The Role of Lymph Node Downstaging Following Neoadjuvant Treatment in a Group of Patients with Advanced Stage Cervical Cancer
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Crosbie, E.J.; Einstein, M.H.; Franceschi, S.; Kitchener, H.C. Human Papillomavirus and Cervical Cancer. Lancet 2013, 382, 889–899. [Google Scholar] [CrossRef]
- Everett, T.; Bryant, A.; Griffin, M.F.; Martin-Hirsch, P.P.; Forbes, C.A.; Jepson, R.G. Interventions Targeted at Women to Encourage the Uptake of Cervical Screening. Cochrane Database Syst. Rev. 2011, 5, CD002834. [Google Scholar] [CrossRef]
- Gilles, C.; Konopnicki, D.; Rozenberg, S. The Recent Natural History of Human Papillomavirus Cervical Infection in Women Living with HIV: A Scoping Review of Meta-analyses and Systematic Reviews and the Construction of a Hypothetical Model. HIV Med. 2023, 24, 877–892. [Google Scholar] [CrossRef]
- Olusola, P.; Banerjee, H.N.; Philley, J.V.; Dasgupta, S. Human Papilloma Virus-Associated Cervical Cancer and Health Disparities. Cells 2019, 8, 622. [Google Scholar] [CrossRef] [PubMed]
- Wichmann, I.A.; Cuello, M.A. Obesity and Gynecological Cancers: A Toxic Relationship. Int. J. Gynecol. Obstet. 2021, 155, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Roura, E.; Castellsagué, X.; Pawlita, M.; Travier, N.; Waterboer, T.; Margall, N.; Bosch, F.X.; de Sanjosé, S.; Dillner, J.; Gram, I.T.; et al. Smoking as a Major Risk Factor for Cervical Cancer and Pre-Cancer: Results from the EPIC Cohort. Int. J. Cancer 2014, 135, 453–466. [Google Scholar] [CrossRef]
- Tekalegn, Y.; Sahiledengle, B.; Woldeyohannes, D.; Atlaw, D.; Degno, S.; Desta, F.; Bekele, K.; Aseffa, T.; Gezahegn, H.; Kene, C. High Parity Is Associated with Increased Risk of Cervical Cancer: Systematic Review and Meta-Analysis of Case-Control Studies. Womens Health 2022, 18, 17455065221075904. [Google Scholar] [CrossRef] [PubMed]
- Dicu-Andreescu, I.-G.; Marincaș, M.-A.; Prunoiu, V.-M.; Dicu-Andreescu, I.; Ionescu, S.-O.; Simionescu, A.-A.; Brătucu, E.; Simion, L. The Impact of Patient Characteristics, Risk Factors, and Surgical Intervention on Survival in a Cohort of Patients Undergoing Neoadjuvant Treatment for Cervical Cancer. Medicina 2023, 59, 2147. [Google Scholar] [CrossRef]
- Ghebre, R.G.; Grover, S.; Xu, M.J.; Chuang, L.T.; Simonds, H. Cervical Cancer Control in HIV-Infected Women: Past, Present and Future. Gynecol. Oncol. Rep. 2017, 21, 101–108. [Google Scholar] [CrossRef]
- Foran, C.; Brennan, A. Prevention and Early Detection of Cervical Cancer in the UK. Br. J. Nurs. 2015, 24, S22–S24, S26, S28–S29. [Google Scholar] [CrossRef]
- Lei, J.; Arroyo-Mühr, L.S.; Lagheden, C.; Eklund, C.; Nordqvist Kleppe, S.; Elfström, M.; Andrae, B.; Sparén, P.; Dillner, J.; Sundström, K. Human Papillomavirus Infection Determines Prognosis in Cervical Cancer. J. Clin. Oncol. 2022, 40, 1522–1528. [Google Scholar] [CrossRef]
- Cervical Cancer Statistics by Age. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/cervical-cancer/incidence#heading-One (accessed on 15 February 2024).
- Hull, R.; Mbele, M.; Makhafola, T.; Hicks, C.; Wang, S.; Reis, R.; Mehrotra, R.; Mkhize-Kwitshana, Z.; Kibiki, G.; Bates, D.; et al. Cervical Cancer in Low and Middle-income Countries (Review). Oncol. Lett. 2020, 20, 2058–2074. [Google Scholar] [CrossRef]
- NCCN Guidelines for Cervical Cancer. Available online: https://www.nccn.org/professionals/physician_gls/pdf/cervical.pdf (accessed on 18 June 2023).
- Marth, C.; Landoni, F.; Mahner, S.; McCormack, M.; Gonzalez-Martin, A.; Colombo, N. Cervical Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2017, 28, iv72–iv83. [Google Scholar] [CrossRef]
- Cibula, D.; Raspollini, M.R.; Planchamp, F.; Centeno, C.; Chargari, C.; Felix, A.; Fischerová, D.; Jahnn-Kuch, D.; Joly, F.; Kohler, C.; et al. ESGO/ESTRO/ESP Guidelines for the Management of Patients with Cervical Cancer—Update 2023*. Int. J. Gynecol. Cancer 2023, 33, 649–666. [Google Scholar] [CrossRef]
- Perelli, F.; Mattei, A.; Scambia, G.; Cavaliere, A.F. Editorial: Methods in Gynecological Oncology. Front. Oncol. 2023, 13, 1167088. [Google Scholar] [CrossRef]
- Áyen, Á.; Jiménez Martínez, Y.; Boulaiz, H. Targeted Gene Delivery Therapies for Cervical Cancer. Cancers 2020, 12, 1301. [Google Scholar] [CrossRef]
- Ramirez, P.T.; Pareja, R.; Rendón, G.J.; Millan, C.; Frumovitz, M.; Schmeler, K.M. Management of Low-Risk Early-Stage Cervical Cancer: Should Conization, Simple Trachelectomy, or Simple Hysterectomy Replace Radical Surgery as the New Standard of Care? Gynecol. Oncol. 2014, 132, 254–259. [Google Scholar] [CrossRef]
- Querleu, D.; Cibula, D.; Abu-Rustum, N.R. 2017 Update on the Querleu-Morrow Classification of Radical Hysterectomy. Ann. Surg. Oncol. 2017, 24, 3406–3412. [Google Scholar] [CrossRef]
- Dicu-Andreescu, I.-G.; Marincaș, A.-M.; Ungureanu, V.-G.; Ionescu, S.-O.; Prunoiu, V.-M.; Brătucu, E.; Simion, L. Current Therapeutic Approaches in Cervical Cancer Based on the Stage of the Disease: Is There Room for Improvement? Medicina 2023, 59, 1229. [Google Scholar] [CrossRef]
- Querleu, D.; Morrow, C.P. Classification of Radical Hysterectomy. Lancet Oncol. 2008, 9, 297–303. [Google Scholar] [CrossRef]
- Palfalvi, L.; Ungar, L. Laterally Extended Parametrectomy (LEP), the Technique for Radical Pelvic Side Wall Dissection: Feasibility, Technique and Results. Int. J. Gynecol. Cancer 2003, 13, 914–917. [Google Scholar] [CrossRef]
- Nagy, V.; Rancea, A.; Coza, O.; Kacso, G.; Aldea, B. Alexandru Eniu Ghid MS Conduita Cancer Col Uterin. Available online: http://old.ms.ro/index.php?pag=181&pg=5 (accessed on 4 March 2024).
- Cao, L.; Kong, W.; Li, J.; Song, D.; Jin, B.; Liu, T.; Han, C. Analysis of Lymph Node Metastasis and Risk Factors in 975 Patients with FIGO 2009 Stage IA–IIA Cervical Cancer. Gynecol. Obstet. Investig. 2023, 88, 30–36. [Google Scholar] [CrossRef]
- Ronsini, C.; Anchora, L.P.; Restaino, S.; Fedele, C.; Arciuolo, D.; Teodorico, E.; Bizzarri, N.; Zannoni, G.F.; Ferrandina, G.; Scambia, G.; et al. The Role of Semiquantitative Evaluation of Lympho-Vascular Space Invasion in Early Stage Cervical Cancer Patients. Gynecol. Oncol. 2021, 162, 299–307. [Google Scholar] [CrossRef]
- Luo, L.; Luo, Q.; Tang, L. Diagnostic Value and Clinical Significance of MRI and CT in Detecting Lymph Node Metastasis of Early Cervical Cancer. Oncol. Lett. 2020, 19, 700–706. [Google Scholar] [CrossRef]
- Zhu, Y.; Shen, B.; Pei, X.; Liu, H.; Li, G. CT, MRI, and PET Imaging Features in Cervical Cancer Staging and Lymph Node Metastasis. Am. J. Transl. Res. 2021, 13, 10536–10544. [Google Scholar]
- Kondo, E.; Yoshida, K.; Tabata, T.; Kobayashi, Y.; Yamagami, W.; Ebina, Y.; Kaneuchi, M.; Nagase, S.; Machida, H.; Mikami, M. Comparison of Treatment Outcomes of Surgery and Radiotherapy, Including Concurrent Chemoradiotherapy for Stage Ib2-IIb Cervical Adenocarcinoma Patients: A Retrospective Study. J. Gynecol. Oncol. 2022, 33, e14. [Google Scholar] [CrossRef]
- Voinea, S.; Herghelegiu, C.; Sandru, A.; Ioan, R.; Bohilțea, R.; Bacalbașa, N.; Chivu, L.; Furtunescu, F.; Stanica, D.; Neacșu, A. Impact of Histological Subtype on the Response to Chemoradiation in Locally Advanced Cervical Cancer and the Possible Role of Surgery. Exp. Ther. Med. 2020, 21, 93. [Google Scholar] [CrossRef]
- Kaidar-Person, O.; Yosefia, S.; Abdah-Bortnyak, R. Response of Adenocarcinoma of the Uterine Cervix to Chemoradiotherapy. Oncol. Lett. 2015, 9, 2791–2794. [Google Scholar] [CrossRef]
- Kang, J.-H.; Cho, W.K.; Yeo, H.J.; Jeong, S.Y.; Noh, J.J.; Shim, J.I.; Lee, Y.-Y.; Kim, T.-J.; Lee, J.-W.; Kim, B.-G.; et al. Prognostic Significance of Tumor Regression Rate during Concurrent Chemoradiotherapy in Locally Advanced Cervix Cancer: Analysis by Radiation Phase and Histologic Type. J. Clin. Med. 2020, 9, 3471. [Google Scholar] [CrossRef]
- Wakatsuki, M.; Ohno, T.; Kato, S.; Ando, K.; Noda, S.-e.; Kiyohara, H.; Shibuya, K.; Karasawa, K.; Kamada, T.; Nakano, T. Impact of Boost Irradiation on Pelvic Lymph Node Control in Patients with Cervical Cancer. J. Radiat. Res. 2014, 55, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Serkies, K.; Badzio, A.; Jassem, J. Clinical Relevance of Hemoglobin Level in Cervical Cancer Patients Administered Definitive Radiotherapy. Acta Oncol. 2006, 45, 695–701. [Google Scholar] [CrossRef]
- Mercadante, S.; Gebbia, V.; Marrazzo, A.; Filosto, S. Anaemia in Cancer: Pathophysiology and Treatment. Cancer Treat. Rev. 2000, 26, 303–311. [Google Scholar] [CrossRef]
- Dunst, J.; Kuhnt, T.; Strauss, H.G.; Krause, U.; Pelz, T.; Koelbl, H.; Haensgen, G. Anemia in Cervical Cancers: Impact on Survival, Patterns of Relapse, and Association with Hypoxia and Angiogenesis. Int. J. Radiat. Oncol. Biol. Phys. 2003, 56, 778–787. [Google Scholar] [CrossRef] [PubMed]
- Caro, J.J.; Salas, M.; Ward, A.; Goss, G. Anemia as an Independent Prognostic Factor for Survival in Patients with Cancer: A Systemic, Quantitative Review. Cancer 2001, 91, 2214–2221. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Kong, W.; Chen, S.; Zhao, X.; Luo, D.; Xie, Y. Surgical Staging of Locally Advanced Cervical Cancer: Current Status and Research Progress. Front. Oncol. 2022, 12, 940807. [Google Scholar] [CrossRef] [PubMed]
- Arbyn, M.; Weiderpass, E.; Bruni, L.; de Sanjosé, S.; Saraiya, M.; Ferlay, J.; Bray, F. Estimates of Incidence and Mortality of Cervical Cancer in 2018: A Worldwide Analysis. Lancet Glob. Health 2020, 8, e191–e203. [Google Scholar] [CrossRef] [PubMed]
- Vora, C.; Gupta, S. Targeted Therapy in Cervical Cancer. ESMO Open 2018, 3, e000462. [Google Scholar] [CrossRef] [PubMed]
- Watkins, D.E.; Craig, D.J.; Vellani, S.D.; Hegazi, A.; Fredrickson, K.J.; Walter, A.; Stanbery, L.; Nemunaitis, J. Advances in Targeted Therapy for the Treatment of Cervical Cancer. J. Clin. Med. 2023, 12, 5992. [Google Scholar] [CrossRef]
- Qin, C.; Chen, X.; Bai, Q.; Davis, M.R.; Fang, Y. Factors Associated with Radiosensitivity of Cervical Cancer. Anticancer Res. 2014, 34, 4649–4656. [Google Scholar]
- Singh, U.; Verma, M.L.; Rahman, Z.; Qureshi, S.; Srivastava, K. Factors Affecting Quality of Life of Cervical Cancer Patients: A Multivariate Analysis. J. Cancer Res. Ther. 2019, 15, 1338–1344. [Google Scholar] [CrossRef]
- Osann, K.; Hsieh, S.; Nelson, E.L.; Monk, B.J.; Chase, D.; Cella, D.; Wenzel, L. Factors Associated with Poor Quality of Life among Cervical Cancer Survivors: Implications for Clinical Care and Clinical Trials. Gynecol. Oncol. 2014, 135, 266–272. [Google Scholar] [CrossRef]
- Mereu, L.; Pecorino, B.; Ferrara, M.; Tomaselli, V.; Scibilia, G.; Scollo, P. Neoadjuvant Chemotherapy plus Radical Surgery in Locally Advanced Cervical Cancer: Retrospective Single-Center Study. Cancers 2023, 15, 5207. [Google Scholar] [CrossRef]
- Cui, H.; Huang, Y.; Wen, W.; Li, X.; Xu, D.; Liu, L. Prognostic Value of Lymph Node Ratio in Cervical Cancer: A Meta-Analysis. Medicine 2022, 101, e30745. [Google Scholar] [CrossRef]
- Sun, C.; Wang, S.; Ye, W.; Wang, R.; Tan, M.; Zhang, H.; Zhou, J.; Li, M.; Wei, L.; Xu, P.; et al. The Prognostic Value of Tumor Size, Volume and Tumor Volume Reduction Rate During Concurrent Chemoradiotherapy in Patients With Cervical Cancer. Front. Oncol. 2022, 12, 934110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, X.; Li, Z.; Wang, W.; Wang, L. Score for the Overall Survival Probability of Patients With First-Diagnosed Distantly Metastatic Cervical Cancer: A Novel Nomogram-Based Risk Assessment System. Front. Oncol. 2019, 9, 1106. [Google Scholar] [CrossRef] [PubMed]
- Berman, M.L.; Keys, H.; Creasman, W.; DiSaia, P.; Bundy, B.; Blessing, J. Survival and Patterns of Recurrence in Cervical Cancer Metastatic to Periaortic Lymph Nodes. Gynecol. Oncol. 1984, 19, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Mileshkin, L.R.; Moore, K.N.; Barnes, E.H.; Lee, Y.C.; Gebski, V.; Narayan, K.; Bradshaw, N.; Diamante, K.; Fyles, A.W.; Small, W.; et al. Staging Locally Advanced Cervical Cancer with FIGO 2018 versus FIGO 2008: Impact on Overall Survival and Progression-Free Survival in the OUTBACK Trial (ANZGOG 0902, RTOG 1174, NRG 0274). J. Clin. Oncol. 2022, 40, 5531. [Google Scholar] [CrossRef]
- Ronsini, C.; De Franciscis, P.; Carotenuto, R.M.; Pasanisi, F.; Cobellis, L.; Colacurci, N. The Oncological Implication of Sentinel Lymph Node in Early Cervical Cancer: A Meta-Analysis of Oncological Outcomes and Type of Recurrences. Medicina 2022, 58, 1539. [Google Scholar] [CrossRef]
- Ronsini, C.; Köhler, C.; De Franciscis, P.; La Verde, M.; Mosca, L.; Solazzo, M.C.; Colacurci, N. Laparo-Assisted Vaginal Radical Hysterectomy as a Safe Option for Minimal Invasive Surgery in Early Stage Cervical Cancer: A Systematic Review and Meta-Analysis. Gynecol. Oncol. 2022, 166, 188–195. [Google Scholar] [CrossRef]
- Ronsini, C.; Solazzo, M.C.; Molitierno, R.; De Franciscis, P.; Pasanisi, F.; Cobellis, L.; Colacurci, N. Fertility-Sparing Treatment for Early-Stage Cervical Cancer ≥ 2 Cm: Can One Still Effectively Become a Mother? A Systematic Review of Fertility Outcomes. Ann. Surg. Oncol. 2023, 30, 5587–5596. [Google Scholar] [CrossRef] [PubMed]
- Ronsini, C.; Solazzo, M.C.; Bizzarri, N.; Ambrosio, D.; La Verde, M.; Torella, M.; Carotenuto, R.M.; Cobellis, L.; Colacurci, N.; De Franciscis, P. Fertility-Sparing Treatment for Early-Stage Cervical Cancer ≥ 2 Cm: A Problem with a Thousand Nuances-A Systematic Review of Oncological Outcomes. Ann. Surg. Oncol. 2022, 29, 8346–8358. [Google Scholar] [CrossRef] [PubMed]
- Scotti, R.J.; Bergman, A.; Bhatia, N.N.; Ostergard, D.R. Urodynamic Changes in Urethrovesical Function After Radical Hysterectomy. Obstet. Gynecol. 1986, 68, 111–120. [Google Scholar] [PubMed]
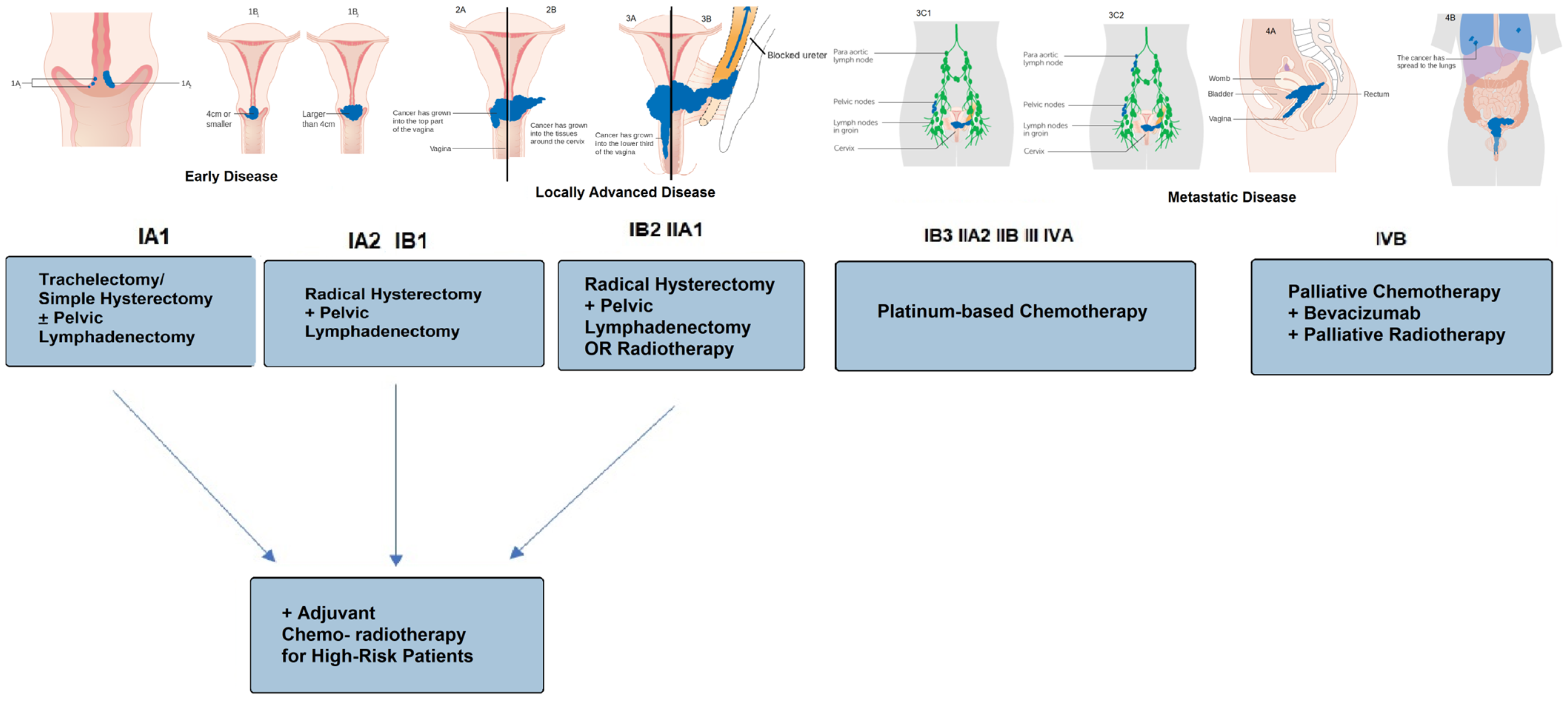
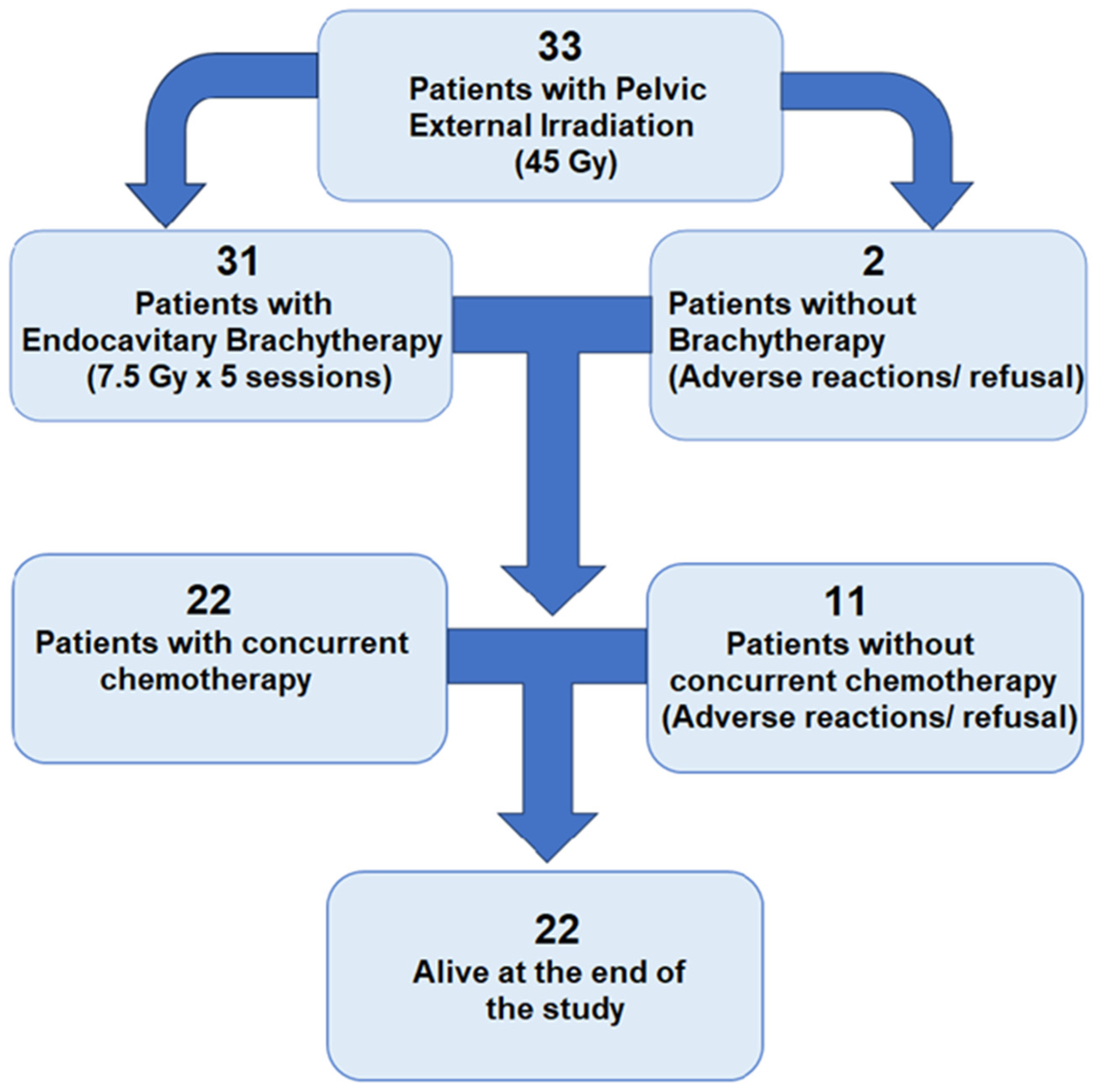
| Total (33) | Alive at 3 Years (22) | Deceased at 3 Years (11) | p-Value | |
|---|---|---|---|---|
| Age, years, median (SD) | 55.3 (11.5) | 56 (14) | 54 (26) | 0.895 |
| Environment, n (%) | urban: 12 (36) rural: 21 (64) | urban: 8 (36) rural: 14 (64) | urban: 4 (36) rural: 7 (64) | 0.801 |
| Histological types of cancer, (biopsy) n (%) | 1. Squamous cell carcinoma 30 (90) 2. Adenocarcinoma-2 (6) 3. Adenosquamous carcinoma-1 (3) | 1. Squamous cell Carcinoma-20 (90) 2. Adenocarcinoma-1 (4) 3. Adenosquamous Carcinoma-1 (4) | 1. Squamous cell carcinoma-10 (90) 2. Adenocarcinoma-1 (9) | 0.687 |
| Pre-RT FIGO stage, n (%) | III C1 33 (100) | IIIC1: 22 (100) | IIIC1: 11 (100) | 1 |
| Pre-RT Parametrial Invasion n (%) | 19 (57) | 11 (50) | 8 (72) | 0.210 |
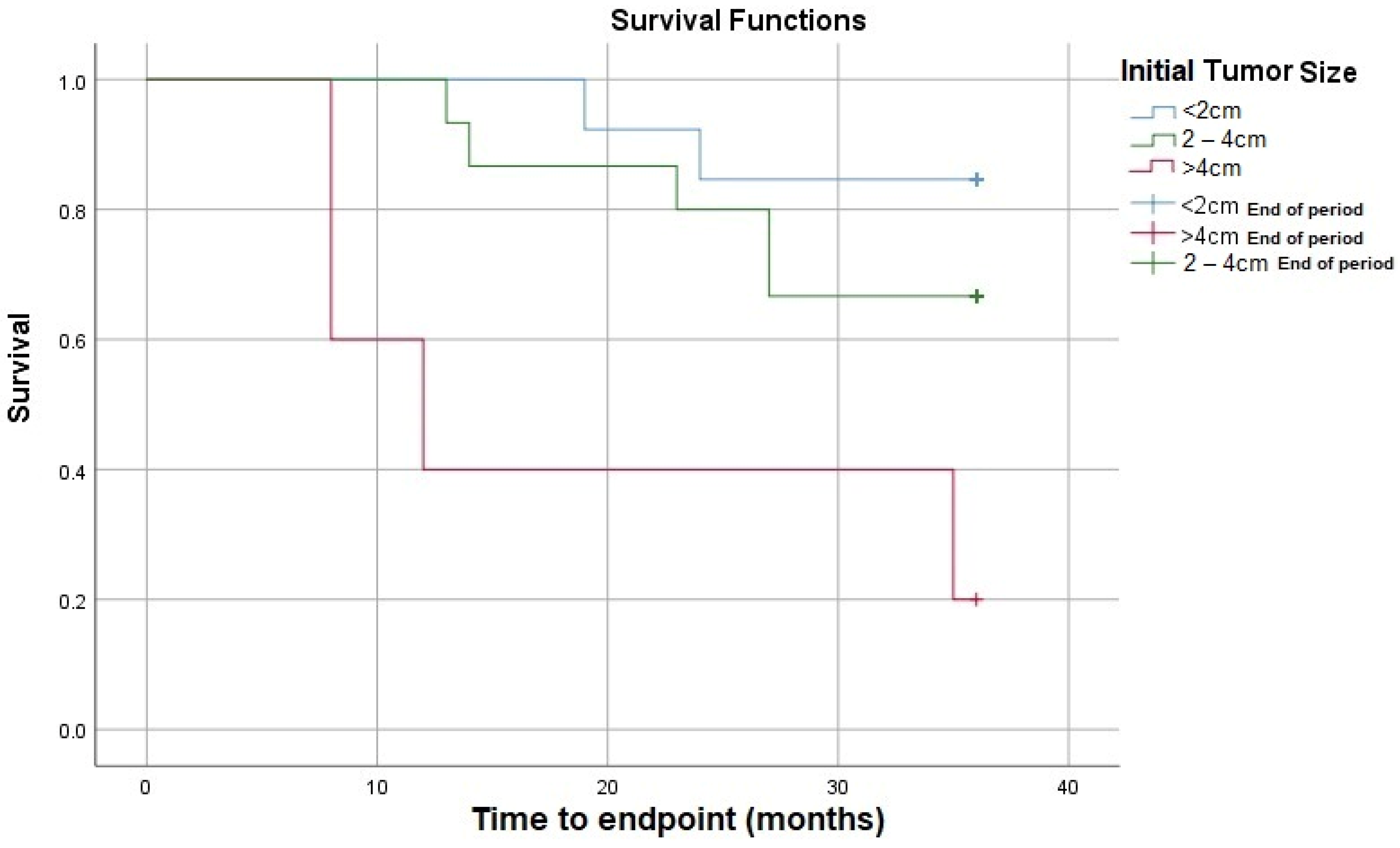
| Initial Tumor size (cm), median (IQR) | 2 (1.6) | 1.95 (0.9) | 3 (2.5) | 0.048 |
| Pre-RT leukocyte count, median (IQR) | 6200 (2000) | 6350 (2100) | 6050 (2700) | 0.825 |
| Pre-RT hemoglobin, mean (SD) | 12.5 (1.4) | 12.8 (1.1) | 11.5 (1.3) | 0.03 |
| Post-Neoadjuvant Treatment Adverse Reactions | Alive at 3 Years after Treatment | Dead at 3 Years after Treatment | p-Value |
|---|---|---|---|
| Radiation colitis | 0 | 1 | 0.33 |
| Radiation cystitis | 0 | 4 | 0.008 |
| Anemia | 6 | 7 | 0.06 |
| Leukopenia | 1 | 3 | 0.09 |
| Total (33) | Alive at 3 Years (22) | Deceased at 3 Years (11) | p-Value | |
|---|---|---|---|---|
| RT dose, median (IQR) | 50 (0.2) | 50 (0.4) | 50 (0) | 0.611 |
| Nr. of RT sessions, median (IQR) | 25 (1) | 25 (1) | 25 (1) | 0.807 |
| Sensitization chemotherapy, median (IQR) | 5 (5) | 5 (5) | 3 (5) | 0.440 |
| Postoperatively chemotherapy, n (%) | 10 (30) | 5 (22) | 5 (45) | 0.181 |
| Total (33) | Alive at 3 Years (22) | Deceased at 3 Years (11) | p-Value | |
|---|---|---|---|---|
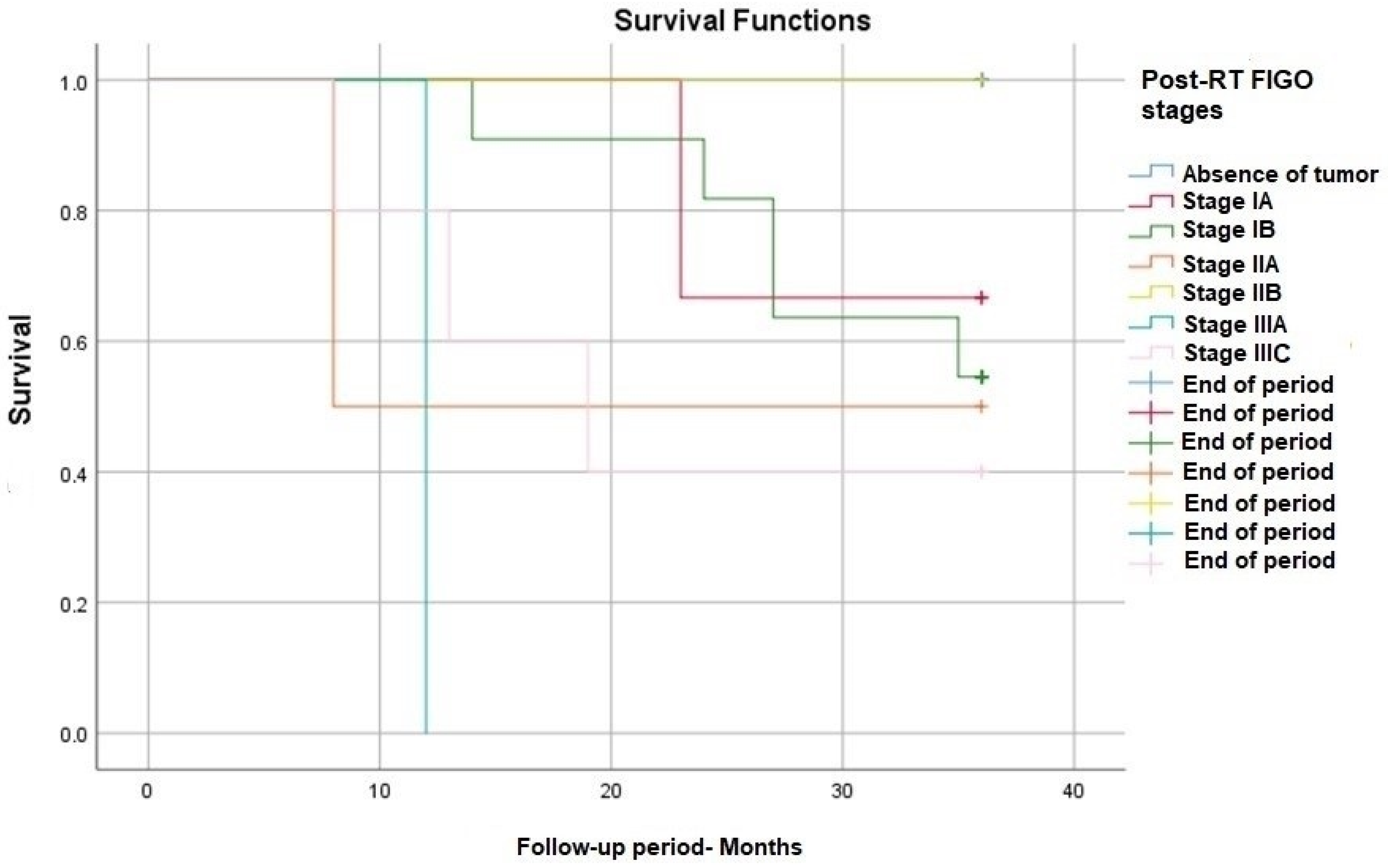
| Post-RT FIGO stage, n (%) | Absence of tumor: 10 (30) IA1-2 (6) IA2-1 (3) IB1-10 (30) IB2-1 (3) IIA1-1 (3) IIA2-1 (3) IIB-1 (3) IIIA-1 (3) IIIC1-5 (15) | Absence of tumor: 10 (45) IA1-2 (9) IA2-0 (0) IB1-6 (27) IB2-0 (0) IIA1-1 (4) IIA2-0 (0) IIB-1 (5) IIIA-0 (0) IIIC1-2 (9) | Absence of tumor: 0 (0) IA1-0 (0) IA2-1 (9) IB1-4 (36) IB2-1 (9) IIA1-0 (0) IIA2-1 (9) IIB-0 (0) IIIA-1 (9) IIIC1-3 (27) | 0.121 |
| Post-RT lymphadenopathy, n (%) | 5 (15) | 2 (9) | 3 (27) | 0.304 |
| Preoperative leukocyte count, median (IQR) | 5600 (2100) | 5630 (2070) | 5600 (3500) | 0.721 |
| Preoperative hemoglobin, mean (SD) | 11.9 (1.4) | 12.3 (1.2) | 11 (1.4) | 0.02 |
| Intraoperative FIGO stage, n (%) | Absence of tumor: 15 (45) IA1-2 (6) IA2-3 (9) IB1-3 (9) IB2-0 (0) IIA1-1 (3) IIA2-1 (3) IIB-1 (3) IIIA-1 (3) IIIC1-4 (12) | Absence of tumor: 13 (59) IA1-1 (4) IA2-2 (9) IB1-1 (4) IB2-0 (0) IIA1-1 (4) IIA2-0 (0) IIB-1 (4) IIIA-0 (0) IIIC1-1 (4) | Absence of tumor: 2 (18) IA1-1 (9) IA2-1 (9) IB1-2 (18) IB2-0 (0) IIA1-0 (0) IIA2-1 (9) IIB-0 (0) IIIA-1 (9) IIIC1-3 (27) | 0.119 |
| Intraoperative histological type of cancer n (%) | Absence of tumor: 15 (45) In situ carcinoma: 1 (3) Squamous cell carcinoma: 15 (45) 2. Adenocarcinoma: 1 (3) 3. Adenosquamous Carcinoma: 1 (3) | Absence of tumor: 13 (59) In situ carcinoma: 0 (0) Squamous cell carcinoma: 8 (36) 2. Adenocarcinoma: 0 (0) 3. Adenosquamous Carcinoma: 1 (4) | Absence of tumor: 2 (18) In situ carcinoma: 1 (9) Squamous cell Carcinoma: 7 (63) 2. Adenocarcinoma: 1 (9) 3. Adenosquamous Carcinoma: 0 (0) | |
| Intraoperative parametrial Invasion n (%) | 2 (6) | 1(4) | 1(9) | 0.601 |
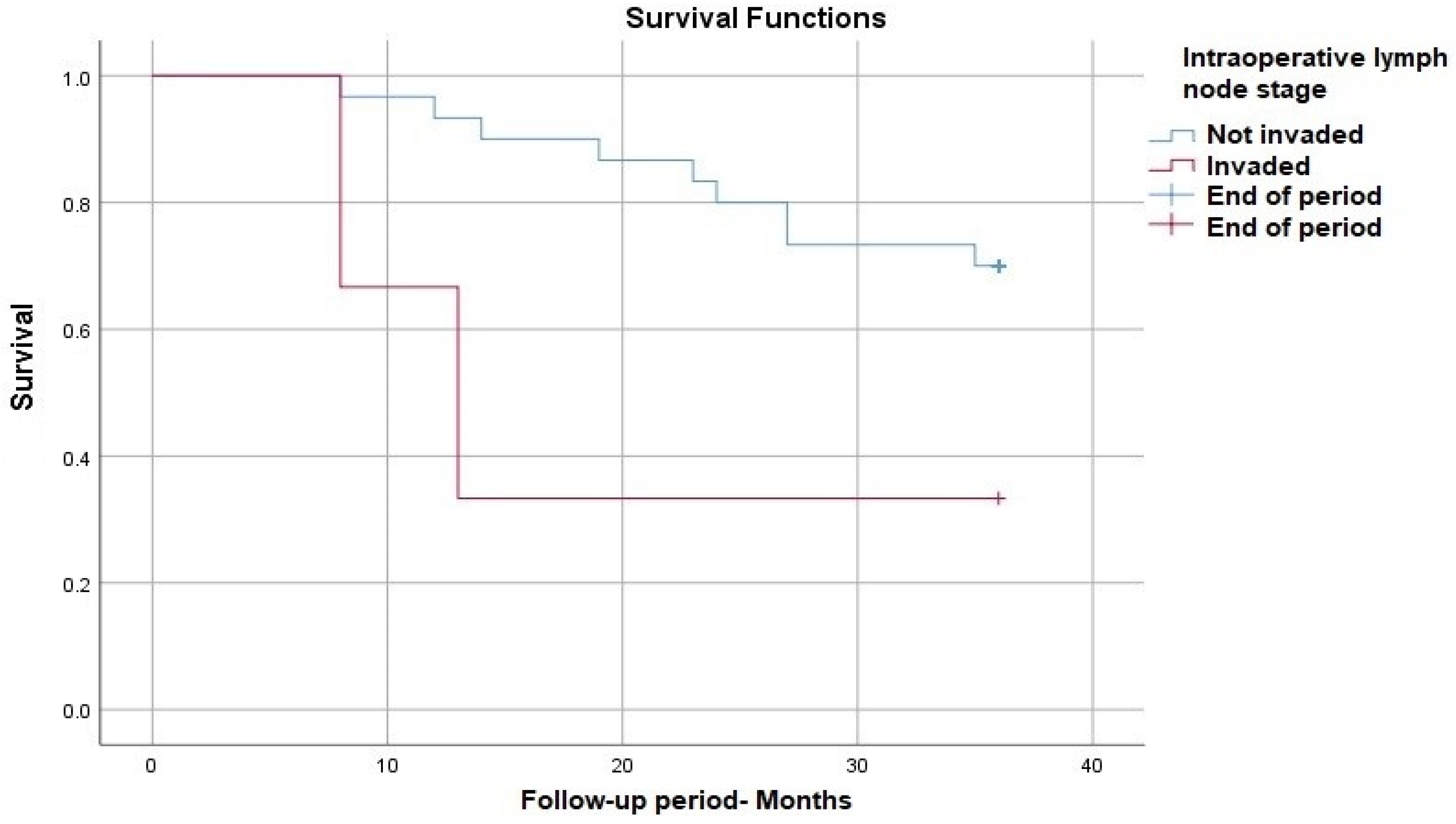
| Positive intraoperative lymph nodes, n (%) | 3 (9) | 1 (4) | 2 (18) | 0.252 |
| Lymphovascular invasion, n (%) | 7 (21) | 3 (13) | 4 (36) | 0.186 |
| Factors | HR | CI | p-Value |
|---|---|---|---|
| Age | 0.987 | 0.935–1.041 | 0.621 |
| Environment | 1.001 | 0.292–3.423 | 0.999 |
| Histological types of cancer, (biopsy) | 1.241 | 0.159–9.706 | 0.979 |
| Pre-RT hemoglobin | 0.702 | 0.397–0.912 | 0.005 |
| Initial Tumor size | 1.746 | 1.248–2.441 | 0.001 |
| Pre-RT parametrial invasion | 2.445 | 0.647–9.227 | 0.187 |
| RT dose | 0.970 | 0.792–1.187 | 0.766 |
| Nr. of RT sessions | 0.971 | 0.689–1.368 | 0.867 |
| Post-RT Lymphadenopathy | 3.186 | 0.835–12.153 | 0.09 |
| Post-RT FIGO | 2.965 | 0.972–7.842 | 0.573 |
| Sensitization chemotherapy | 0.937 | 0.761–1.155 | 0.543 |
| Pre-RT hemoglobin | 0.702 | 0.397–0.912 | 0.005 |
| Preoperative hemoglobin | 0.506 | 0.325–0.789 | 0.003 |
| Preoperative leukocyte count | 1.153 | 0.895–1.486 | 0.271 |
| Intraoperative FIGO | 47.447 9.203 | 3.078–731.400 1.529–55.372 | Stage IIIA: 0.006 Stage IIIC1: 0.01 |
| Intraoperative parametrial invasion | 2.162 | 0.275–16.970 | 0.600 |
| Positive intraoperative lymph nodes | 4.064 | 0.862–19.164 | 0.076 |
| Postoperatively chemotherapy | 2.617 | 0.795–8.610 | 0.113 |
| Lymphovascular invasion | 3.04 | 0.885–10.442 | 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dicu-Andreescu, I.-G.; Marincaș, M.-A.; Simionescu, A.-A.; Dicu-Andreescu, I.; Ionescu, S.-O.; Prunoiu, V.-M.; Brătucu, E.; Simion, L. The Role of Lymph Node Downstaging Following Neoadjuvant Treatment in a Group of Patients with Advanced Stage Cervical Cancer. Medicina 2024, 60, 871. https://doi.org/10.3390/medicina60060871
Dicu-Andreescu I-G, Marincaș M-A, Simionescu A-A, Dicu-Andreescu I, Ionescu S-O, Prunoiu V-M, Brătucu E, Simion L. The Role of Lymph Node Downstaging Following Neoadjuvant Treatment in a Group of Patients with Advanced Stage Cervical Cancer. Medicina. 2024; 60(6):871. https://doi.org/10.3390/medicina60060871
Chicago/Turabian StyleDicu-Andreescu, Irinel-Gabriel, Marian-Augustin Marincaș, Anca-Angela Simionescu, Ioana Dicu-Andreescu, Sînziana-Octavia Ionescu, Virgiliu-Mihail Prunoiu, Eugen Brătucu, and Laurențiu Simion. 2024. "The Role of Lymph Node Downstaging Following Neoadjuvant Treatment in a Group of Patients with Advanced Stage Cervical Cancer" Medicina 60, no. 6: 871. https://doi.org/10.3390/medicina60060871
APA StyleDicu-Andreescu, I.-G., Marincaș, M.-A., Simionescu, A.-A., Dicu-Andreescu, I., Ionescu, S.-O., Prunoiu, V.-M., Brătucu, E., & Simion, L. (2024). The Role of Lymph Node Downstaging Following Neoadjuvant Treatment in a Group of Patients with Advanced Stage Cervical Cancer. Medicina, 60(6), 871. https://doi.org/10.3390/medicina60060871








