Project Viva) | Pregnant women (20–40 y) | n = 1038 mother-child pairs | Validated food frequency questionnaire during pregnancy | Child cognition at age 7 years | Highest choline intake quartile (>449 mg/day) vs. lowest (<237 mg/day): better visual memory |
| Mellott [130] | Animal model (rat), controlled diet | Pregnant rats | n = 45 (15 per group: supplemented/control/deficient) | Controlled dietary choline: 5.0 g/kg (supplemented), 1.1 g/kg (control), 0 g/kg (deficient); plasma choline | Offspring hippocampal development, MAPK/CREB activation, spatial memory | Supplementation enhanced hippocampal maturation, increased MAPK/CREB phosphorylation (p < 0.001), improved memory |
| Jadavji [131] | Animal model (mouse), controlled diet | Pregnant mice (MTHFR+/+ and MTHFR+/-) | n = 80 (20 per group) | Controlled dietary choline: 0.6 g/kg (deficient) vs. 1.2 g/kg (control); maternal/fetal choline and DNA methylation measured | Offspring hippocampal neurons, apoptosis, memory, DNA methylation | Deficiency: 15–20% fewer neurons (p < 0.001), increased apoptosis, impaired memory (p < 0.01), altered BDNF/CREB methylation |
| Wong-Goodrich [132] | Animal model (rat), controlled diet, lifespan study | Pregnant rats with prenatal and adult choline manipulation | n = 64 offspring | Controlled dietary choline: 5 g/kg (supplemented) vs. 1.1 g/kg (control); plasma and brain tissue choline measured | Adult hippocampal plasticity, neurogenesis, spatial memory | Prenatal supplementation: 25% more progenitor cells (p < 0.001), enhanced LTP (p < 0.01), superior memory performance |
| Moreno [133] | Animal model (rat), controlled diet | Pregnant rats | n = 40 litters | Controlled dietary choline: 5 g/kg vs. 1.1 g/kg; offspring plasma choline measured | Developmental trajectory of memory function | Supplementation accelerated memory emergence by 3–5 days (p < 0.001), enhanced hippocampal-dependent learning |
| Baumgartner [2] | Human tissue analysis | Placentas from pregnancies (20–40 y) | n = 29 placentas (8–40 weeks gestation) | Placental tissue: CTL1/CTL2 mRNA (qRT-PCR) and protein (Western blot) | Choline transporter expression across gestation | CTL1 increases throughout gestation; localized to syncytiotrophoblast microvillous membrane; peak expression at term |
| Bernhard [106] | Prospective cohort | Preterm and term infants with mothers | n = 88 mother-infant pairs | Maternal plasma and cord blood choline (LC-MS/MS) at delivery | Maternal–fetal choline concentration gradient | Median cord blood choline (16.3 μmol/L) > maternal plasma (8.9 μmol/L); ratio 1.83:1 (p < 0.001); preterm infants lower |
| Taesuwan [107] | RCT, metabolomics study | Pregnant women (22–35 y) | n = 26 (13 per group) | Comprehensive choline metabolome in maternal plasma, cord blood, placenta (LC-MS/MS) | Choline metabolite profiles across pregnancy and delivery | Supplementation (550 mg/day) increased maternal betaine; altered placental choline partitioning; increased cord blood phosphocholine |
| Shaw [70] | Case–control study | Women with NTD-affected pregnancies vs. controls | n = 424 cases, 440 controls | Periconceptional dietary choline and betaine intake (FFQ) | Neural tube defects in offspring | Highest quartile choline intake (>498 mg/day) vs. lowest (<290 mg/day): 51% reduced NTD risk (OR 0.49, 95% CI 0.30–0.81) |
| Shaw [17] | Case–control study | Women with NTD-affected pregnancies vs. controls | n = 330 cases, 680 controls | Maternal plasma total choline, betaine, methionine, vitamers (LC-MS/MS) | Neural tube defects in offspring | Low plasma choline (<5.1 μmol/L) associated with 2.0-fold increased NTD risk (OR 2.0, 95% CI 1.2–3.4), independent of folate |
| Das [11] | Narrative review | Adolescents (10–19 y) | Review of 89 studies | Systematic literature review | Adolescent nutritional physiology, metabolism, requirements | Adolescents have unique nutritional needs due to ongoing growth; pregnancy compounds demands; many deficiencies documented |
| Cusick [134] | Narrative review | Prenatal through age 2 years | Review of 127 studies | Systematic literature review | Nutrition and brain development in first 1000 days | Critical periods for nutritional influence on brain development; choline, iron, iodine, folate essential; deficiencies have lasting effects |
5. Clinical Implications and Current Evidence
5.1. Cognitive and Neurological Outcomes in Offspring
5.2. Maternal Health Outcomes
5.3. Pregnancy Complications and Birth Outcomes
| Parameter | Adolescents (15–19 years) | Adults (20–35 years) | Clinical Significance | Ref. |
|---|---|---|---|---|
| Plasma choline (μmol/L) | 6.8 ± 1.0 | 8.5 ± 1.2 | Increased deficiency risk | [78,129] |
| Choline clearance (mL/min/kg) | 0.8 ± 0.2 | 1.2 ± 0.3 | Reduced renal handling | [87,149] |
| Hepatic choline reserves (% of adult) | 55 ± 12 | 100 (reference) | Limited storage capacity | [96,144] |
| CHKA activity (% increase) | 150–200 | 200–300 | Impaired PC synthesis | [3,113,114] |
| CTL1 expression (% of adult) | 80 ± 15 | 100 (reference) | Reduced placental transport | [3,6] |
| Methylation capacity (SAM/SAH ratio) | 2.8 ± 0.4 | 3.6 ± 0.5 | Compromised epigenetic regulation | [140,150] |
5.4. Evidence from Animal Models
5.4.1. Rodent Models of Prenatal Choline Supplementation and Deficiency
- Increases in hippocampal progenitor cell proliferation during the critical period of neurogenesis (equivalent to human second trimester)
- Enhanced dendritic spine density in CA1 and CA3 pyramidal neurons, with more dendritic spines per unit dendrite length persisting into adulthood
- Larger hippocampal volume maintained throughout the lifespan
- Reduced age-related hippocampal atrophy, suggesting neuroprotective effects extending into senescence
- Conversely, prenatal choline deficiency (typically <25% of adequate intake) produces opposite effects, with smaller hippocampi, reduced neuronal numbers, and impaired neurogenesis.
- The structural changes induced by prenatal choline availability translate into functional differences in neurotransmitter systems and synaptic plasticity. Offspring of choline-supplemented dams show greater acetylcholine release capacity in hippocampal synapses in response to depolarizing stimuli. This enhanced cholinergic function correlates with improved performance on hippocampus-dependent spatial memory tasks.
5.4.2. Epigenetic Mechanisms
5.4.3. Translational Limitations and Considerations
- Species differences in choline metabolism: Rodents have higher rates of endogenous phosphatidylcholine synthesis via PEMT compared to humans, potentially making them less dependent on dietary choline
- Lack of true adolescent developmental stage: Rodents transition more abruptly from juvenility to reproductive maturity without the extended adolescent growth phase characteristic of humans
- Compressed gestation period: Rat gestation lasts only 21 days versus 40 weeks in humans, compressing developmental events into a much shorter timeframe
- Genetic homogeneity: Inbred laboratory rodent strains lack the genetic diversity of human populations
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PC | Phosphatidylcholine |
| CK | Choline Kinase |
| CHKA | Choline Kinase α (Alpha) |
| CCT | Phosphocholine Cytidylyltransferase |
| ACh | Acetylcholine |
| ChAT | Choline acetyltransferase |
| SAM | S-adenosylmethionine |
| SAH | S-adenosylhomocysteine |
| PEMT | Phosphatidylethanolamine N-methyltransferase |
| VLDL | Very Low-Density Lipoprotein |
| SM | Sphingomyelin |
| CTL1 | Choline Transporter-Like Protein 1 (SLC44A1) |
| CTL2 | Choline Transporter-Like Protein 2 (SLC44A2) |
| OCT1 | Organic Cation Transporter 1 (SLC22A1) |
| OCT2 | Organic Cation Transporter 2 (SLC22A2) |
| OCT3 | Organic Cation Transporter 3 (SLC22A3) |
| Km | Michaelis-Menten Constant |
| Vmax | Maximum Enzymatic Velocity |
| BDNF | Brain-Derived Neurotrophic Factor |
| CREB | cAMP Response Element-Binding Protein |
| NTDs | Neural Tube Defects |
| IUGR | Intrauterine Growth Restriction |
References
- W.H.O. Adolescent Pregnancy: Unmet Needs and Undone Deeds; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Baumgartner, H.K.; Trinder, K.M.; Galimanis, C.E.; Post, A.; Wilcox, J.; Dawson, P.A. Characterization of choline transporters in the human placenta over gestation. Placenta 2015, 36, 1362–1369. [Google Scholar] [CrossRef]
- Michel, V.; Yuan, Z.; Ramsubir, S.; Bakovic, M. Choline transport for phospholipid synthesis. Exp. Biol. Med. 2006, 231, 490–504. [Google Scholar] [CrossRef]
- Blusztajn, J.K.; Mellott, T.J. Choline nutrition programs brain development via DNA and histone methylation. Cent. Nerv. Syst. Agents Med. Chem. 2012, 12, 82–94. [Google Scholar] [CrossRef]
- Medicine, I. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline; National Academies Press: Washington, DC, USA, 1998. [Google Scholar]
- Hedtke, V.; Bakovic, M. Choline transport for phospholipid synthesis: An emerging role of choline transporter-like protein 1. Exp. Biol. Med. 2019, 244, 655–662. [Google Scholar] [CrossRef]
- Ridgway, N.D. The role of phosphatidylcholine and choline metabolites to cell proliferation and survival. Crit. Rev. Biochem. Mol. Biol. 2013, 48, 20–38. [Google Scholar] [CrossRef]
- Paoletti, L.; Elena, C.; Domizi, P.; Banchio, C. Role of phosphatidylcholine during neuronal differentiation. IUBMB Life 2011, 63, 714–720. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Tie, A.; Tarnopolsky, M.; Bakovic, M. Genomic organization, promoter activity, and expression of the human choline transporter-like protein 1. Physiol. Genom. 2006, 26, 76–90. [Google Scholar] [CrossRef] [PubMed]
- Marvin-Dowle, K.; Burley, V.J.; Soltani, H. Nutrient intakes and nutritional biomarkers in pregnant adolescents: A systematic review of studies in developed countries. BMC Pregnancy Childbirth 2016, 16, 268. [Google Scholar] [CrossRef]
- Das, J.K.; Salam, R.A.; Thornburg, K.L.; Prentice, A.M.; Campisi, S.; Lassi, Z.S.; Koletzko, B.; Bhutta, Z.A. Nutrition in adolescents: Physiology, metabolism, and nutritional needs. Ann. N. Y. Acad. Sci. 2017, 1393, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H. Choline: Critical role during fetal development and dietary requirements in adults. Annu. Rev. Nutr. 2006, 26, 229–250. [Google Scholar] [CrossRef]
- Innis, S.M. Dietary omega 3 fatty acids and the developing brain. Brain Res. 2008, 1237, 35–43. [Google Scholar] [CrossRef]
- Boeke, C.E.; Gillman, M.W.; Hughes, M.D.; Rifas-Shiman, S.L.; Villamor, E.; Oken, E. Choline intake during pregnancy and child cognition at age 7 years. Am. J. Epidemiol. 2013, 177, 1338–1347. [Google Scholar] [CrossRef] [PubMed]
- Veena, S.R.; Gale, C.R.; Krishnaveni, G.V.; Kehoe, S.H.; Srinivasan, K.; Fall, C.H. Association between maternal nutritional status in pregnancy and offspring cognitive function during childhood and adolescence; a systematic review. BMC Pregnancy Childbirth 2016, 16, 220. [Google Scholar] [CrossRef] [PubMed]
- Caudill, M.A. Pre- and postnatal health: Evidence of increased choline needs. J. Am. Diet. Assoc. 2010, 110, 1198–1206. [Google Scholar] [CrossRef]
- Shaw, G.M.; Carmichael, S.L.; Yang, W.; Selvin, S.; Schaffer, D.M. Periconceptional dietary intake of choline and betaine and neural tube defects in offspring. Am. J. Epidemiol. 2004, 160, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.C.; Zeisel, S.H.; Mar, M.H.; Sadler, T.W. Inhibitors of choline uptake and metabolism cause developmental abnormalities in neurulating mouse embryos. Teratology 2001, 64, 114–122. [Google Scholar] [CrossRef]
- Hochberg, Z.; Belsky, J. Evo-devo of human adolescence: Beyond disease models of early puberty. BMC Med. 2013, 11, 113. [Google Scholar] [CrossRef]
- Wallace, T.C.; Blusztajn, J.K.; Caudill, M.A.; Klatt, K.C.; Natker, E.; Zeisel, S.H.; Zelman, K.M. Choline: The neurocognitive essential nutrient of interest to obstetricians and gynecologists. J. Diet. Suppl. 2020, 17, 733–752. [Google Scholar] [CrossRef]
- Craciunescu, C.N.; Albright, C.D.; Mar, M.H.; Song, J.; Zeisel, S. Choline availability during embryonic development alters progenitor cell mitosis in developing mouse hippocampus. J. Nutr. 2003, 133, 3614–3618. [Google Scholar] [CrossRef]
- Meck, W.H.; Williams, C.L. Metabolic imprinting of choline by its availability during gestation: Implications for memory and attentional processing across the lifespan. Neurosci. Biobehav. Rev. 2003, 27, 385–399. [Google Scholar] [CrossRef]
- Niculescu, M.D.; Craciunescu, C.N.; Zeisel, S.H. Dietary choline deficiency alters global and gene-specific DNA methylation in the developing hippocampus of mouse fetal brains. FASEB J. 2006, 20, 43–49. [Google Scholar] [CrossRef]
- Fullerton, M.D.; Wagner, L.; Yuan, Z.; Bakovic, M. Impaired trafficking of choline transporter-like protein-1 at plasma membrane and inhibition of choline transport in THP-1 monocyte-derived macrophages. Am. J. Physiol. Cell Physiol. 2006, 290, C1230–C1238. [Google Scholar] [CrossRef]
- Marcucci, H.; Paoletti, L.; Jackowski, S.; Banchio, C. Phosphatidylcholine biosynthesis during neuronal differentiation and its role in cell fate determination. J. Biol. Chem. 2010, 285, 25382–25393. [Google Scholar] [CrossRef] [PubMed]
- Kwan, S.T.C.; King, J.H.; Yan, J.; Wang, Z.; Jiang, X.; Hutzler, J.S.; Klein, H.R.; Brenna, J.T.; Roberson, M.S.; Caudill, M.A. Maternal choline supplementation modulates placental nutrient transport and metabolism in late gestation of mouse pregnancy. J. Nutr. 2017, 147, 2083–2092. [Google Scholar] [CrossRef]
- Lewis, E.D.; Richard, C.; Larsen, B.M.; Field, C.J. The importance of human milk for immunity in preterm infants. Clin. Perinatol. 2017, 44, 23–47. [Google Scholar] [CrossRef]
- Larqué, E.; Gil-Sánchez, A.; Prieto-Sánchez, M.T.; Koletzko, B. Omega 3 fatty acids, gestation and pregnancy outcomes. Br. J. Nutr. 2012, 107 (Suppl. 2), S77–S84. [Google Scholar] [CrossRef]
- Bernardi, J.R.; Escobar, R.S.; Ferreira, C.F.; Silveira, P.P. Fetal and neonatal levels of omega-3: Effects on neurodevelopment, nutrition, and growth. Sci. World J. 2012, 2012, 202473. [Google Scholar] [CrossRef]
- Yan, J.; Jiang, X.; West, A.A.; Perry, C.A.; Malysheva, O.V.; Devapatla, S.; Pressman, E.; Vermeylen, F.; Caudill, M.A. Maternal choline intake modulates maternal and fetal biomarkers of choline metabolism in humans. Am. J. Clin. Nutr. 2012, 95, 1060–1071. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H. Gene response elements, genetic polymorphisms and epigenetics influence the human dietary requirement for choline. IUBMB Life 2007, 59, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Sherzai, D.; Moness, R.; Sherzai, S.; Sherzai, A. A systematic review of omega-3 fatty acid consumption and cognitive outcomes in neurodevelopment. Am. J. Lifestyle Med. 2023, 17, 228–243. [Google Scholar] [CrossRef]
- Hacker, A.N.; Fung, E.B.; King, J.C. Role of calcium during pregnancy: Maternal and fetal needs. Nutr. Rev. 2012, 70, 397–409. [Google Scholar] [CrossRef]
- Golden, N.H.; Nutrition, A.S.C. on: Optimizing bone health in children and adolescents. Pediatrics 2014, 134, e1229–e1243. [Google Scholar] [CrossRef]
- Kovacs, C.S. Calcium and bone metabolism in pregnancy and lactation. J. Clin. Endocrinol. Metab. 2001, 86, 2344–2348. [Google Scholar] [CrossRef]
- Moran, V.H. Nutritional status in pregnant adolescents: A systematic review of biochemical markers. Matern. Child Nutr. 2007, 3, 74–93. [Google Scholar] [CrossRef]
- Nupo, S.S.; Martínez-De la Fuente, V.; Cruz, G.O.; Cortes-Hernandez, J.L. Teenage pregnancy and micronutrient deficiency: A critical review. Tecnociencia Chihuah. 2024, 18, 3. [Google Scholar] [CrossRef]
- Rosenberg, K.; McEwan, H.P. Teenage pregnancy in Scotland: Trends and risks. Scott. Med. J. 1991, 36, 172–174. [Google Scholar] [CrossRef] [PubMed]
- Williamson, C.S. Nutrition in pregnancy. Nutr. Bull. 2006, 31, 28–59. [Google Scholar] [CrossRef]
- Chantry, C.J.; Auinger, P.; Byrd, R.S. Lactation among adolescent mothers and subsequent bone mineral density. Arch. Pediatr. Adolesc. Med. 2004, 158, 650–656. [Google Scholar] [CrossRef]
- Behere, R.V.; Deshmukh, A.S.; Otiv, S.; Gupte, M.D.; Yajnik, C.S. Maternal vitamin B12 status during pregnancy and its association with outcomes of pregnancy and health of the offspring: A systematic review and implications for policy in India. Front. Endocrinol. 2021, 12, 619176. [Google Scholar] [CrossRef]
- Mamabolo, R.L.; Alberts, M.; Steyn, N.P.; Levitt, N.S. The effect of maternal glucose metabolism, iron, vitamin B12 and folate status on pregnancy outcomes. S. Afr. J. Clin. Nutr. 2006, 19, 120–126. [Google Scholar]
- Wellinghausen, N. Immunobiology of gestational zinc deficiency. Br. J. Nutr. 2001, 85, 81–86. [Google Scholar] [CrossRef]
- de Moraes, M.L.; Barbosa, R.d.F.; Santo, R.E.; Santos, F.d.S.; de Jesus, E.F.O.; Sardinha, F.L.d.C.; Carmo, M.d.G.T.D. Maternal-fetal distribution of calcium, iron, copper, and zinc in pregnant teenagers and adults. Biol. Trace Elem. Res. 2011, 139, 139–147. [Google Scholar] [CrossRef]
- Swanson, C.A.; King, J.C. Zinc and pregnancy outcome. Am. J. Clin. Nutr. 1987, 46, 763–771. [Google Scholar] [CrossRef]
- Deshpande, J.D.; Joshi, M.M.; Giri, P.A. Zinc: The trace element of major importance in human nutrition and health. Int. J. Med. Sci. Public Health 2013, 2, 1–6. [Google Scholar] [CrossRef]
- Woo, Q.Y.; Lee, B.T.K.; Lim, L.W.; Zhang, J.; Tashiro, A.; Lau, P.K.; Thibault, G.; Wang, Y.; Lin, V.C.L. Choline intake during pregnancy influences maternal cognitive function and hippocampal gene expression in late adulthood. Nutr. Neurosci. 2025, 28, 1–15. [Google Scholar] [CrossRef]
- Ray, J.G.; Wyatt, P.R.; Thompson, M.D.; Vermeulen, M.J.; Meier, C.; Wong, P.-Y.; Farrell, S.A.; Cole, D.E.C. Vitamin B12 and the risk of neural tube defects in a folic-acid-fortified population. Epidemiology 2007, 18, 362–366. [Google Scholar] [CrossRef]
- Molloy, A.M.; Kirke, P.N.; Troendle, J.F.; Burke, H.; Sutton, M.; Brody, L.C.; Scott, J.M.; Mills, J.L. Maternal vitamin B12 status and risk of neural tube defects in a population with high neural tube defect prevalence and no folic Acid fortification. Pediatrics 2009, 123, 917–923. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, J.L.; Layden, A.J.; Stover, P.J. Vitamin B-12 and perinatal health. Adv. Nutr. 2015, 6, 552–563. [Google Scholar] [CrossRef] [PubMed]
- Derbyshire, E.; Obeid, R.; Schön, C. Habitual choline intakes across the childbearing years: A review. Nutrients 2021, 13, 4390. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Oktayani, P.P.I.; Lee, S.-D.; Huang, L.-C. Choline in pregnant women: A systematic review and meta-analysis. Nutr. Rev. 2025, 83, e2009–e2014. [Google Scholar] [CrossRef] [PubMed]
- Spoelstra, S.K.; Eijsink, J.J.H.; Hoenders, H.J.R.; Knegtering, H. Maternal choline supplementation during pregnancy to promote mental health in offspring. Early Interv. Psychiatry 2023, 17, 947–958. [Google Scholar] [CrossRef]
- Caudill, M.A.; Strupp, B.J.; Muscalu, L.; Nevins, J.E.H.; Canfield, R.L. Maternal choline supplementation during the third trimester of pregnancy improves infant information processing speed: A randomized, double-blind, controlled feeding study. FASEB J. 2018, 32, 2172–2180. [Google Scholar] [CrossRef]
- Jacobson, S.W.; Carter, R.C.; Molteno, C.D.; Stanton, M.E.; Herbert, J.S.; Lindinger, N.M.; Lewis, C.E.; Dodge, N.C.; Hoyme, H.E.; Zeisel, S.H.; et al. Efficacy of maternal choline supplementation during pregnancy in mitigating adverse effects of prenatal alcohol exposure on growth and cognitive function: A randomized, double-blind, placebo-controlled clinical trial. Alcohol. Clin. Exp. Res. 2018, 42, 1327–1341. [Google Scholar] [CrossRef]
- Ross, R.G.; Hunter, S.K.; McCarthy, L.; Beuler, J.; Hutchison, A.K.; Wagner, B.D.; Leonard, S.; Stevens, K.E.; Freedman, R. Perinatal choline effects on neonatal pathophysiology related to later schizophrenia risk. Am. J. Psychiatry 2013, 170, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Mudd, A.T.; Getty, C.M.; Sutton, B.P.; Dilger, R.N. Perinatal choline deficiency delays brain development and alters metabolite concentrations in the young pig. Nutr. Neurosci. 2016, 19, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Ganz, A.B.; Klatt, K.C.; Caudill, M.A. Common genetic variants alter metabolism and influence dietary choline requirements. Nutrients 2017, 9, 837. [Google Scholar] [CrossRef] [PubMed]
- Korsmo, H.W.; Jiang, X.; Caudill, M.A. Choline: Exploring the growing science on its benefits for moms and babies. Nutrients 2019, 11, 1823. [Google Scholar] [CrossRef]
- Rubinchik-Stern, M.; Shmuel, M.; Bar, J.; Kovo, M.; Eyal, S. Adverse placental effects of valproic acid: Studies in perfused human placentas. Epilepsia 2018, 59, 993–1003. [Google Scholar] [CrossRef]
- Taylor, A. SLC44A1 Transport of Choline and Ethanolamine in Disease. Ph.D. Thesis, University of Guelph, Guelph, ON, Canada, 2019. [Google Scholar]
- Zeisel, S.H.; Klatt, K.C.; Caudill, M.A. Choline. Adv. Nutr. 2018, 9, 58–60. [Google Scholar] [CrossRef]
- Wallace, T.C.; Fulgoni, V.L. Assessment of total choline intakes in the United States. J. Am. Coll. Nutr. 2016, 35, 108–112. [Google Scholar] [CrossRef]
- Chester, D.N.; Goldman, J.D.; Ahuja, J.K.; Moshfegh, A.J. Dietary intakes of choline: What we eat in America, NHANES 2007–2008. In Food Surveys Research Group Dietary Data Brief; United States Department of Agriculture: Beltsville, MD, USA, 2007. [Google Scholar]
- Bailey, R.L.; Pac, S.G.; Fulgoni, V.L.; Reidy, K.C.; Catalano, P.M. Estimation of total usual dietary intakes of pregnant women in the United States. JAMA Netw. Open 2019, 2, e195967. [Google Scholar] [CrossRef]
- Klatt, K.C.; McDougall, M.Q.; Malysheva, O.V.; Taesuwan, S.; Loinard-González, A.A.P.; Nevins, J.E.H.; Beckman, K.; Bhawal, R.; Anderson, E.; Zhang, S.; et al. Prenatal choline supplementation improves biomarkers of maternal docosahexaenoic acid status among pregnant participants consuming supplemental DHA: A randomized controlled trial. Am. J. Clin. Nutr. 2022, 116, 1357–1368. [Google Scholar] [CrossRef] [PubMed]
- Wiedeman, A.M.; Barr, S.I.; Green, T.J.; Xu, Z.; Innis, S.M.; Kitts, D.D. Dietary choline intake: Current state of knowledge across the life cycle. Nutrients 2018, 10, 1513. [Google Scholar] [CrossRef]
- Lewis, E.D.; Subhan, F.B.; Bell, R.C.; McCargar, L.J.; Curtis, J.M.; Jacobs, R.L.; Field, C.J.; APrON team. Estimation of choline intake from 24 h dietary intake recalls and contribution of egg and milk consumption to intake among pregnant and breastfeeding women in Alberta. Br. J. Nutr. 2014, 112, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Masih, S.P.; Plumptre, L.; Ly, A.; Berger, H.; Lausman, A.Y.; Croxford, R.; Kim, Y.-I.; O’cOnnor, D.L. Pregnant Canadian women achieve recommended intakes of one-carbon nutrients through prenatal supplementation but the supplement composition, including choline, requires reconsideration. J. Nutr. 2015, 145, 1824–1834. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, S.L.; Yang, W.; Shaw, G.M. Periconceptional nutrient intakes and risks of neural tube defects in California. Birth Defects Res. A Clin. Mol. Teratol. 2010, 88, 670–678. [Google Scholar] [CrossRef]
- Poly, C.; Massaro, J.M.; Seshadri, S.; Wolf, P.A.; Cho, E.; Krall, E.; Jacques, P.F.; Au, R. The relation of dietary choline to cognitive performance and white-matter hyperintensity in the Framingham Offspring Cohort. Am. J. Clin. Nutr. 2011, 94, 1584–1591. [Google Scholar] [CrossRef]
- Bidulescu, A.; Chambless, L.E.; Siega-Riz, A.M.; Zeisel, S.H.; Heiss, G. Usual choline and betaine dietary intake and incident coronary heart disease: The Atherosclerosis Risk in Communities (ARIC) study. BMC Cardiovasc. Disord. 2007, 7, 20. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, Y.; Rimm, E.B.; Hu, F.B.; Albert, C.M.; Rexrode, K.M.; Manson, J.E.; Qi, L. Dietary phosphatidylcholine and risk of all-cause and cardiovascular-specific mortality among US women and men. Am. J. Clin. Nutr. 2016, 104, 173–180. [Google Scholar] [CrossRef]
- Mills, J.L.; Fan, R.; Brody, L.C.; Liu, A.; Ueland, P.M.; Wang, Y.; Kirke, P.N.; Shane, B.; Molloy, A.M. Maternal choline concentrations during pregnancy and choline-related genetic variants as risk factors for neural tube defects. Am. J. Clin. Nutr. 2014, 100, 1069–1074. [Google Scholar] [CrossRef]
- Obeid, R.; Derbyshire, E.; Schön, C. Association between maternal choline, fetal brain development, and child neurocognition: Systematic review and meta-analysis of human studies. Adv. Nutr. 2022, 13, 2445–2457. [Google Scholar] [CrossRef]
- Bahnfleth, C.L.; Strupp, B.J.; Caudill, M.A.; Canfield, R.L. Prenatal choline supplementation improves child color-word inhibition performance aged 7 years in a randomized trial. J. Nutr. 2022, 152, 1524–1534. [Google Scholar] [CrossRef]
- Freedman, R.; Hunter, S.K.; Law, A.J.; Wagner, B.D.; D’Alessandro, A.; Christians, U.; Noonan, K.; Wyrwa, A.; Hoffman, M.C. Higher gestational choline levels in maternal infection are protective for infant brain development. J. Pediatr. 2019, 208, 198–206.e2. [Google Scholar] [CrossRef]
- Velzing-Aarts, F.V.; Holm, P.I.; Fokkema, M.R.; van der Dijs, F.P.; Ueland, P.M.; Muskiet, F.A. Plasma choline and betaine and their relation to plasma homocysteine in normal pregnancy. Am. J. Clin. Nutr. 2005, 81, 1383–1389. [Google Scholar] [CrossRef]
- Ilcol, Y.O.; Ozbek, R.; Hamurtekin, E.; Ulus, I.H. Choline status in newborns, infants, children, breast-feeding women, breast-fed infants and human breast milk. J. Nutr. Biochem. 2005, 16, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Holmes-McNary, M.Q.; Cheng, W.L.; Mar, M.H.; Fussell, S.; Zeisel, S.H. Choline and choline esters in human and rat milk and in infant formulas. Am. J. Clin. Nutr. 1996, 64, 572–576. [Google Scholar] [CrossRef]
- Davenport, C.; Yan, J.; Taesuwan, S.; Shields, K.; West, A.A.; Jiang, X.; Perry, C.A.; Malysheva, O.V.; Stabler, S.P.; Allen, R.H.; et al. Choline intakes exceeding recommendations during human lactation improve breast milk choline content by increasing PEMT pathway metabolites. J. Nutr. Biochem. 2015, 26, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Stammers, A.-L.; Lowe, N.M.; Medina, M.W.; Patel, S.; Dykes, F.; Pérez-Rodrigo, C.; Serra-Majam, L.; Nissensohn, M.; Moran, V.H. The relationship between zinc intake and growth in children aged 1-8 years: A systematic review and meta-analysis. Eur. J. Clin. Nutr. 2015, 69, 147–153. [Google Scholar] [CrossRef]
- Leung, B.M.Y.; Kaplan, B.J. Perinatal depression: Prevalence, risks, and the nutrition link—A review of the literature. J. Am. Diet. Assoc. 2009, 109, 1566–1575. [Google Scholar] [CrossRef] [PubMed]
- Christian, P.; Murray-Kolb, L.E.; Khatry, S.K.; Katz, J.; Schaefer, B.A.; Cole, P.M.; LeClerq, S.C.; Tielsch, J.M. Prenatal micronutrient supplementation and intellectual and motor function in early school-aged children in Nepal. JAMA 2010, 304, 2716–2723. [Google Scholar] [CrossRef]
- Sweiry, J.H.; Page, K.R.; Dacke, C.G.; Abramovich, D.R.; Yudilevich, D.L. Evidence of saturable uptake mechanisms at maternal and fetal sides of the perfused human placenta by rapid paired-tracer dilution: Studies with calcium and choline. J. Dev. Physiol. 1986, 8, 435–445. [Google Scholar]
- Traiffort, E.; O’Regan, S.; Ruat, M. The choline transporter-like family SLC44: Properties and roles in human diseases. Mol. Asp. Med. 2013, 34, 646–654. [Google Scholar] [CrossRef]
- Sanders, L.M.; Zeisel, S.H. Choline: Dietary requirements and role in brain development. Nutr. Today 2007, 42, 181–186. [Google Scholar] [CrossRef]
- West, A.A.; Yan, J.; Jiang, X.; Perry, C.A.; Innis, S.M.; Caudill, M.A. Choline intake influences phosphatidylcholine DHA enrichment in nonpregnant women but not in pregnant women in the third trimester. Am. J. Clin. Nutr. 2013, 97, 718–727. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Dietary reference values for choline. EFSA J. 2016, 14, 4484. [Google Scholar] [CrossRef]
- Casey, B.J.; Jones, R.M.; Somerville, L.H. Braking and accelerating of the adolescent brain. J. Res. Adolesc. 2011, 21, 21–33. [Google Scholar] [CrossRef]
- Hayward, C.E.; Greenwood, S.L.; Sibley, C.P.; Baker, P.N.; Jones, R.L. Effect of maternal age and growth on placental nutrient transport: Potential mechanisms for teenagers’ predisposition to small-for-gestational-age birth? Am. J. Physiol.-Endocrinol. Metab. 2012, 302, E233–E242. [Google Scholar] [CrossRef]
- Zhu, X.Z.; Deng, Z.M.; Dai, F.F.; Liu, H.; Cheng, Y.X. The impact of early pregnancy metabolic disorders on pregnancy outcome and the specific mechanism. Eur. J. Med. Res. 2023, 28, 187. [Google Scholar] [CrossRef] [PubMed]
- Black, M.M. Effects of vitamin B12 and folate deficiency on brain development in children. Food Nutr. Bull. 2008, 29, S126–S131. [Google Scholar] [CrossRef] [PubMed]
- Rush, D. Nutrition and maternal mortality in the developing world. Am. J. Clin. Nutr. 2000, 72, 212S–240S. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.H. Multiple micronutrients in pregnancy and lactation: An overview. Am. J. Clin. Nutr. 2005, 81, 1206. [Google Scholar] [CrossRef]
- Yaworski, R. The Regulation of Hepatic Choline Transport. Master’s Thesis, The University of Ottawa, Ottawa, ON, Canada, 2017. [Google Scholar]
- Craciunescu, C.N.; Brown, E.C.; Mar, M.-H.; Albright, C.D.; Nadeau, M.R.; Zeisel, S.H. Folic acid deficiency during late gestation decreases progenitor cell proliferation and increases apoptosis in fetal mouse brain. J. Nutr. 2004, 134, 162–166. [Google Scholar] [CrossRef]
- Zeisel, S.H. Nutrition in pregnancy: The argument for including a source of choline. Int. J. Womens Health 2013, 5, 193–199. [Google Scholar] [CrossRef]
- Christifano, D.N.; Chollet-Hinton, L.; Hoyer, D.; Schmidt, K.A.; Wasser, H.M.; McRitchie, S.L.; Sumner, S.; Newby, P.K.; Thompson, A.L.; Siega-Riz, A.M.; et al. Intake of eggs, choline, lutein, zeaxanthin, and DHA during pregnancy and their relationship to fetal neurodevelopment. Nutr. Neurosci. 2023, 26, 595–609. [Google Scholar] [CrossRef] [PubMed]
- Barnea-Goraly, N.; Menon, V.; Eckert, M.; Tamm, L. White matter development during childhood and adolescence: A cross-sectional diffusion tensor imaging study. Cereb. Cortex 2005, 15, 1848–1854. [Google Scholar] [CrossRef]
- Nagy, Z.; Westerberg, H.; Klingberg, T. Maturation of white matter is associated with the development of cognitive functions during childhood. J. Cogn. Neurosci. 2004, 16, 1227–1233. [Google Scholar] [CrossRef]
- Sousa, S.S.; Amaro, E., Jr.; Crego, A.; Gonçalves, Ó.F.; Sampaio, A. Developmental trajectory of the prefrontal cortex: A systematic review of diffusion tensor imaging studies. Brain Imaging Behav. 2018, 12, 1197–1210. [Google Scholar] [CrossRef]
- Shaw, G.A.; Dupree, J.L.; Neigh, G.N. Adolescent maturation of the prefrontal cortex: Role of stress and sex in shaping adult risk for compromise. Genes Brain Behav. 2020, 19, e12626. [Google Scholar] [CrossRef] [PubMed]
- Vanes, L.D.; Moutoussis, M.; Ziegler, G. White matter tract myelin maturation and its association with general psychopathology in adolescence and early adulthood. Hum. Brain Mapp. 2020, 41, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Klingberg, T.; Vaidya, C.J.; Gabrieli, J.D.E.; Moseley, M.E.; Hedehus, M. Myelination and organization of the frontal white matter in children: A diffusion tensor MRI study. Neuroreport 1999, 10, 2817–2821. [Google Scholar] [CrossRef]
- Bernhard, W.; Raith, M.; Kunze, R.; Koch, V. Choline concentrations are lower in postnatal plasma of preterm infants than in cord plasma. Eur. J. Nutr. 2015, 54, 733–741. [Google Scholar] [CrossRef]
- Taesuwan, S.; McDougall, M.Q.; Malysheva, O.V.; Bender, E. Choline metabolome response to prenatal choline supplementation across pregnancy: A randomized controlled trial. FASEB J. 2021, 35, e21966. [Google Scholar] [CrossRef]
- Zeisel, S.H.; Niculescu, M.D. Perinatal choline influences brain structure and function. Nutr. Rev. 2006, 64, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Zeisel, S.H. The fetal origins of memory: The role of dietary choline in optimal brain development. J. Pediatr. 2006, 149, S131–S136. [Google Scholar] [CrossRef]
- Chmurzynska, A.; Seremak-Mrozikiewicz, A.; Malinowska, A.M. Associations between folate and choline intake, homocysteine metabolism, and genetic polymorphism of MTHFR, BHMT and PEMT in healthy pregnant Polish women. Nutr. Diet. 2020, 77, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Michel, V.; Bakovic, M. The ubiquitous choline transporter SLC44A1. Cent. Nerv. Syst. Agents Med. Chem. 2012, 12, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Garner, S.C.; Mar, M.H.; Zeisel, S.H. Choline distribution and metabolism in pregnant rats and fetuses are influenced by the choline content of the maternal diet. J. Nutr. 1995, 125, 2851–2858. [Google Scholar] [CrossRef]
- Albright, C.D.; Friedrich, C.B.; Brown, E.C.; Mar, M.-H.; Zeisel, S.H. Maternal dietary choline availability alters mitosis, apoptosis and the localization of TOAD-64 protein in the developing fetal rat septum. Brain Res. Dev. Brain Res. 1999, 115, 123–129. [Google Scholar] [CrossRef]
- Napoli, I.; Blusztajn, J.K.; Mellott, T.J. Prenatal choline supplementation in rats increases the expression of IGF2 and its receptor IGF2R and enhances IGF2-induced acetylcholine release in hippocampus and frontal cortex. Brain Res. 2008, 1237, 124–135. [Google Scholar] [CrossRef]
- Moon, J.; Chen, M.; Gandhy, S.U.; Strawderman, M.; Levitsky, D.A.; Maclean, K.N.; Strupp, B.J. Perinatal choline supplementation improves cognitive functioning and emotion regulation in the Ts65Dn mouse model of Down syndrome. Behav. Neurosci. 2010, 124, 346–361. [Google Scholar] [CrossRef]
- Thomas, J.D.; Garrison, M.; O’Neill, T.M. Perinatal choline supplementation attenuates behavioral alterations associated with neonatal alcohol exposure in rats. Neurotoxicology Teratol. 2004, 26, 35–45. [Google Scholar] [CrossRef]
- Ryan, S.H.; Williams, J.K.; Thomas, J.D. Choline supplementation attenuates learning deficits associated with neonatal alcohol exposure in the rat: Effects of varying the timing of choline administration. Brain Res. 2008, 1237, 91–100. [Google Scholar] [CrossRef]
- Glenn, M.J.; Gibson, E.M.; Kirby, E.D.; Mellott, T.J.; Blusztajn, J.K.; Williams, C.L. Prenatal choline availability modulates hippocampal neurogenesis and neurogenic responses to enriching experiences in adult female rats. Eur. J. Neurosci. 2007, 25, 2473–2482. [Google Scholar] [CrossRef]
- Holler, T.; Cermak, J.M.; Blusztajn, J.K. Dietary choline supplementation in pregnant rats increases hippocampal phospholipase D activity of the offspring. FASEB J. 1996, 10, 1653–1659. [Google Scholar] [CrossRef]
- Blusztajn, J.K.; Cermak, J.M.; Holler, T.; Jackson, D.A. Imprinting of hippocampal metabolism of choline by its availability during gestation: Implications for cholinergic neurotransmission. J. Physiol. 1998, 92, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Cermak, J.M.; Holler, T.; Jackson, D.A.; Blusztajn, J.K. Prenatal availability of choline modifies development of the hippocampal cholinergic system. FASEB J. 1998, 12, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Pyapali, G.K.; Turner, D.A.; Williams, C.L.; Meck, W.H.; Swartzwelder, H.S. Prenatal dietary choline supplementation decreases the threshold for induction of long-term potentiation in young adult rats. J. Neurophysiol. 1998, 79, 1790–1796. [Google Scholar] [CrossRef]
- Montoya, D.A.; White, A.M.; Williams, C.L.; Blusztajn, J.K.; Meck, W.H.; Swartzwelder, H. Prenatal choline exposure alters hippocampal responsiveness to cholinergic stimulation in adulthood. Brain Res. Dev. Brain Res. 2000, 123, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.L.; Meck, W.H.; Heyer, D.D.; Loy, R. Hypertrophy of basal forebrain neurons and enhanced visuospatial memory in perinatally choline-supplemented rats. Brain Res. 1998, 794, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Meck, W.H.; Smith, R.A.; Williams, C.L. Pre- and postnatal choline supplementation produces long-term facilitation of spatial memory. Dev. Psychobiol. 1988, 21, 339–353. [Google Scholar] [CrossRef]
- McCann, J.C.; Hudes, M.; Ames, B.N. An overview of evidence for a causal relationship between dietary availability of choline during development and cognitive function in offspring. Neurosci. Biobehav. Rev. 2006, 30, 696–712. [Google Scholar] [CrossRef]
- Zhu, X.; Mar, M.H.; Song, J.; Zeisel, S.H. Deletion of the Pemt gene increases progenitor cell mitosis, DNA and protein methylation and decreases calretinin expression in embryonic day 17 mouse hippocampus. Brain Res. Dev. Brain Res. 2004, 149, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Niculescu, M.D.; Yamamuro, Y.; Zeisel, S.H. Choline availability modulates human neuroblastoma cell proliferation and alters the methylation of the promoter region of the cyclin-dependent kinase inhibitor 3 gene. J. Neurochem. 2004, 89, 1252–1259. [Google Scholar] [CrossRef]
- Wu, B.T.F.; Dyer, R.A.; King, D.J.; Richardson, K.J.; Innis, S.M. Early second trimester maternal plasma choline and betaine are related to measures of early cognitive development in term infants. PLoS ONE 2012, 7, e43448. [Google Scholar] [CrossRef] [PubMed]
- Mellott, T.J.; Williams, C.L.; Meck, W.H.; Blusztajn, J.K. Prenatal choline supplementation advances hippocampal development and enhances MAPK and CREB activation. FASEB J. 2004, 18, 545–547. [Google Scholar] [CrossRef]
- Jadavji, N.M.; Deng, L.; Malysheva, O.; Caudill, M.A.; Rozen, R. MTHFR deficiency or reduced intake of folate or choline in pregnant mice results in impaired short-term memory and increased apoptosis in the hippocampus of wild-type offspring. Neuroscience 2015, 300, 1–9. [Google Scholar] [CrossRef]
- Wong-Goodrich, S.J.E.; Glenn, M.J.; Mellott, T.J.; Blusztajn, J.K. Spatial memory and hippocampal plasticity are differentially sensitive to the availability of choline in adulthood as a function of choline supply in utero. Brain Res. 2008, 1237, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Moreno, H.; de Brugada, I. Prenatal dietary choline supplementation modulates long-term memory development in rat offspring. Nutr. Neurosci. 2021, 24, 552–561. [Google Scholar] [CrossRef]
- Cusick, S.E.; Georgieff, M.K. The role of nutrition in brain development: The golden opportunity of the “first 1000 days". J. Pediatr. 2016, 175, 16–21. [Google Scholar] [CrossRef]
- Prado, E.L.; Dewey, K.G. Nutrition and brain development in early life. Nutr. Rev. 2014, 72, 267–284. [Google Scholar] [CrossRef]
- Zeisel, S.H. Choline: Needed for normal development of memory. J. Am. Coll. Nutr. 2000, 19, 528S–531S. [Google Scholar] [CrossRef]
- Sandstrom, N.J.; Loy, R.; Williams, C.L. Prenatal choline supplementation increases NGF levels in the hippocampus and frontal cortex of young and adult rats. Brain Res. 2002, 947, 9–16. [Google Scholar] [CrossRef]
- Resseguie, M.E.; da Costa, K.-A.; Galanko, J.A.; Patel, M.; Davis, I.J.; Zeisel, S.H. Aberrant estrogen regulation of PEMT results in choline deficiency-associated liver dysfunction. J. Biol. Chem. 2011, 286, 1649–1658. [Google Scholar] [CrossRef]
- Corbin, K.D.; Zeisel, S.H. Choline metabolism provides novel insights into nonalcoholic fatty liver disease and its progression. Curr. Opin. Gastroenterol. 2012, 28, 159–165. [Google Scholar] [CrossRef]
- Davison, J.M.; Mellott, T.J.; Kovacheva, V.P.; Blusztajn, J.K. Gestational choline supply regulates methylation of histone H3, expression of histone methyltransferases G9a (Kmt1c) and Suv39h1 (Kmt1a), and DNA methylation of their genes in rat fetal liver and brain. J. Biol. Chem. 2009, 284, 1982–1989. [Google Scholar] [CrossRef]
- Imdad, A.; Lassi, Z.; Salaam, R.; Bhutta, Z.A. Prenatal nutrition and nutrition in pregnancy: Effects on long-term growth and development. In Early Nutrition and Long-Term Health; Woodhead Publishing: Cambridge, UK, 2017; pp. 3–24. [Google Scholar] [CrossRef]
- Irvine, N.; England-Mason, G.; Field, C.J.; Dewey, D.; Aghajafari, F. Prenatal folate and choline levels and brain and cognitive development in children: A critical narrative review. Nutrients 2022, 14, 364. [Google Scholar] [CrossRef]
- Morales, M.F.; Girard, L.-C.; Raouna, A.; MacBeth, A. The association of different presentations of maternal depression with children’s socio-emotional development: A systematic review. PLoS Glob. Public Health 2023, 3, e0001649. [Google Scholar] [CrossRef] [PubMed]
- Buchman, A.L.; Dubin, M.D.; Moukarzel, A.A.; Jenden, D.J.; Roch, M.; Rice, K.M.; Gornbein, J.; Ament, M.E. Choline deficiency: A cause of hepatic steatosis during parenteral nutrition that can be reversed with intravenous choline supplementation. Hepatology 1995, 22, 1399–1403. [Google Scholar]
- Zhao, N.; Yang, S.; Hu, Y.; Dong, H.; Zhao, R. Maternal betaine protects rat offspring from maternal high-fat diet-induced visceral adiposity. J. Nutr. Biochem. 2014, 25, 1320–1325. [Google Scholar] [CrossRef]
- Mehedint, M.G.; Niculescu, M.D.; Craciunescu, C.N.; Zeisel, S.H. Choline deficiency alters global histone methylation and epigenetic marking at the Re1 site of the calbindin 1 gene. FASEB J. 2010, 24, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Uzunov, A.V.; Secara, D.C.; Constantin, A.E.; Mehedintu, C.; Cirstoiu, M.M. Difference between Preterm Birth in Adolescent and Adult Patients. Maedica 2022, 17, 789–794. [Google Scholar] [CrossRef]
- Lees, C.C.; Marlow, N.; van Wassenaer-Leemhuis, A. 2 year neurodevelopmental and intermediate perinatal outcomes in infants with very preterm fetal growth restriction (TRUFFLE): A randomised trial. Lancet 2015, 385, 2162–2172. [Google Scholar] [CrossRef]
- Zeisel, S.H.; Da Costa, K.A. Choline: An essential nutrient for public health. Nutr. Rev. 2009, 67, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Bekdash, R.A.; Zhang, C.; Sarkar, D.K. Gestational choline supplementation normalized fetal alcohol-induced alterations in histone modifications, DNA methylation, and proopiomelanocortin (POMC) gene expression in β-endorphin-producing POMC neurons of the hypothalamus. Alcohol. Clin. Exp. Res. 2013, 37, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- King, C.; Plakke, B. Maternal choline supplementation in neurodevelopmental disorders: Mechanistic insights from animal models and future directions. Nutr. Neurosci. 2025, 28, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Korsmo, H.W.; Dave, B.; Trasino, S.E.; Holscher, H.; Ivanov, I.; Stackhouse, C.; Saxena, A.; Jiang, X. Prenatal choline supplementation during high-fat feeding improves long-term blood glucose control in male mouse offspring. Nutrients 2020, 12, 144. [Google Scholar] [CrossRef]
- World Health Organization. Sexual, Reproductive, Maternal, Newborn, Child and Adolescent Health: Report on the 2023 Policy Survey; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- Jaiswal, A.; Dewani, D.; Reddy, L.S.; Patel, A.; Jindam, A. Choline supplementation in pregnancy: Current evidence and implications. Cureus 2023, 15, e47073. [Google Scholar] [CrossRef]

| Enzyme | Function | Adult Activity (% Change) | Adolescent Activity (% Change) | Km (μM) | Ref. |
|---|---|---|---|---|---|
| Choline Kinase α | PC synthesis | +200–300% | +150–200% | 75–100 | [76,77] |
| CTP:PC Cytidylyltransferase | PC synthesis | +150–200% | +80–120% | 25–40 | [78,79] |
| Choline Acetyltransferase | ACh synthesis | +120–150% | +80–100% | 300–450 | [30,80,81] |
| Choline Dehydrogenase | Betaine synthesis | +50–75% | +100–125% | 150–250 | [30,82] |
| PEMT | Alternative PC synthesis | +100–150% | +50–80% | - | [83,84] |
| Transporter | Gene | Protein (kDa) | Choline (μM) | Vmax (Relative) | Placental Localization | Adolescent Expression | References |
|---|---|---|---|---|---|---|---|
| CTL1 | SLC44A1 | 70 | 5–20 | 100% (reference) | Microvillous membrane | 80–85% of adult | [86] |
| CTL2 | SLC44A2 | 68 | 50–100 | 30–40% | Both membranes | 85–90% of adult | [6] |
| OCT1 | SLC22A1 | 61 | 100–500 | 15–20% | Basal membrane | 90–95% of adult | [87] |
| OCT2 | SLC22A2 | 62 | 200–400 | 10–15% | Maternal endothelium | 95–100% of adult | [87,88] |
| OCT3 | SLC22A3 | 58 | 30–80 | 25–30% | Syncytiotrophoblast | 75–80% of adult | [12,89] |
| System | Pathophysiological Mechanism | Clinical Manifestations | Reversibility | Ref. |
|---|---|---|---|---|
| Nervous System | Reduced PC synthesis, impaired ACh production, altered methylation | Cognitive deficits, NTDs, reduced memory function | Partially reversible | [22,127] |
| Hepatic System | Decreased VLDL synthesis, triglyceride accumulation | Fatty liver, elevated transaminases | Reversible | [144] |
| Cardiovascular | Endothelial dysfunction, altered lipid metabolism | Hypertension, preeclampsia risk | Partially reversible | [145] |
| Immune System | Altered lymphocyte function, cytokine dysregulation | Increased infection risk, inflammatory complications | Reversible | [146] |
| Placental Function | Membrane instability, impaired angiogenesis | IUGR, preterm birth, abruption | Partially reversible | [26,62,74] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khudor, A.J.; Moga, M.A.; Dimienescu, O.G.; Nicolau, A.C.; Arvătescu, C.A.; Hogea, M.D.; Ciobanu, N. Choline in Adolescent Pregnancy: The Impact on Fetal Brain Development and Long-Term Cognitive Outcomes of Offspring. Medicina 2025, 61, 2057. https://doi.org/10.3390/medicina61112057
Khudor AJ, Moga MA, Dimienescu OG, Nicolau AC, Arvătescu CA, Hogea MD, Ciobanu N. Choline in Adolescent Pregnancy: The Impact on Fetal Brain Development and Long-Term Cognitive Outcomes of Offspring. Medicina. 2025; 61(11):2057. https://doi.org/10.3390/medicina61112057
Chicago/Turabian StyleKhudor, Abdul Jabar, Marius Alexandru Moga, Oana Gabriela Dimienescu, Andrada Camelia Nicolau, Cristian Andrei Arvătescu, Mircea Daniel Hogea, and Natalia Ciobanu. 2025. "Choline in Adolescent Pregnancy: The Impact on Fetal Brain Development and Long-Term Cognitive Outcomes of Offspring" Medicina 61, no. 11: 2057. https://doi.org/10.3390/medicina61112057
APA StyleKhudor, A. J., Moga, M. A., Dimienescu, O. G., Nicolau, A. C., Arvătescu, C. A., Hogea, M. D., & Ciobanu, N. (2025). Choline in Adolescent Pregnancy: The Impact on Fetal Brain Development and Long-Term Cognitive Outcomes of Offspring. Medicina, 61(11), 2057. https://doi.org/10.3390/medicina61112057







