EVD-Associated Infections in Subarachnoid Hemorrhage: Risk Factors and Clinical Predictions—A Retrospective Single-Center Study
Abstract
1. Introduction
1.1. The SAH-Specific Challenge
1.2. Study Rationale and Objectives
2. Materials and Methods
2.1. Study Design and Population
2.2. Inclusion and Exclusion Criteria
2.3. External Ventricular Drain Specifications
Infection Surveillance Protocol
- (1)
- Laboratory Monitoring: Cerebrospinal fluid samples were obtained three times weekly for all EVD patients; additional CSF sampling was performed at increased frequency at the physician’s discretion for clinically deteriorating patients. Serum biomarkers (C-reactive protein, procalcitonin, white blood cell count, interleukin-6) were obtained daily on a regular basis, with increased frequency in patients demonstrating clinical or laboratory signs of infection.
- (2)
- Clinical Monitoring: Bedside clinical assessments were performed daily by the ICU nursing and physician team. Clinical parameters documented included the following: presence of fever (≥38.5 °C), meningeal signs (neck stiffness, photophobia), altered mental status, and focal neurological changes. Patients demonstrating clinical deterioration underwent more frequent assessments (per shift or continuous monitoring, as clinically indicated).
- (3)
- Case Selection: Each suspected CNS infection case was reviewed jointly by neurosurgery, infectious diseases, and neurocritical care team members. Classification of confirmed infection (culture-positive) or presumed infection (abnormal CSF/blood parameters and clinical findings consistent with infection per published diagnostic criteria) was made by consensus using the documentation of classification rationale from the medical record.
2.4. Definition of EVD-Associated Infection
2.5. Data Collection
2.6. Statistical Analysis
2.7. Software
2.8. Ethical Considerations
3. Results
3.1. Study Population and Baseline Characteristics
3.2. Infection Epidemiology and Microbiological Profile
3.3. Univariate Risk Factor Analysis
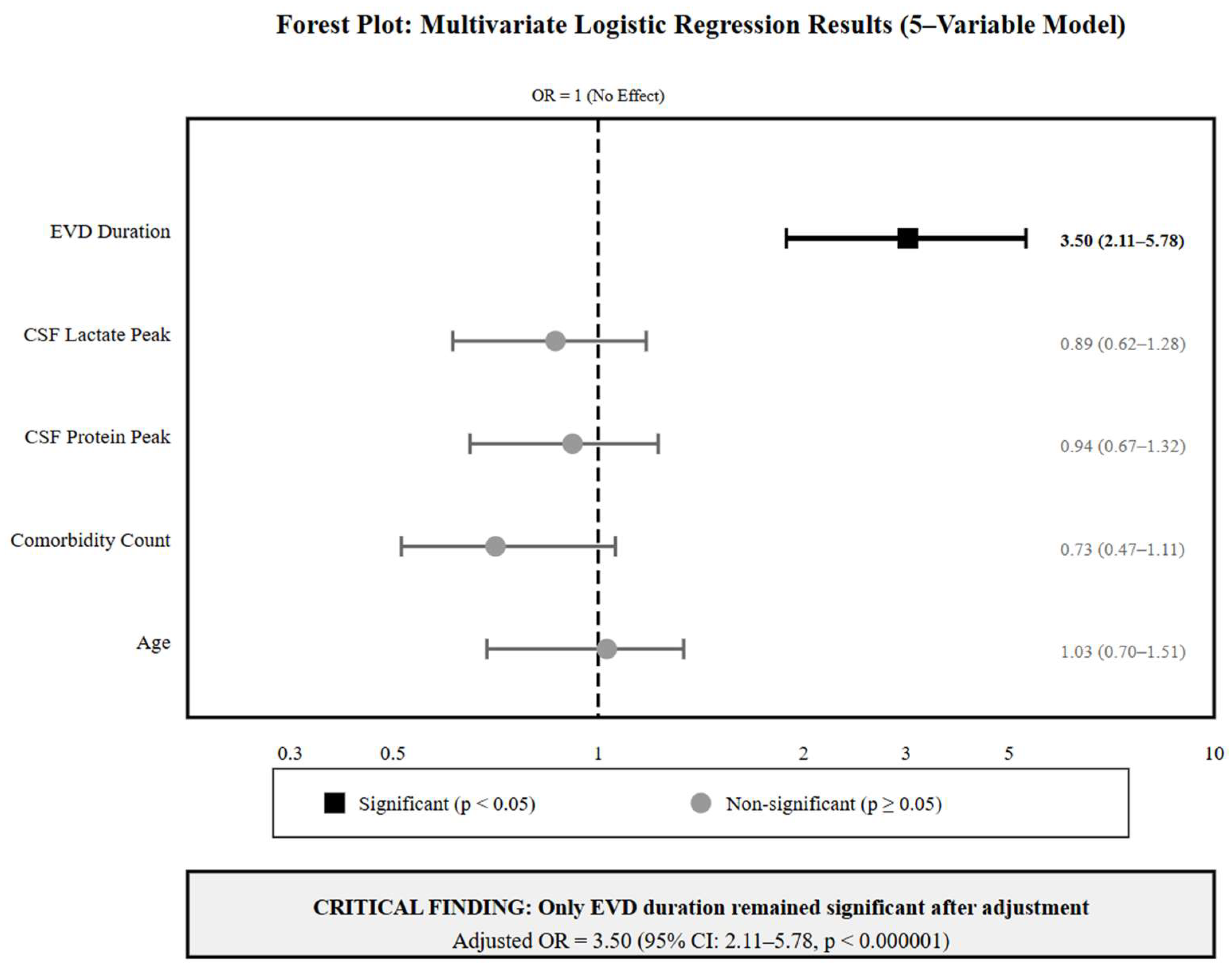
3.4. Multivariate Analysis: The Dominance of EVD Duration
3.5. Clinical Risk Stratification and Threshold Optimization
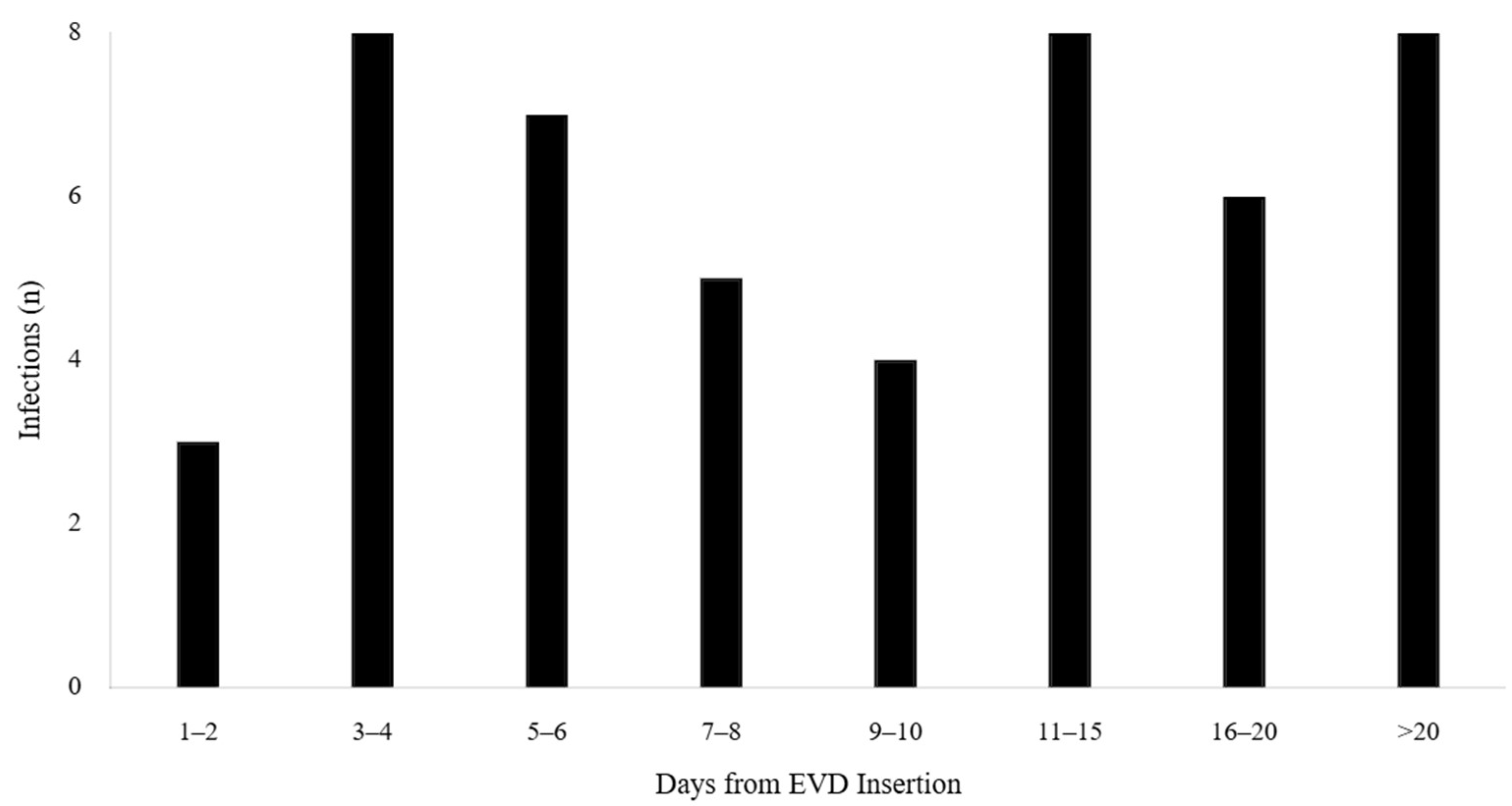
3.6. Temporal Trend Analysis
4. Discussion
4.1. Principal Findings
4.2. EVD Duration as the Dominant Risk Factor
4.2.1. Comparison with the Existing Literature
4.2.2. Mechanistic Explanations
4.3. Clinical Risk Stratification and Implementation
4.3.1. Evidence-Based Duration Phases
4.3.2. Selection Bias and Exclusion Criteria
4.3.3. Diagnostic Misclassification and Culture-Negative Infections
4.3.4. Temporal Collinearity and Model Specification
4.4. SAH-Specific Considerations
4.4.1. Unique Challenges in SAH Population
4.4.2. Implications for SAH Management
4.5. Study Limitations and Methodological Considerations
4.5.1. Single-Center Design
4.5.2. Temporal Changes in Practice
4.6. Clinical Implementation and Future Directions
4.6.1. Integration into Clinical Practice
4.6.2. Research Priorities
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AUC | Area Under the Curve |
| BMI | Body Mass Index |
| CI | Confidence Interval |
| CNS | Central Nervous System |
| CRP | C-Reactive Protein |
| CSF | Cerebrospinal Fluid |
| EVD | External Ventricular Drain |
| GCS | Glasgow Coma Scale |
| H&H | Hunt & Hess |
| IL-6 | Interleukin-6 |
| OR | Odds Ratio |
| SAH | Subarachnoid Hemorrhage |
| TUM | Technical University of Munich |
| VIF | Variance Inflation Factor |
| WBC | White Blood Cell |
References
- Connolly, E.S., Jr.; Rabinstein, A.A.; Carhuapoma, J.R.; Derdeyn, C.P.; Dion, J.; Higashida, R.T.; Hoh, B.L.; Kirkness, C.J.; Naidech, A.M.; Ogilvy, C.S.; et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2012, 43, 1711–1737. [Google Scholar] [CrossRef] [PubMed]
- Steiner, T.; Juvela, S.; Unterberg, A.; Jung, C.; Forsting, M.; Rinkel, G. European Stroke Organisation guidelines for the management of intracranial aneurysms and subarachnoid haemorrhage. Cerebrovasc. Dis. 2013, 35, 93–112. [Google Scholar] [CrossRef] [PubMed]
- Kelemen, J.; Szterman, M.; Dakos, E.; Barzó, P.; Bella, Z.; Csomós, Á. Risk Assessment and Recommended Approaches to Optimize Infection Control and Antibiotic Stewardship to Reduce External Ventricular Drain Infection: A Single-Center Study. Antibiotics 2024, 13, 1093. [Google Scholar] [CrossRef] [PubMed]
- Dorresteijn, K.R.; Jellema, K.; van de Beek, D.; Brouwer, M.C. Factors predicting external CSF drain-associated ventriculitis. Neurology 2019, 93, e2027–e2036. [Google Scholar] [CrossRef]
- Ramanan, M.; Lipman, J.; Shorr, A.; Shankar, A. A meta-analysis of ventriculostomy-associated cerebrospinal fluid infections. BMC Infect. Dis. 2015, 15, 3. [Google Scholar] [CrossRef]
- Tunkel, A.R.; Hasbun, R.; Bhimraj, A.; Byers, K.; Kaplan, S.L.; Scheld, W.M.; van de Beek, D.; Bleck, T.P.; Garton, H.J.L.; Zunt, J.R. 2017 Infectious Diseases Society of America’s Clinical Practice Guidelines for Healthcare-Associated Ventriculitis and Meningitis. Clin. Infect. Dis. 2017, 64, e34–e65. [Google Scholar] [CrossRef]
- Van de Beek, D.; Drake, J.M.; Tunkel, A.R. Nosocomial bacterial meningitis. N. Engl. J. Med. 2010, 362, 146–154. [Google Scholar] [CrossRef]
- Negrini, B.; Kelleher, K.J.; Wald, E.R. Cerebrospinal fluid findings in aseptic versus bacterial meningitis. Pediatrics 2000, 105, 316–319. [Google Scholar] [CrossRef]
- Fried, H.I.; Nathan, B.R.; Rowe, A.S.; Zabramski, J.M.; Andaluz, N.; Bhimraj, A.; Guanci, M.M.; Seder, D.B.; Singh, J.M. The insertion and management of external ventricular drains: An evidence-based consensus statement. Neurocrit. Care 2016, 24, 61–81. [Google Scholar] [CrossRef]
- Chung, D.Y.; Thompson, B.B.; Kumar, M.A.; Mahta, A.; Rao, S.S.; Lai, J.H.; Tadevosyan, A.; Kessler, K.; Locascio, J.J.; Patel, A.B.; et al. Association of External Ventricular Drain Wean Strategy with Shunt Placement and Length of Stay in Subarachnoid Hemorrhage: A Prospective Multicenter Study. Neurocritical Care 2022, 36, 536–545. [Google Scholar] [CrossRef]
- Hagel, S.; Bruns, T.; Pletz, M.W.; Engel, C.; Kalff, R.; Ewald, C. External ventricular drain infections: Risk factors and outcome. Interdiscip. Perspect. Infect. Dis. 2014, 2014, 708531. [Google Scholar] [CrossRef]
- Frontera, J.A.; Fernandez, A.; Schmidt, J.M.; Claassen, J.; Wartenberg, K.E.; Badjatia, N.; Connolly, E.S.; Mayer, S.A. Defining vasospasm after subarachnoid hemorrhage: What is the most clinically relevant definition? Stroke 2009, 40, 1963–1968. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilm elimination on intravascular catheters: Important considerations for the infectious disease practitioner. Clin. Infect. Dis. 2011, 52, 1038–1045. [Google Scholar] [CrossRef]
- Oliveira, W.F.; Silva, P.M.S.; Silva, R.C.S.; Silva, G.M.; Machado, G.; Coelho, L.C.B.B.; Correia, M.T.S. Staphylococcus epidermidis biofilms undergo metabolic and matrix remodeling under nitrosative stress. Front. Cell. Infect. Microbiol. 2023, 13, 1200923. [Google Scholar] [CrossRef]
- Hunt, W.E.; Hess, R.M. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J. Neurosurg. 1968, 28, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Treggiari, M.M.; Rabinstein, A.A.; Busl, K.M.; Connolly, E.S.; Cockroft, K.M.; Danziger, J.; Grzybowski, A.; Hall, E.S.; Hoh, B.L.; Kirkness, C.J.; et al. Guidelines for the Neurocritical Care Management of Aneurysmal Subarachnoid Hemorrhage. Neurocrit. Care 2023, 39, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Collins, G.S.; Reitsma, J.B.; Altman, D.G.; Moons, K.G. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): The TRIPOD statement. BMJ 2015, 350, g7594. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, P.; Olsen, M.H.; Willer-Hansen, R.S.; Hauerberg, J.; Johansen, H.K.; Andersen, A.B.; Knudsen, J.D.; Møller, K. Ventriculostomy-associated infection (VAI) in patients with acute brain injury—A retrospective study. Acta Neurochir. 2024, 166, 128. [Google Scholar] [CrossRef]
- Rammos, S.; Klopfenstein, J.; Augspurger, L.; Wang, H.; Wagenbach, A.; Poston, J.; Lanzino, G. Conversion of external ventricular drains to ventriculoperitoneal shunts after aneurysmal subarachnoid hemorrhage: Effects of site and protein/red blood cell counts on shunt infection and malfunction. J. Neurosurg. 2008, 109, 1001–1004. [Google Scholar] [CrossRef]
- Hoefnagel, D.; Dammers, R.; Ter Laak-Poort, M.P.; Avezaat, C.J. Risk factors for infections related to external ventricular drainage. Acta Neurochir. 2008, 150, 209–214. [Google Scholar] [CrossRef]
- Dubourg, J.; Javouhey, E.; Geeraerts, T.; Messerer, M.; Kassai, B. Ultrasonography of optic nerve sheath diameter for detection of raised intracranial pressure: A systematic review and meta-analysis. Intensive Care Med. 2011, 37, 1059–1068. [Google Scholar] [CrossRef]
- Park, P.; Garton, H.J.; Kocan, M.J.; Thompson, B.G. Risk of infection with prolonged ventricular catheterization. Neurosurgery 2004, 55, 594–601. [Google Scholar] [CrossRef]
- Leverstein-van Hall, M.A.; Hopmans, T.E.; van der Sprenkel, J.W.; Blok, H.E.; van der Mark, W.A.; Hanlo, P.W.; Geerts, A.T.; Fleer, A.; Verhoef, J. A bundle approach to reduce the incidence of external ventricular and lumbar drain-related infections. J. Neurosurg. 2010, 112, 345–353. [Google Scholar] [CrossRef]
- Woo, P.Y.; Wong, H.T.; Pu, J.K.; Wong, L.Y.; Lam, W.S.; Tang, A.; Fung, L.M.; Ng, S.C.; Poon, W.S. Moving the goalposts: A comparison of different definitions for primary external ventricular drain infection and its risk factors: A multi-center study of 2575 patients. J. Clin. Neurosci. 2017, 45, 67–72. [Google Scholar] [CrossRef]
- Wagner, A.; Wostrack, M.; Hartz, F.; Meyer, B.; Gempt, J. The role of extended coagulation screening in adult cranial neurosurgery. Brain Spine 2023, 3, 101756. [Google Scholar] [CrossRef]
- Mandel, J.C.; Kreda, D.A.; Mandl, K.D.; Kohane, I.S.; Ramoni, R.B. SMART on FHIR: A standards-based, interoperable apps platform for electronic health records. J. Am. Med. Inform. Assoc. 2016, 23, 899–908. [Google Scholar] [CrossRef] [PubMed]



| Characteristic | All Patients (N = 198) | No CNS Infection (N = 149) | CNS Infection (N = 49) | p-Value |
|---|---|---|---|---|
| Demographics | ||||
| Age, years (mean ± SD) | 61.6 ± 18.2 | 62.1 ± 18.5 | 60.2 ± 17.3 | 0.534 |
| Female sex, n (%) | 113 (57.1) | 88 (59.1) | 25 (51.0) | 0.327 |
| BMI (mean ± SD) | 27.2 ± 5.8 | 27.0 ± 5.6 | 27.8 ± 6.3 | 0.412 |
| Clinical Severity Scores | ||||
| Hunt & Hess grade ≥ 4, n (%) | 21 (10.6) | 13 (8.7) | 8 (16.3) | 0.011 * |
| GCS score (mean ± SD) | 11.2 ± 3.8 | 11.5 ± 3.7 | 10.3 ± 4.0 | 0.058 |
| EVD Characteristics | ||||
| EVD duration, days (mean ± SD) | 17.5 ± 12.8 | 14.6 ± 10.2 | 22.4 ± 15.7 | <0.001 * |
| EVD revisions, n (%) | 36 (18.2) | 20 (13.4) | 16 (32.7) | 0.003 * |
| CSF leak present, n (%) | 12 (6.1) | 7 (4.7) | 5 (10.2) | 0.172 |
| Microbiological Findings | n (%) |
|---|---|
| Culture Results (N = 49 infected patients) | |
| CSF culture performed | 48 (98.0) |
| CSF culture positive | 29 (59.2) |
| Culture-negative CNS infection | 19 (38.8) |
| Isolated Organisms (N = 29 culture-positive) | |
| Gram-positive cocci | 13 (26.5) |
| Coagulase-negative Staphylococci | 8 (16.3) |
| Staphylococcus aureus | 3 (6.1) |
| Enterococcus species | 2 (4.1) |
| Gram-negative bacilli | 5 (10.2) |
| Klebsiella species | 2 (4.1) |
| Other Gram-negatives | 3 (6.1) |
| Fungal organisms | 2 (4.1) |
| MRSA | 1 (2.0) |
| Polymicrobial infection | 4 (8.2) |
| Variable Category | Specific Variable | Infected Group (n = 49) | Non-Infected Group (n = 149) | p-Value | Cohen’s d | AUC e | ||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||||
| Clinical Factors c | ||||||||
| EVD Duration (days) a,d | 14.3 | 6.2 | 7.5 | 4.1 | <0.001 | 1.00 | 0.81 | |
| EVD Revisions (n) a,d | 2.1 | 1.3 | 0.8 | 0.9 | <0.001 | 1.11 | 0.76 | |
| Age (years) d | 58.2 | 14.1 | 55.8 | 13.2 | 0.342 | 0.18 | 0.55 | |
| Hunt & Hess Grade | 3.2 | 1.1 | 2.8 | 1.0 | 0.011 | 0.38 | 0.62 | |
| CSF Biomarkers (Peak Values) b,c | ||||||||
| Lactate (mmol/L) a,d | 5.8 | 2.1 | 3.2 | 1.3 | <0.001 | 0.53 | 0.79 | |
| Protein (mg/dL) a,d | 184.2 | 78.3 | 98.4 | 52.1 | <0.001 | 0.52 | 0.74 | |
| Glucose (mg/dL) a | 52.1 | 18.7 | 64.8 | 21.3 | <0.001 | 0.63 | 0.73 | |
| Cell Count (cells/μL) | 234.5 | 189.3 | 125.7 | 98.2 | 0.002 | 0.28 | 0.68 | |
| Blood Biomarkers (Peak Values) b,c | ||||||||
| CRP (mg/L) d | 142.8 | 68.4 | 98.2 | 45.6 | 0.001 | 0.37 | 0.69 | |
| WBC (×103/μL) d | 14.2 | 5.3 | 11.8 | 4.1 | 0.014 | 0.42 | 0.63 | |
| Procalcitonin (ng/mL) a,d | 2.8 | 1.9 | 1.2 | 0.8 | <0.001 | 0.58 | 0.75 | |
| IL-6 (pg/mL) a,d | 145.6 | 89.2 | 78.3 | 52.1 | <0.001 | 0.61 | 0.75 | |
| Monitoring Intensity c | ||||||||
| CSF Tests (n) a,d | 8.4 | 3.2 | 3.2 | 1.8 | <0.001 | 0.71 | 0.77 | |
| Blood Tests (n) a,d | 24.3 | 8.4 | 12.8 | 6.2 | <0.001 | 0.68 | 0.74 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarkis, H.; Kerhani, A.A.; Joerger, A.-K.; Albrecht, C.; Negwer, C.; Wostrack, M.; Wagner, A.; Meyer, B. EVD-Associated Infections in Subarachnoid Hemorrhage: Risk Factors and Clinical Predictions—A Retrospective Single-Center Study. Medicina 2025, 61, 2058. https://doi.org/10.3390/medicina61112058
Sarkis H, Kerhani AA, Joerger A-K, Albrecht C, Negwer C, Wostrack M, Wagner A, Meyer B. EVD-Associated Infections in Subarachnoid Hemorrhage: Risk Factors and Clinical Predictions—A Retrospective Single-Center Study. Medicina. 2025; 61(11):2058. https://doi.org/10.3390/medicina61112058
Chicago/Turabian StyleSarkis, Hraq, Abed Alrazzak Kerhani, Ann-Kathrin Joerger, Carolin Albrecht, Chiara Negwer, Maria Wostrack, Arthur Wagner, and Bernhard Meyer. 2025. "EVD-Associated Infections in Subarachnoid Hemorrhage: Risk Factors and Clinical Predictions—A Retrospective Single-Center Study" Medicina 61, no. 11: 2058. https://doi.org/10.3390/medicina61112058
APA StyleSarkis, H., Kerhani, A. A., Joerger, A.-K., Albrecht, C., Negwer, C., Wostrack, M., Wagner, A., & Meyer, B. (2025). EVD-Associated Infections in Subarachnoid Hemorrhage: Risk Factors and Clinical Predictions—A Retrospective Single-Center Study. Medicina, 61(11), 2058. https://doi.org/10.3390/medicina61112058






