Insights into the Use of Erythrocyte and Platelet Distribution Indices for Assessing the Extent of Coronary Lesions
Abstract
1. Introduction
2. Materials and Methods
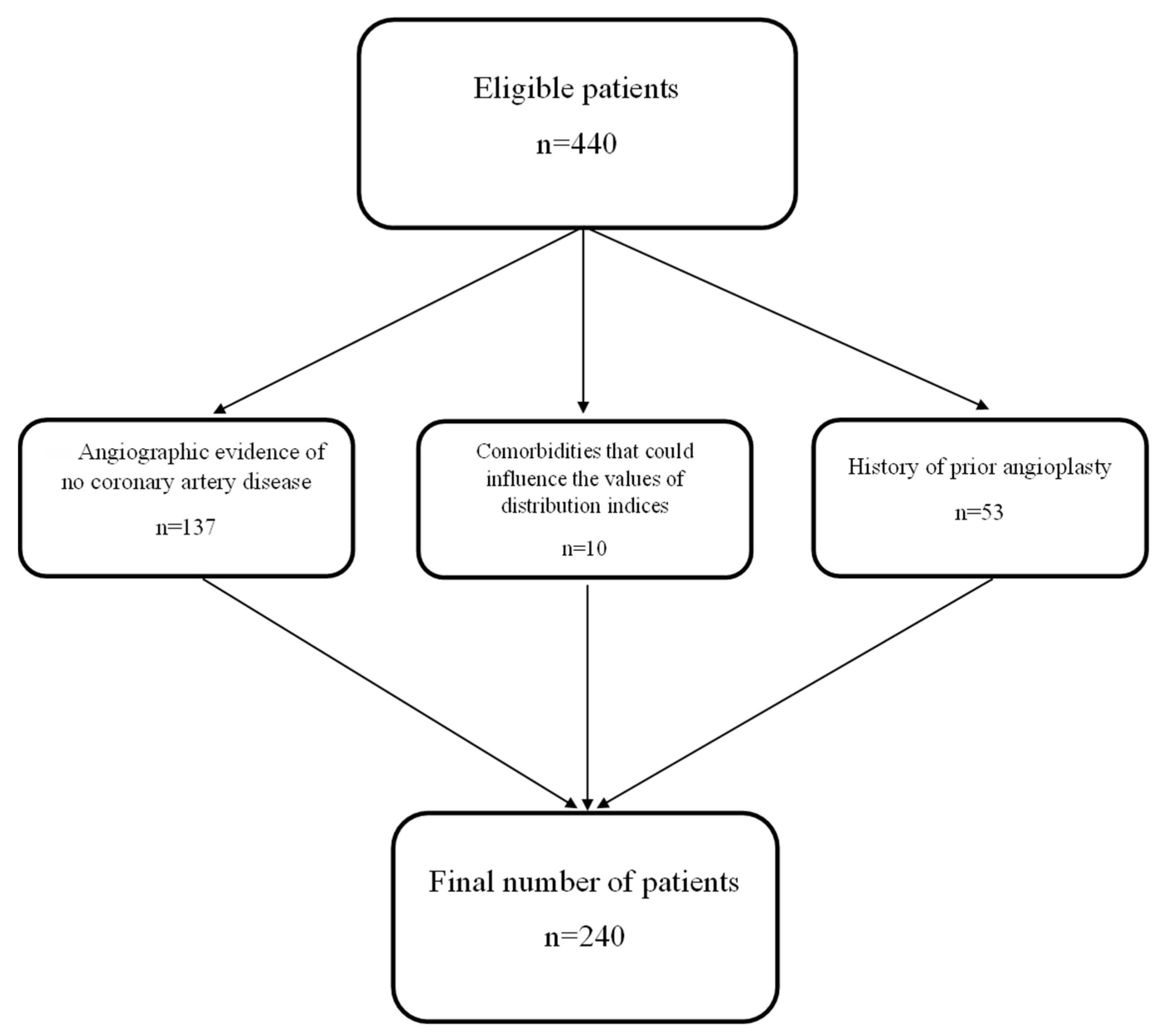
2.1. Study Design and Population
2.2. Angiographic Examination
2.3. Statistical Analyses
3. Results
3.1. Baseline Clinical Characteristics
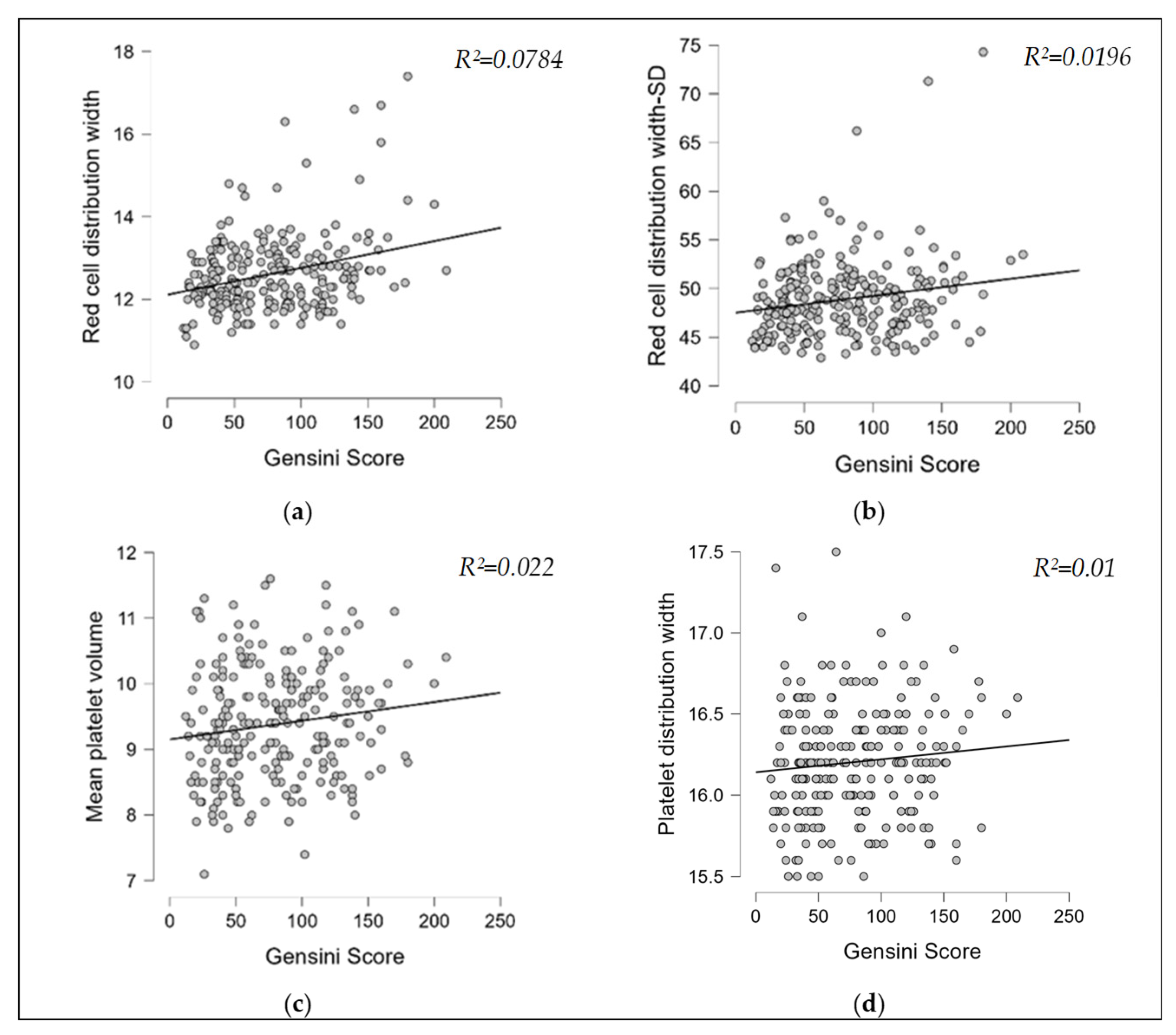
3.2. Correlation Analyses
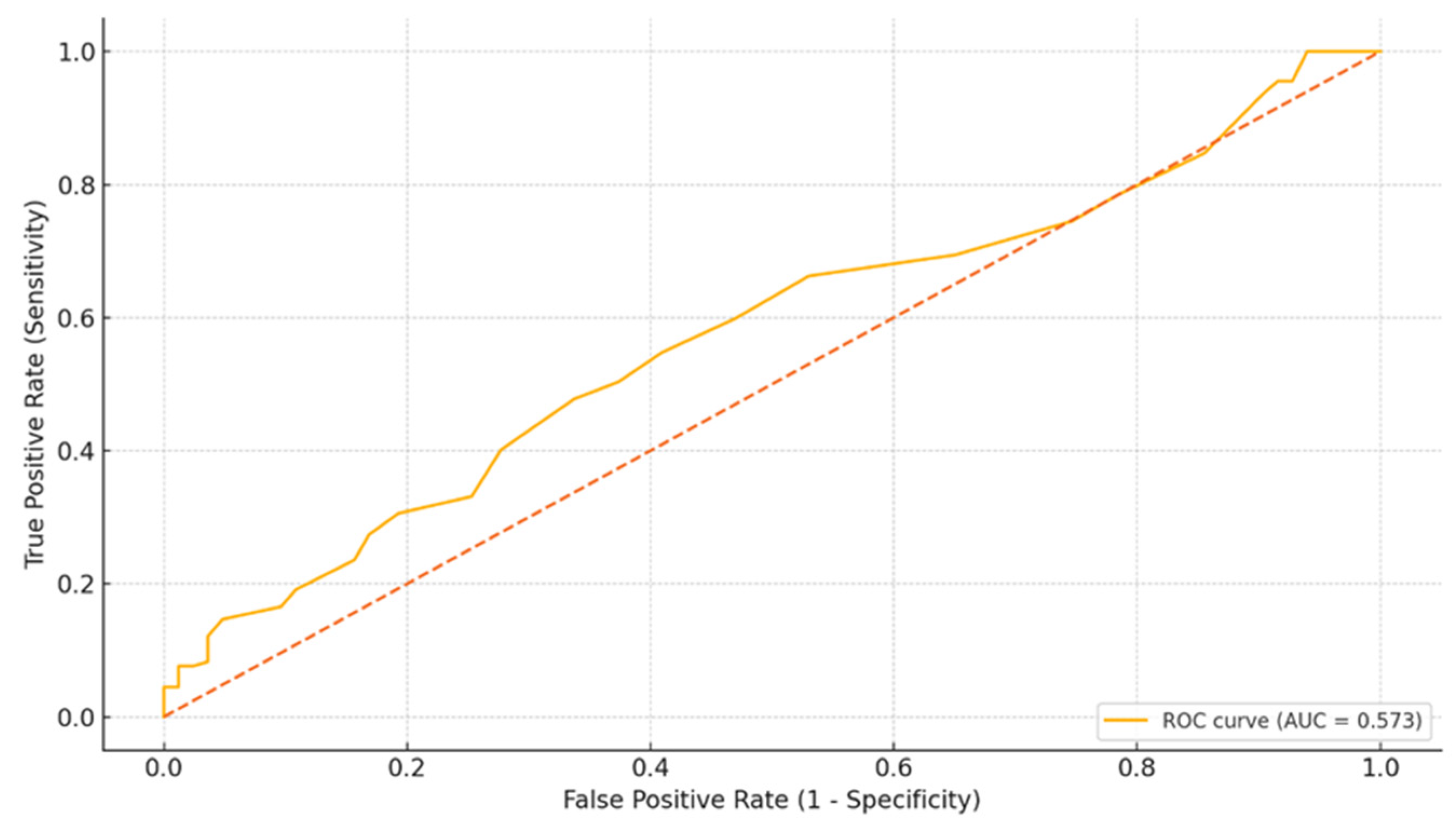
3.3. Receiver Operating Characteristic (ROC) Curve
3.4. Univariate and Multivariate Regression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MCV | Mean corpuscular volume |
| RDW | Red cell distribution width |
| RDW-SD | Red cell distribution width–standard deviation |
| PDW | Platelet distribution width |
| MPV | Mean platelet volume |
| CAD | Coronary artery disease |
| ROC | Receiver operating characteristics |
| AST | Aspartate Aminotransferase |
| ALT | Alanine Aminotransferase |
| TAPSE | Tricuspid annular plane systolic excursion |
| STEMI | ST-elevation myocardial infarction |
| LMCA | Left main coronary artery |
| LAD | Left anterior descending artery |
| LCX | Left circumflex artery |
| AUC | Area under the curve |
References
- Qiu, Q.; Zhu, G.; Peng, G.; Wang, L.; Lu, H.; Xu, Y.; Zhang, W.; Shen, Y.; Ye, Y.; Lv, Q.; et al. Correlation between Mean Platelet Volume and Gensini Score in Patients with Coronary Heart Disease in Different Diabetic States. Heart Surg. Forum 2023, 26, E680–E686. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, B.; Uysal, O.K.; Duran, M.; Sahin, D.Y.; Elbasan, Z.; Tekin, K.; Cagliyan, C.E.; Cayli, M. Relationship between Mean Platelet Volume and Atherosclerosis in Young Patients with ST Elevation Myocardial Infarction. Angiology 2013, 64, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Isik, T.; Ayhan, E.; Kurt, M.; Tanboga, I.H.; Kaya, A.; Aksakal, E. Is Red Cell Distribution Width a Marker for the Presence and Poor Prognosis of Cardiovascular Disease? Eurasian J. Med. 2012, 44, 169. [Google Scholar] [CrossRef]
- Wang, H.; Li, H.; Wang, Y.; Zhao, C.; Tian, Q.W.; Wang, Q.; He, G.W.; Lun, L.M.; Xuan, C. Hematological Parameters and Early-Onset Coronary Artery Disease: A Retrospective Case-Control Study Based on 3366 Participants. Ther. Adv. Chronic Dis. 2023, 14, 20406223221142670. [Google Scholar] [CrossRef]
- Vatankulu, M.A.; Sonmez, O.; Ertas, G.; Bacaksiz, A.; Turfan, M.; Erdogan, E.; Tasal, A.; Kul, S.; Uyarel, H.; Goktekin, O. A New Parameter Predicting Chronic Total Occlusion of Coronary Arteries: Platelet Distribution Width. Angiology 2014, 65, 60–64. [Google Scholar] [CrossRef]
- Khalil, A.; Shehata, M.; Abdeltawab, A.; Onsy, A. Red Blood Cell Distribution Width and Coronary Artery Disease Severity in Diabetic Patients. Future Cardiol. 2019, 15, 355–366. [Google Scholar] [CrossRef]
- Jiang, F.; Tang, Y.; Zhang, L.; Xiang, J.; Lei, J.; Feng, J.; Xu, W.; Tan, Y.; Yang, B.; Zhu, Y. Predictive Value of Red Blood Cell Distribution Width and Platelet-to-Lymphocyte Ratio for Acute Kidney Injury in Critically Ill Patients. Clin. Nephrol. 2023, 100, 157–164. [Google Scholar] [CrossRef]
- Jia, L.; Cui, S.; Yang, J.; Jia, Q.; Hao, L.; Jia, R.; Zhang, H. Red Blood Cell Distribution Width Predicts Long-Term Mortality in Critically Ill Patients with Acute Kidney Injury: A Retrospective Database Study. Sci. Rep. 2020, 10, 4563. [Google Scholar] [CrossRef]
- Rafiei Sorouri, Z.; Kabodmehri, R.; Milani, F.; Parvari, P. Red Cell Distribution Width and Mean Platelet Volume Detection in Patients with Endometrial Cancer and Endometrial Hyperplasia. Health Sci. Rep. 2024, 7, e70109. [Google Scholar] [CrossRef]
- Celik, A.; Karayakali, M.; Altunkas, F.; Karaman, K.; Arisoy, A.; Ceyhan, K.; Kadi, H.; Koc, F. Red Cell Distribution Width Is Correlated with Extensive Coronary Artery Disease in Patients with Diabetes Mellitus. Cardiovasc. J. Afr. 2017, 28, 319. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Feng, G.H.; Li, H.P.; Li, Q.L.; Fu, Y.; Huang, R. Bin Red Blood Cell Distribution Width and Ischaemic Stroke. Stroke Vasc. Neurol. 2017, 2, 172–175. [Google Scholar] [CrossRef]
- Li, N.; Zhou, H.; Tang, Q. Red Blood Cell Distribution Width: A Novel Predictive Indicator for Cardiovascular and Cerebrovascular Diseases. Dis. Markers 2017, 2017, 7089493. [Google Scholar] [CrossRef]
- Botros, L.; Qayyum, R. Association of Platelet Distribution Width with All-Cause and Cause-Specific Mortality in US Adults. Int. J. Cardiol. 2024, 407, 132100. [Google Scholar] [CrossRef]
- Khandekar, M.M.; Khurana, A.S.; Deshmukh, S.D.; Kakrani, A.L.; Katdare, A.D.; Inamdar, A.K. Platelet Volume Indices in Patients with Coronary Artery Disease and Acute Myocardial Infarction: An Indian Scenario. J. Clin. Pathol. 2006, 59, 146. [Google Scholar] [CrossRef] [PubMed]
- Demir, A.; Yarali, N.; Fisgin, T.; Duru, F.; Kara, A. Most Reliable Indices in Differentiation between Thalassemia Trait and Iron Deficiency Anemia. Pediatr. Int. 2002, 44, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Rampidis, G.P.; Benetos, G.; Benz, D.C.; Giannopoulos, A.A.; Buechel, R.R. A Guide for Gensini Score Calculation. Atherosclerosis 2019, 287, 181–183. [Google Scholar] [CrossRef] [PubMed]
- Bundhun, P.K.; Sookharee, Y.; Bholee, A.; Huang, F. Application of the SYNTAX Score in Interventional Cardiology: A Systematic Review and Meta-Analysis. Medicine 2017, 96, e7410. [Google Scholar] [CrossRef]
- Boyraz, B.; Peker, T. Comparison of SYNTAX and Gensini Scores in the Decision of Surgery or Percutaneous Revascularization in Patients with Multivessel Coronary Artery Disease. Cureus 2022, 14, e22482. [Google Scholar] [CrossRef]
- Gai, M.T.; Yan, S.Q.; Wang, M.Y.; Ruze, A.; Zhao, L.; Li, Q.L.; Zhao, B.H.; Deng, A.X.; Hu, S.; Gao, X.M. Comparison of Gensini Score and SYNTAX Score for Predicting In-Stent Restenosis in Patients with Coronary Artery Disease and Drug-Eluting Stent Implantation. Sci. Rep. 2025, 15, 1077. [Google Scholar] [CrossRef]
- Ganguly, P.; Alam, S.F. Role of Homocysteine in the Development of Cardiovascular Disease. Nutrients 2015, 14, 6. [Google Scholar] [CrossRef]
- Mueller, T.; Haltmayer, M.; Haidinger, D.; Luft, C.; Horvath, W.; Poelz, W. Association between Erythrocyte Mean Corpuscular Volume and Peripheral Arterial Disease in Male Subjects. Angiology 2001, 52, 605–613. [Google Scholar] [CrossRef]
- Liu, Y.; Geng, T.; Wan, Z.; Lu, Q.; Zhang, X.; Qiu, Z.; Li, L.; Zhu, K.; Liu, L.; Pan, A.; et al. Associations of Serum Folate and Vitamin B12 Levels with Cardiovascular Disease Mortality Among Patients with Type 2 Diabetes. JAMA Netw. Open 2022, 5, e2146124. [Google Scholar] [CrossRef]
- Peng, Y.F.; Pan, G.G. Red Blood Cell Distribution Width Predicts Homocysteine Levels in Adult Population without Vitamin B 12 and Folate Deficiencies. Int. J. Cardiol. 2017, 227, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Semba, R.D.; Patel, K.V.; Ferrucci, L.; Sun, K.; Roy, C.N.; Guralnik, J.M.; Fried, L.P. Serum Antioxidants and Inflammation Predict Red Cell Distribution Width in Older Women: The Women’s Health and Aging Study I. Clin. Nutr. 2010, 29, 600–604. [Google Scholar] [CrossRef]
- Kamath, S.; Blann, A.D.; Lip, G.Y.H. Platelet Activation: Assessment and Quantification. Eur. Heart J. 2001, 22, 1561–1571. [Google Scholar] [CrossRef] [PubMed]
- Giacco, F.; Brownlee, M. Oxidative Stress and Diabetic Complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Cetin, M.S.; Cetin, E.H.O.; Akdi, A.; Aras, D.; Topaloglu, S.; Temizhan, A.; Aydogdu, S. Platelet Distribution Width and Plateletcrit: Novel Biomarkers of ST Elevation Myocardial Infarction in Young Patients. Kardiol. Pol. 2017, 75, 1005–1012. [Google Scholar] [CrossRef]
- De Luca, G.; Venegoni, L.; Iorio, S.; Secco, G.G.; Cassetti, E.; Verdoia, M.; Schaffer, A.; Coppo, L.; Bellomo, G.; Marino, P. Platelet Distribution Width and the Extent of Coronary Artery Disease: Results from a Large Prospective Study. Platelets 2010, 21, 508–514. [Google Scholar] [CrossRef]
- Bekler, A.; Ozkan, M.T.A.; Tenekecioglu, E.; Gazi, E.; Yener, A.U.; Temiz, A.; Altun, B.; Barutcu, A.; Erbag, G.; Binnetoglu, E. Increased Platelet Distribution Width Is Associated with Severity of Coronary Artery Disease in Patients with Acute Coronary Syndrome. Angiology 2015, 66, 638–643. [Google Scholar] [CrossRef]
- Tzur, I.; Barchel, D.; Izhakian, S.; Swarka, M.; Garach-Jehoshua, O.; Krutkina, E.; Plotnikov, G.; Gorelik, O. Platelet Distribution Width: A Novel Prognostic Marker in an Internal Medicine Ward. J. Community Hosp. Intern. Med. Perspect. 2019, 9, 464–470. [Google Scholar] [CrossRef]
- Qiu, X.; Nair, M.G.; Jaroszewski, L.; Godzik, A. Deciphering Abnormal Platelet Subpopulations in COVID-19, Sepsis and Systemic Lupus Erythematosus through Machine Learning and Single-Cell Transcriptomics. Int. J. Mol. Sci. 2024, 25, 5941. [Google Scholar] [CrossRef] [PubMed]
- Vaseghi, G.; Heshmat-Ghahdarijani, K.; Taheri, M.; Ghasempoor, G.; Hajian, S.; Haghjooy-Javanmard, S.; Aliyari, R.; Shafiee, Z.; Shekarchizadeh, M.; Pourmoghadas, A.; et al. Hematological Inflammatory Markers in Patients with Clinically Confirmed Familial Hypercholesterolemia. Biomed. Res. Int. 2022, 2022, 5051434. [Google Scholar] [CrossRef]
- Icli, A.; Aksoy, F.; Nar, G.; Kaymaz, H.; Alpay, M.F.; Nar, R.; Guclu, A.; Arslan, A.; Dogan, A. Increased Mean Platelet Volume in Familial Hypercholesterolemia. Angiology 2016, 67, 146–150. [Google Scholar] [CrossRef]
- Cholidou, K.; Anagnostopoulos, N.; Bartziokas, K.; Vafeiadis, K.; Bakakos, A.; Vontetsianos, A.; Gogou, V.; Sotiropoulou, Z.; Anagnostopoulou, C.; Papasarantou, A.; et al. Correlation of Mean Platelet Volume and Red Blood Cell Distribution Width with Obstructive Sleep Apnoea Syndrome Severity. Lung India 2025, 42, 179–185. [Google Scholar] [CrossRef]
- Lin, G.; Dai, C.; Xu, K.; Wu, M. Predictive Value of Red Blood Cell Distribution Width and Atrial Diameter in Paroxysmal Atrial Fibrillation: A Cross-Sectional Study. Med. Sci. Monit. 2022, 28, e937802. [Google Scholar] [CrossRef]
- Nageen, S.; Shah, R.; Sharif, S.; Jamgochian, M.; Waqas, N.; Rao, B. Platelet Count, Mean Platelet Volume, and Red Cell Distribution Width as Markers for Psoriasis Severity. J. Drugs Dermatol. 2022, 21, 156–161. [Google Scholar] [CrossRef]
- Erkus, E.; Aktas, G.; Atak, B.M.; Kocak, M.Z.; Duman, T.T.; Savli, H. Haemogram Parameters in Vitamin D Deficiency. J. Coll. Physicians Surg. Pak. 2018, 28, 779–782. [Google Scholar]
- Kassa, E.; Enawgaw, B.; Gelaw, A.; Gelaw, B. Effect of Anti-Tuberculosis Drugs on Hematological Profiles of Tuberculosis Patients Attending at University of Gondar Hospital, Northwest Ethiopia. BMC Hematol. 2016, 16, 1. [Google Scholar] [CrossRef]
- Agrawal, A.; Bhardwaj, A.; Singh, S.; Budania, A.; Bains, A.; Sharma, S.; Purohit, A.; Rajan, M.B.; Rajagopal, V. Effect of Oral Doxycycline, Azithromycin and Isotretinoin on Haematological Inflammatory Markers and Interleukin-17A Levels in Acne Vulgaris: A Single Blinded Randomised Interventional Study. Arch. Dermatol. Res. 2024, 316, 697. [Google Scholar] [CrossRef] [PubMed]
- Demirbas, A.; Demirbas, G.U.; Diremsizoglu, E.; Islamoglu, G. Systemic Anti-Inflammatory Effects of Isotretinoin: Evaluation of Red Cell Distribution Width to Lymphocyte and Platelet Ratios as New Hematological Markers and Clinical Outcomes in Acne Vulgaris. J. Cosmet. Dermatol. 2025, 24, e70108. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | N = 240 |
|---|---|
| Male, % (N) | 81% (195) |
| Female, % (N) | 19% (45) |
| Smoker, % (N) | 38% (92) |
| Diabetes mellitus, % (N) | 30.83% (74) |
| Hypertension, % (N) | 95% (228) |
| Age, years–median (IQR) | 64 (56–70) |
| Gensini score–median (IQR) | 75 (40–110) |
| Diastolic dysfunction, % (N) | 81% (195) |
| Wall motion abnormality, % (N) | 55% (133) |
| Hypokinesia, % (N) | 29% (70) |
| Akinesia, % (N) | 26% (63) |
| Left ventricular ejection fraction, %, mean ± SD | 48.96 ± 7.12 |
| TAPSE, cm, mean ± SD | 2.18 ± 0.28 |
| AST, U/L, mean ± SD | 27.5 ± 12.2 |
| ALT, U/L–median (IQR) | 34 (27–46) |
| Creatinine, µmol/L–median (IQR) | 90.17 (76.02–106.96) |
| Natrium, mmol/L, mean ± SD | 139.73 ± 2.8 |
| Potassium, mmol/L, mean ± SD | 4.15 ± 0.45 |
| Chloride, mmol/L, mean ± SD | 101.89 ± 3.29 |
| Fasting blood glucose, mmol/L–median (IQR) | 6.1 (5.5–7.5) |
| Total cholesterol, mmol/L-median (IQR) | 3.98 (3.21–4.60) |
| Low-density lipoprotein, mmol/L-median (IQR) | 2.22 (1.60–2.77) |
| Triglyceride, mmol/L–median (IQR) | 1.16 (0.86–1.63) |
| Hemoglobin, g/dL, mean ± SD | 14.17 ± 1.5 |
| Mean corpuscular volume, fL, mean ± SD | 92.24 ± 4.97 |
| Red cell distribution width, %, mean ± SD | 12.61 ± 0.92 |
| Red cell distribution width-SD, fL, mean ± SD | 48.87 ± 4 |
| Mean platelet volume, fL, mean ± SD | 9.37 ± 0.86 |
| Platelet distribution width, %, mean ± SD | 16.2 ± 0.34 |
| Characteristics | Low Score < 50 N = 79 | High Score ≥ 50 N = 161 | p Value | CI (95%) |
|---|---|---|---|---|
| Male, % (N) | 74% (59) | 84% (136) | 0.06 | −0.58–21.68% |
| Smoker, % (N) | 45% (36) | 34% (56) | 0.10 | −1.95–23.92% |
| Age, years–median (IQR) | 62 (54–69) | 65 (56–71) | 0.04 | −6.0 to −0.2 |
| Diabetes mellitus, % (N) | 29% (23) | 31% (51) | 0.75 | −10.66–13.60% |
| Hypertension, % (N) | 94% (75) | 95% (153) | 0.74 | −4.70–8.93% |
| Diastolic dysfunction, % (N) | 67% (53) | 88% (142) | <0.001 * | 9.91–32.70% |
| Wall motion abnormality, % (N) | 46% (37) | 59% (96) | 0.057 | −0.37–25.82% |
| Left ventricular ejection fraction, %, mean ± SD | 51.51 ± 5.84 | 47.71 ± 7.36 | <0.001 * | 1.93–5.67 |
| TAPSE, cm, mean ± SD | 2.20 ± 0.29 | 2.18 ± 0.27 | 0.61 | −0.05–0.09 |
| AST, U/L, mean ± SD | 27.45 ± 12.39 | 27.52 ± 12.14 | 0.96 | −3.37–3.24 |
| ALT, U/L-median (IQR) | 35 (28–45) | 34 (27–46) | 0.68 | −3.00–5.00 |
| Creatinine, µmol/L–median (IQR) | 86 (73–103) | 93 (79–119) | 0.008 | −15.02 to −1.76 |
| Natrium, mmol/L, mean ± SD | 139.74 ± 2.44 | 139.72 ± 2.97 | 0.95 | −0.74–0.78 |
| Potassium, mmol/L, mean ± SD | 4.06 ± 0.37 | 4.19 ± 0.48 | 0.02 * | −0.23 to −0.01 |
| Chloride, mmol/L, mean ± SD | 101.94 ± 3.51 | 101.87 ± 3.19 | 0.86 | −0.81–0.97 |
| Fasting blood glucose, mmol/L–median (IQR) | 6.17 (5.39–7.17) | 6.11 (5.50–7.67) | 0.64 | −0.44–0.28 |
| Total cholesterol, mmol/L-median (IQR) | 4.09 (3.39–4.73) | 3.88 (3.08–4.55) | 0.10 | −0.07–0.59 |
| Low-density lipoprotein, mmol/L-median (IQR) | 2.22 (1.68–2.85) | 2.12 (1.60–2.74) | 0.37 | −0.13–0.36 |
| Triglyceride, mmol/L-median (IQR) | 1.17 (0.86–1.78) | 1.15 (0.86–1.52) | 0.42 | −0.09–0.25 |
| Hemoglobin, g/dL, mean ± SD | 14.18 ± 1.35 | 14.17 ± 1.57 | 0.97 | −0.40–0.41 |
| Mean corpuscular volume, fL, mean ± SD | 92.43 ± 4.85 | 92.14 ± 5.05 | 0.68 | −1.06–1.63 |
| Red cell distribution width,%, mean ± SD | 12.43 ± 0.68 | 12.70 ± 1.01 | 0.03 * | −0.52 to −0.02 |
| Red cell distribution width-SD, fL, mean ± SD | 48.28 ± 3.03 | 49.16 ± 4.37 | 0.11 | −1.95–0.20 |
| Mean platelet volume, fL, mean ± SD | 9.20 ± 0.88 | 9.45 ± 0.84 | 0.03 * | −0.48 to −0.02 |
| Platelet distribution width, %, mean ± SD | 16.16 ± 0.36 | 16.22 ± 0.33 | 0.20 | −0.15–0.03 |
| Spearman’s Rho (CI 95%) | p | ||
|---|---|---|---|
| Gensini score | RDW | 0.28 * (0.16–0.39) | 0.001 |
| RDW-SD | 0.14 * (0.01–0.26) | 0.028 | |
| MPV | 0.15 * (0.02–0.27) | 0.018 | |
| PDW | 0.10 (–0.03–0.22) | 0.100 | |
| MCV | −0.08 (–0.20–0.05) | 0.212 |
| Variable | Univariate β (CI 95%) p | Multivariate β (CI 95%) p | ||
|---|---|---|---|---|
| MCV | −0.953 (−2.04–0.13) | 0.087 | ||
| RDW | 13.829 (8.20–19.45) | <0.001 * | 16.44 (8.42–24.46) | <0.001 * |
| RDW-SD | 1.996 (0.65–3.33) | 0.004 * | −1.37 (−3.24–0.50) | 0.15 |
| MPV | 6.988 (0.71–13.26) | 0.029 * | 7.18 (1.24–13.11) | 0.01 * |
| PDW | 11.926 (−3.66–27.51) | 0.133 | ||
| Left ventricular ejection fraction | −1.336 (−2.08–−0.58) | <0.001 * | −0.852 (−1.77–0.06) | 0.06 |
| Diastolic dysfunction | 20.441 (6.70–34.17) | 0.004 * | 16.63 (3.11–30.15) | 0.01 * |
| Wall motion abnormality | 13.801 (2.96–24.63) | 0.013 * | 0.06 (−12.94–13.07) | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the Lithuanian University of Health Sciences. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zavragiu, A.-C.; Sutoi, D.; Radbea, O.-R.; Chiu, B.; Mailat, D.-E.; Ardelean, S.; Barzache, P.-A.; Dudau, I.; Mederle, O.-A.; Andor, M. Insights into the Use of Erythrocyte and Platelet Distribution Indices for Assessing the Extent of Coronary Lesions. Medicina 2025, 61, 1939. https://doi.org/10.3390/medicina61111939
Zavragiu A-C, Sutoi D, Radbea O-R, Chiu B, Mailat D-E, Ardelean S, Barzache P-A, Dudau I, Mederle O-A, Andor M. Insights into the Use of Erythrocyte and Platelet Distribution Indices for Assessing the Extent of Coronary Lesions. Medicina. 2025; 61(11):1939. https://doi.org/10.3390/medicina61111939
Chicago/Turabian StyleZavragiu, Andrei-Catalin, Dumitru Sutoi, Oana-Raluca Radbea, Bogdan Chiu, Diana-Evelyne Mailat, Samuel Ardelean, Petre-Adrian Barzache, Ionut Dudau, Ovidiu-Alexandru Mederle, and Minodora Andor. 2025. "Insights into the Use of Erythrocyte and Platelet Distribution Indices for Assessing the Extent of Coronary Lesions" Medicina 61, no. 11: 1939. https://doi.org/10.3390/medicina61111939
APA StyleZavragiu, A.-C., Sutoi, D., Radbea, O.-R., Chiu, B., Mailat, D.-E., Ardelean, S., Barzache, P.-A., Dudau, I., Mederle, O.-A., & Andor, M. (2025). Insights into the Use of Erythrocyte and Platelet Distribution Indices for Assessing the Extent of Coronary Lesions. Medicina, 61(11), 1939. https://doi.org/10.3390/medicina61111939







