Atypical Morphological Variations of the Sacrum in the Korean Population: A PMCT-Based 3D Reconstruction Study
Abstract
1. Introduction
2. Materials and Methods
2.1. PMCT Image and Data Acquisition
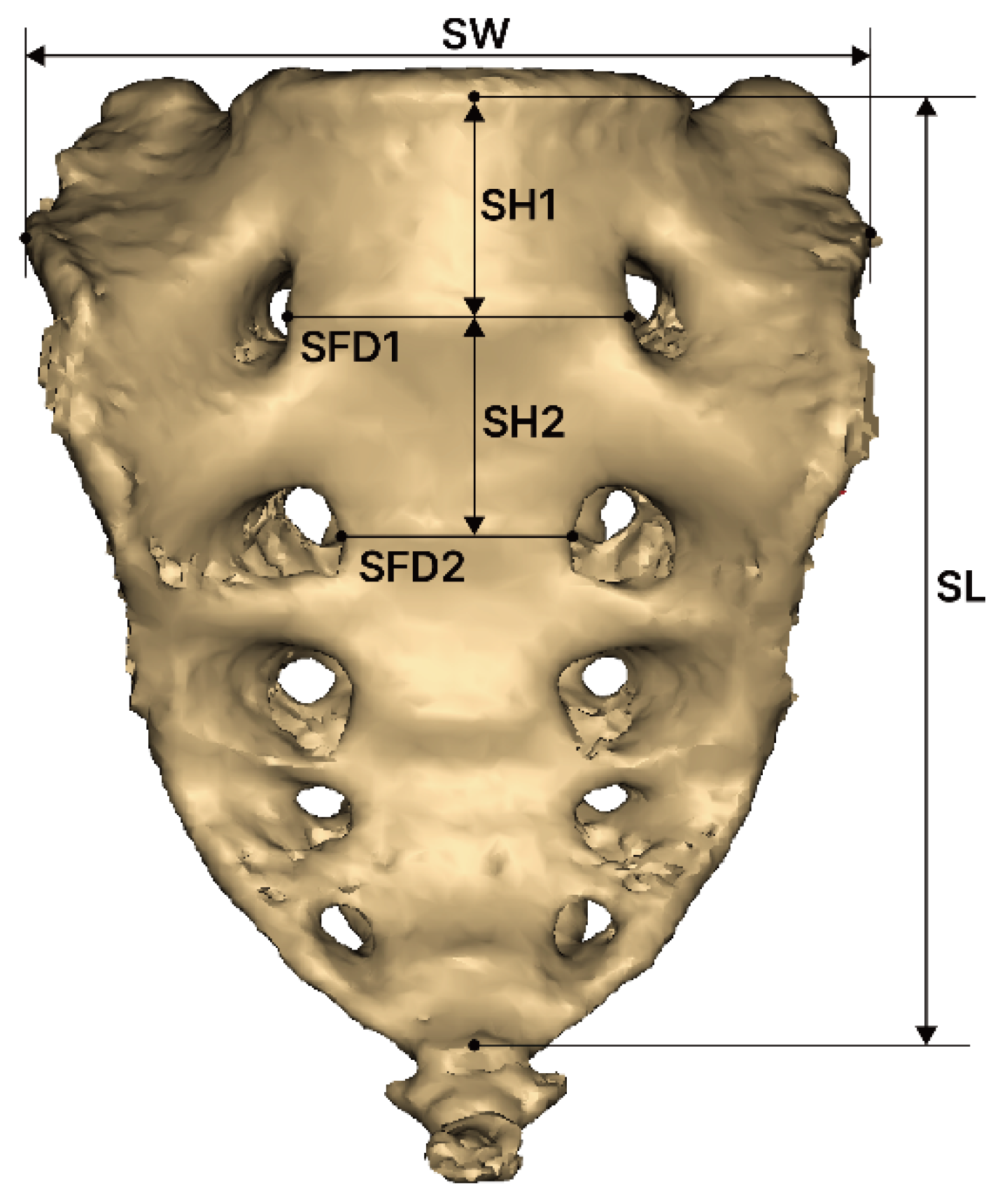
2.2. Measurement of Sacrum Morphology
- SW (sacral width): Measured between the auricular surfaces at the posterosuperior point of the auricular surface.
- SL (sacral length): Measured from the upper midpoint of the promontory to the apex of the sacrum in the sagittal plane.
- SFD1 (sacral foramina distance of first): Measured between the left and right first anterior sacral foramina.
- SFD2 (sacral foramina distance of second): Measured between the left and right second anterior sacral foramina.
- SH1 (sacrum vertebrae height of first): Measured the height of the first sacral vertebra at the midline.
- SH2 (sacrum vertebrae height of second): Measured the height of the second sacral vertebra at the midline.
- SC (sacral curvature): Calculated as the quotient of the sacral anterior surface distance divided by SL.
- SI (sacrum index): Calculated as the quotient of SL divided by SW.
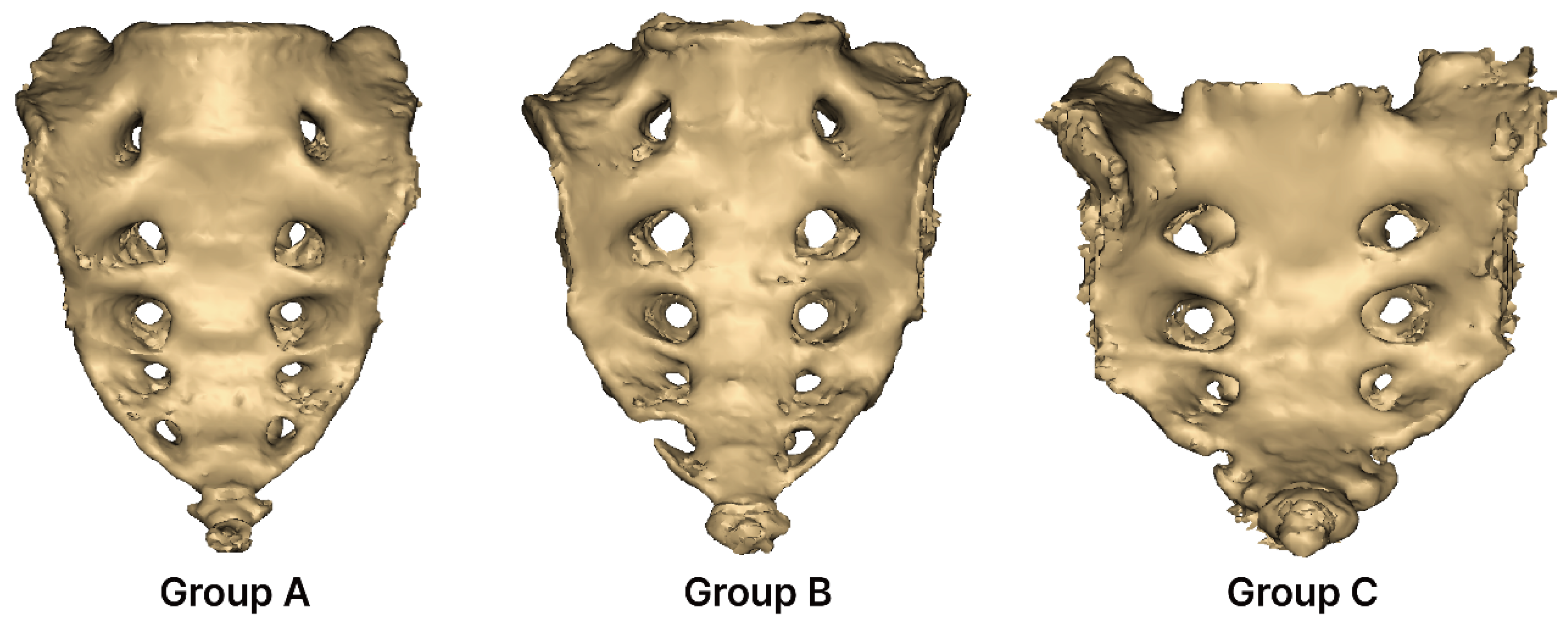
2.3. Grouping of Sacral Foraminal Variations
- Group A (complete five pairs): Consists of five complete pairs of foramina. In Group A, none of the foramina are perforated or separated; all sides are completely closed.
- Group B (incomplete five pairs): Contains five incomplete pairs of foramina. In Group B, the foramina are not fully closed on either the right or left side and remain open.
- Group C (complete three pairs): Consists of three complete pairs of foramina. In Group C, only three pairs of foramina are completely closed.
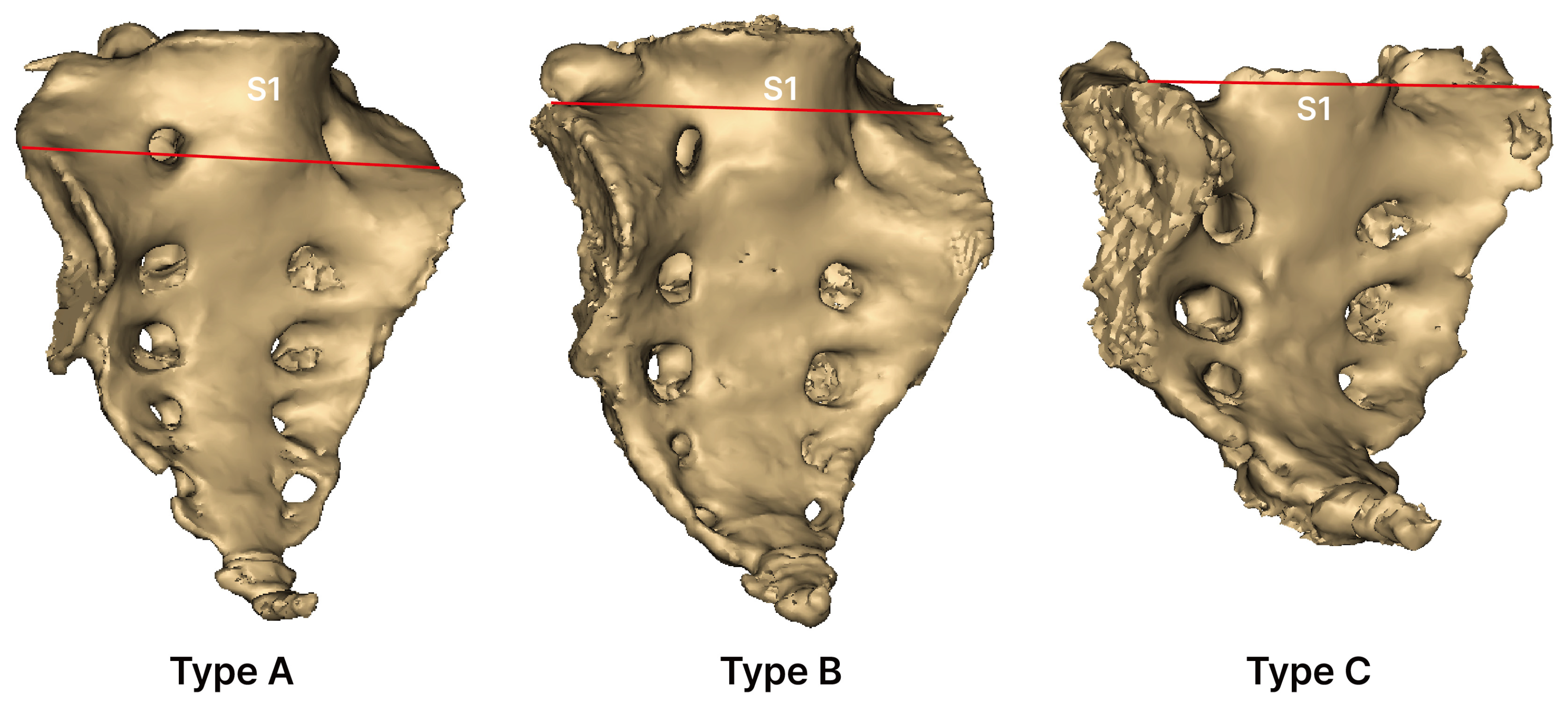
2.4. Type of Sacral Auricular Surface
- Type A (low-down): Auricular surface extending from the lower part of S1.
- Type B (standard): Auricular surface extending from the upper part of S1.
- Type C (high-up): Auricular surface extending from a level above the upper part of S1.
2.5. Statistical Analysis
3. Result
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PMCT | Postmortem computed tomography |
| LSTV | Lumbosacral transitional vertebra |
| CT | Computed tomography |
| SW | Sacral width |
| SL | Sacral length |
| SFD1 | Distance between the first pair of sacral foramina |
| SFD2 | Distance between the second pair of sacral foramina |
| SH1 | Height of the first sacral vertebra |
| SH2 | Height of the second sacral vertebra |
| SC | Sacral curvature |
| SI | Sacral index |
References
- Standring, S.; Ellis, H.; Healy, J.; Johnson, D.; Williams, A.; Collins, P.; Wigley, C. Gray’s Anatomy: The Anatomical Basis of Clinical Practice. Am. J. Neuroradiol. 2005, 26, 2703. [Google Scholar]
- Kubavat, D.M.; Nagar, S.K.; Lakhani, C.; Ruparelia, S.S.; Patel, S.; Varlekar, P. A Study of Sacrum with Three Pairs of Sacral Foramina in Western India. Int. J. Med. Sci. Public Health 2012, 1, 127–131. [Google Scholar] [CrossRef]
- Cheng, J.S.; Song, J.K. Anatomy of the Sacrum. J. Neurosurg. Focus 2003, 15, 1–4. [Google Scholar] [CrossRef]
- Krenn, V.A.; Fornai, C.; Webb, N.M.; Woodert, M.A.; Prosch, H.; Haeusler, M. The Morphological Consequences of Segmentation Anomalies in the Human Sacrum. Am. J. Biol. Anthropol. 2022, 177, 690–707. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.G.; Moon, M.S.; Park, B.K.; Lee, H.; Kim, D.H. Analysis of Sacrococcygeal Morphology in Koreans Using Computed Tomography. Clin. Orthop. Surg. 2016, 8, 412–419. [Google Scholar] [CrossRef]
- Ajai, S. Bertolloti Syndrome—Musculoskeletal Case of the Month; London Bridge Sports Medicine Group: London, UK, 2023. [Google Scholar]
- Lin, J.D.; Tan, L.A.; Wei, C.; Shillingford, J.N.; Laratta, J.L.; Lombardi, J.M.; Lenke, L.G. The Posterior Superior Iliac Spine and Sacral Laminar Slope: Key Anatomical Landmarks for Freehand S2-Alar-Iliac Screw Placement. J. Neurosurg. Spine 2018, 29, 429–434. [Google Scholar] [CrossRef]
- Wu, L.P.; Li, Y.K.; Li, Y.M.; Zhang, Y.Q.; Zhong, S.Z. Variable Morphology of the Sacrum in a Chinese Population. Clin. Anat. 2009, 22, 619–626. [Google Scholar] [CrossRef]
- Kamal, A.M.; Ara, S.; Begum, S.; Hoque, M.M.; Khatun, K. Sacralization: Sacrum with Five Pairs of Sacral Foramina. Bangladesh J. Anat. 2013, 11, 54–57. [Google Scholar] [CrossRef]
- Shaikh, S.T. A Morphometric Study of Sacrum with Five Pairs of Sacral Foramina. GAIMS J. Med. Sci. 2023, 3, 80–83. [Google Scholar]
- Lee, H.Y.; Chung, I.H. A Morphologieal Study of the Sacrum in Korean Adult. Korean J. Phys. Anthropol. 1989, 2, 101–112. [Google Scholar] [CrossRef]
- Beckmann, N.M.; Chinapuvvula, N.R. Sacral Fractures: Classification and Management. Emerg. Radiol. 2017, 24, 605–617. [Google Scholar] [CrossRef]
- Sieper, J.; Rudwaleit, M.; Baraliakos, X.; Brandt, J.; Braun, J.; Burgos-Vargas, R.; Van Der Heijde, D. The Assessment of Spondyloarthritis International Society (Asas) Handbook: A Guide to Assess Spondyloarthritis. Ann. Rheum. Dis. 2009, 68, ii1–ii44. [Google Scholar] [CrossRef]
- Gerber, S.; Ollivier, L.; Leclère, J.; Vanel, D.; Missenard, G.; Brisse, H.; Neuenschwander, S. Imaging of Sacral Tumours. Skeletal Radiol. 2008, 37, 277–289. [Google Scholar] [CrossRef]
- Vrtovec, T.; Janssen, M.M.; Pernuš, F.; Castelein, R.M.; Viergever, M.A. Analysis of Pelvic Incidence from 3-Dimensional Images of a Normal Population. Spine 2012, 37, E479–E485. [Google Scholar] [CrossRef]
- Jakka, L.D.; Chandrika, P.V.; Devi, A.V.L.; Madhavi, D.; Syamala, G. Morphometric Study of Sacrum and Its Clinical Implications. Eur. J. Cardiovasc. Med. 2025, 15, 3336. [Google Scholar] [CrossRef]
- Bernardi, S.; Marchetti, E.; Torge, D.; Simeone, D.; Macchiarelli, G.; Bianchi, S. Ultrastructural Assessment of Human Periodontal Ligament Fibroblast Interaction with Bovine Pericardium Membranes: An In Vitro Study. Histol. Histopathol. 2025, 40, 860. [Google Scholar] [CrossRef]
- van Schalkwyk, J.; Matshidza, S.; Mogale, N. The Classification of Sacral Foramina in a South African Sample Using Cadaveric and Osteological Remains. Transl. Res. Anat. 2024, 37, 100347. [Google Scholar] [CrossRef]
- Mahato, N.K. Implications of Structural Variations in the Human Sacrum: Why Is an Anatomical Classification Crucial? Surg. Radiol. Anat. 2016, 38, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Mahato, N.K. Re-Examining the Spectrum of Lumbosacral Transitional Dysmorphisms: Quantifying Joint Asymmetries and Evaluating the Anatomy of Screw Fixation Corridors. Neurospine 2020, 17, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Gil, Y.C.; Shin, K.J.; Kim, J.N.; Joo, S.H.; Koh, K.S.; Song, W.C. An Anatomical and Morphometric Study of the Coccyx Using Three-Dimensional Reconstruction. Anat. Rec. 2016, 299, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, R.; Sharadkumar, P.S. Coccygeal Sacralisation–a Study. Int. J. Pharmacol. Res. 2021, 21, 14–18. [Google Scholar]
- Castellvi, A.E.; Goldstein, L.A.; Chan, D.P. Lumbosacral Transitional Vertebrae and Their Relationship with Lumbar Extradural Defects. Spine 1984, 9, 493–495. [Google Scholar] [CrossRef]
- Mahato, N.K. Variable Positions of the Sacral Auricular Surface: Classification and Importance. Neurosurg. Focus 2010, 28, E12. [Google Scholar] [CrossRef]
- Saluja, S.; Agarwal, S.; Tuli, A.N.I.T.A.; Raheja, S.; Tigga, S.R.; Paul, S. Morphometric Analysis of the Sacrum and Its Surgical Implications. Clin. Diagnostic Res. 2018, 12, AC01–AC06. [Google Scholar] [CrossRef]

| Parameter | Interclass Correlation | |
|---|---|---|
| ICC | 95% CI | |
| SW | 0.980 | 0.98~0.991 |
| SL | 0.998 | 0.996~0.999 |
| SFD1 | 0.997 | 0.994~0.999 |
| SFD2 | 0.977 | 0.950~0.989 |
| SH1 | 0.979 | 0.955~0.990 |
| SH2 | 0.991 | 0.982~0.996 |
| SC | 0.977 | 0.950~0.989 |
| SI | 0.996 | 0.992~0.998 |
| Male (n = 10) | Female (n = 19) | Total (n = 29) | ||||
|---|---|---|---|---|---|---|
| Parameter | Median (IQR) | Range | Median (IQR) | Range | Median (IQR) | Range |
| Age | 51.5 (46.7, 46.5) | 30 to 61 | 59.0 (44.0, 68.0) | 41 to 81 | 54.0 (46.5, 64.0) | 30.0 to 78.0 |
| Stature (cm) | 173.0 (172.2, 176.2) | 168.0 to 1800.0 | 155.0 (150.0, 166.0) | 146.0 to 168.0 | 166.0 (153.5, 173.0) | 146.0 to 180.0 |
| Weight (kg) | 76.5 (67.7, 83.2) | 56.0 to 90.2 | 54.0 (51.0, 65.0) | 37.0 to 89.0 | 62.0 (53.5, 79.5) | 37.0 to 90.2 |
| SW (mm) | 93.2 (87.6, 97.4) | 85.9 to 101.4 | 96.8 (92.2, 102.9) | 90.2 to 105.8 | 95.3 (91.6, 100.2) | 85.9 to 105.8 |
| SL (mm) | 126.6 (115.9, 129.1) | 114.7 to 131.2 | 118.6 (95.8, 131.1) | 86.1 to 142.19 | 118.6 (105.1, 129.8) | 86.1 to 142.1 |
| SFD1 (mm) | 37.7 (34.2, 41.2) | 29.6 to 46.1 | 35.9 (30.4, 40.7) | 25.9 to 45.4 | 36.1 (31.1, 40.5) | 25.9 to 46.1 |
| SFD2 (mm) | 30.3 (28.2, 31.2) | 27.5 to 31.8 | 28.7 (27.6, 30.2) | 23.9 to 35.6 | 28.8 (27.2, 30.9) | 23.9 to 35.6 |
| SH1 (mm) | 28.5 (24.1, 32.6) | 22.1 to 35.3 | 28.0 (23.2, 30.6) | 19.2 to 34.8 | 28.0 (23.3, 31.3) | 19.2 to 35.3 |
| SH2 (mm) | 28.5 (25.2, 33.8) | 22.9 to 34.7 | 30.7 (25.7, 35.1) | 20.2 to 36.2 | 29.7 (25.6, 34.3) | 20.2 to 36.2 |
| SC | 0.93 (0.91, 0.95) | 0.91 to 0.96 | 0.91 (0.89, 0.95) | 80.0 to 98.0 | 0.92 (0.90, 0.95) | 0.80 to 0.98 |
| SI | 0.75 (0.71, 0.77) | 0.65 to 0.85 | 0.79 (0.76, 1.01) | 0.65 to 1.13 | 0.78 (0.74, 0.91) | 0.65 to 1.13 |
| Group | Male | Female | Total | p |
|---|---|---|---|---|
| Group A (Complete five pairs) | 7 (70.0) | 9 (47.4) | 16 (55.2) | 0.629 |
| Group B (Unilateral five pairs) | 3 (30.0) | 9 (47.4) | 12 (41.4) | |
| Group C (Complete three pairs) | 0 (0.0) | 1 (5.3) | 1 (3.4) | |
| Total | 10 (100.0) | 19 (100.0) | 29 (100.0) |
| Type | Male | Female | Total | p |
|---|---|---|---|---|
| Type A (Low-down) | 7 (70.0) | 11 (57.9) | 18 (62.1) | 0.805 |
| Type B (Normal) | 3 (30.0) | 7 (36.8) | 10 (34.5) | |
| Type C (High-up) | 0 (0.0) | 1 (5.3) | 1 (3.4) | |
| Total | 10 (100.0) | 19 (100.0) | 29 (100.0) |
| Parameter (mm) | Male (n = 10) | Female (n = 19) | Total (n = 29) | p |
|---|---|---|---|---|
| SW | 93.0 ± 5.0 | 97.2 ± 5.4 | 95.8 ± 5.5 | 0.049 |
| SL | 124.0 ± 6.2 | 115.1 ± 17.9 | 118.2 ± 15.4 | 0.064 |
| SFD1 | 37.6 ± 5.0 | 35.1 ± 5.9 | 35.9 ± 5.6 | 0.271 |
| SFD2 | 29.8 ± 1.5 | 28.9 ± 2.7 | 29.2 ± 2.4 | 0.352 |
| SH1 | 28.5 ± 4.6 | 27.3 ± 4.5 | 27.8 ± 4.5 | 0.506 |
| SH2 | 29.1 ± 4.3 | 29.7 ± 5.5 | 29.5 ± 5.1 | 0.753 |
| SC | 0.93 ± 0.01 | 0.90 ± 0.05 | 0.91 ± 0.04 | 0.070 |
| SI | 0.75 ± 0.05 | 0.86 ± 0.15 | 0.82 ± 0.13 | 0.007 |
| Stature | Weight | SW | SL | SFD1 | SFD2 | SH1 | SH2 | SC | |
|---|---|---|---|---|---|---|---|---|---|
| Weight | 0.710 ** | ||||||||
| SW | −0.273 | −0.237 | |||||||
| SL | 0.635 ** | 0.645 ** | −0.129 | ||||||
| SFD1 | 0.157 | 0.276 | 0.032 | 0.332 | |||||
| SFD2 | 0.103 | 0.164 | 0.082 | 0.193 | 0.704 ** | ||||
| SH1 | 0.242 | −0.156 | 0.149 | 0.003 | −0.654 ** | −0.441 * | |||
| SH2 | 0.184 | 0.338 | −0.02 | 0.605 ** | 0.683 ** | 0.377 * | −0.505 ** | ||
| SC | 0.543 ** | 0.666 ** | −0.463 * | 0.764 ** | 0.181 | 0.145 | −0.063 | 0.406 * | |
| SI | −0.663 ** | −0.661 ** | 0.493 ** | −0.921 ** | −0.303 | −0.14 | 0.065 | −0.547 ** | −0.845 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.-H.; Park, E.-S.; Cho, J.; Choi, Y.-J.; Kwon, H.-W.; Kim, D.; Choe, Y.; Lee, G.; Park, K.-R. Atypical Morphological Variations of the Sacrum in the Korean Population: A PMCT-Based 3D Reconstruction Study. Medicina 2025, 61, 1942. https://doi.org/10.3390/medicina61111942
Park J-H, Park E-S, Cho J, Choi Y-J, Kwon H-W, Kim D, Choe Y, Lee G, Park K-R. Atypical Morphological Variations of the Sacrum in the Korean Population: A PMCT-Based 3D Reconstruction Study. Medicina. 2025; 61(11):1942. https://doi.org/10.3390/medicina61111942
Chicago/Turabian StylePark, Jeong-Hyun, Eun-Seo Park, Jaeho Cho, Yu-Jin Choi, Hyung-Wook Kwon, Digud Kim, Yunil Choe, Goeun Lee, and Kwang-Rak Park. 2025. "Atypical Morphological Variations of the Sacrum in the Korean Population: A PMCT-Based 3D Reconstruction Study" Medicina 61, no. 11: 1942. https://doi.org/10.3390/medicina61111942
APA StylePark, J.-H., Park, E.-S., Cho, J., Choi, Y.-J., Kwon, H.-W., Kim, D., Choe, Y., Lee, G., & Park, K.-R. (2025). Atypical Morphological Variations of the Sacrum in the Korean Population: A PMCT-Based 3D Reconstruction Study. Medicina, 61(11), 1942. https://doi.org/10.3390/medicina61111942









