Association of Advanced Glycation End Products with Diabetic Retinopathy Severity in Lithuanian Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sample
2.2. Ophthalmological Assessment
2.3. General Assessment
2.4. Statistical Analyses
3. Results
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A/C ratio | Albumin/creatinine ratio |
| AF | Autofluorescence |
| AGE | Advanced glycation end products |
| AH | Arterial hypertension |
| AUC | Area under the curve |
| BMI | Body mass index |
| CI | Confidence interval |
| cm | Centimeters |
| DBP | Diastolic blood pressure |
| DM | Diabetes mellitus |
| DR | Diabetic retinopathy |
| HbA1c | Glycated hemoglobin |
| HDL-C | High-density lipoprotein cholesterol |
| HR | Heart rate |
| kg | kilograms |
| LDL-C | Low-density lipoprotein cholesterol |
| LUHS | Lithuanian University of Health Sciences |
| NPDR | Non-proliferative diabetic retinopathy |
| OR | Odds ratio |
| PDR | Proliferative diabetic retinopathy |
| ROC | Receiver operating characteristic |
| SBP | Systolic blood pressure |
| SD | Standard deviation |
| TC | Total cholesterol |
| TG | Triglycerides |
| T1D | Type 1 diabetes |
| T2D | Type 2 diabetes |
References
- Spital, G.; Faatz, H. Volkskrankheit diabetische Retinopathie. Klin. Monbl. Augenheilkd. 2023, 240, 1060–1070. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.E.; Wong, T.Y. Diabetic retinopathy: Looking forward to 2030. Front. Endocrinol. 2022, 13, 1077669. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, S.H.; Schwartz, S.S. Diabetic Retinopathy–An Underdiagnosed and Undertreated Inflammatory, Neuro-Vascular Complication of Diabetes. Front. Endocrinol. 2019, 10, 843. [Google Scholar] [CrossRef]
- Al Ghamdi, A.H. Clinical Predictors of Diabetic Retinopathy Progression; A Systematic Review. Curr. Diabetes Rev. 2019, 16, 242–247. [Google Scholar]
- Rao, H.; Jalali, J.A.; Johnston, T.P.; Koulen, P. Emerging Roles of Dyslipidemia and Hyperglycemia in Diabetic Retinopathy: Molecular Mechanisms and Clinical Perspectives. Front. Endocrinol. 2021, 12, 620045. [Google Scholar] [CrossRef]
- Ahuja, P.; Waris, A.; Siddiqui, S.S.; Mukherjee, A. Single nucleotide variants of receptor for advanced glycation end-products (AGER) gene is it a new opening in the risk assessment of diabetic retinopathy review. J. Genet. Eng. Biotechnol. 2022, 20, 17. [Google Scholar] [CrossRef] [PubMed]
- Dariya, B.; Nagaraju, G.P. Advanced glycation end products in diabetes, cancer and phytochemical therapy. Drug Discov. Today 2020, 25, 1614–1623. [Google Scholar] [CrossRef]
- Hirano, T.; Iesato, Y.; Toriyama, Y.; Imai, A.; Chiba, D.; Murata, T. Correlation between diabetic retinopathy severity and elevated skin autofluorescence as a marker of advanced glycation end-product accumulation in type 2 diabetic patients. J. Diabetes Complicat. 2014, 28, 729–734. [Google Scholar] [CrossRef]
- Januszewski, A.S.; Sachithanandan, N.; Karschimkus, C.; O’Neal, D.N.; Yeung, C.K.; Alkatib, N.; Jenkins, A.J. Non-invasive measures of tissue autofluorescence are increased in Type 1 diabetes complications and correlate with a non-invasive measure of vascular dysfunction. Diabet. Med. 2012, 29, 726–733. [Google Scholar] [CrossRef]
- Monnier, V.M.; Bautista, O.; Kenny, D.; Sell, D.R.; Fogarty, J.; Dahms, W.; A Cleary, P.; Lachin, J.; Genuth, S. Skin collagen glycation, glycoxidation, and crosslinking are lower in subjects with long-term intensive versus conventional therapy of type 1 diabetes: Relevance of glycated collagen products versus HbA1c as markers of diabetic complications. DCCT Skin Collagen Ancillary Study Group. Diabetes Control and Complications Trial. Diabetes 1999, 48, 870–880. [Google Scholar]
- Flaxel, C.J.; Adelman, R.A.; Bailey, S.T.; Kovach, J.L.; Vemulakonda, G.A.; Ying, G.-S.; Flaxel, C.J.; American Academy of Ophthalmology Preferred Practice Pattern Retina/Vitreous Committee. Diabetic Retinopathy Preferred Practice Pattern. Ophthalmology 2020, 127, P66–P145, Erratum in Ophthalmology 2020, 127, 1279. [Google Scholar] [CrossRef] [PubMed]
- WHO. Physical Status: The Use and Interpretation of Anthropometry. Report of a WHO Expert Committee; (WHO Technical Report Series, No. 854); World Health Organization: Geneva, Switzerland, 1995; pp. 1–36. [Google Scholar]
- Lin, K.Y.; Hsih, W.H.; Lin, Y.B.; Wen, C.Y.; Chang, T.J. Update in the epidemiology, risk factors, screening, and treatment of diabetic retinopathy. J. Diabetes Investig. 2021, 12, 1322–1325. [Google Scholar] [CrossRef]
- Zhu, H.; Li, B.; Huang, T.; Wang, B.; Li, S.; Yu, K.; Cai, L.; Ye, Y.; Chen, S.; Zhu, H.; et al. Update in the molecular mechanism and biomarkers of diabetic retinopathy. Biochim. Biophys. Acta Mol. Basis Dis. 2025, 1871, 167758. [Google Scholar] [CrossRef]
- Milne, R.; Brownstein, S. Advanced glycation end products and diabetic retinopathy. Amino Acids 2013, 44, 1397–1407. [Google Scholar] [CrossRef] [PubMed]
- Osawa, S.; Katakami, N.; Sato, I.; Ninomiya, H.; Omori, K.; Yamamoto, Y.; Takahara, M.; Miyashita, K.; Sakamoto, F.; Kawamori, D.; et al. Skin autofluorescence is associated with vascular complications in patients with type 2 diabetes. J. Diabet Complicat. 2018, 32, 839–844. [Google Scholar] [CrossRef]
- Cho, Y.H.; Craig, M.E.; Januszewski, A.S.; Benitez-Aguirre, P.; Hing, S.; Jenkins, A.J.; Donaghue, K.C. Higher skin autofluorescence in young people with Type 1 diabetes and microvascular complications. Diabet. Med. 2017, 34, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Martínez-García, I.; Cavero-Redondo, I.; Álvarez-Bueno, C.; Pascual-Morena, C.; Gómez-Guijarro, M.D.; Saz-Lara, A. Non-invasive skin autofluorescence as a screening method for diabetic retinopathy. Diabetes Metab. Res. Rev. 2024, 40, e3721. [Google Scholar] [CrossRef]
- Wu, M.Y.; Yiang, G.T.; Lai, T.T.; Li, C.J. The oxidative stress and mitochondrial dysfunction during the pathogenesis of diabetic retinopathy. Oxid. Med. Cell. Longev. 2018, 2018, 3420187. [Google Scholar] [CrossRef]
- Twarda-Clapa, A.; Olczak, A.; Białkowska, A.M.; Koziołkiewicz, M. Advanced Glycation End-Products (AGEs): Formation, Chemistry, Classification, Receptors, and Diseases Related to AGEs. Cells 2022, 11, 1312. [Google Scholar] [CrossRef]
- Asmamaw, M.M.; Endeshaw, C.A.; Awgichew, B.T.; Mulu, A.T.; Agidew, M.M.; Azezew, M.T.; Zewde, E.A.; Teshome, A.A. Endogenous advanced glycation end products in the pathogenesis of chronic diabetic complications. Front. Mol. Biosci. 2022, 9, 1002710. [Google Scholar] [CrossRef]
- Ying, L.; Shen, Y.; Zhang, Y.; Wang, Y.; Liu, Y.; Yin, J.; Wang, Y.; Yin, J.; Zhu, W.; Bao, Y.; et al. Association of advanced glycation end products with diabetic retinopathy in type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 2021, 177, 108880. [Google Scholar] [CrossRef]
- Semba, R.D.; Nicklett, E.J.; Ferrucci, L. Does accumulation of advanced glycation end products contribute to the aging phenotype? J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 963–975. [Google Scholar] [CrossRef]
- VanderBeek, B.L.; Yu, Y.; Cardillo, S.; Hubbard, R. Twenty-Year Trends in Prevalence and Incidence of Diabetic Retinal Disease. Ophthalmology 2025, 132, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Lundeen, E.A.; Burke-Conte, Z.; Rein, D.B.; Wittenborn, J.S.; Saaddine, J.; Lee, A.Y.; Flaxman, A.D. Prevalence of Diabetic Retinopathy in the US in 2021. JAMA Ophthalmol. 2023, 141, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Medina-Ramirez, S.A.; Soriano-Moreno, D.R.; Tuco, K.G.; Castro-Diaz, S.D.; Alvarado-Villacorta, R.; Pacheco-Mendoza, J.; Yovera-Aldana, M. Prevalence and incidence of diabetic retinopathy in patients with diabetes of Latin America and the Caribbean: A systematic review and meta-analysis. PLoS ONE. 2024, 19, e0296998. [Google Scholar] [CrossRef]
- Cannizzaro, L.; Rossoni, G.; Savi, F.; Altomare, A.; Marinello, C.; Saethang, T.; Carini, M.; Payne, D.M.; Pisitkun, T.; Aldini, G.; et al. Regulatory landscape of AGE-RAGE-oxidative stress axis and its modulation by PPARγ activation in high fructose diet-induced metabolic syndrome. Nutr. Metab. 2017, 14, 5. [Google Scholar] [CrossRef] [PubMed]
- Bains, Y.; Gugliucci, A. Ilex paraguariensis and its main component chlorogenic acid inhibit fructose formation of advanced glycation endproducts with amino acids at conditions compatible with those in the digestive system. Fitoterapia 2017, 117, 6–10. [Google Scholar] [CrossRef]
- Aragno, M.; Mastrocola, R. Dietary sugars and endogenous formation of advanced glycation endproducts: Emerging mechanisms of disease. Nutrients 2017, 9, 385. [Google Scholar] [CrossRef]
- Vlassara, H.; Cai, W.; Tripp, E.; Pyzik, R.; Yee, K.; Goldberg, L.; Tansman, L.; Chen, X.; Mani, V.; Fayad, Z.A.; et al. Oral AGE restriction ameliorates insulin resistance in obese individuals with the metabolic syndrome: A randomised controlled trial. Diabetologia 2016, 59, 2181–2192. [Google Scholar] [CrossRef]
| Variable | All (n = 152) | No DR (n = 69) | Mild-to-Moderate NPDR (n = 56) | Advanced DR (Severe NPDR to PDR) (n = 27) | p Value |
|---|---|---|---|---|---|
| Male, n (%) | 69 (45.4) | 29 (42.0) | 26 (46.4) | 14 (51.9) | 0.672 |
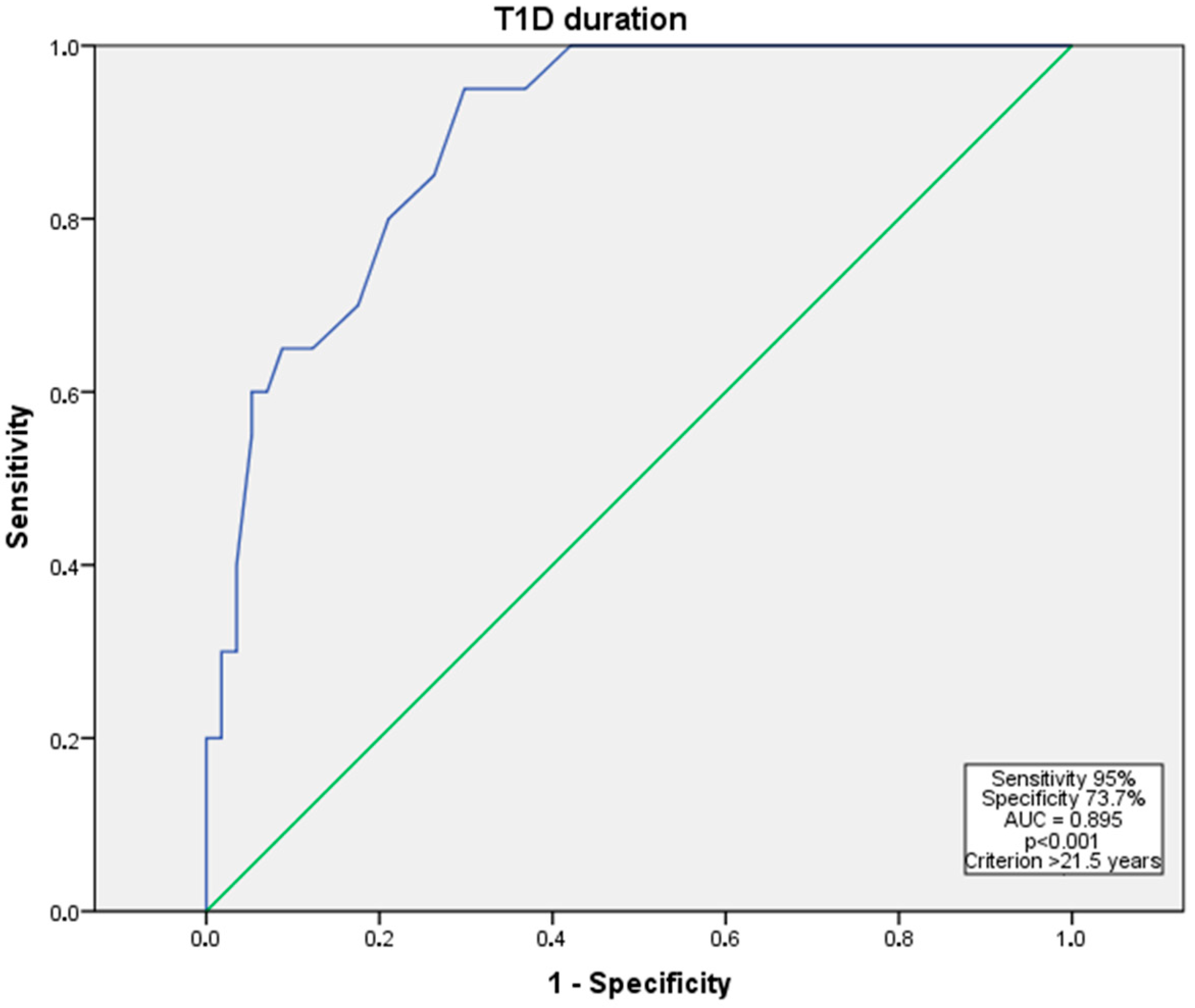
| T1D, n (%) | 77 (50.7) | 20 (29.0) | 33 (58.9) | 24 (88.9) | <0.001 * |
| Presence of AH, n (%) | 89 (58.6) | 44 (63.8) | 33 (58.9) | 12 (44.4) | 0.224 |
| Presence of dyslipidemia, n (%) | 52 (34.2) | 28 (40.6) | 16 (28.6) | 8 (29.6) | 0.319 |
| Smokers, n (%) | 40 (26.3) | 18 (26.1) | 15 (26.8) | 7 (25.9) | 0.803 |
| Age, years | 51.85 ± 16.63 | 54.81 ± 15.50 | 50.66 ± 18.16 | 46.74 ± 15.05 | 0.08 |
| Duration of DM, years | 15.99 ± 11.18 | 9.67 ± 8.46 | 17.32 ± 7.87 | 29.41 ± 10.52 | <0.001 * |
| SBP, mmHg | 137.18 ± 18.58 | 136.96 ± 17.23 | 137.02 ± 20.02 | 138.11 ± 19.30 | 0.960 |
| DBP, mmHg | 83.96 ± 11.07 | 84.04 ± 9.98 | 84.30 ± 12.74 | 83.04 ± 10.33 | 0.886 |
| HR, beats/min | 80.80 ± 12.40 | 79.70 ± 10.31 | 81.23 ± 14.08 | 82.74 ± 13.72 | 0.531 |
| BMI, kg/m2 | 29.86 ± 6.69 | 32.05 ± 6.84 | 28.96 ± 6.32 | 26.13 ± 4.95 | <0.001 * |
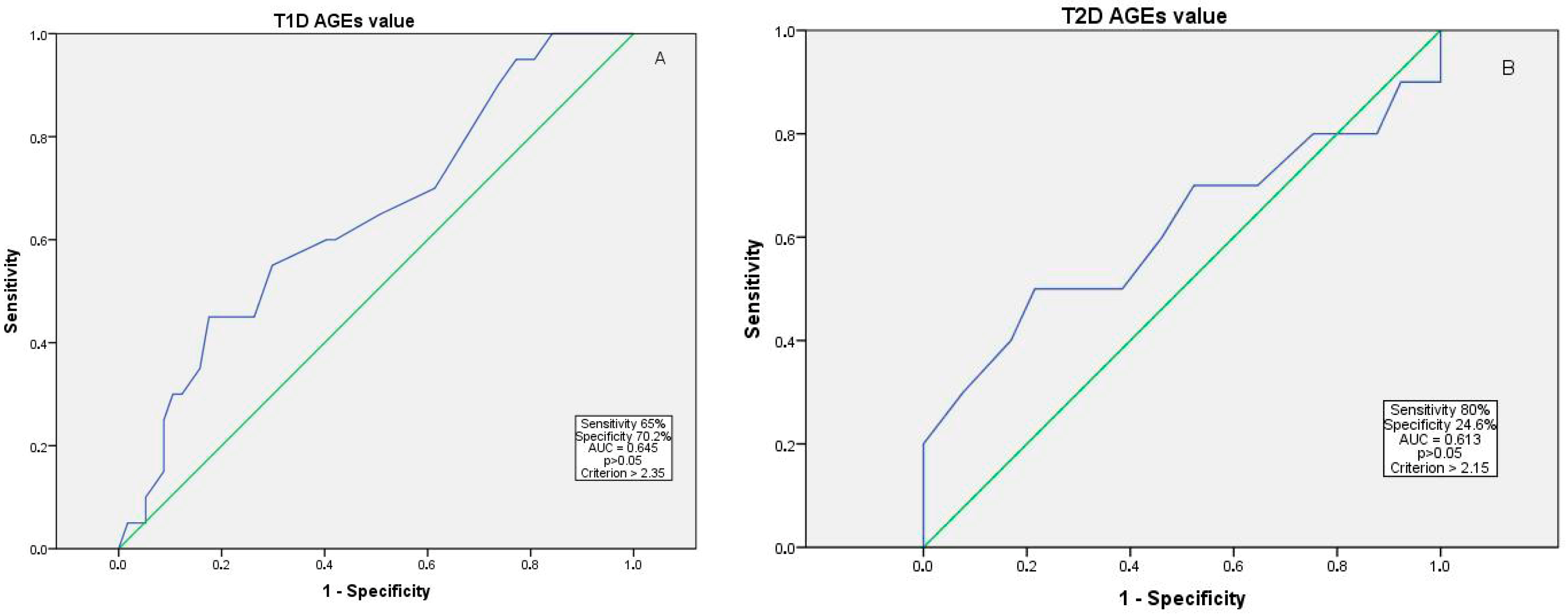
| AGE value | 2.32 ± 0.52 | 2.26 ± 0.51 | 2.37 ± 0.47 | 2.38 ± 0.63 | 0.386 |
| HbA1c, % | 8.34 ± 1.99 | 8.03 ± 1.98 | 8.54 ± 2.09 | 8.74 ± 1.71 | 0.204 |
| TC, mmol/L | 5.13 ± 1.44 | 5.37 ± 1.63 | 4.93 ± 1.09 | 4.96 ± 1.53 | 0.190 |
| LDL-C, mmol/L | 3.14 ± 1.00 | 3.26 ± 1.17 | 3.04 ± 0.90 | 3.06 ± 0.76 | 0.422 |
| HDL-C, mmol/L | 1.34 ± 0.37 | 1.26 ± 0.37 | 1.34 ± 0.31 | 1.54 ± 0.39 | 0.002 * |
| TG, mmol/L | 2.03 ± 3.39 | 2.62 ± 4.79 | 1.61 ± 0.96 | 1.42 ± 1.77 | 0.152 |
| Creatinine, μmol/L | 75.14 ± 25.80 | 73.60 ± 24.33 | 78.94 ± 26.19 | 71.22 ± 28.52 | 0.355 |
| A/C ratio, mg/mmol | 8.24 ± 27.94 | 3.82 ± 11.13 | 6.09 ± 27.03 | 23.83 ± 48.42 | 0.005 * |
| Variable | T1D | T2D | ||
|---|---|---|---|---|
| p Value | OR [95% CI] for Severe NPDR to PDR | p Value | OR [95% CI] for Severe NPDR to PDR | |
| Duration of the DM | 0.003 * | 0.668 [0.511–0.872] | 0.082 | 0.874 [0.788–1.014] |
| AGE value | 0.366 | 0.372 [0.044–3.73] | 0.136 | 0.175 [0.018–1.727] |
| Total cholesterol | 0.887 | 1.263 [0.050–31.909] | 0.027 * | 0.079 [0.008–0.745] |
| A/C ratio | 0.355 | 0.986 [0.957–1.016] | 0.023 * | 0.965 [0.935–0.995] |
| Comparison (vs. Advanced DR) | Predictor | Odds Ratio | 95% CI (Lower–Upper) | p Value |
|---|---|---|---|---|
| No DR | AGE value | 0.118 | 0.025–0.566 | 0.008 * |
| Mild-to-moderate NPDR | AGE value | 0.587 | 0.208–1.656 | 0.314 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varoniukaite, A.; Poskiene, U.; Paskeviciene, D.; Simoniene, D.; Radzeviciene, L.; Verkauskiene, R.; Balciuniene, V.J. Association of Advanced Glycation End Products with Diabetic Retinopathy Severity in Lithuanian Patients. Medicina 2025, 61, 1956. https://doi.org/10.3390/medicina61111956
Varoniukaite A, Poskiene U, Paskeviciene D, Simoniene D, Radzeviciene L, Verkauskiene R, Balciuniene VJ. Association of Advanced Glycation End Products with Diabetic Retinopathy Severity in Lithuanian Patients. Medicina. 2025; 61(11):1956. https://doi.org/10.3390/medicina61111956
Chicago/Turabian StyleVaroniukaite, Aiste, Ugne Poskiene, Deimante Paskeviciene, Diana Simoniene, Lina Radzeviciene, Rasa Verkauskiene, and Vilma Jurate Balciuniene. 2025. "Association of Advanced Glycation End Products with Diabetic Retinopathy Severity in Lithuanian Patients" Medicina 61, no. 11: 1956. https://doi.org/10.3390/medicina61111956
APA StyleVaroniukaite, A., Poskiene, U., Paskeviciene, D., Simoniene, D., Radzeviciene, L., Verkauskiene, R., & Balciuniene, V. J. (2025). Association of Advanced Glycation End Products with Diabetic Retinopathy Severity in Lithuanian Patients. Medicina, 61(11), 1956. https://doi.org/10.3390/medicina61111956






