Arrhythmogenic Risk in iPSC-Derived Cardiomyocytes: Current Limitations and Therapeutic Perspectives
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search Strategy
2.2. Selection Criteria
- (1)
- experimental research involving human or animal iPSC-CMs addressing electrophysiological, structural, or metabolic correlates of arrhythmogenicity;
- (2)
- interventional studies evaluating strategies to mitigate arrhythmic risk through maturation protocols, genetic or epigenetic editing, co-culture systems, or pharmacological modulation; and
- (3)
- reviews or meta-analyses that provide mechanistic or translational insights into iPSC-CM electrophysiology and bioenergetics.
2.3. Data Analysis
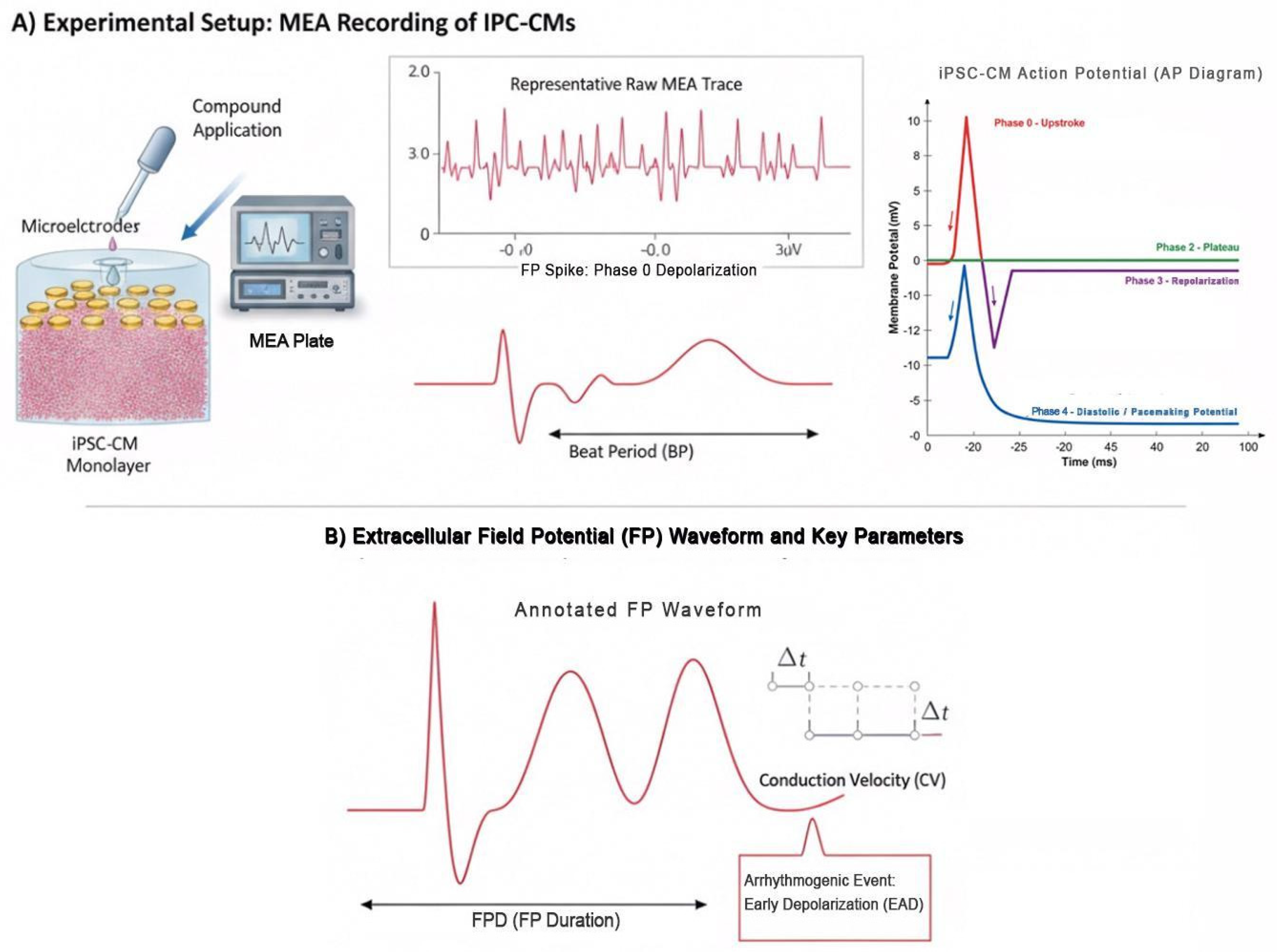
3. Arrhythmogenic Risks in iPSC-CMs
3.1. Electrophysiological Characteristics
3.2. Cellular Heterogeneity
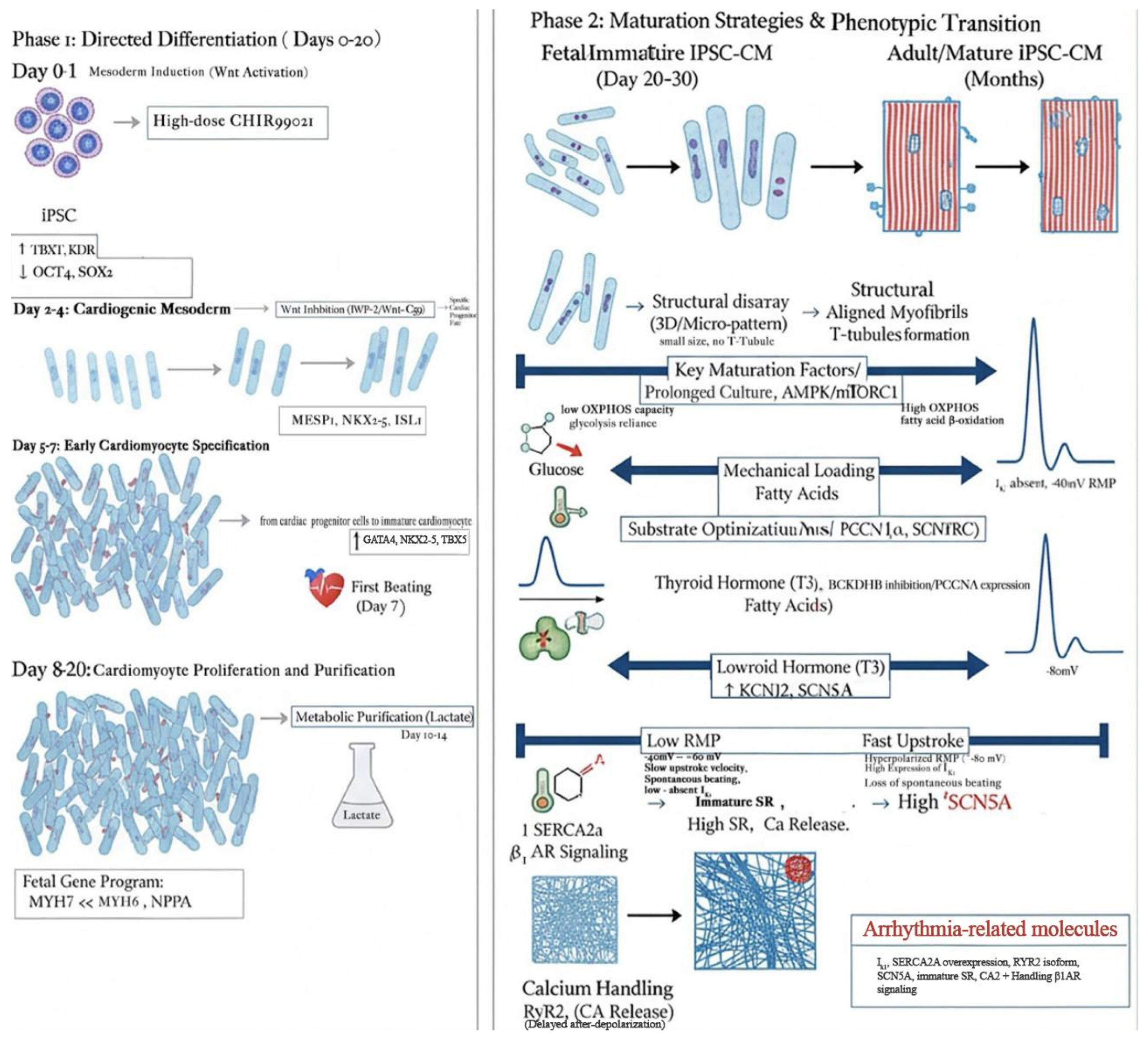
3.3. Immaturity of iPSC-CMs
3.4. Integrative Perspective
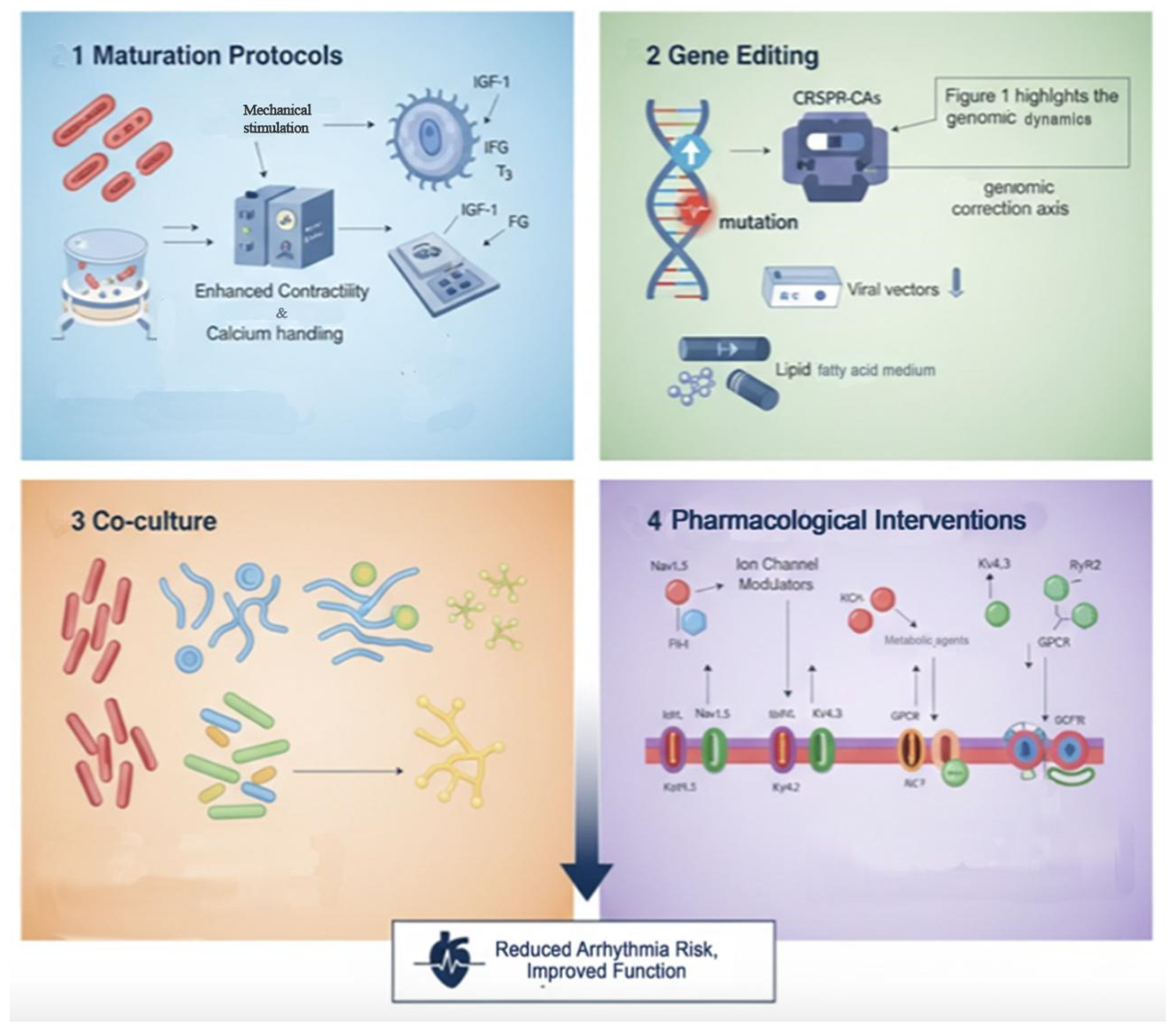
4. Therapeutic Strategies to Mitigate Arrhythmogenic Risks
4.1. Maturation Protocols
4.2. Gene Editing Techniques
4.3. Co-Culture Systems
4.4. Pharmacological Interventions
4.5. Integrative Outlook
5. Discussion
5.1. Integration of Findings
5.2. Clinical Implications
5.3. Future Directions
- Standardization of Maturation Platforms: Electrical pacing, metabolic reprogramming, and 3D tissue engineering have each shown partial success. The next frontier lies in combining these into unified, GMP-compliant bioreactor systems capable of producing mature, arrhythmia-resistant iPSC-CMs at scale. Longitudinal studies should evaluate not only electrophysiological endpoints but also the durability of maturation post-transplantation [2,3].
- Next-Generation Gene Editing: While CRISPR/Cas9 correction has established feasibility, the emergence of prime editing and epigenetic reprogramming offers the possibility of correcting polygenic arrhythmogenic substrates with reduced off-target risk [155,156,157]. Pairing gene editing with real-time functional readouts, such as optical mapping of conduction and calcium transients, could provide a closed-loop framework for tailoring therapies at the single-cell level [158,159,160,161,162].
- Bioengineered Multicellular Niches: Co-culture approaches need to evolve into fully bioengineered myocardial constructs, where iPSC-CMs are integrated with fibroblasts, endothelial cells, and autonomic inputs in 3D microenvironments that replicate the physiological conduction hierarchy. Integration of vascularization strategies—such as endothelialized scaffolds or angiogenic extracellular vesicles—may further reduce arrhythmic substrates by optimizing oxygen and nutrient supply [2,3,5,15].
- Pharmacological-Genetic Hybrids: There is untapped potential in designing therapies that combine transient pharmacological stabilization with long-term genomic correction. For instance, patients receiving iPSC-CM grafts may initially be treated with ion channel modulators or calcium stabilizers until gene-edited, matured grafts achieve stable conduction synchrony.
- Integration with Bioenergetics Therapies: Our previous findings on mitochondria-enriched extracellular vesicles underscore how metabolic integrity underpins electrical stability. A critical research direction is the co-application of metabolic modulators—whether vesicle-based, small-molecule, or gene-driven—alongside iPSC-CM transplantation. By restoring mitochondrial architecture and oxidative phosphorylation, one can reduce delayed afterdepolarizations and stabilize excitation–contraction coupling.
Unresolved Challenges and Limitations
5.4. Concluding Perspective
6. Conclusions
6.1. Recommendations
6.2. Final Thoughts
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sugiura, T.; Shahannaz, D.C.; Ferrell, B.E. Current status of cardiac regenerative therapy using induced pluripotent stem cells. Int. J. Mol. Sci. 2024, 25, 5772. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, T.; Nawaz, S.; Shahannaz, D.C.; Ferrell, B.E.; Yoshida, T. From injury to repair: The therapeutic potential of induced pluripotent stem cells in heart failure. Regen. Med. Rep. 2025, 2, 22–30. [Google Scholar] [CrossRef]
- Sugiura, T.; Shahannaz, D.C.; Ferrell, B.E.; Yoshida, T. Advancements in cardiac regenerative therapy: Scalable human iPSC-derived cardiomyocyte differentiation and maturation. Glob. Transl. Med. 2025, 4, 5745. [Google Scholar] [CrossRef]
- Luce, E.; Duclos-Vallee, J.C. Stem Cells and Organoids: A paradigm shift in preclinical models toward personalized medicine. Pharmaceuticals 2025, 18, 992. [Google Scholar] [CrossRef]
- Shahannaz, D.C.; Sugiura, T.; Ferrell, B.E. Enhancing mitochondrial maturation in IPSC-DerivedCardiomyocytes: Strategies for Metabolic Optimization. BioChem 2025, 5, 23. [Google Scholar] [CrossRef]
- Navarrete, E.G.; Liang, P.; Lan, F.; Sanchez-Freire, T.; Simmons, C.; Gong, T.; Sharma, A.; Burridge, P.W.; Patlolia, B.; Lee, A.S.; et al. Screening Drug-Induced arrhythmia using human induced pluripotent stem Cell–Derived cardiomyocytes and Low-Impedance microelectrode arrays. Circulation 2013, 128 (Suppl. S1), S1–S13. [Google Scholar] [CrossRef] [PubMed]
- Joshi, J.; Albers, C.; Smole, N.; Guo, S.; Smith, S.A. Human induced pluripotent stem cell-derived cardiomyocytes (iPSC-CMs) for modeling cardiac arrhythmias: Strengths, challenges and potential solutions. Front. Physiol. 2024, 15, 1475152. [Google Scholar] [CrossRef]
- Reisqs, J.B.; Moreau, A.; Sleiman, Y.; Boutjdir, M.; Richard, S.; Chevalier, P. Arrhythmogenic cardiomyopathy as a myogenic disease: Highlights from cardiomyocytes derived from human induced pluripotent stem cells. Front. Physiol. 2023, 14, 1191965. [Google Scholar] [CrossRef]
- Garg, P.; Oikonomopoulos, A.; Chen, H.; Li, Y.; Lam, C.K.; Sallam, K.; Perez, M.; Lux, R.L.; Sanguinetti, M.C.; Wu, J.C. Genome Editing of Induced Pluripotent Stem Cells to Decipher Cardiac Channelopathy Variant. J. Am. Coll. Cardiol. 2018, 72, 62–75. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Y.F.; Wang, B.; Lv, T.T.; Zhang, P. Induced pluripotent stem cells in congenital long QT syndrome: Research progress and clinical applications. Rev. Cardiovasc. Med. 2025, 26, 28251. [Google Scholar] [CrossRef]
- Itzhaki, I.; Maizels, L.; Huber, I.; Gepstein, A.; Arbel, G.; Caspi, O.; Miller, L.; Belhassen, B.; Nof, E.; Glikson, M.; et al. Modeling of Catecholaminergic Polymorphic Ventricular Tachycardia With Patient-Specific Human-Induced Pluripotent Stem Cells. J. Am. Coll. Cardiol. 2012, 60, 990–1000. [Google Scholar] [CrossRef]
- Zhang, X.H.; Haviland, S.; Wei, H.; Gepstein, A.; Arbel, G.; Caspi, O.; Miller, L.; Belhassen, B.; Nof, E.; Glikson, M.; et al. Ca2+ signaling in human induced pluripotent stem cell-derived cardiomyocytes (iPS-CM) from normal and catecholaminergic polymorphic ventricular tachycardia (CPVT)-afflicted subjects. Cell Calcium 2013, 54, 57–70. [Google Scholar] [CrossRef]
- Li, Y.; Lang, S.; Akin, I.; Zhou, X.; El-Battrawy, I. Brugada Syndrome: Different experimental models and the role of human cardiomyocytes from induced pluripotent stem cells. J. Am. Heart Assoc. 2022, 11, e024410. [Google Scholar] [CrossRef]
- Seibertz, F.; Rubio, T.; Springer, R.; Popp, F.; Ritter, M.; Liutkute, A.; Bartelt, L.; Stelzer, L.; Haghighi, F.; Pietras, J.; et al. Atrial fibrillation-associated electrical remodelling in human induced pluripotent stem cell-derived atrial cardiomyocytes: A novel pathway for antiarrhythmic therapy development. Cardiovasc. Res. 2023, 119, 2623–2637. [Google Scholar] [CrossRef]
- Shahannaz, D.C.; Sugiura, T.; Yoshida, T. Mitochondria-Enriched Extracellular Vesicles (EVs) for Cardiac Bioenergetics Restoration: A Scoping Review of Preclinical Mechanisms and Source-Specific Strategies. Int. J. Mol. Sci. 2025, 26, 11052. [Google Scholar] [CrossRef]
- Shahannaz, D.C.; Sugiura, T.; Ferrell, B.E.; Yoshida, T. Targetting Mitochondrial Dynamics via EV Delivery in Regenerative Cardiology: Mechanistic and Therapeutic Perspectives. Cells 2025, 14, 1738. [Google Scholar] [CrossRef]
- Shahannaz, D.C.; Sugiura, T.; Ferrell, B.E. The role of large language models in induced pluripotent stem cell-derived cardiomyocytes research and clinical translation. J. Clin. Transl. Res. 2025, 11, 4–28. [Google Scholar] [CrossRef]
- Charneca, J.; Matias, A.C.; Escapa, A.L.; Fernandes, C.; Alves, A.; Santos, J.M.; Nascimento, R.; Bragança, J. Ectopic expression of CITED2 prior to reprogramming, promotes and homogenises the conversion of somatic cells into induced pluripotent stem cells. Exp. Cell Res. 2017, 358, 290–300. [Google Scholar] [CrossRef]
- Nakanishi, H.; Lee, J.K.; Miwa, K.; Masuyama, K.; Yasutake, H.; Li, J.; Tomoyama, S.; Honda, Y.; Deguchi, J.; Tsujimoto, S.; et al. Geometrical Patterning and Constituent Cell Heterogeneity Facilitate Electrical Conduction Disturbances in a Human Induced Pluripotent Stem Cell-Based Platform: An In vitro Disease Model of Atrial Arrhythmias. Front. Physiol. 2019, 10, 818. [Google Scholar] [CrossRef] [PubMed]
- Treat, J.A.; Goodrow, R.J.; Bot, C.T.; Haedo, R.J.; Cordeiro, J.M. Pharmacological enhancement of repolarization reserve in human induced pluripotent stem cells derived cardiomyocytes. Biochem. Pharmacol. 2019, 169, 113608. [Google Scholar] [CrossRef] [PubMed]
- Giannetti, F.; Benzoni, P.; Campostrini, G.; Milanesi, R.; Bucchi, A.; Baruscotti, M.; Dell’Era, P.; Rossini, A.; Barbuti, A. A detailed characterization of the hyperpolarization-activated “funny” current (If) in human-induced pluripotent stem cell (iPSC)-derived cardiomyocytes with pacemaker activity. Pflug. Arch. 2021, 473, 1009–1021. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saito, Y.; Nakamura, K.; Yoshida, M.; Sugiyama, H.; Akagi, S.; Miyoshi, T.; Morita, H.; Ito, H. Enhancement of pacing function by HCN4 overexpression in human pluripotent stem cell-derived cardiomyocytes. Stem Cell Res. Ther. 2022, 13, 141. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, J.J.; Yang, L.; Lin, B.; Zhu, X.; Sun, B.; Kaplan, A.D.; Bett, G.C.; Rasmusson, R.L.; London, B.; Salama, G. Mechanism of automaticity in cardiomyocytes derived from human induced pluripotent stem cells. J. Mol. Cell. Cardiol. 2015, 81, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Cruz, F.M.; Macías, Á.; Moreno-Manuel, A.I.; Gutiérrez, L.K.; Vera-Pedrosa, M.L.; Martínez-Carrascoso, I.; Pérez, P.S.; Robles, J.M.R.; Bermúdez-Jiménez, F.J.; Díaz-Agustín, A.; et al. Extracellular KIR2.1 C122Y mutant upsets KIR2.1-PIP 2 bonds and is arrhythmogenic in Andersen-Tawil syndrome. Circ. Res. 2024, 134, e52–e71. [Google Scholar] [CrossRef] [PubMed]
- Trum, M.; Islam, M.M.T.; Lebek, S.; Baier, M.; Hegner, P.; Eaton, P.; Maier, L.S.; Wagner, S. Inhibition of cardiac potassium currents by oxidation-activated protein kinase A contributes to early afterdepolarizations in the heart. AJP Heart Circ. Physiol. 2020, 319, H1347–H1357. [Google Scholar] [CrossRef]
- Cabo, C. Positive rate-dependent action potential prolongation by modulating potassium ion channels. Physiol. Rep. 2022, 10, e15356. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Steinberg, C.; Roston, T.M.; Van Der Werf, C.; Sanatani, S.; Chen, S.R.W.; Wilde, A.A.M.; Krahn, A.D. RYR2-ryanodinopathies: From calcium overload to calcium deficiency. Europace 2023, 25, euad156. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Cao, Y.; Jardin, B.D.; Zhang, X.; Zhou, P.; Guatimosim, S.; Lin, J.; Chen, Z.; Zhang, Y.; Mazumdar, N.; et al. Ryanodine receptor 2 (RYR2) dysfunction activates the unfolded protein response and perturbs cardiomyocyte maturation. Cardiovasc. Res. 2022, 119, 221–235. [Google Scholar] [CrossRef]
- Zaffran, S.; Kraoua, L.; Jaouadi, H. Calcium handling in inherited cardiac diseases: A focus on catecholaminergic polymorphic ventricular tachycardia and hypertrophic cardiomyopathy. Int. J. Mol. Sci. 2023, 24, 3365. [Google Scholar] [CrossRef]
- Starnes, L.; Hall, A.; Etal, D.; Cavallo, A.-L.; Grabowski, P.; Gallon, J.; Kha, M.; Hicks, R.; Pointon, A. RYR2 deficient human model identifies calcium handling and metabolic dysfunction impacting pharmacological responses. Front. Cardiovasc. Med. 2024, 11, 1357315. [Google Scholar] [CrossRef]
- Itzhaki, I.; Maizels, L.; Huber, I.; Zwi-Dantsis, L.; Caspi, O.; Winterstern, A.; Feldman, O.; Gepstein, A.; Arbel, G.; Hammerman, H.; et al. Modelling the long QT syndrome with induced pluripotent stem cells. Nature 2011, 471, 225–229. [Google Scholar] [CrossRef]
- Yu, Y.; Deschenes, I.; Zhao, M.T. Precision medicine for long QT syndrome: Patient-specific iPSCs take the lead. Expert Rev. Mol. Med. 2023, 25, e5. [Google Scholar] [CrossRef] [PubMed]
- Schneider, L.; Begovic, M.; Zhou, X.; Hamdani, N.; Akin, I.; El-Battrawy, I. Catecholaminergic Polymorphic Ventricular Tachycardia: Advancing from molecular insights to preclinical models. J. Am. Heart Assoc. 2025, 14, e038308. [Google Scholar] [CrossRef]
- Van Bavel, J.J.A.; Beekman, H.D.M.; Van Weperen, V.Y.H.; Van Der Linde, H.J.; Van Der Heyden, M.A.G.; Vos, M.A. IKs inhibitor JNJ303 prolongs the QT interval and perpetuates arrhythmia when combined with enhanced inotropy in the CAVB dog. Eur. J. Pharmacol. 2022, 932, 175218. [Google Scholar] [CrossRef]
- Harmer, S.C.; Mohal, J.S.; Royal, A.A.; McKenna, W.J.; Lambiase, P.D.; Tinker, A. Cellular mechanisms underlying the increased disease severity seen for patients with long QT syndrome caused by compound mutations in KCNQ1. Biochem. J. 2014, 462, 133–142. [Google Scholar] [CrossRef]
- Wilde, A.A.M.; Amin, A.S.; Postema, P.G. Diagnosis, management and therapeutic strategies for congenital long QT syndrome. Heart 2022, 108, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Zhang, J.; Lee, C.; Kim, G.; Liu, F.; Petersen, A.J.; Lim, E.; Anderson, C.L.; Orland, K.M.; Robertson, G.A.; et al. Long QT syndrome KCNH2 variant induces HERG1A/1B subunit imbalance in Patient-Specific induced pluripotent STEM Cell–Derived cardiomyocytes. Circ. Arrhythmia Electrophysiol. 2021, 14, 424–439. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.R.; Mondéjar-Parreño, G.; Li, D.; Shen, M.; Wu, J.C. Technical applications of microelectrode array and patch clamp recordings on human induced pluripotent stem Cell-Derived cardiomyocytes. J. Vis. Exp. 2022, 186, e64265. [Google Scholar] [CrossRef]
- Nijak, A.; Saenen, J.; Labro, A.J.; Schepers, D.; Loeys, B.L.; Alaerts, M. IPSC-Cardiomyocyte Models of Brugada Syndrome—Achievements, Challenges and Future Perspectives. Int. J. Mol. Sci. 2021, 22, 2825. [Google Scholar] [CrossRef]
- Galanti, K.; Iezzi, L.; Rizzuto, M.L.; Falco, D.; Negri, G.; Pham, H.N.; Mansour, D.; Giansante, R.; Stuppia, L.; Mazzocchetti, L.; et al. Desmosomal versus Non-Desmosomal Arrhythmogenic Cardiomyopathies: A State-of-the-Art Review. Cardiogenetics 2025, 15, 22. [Google Scholar] [CrossRef]
- Pergola, V.; Trancuccio, A.; Kukavica, D.; Mazzanti, A.; Napolitano, C.; Scilabra, G.G.; Steele, K.; Memmi, M.; Gambelli, P.; Sugamiele, A.; et al. Genotype-Specific outcomes of desmosomal cardiomyopathies. Circulation 2025, 152, 233–245. [Google Scholar] [CrossRef]
- Mohammed, F.; Chidgey, M. Desmosomal protein structure and function and the impact of disease-causing mutations. J. Struct. Biol. 2021, 213, 107749. [Google Scholar] [CrossRef]
- Beltrami, M.; Fedele, E.; Fumagalli, C.; Mazzarotto, F.; Girolami, F.; Ferrantini, C.; Coppini, R.; Tofani, L.; Bertaccini, B.; Poggesi, C.; et al. Long-Term prevalence of systolic dysfunction in MYBPC3 versus MYH7-Related hypertrophic cardiomyopathy. Circ. Genom. Precis. Med. 2023, 16, 363–371. [Google Scholar] [CrossRef]
- Sewanan, L.R.; Campbell, S.G. Modelling sarcomeric cardiomyopathies with human cardiomyocytes derived from induced pluripotent stem cells. J. Physiol. 2019, 598, 2909–2922. [Google Scholar] [CrossRef]
- Li, J.; Feng, X.; Wei, X. Modeling hypertrophic cardiomyopathy with human cardiomyocytes derived from induced pluripotent stem cells. Stem Cell Res. Ther. 2022, 13, 232. [Google Scholar] [CrossRef]
- Lennermann, D.; Backs, J.; van den Hoogenhof, M.M.G. New Insights in RBM20 Cardiomyopathy. Curr. Heart Fail. Rep. 2020, 17, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Briganti, F.; Sun, H.; Wei, W.; Wu, J.; Zhu, C.; Liss, M.; Karakikes, I.; Rego, S.; Cipriano, A.; Snyder, M.; et al. IPSC Modeling of RBM20-Deficient DCM identifies upregulation of RBM20 as a therapeutic strategy. Cell Rep. 2020, 32, 108117. [Google Scholar] [CrossRef] [PubMed]
- Cubeddu, L. Drug-induced Inhibition and Trafficking Disruption of ion Channels: Pathogenesis of QT Abnormalities and Drug-induced Fatal Arrhythmias. Curr. Cardiol. Rev. 2016, 12, 141–154. [Google Scholar] [CrossRef]
- Goversen, B.; Van Der Heyden, M.A.G.; Van Veen, T.A.B.; De Boer, T.P. The immature electrophysiological phenotype of iPSC-CMs still hampers in vitro drug screening: Special focus on I K1. Pharmacol. Ther. 2017, 183, 127–136. [Google Scholar] [CrossRef]
- Huang, M.; Liao, Z.; Li, X.; Yang, Z.; Fan, X.; Li, Y.; Zhao, Z.; Lang, S.; Cyganek, L.; Zhou, X.; et al. Effects of antiarrhythmic drugs on HERG gating in Human-Induced pluripotent stem Cell-Derived cardiomyocytes from a patient with short QT syndrome type 1. Front. Pharmacol. 2021, 12, 675003. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.X.; Kristiansen, C.K.; Mostafavi, S.; Vatne, G.H.; Zantingh, G.A.; Kianian, A.; Tzoulis, C.; Høyland, L.E.; Ziegler, M.; Perez, R.M.; et al. Disease-specific phenotypes in iPSC -derived neural stem cells with POLG mutations. EMBO Mol. Med. 2020, 12, e12146. [Google Scholar] [CrossRef]
- Chen, A.; Kristiansen, C.K.; Høyland, L.E.; Ziegler, M.; Wang, J.; Sullivan, G.J.; Li, X.; Bindoff, L.A.; Liang, K.X. POLG mutations lead to abnormal mitochondrial remodeling during neural differentiation of human pluripotent stem cells via SIRT3/AMPK pathway inhibition. Cell Cycle 2022, 21, 1178–1193. [Google Scholar] [CrossRef]
- McKnight, C.L.; Low, Y.C.; Elliott, D.A.; Thorburn, D.R.; Frazier, A.E. Modelling mitochondrial disease in human pluripotent stem cells: What have we learned? Int. J. Mol. Sci. 2021, 22, 7730. [Google Scholar] [CrossRef]
- Wang, X.; Tan, X.; Zhang, T.; Xu, S.; Zeng, Y.; Xu, A.; Li, X.; Zhang, G.; Jiang, Y.; Jiang, H.; et al. Modeling Diabetic cardiomyopathy using human cardiac organoids: Effects of high glucose and lipid conditions. Chem. Biol. Interact. 2025, 411, 111421. [Google Scholar] [CrossRef] [PubMed]
- Carter, R.D.; Purnama, U.; Castro-Guarda, M.; Montes-Aparicio, C.N.; Chandran, A.; Mbasu, R.; Ruby, M.; Daly, C.; Buffa, F.M.; Heather, L.C.; et al. Multiomics-based assessment of 2D and 3D human iPSC-cardiomyocyte models of insulin resistance demonstrate metabolic and contractile dysfunction that recapitulates diabetic cardiomyopathy. bioRxiv 2024. bioRxiv:2024.11.20.624467. [Google Scholar] [CrossRef]
- Purnama, U.; Castro-Guarda, M.; Sahoo, O.S.; Carr, C.A. Modelling diabetic cardiomyopathy: Using human stem Cell-Derived cardiomyocytes to complement animal models. Metabolites 2022, 12, 832. [Google Scholar] [CrossRef]
- Kistamás, K.; Lamberto, F.; Vaiciuleviciute, R.; Leal, F.; Muenthaisong, S.; Marte, L.; Subías-Beltrán, P.; Alaburda, A.; Arvanitis, D.N.; Zana, M.; et al. The current state of realistic heart models for disease modelling and cardiotoxicity. Int. J. Mol. Sci. 2024, 25, 9186. [Google Scholar] [CrossRef] [PubMed]
- Parrotta, E.I.; Lucchino, V.; Scaramuzzino, L.; Scalise, S.; Cuda, G. Modeling cardiac disease mechanisms using Induced Pluripotent Stem Cell-Derived cardiomyocytes: Progress, promises and challenges. Int. J. Mol. Sci. 2020, 21, 4354. [Google Scholar] [CrossRef]
- Kotadia, I.; Whitaker, J.; Roney, C.; Niederer, S.; O’neill, M.; Bishop, M.; Wright, M. Anisotropic cardiac conduction. Arrhythmia Electrophysiol. Rev. 2020, 9, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Biendarra-Tiegs, S.M.; Secreto, F.J.; Nelson, T.J. Addressing variability and heterogeneity of induced Pluripotent Stem Cell-Derived cardiomyocytes. Adv. Exp. Med. Biol. 2019, 1212, 1–29. [Google Scholar] [CrossRef]
- Varela, M.; Colman, M.A.; Hancox, J.C.; Aslanidi, O.V. Atrial Heterogeneity Generates Re-entrant Substrate during Atrial Fibrillation and Anti-arrhythmic Drug Action: Mechanistic Insights from Canine Atrial Models. PLoS Comput. Biol. 2016, 12, e1005245. [Google Scholar] [CrossRef]
- Iwamiya, S.; Ihara, K.; Nitta, G.; Sasano, T. Atrial fibrillation and underlying structural and electrophysiological heterogeneity. Int. J. Mol. Sci. 2024, 25, 10193. [Google Scholar] [CrossRef]
- Cabo, C.; Yao, J.; Boyden, P.; Chen, S.; Hussain, W.; Duffy, H.; Ciaccio, E.; Peters, N.; Wit, A. Heterogeneous gap junction remodeling in reentrant circuits in the epicardial border zone of the healing canine infarct. Cardiovasc. Res. 2006, 72, 241–249. [Google Scholar] [CrossRef]
- Zaniboni, M.; Cacciani, F.; Lux, R.L. Beat-to-Beat Cycle Length Variability of Spontaneously Beating Guinea Pig Sinoatrial Cells: Relative Contributions of the Membrane and Calcium Clocks. PLoS ONE 2014, 9, e100242. [Google Scholar] [CrossRef] [PubMed]
- Heijman, J.; Zaza, A.; Johnson, D.M.; Rudy, Y.; Peeters, R.L.M.; Volders, P.G.A.; Westra, R.L. Determinants of Beat-to-Beat Variability of Repolarization Duration in the Canine Ventricular Myocyte: A Computational Analysis. PLoS Comput. Biol. 2013, 9, e1003202. [Google Scholar] [CrossRef]
- Lemay, M.; De Lange, E.; Kucera, J.P. Effects of stochastic channel gating and distribution on the cardiac action potential. J. Theor. Biol. 2011, 281, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, V.E.; Verkerk, A.O.; Amin, A.S.; De Bakker, J.M. Intracardiac Origin of Heart Rate Variability, Pacemaker Funny Current and their Possible Association with Critical Illness. Curr. Cardiol. Rev. 2013, 9, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Spach, M.S.; Heidlage, J.F. The stochastic nature of cardiac propagation at a microscopic level. Circ. Res. 1995, 76, 366–380. [Google Scholar] [CrossRef]
- Lerma, C.; Krogh-Madsen, T.; Guevara, M.; Glass, L. Stochastic Aspects of Cardiac Arrhythmias. J. Stat. Phys. 2007, 128, 347–374. [Google Scholar] [CrossRef]
- Piscaglia, A.C. Stem cells, a two-edged sword: Risks and potentials of regenerative medicine. World J. Gastroenterol. 2008, 14, 4273. [Google Scholar] [CrossRef]
- Tajnšek, U.; Motaln, H.; Levičar, N.; Rotter, A.; Lah, T.T. The Duality of Stem Cells: Double-Edged Sword in tumor Evolution and Treatment. In Trends in Stem Cell Proliferation and Cancer Research; Resende, R., Ulrich, H., Eds.; Springer: Dordrecht, The Netherlands, 2013. [Google Scholar] [CrossRef]
- Selvakumar, D.; Clayton, Z.E.; Prowse, A.; Dingwall, S.; Kim, S.K.; Reyes, L.; George, J.; Shah, H.; Chen, S.; Leung, H.H.L.; et al. Cellular heterogeneity of pluripotent stem cell-derived cardiomyocyte grafts is mechanistically linked to treatable arrhythmias. Nat. Cardiovasc. Res. 2024, 3, 145–165. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Liu, Y.; Yan, Y.; Fooladi, S.; Qyang, Y. Advancements in techniques for human iPSC-derived cardiomyocytes maturation: Mechanical and electrical stimulation approaches. Biophys. Rev. 2025, 17, 169–183. [Google Scholar] [CrossRef]
- Wei, F.; Pourrier, M.; Strauss, D.G.; Stockbridge, N.; Pang, L. Effects of electrical stimulation on HIPSC-CM responses to classic ion channel blockers. Toxicol. Sci. 2020, 174, 254–265. [Google Scholar] [CrossRef]
- Vo, Q.D.; Nakamura, K.; Saito, Y.; Iida, T.; Yoshida, M.; Amioka, N.; Akagi, S.; Miyoshi, T.; Yuasa, S. IPSC-Derived Biological Pacemaker—From bench to bedside. Cells 2024, 13, 2045. [Google Scholar] [CrossRef]
- Van De Sande, D.V.; Kopljar, I.; Alaerts, M.; Teisman, A.; Gallacher, D.J.; Loeys, B.; Snyders, D.J.; Leybaert, L.; Lu, H.R.; Labro, A.J. The resting membrane potential of hSC-CM in a syncytium is more hyperpolarised than that of isolated cells. Channels 2021, 15, 239–252. [Google Scholar] [CrossRef]
- Mahoney, V.M.; Mezzano, V.; Mirams, G.R.; Maass, K.; Li, Z.; Cerrone, M.; Vasquez, C.; Bapat, A.; Delmar, M.; Morley, G.E. Connexin43 contributes to electrotonic conduction across scar tissue in the intact heart. Sci. Rep. 2016, 6, 26744. [Google Scholar] [CrossRef]
- Zhu, Y. Gap Junction-Dependent and -Independent Functions of Connexin43 in Biology. Biology 2022, 11, 283. [Google Scholar] [CrossRef]
- Sims, J.J.; Miller, A.W.; Ujhelyi, M.R. Electrical Heterogeneity and Arrhythmogenesis: Importance of Conduction Velocity Dispersion. J. Cardiovasc. Pharmacol. 2003, 41, 795–803. [Google Scholar] [CrossRef]
- Heida, A.; van der Does, W.; van Staveren, L.; Taverne, Y.J.; Roos-Serote, M.C.; Bogers, A.J.; de Groot, N.M. Conduction Heterogeneity: Impact of Underlying Heart Disease and Atrial Fibrillation. JACC Clin. Electrophysiol. 2020, 6, 1844–1854. [Google Scholar] [CrossRef] [PubMed]
- Fassina, D.; Costa, C.M.; Longobardi, S.; Karabelas, E.; Plank, G.; Harding, S.E.; Niederer, S.A. Modelling the interaction between stem cells derived cardiomyocytes patches and host myocardium to aid non-arrhythmic engineered heart tissue design. PLoS Comput. Biol. 2022, 18, e1010030. [Google Scholar] [CrossRef] [PubMed]
- Lemme, M.; Braren, I.; Prondzynski, M.; Aksehirlioglu, B.; Ulmer, B.M.; Schulze, M.L.; Ismaili, D.; Meyer, C.; Hansen, A.; Christ, T.; et al. Chronic intermittent tachypacing by an optogenetic approach induces arrhythmia vulnerability in human engineered heart tissue. Cardiovasc. Res. 2019, 116, 1487–1499. [Google Scholar] [CrossRef]
- Seguret, M.; Davidson, P.; Robben, S.; Jouve, C.; Pereira, C.; Lelong, Q.; Deshayes, L.; Cerveau, C.; Le Berre, M.; Ribeiro, R.S.R.; et al. A versatile high-throughput assay based on 3D ring-shaped cardiac tissues generated from human induced pluripotent stem cell-derived cardiomyocytes. eLife 2024, 12, RP87739. [Google Scholar] [CrossRef]
- Xu, Y.; Qi, J.; Zhou, W.; Liu, X.; Zhang, L.; Yao, X.; Wu, H. Generation of ring-shaped human iPSC-derived functional heart microtissues in a Möbius strip configuration. Bio Des. Manuf. 2022, 5, 687–699. [Google Scholar] [CrossRef]
- Andrée, B.; Voß, N.; Kriedemann, N.; Triebert, W.; Teske, J.; Mertens, M.; Witte, M.; Szádocka, S.; Hilfiker, A.; Aper, T.; et al. Fabrication of heart tubes from iPSC derived cardiomyocytes and human fibrinogen by rotating mold technology. Sci. Rep. 2024, 14, 13174. [Google Scholar] [CrossRef]
- Vanderslice, E.J.; Golding, S.G.H.; Jacot, J.G. Vascularization of PEGylated fibrin hydrogels increases the proliferation of human iPSC-cardiomyocytes. J. Biomed. Mater. Res. Part A 2023, 112, 625–634. [Google Scholar] [CrossRef]
- Li, R.A.; Keung, W.; Cashman, T.J.; Backeris, P.C.; Johnson, B.V.; Bardot, E.S.; Wong, A.O.; Chan, P.K.; Chan, C.W.; Costa, K.D. Bioengineering an electro-mechanically functional miniature ventricular heart chamber from human pluripotent stem cells. Biomaterials 2018, 163, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Goldfracht, I.; Protze, S.; Shiti, A.; Setter, N.; Gruber, A.; Shaheen, N.; Nartiss, Y.; Keller, G.; Gepstein, L. Generating ring-shaped engineered heart tissues from ventricular and atrial human pluripotent stem cell-derived cardiomyocytes. Nat. Commun. 2020, 11, 75. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ikeda, K.; Nagata, S.; Okitsu, T.; Takeuchi, S. Cell fiber-based three-dimensional culture system for highly efficient expansion of human induced pluripotent stem cells. Sci. Rep. 2017, 7, 2850. [Google Scholar] [CrossRef]
- Gartner, T.C.L.B.; Crnko, S.; Leiteris, L.; van Adrichem, I.; van Laake, L.W.; Bouten, C.V.C.; Goumans, M.J.; Suyker, W.J.L.; Sluijter, J.P.G.; Hjortnaes, J. Pirfenidone has anti-fibrotic effects in a Tissue-Engineered model of human cardiac fibrosis. Front. Cardiovasc. Med. 2022, 9, 854314. [Google Scholar] [CrossRef]
- Tadano, K.; Miyagawa, S.; Takeda, M.; Tsukamoto, Y.; Kazusa, K.; Takamatsu, K.; Akashi, M.; Sawa, Y. Cardiotoxicity assessment using 3D vascularized cardiac tissue consisting of human iPSC-derived cardiomyocytes and fibroblasts. Mol. Ther. Methods Clin. Dev. 2021, 22, 338–349. [Google Scholar] [CrossRef]
- Campostrini, G.; Kosmidis, G.; Ward-van Oostwaard, D.; Davis, R.P.; Yiangou, L.; Ottaviani, D.; Veerman, C.C.; Mei, H.; Orlova, V.V.; Wilde, A.A.M.; et al. Maturation of hiPSC-derived cardiomyocytes promotes adult alternative splicing of SCN5A and reveals changes in sodium current associated with cardiac arrhythmia. Cardiovasc. Res. 2023, 119, 167–182. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liang, P.; Sallam, K.; Wu, H.; Li, Y.; Itzhaki, I.; Garg, P.; Zhang, Y.; Termglichan, V.; Lan, F.; Gu, M.; et al. Patient-Specific and Genome-Edited induced pluripotent stem Cell–Derived cardiomyocytes elucidate Single-Cell phenotype of Brugada syndrome. J. Am. Coll. Cardiol. 2016, 68, 2086–2096. [Google Scholar] [CrossRef] [PubMed]
- Lemoine, M.D.; Mannhardt, I.; Breckwoldt, K.; Prondzynski, M.; Flenner, F.; Ulmer, B.; Hirt, M.N.; Neuber, C.; Horváth, A.; Kloth, B.; et al. Human iPSC-derived cardiomyocytes cultured in 3D engineered heart tissue show physiological upstroke velocity and sodium current density. Sci. Rep. 2017, 7, 5464. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pourchet, L.; Casado-Medina, L.; Richaud-Patin, Y.; Tadevosyan, K.; Morillas-García, A.; Lorenzo, E.; Lazis, I.; Ventura, A.; Litowczenko, J.; Guiu, J.; et al. 3D bioprinting of human iPSC-derived cardiac constructs with microvascular network support for improved graft survival in vivo. Biofabrication 2025, 17, 035010. [Google Scholar] [CrossRef] [PubMed]
- Esser, T.U.; Anspach, A.; Muenzebrock, K.A.; Kah, D.; Schrüfer, S.; Schenk, J.; Heinze, K.G.; Schubert, D.W.; Fabry, B.; Engel, F.B. Direct 3D-Bioprinting of HIPSC-Derived cardiomyocytes to generate functional cardiac tissues. Adv. Mater. 2023, 35, e2305911. [Google Scholar] [CrossRef]
- Bliley, J.; Tashman, J.; Stang, M.; Coffin, B.; Shiwarski, D.; Lee, A.; Hinton, T.; Feinberg, A. FRESH 3D bioprinting a contractile heart tube using human stem cell-derived cardiomyocytes. Biofabrication 2022, 14, 024106. [Google Scholar] [CrossRef]
- Miklas, J.W.; Nunes, S.S.; Sofla, A.; Reis, L.A.; Pahnke, A.; Xiao, Y.; Laschinger, C.; Radisic, M. Bioreactor for modulation of cardiac microtissue phenotype by combined static stretch and electrical stimulation. Biofabrication 2014, 6, 024113. [Google Scholar] [CrossRef]
- Marino, S.; Alheijailan, R.; Alonaizan, R.; Gabetti, S.; Massai, D.; Pesce, M. Cardiac tissue bioprinting: Integrating structure and functions through biomimetic design, bioINKs, and stimulation. Gels 2025, 11, 593. [Google Scholar] [CrossRef]
- Afjeh-Dana, E.; Naserzadeh, P.; Moradi, E.; Hosseini, N.; Seifalian, A.M.; Ashtari, B. Stem Cell Differentiation into Cardiomyocytes: Current Methods and Emerging Approaches. Stem Cell Rev. Rep. 2022, 18, 2566–2592. [Google Scholar] [CrossRef]
- Sesena-Rubfiaro, A.; Prajapati, N.J.; Paolino, L.; Lou, L.; Cotayo, D.; Pandey, P.; Shaver, M.; Hutcheson, J.D.; Agarwal, A.; He, J. Membrane remodeling of Human-Engineered cardiac tissue by chronic electric stimulation. ACS Biomater. Sci. Eng. 2023, 9, 1644–1655. [Google Scholar] [CrossRef]
- Terrar, D.A. Timing mechanisms to control heart rhythm and initiate arrhythmias: Roles for intracellular organelles, signalling pathways and subsarcolemmal Ca2. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 2023, 378, 20220170. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Prusty, D.; Nayak, A.R. Pacing and ionic conductance effects on excitation waves in Early-Afterdepolarization cardiac models. J. Comput. Nonlinear Dyn. 2025, 20, 111007. [Google Scholar] [CrossRef]
- Leclech, C.; Villard, C. Cellular and Subcellular Contact Guidance on Microfabricated Substrates. Front. Bioeng. Biotechnol. 2020, 8, 551505. [Google Scholar] [CrossRef]
- Hu, X.; Bao, M. Advances in micropatterning technology for mechanotransduction research. Mechanobiol. Med. 2024, 2, 100066. [Google Scholar] [CrossRef]
- Song, Y.; Zheng, Z.; Lian, J. Deciphering Common Long QT Syndrome Using CRISPR/Cas9 in Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Front. Cardiovasc. Med. 2022, 9, 889519. [Google Scholar] [CrossRef]
- Li, W.; Han, J.L.; Entcheva, E. Syncytium cell growth increases Kir2.1 contribution in human iPSC-cardiomyocytes. Am. J. Physiol.-Heart Circ. Physiol. 2020, 319, H1112–H1122. [Google Scholar] [CrossRef]
- Saber Sichani, A.; Ranjbar, M.; Baneshi, M.; Zadeh, F.T.; Fallahi, J. A Review on Advanced CRISPR-Based Genome-Editing Tools: Base Editing and Prime Editing. Mol. Biotechnol. 2023, 65, 849–860. [Google Scholar] [CrossRef]
- Pellman, J.; Zhang, J.; Sheikh, F. Myocyte-fibroblast communication in cardiac fibrosis and arrhythmias: Mechanisms and model systems. J. Mol. Cell. Cardiol. 2016, 94, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Kirabo, A.; Ryzhov, S.; Gupte, M.; Sengsayadeth, S.; Gumina, R.J.; Sawyer, D.B.; Galindo, C.J. Neuregulin-1β induces proliferation, survival and paracrine signaling in normal human cardiac ventricular fibroblasts. J. Mol. Cell. Cardiol. 2017, 105, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Antzelevitch, C.; Burashnikov, A.; Sicouri, S.; Belardinelli, L. Electrophysiologic basis for the antiarrhythmic actions of ranolazine. Heart Rhythm. 2011, 8, 1281–1290. [Google Scholar] [CrossRef]
- Verrier, R.L.; Kumar, K.; Nieminen, T.; Belardinelli, L. Mechanisms of ranolazine’s dual protection against atrial and ventricular fibrillation. Europace 2013, 15, 317–324. [Google Scholar] [CrossRef]
- Rouhana, S.; Virsolvy, A.; Fares, N.; Richard, S.; Thireau, J. Ranolazine: An Old Drug with Emerging Potential; Lessons from Pre-Clinical and Clinical Investigations for Possible Repositioning. Pharmaceuticals 2022, 15, 31. [Google Scholar] [CrossRef] [PubMed]
- Lenaeus, M.; Gamal El-Din, T.M.; Tonggu, L.; Zheng, N.; Catterall, W.A. Structural basis for inhibition of the cardiac sodium channel by the atypical antiarrhythmic drug ranolazine. Nat. Cardiovasc. Res. 2023, 2, 587–594. [Google Scholar] [CrossRef]
- Perry, M.D.; Ng, C.A.; Mangala, M.M.; Ng, T.Y.M.; Hines, A.D.; Liang, W.; Xu, M.J.O.; Hill, A.P.; Vandenberg, J.I. Pharmacological activation of IKr in models of long QT Type 2 risks overcorrection of repolarization. Cardiovasc. Res. 2020, 116, 1434–1445. [Google Scholar] [CrossRef] [PubMed]
- Tadini-Buoninsegni, F.; Smeazzetto, S.; Gualdani, R.; Moncelli, M.R. Drug interactions with the CA2+-ATPase from Sarco(Endo)Plasmic reticulum (SERCA). Front. Mol. Biosci. 2018, 5, 36. [Google Scholar] [CrossRef]
- McKeithan, W.L.; Feyen, D.A.M.; Bruyneel, A.A.N.; Okolotowicz, K.J.; Ryan, D.A.; Sampson, K.J.; Potet, F.; Savchenko, A.; Gómez-Galeno, J.; Vu, M.; et al. Reengineering an antiarrhythmic drug using patient HIPSC cardiomyocytes to improve therapeutic potential and reduce toxicity. Cell Stem Cell 2020, 27, 813–821.e6. [Google Scholar] [CrossRef] [PubMed]
- Teles, D.; Fine, B.M. Using induced pluripotent stem cells for drug discovery in arrhythmias. Expert Opin. Drug Discov. 2024, 19, 827–840. [Google Scholar] [CrossRef]
- Sahoglu, S.G.; Kazci, Y.E.; Tuncay, E.; Torun, T.; Akdeniz, C.; Tuzcu, V.; Cagavi, E. Functional evaluation of the tachycardia patient-derived iPSC cardiomyocytes carrying a novel pathogenic SCN5A variant. J. Cell. Physiol. 2022, 237, 3900–3911. [Google Scholar] [CrossRef]
- Johnson, M.; Gomez-Galeno, J.; Ryan, D.; Okolotowicz, K.; McKeithan, W.L.; Sampson, K.J.; Kass, R.S.; Mercola, M.; Cashman, J.R. Human iPSC-derived cardiomyocytes and pyridyl-phenyl mexiletine analogs. Bioorganic Med. Chem. Lett. 2021, 46, 128162. [Google Scholar] [CrossRef]
- Cashman, J.R. Reengineering Mexiletine by chemical synthesis to decrease toxicity and improve pharmacological properties with patient-derived iPSC cardiomyocytes. Arch. Clin. Toxicol. 2022, 4, 5–10. [Google Scholar] [CrossRef]
- Crotti, L.; Neves, R.; Dagradi, F.; Musu, G.; Giannetti, F.; Bos, J.M.; Barbieri, M.; Cerea, P.; Giovenzana, F.L.; Torchio, M.; et al. Therapeutic efficacy of mexiletine for long QT syndrome Type 2: Evidence from human induced Pluripotent Stem Cell–Derived cardiomyocytes, transgenic rabbits, and patients. Circulation 2024, 150, 531–543. [Google Scholar] [CrossRef]
- Horváth, A.; Lemoine, M.D.; Löser, A.; Mannhardt, I.; Flenner, F.; Uzun, A.U.; Neuber, C.; Breckwoldt, K.; Hansen, A.; Girdauskas, E.; et al. Low resting membrane potential and low inward rectifier potassium currents are not inherent features of HIPSC-Derived cardiomyocytes. Stem Cell Rep. 2018, 10, 822–833. [Google Scholar] [CrossRef] [PubMed]
- Doss, M.X.; Di Diego, J.M.; Goodrow, R.J.; Wu, Y.; Cordeiro, J.M.; Nesterenko, V.V.; Barajas-Martínez, H.; Hu, D.; Urrutia, J.; Desai, M.; et al. Maximum Diastolic Potential of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes Depends Critically on IKr. PLoS ONE 2012, 7, e40288. [Google Scholar] [CrossRef] [PubMed]
- Vaidyanathan, R.; Markandeya, Y.S.; Kamp, T.J.; Makielski, J.C.; January, C.T.; Eckhardt, L.L. IK1-enhanced human-induced pluripotent stem cell-derived cardiomyocytes: An improved cardiomyocyte model to investigate inherited arrhythmia syndromes. AJP Heart Circ. Physiol. 2016, 310, H1611–H1621. [Google Scholar] [CrossRef]
- Ma, J.; Guo, L.; Fiene, S.J.; Anson, B.D.; Thomson, J.A.; Kamp, T.J.; Kolaja, K.L.; Swanson, B.J.; January, C.T. High purity human-induced pluripotent stem cell-derived cardiomyocytes: Electrophysiological properties of action potentials and ionic currents. AJP Heart Circ. Physiol. 2011, 301, H2006–H2017. [Google Scholar] [CrossRef]
- Quach, B.; Krogh-Madsen, T.; Entcheva, E.; Christini, D.J. Light-Activated Dynamic Clamp using IPSC-Derived Cardiomyocytes. Biophys. J. 2018, 115, 2206–2217. [Google Scholar] [CrossRef]
- Koivumäki, J.T.; Naumenko, N.; Tuomainen, T.; Takalo, J.; Oksanen, M.; Puttonen, K.A.; Lehtonen, Š.; Kuusisto, J.; Laakso, M.; Koistinaho, J.; et al. Structural immaturity of human IPSC-Derived Cardiomyocytes: In Silico Investigation of Effects on Function and Disease Modeling. Front. Physiol. 2018, 9, 80. [Google Scholar] [CrossRef] [PubMed]
- Pourrier, M.; Fedida, D. The Emergence of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes (hiPSC-CMs) as a Platform to Model Arrhythmogenic Diseases. Int. J. Mol. Sci. 2020, 21, 657. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Paci, M.; Hyttinen, J.; Rodriguez, B.; Severi, S. Human induced pluripotent stem cell-derived versus adult cardiomyocytes: An in silico electrophysiological study on effects of ionic current block. Br. J. Pharmacol. 2015, 172, 5147–5160. [Google Scholar] [CrossRef]
- Karakikes, I.; Ameen, M.; Termglinchan, V.; Wu, J.C. Human induced Pluripotent Stem Cell–Derived cardiomyocytes. Circ. Res. 2015, 117, 80–88. [Google Scholar] [CrossRef]
- Wulkan, F.; Romagnuolo, R.; Qiang, B.; Sadikov, T.V.; Kim, K.-P.; Quesnel, E.; Jiang, W.; Andharia, N.; Weyers, J.J.; Ghugre, N.R.; et al. Stem cell-derived cardiomyocytes expressing a dominant negative pacemaker HCN4 channel do not reduce the risk of graft-related arrhythmias. Front. Cardiovasc. Med. 2024, 11, 1374881. [Google Scholar] [CrossRef] [PubMed]
- Protze, S.I.; Liu, J.; Nussinovitch, U.; Ohana, L.; Backx, P.H.; Gepstein, L.; Keller, G.M. Sinoatrial node cardiomyocytes derived from human pluripotent cells function as a biological pacemaker. Nat. Biotechnol. 2016, 35, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.P.; Casini, S.; Van Den Berg, C.W.; Hoekstra, M.; Remme, C.A.; Dambrot, C.; Salvatori, D.; Oostwaard, D.W.-V.; Wilde, A.A.; Bezzina, C.R.; et al. Cardiomyocytes derived from pluripotent stem cells recapitulate electrophysiological characteristics of an overlap syndrome of cardiac sodium channel disease. Circulation 2012, 125, 3079–3091. [Google Scholar] [CrossRef]
- Goodrow, R.J.; Desai, S.; Treat, J.A.; Panama, B.K.; Desai, M.; Nesterenko, V.V.; Cordeiro, J.M. Biophysical comparison of sodium currents in native cardiac myocytes and human induced pluripotent stem cell-derived cardiomyocytes. J. Pharmacol. Toxicol. Methods 2017, 90, 19–30. [Google Scholar] [CrossRef]
- Blazeski, A.; Zhu, R.; Hunter, D.W.; Weinberg, S.H.; Boheler, K.R.; Zambidis, E.T.; Tung, L. Electrophysiological and contractile function of cardiomyocytes derived from human embryonic stem cells. Prog. Biophys. Mol. Biol. 2012, 110, 178–195. [Google Scholar] [CrossRef]
- Dhamoon, A.S.; Jalife, J. The inward rectifier current (IK1) controls cardiac excitability and is involved in arrhythmogenesis. Heart Rhythm. 2005, 2, 316–324. [Google Scholar] [CrossRef]
- Deo, M.; Akwaboah, A.; Tsevi, B.; Treat, J.A.; Cordeiro, J.M. Role of the rapid delayed rectifier K current in human induced pluripotent stem cells derived cardiomyocytes. Arch. Stem Cell Ther. 2020, 1, 14–18. [Google Scholar] [CrossRef]
- Karbassi, E.; Fenix, A.; Marchiano, S.; Muraoka, N.; Nakamura, K.; Yang, X.; Murry, C.E. Cardiomyocyte maturation: Advances in knowledge and implications for regenerative medicine. Nat. Rev. Cardiol. 2020, 17, 341–359. [Google Scholar] [CrossRef]
- Ahmed, R.E.; Anzai, T.; Chanthra, N.; Uosaki, H. A brief review of current maturation methods for human induced Pluripotent Stem Cells-Derived cardiomyocytes. Front. Cell Dev. Biol. 2020, 8, 178. [Google Scholar] [CrossRef]
- Ernst, P.; Bidwell, P.A.; Dora, M.; Thomas, D.D.; Kamdar, F. Cardiac calcium regulation in human induced pluripotent stem cell cardiomyocytes: Implications for disease modeling and maturation. Front. Cell Dev. Biol. 2023, 10, 986107. [Google Scholar] [CrossRef] [PubMed]
- Itzhaki, I.; Rapoport, S.; Huber, I.; Mizrahi, I.; Zwi-Dantsis, L.; Arbel, G.; Schiller, J.; Gepstein, L. Calcium Handling in Human Induced Pluripotent Stem Cell Derived Cardiomyocytes. PLoS ONE 2011, 6, e18037. [Google Scholar] [CrossRef]
- Minor, A.J.; Coulombe, K.L.K. Stimulating calcium handling in HIPSC-Derived engineered cardiac tissues enhances force production. Stem Cells Transl. Med. 2022, 11, 97–106. [Google Scholar] [CrossRef]
- Seibertz, F.; Sutanto, H.; Dülk, R.; Pronto, J.R.D.; Springer, R.; Rapedius, M.; Liutkute, A.; Ritter, M.; Jung, P.; Stelzer, L.; et al. Electrophysiological and calcium-handling development during long-term culture of human-induced pluripotent stem cell-derived cardiomyocytes. Basic Res. Cardiol. 2023, 118, 14. [Google Scholar] [CrossRef]
- Hwang, H.S.; Kryshtal, D.O.; Feaster, T.K.; Sánchez-Freire, V.; Zhang, J.; Kamp, T.J.; Hong, C.C.; Wu, J.C.; Knollmann, B.C. Comparable calcium handling of human iPSC-derived cardiomyocytes generated by multiple laboratories. J. Mol. Cell. Cardiol. 2015, 85, 79–88. [Google Scholar] [CrossRef]
- Al-Attar, R.; Jargstorf, J.; Romagnuolo, R.; Jouni, M.; Alibhai, F.J.; Lampe, P.D.; Solan, J.L.; Laflamme, M.A. Casein kinase 1 Phosphomimetic mutations negatively impact connexin-43 gap junctions in human pluripotent stem Cell-Derived cardiomyocytes. Biomolecules 2024, 14, 61. [Google Scholar] [CrossRef]
- Kiss, E.; Fischer, C.; Sauter, J.M.; Sun, J.; Ullrich, N.D. The structural and the functional aspects of intercellular communication in IPSC-Cardiomyocytes. Int. J. Mol. Sci. 2022, 23, 4460. [Google Scholar] [CrossRef] [PubMed]
- Wahl, C.M.; Schmidt, C.; Hecker, M.; Ullrich, N.D. Distress-Mediated remodeling of cardiac connexin-43 in a novel cell model for arrhythmogenic heart diseases. Int. J. Mol. Sci. 2022, 23, 10174. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Huang, C.; Li, H.; Wu, F.; Luo, J.; Lu, W.; Lan, F. A net-shaped multicellular formation facilitates the maturation of hPSC-derived cardiomyocytes through mechanical and electrophysiological stimuli. Aging 2018, 10, 532–548. [Google Scholar] [CrossRef]
- Chen, G.; Li, S.; Karakikes, I.; Ren, L.; Chow, M.Z.-Y.; Chopra, A.; Keung, W.; Yan, B.; Chan, C.W.; Costa, K.D.; et al. Phospholamban as a crucial determinant of the inotropic response of human pluripotent stem Cell–Derived ventricular cardiomyocytes and engineered 3-Dimensional tissue constructs. Circ. Arrhythmia Electrophysiol. 2014, 8, 193–202. [Google Scholar] [CrossRef]
- Okai, Y.; Kaushik, E.P.; Sameshima, T.; Feric, N.; Singh, R.; Pallotta, I.; Bogdanowicz, D.R.; Gustilo, M.M.; Harada, K.; Baker, K.S.; et al. Establishing a context of use for three-dimensional cardiac tissue derived from human induced pluripotent stem cell-derived cardiomyocytes using inotropes. Toxicol. Sci. 2025, 205, 401–416. [Google Scholar] [CrossRef] [PubMed]
- Huebsch, N.; Charrez, B.; Neiman, G.; Siemons, B.; Boggess, S.C.; Wall, S.; Charwat, V.; Jæger, K.H.; Cleres, D.; Telle, Å.; et al. Metabolically driven maturation of human-induced-pluripotent-stem-cell-derived cardiac microtissues on microfluidic chips. Nat. Biomed. Eng. 2022, 6, 372–388. [Google Scholar] [CrossRef]
- Fu, Z.; Dong, R.; Zheng, H.; Wang, Z.; Cao, B.; Bai, J.; Ma, M.; Song, Z.; Pan, F.; Xia, L.; et al. Progress of conductivity and conduction velocity measured in human and animal hearts. Rev. Cardiovasc. Med. 2024, 25, 364. [Google Scholar] [CrossRef]
- Wu, P.; Deng, G.; Sai, X.; Guo, H.; Huang, H.; Zhu, P. Maturation strategies and limitations of induced pluripotent stem cell-derived cardiomyocytes. Biosci. Rep. 2020, 41, BSR20200833. [Google Scholar] [CrossRef] [PubMed]
- Yao, B.; Lei, Z.; Gonçalves Ma, F.V.; Sluijter, J.P.G. Integrating prime editing and cellular reprogramming as novel strategies for genetic cardiac disease modeling and treatment. Curr. Cardiol. Rep. 2024, 26, 1197–1208. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.B.; Parikh, V.N. Toward Precision Medicine in the Treatment of Arrhythmogenic Cardiomyopathy. Curr. Treat. Options Cardiovasc. Med. 2024, 26, 317–330. [Google Scholar] [CrossRef]
- Han, J.L.; Entcheva, E. Gene Modulation with CRISPR-based Tools in Human iPSC-Cardiomyocytes. Stem Cell Rev. Rep. 2023, 19, 886–905. [Google Scholar] [CrossRef]
- Dou, W.; Zhao, Q.; Malhi, M.; Liu, X.; Zhang, Z.; Wang, L.; Masse, S.; Nanthakumar, K.; Hamilton, R.; Maynes, J.T.; et al. Label-free conduction velocity mapping and gap junction assessment of functional iPSC-Cardiomyocyte monolayers. Biosens. Bioelectron. 2020, 167, 112468. [Google Scholar] [CrossRef]
- Liu, W.; Han, J.L.; Tomek, J.; Bub, G.; Entcheva, E. Simultaneous widefield voltage and Dye-Free optical mapping quantifies electromechanical waves in human induced pluripotent stem Cell-Derived cardiomyocytes. ACS Photonics 2023, 10, 1070–1083. [Google Scholar] [CrossRef]
- Baines, O.; Sha, R.; Kalla, M.; Holmes, A.P.; Efimov, I.R.; Pavlovic, D.; O’sHea, C. Optical mapping and optogenetics in cardiac electrophysiology research and therapy: A state-of-the-art review. Europace 2024, 26, euae017. [Google Scholar] [CrossRef]
- Alibhai, F.J.; Masoumi, A.; Kim, H.J.; Kim, N.; Kim, W.; Gomez-Garcia, J.; Lee, P.; Laflamme, M.A. Protocol for dual-optical mapping of voltage and calcium sensors in human pluripotent stem cell-derived cardiomyocytes. STAR Protoc. 2025, 6, 104044. [Google Scholar] [CrossRef]
- Guragain, B.; Zhang, H.; Wu, Y.; Wang, Y.; Wei, Y.; Wood, G.A.; Ye, L.; Walcott, G.P.; Zhang, J.; Rogers, J.M. Optogenetic stimulation and simultaneous optical mapping of membrane potential and calcium transients in human engineered cardiac spheroids. J. Mol. Cell. Cardiol. 2024, 199, 51–59. [Google Scholar] [CrossRef] [PubMed]
| Disease Model | Primary Genetic/Pathogenic Mechanism | Representative Phenotype (Electrophysiological & Metabolic) | Interventions & Remaining Limitations | References |
|---|---|---|---|---|
| Long QT Syndrome (LQTS) * | Mutations in KCNQ1, KCNH2, SCN5A causing impaired IKs, IKr, or enhancing late INa | Prolonged APD, early afterdepolarizations, exaggerated β-adrenergic response; elevated ROS, glycolytic bias | Mexiletine or CRISPR correction shorten APD; immaturity of IK1 and Ca2+ cycling persists | [1,34,35,36,37,38] |
| Catecholaminergic Polymorphic Ventricular Tachycardia (CPVT) | RYR2, CASQ2 mutations destabilizing SR Ca2+ release | Triggered activity and delayed afterdepolarizations; fragmented mitochondria, low ATP/ADP ratio | Flecainide restores Ca2+ stability; incomplete excitation–energy coupling remains | [2,29] |
| Brugada Syndrome | SCN5A loss-of-function reducing INa | Slowed conduction and conduction block; mitochondrial depolarization under stress | Sodium current enhancers normalize upstroke; gap junction immaturity promotes reentry | [13,39,40] |
| Arrhythmogenic Right Ventricular Cardiomyopathy (ARVC) | PKP2, DSP, DSG2 mutations impairing desmosomes | Reduced adhesion, slowed conduction, Ca2+ wave heterogeneity; lipid accumulation | PPAR/Wnt modulation lowers lipogenesis; structural syncytium loss in 2D persists | [3,41,42] |
| Hypertrophic Cardiomyopathy (HCM) | MYH7, MYBPC3 mutations altering sarcomeric contractility | Prolonged APD, Ca2+ alternans, oxidative stress; hyperfused mitochondria | Antioxidants reduce EADs; lack of chronic mechanical conditioning remains | [17,43,44,45] |
| Dilated Cardiomyopathy (DCM) | TTN, LMNA, RBM20 defects weakening sarcomeres | Slowed conduction, prolonged Ca2+ decay; reduced mitochondrial mass, ATP deficit | Gene correction and T3 maturation improve APs; contractile recovery incomplete | [17,46,47] |
| Drug-Induced QT Models | Pharmacological IKr or INa blockade | Dose-dependent APD prolongation, Ca2+ instability, ROS accumulation | Ranolazine reverses QT prolongation; maturity variability limits predictivity | [48,49,50] |
| Mitochondrial Cardiomyopathies | POLG, mt-tRNA mutations impairing respiration | Depolarized mitochondria, reduced Ca2+ uptake, EADs/DADs | Mitochondria-enriched EVs restore stability; mtDNA heteroplasmy not modeled | [15,16,51,52,53] |
| Metabolic Arrhythmia (Diabetic/Stress) | Hyperglycemia, lipotoxicity, oxidative injury | APD variability, Ca2+ leak, ROS-driven triggered activity | Antioxidants and EV rescue reduce arrhythmia; chronic stress effects untested | [16,54,55,56,57] |
| Ischemia/Reperfusion Injury | Hypoxia–reoxygenation injury | Afterdepolarizations, Ca2+ alternans, ATP depletion | EV-based mitochondrial transfer restores stability; lack of microvascular coupling persists | [2,3,5] |
| Study/Model | Cell Source& Subtype Composition | 3D Platform/Scaffold | Electrophysiology Highlights | Arrhythmogenic Observations | Mitigation Strategies |
|---|---|---|---|---|---|
| Fassina et al. (2022) [81] | iPSC-derived ventricular, atrial, nodal-like cells (mixed) | Engineered heart tissue (EHT) | Spontaneous automaticity; prolonged APD; low IK1 | Early afterdepolarizations (EADs); beat-to-beat variability | Electrical pacing, T3 hormone supplementation |
| Lemme et al. (2019) [82] | Ventricular-biased iPSC-CMs | Biomimetic mechanical laid + 3D EHT | Increased conduction velocity; improved Ca2+ handling | Reduced DADs, but minor reentry circuits persisted | Chronic electrical pacing, mechanical stretch, metabolic shift |
| Xu et al. (2022) [83] & Seguret et al. (2024) [84] | Mixed ventricular-atrial iPSC-CMs | 3D ring-shaped microtissues | Action potential heterogeneity; slow conduction | Reentry-like propagation in ring model | Micro-patterned substrate alignment |
| Andrée et al. (2024) [85] and Vanderslice et al. (2024) [86] | Patient-specific iPSC-CMs (Long QT) | Fibrin-based 3D tissues | Prolonged APD; arrhythmic Ca2+ transients under adrenergic stimulation | Catecholaminergic polymorphic ventricular tachycardia (CPVT)-like events | β-adrenergic blockers, CRISPR correction of KCNH2 |
| Li et al. (2018) [87] and Goldfracht et al. (2020) [88] | Ventricular iPSC-CM | Hydrogel-embedded 3D EHT | Improved conduction velocity with cell alignment | Reduced spontaneous arrhythmias | Electrical stimulation + fatty acid metabolic maturation |
| Ikeda et al. (2021) [89] | Heterogenous MSC/iPSC-CMs (Atrial, ventricular, nodal) | 3D scaffold + mitochondria-enriched EV supplementation | Normalized APD, improved Ca2+ transient synchronization | DADs reduced; conduction dispersion minimized | EV-mediated metabolic enhancement, electrical pacing, subtype alignment |
| Gartner (2022) [90] and Tadano (2021) [91] | Ventricular iPSC-CMs | Engineered cardiac tissues with fibroblast/endothelial co-culture | Enhanced APD uniformity; improved conduction | Minor ectopic activity; lower incidence of reentry | Co-culture with fibroblasts and endothelial cells; extracellular matrix optimization |
| Campostrini et al. (2023) [92], Liang et al. (2016) [93], and Lemoine et al. (2017) [94] | iPSC-CMs with SCN5A mutation | 3D EHT | Slow Na+ current; conduction velocity deficit | Ectopic pacemaking; triggered activity | CRISPR correction; pharmacological sodium channel modulators |
| Pourchet et al. (2025) [95], Esser et al. (2023) [96], and Bliley (2022) [97] | Ventricular iPSC-CMs | 3D bioprinted tissues with microvascular perfusion | Reduced APD variability; stable Ca2+ transients | Minimal spontaneous arrhythmias | Perfusion-enhanced metabolic maturation; mechanical and electrical cues |
| Parameter | iPSC-CMs | Adult Ventricular CMs | Functional Consequences/Clinical Implication | References |
|---|---|---|---|---|
| Resting Membrane Potential (RMP) | Depolarized (−50 to −65 mV) due to low IK1 density (↓ KCNJ2 expression) | Stable (−80 to −90 mV) via robust IK1 conductance | Depolarized RMP increases automaticity and ectopic firing | [49,123,124,125,126,127] |
| Action Potential Duration (APD) | Prolonged and variable (200–500 ms); dependent on immature IKs and IKr | Stable, shorter APD (150–250 ms) | Promotes early afterdepolarizations (EADs) and QT prolongation | [128,129,130,131] |
| Automaticity/Pacemaker Activity | Spontaneous beating via persistent funny current (↑ HCN4) | Quiescent without sinoatrial input | Uncontrolled pacemaking contributes to ectopic rhythm generation post-transplant | [2,21,22,132,133] |
| Sodium Current (INa) | Reduced peak INa density; slow upstroke velocity (Vmax ↓) | High amplitude INa ensures rapid depolarization | Slower conduction velocity, higher conduction block risk | [2,3,5,94,134,135,136] |
| Inward Rectifier K+ Current (IK1) | Severely diminished or absent | Prominent, stabilizes RMP | Destabilized RMP → spontaneous depolarization & triggered activity | [16,49,125,137] |
| Repolarizing K+ Currents (IKr, IKs) | Low expression and incomplete maturation | Well-developed, ensuring phase 3 repolarization | Prolonged APD and increased dispersion of refractoriness | [5,130,138,139,140] |
| Calcium Handling | Immature SR; ↓ RyR2 and SERCA2a expression, asynchronous Ca2+ transients | Mature SR; synchronized Ca2+-induced Ca2+ release | Delayed afterdepolarizations (DADs), alternans, and instability | [1,3,5,141,142,143,144,145] |
| Conexxin 43 (Cx43) Expression | Reduced, disorganized gap junctions | Dense, polarized intercalated disks | Impaired coupling and anisotropic conduction → reentry potential | [1,3,5,146,147,148,149] |
| Metabolic Profile | Glycolytic dominance; low oxidative phosphorylation, fragmented mitochondria | Fatty acid oxidation; dense cristae and efficient ATP delivery | Energetic mismatch promotes Ca2+ instability and arrhythmogenic stress | [2,5,16] |
| Response to β-Adrenergic Stimulation | Exaggerated or erratic chronotropic response; limited inotropy | Physiological HR increase, synchronized contraction | Enhanced adrenergic sensitivity → catecholaminergic arrhythmias | [49,128,150,151,152,153] |
| Electrical Conduction Velocity | Slower (10–20 cm/s) | Rapid (40–60 cm/s) | Facilitates reentrant circuit formation | [1,2,3,5,128,140,153,154] |
| Maturation Response to Mechanical/Electrical Cues | Improves with pacing, 3D culture, and metabolic conditioning | Fully Mature, Stable | External conditioning partially restores adult-like AP but not full fidelity | [1,2,3,5,15,16,17] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahannaz, D.C.; Sugiura, T.; Ferrell, B.E.; Yoshida, T. Arrhythmogenic Risk in iPSC-Derived Cardiomyocytes: Current Limitations and Therapeutic Perspectives. Medicina 2025, 61, 2056. https://doi.org/10.3390/medicina61112056
Shahannaz DC, Sugiura T, Ferrell BE, Yoshida T. Arrhythmogenic Risk in iPSC-Derived Cardiomyocytes: Current Limitations and Therapeutic Perspectives. Medicina. 2025; 61(11):2056. https://doi.org/10.3390/medicina61112056
Chicago/Turabian StyleShahannaz, Dhienda C., Tadahisa Sugiura, Brandon E. Ferrell, and Taizo Yoshida. 2025. "Arrhythmogenic Risk in iPSC-Derived Cardiomyocytes: Current Limitations and Therapeutic Perspectives" Medicina 61, no. 11: 2056. https://doi.org/10.3390/medicina61112056
APA StyleShahannaz, D. C., Sugiura, T., Ferrell, B. E., & Yoshida, T. (2025). Arrhythmogenic Risk in iPSC-Derived Cardiomyocytes: Current Limitations and Therapeutic Perspectives. Medicina, 61(11), 2056. https://doi.org/10.3390/medicina61112056







