Severe Sunburns and Sunbed Use Risk with Cutaneous Melanoma: A Case–Control Study in Lithuania †
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Data Collection
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMN | Atypical Melanocytic Nevi |
| CI | Confidence Interval |
| CM | Cutaneous Melanoma |
| DNA | Deoxyribonucleic Acid |
| IBM SPSS | International Business Machines Statistical Package for the Social Sciences |
| LSMU | Lithuanian University of Health Sciences |
| LSMUL KK | Lithuanian University of Health Sciences Kauno Klinikos (Hospital of LSMU) |
| MN | Melanocytic Nevi |
| OR | Odds Ratio |
| ROS | Reactive Oxygen Species |
| SD | Standard Deviation |
| SPF | Sun Protection Factor |
| UV | Ultraviolet |
| χ2 | Chi-Square |
References
- Gosman, L.M.; Țăpoi, D.A.; Costache, M. Cutaneous Melanoma: A Review of Multifactorial Pathogenesis, Immunohistochemistry, and Emerging Biomarkers for Early Detection and Management. Int. J. Mol. Sci. 2023, 24, 15881. [Google Scholar] [CrossRef]
- Yuan, J.; Li, X.; Yu, S. Global, Regional, and National Incidence Trend Analysis of Malignant Skin Melanoma Between 1990 and 2019, and Projections Until 2034. Cancer Control 2024, 31, 10732748241227340. [Google Scholar] [CrossRef]
- Viksna, L.; Ozola, E.; Karls, R. Skin cancer incidence in Latvia 2007–2017: Comparison with Estonia and Lithuania. In Proceedings of the 24th World Congress of Dermatology, Milan, Italy, 10–15 June 2019. [Google Scholar]
- European Cancer Information System. Data Explorer [Internet]. JRC, European Commission. Available online: https://ecis.jrc.ec.europa.eu/data-explorer#/historical (accessed on 5 October 2025).
- Dennis, L.K. Cumulative Sun Exposure and Melanoma in a Population-Based Case-Control Study: Does Sun Sensitivity Matter? Cancers 2022, 14, 1008. [Google Scholar] [CrossRef]
- Kulichová, D.; Dáňová, J.; Kunte, C.; Ruzicka, T.; Celko, A.M. Risk Factors for Malignant Melanoma and Preventive Methods. Cutis 2014, 94, 241–248. [Google Scholar] [PubMed]
- Farley, C.; Alimi, Y.; Espinosa, L.R.; Perez, S.; Knechtle, W.; Hestley, A.; Carlson, G.W.; Russell, M.C.; Delman, K.A.; Rizzo, M. Tanning Beds: A Call to Action for Further Educational and Legislative Efforts. J. Surg. Oncol. 2015, 112, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Pagliarello, C.; Girardelli, C.R.; Feliciani, C.; Stanganelli, I. Melanoma Likelihood and Relationship with Nevi Distribution: A Case-Control Study Among Italian Patients. Int. J. Dermatol. 2022, 61, e443–e445. [Google Scholar] [CrossRef] [PubMed]
- Ghiasvand, R.; Robsahm, T.E.; Green, A.C.; Rueegg, C.S.; Weiderpass, E.; Lund, E.; Veierød, M.B. Association of Phenotypic Characteristics and UV Radiation Exposure With Risk of Melanoma on Different Body Sites. JAMA Dermatol. 2019, 155, 39–49. [Google Scholar] [CrossRef]
- Olsen, C.M.; Pandeya, N.; Thompson, B.S.; Dusingize, J.C.; Green, A.C.; Neale, R.E.; Whiteman, D.C.; QSkin Study. Association Between Phenotypic Characteristics and Melanoma in a Large Prospective Cohort Study. J. Investig. Dermatol. 2019, 139, 665–672. [Google Scholar] [CrossRef]
- Hübner, J.; Waldmann, A.; Eisemann, N.; Noftz, M.; Geller, A.C.; Weinstock, M.A.; Volkmer, B.; Greinert, R.; Breitbart, E.W.; Katalinic, A. Association Between Risk Factors and Detection of Cutaneous Melanoma in the Setting of a Population-Based Skin Cancer Screening. Eur. J. Cancer Prev. 2018, 27, 563–569. [Google Scholar] [CrossRef]
- Olsen, C.M.; Pandeya, N.; Thompson, B.S.; Dusingize, J.C.; Webb, P.M.; Green, A.C.; Neale, R.E.; Whiteman, D.C.; QSkin Study. Risk Stratification for Melanoma: Models Derived and Validated in a Purpose-Designed Prospective Cohort. J. Natl. Cancer Inst. 2018, 110, 1075–1083. [Google Scholar] [CrossRef]
- Wei, E.X.; Li, X.; Nan, H. Extremity Nevus Count is an Independent Risk Factor for Basal Cell Carcinoma and Melanoma, but Not Squamous Cell Carcinoma. J. Am. Acad. Dermatol. 2019, 80, 970–978. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska, A.; Lewandowski, T.; Rudzki, G.; Próchnicki, M.; Laskowska, B.; Pavlov, S.; Vlasenko, O.; Rudzki, S.; Wójcik, W. The Risk of Melanoma Due to Exposure to Sun and Solarium Use in Poland: A Large-Scale, Hospital-Based Case-Control Study. Asian Pac. J. Cancer Prev. 2023, 24, 2259–2269. [Google Scholar] [CrossRef] [PubMed]
- Stenehjem, J.S.; Robsahm, T.E.; Bråtveit, M.; Samuelsen, S.O.; Kirkeleit, J.; Grimsrud, T.K. Ultraviolet Radiation and Skin Cancer Risk in Offshore Workers. Occup. Med. 2017, 67, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Lergenmuller, S.; Rueegg, C.S.; Perrier, F.; Robsahm, T.E.; Green, A.C.; Lund, E.; Ghiasvand, R.; Veierød, M.B. Lifetime Sunburn Trajectories and Associated Risks of Cutaneous Melanoma and Squamous Cell Carcinoma Among a Cohort of Norwegian Women. JAMA Dermatol. 2022, 158, 1367–1377. [Google Scholar] [CrossRef]
- Wu, S.; Cho, E.; Li, W.Q.; Weinstock, M.A.; Han, J.; Qureshi, A.A. History of Severe Sunburn and Risk of Skin Cancer Among Women and Men in 2 Prospective Cohort Studies. Am. J. Epidemiol. 2016, 183, 824–833. [Google Scholar] [CrossRef]
- Savoye, I.; Olsen, C.M.; Whiteman, D.C.; Bijon, A.; Wald, L.; Dartois, L.; Clavel-Chapelon, F.; Boutron-Ruault, M.C.; Kvaskoff, M. Patterns of Ultraviolet Radiation Exposure and Skin Cancer Risk: The E3N-SunExp Study. J. Epidemiol. 2018, 28, 27–33. [Google Scholar] [CrossRef]
- Tran, M.M.; George-Washburn, E.A.; Rhee, J.; Li, W.Q.; Qureshi, A.; Cho, E. A Prospective Cohort Study Exploring the Joint Influence of Sunlight Exposure and Tanning Bed Use on Basal Cell Carcinoma, Squamous Cell Carcinoma, and Melanoma Risk. Arch. Dermatol. Res. 2024, 316, 281. [Google Scholar] [CrossRef]
- Ghiasvand, R.; Rueegg, C.S.; Weiderpass, E.; Green, A.C.; Lund, E.; Veierød, M.B. Indoor Tanning and Melanoma Risk: Long-Term Evidence from a Prospective Population-Based Cohort Study. Am. J. Epidemiol. 2017, 185, 147–156. [Google Scholar] [CrossRef]
- Pedersen, J.E.; Hansen, J. Incident Skin Melanoma in Danish Male Military Pilots: A Nested Case-Control Study. Occup. Environ. Med. 2023, 80, 239–245. [Google Scholar] [CrossRef]
- Langston, M.E.; Brown, H.E.; Lynch, C.F.; Roe, D.J.; Dennis, L.K. Ambient UVR and Environmental Arsenic Exposure in Relation to Cutaneous Melanoma in Iowa. Int. J. Environ. Res. Public Health 2022, 19, 1742. [Google Scholar] [CrossRef]
- Vuong, K.; McGeechan, K.; Armstrong, B.K.; Cust, A.E.; AMFS Investigators; GEM Investigators. Occupational Sun Exposure and Risk of Melanoma According to Anatomical Site. Int. J. Cancer 2014, 134, 2735–2741. [Google Scholar] [CrossRef]
- Collatuzzo, G.; Boffetta, P.; Dika, E.; Visci, G.; Zunarelli, C.; Mastroeni, S.; Antonelli, G.; Fortes, C. Occupational Exposure to Arsenic, Mercury and UV Radiation and Risk of Melanoma: A Case-Control Study from Italy. Int. Arch. Occup. Environ. Health 2023, 96, 443–449. [Google Scholar] [CrossRef]
- Wu, S.; Han, J.; Vleugels, R.A.; Puett, R.; Laden, F.; Hunter, D.J.; Qureshi, A.A. Cumulative Ultraviolet Radia-tion Flux in Adulthood and Risk of Incident Skin Cancers in Women. Br. J. Cancer 2014, 110, 1855–1861. [Google Scholar] [CrossRef] [PubMed]
- Ransohoff, K.J.; Ally, M.S.; Stefanick, M.L.; Keiser, E.; Spaunhurst, K.; Kapphahn, K.; Pagoto, S.; Messina, C.; Hedlin, H.; Manson, J.E.; et al. Impact of Residential UV Exposure in Childhood Versus Adulthood on Skin Cancer Risk in Caucasian, Postmenopausal Women in the Women’s Health Initiative. Cancer Causes Control 2016, 27, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Cust, A.E.; Drummond, M.; Bishop, D.T.; Azizi, L.; Schmid, H.; Jenkins, M.A.; Hopper, J.L.; Armstrong, B.K.; Aitken, J.F.; Kefford, R.F.; et al. Associations of Pigmentary and Naevus Phenotype with Melanoma Risk in Two Populations with Comparable Ancestry but Contrasting Levels of Ambient Sun Exposure. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 1874–1885. [Google Scholar] [CrossRef] [PubMed]
- Valiukeviciene, S.; Miseviciene, I.; Gollnick, H. The Prevalence of Common Acquired Melanocytic Nevi and the Relationship with Skin Type Characteristics and Sun Exposure Among Children in Lithuania. Arch. Dermatol. 2005, 141, 579–586. [Google Scholar] [CrossRef][Green Version]
- Kontautiene, S.; Stang, A.; Gollnick, H.; Valiukeviciene, S. The Role of Phenotype, Body Mass Index, Parental and Sun Exposure Factors in the Prevalence of Melanocytic Nevi Among Schoolchildren in Lithuania. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1506–1516. [Google Scholar] [CrossRef]
- Šemeklis, L.; Kapitanovaitė, L.; Butrimas, G.; Briedė, K.; Račkauskaitė, L.; Žemaitienė, R.; Valiukevičienė, S. Cataract Prevalence in Patients with Cutaneous Melanoma in Lithuanian Population. J. Clin. Med. 2024, 13, 6717. [Google Scholar] [CrossRef]
- Šemeklis, L.; Kapitanovaitė, L.; Butrimas, G.; Briedė, K.; Dubinskaitė, A.; Žemaitienė, R.; Valiukevičienė, S. Iris Pigmented Lesions and Risk of Cutaneous Melanoma: Case–Control Study in Lithuania. J. Pers. Med. 2024, 14, 530. [Google Scholar] [CrossRef]
- Daniūtė, G.; Pranckėnienė, L.; Pakerys, J.; Kloviņš, J.; Kučinskas, V.; Urnikytė, A. Populations of Latvia and Lithuania in the context of some Indo-European and non-Indo-European speaking populations of Europe and India: Insights from genetic structure analysis. Front. Genet. 2024, 15, 1493270. [Google Scholar] [CrossRef]
- Urnikyte, A.; Molyte, A.; Kučinskas, V. Genome-Wide Landscape of North-Eastern European Populations: A View from Lithuania. Genes 2021, 12, 1730. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Institute of Hygiene. Lithuanian Health Statistics. Available online: https://stat.hi.lt (accessed on 19 February 2025).
- Lui, H.; Ahad, T.; Kalia, S. The Use and Misuse of Fitzpatrick’s Skin Types. J. Cutan. Med. Surg. 2022, 26, 15–16. [Google Scholar] [CrossRef] [PubMed]
- Arisi, M.; Zane, C.; Caravello, S.; Rovati, C.; Zanca, A.; Venturini, M.; Calzavara-Pinton, P. Sun Exposure and Melanoma, Certainties and Weaknesses of the Present Knowledge. Front. Med. 2018, 5, 235. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhang, N.; Yin, C.; Zhu, B.; Li, X. Ultraviolet Radiation and Melanomagenesis: From Mechanism to Immunotherapy. Front. Oncol. 2020, 10, 951. [Google Scholar] [CrossRef]
- Gieniusz, E.; Skrzydlewska, E.; Łuczaj, W. Current Insights into the Role of UV Radiation-Induced Oxidative Stress in Melanoma Pathogenesis. Int. J. Mol. Sci. 2024, 25, 11651. [Google Scholar] [CrossRef]
- Nurla, L.-A.; Wafi, G.; Tatar, R.; Dorobanțu, A.M.; Chivu, M.; Popa, L.G.; Giurcăneanu, C.; Orzan, O.A. Recent-Onset Melanoma and the Implications of the Excessive Use of Tanning Devices—Case Report and Review of the Literature. Medicina 2024, 60, 187. [Google Scholar] [CrossRef]
- Ghiasvand, R.; Weiderpass, E.; Green, A.C.; Lund, E.; Veierød, M.B. Sunscreen Use and Subsequent Melanoma Risk: A Population-Based Cohort Study. J. Clin. Oncol. 2016, 34, 3976–3983. [Google Scholar] [CrossRef]
- Butrimas, G.; Šemeklis, L.; Paukštaitienė, R.; Dubinskaitė, A.; Cibulskaitė, R.; Janonytė, U.; Valiukevičienė, S. The Influence of Sun Exposure, Melanocytic Nevi, and Phenotypic Factors on the Development of Cutaneous Melanoma: A Case-Control Study from Lithuania. In JDDG: Journal der Deutschen Dermatologischen Gesellschaft, Proceedings of the 53rd Tagung der Deutschen Dermatologischen Gesellschaft, Berlin, Germany, 30 April–3 May 2025; Wiley: Hoboken, NJ, USA, 2025. [Google Scholar]
- Butrimas, G.; Šemeklis, L.; Paukštaitienė, R.; Dubinskaitė, A.; Janonytė, U.; Valiukevičienė, S. Melanocytic nevi and sun exposure as major risk factors for CM. Hospital-based study in Lithuania. In Proceedings of the 11th World Congress of Melanoma in Conjunction with 21st EADO Congress, Athens, Greece, 3–5 April 2025. [Google Scholar]
| Characteristics | Control Group | Case Group | p-Value |
|---|---|---|---|
| N = 182 | N = 180 | ||
| Sex, % (n) | |||
| Male | 37.9 (69) | 37.2 (67) | 0.892 |
| Female | 62.1 (113) | 62.8 (113) | |
| Total | 182 | 180 | |
| Age (years), mean (SD) | |||
| Male | 57.97 (8.20) | 60.70 (13.50) | 0.155 |
| Female | 57.34 (7.83) | 58.88 (11.89) | 0.249 |
| Total | 57.58 (7.95) | 59.56 (12.51) | 0.072 a |
| Number of melanocytic nevi, median (range) | |||
| Diameter 2–5 mm | 4 (0–131) | 19.5 (0–194) | <0.001 b |
| Diameter ≥ 5 mm | 0 (0–25) | 3 (0–51) | <0.001 b |
| Number of MN > 2 mm across varying sun-exposure areas, median (range) | |||
| Minimal | 0 (0–40) | 1 (0–28) | <0.001 b |
| Intermittent or maximal | 5 (0–135) | 23 (0–188) | |
| Freckles on face, shoulders, and dorsal surface of the hands, % (n) | |||
| <30 | 64.3 (117) | 42.8 (77) | <0.001 |
| ≥30 | 35.7 (65) | 57.2 (103) | |
| Skin colour, % (n) | |||
| Fair | 30.2 (54) | 48.6 (87) | <0.001 |
| Medium/olive | 69.8 (125) | 51.4 (92) | |
| Hair colour, % (n) | |||
| Blond/red | 10.1 (18) | 16.8 (30) | 0.069 |
| Light brown | 41.9 (75) | 45.3 (81) | |
| Dark brown/black | 48.0 (86) | 38.0 (68) | |
| Eye colour, % (n) | |||
| Blue/grey | 66.7 (118) | 65.9 (118) | 0.863 |
| Green | 19.2 (34) | 21.2 (38) | |
| Brown | 14.1 (25) | 12.8 (23) | |
| Skin type (Fitzpatrick scale), % (n) | |||
| Type I/Type II | 44.7 (80) | 41.3 (74) | 0.552 |
| Type III/Type IV | 55.3 (99) | 58.7 (105) | |
| Biological parent with ≥50 pigmented moles, % (n) | |||
| At least one | 31.7 (40) | 32.9 (46) | 0.847 |
| Neither | 68.3 (86) | 67.1 (94) | |
| Tanning bed use (≥1 time), % (n) | |||
| No | 95.6 (174) | 82.8 (149) | <0.001 |
| Yes | 4.4 (8) | 17.2 (31) | |
| Not using sunscreen outside on sunny days, % (n) | |||
| Yes | 66.7 (118) | 88.1 (156) | <0.001 |
| No | 33.3 (59) | 11.9 (21) | |
| Time spent daily at the beach while vacationing, % (n) | |||
| <2 h | 41.4 (70) | 23.9 (38) | <0.001 |
| ≥2 h | 58.6 (99) | 76.1 (121) | |
| Number of sunburns, median (range) | 0 (0–6) | 2 (0–6) | <0.001 b |
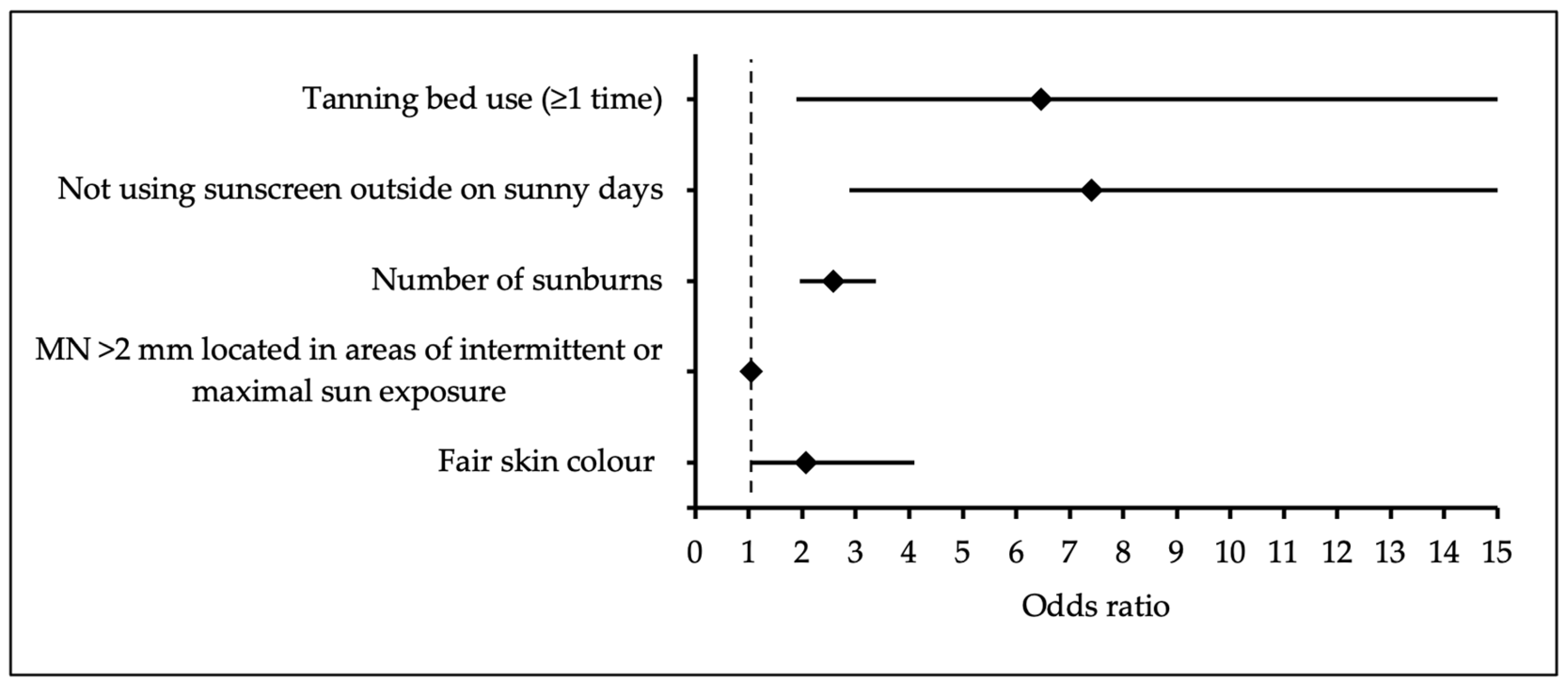
| Variable | B | Odds Ratio | 95% Confidence Interval | p-Value | |
|---|---|---|---|---|---|
| Lower Bound | Upper Bound | ||||
| Tanning bed use (≥1 time) | 1.87 | 6.46 | 1.89 | 0.004 | |
| 22.96 | |||||
| Not using sunscreen outside on sunny days | 2.003 | 7.41 | 2.88 | <0.001 | |
| 19.09 | |||||
| Number of sunburns | 0.946 | 2.57 | 1.96 | <0.001 | |
| 3.38 | |||||
| MN >2 mm located in areas of intermittent or maximal sun exposure | 0.051 | 1.05 | 1.03 | <0.001 | |
| 1.07 | |||||
| Fair skin colour | 0.722 | 2.06 | 1.03 | 0.040 | |
| 4.09 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butrimas, G.; Šemeklis, L.; Paukštaitienė, R.; Dubinskaitė, A.; Janonytė, U.; Lukšienė, D.; Valiukevičienė, S. Severe Sunburns and Sunbed Use Risk with Cutaneous Melanoma: A Case–Control Study in Lithuania. Medicina 2025, 61, 1941. https://doi.org/10.3390/medicina61111941
Butrimas G, Šemeklis L, Paukštaitienė R, Dubinskaitė A, Janonytė U, Lukšienė D, Valiukevičienė S. Severe Sunburns and Sunbed Use Risk with Cutaneous Melanoma: A Case–Control Study in Lithuania. Medicina. 2025; 61(11):1941. https://doi.org/10.3390/medicina61111941
Chicago/Turabian StyleButrimas, Grinvydas, Lukas Šemeklis, Renata Paukštaitienė, Augustė Dubinskaitė, Ugnė Janonytė, Dalia Lukšienė, and Skaidra Valiukevičienė. 2025. "Severe Sunburns and Sunbed Use Risk with Cutaneous Melanoma: A Case–Control Study in Lithuania" Medicina 61, no. 11: 1941. https://doi.org/10.3390/medicina61111941
APA StyleButrimas, G., Šemeklis, L., Paukštaitienė, R., Dubinskaitė, A., Janonytė, U., Lukšienė, D., & Valiukevičienė, S. (2025). Severe Sunburns and Sunbed Use Risk with Cutaneous Melanoma: A Case–Control Study in Lithuania. Medicina, 61(11), 1941. https://doi.org/10.3390/medicina61111941







