Association Between Thyroid-Stimulating Hormone and Estimated Glomerular Filtration Rate in the Third Trimester of Pregnancy: A Retrospective Cross-Sectional Study in Euthyroid Women
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
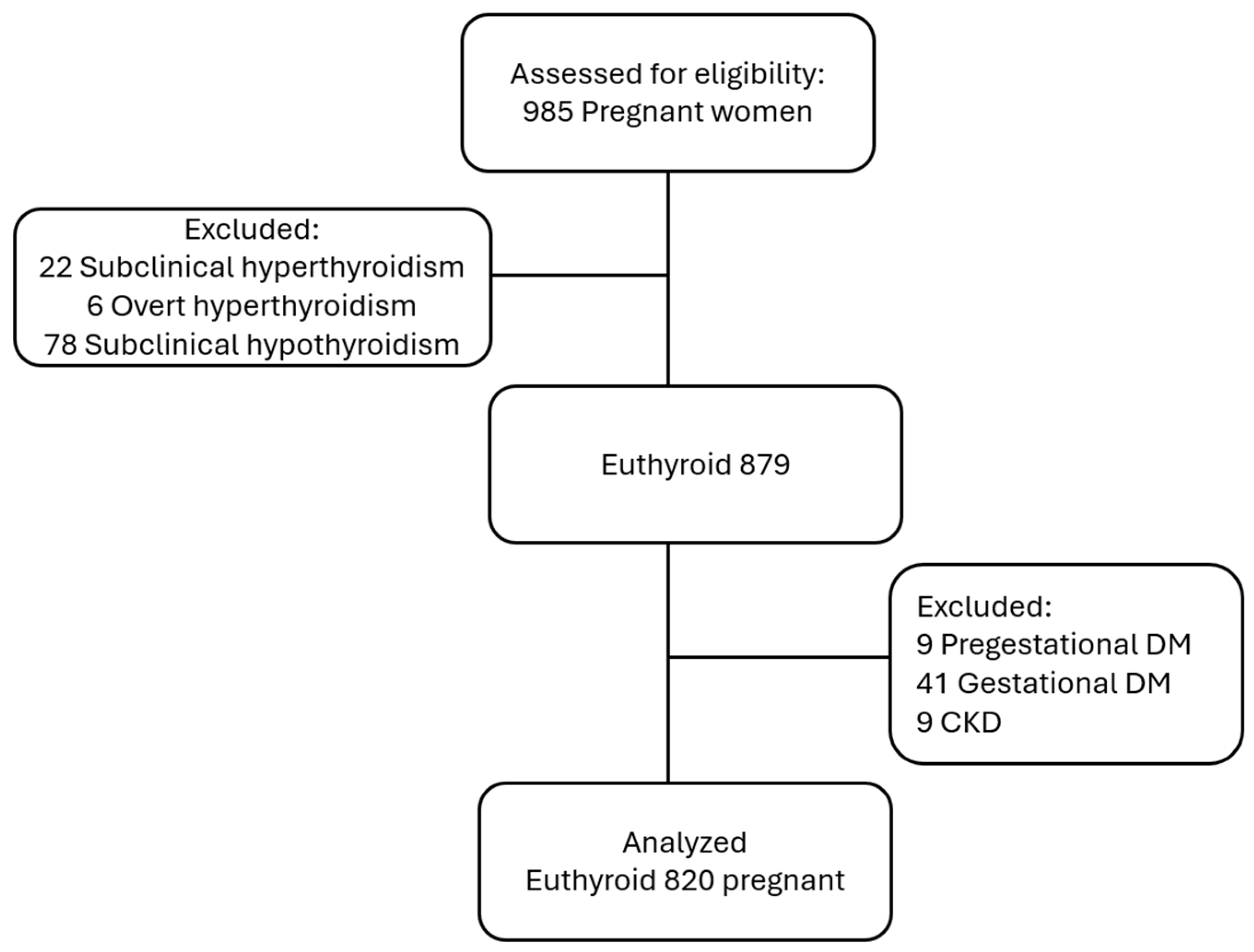
2.2. Participants and Data Collection
- Maternal age between 18 and 45 years;
- Gestational age ≥ 28 weeks (third trimester);
- Euthyroid status, defined as TSH, fT3, and fT4 levels within the reference ranges.
- Maternal age < 18 or >45 years;
- Gestational age < 28 weeks;
- Any form of thyroid dysfunction (subclinical or overt hypothyroidism or hyperthyroidism);
- Hypertensive disorders;
- Gestational or pregestational diabetes mellitus;
- Chronic kidney disease;
- Muscular or urinary tract disorders.
2.3. Sample Size
2.4. Laboratory Measurements
2.5. Statistical Analysis
3. Results
4. Discussion
Clinical Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, S.Y.; Pearce, E.N. Assessment and treatment of thyroid disorders in pregnancy and the postpartum period. Nat. Rev. Endocrinol. 2022, 18, 158–171. [Google Scholar] [CrossRef]
- Leung, A.M. Thyroid function in pregnancy. J. Trace Elem. Med. Biol. 2012, 26, 137–140. [Google Scholar] [CrossRef]
- Glinoer, D.; de Nayer, P.; Bourdoux, P.; Lemone, M.; Robyn, C.; van Steirteghem, A.; Kinthaert, J.; Lejeune, B. Regulation of maternal thyroid during pregnancy. J. Clin. Endocrinol. Metab. 1990, 71, 276–287. [Google Scholar] [CrossRef]
- Lee, R.H.; Spencer, C.A.; Mestman, J.H.; Miller, E.A.; Petrovic, I.; Braverman, L.E.; Goodwin, T.M. Free T4 immunoassays are flawed during pregnancy. Am. J. Obstet. Gynecol. 2009, 200, 260.e1–260.e6. [Google Scholar] [CrossRef]
- Alexander, E.K.; Pearce, E.N.; Brent, G.A.; Brown, R.S.; Chen, H.; Dosiou, C.; Grobman, W.A.; Laurberg, P.; Lazarus, J.H.; Mandel, S.J.; et al. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid 2017, 27, 315–389. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.L.; Lafayette, R.A. Renal physiology of pregnancy. Adv. Chronic Kidney Dis. 2013, 20, 209–214. [Google Scholar] [CrossRef]
- Wu, L.; Wu, Q.; Li, Q.; Cao, S.; Zhang, Y.; Liu, Y.; Qin, X. Consecutive reference intervals for biochemical indices related to serum lipid levels and renal function during normal pregnancy. BMC Pregnancy Childbirth 2022, 22, 642. [Google Scholar] [CrossRef] [PubMed]
- Korevaar, T.I.M.; Medici, M.; Visser, T.J.; Peeters, R.P. Thyroid function and pregnancy: What is normal? Lancet Diabetes Endocrinol. 2017, 5, 561–563. [Google Scholar]
- Smith, C.; Patel, N.; Shah, M.; Chen, Q. Thyroid hormone physiology and renal adaptation in pregnancy. Front. Endocrinol. 2023, 14, 1156721. [Google Scholar]
- Lippi, G.; Montagnana, M.; Targher, G.; Salvagno, G.L.; Guidi, G.C. Relationship between thyroid status and renal function in a general population of unselected outpatients. Clin. Biochem. 2008, 41, 625–627. [Google Scholar] [CrossRef]
- Anderson, J.L.C.; Gruppen, E.G.; van Tienhoven Wind, L.; Eisenga, M.F.; de Vries, H.; Gansevoort, R.T.; Bakker, S.J.L.; Dullaart, R.P.F. Glomerular filtration rate is associated with free triiodothyronine in euthyroid subjects: Comparison between various equations to estimate renal function and creatinine clearance. Eur. J. Intern. Med. 2018, 48, 94–99. [Google Scholar] [CrossRef]
- Tsuda, A.; Inaba, M.; Ichii, M.; Ochi, A.; Ohno, Y.; Nakatani, S.; Yamada, S.; Mori, K.; Tahara, H.; Ishimura, E. Relationship between serum TSH levels and intrarenal hemodynamic parameters in euthyroid subjects. Eur. J. Endocrinol. 2013, 169, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Maynard, S.E.; Thadhani, R. Pregnancy and the kidney. J. Am. Soc. Nephrol. JASN 2009, 20, 14–22. [Google Scholar] [CrossRef]
- Asvold, B.O.; Bjøro, T.; Vatten, L.J. Association of thyroid function with estimated glomerular filtration rate in a population based study: The HUNT study. Eur. J. Endocrinol. 2011, 164, 101–105. [Google Scholar] [CrossRef]
- Sun, M.T.; Hsiao, F.C.; Su, S.C.; Pei, D.; Hung, Y.J. Thyrotropin as an independent factor of renal function and chronic kidney disease in normoglycemic euthyroid adults. Endocr. Res. 2012, 37, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Keskin, H.; Cadirci, K.; Gungor, K.; Karaaslan, T.; Usta, T.; Ozkeskin, A.; Musayeva, A.; Yesildal, F.; Isman, F.; Zengin, H.Y. Association between TSH values and GFR levels in euthyroid cases with metabolic syndrome. Int. J. Endocrinol. 2021, 2021, 8891972. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, H.; Tan, M.; Gao, G.; Zhang, Y.; Ding, B.; Su, X.; Kong, X.; Ma, J. Association between thyroid function and diabetic nephropathy in euthyroid subjects with type 2 diabetes mellitus: A cross sectional study in China. Oncotarget 2019, 10, 88–97. [Google Scholar] [CrossRef]
- Peixoto de Miranda, É.J.F.; Bittencourt, M.S.; Goulart, A.C.; Santos, I.S.; de Oliveira Titan, S.M.; Ladeira, R.M.; Barreto, S.M.; Lotufo, P.A.; Benseñor, I.J.M. Thyrotropin levels are associated with chronic kidney disease among healthy subjects in cross sectional analysis of the Brazilian Longitudinal Study of Adult Health (ELSA Brasil). Clin. Exp. Nephrol. 2017, 21, 1035–1043. [Google Scholar] [CrossRef]
- Patil, V.P.; Shilpasree, A.S.; Patil, V.S.; Pravinchandra, K.R.; Ingleshwar, D.G.; Vani, A.C. Evaluation of renal function in subclinical hypothyroidism. J. Lab. Physicians 2018, 10, 50–55. [Google Scholar] [CrossRef]
- Liu, Z.X.; Lv, J.L.; Xiang, Y.L.; Deng, W.; Huang, H.; Sun, Y.H.; Li, L.H. The association between thyroid hormones and renal function in euthyroid Chinese individuals: A population based cross sectional study. Cureus 2024, 16, e55682. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chang, Y.; Ryu, S.; Cho, J.; Lee, W.-Y.; Rhee, E.-J.; Kwon, M.-J.; Pastor-Barriuso, R.; Rampal, S.; Han, W.K.; et al. Thyroid hormone levels and incident chronic kidney disease in euthyroid individuals: The Kangbuk Samsung Health Study. Int. J. Epidemiol. 2014, 43, 1624–1632. [Google Scholar] [CrossRef] [PubMed]

| Variable | Mean ± SD |
|---|---|
| Age (years) | 28.66 ± 6.02 |
| TSH (μIU/mL) | 2.18 ± 0.86 |
| FT3 (ng/L) | 2.72 ± 0.47 |
| FT4 (ng/dL) | 0.98 ± 0.23 |
| eGFR (mL/min/1.73 m2) | 132.36 ± 10.96 |
| Creatinine (mg/dL) | 0.50 ± 0.09 |
| Gravida (n) | 2.53 ± 1.57 |
| Gestational age (weeks) | 38.69 ± 2.21 |
| Birth Weight (g) | 3199.67 ± 588.75 |
| Birth Height (cm) | 49.76 ± 22.85 |
| Neonatal Head Circumference (cm) | 34.86 ± 2.5 |
| Cesarean Section Rate | 487 (49.4%) |
| Apgar 1 | 7.33 ± 1.76 |
| Apgar 5 | 9.04 ± 1.70 |
| eGFR (mL/min/1.73 m2) | p | Creatinine (mg/dL) | p | ||
|---|---|---|---|---|---|
| TSH | 1. Tertile median (IQR) | 133 (13) | 0.187 | 0.47(0.13) | 0.011 |
| 2. Tertile median (IQR) | 132 (14) | 0.50 (0.06) | |||
| 3. Tertile median (IQR) | 131 (14) | 0.50 (0.13) | |||
| fT3 | 1. Tertile median (IQR) | 131 (14) | 0.034 | 0.51 (0.12) | 0.066 |
| 2. Tertile median (IQR) | 132 (14) | 0.49 (0.12) | |||
| 3. Tertile median (IQR) | 133 (14) | 0.48 (0.08) | |||
| fT4 | 1. Tertile median (IQR) | 131 (14) | 0.038 | 0.49 (0.12) | 0.633 |
| 2. Tertile median (IQR) | 132 (14) | 0.49 (0.11) | |||
| 3. Tertile median (IQR) | 134 (12) | 0.50 (0.11) |
| Variable | eGFR (mL/min/1.73 m2) | Creatinine (mg/dL) | ||
|---|---|---|---|---|
| r | p-Value | r | p-Value | |
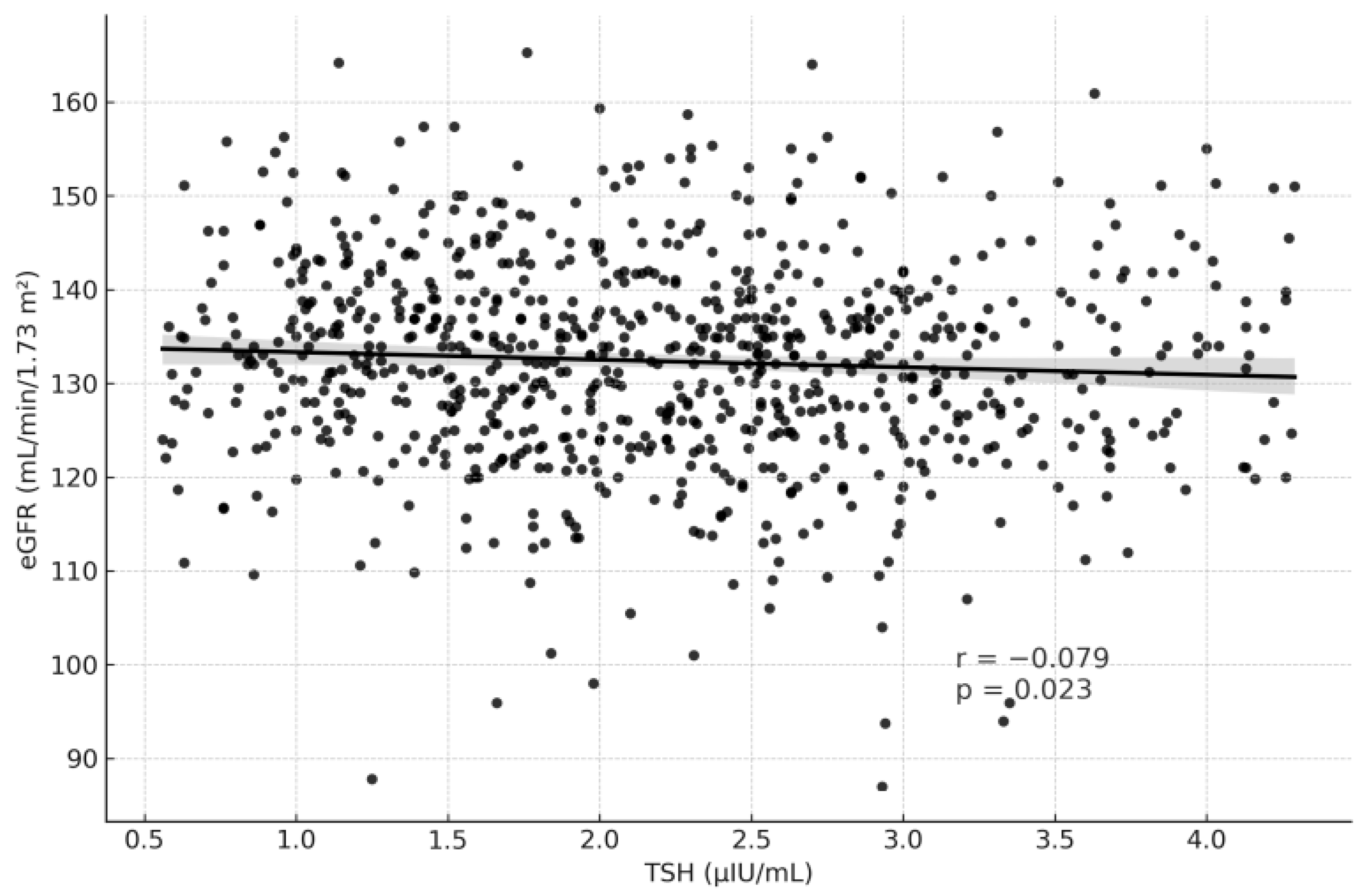
| TSH (μIU/mL) | −0.079 | 0.023 | 0.097 | 0.005 |
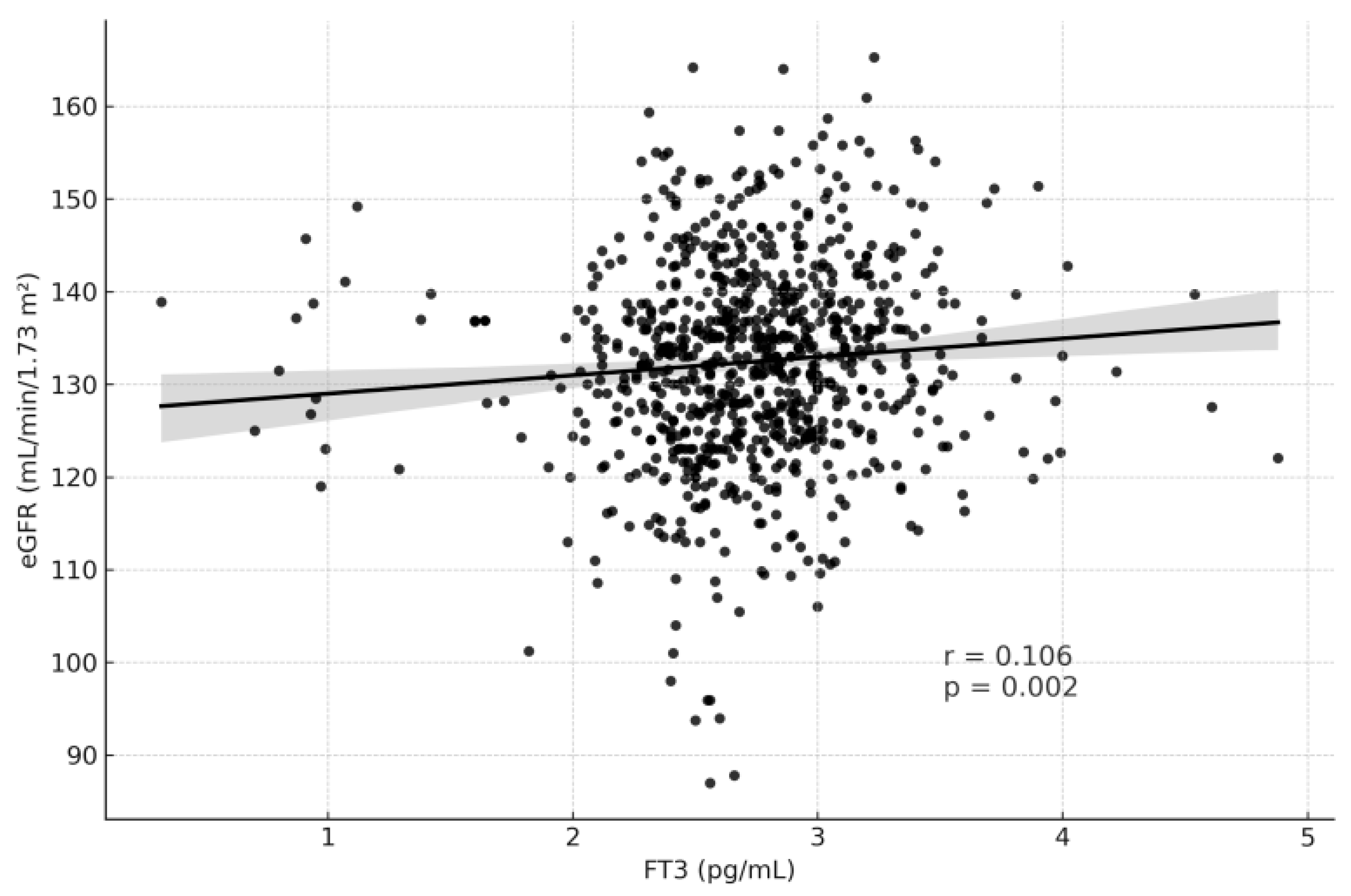
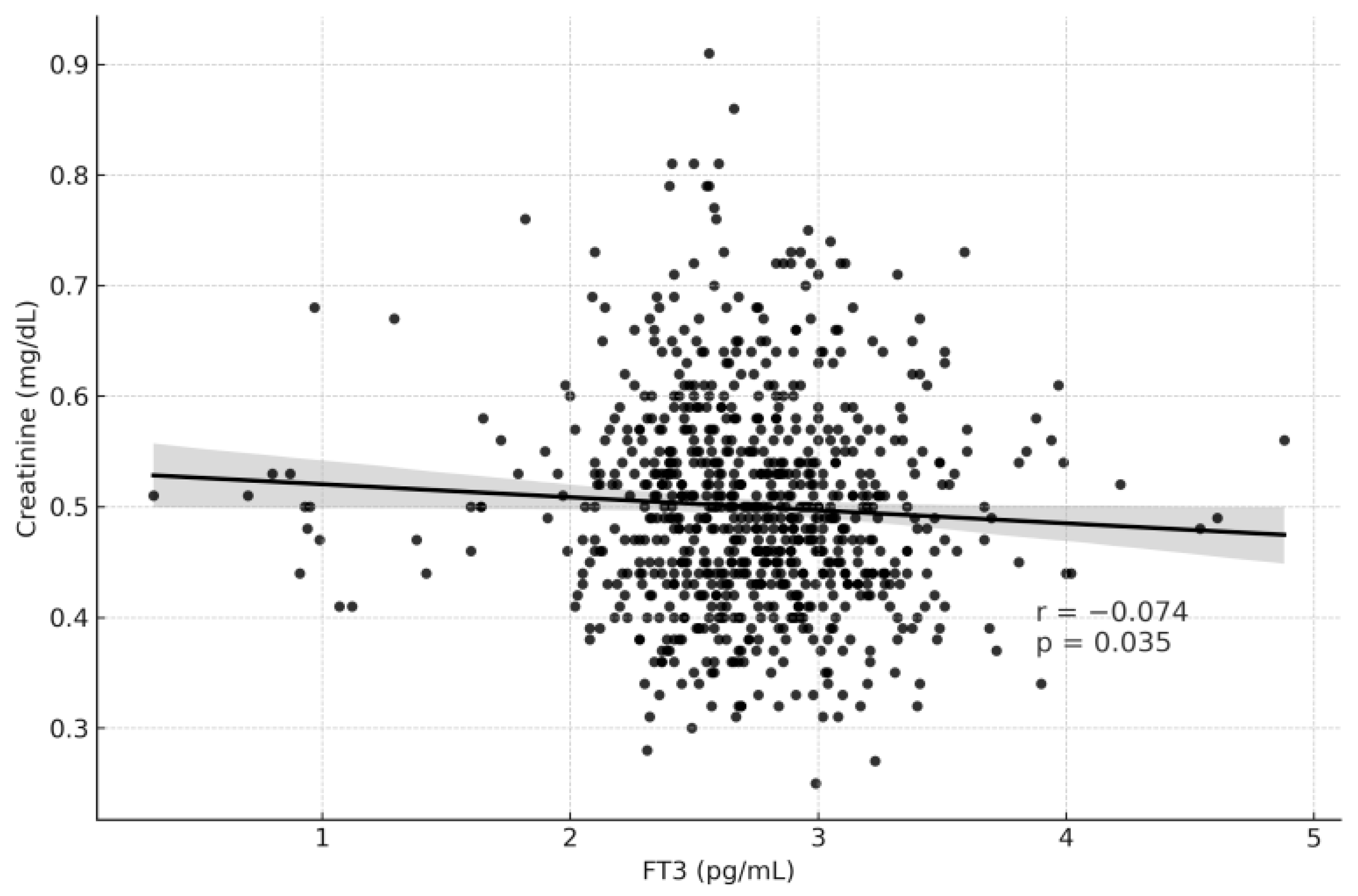
| fT3 (ng/L) | 0.106 | 0.002 | −0.074 | 0.035 |
| fT4 (ng/dL) | 0.067 | 0.058 | 0.045 | 0.196 |
| Predictor | B | SE | β | t | p-Value |
|---|---|---|---|---|---|
| Constant | 124.044 | 3.180 | — | 39.009 | <0.001 |
| TSH (0.5–5.1 mIU/L) | −0.811 | 0.441 | −0.064 | −1.837 | 0.067 |
| fT3 (2.04–4.4 ng/L) | 2.316 | 0.830 | 0.099 | 2.790 | 0.005 |
| fT4 (0.93–1.71 ng/dL) | 3.901 | 1.659 | 0.083 | 2.351 | 0.019 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Satır Özel, C.; Sertbaş, Y.; Taştekin, Ş.; Tancer Özçelik, A.; Sertbaş, M.; Kınlı Yıldız, Ö.; Turgut, A. Association Between Thyroid-Stimulating Hormone and Estimated Glomerular Filtration Rate in the Third Trimester of Pregnancy: A Retrospective Cross-Sectional Study in Euthyroid Women. Medicina 2025, 61, 2046. https://doi.org/10.3390/medicina61112046
Satır Özel C, Sertbaş Y, Taştekin Ş, Tancer Özçelik A, Sertbaş M, Kınlı Yıldız Ö, Turgut A. Association Between Thyroid-Stimulating Hormone and Estimated Glomerular Filtration Rate in the Third Trimester of Pregnancy: A Retrospective Cross-Sectional Study in Euthyroid Women. Medicina. 2025; 61(11):2046. https://doi.org/10.3390/medicina61112046
Chicago/Turabian StyleSatır Özel, Canan, Yaşar Sertbaş, Şeyma Taştekin, Asya Tancer Özçelik, Meltem Sertbaş, Özge Kınlı Yıldız, and Abdulkadir Turgut. 2025. "Association Between Thyroid-Stimulating Hormone and Estimated Glomerular Filtration Rate in the Third Trimester of Pregnancy: A Retrospective Cross-Sectional Study in Euthyroid Women" Medicina 61, no. 11: 2046. https://doi.org/10.3390/medicina61112046
APA StyleSatır Özel, C., Sertbaş, Y., Taştekin, Ş., Tancer Özçelik, A., Sertbaş, M., Kınlı Yıldız, Ö., & Turgut, A. (2025). Association Between Thyroid-Stimulating Hormone and Estimated Glomerular Filtration Rate in the Third Trimester of Pregnancy: A Retrospective Cross-Sectional Study in Euthyroid Women. Medicina, 61(11), 2046. https://doi.org/10.3390/medicina61112046






