Avocado–Soybean Unsaponifiables Enhance Tendon Healing via Anti-Inflammatory and Antioxidant Mechanisms in a Rat Achilles Injury Model
Abstract
1. Introduction
2. Materials and Methods
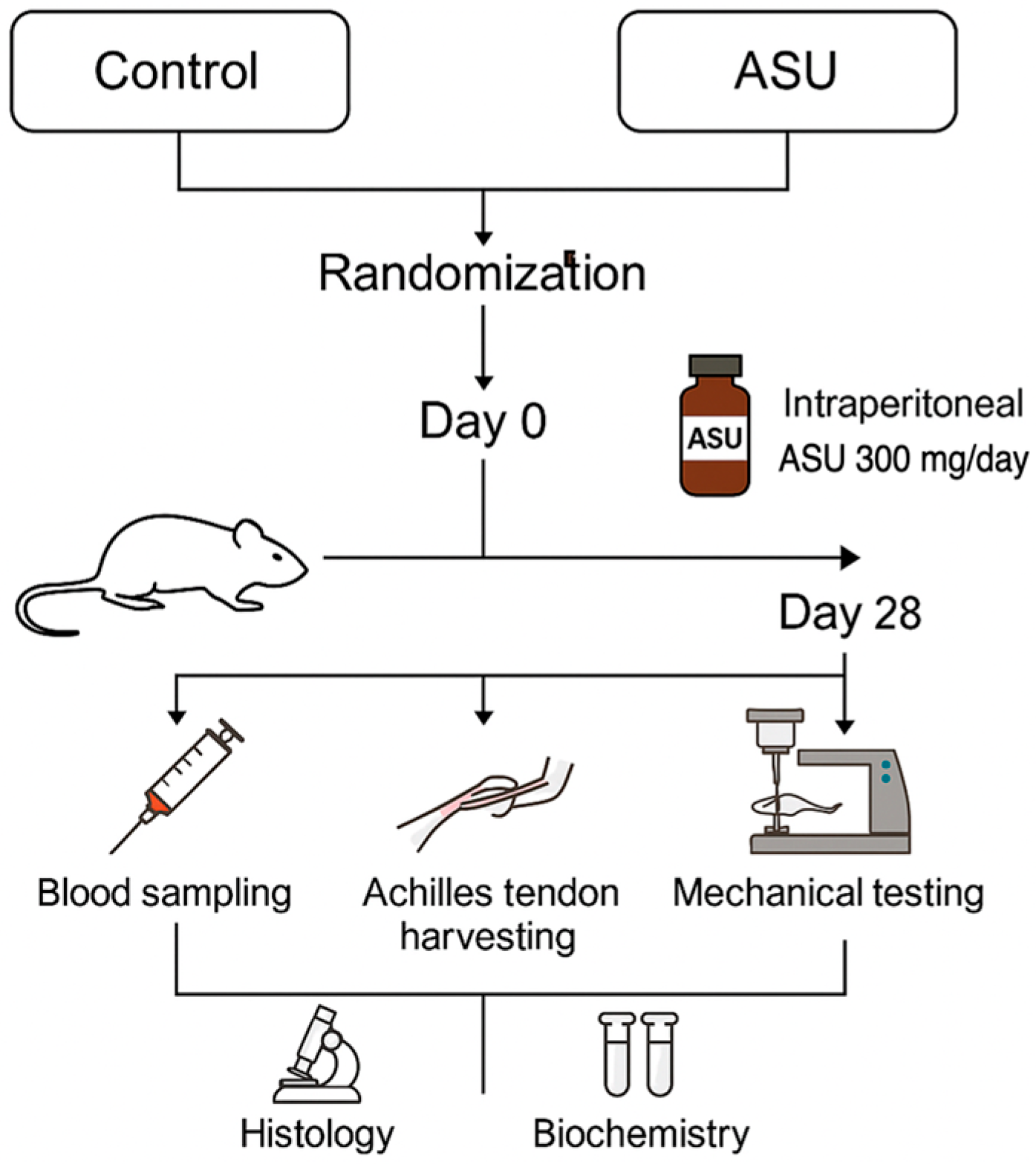
2.1. Study Design
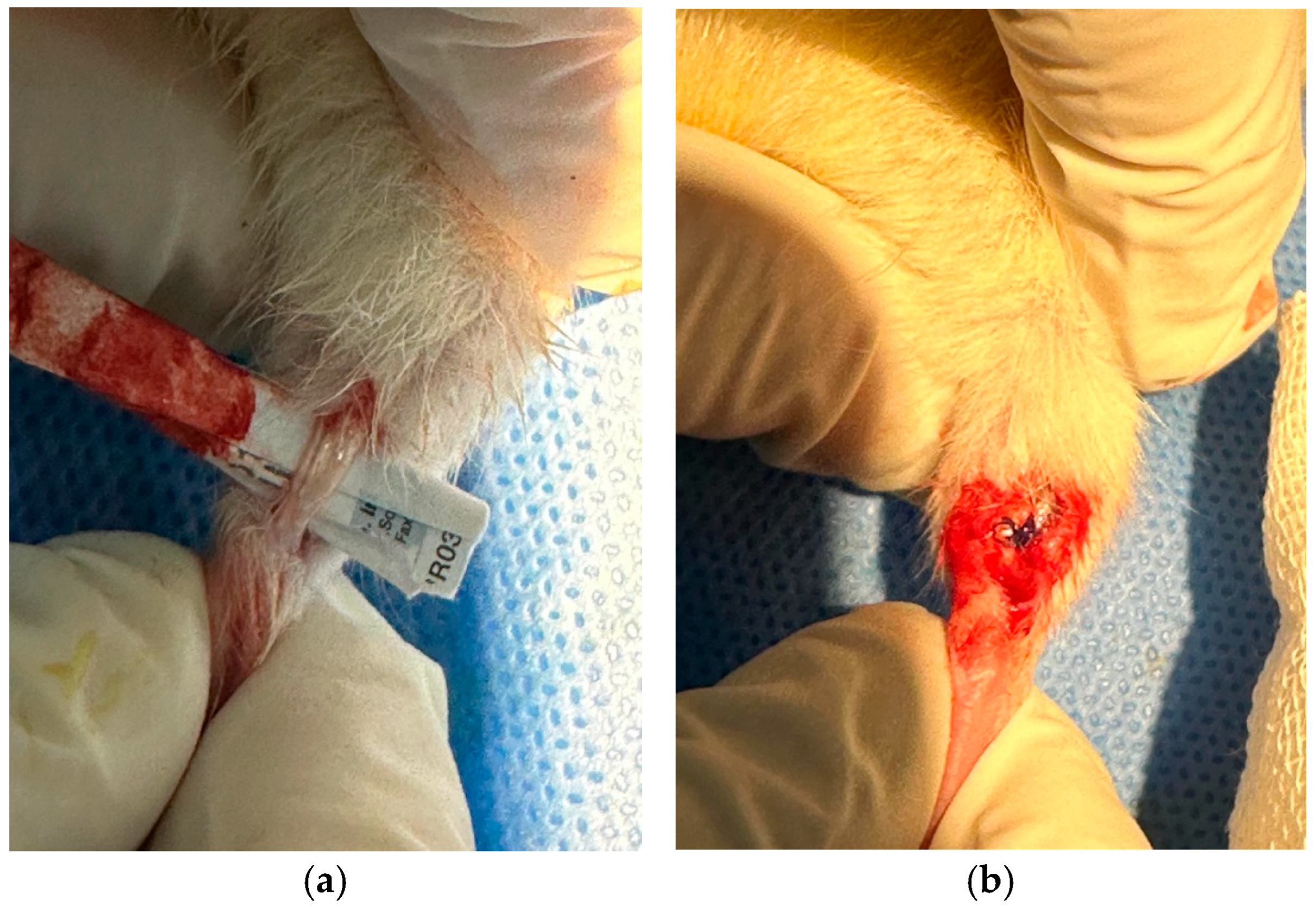
2.2. Surgical Procedure
2.3. Treatment Protocol
2.4. Sample Collection and Processing
2.4.1. Blood Sampling
2.4.2. Tendon Tissue Collection
2.5. Histological Analysis
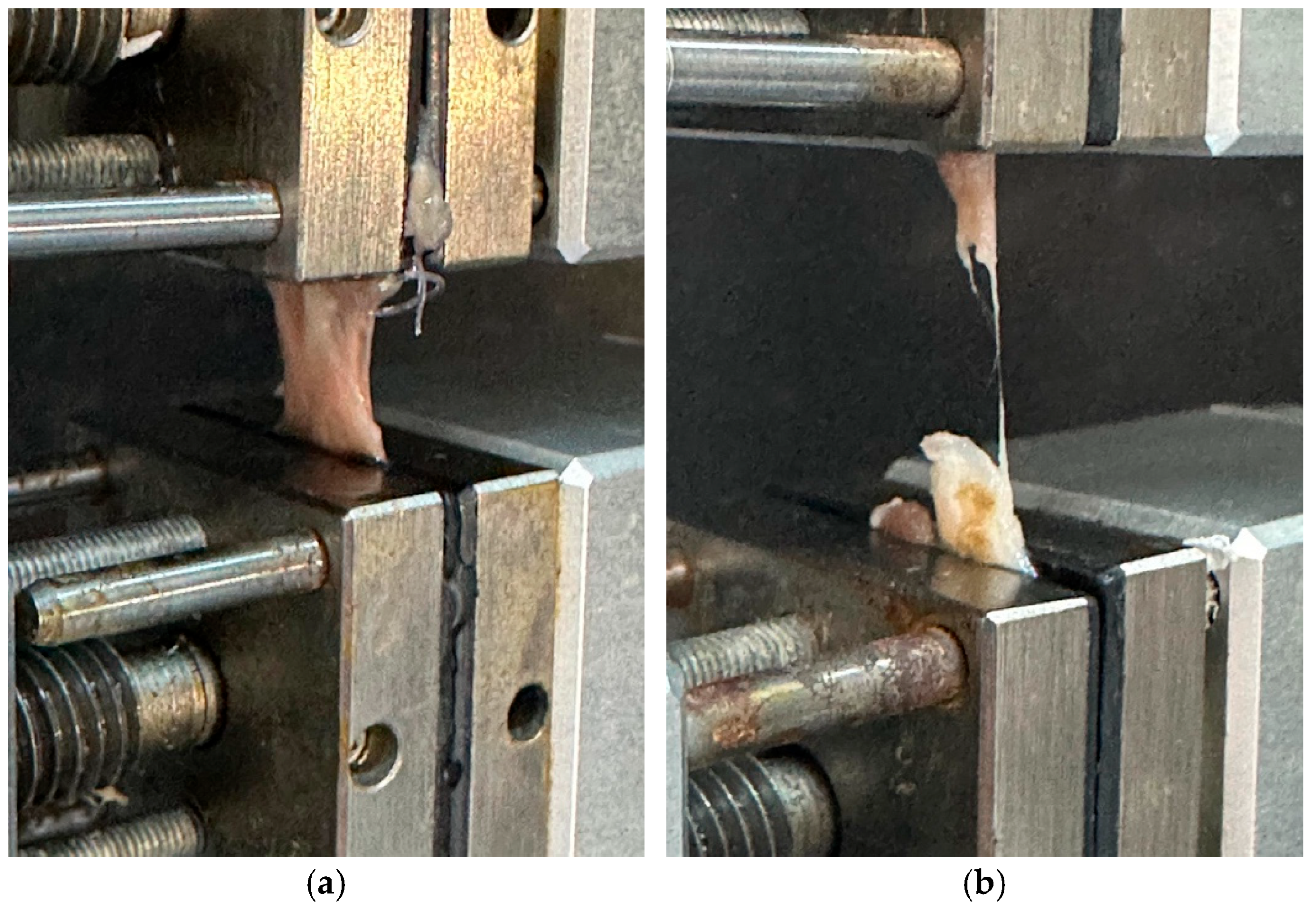
2.6. Biomechanical Testing
2.7. ELISA Analysis
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASU | Avocado–Soybean Unsaponifiables |
| COX-2 | Cyclooxygenase-2 |
| DNA | Deoxyribonucleic Acid |
| ECM | Extracellular Matrix |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| GAG | Glycosaminoglycan |
| GPx | Glutathione Peroxidase |
| H&E | Hematoxylin and Eosin |
| IL-1β | Interleukin-1 beta |
| IL-6 | Interleukin-6 |
| iNOS | Inducible Nitric Oxide Synthase |
| MMP-3 | Matrix Metalloproteinase-3 |
| OSI | Oxidative Stress Index |
| ROS | Reactive Oxygen Species |
| SD | Standard Deviation |
| SOD | Superoxide Dismutase |
| TAS | Total Antioxidant Status |
| TNF-α | Tumor Necrosis Factor-alpha |
| TOS | Total Oxidant Status |
References
- Jiang, D.; Gao, P.; Lin, H.; Geng, H. Curcumin improves tendon healing in rats: A histological, biochemical, and functional evaluation. Connect. Tissue Res. 2016, 57, 20–27. [Google Scholar] [CrossRef]
- Jiang, F.; Zhao, H.; Zhang, P.; Bi, Y.; Zhang, H.; Sun, S.; Yao, Y.; Zhu, X.; Yang, F.; Liu, Y. Challenges in tendon–bone healing: Emphasizing inflammatory modulation mechanisms and treatment. Front. Endocrinol. 2024, 15, 1485876. [Google Scholar] [CrossRef]
- Müller, S.A.; Todorov, A.; Heisterbach, P.E.; Martin, I.; Majewski, M. Tendon healing: An overview of physiology, biology, and pathology of tendon healing and systematic review of state of the art in tendon bioengineering. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 2097–2105. [Google Scholar] [CrossRef]
- Nichols, A.E.; Best, K.T.; Loiselle, A.E. The cellular basis of fibrotic tendon healing: Challenges and opportunities. Transl. Res. 2019, 209, 156–168. [Google Scholar] [CrossRef]
- Büyükdoğan, H.; Ertürk, C.; Eren, E.; Öztürk, Ç.; Yıldırım, B.; Sarıtaş, T.B.; Demirkol, M. The impact of N-acetylcysteine on early periods of tendon healing: Histopathologic, immunohistochemical, and biomechanical analysis in a rat model. Connect. Tissue Res. 2025, 66, 161–174. [Google Scholar] [CrossRef]
- Macchi, M.; Spezia, M.; Elli, S.; Schiaffini, G.; Chisari, E. Obesity increases the risk of tendinopathy, tendon tear and rupture, and postoperative complications: A systematic review of clinical studies. Clin. Orthop. Relat. Res. 2020, 478, 1839–1847. [Google Scholar] [CrossRef]
- Nichols, A.E.; Oh, I.; Loiselle, A.E. Effects of type II diabetes mellitus on tendon homeostasis and healing. J. Orthop. Res. 2020, 38, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Rejeski, W.J.; Marsh, A.P.; Fanning, J.; Ambrosius, W.T.; Walkup, M.P.; Nicklas, B.J. Dietary weight loss, exercise, and inflammation in older adults with overweight or obesity and cardiometabolic disease. Obesity 2019, 27, 1805–1811. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, J.; Ji, Y.; Liu, Y.; Jiang, J.; Wang, Y.; Cui, X.; Wan, Y.; Guo, B.; Yu, H. The impact of diabetes mellitus on tendon pathology: A review. Front. Pharmacol. 2024, 15, 1491633. [Google Scholar] [CrossRef]
- Cesanelli, L.; Minderis, P.; Balnyte, I.; Ratkevicius, A.; Degens, H.; Satkunskiene, D. Obesity-driven musculotendinous remodeling impairs tissue resilience to mechanical damage. Cell Tissue Res. 2025, 400, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Campisi, J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2014, 69, S4–S9. [Google Scholar] [CrossRef]
- Chisari, E.; Rehak, L.; Khan, W.S.; Maffulli, N. Tendon healing is adversely affected by low-grade inflammation. J. Orthop. Surg. Res. 2021, 16, 700. [Google Scholar] [CrossRef]
- Dakin, S.G.; Newton, J.; Martinez, F.O.; Hedley, R.; Gwilym, S.; Jones, N.; Reid, H.A.; Wood, S.; Wells, G.; Appleton, L. Chronic inflammation is a feature of Achilles tendinopathy and rupture. Br. J. Sports Med. 2018, 52, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.J.; Beaumont, R.E.; McClellan, A.; Sze, C.; Palomino Lago, E.; Hazelgrove, L.; Dudhia, J.; Smith, R.K.; Guest, D.J. Tumour necrosis factor alpha, interleukin 1 beta and interferon gamma have detrimental effects on equine tenocytes that cannot be rescued by IL-1RA or mesenchymal stromal cell–derived factors. Cell Tissue Res. 2023, 391, 523–544. [Google Scholar] [CrossRef]
- Al-Sadi, O.; Schulze-Tanzil, G.; Kohl, B.; Lohan, A.; Lemke, M.; Ertel, W.; John, T. Tenocytes, pro-inflammatory cytokines and leukocytes: A relationship? Muscles Ligaments Tendons J. 2012, 1, 68. [Google Scholar] [PubMed]
- Kračun, D.; Görlach, A.; Snedeker, J.G.; Buschmann, J. Reactive oxygen species in tendon injury and repair. Redox Biol. 2025, 81, 103568. [Google Scholar] [CrossRef]
- Shahid, H.; Morya, V.K.; Oh, J.-U.; Kim, J.-H.; Noh, K.-C. Hypoxia-inducible factor and oxidative stress in tendon degeneration: A molecular perspective. Antioxidants 2024, 13, 86. [Google Scholar] [CrossRef]
- Yurteri, A.; Mercan, N.; Çelik, Z.E.; Yaykaşlı, H.; Yıldırım, A. Does quercetin affect tendon healing? An experimental study in a rat model of Achilles tendon injury. Front. Med. 2025, 12, 1522517. [Google Scholar] [CrossRef]
- Peng, C.; Kang, S.; Jiang, M.; Yang, M.; Gong, X. Antioxidant carbon dots and ursolic acid Co-encapsulated liposomes composite hydrogel for alleviating adhesion formation and enhancing tendon healing in tendon injury. Int. J. Nanomed. 2024, 19, 8709–8727. [Google Scholar] [CrossRef]
- Uehara, H.; Itoigawa, Y.; Morikawa, D.; Koga, A.; Tsurukami, H.; Ishijima, M. Antioxidants accelerate tendon-to-bone healing after rotator cuff repair surgery. J. Shoulder Elb. Surg. 2024, 33, e283. [Google Scholar] [CrossRef]
- Christiansen, B.; Bhatti, S.; Goudarzi, R.; Emami, S. Management of osteoarthritis with avocado/soybean unsaponifiables. Cartilage 2015, 6, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Sabucedo-Suárez, A.; López-Peña, M.; Permuy, M.; Muñóz, F. Soybean and avocado unsaponifiables: A review of their potential use in the treatment of osteoarthritis. Front. Vet. Sci. 2025, 11, 1473688. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Rescigno, A.; Dettori, T.; Calina, D.; Docea, A.O.; Singh, L.; Cebeci, F.; Özçelik, B.; Bhia, M.; Dowlati Beirami, A. Avocado–soybean unsaponifiables: A panoply of potentialities to be exploited. Biomolecules 2020, 10, 130. [Google Scholar] [CrossRef] [PubMed]
- Au, R.; Al-Talib, T.; Au, A.; Phan, P.; Frondoza, C. Avocado soybean unsaponifiables (ASU) suppress TNF-α, IL-1β, COX-2, iNOS gene expression, and prostaglandin E2 and nitric oxide production in articular chondrocytes and monocyte/macrophages. Osteoarthr. Cartil. 2007, 15, 1249–1255. [Google Scholar] [CrossRef]
- Jangravi, Z.; Basereh, S.; Mahmoudabadi, A.Z.; Saberi, M.; Alishiri, G.H.; Korani, M. Avocado/soy unsaponifiables can redress the balance between serum antioxidant and oxidant levels in patients with osteoarthritis: A double-blind, randomized, placebo-controlled, cross-over study. J. Complement. Integr. Med. 2021, 18, 769–774. [Google Scholar] [CrossRef]
- Simental-Mendía, M.; Sánchez-García, A.; Acosta-Olivo, C.A.; Vilchez-Cavazos, F.; Osuna-Garate, J.; Peña-Martínez, V.M.; Simental-Mendía, L.E. Efficacy and safety of avocado-soybean unsaponifiables for the treatment of hip and knee osteoarthritis: A systematic review and meta-analysis of randomized placebo-controlled trials. Int. J. Rheum. Dis. 2019, 22, 1607–1615. [Google Scholar] [CrossRef]
- Christensen, R.; Bartels, E.; Astrup, A.; Bliddal, H. Symptomatic efficacy of avocado–soybean unsaponifiables (ASU) in osteoarthritis (OA) patients: A meta-analysis of randomized controlled trials. Osteoarthr. Cartil. 2008, 16, 399–408. [Google Scholar] [CrossRef]
- Maheu, E.; Mazières, B.; Valat, J.P.; Loyau, G.; Loët, X.L.; Bourgeois, P.; Grouin, J.M.; Rozenberg, S. Symptomatic efficacy of avocado/soybean unsaponifiables in the treatment of osteoarthritis of the knee and hip: A prospective, randomized, double-blind, placebo-controlled, multicenter clinical trial with a six-month treatment period and a two-month followup demonstrating a persistent effect. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 1998, 41, 81–91. [Google Scholar]
- Henrotin, Y. Avocado/Soybean Unsaponifiables (Piacledine® 300) show beneficial effect on the metabolism of osteoarthritic cartilage, synovium and subchondral bone: An overview of the mechanisms. AIMS Med. Sci. 2018, 5, 33–52. [Google Scholar] [CrossRef]
- Lambert, C.; Bellemère, G.; Boyer, G.; Ponelle, F.; Bauer, T.; Legeny, M.-C.; Baudouin, C.; Henrotin, Y. Composition Analysis and Pharmacological Activity of Avocado/Soybean Unsaponifiable Products Used in the Treatment of Osteoarthritis. Front. Pharmacol. 2022, 12, 781389. [Google Scholar] [CrossRef]
- Jimenez-Lopez, C.; Carpena, M.; Lourenço-Lopes, C.; Gallardo-Gomez, M.; Lorenzo, J.M.; Barba, F.J.; Prieto, M.A.; Simal-Gandara, J. Bioactive compounds and quality of extra virgin olive oil. Foods 2020, 9, 1014. [Google Scholar] [CrossRef]
- Lerma-García, M.; Herrero-Martínez, J.; Simó-Alfonso, E.; Mendonça, C.R.; Ramis-Ramos, G. Composition, industrial processing and applications of rice bran γ-oryzanol. Food Chem. 2009, 115, 389–404. [Google Scholar] [CrossRef]
- Kumar, G.S.; Krishna, A.G. Studies on the nutraceuticals composition of wheat derived oils wheat bran oil and wheat germ oil. J. Food Sci. Technol. 2015, 52, 1145–1151. [Google Scholar] [CrossRef]
- Misir, A.; Kizkapan, T.B.; Arikan, Y.; Akbulut, D.; Onder, M.; Yildiz, K.I.; Ozkocer, S.E. Repair within the first 48 h in the treatment of acute Achilles tendon ruptures achieves the best biomechanical and histological outcomes. Knee Surg. Sports Traumatol. Arthrosc. 2020, 28, 2788–2797. [Google Scholar] [CrossRef] [PubMed]
- Sarı, A.; Dinçel, Y.M.; Karabağ, S.; Çetin, M.Ü. Histopathological and immunohistochemical investigation of the local and systemic effects of tranexamic acid on the healing of the Achilles tendon in rats. Jt. Dis. Relat. Surg. 2021, 32, 152. [Google Scholar] [CrossRef]
- Al-Afify, A.S.; El-Akabawy, G.; El-Sherif, N.M.; El-Safty, F.E.-N.A.; El-Habiby, M.M. Avocado soybean unsaponifiables ameliorates cartilage and subchondral bone degeneration in mono-iodoacetate-induced knee osteoarthritis in rats. Tissue Cell 2018, 52, 108–115. [Google Scholar] [CrossRef]
- Oliveira, G.; Paula, L.; Souza, J.; Spin-Neto, R.; Stavropoulos, A.; Marcantonio, R. Effect of avocado/soybean unsaponifiables on ligature-induced bone loss and bone repair after ligature removal in rats. J. Periodontal Res. 2016, 51, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Freitas de Paula, L.G.; Lopes de Oliveira, G.J.P.; Pinotti, F.E.; Grecchi, B.B.; Garcia de Aquino, S.; Chierici Marcantonio, R.A. Effect of Avocado/Soybean Unsaponifiables (ASU) on Osseointegration in Rats with Experimental Arthritis. Int. J. Oral Maxillofac. Implant. 2018, 33, 603. [Google Scholar] [CrossRef]
- Al Shoyaib, A.; Archie, S.R.; Karamyan, V.T. Intraperitoneal route of drug administration: Should it be used in experimental animal studies? Pharm. Res. 2020, 37, 12. [Google Scholar] [CrossRef]
- Sekihashi, K.; Sasaki, T.; Yamamoto, A.; Kawamura, K.; Ikka, T.; Tsuda, S.; Sasaki, Y.F. A comparison of intraperitoneal and oral gavage administration in comet assay in mouse eight organs. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2001, 493, 39–54. [Google Scholar] [CrossRef]
- Damsch, S.; Eichenbaum, G.; Tonelli, A.; Lammens, L.; Bulck, K.V.d.; Feyen, B.; Vandenberghe, J.; Megens, A.; Knight, E.; Kelley, M. Gavage-related reflux in rats: Identification, pathogenesis, and toxicological implications. Toxicol. Pathol. 2011, 39, 348–360. [Google Scholar] [CrossRef]
- Radice, C.; Korzekwa, K.; Nagar, S. Predicting impact of food and feeding time on oral absorption of drugs with a novel rat continuous intestinal absorption model. Drug Metab. Dispos. 2022, 50, 750–761. [Google Scholar] [CrossRef]
- Yurteri, A.; Mercan, N.; Celik, M.; Doğar, F.; Kılıç, M.; Yıldırım, A. The effect of caffeic acid on tendon healing in rats with an Achilles tendon injury model. Jt. Dis. Relat. Surg. 2023, 34, 669. [Google Scholar] [CrossRef]
- Can, E.; Dincel, Y.M.; Karabulut, D.; Karabag, S.; Arslan, Y.Z. The effect of papaverine on tendon healing and adhesion in rats following Achilles tendon repair. Jt. Dis. Relat. Surg. 2024, 35, 368. [Google Scholar] [CrossRef]
- Kuşcu, B.; Bilal, Ö.; Doğar, F.; Topak, D.; Gürbüz, K.; Dere, K.İ.; Telek, M.; Karadeniz, A.A.; Seyithanoğlu, M.; Kocaslan, S. Effects of L-carnitine on healing of Achilles tendon in rats. Jt. Dis. Relat. Surg. 2022, 34, 84. [Google Scholar] [CrossRef]
- Köker, Y.; Armangil, M.; Karaduman, M.; Tenekeci, G.Y.; Acar, B.; Akan, B. Investigation into the effect of systemic single high-dose erythropoietin on the healing of Achilles tendons in rats. Acta Orthop. Traumatol. Turc. 2022, 56, 357. [Google Scholar] [CrossRef]
- Johnson, W.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W. Amended safety assessment of Sesamum indicum (sesame) seed oil, hydrogenated sesame seed oil, Sesamum indicum (sesame) oil unsaponifiables, and sodium sesameseedate. Int. J. Toxicol. 2011, 30, 40S–53S. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.T.; Wanzeler, A.M.V.; Júnior, S.M.; Soares, R.H.F.C.; de Oliveira, C.P.; de M. Rodrigues, E.; Soares, B.M.; Alcantara, D.D.; Burbano, R.M.; Tuji, F.M. The chromatographic constitution of andiroba oil and his healing effects, compared to the LLLT outcomes, in oral mucositis induced in golden Syrian hamsters: A new treatment option. Oncotarget 2023, 14, 23. [Google Scholar] [CrossRef] [PubMed]
- Fezai, M.; Senovilla, L.; Jemaà, M.; Ben-Attia, M. Analgesic, Anti-Inflammatory and Anticancer Activities of Extra Virgin Olive Oil. J. Lipids 2013, 2013, 129736. [Google Scholar] [CrossRef]
- Malatesta, M. Histological and histochemical methods-theory and practice. Eur. J. Histochem. EJH 2016, 60, 2639. [Google Scholar] [CrossRef]
- Lau, S.K. Histological and Histochemical Methods: Theory and Practice; Taylor & Francis: Abingdon, UK, 2015. [Google Scholar]
- Fischer, A.H.; Jacobson, K.A.; Rose, J.; Zeller, R. Hematoxylin and eosin staining of tissue and cell sections. Cold Spring Harb. Protoc. 2008, 2008, pdb-prot4986. [Google Scholar] [CrossRef] [PubMed]
- da Silva Rosa, B.L.; Silva Pompêo, G.H.; Melo Ramos, C.; Ferreira, J.H.; Ruginsk, S.G.; Pires Marques, P.; Da Ré Guerra, F. Evaluation of the Effect of Melatonin on the Calcaneal Tendon of Ovariectomized Wistar Rats. Muscles Ligaments Tendons J. 2023, 13, 517. [Google Scholar] [CrossRef]
- Curtis, R.J.; Delee, J.C.; Drez JR, D.J. Reconstruction of the anterior cruciate ligament with freeze dried fascia lata allografts in dogs: A preliminary report. Am. J. Sports Med. 1985, 13, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Sufian, N.; Behfar, M.; Hobbenaghi, R.; Asri-Rezaei, S. Effect of curcumin-loaded polycaprolactone scaffold on Achilles tendon repair in rats. Vet. Res. Forum 2024, 15, 621–627. [Google Scholar]
- Zabrzyński, J.; Gagat, M.; Huri, G.; Łapaj, Ł.; Paczesny, Ł.; Zielińska, W.; Zabrzyńska, M.; Szwedowski, D.; Kruczyński, J. Therapeutic Advances in Tendinopathy Quantified Microscopically Using Bonar Score, with a Special Reference to PRP Therapy—A Systematic Review of Experimental Studies. Appl. Sci. 2021, 11, 4973. [Google Scholar] [CrossRef]
- Cook, J.; Feller, J.; Bonar, S.; Khan, K. Abnormal tenocyte morphology is more prevalent than collagen disruption in asymptomatic athletes’ patellar tendons. J. Orthop. Res. 2004, 22, 334–338. [Google Scholar] [CrossRef]
- Maffulli, N.; Longo, U.G.; Franceschi, F.; Rabitti, C.; Denaro, V. Movin and Bonar scores assess the same characteristics of tendon histology. Clin. Orthop. Relat. Res. 2008, 466, 1605–1611. [Google Scholar] [CrossRef]
- Loppini, M.; Giuseppe Longo, U.; Niccoli, G.; S Khan, W.; Maffulli, N.; Denaro, V. Histopathological scores for tissue-engineered, repaired and degenerated tendon: A systematic review of the literature. Curr. Stem Cell Res. Ther. 2015, 10, 43–55. [Google Scholar] [CrossRef]
- Soslowsky, L.J.; Carpenter, J.E.; DeBano, C.M.; Banerji, I.; Moalli, M.R. Development and use of an animal model for investigations on rotator cuff disease. J. Shoulder Elb. Surg. 1996, 5, 383–392. [Google Scholar] [CrossRef]
- Oliva, F.; Maffulli, N.; Gissi, C.; Veronesi, F.; Calciano, L.; Fini, M.; Brogini, S.; Gallorini, M.; Antonetti Lamorgese Passeri, C.; Bernardini, R. Combined ascorbic acid and T3 produce better healing compared to bone marrow mesenchymal stem cells in an Achilles tendon injury rat model: A proof of concept study. J. Orthop. Surg. Res. 2019, 14, 54. [Google Scholar] [CrossRef]
- Kurt, V.; Guner, S.; Kayacan, A.M.; Eronat, O. The effect of Sildenafil, a phosphodiesterase-5 inhibitor, on tendon healing: An experimental study in rat model of achilles tendon injury. Arch. Orthop. Trauma Surg. 2024, 144, 1107–1115. [Google Scholar] [CrossRef]
- Erel, O. A new automated colorimetric method for measuring total oxidant status. Clin. Biochem. 2005, 38, 1103–1111. [Google Scholar] [CrossRef]
- Maheu, E.; Cadet, C.; Marty, M.; Moyse, D.; Kerloch, I.; Coste, P.; Dougados, M.; Mazières, B.; Spector, T.D.; Halhol, H. Randomised, controlled trial of avocado–soybean unsaponifiable (Piascledine) effect on structure modification in hip osteoarthritis: The ERADIAS study. Ann. Rheum. Dis. 2014, 73, 376–384. [Google Scholar] [CrossRef]
- Msika, P.; Baudouin, C.; Saunois, A.; Bauer, T. Avocado/soybean unsaponifiables, ASU EXPANSCIENCE™, are strictly different from the nutraceutical products claiming ASU appellation. Osteoarthr. Cartil. 2008, 16, 1275–1276. [Google Scholar] [CrossRef]
- Turner, P.V.; Brabb, T.; Pekow, C.; Vasbinder, M.A. Administration of substances to laboratory animals: Routes of administration and factors to consider. J. Am. Assoc. Lab. Anim. Sci. 2011, 50, 600–613. [Google Scholar]
- Teixeira-Santos, L.; Albino-Teixeira, A.; Pinho, D. An alternative method for oral drug administration by voluntary intake in male and female mice. Lab. Anim. 2021, 55, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Sun, J.; Zhang, T.t.; Ma, B.; Cui, S.m.; Chen, D.w.; He, Z.g. Pharmacokinetic behaviors and oral bioavailability of oridonin in rat plasma. Acta Pharmacol. Sin. 2006, 27, 1642–1646. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Yang, H.; Li, J.; Wang, H. Optimization of phytosterols recovery from soybean oil deodorizer distillate. J. Am. Oil Chem. Soc. 2012, 89, 1363–1370. [Google Scholar] [CrossRef]
- Flores, M.; Saravia, C.; Vergara, C.E.; Avila, F.; Valdés, H.; Ortiz-Viedma, J. Avocado oil: Characteristics, properties, and applications. Molecules 2019, 24, 2172. [Google Scholar] [CrossRef]
- Barp, L.; Miklavčič Višnjevec, A.; Moret, S. Analytical determination of squalene in extra virgin olive oil and olive processing by-products, and its valorization as an ingredient in functional food—A critical review. Molecules 2024, 29, 5201. [Google Scholar] [CrossRef]
- Li, P.; Zhou, H.; Tu, T.; Lu, H. Dynamic exacerbation in inflammation and oxidative stress during the formation of peritendinous adhesion resulted from acute tendon injury. J. Orthop. Surg. Res. 2021, 16, 293. [Google Scholar] [CrossRef] [PubMed]
- Ellis, I.M.; Schnabel, L.V.; Berglund, A.K. Defining the profile: Characterizing cytokines in tendon injury to improve clinical therapy. J. Immunol. Regen. Med. 2022, 16, 100059. [Google Scholar] [CrossRef]
- Legerlotz, K.; Jones, E.R.; Screen, H.R.; Riley, G.P. Increased expression of IL-6 family members in tendon pathology. Rheumatology 2012, 51, 1161–1165. [Google Scholar] [CrossRef] [PubMed]
- Najafi, Z.; Moosavi, Z.; Rahimi, V.B.; Hashemitabar, G.; Askari, V.R. Evaluation of Anti-Nociceptive, Anti-Inflammatory, and Anti-Fibrotic effects of noscapine against a rat model of Achilles tendinopathy. Int. Immunopharmacol. 2024, 130, 111704. [Google Scholar] [CrossRef] [PubMed]
- Ownby, S.L.; Fortuno, L.V.; Au, A.Y.; Grzanna, M.W.; Rashmir-Raven, A.M.; Frondoza, C.G. Expression of pro-inflammatory mediators is inhibited by an avocado/soybean unsaponifiables and epigallocatechin gallate combination. J. Inflamm. 2014, 11, 8. [Google Scholar] [CrossRef]
- Henrotin, Y.; Labasse, A.; Jaspar, J.; De Groote, D.; Zheng, S.; Guillou, G.; Reginster, J. Effects of three avocado/soybean unsaponifiable mixtures on metalloproteinases, cytokines and prostaglandin E2 production by human articular chondrocytes. Clin. Rheumatol. 1998, 17, 31–39. [Google Scholar] [CrossRef]
- Prasetia, R.; Purwana, S.Z.B.; Lesmana, R.; Herman, H.; Chernchujit, B.; Rasyid, H.N. The pathology of oxidative stress-induced autophagy in a chronic rotator cuff enthesis tear. Front. Physiol. 2023, 14, 1222099. [Google Scholar] [CrossRef]
- Semis, H.S.; Gur, C.; Ileriturk, M.; Kandemir, F.M.; Kaynar, O. Evaluation of therapeutic effects of quercetin against Achilles tendinopathy in rats via oxidative stress, inflammation, apoptosis, autophagy, and metalloproteinases. Am. J. Sports Med. 2022, 50, 486–498. [Google Scholar] [CrossRef]
- Lui, P.P.Y.; Zhang, X.; Yao, S.; Sun, H.; Huang, C. Roles of oxidative stress in acute tendon injury and degenerative tendinopathy—A target for intervention. Int. J. Mol. Sci. 2022, 23, 3571. [Google Scholar] [CrossRef]
- Irani, M.; Farzizadeh, R.; Abdolmaleki, A.; Asadi, A. The Effect of Aerobic Exercise With an Omega-3 Supplement on the Tendon Healing Process. Am. J. Sports Med. 2025, 53, 1650–1661. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Wang, H.; Li, B.; Cao, R.; Li, Y.; Cui, S.; Zhang, W. Curcumin Improves Functional Recovery of Ruptured Tendon by Promoting Tenogenesis via PI3K/Akt Signaling. Stem Cells Transl. Med. 2024, 13, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.S.; Kim, H.J.; Kang, T.U.; Kazmi, S.Z.; Suh, J.S.; Young Choi, J. Dyslipidemia Is associated with increased risk of Achilles tendon disorders in underweight individuals to a greater extent than obese individuals: A nationwide, population-based, longitudinal cohort study. Orthop. J. Sports Med. 2021, 9, 23259671211042599. [Google Scholar] [CrossRef] [PubMed]
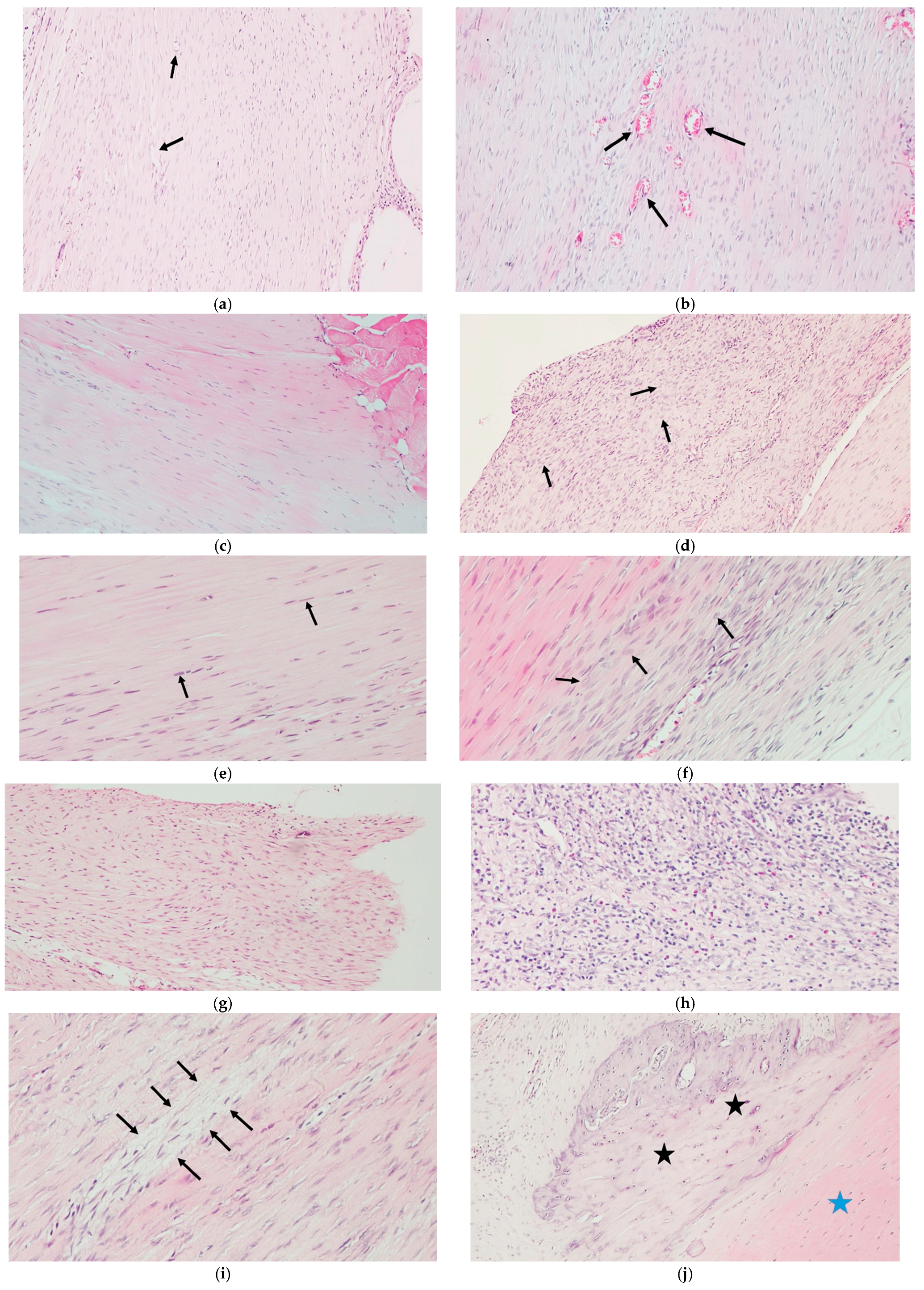
| Parameter | Scoring Range | Reference Criteria | Description |
|---|---|---|---|
| Cartilage Formation | 0–3 | Modified Soslowsky | Degree of ectopic cartilage-like tissue in the tendon. 0 = none; 1 = minimal cartilage-like tissue (<25% of area); 2 = moderate cartilage formation (25–50% of area); 3 = extensive cartilage formation (>50% of area) |
| Tenocyte Morphology | 0–3 | Bonar | Cell shape, nuclear changes, and tenocyte density. 0 = normal elongated spindle-shaped nuclei; 1 = slightly rounded nuclei; 2 = rounded nuclei with nuclear enlargement/changes; 3 = degenerated cells with loss of normal morphology |
| Ground Substance Accumulation | 0–3 | Bonar | Amount of basophilic matrix and proteoglycan presence. 0 = absent; 1 = mild basophilic matrix; 2 = moderate accumulation of proteoglycan-rich matrix; 3 = extensive ground substance deposition |
| Inflammatory Cell Infiltration | 0–1 | Curtis and DeLee | Presence or absence of inflammatory cells. 0 = absent; 1 = present |
| Neovascularization | 0–3 | Curtis and DeLee | Number and density of newly formed blood vessels. 0 = no capillary vessels; 1 = 1–5 capillary vessels; 2 = 6–10 capillary vessels; 3 = >10 capillary vessels |
| Fibroblastic Activity | 0–2 | Curtis and DeLee | Extent of fibroblast proliferation and activity. 0 = none; 1 = mild proliferation; 2 = extensive fibroblastic activity |
| Collagen Fiber Alignment | 0–1 | Curtis and DeLee | Organization and orientation of collagen fibers. 0 = scattered, irregular collagen fiber sequencing; 1 = regular, parallel collagen fiber sequencing |
| Variable | ASU (n = 10) | Control (n = 10) | p Value |
|---|---|---|---|
| Cartilage Formation Grade | 0.0 (0.0–0.25) | 2.0 (1.0–3.0) | <0.001 * |
| Tenocyte Grade | 0.0 (0.0–1.0) | 2.0 (1.0–2.25) | <0.001 * |
| Ground Substance Grade | 0.0 (0.0–1.0) | 1.5 (1.0–2.25) | 0.001 * |
| Inflammation Score | 0.0 (0.0–0.25) | 1.0 (0.75–1.0) | 0.009 * |
| Neovascularization Score | 1.0 (1.0–2.0) | 2.0 (1.0–3.0) | 0.100 * |
| Fibroblastic Activity Score | 1.0 (1.0–1.0) | 2.0 (1.0–2.0) | 0.004 * |
| Collagen Sequencing Score | 1.0 (1.0–1.0) | 0.0 (0.0–0.25) | 0.002 * |
| Histopathological Total Score | 3.0 (3.0–6.3) | 9.5 (6.0–13.8) | 0.002 * |
| Variable | ASU (n = 10) | Control (n = 10) | p Value |
|---|---|---|---|
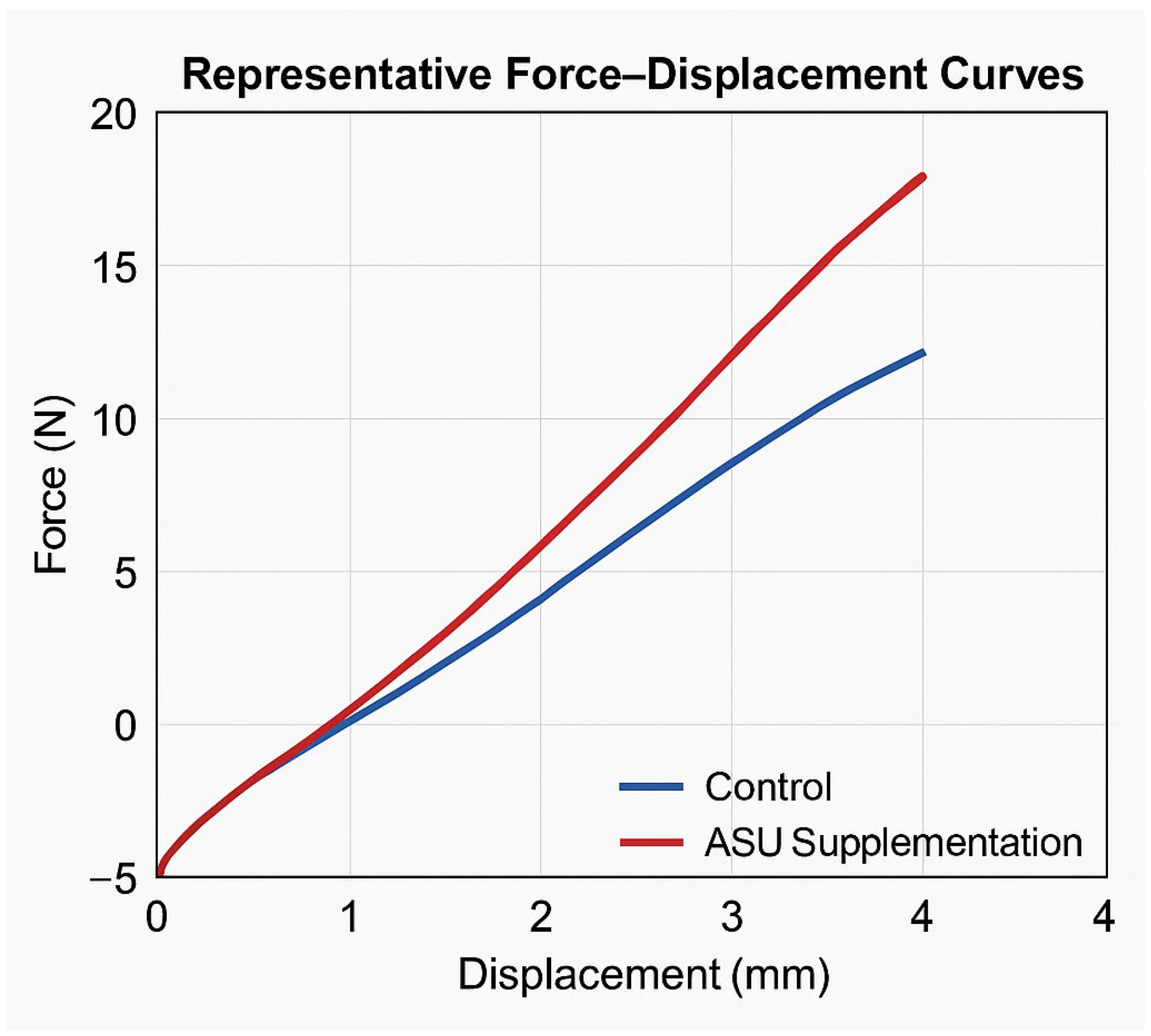
| Max force (N) | 20.5 (19.6–20.7) | 14.25 (13.4–15.4) | <0.001 * |
| Displacement at max force (mm) | 3.75 (3.7–3.83) | 3.05 (2.6–3.3) | 0.004 * |
| Stress (MPa) | 401.94 ± 36.48 | 342.31 ± 53.96 | 0.01 ** |
| Energy at max force (mJ) | 32.86 ± 2.18 | 24.72 ± 2.89 | <0.001 ** |
| Total energy (J) | 82.40 ± 5.90 | 55.78 ± 6.59 | <0.001 ** |
| Stiffness (N/mm) | 106.3 (103.2–114.1) | 80.2 (76.7–82.9) | <0.001 * |
| Variable | ASU Group n = 10 | Control Group n = 10 | p Value |
|---|---|---|---|
| IL1β (pg/mL) | 32.24 ± 3.62 | 57.52 ± 9.16 | <0.001 ** |
| IL6 (pg/mL) | 38.75 (34.5–42.7) | 80.9 (72.8–90.0) | <0.001 * |
| TNFα (pg/mL) | 20.22 (17.9–22.5) | 50.1 (46.9–55.9) | <0.001 * |
| TAS (mmol Trolox equiv./L) | 2.12 ± 0.23 | 1.33 ± 0.17 | <0.001 ** |
| TOS (µmol H2O2 equiv./L) | 10.65 ± 1.95 | 16.44 ± 2.12 | <0.001 ** |
| OSI | 48.8 (42.6–60.5) | 127.2 (97.4–153.2) | <0.001 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dinç, M.; Soydemir, Ö.C.; Bayrak, H.Ç.; Karasu, R.; Aykaç, B.; Topcu, M.E. Avocado–Soybean Unsaponifiables Enhance Tendon Healing via Anti-Inflammatory and Antioxidant Mechanisms in a Rat Achilles Injury Model. Medicina 2025, 61, 2035. https://doi.org/10.3390/medicina61112035
Dinç M, Soydemir ÖC, Bayrak HÇ, Karasu R, Aykaç B, Topcu ME. Avocado–Soybean Unsaponifiables Enhance Tendon Healing via Anti-Inflammatory and Antioxidant Mechanisms in a Rat Achilles Injury Model. Medicina. 2025; 61(11):2035. https://doi.org/10.3390/medicina61112035
Chicago/Turabian StyleDinç, Mustafa, Ömer Cevdet Soydemir, Hünkar Çağdaş Bayrak, Recep Karasu, Bilal Aykaç, and Mehmet Emre Topcu. 2025. "Avocado–Soybean Unsaponifiables Enhance Tendon Healing via Anti-Inflammatory and Antioxidant Mechanisms in a Rat Achilles Injury Model" Medicina 61, no. 11: 2035. https://doi.org/10.3390/medicina61112035
APA StyleDinç, M., Soydemir, Ö. C., Bayrak, H. Ç., Karasu, R., Aykaç, B., & Topcu, M. E. (2025). Avocado–Soybean Unsaponifiables Enhance Tendon Healing via Anti-Inflammatory and Antioxidant Mechanisms in a Rat Achilles Injury Model. Medicina, 61(11), 2035. https://doi.org/10.3390/medicina61112035






